Abstract
Introduction:
Diabetes is associated with anxiety and depression. Resveratrol, one of the most potent natural polyphenols with antioxidant properties, has been demonstrated to have benefits against diabetes. In the current study, we investigated the effects of resveratrol on depression and anxiety-like behaviors in diabetic rats.
Methods:
Adult male Wistar albino rats were assigned for control and diabetic groups, and these groups were divided into four subgroups as follows: Saline-treated, DMSO-treated, resveratrol-treated and imipramine-treated animals (n=10). Diabetes was induced by a single intraperitoneal injection of streptozotocin (STZ) (50 mg/kg), and 2 days after the STZ injection the rats having hyperglycemia (>300 mg/dl) were assigned to be diabetic. Rats in treatment groups were injected intraperitoneally with resveratrol (20 mg/kg) and imipramine (10 mg/kg) for 4 weeks. After 4-week-treatment period, tail suspension test (TST), forced swimming test (FST), elevated plus maze test (EPM) and locomotor activity test were performed. Blood samples were collected to estimate serum superoxide dismutase (SOD) and NADPH oxidase (Nox) levels.
Results:
Diabetic rats displayed depressive-like behaviors in the FST and TST, and anxiety-like behaviors in the EPM. Resveratrol and imipramine decreased anxiety-like and depressive-like behaviors without affecting locomotor activity in diabetic rats. A significant reduction in SOD levels and a marked increase in Nox levels were observed in diabetic rats. Resveratrol treatment normalized these levels, while imipramine did not affect neither SOD nor Nox levels.
Conclusion:
This study indicates that chronic resveratrol treatment may able to treat comorbid anxiety-and depressive-like behaviors in diabetes through inhibition of oxidative stress.
Keywords: Diabetes, depression, anxiety, resveratrol
INTRODUCTION
Diabetes mellitus is a chronic and life-threatening global health problem with its growing prevalence in the modern era. Patients with diabetes frequently suffer about twice from depression and anxiety compared with nondiabetic populations (1). Multiple studies have reported that psychosomatic processes contribute to several consequences of diabetes, and depression has been shown to negatively influence the prognosis and management of diabetes through its effects on factors such as metabolic control (2) and body mass index (3). Accordingly, the presence of comorbid depression in patients with diabetes generally leads to poor quality of life, reduced treatment adherence, excess morbidity and mortality rates (4).
Diabetes-induced depression may emerge due to reduced quality of life depending on the treatment, or may be an outcome of the biochemical alterations accompanying the disease (4). Many studies have aimed to uncover the pathophysiological relationship between diabetes and depression. It has been proposed that decreased brain monoamine levels, the alterations in the function of HPA axis, neuronal loss, impaired synaptic plasticity, enhanced oxidative stress and immune system plays a crucial role in the development of diabetes associated depression (5–7). Among them, elevated oxidative stress due to persistent hyperglycemia, which impairs the antioxidant defense system and thereby induces denovo free radical formation, is one of the main features of diabetes and has been established to be involved in the pathogenesis of some types of diabetes (8). Moreover, there is growing evidence showing increased oxidative stress in patients with major depression (9). On the other hand, inflammatory alterations due to chronic hyperglycemia have been reported to affect psychoneuroimmune process. Several studies revealed that depression is related with the upregulation of the immune system, including enhanced generation of proinflammatory cytokines (10). Thus, it is logical to investigate the effects of a compound with antioxidant, anti-inflammatory properties on diabetes-induced depression.
Resveratrol is one of the most potent natural polyphenols enriched in the skin of red wine and grapes. It is able to cross the blood-brain barrier and elicits several biological and pharmacological effects such as antioxidant, anti-inflammatory and neuroprotective (11). Moreover, resveratrol has been reported to have benefits against diabetes and its complications (12). There is also increasing evidence indicating that resveratrol exhibits antidepressant-like effects in animal experimental models through the regulation of oxidative stress and mTOR pathway (13), modulation of inflammatory cytokines (14), regulation of central serotonin/noradrenalin levels (15) and activation of BDNF levels in brain (16). Besides, the antidepressant effects of resveratrol have been demonstrated in humans (17). However, to date, no studies assessed the effects of resveratrol on diabetes-induced anxiety and depression. Therefore, the goal of our research was to explore the effects of resveratrol on depression-and anxiety-like behaviors in streptozotocin (STZ)-induced diabetic rats. We hypothesized that resveratrol may attenuate oxidative stress levels, improving depressive and anxious behavior in STZ-induced diabetes. So we evaluated superoxide dismutase (SOD) and NADPH oxidase (Nox) levels of the rats.
METHODS
Animals and Experimental Design
Adult male Wistar albino rats provided from the Experimental Medical Research and Application Center of our institution. The rats were maintained under standard laboratory conditions of controlled humidity (45%), temperature (22±2oC) and lighting (from 07:00 to 19:00). The animals were housed in an animal colony (~5 to 6 per cage) starting at least one week before the experiments. Behavioral tests were performed between 09:00 and 12:00. Food and water were provided without restrictions. The experimental protocol in the current study was performed in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (NIH publication No. 8023) and Regulation of Animal Research Ethics Committee in our country (July 6, 2006, Number 26220), and approved by Animal Experiments Local Ethics Committee of our institution (Decision No: KOÜ HADYEK 2/1–2017; decision date: 09/02/2017).
Wistar rats were randomly assigned into two main groups (n=10): control and diabetic (STZ) groups. Control groups were divided into four subgroups as follow: Saline-control, DMSO-control, imipramine-control and resveratrol-control. Diabetic groups were also separated into four subgroups: Saline-STZ, DMSO-STZ, imipramine-STZ and resveratrol-STZ. Diabetes was induced by a single intraperitoneal injection of STZ (50 mg/kg). Blood samples were taken from the tail vein 2 days (48 h) after the STZ injection and the animals having hyperglycemia (>300 mg/dl) were assigned to be diabetic. Saline-control and saline-STZ groups received physiological saline. DMSO-control and DMSO-STZ groups received 5% DMSO. Animals in treatment groups were injected intraperitoneally with resveratrol (20 mg/kg) and imipramine (10 mg/kg) for 4 weeks. Resveratrol was dissolved in 5% DMSO and imipramine was dissolved in physiological saline. All injections were administered in a volume of 2 ml/kg body weight of the rats. After 4-week-treatment period, to evaluate the antidepressant-and anxiolytic-like effects of resveratrol in STZ-induced diabetic rats; tail suspension test (TST), forced swimming test (FST), elevated plus maze test (EPM) and locomotor activity test were performed. 24 h after the last test, rats were decapitated under ketamine/xylazine anaesthesia (90 mg/kg/10 mg/kg) and blood samples were collected for the biochemical analyses.
Tail Suspension Test
The TST was conducted as previously reported (18). Briefly, animals were separated from each other and suspended by the tail to a horizontal bar (50 cm above the floor) using a hook that was both acoustically and visually isolated. The test was videotaped and the immobility time was recorded over 6 min. Absence of any limb or body movement was considered as immobility.
Forced Swimming Test
The test was performed as previously reported by Porsolt et al (19). The experimental procedure in the FST comprised two trials (the pretest and the test) with identical apparatus and conditions (38 cm diameter, 47 cm height, containing of water maintained at 22±1°C). During the pretest trial, animals were placed into the cylinder and forced to swim for 15 min. After the pretest trial, animals were placed into a warm cage before being returned to their home cages. 24 h after the pretest trial, the test trial was performed. Animals were placed in the same apparatus again and left for a 5-min test trial. During the test trial, the duration of immobility and the onset of immobility (latency) were recorded by an observer blind to the treatment conditions.
Elevated Plus Maze Test
The test was performed as previously reported (20). The EPM apparatus consists of 2 opposing closed arms (10 × 50 cm) and 2 opposing open arms (50 × 10 x 50 cm) connected by a central square (10 × 10 cm). During test session animals were gently placed on the central square, facing an open arm and allowed to freely explore the maze for 5 min. The animal was assessed to have entered an arm when all 4 limbs were inside the arm. After each trial, the EPM apparatus was wiped clean. The parameters such as time spent in and the percentage of entries into the open arms were evaluated.
Locomotor Activity
Locomotor activity was measured in a Plexiglas chamber (Commat Ltd., Ankara, Turkey) equipped with a 15-beam array of infrared horizontal (mounted every 2.5 cm, bottom) and vertical (mounted every 4.5 cm, upper) movement detectors. We evaluated the total locomotor activity as the sum of vertical, ambulatory and stereotypic activities. The activity was monitored continuously for 10 min following acclimation to the test room for a period of an hour.
Biochemical Analysis of Superoxide Dismutase and NADPH Oxidase
Blood samples were collected from terminally anaesthetized rats. The serum was prepared by centrifugation at 1000 g for 15 min at 4°C and stored at −40°C for biochemical analysis. The circulating levels of SOD and Nox were analyzed with ELISA kits by Alisei Quality System Seac Radin Company analyzer. Assays were performed strictly according to the manufacturer’s protocol (Elabscience, Bethesda, USA).
Statistical Analysis
Data in this study were expressed as mean ± standard error of the mean (SEM). The program Graph Pad Prism 5.0 (GraphPad Software Inc., San Diego, CA, USA) was used for statistical analysis. Statistical comparisons between the groups were performed by one-way ANOVA plus Tukey’s post hoc test. P<0.05 was assumed to be statistically significant.
Drugs
Resveratrol, imipramine, STZ and DMSO were obtained (purchased) from Sigma-Aldrich Chemical Co. (St. Louis, USA).
RESULTS
Resveratrol Alleviated Blood Glucose Levels and Body Weight Loss in STZ-Induced Diabetic Rats
We observed markedly increased blood glucose levels in all diabetic groups 48 h after the STZ injection (p<0.0001; Fig. 1). These levels remained markedly high in the diabetic groups compared to control groups at day 30 (p<0.0001; Fig. 1), but resveratrol treatment significantly decreased the blood glucose levels in diabetic rats compared to the STZ-alone-treated diabetic rats (p<0.05; Fig. 1). Imipramine did not alter these levels in diabetic rats and no significant differences were observed between all control groups in terms of blood glucose levels (p>0.05; Fig. 1).
Figure 1.
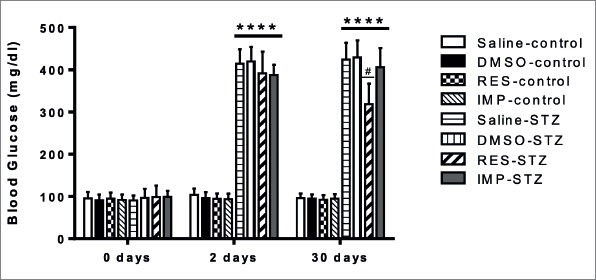
Effects of resveratrol and imipramine on blood glucose levels. After STZ (50 mg/kg) injection, blood glucose levels at day 2 and day 30 were determined. Each value represents the the mean ± SEM. ****P<0.0001 versus control groups and #P<0.05 versus saline-STZ and DMSO-STZ groups.
The initial body weights at the beginning of the experiments were similar in all experimental groups (p>0.05; Fig. 2). The final body weights of four diabetic groups displayed a significant decrease compared with the control groups (p<0.0001; Fig. 2). Weight loss in resveratrol-treated-diabetic rats was significantly less compared with the STZ-alone-treated diabetic group (p<0.05; Fig. 2). No significant difference was observed in final body weights between all control groups (p>0.05; Fig. 2).
Figure 2.
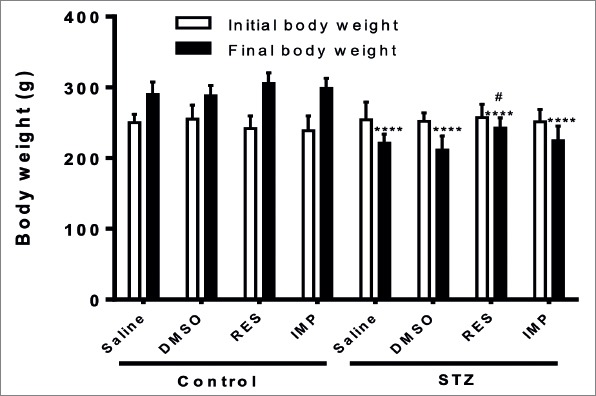
Effects of resveratrol and imipramine on body weight. Initial (day 0) and final (day 30) body weights were measured. Each value represents the mean ± SEM. ****P<0.0001 versus control groups and #P<0.05 versus saline-STZ and DMSO-STZ groups.
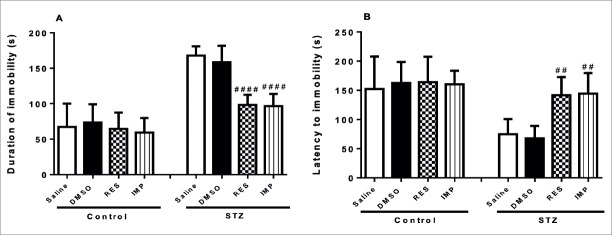
Resveratrol Decreased Depressive-Like Behaviors in the TST in STZ-Induced Diabetic Rats
In the TST, the immobility time were similar in all control groups. DMSO, chronic treatment with resveratrol and imipramine showed no effects on immobility time in non-diabetic rats (p>0.05; Fig. 3). However, resveratrol and imipramine significantly decreased the immobility time in diabetic animals (p<0.001 and p<0.01, respectively; Fig. 3). Moreover, DMSO had no effects on immobility time in diabetic rats (p>0.05; Fig. 3).
Figure 3.
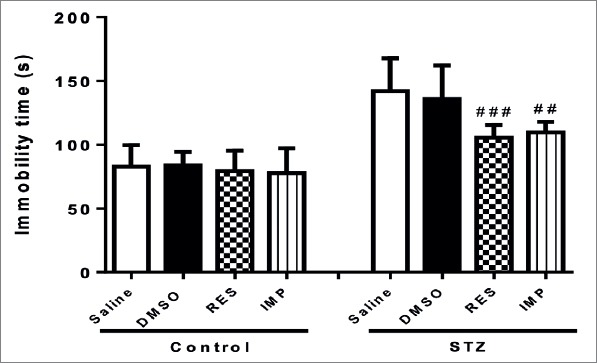
Effects of resveratrol and imipramine in diabetic rats on the immobility time evaluated during the tail suspension test (TST). Each value represents the mean ± SEM. ##P<0.01 and ###P<0.001 versus saline-STZ and DMSO-STZ groups.
Resveratrol Decreased Depressive-Like Behaviors in the FST in STZ-Induced Diabetic Rats
As shown in Fig. 4A, FST results showed that DMSO, chronic treatment with resveratrol and imipramine did not affect the duration of immobility in the control rats (p>0.05; Fig. 4A). However, treatment with resveratrol markedly decreased the duration of immobility in diabetic rats (p<0.0001; Fig. 4A). Similar results were obtained with the imipramine administration (p<0.0001; Fig. 4A). DMSO had no effects on the duration of immobility in diabetic rats (p>0.05; Fig. 4A). As indicated in Fig. 4B, in the experiment performed with non-diabetic rats, DMSO, resveratrol and imipramine treatment showed no effects on the latency to be immobile (p>0.05; Fig. 4B). However, a significant increase was observed in latency to immobility in resveratrol-treated and imipramine-treated diabetic rats (p<0.01; Fig. 4B), while DMSO showed no effects on latency to immobility in diabetic rats (p>0.05; Fig. 4B).
Figure 4.
Effects of resveratrol and imipramine in diabetic rats on the duration of immobility (A) and latency to immobility (B) evaluated during the forced swimming test (FST). Each value represents the mean ± SEM. ##P<0.01 and archneuro-56-144-g004.jpgP<0.0001 versus saline-STZ and DMSO-STZ groups.
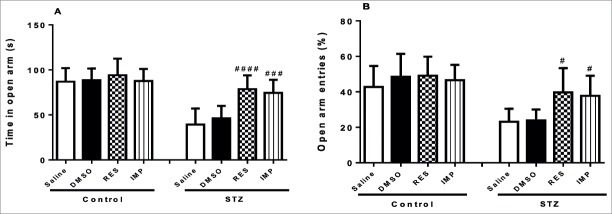
Resveratrol Diminished Anxiety-Like Behaviors in the EPM in STZ-Induced Diabetic Rats
In the EPM, DMSO, treatment with resveratrol and imipramine in non-diabetic rats had no influence on the the time spent in (p>0.05; Fig. 5A) as well as the percentage of entries into the open arms (p>0.05; Fig. 5B). However, chronic resveratrol and imipramine treatment significantly enhanced the time spent in (p<0.0001 and p<0.001, respectively; Fig. 5A) and the percentage of entries into (p<0.05; Fig. 5B) the open arms in the STZ-induced diabetic animals, while DMSO did not affect these parameters in these animals (p>0.05; Fig 5A and Fig. 5B).
Figure 5.
Effects of resveratrol and imipramine in diabetic rats on the time spent in (A) and the percentage of entries into (B) the open arms evaluated during the elevated plus maze test (EPM). Each value represents the mean ± SEM. #P<0.05, ###P<0.001 and archneuro-56-144-g006.jpgP<0.0001 versus saline-STZ and DMSO-STZ groups.
Resveratrol Did Not Affect on Locomotor Activity in STZ-Induced Diabetic Rats
There was no significant difference between animals treated with DMSO, resveratrol and imipramine in terms of the total locomotor activity (p>0.05; Fig. 6), showing that alterations in behavioral tests did not observe due to the changes in locomotor activity.
Figure 6.
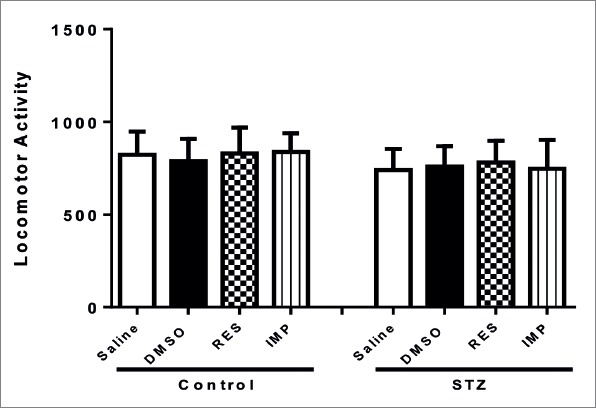
Effects of resveratrol and imipramine in diabetic rats on the locomotor activity test. Each value represents the mean ± SEM.
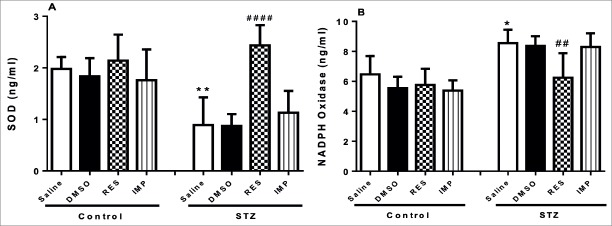
Resveratrol Increased Superoxide Dismutase and Decreased NADPH Oxidase Levels in STZ-Induced Diabetic Rats
STZ-alone-treated animals exerted a significant reduction in circulating levels of SOD (p<0.01; Fig. 7A). These levels in diabetic rats were significantly increased by resveratrol treatment (p<0.0001; Fig. 7A). However, DMSO and imipramine did not alter SOD levels in diabetic rats (p>0.05; Fig. 7A). Additionally, DMSO, treatment with resveratrol and imipramine in control animals did not affect the basal levels of SOD (p>0.05; Fig. 7A).
Figure 7.
Effects of resveratrol and imipramine in diabetic rats on the levels of superoxide dismutase (SOD) (A) and NADPH Oxidase (B). Each value represents the mean ± SEM. *P<0.05, **P<0.01 versus saline-control group and ##P<0.01, archneuro-56-144-g###.jpgP<0.0001 versus saline–STZ and DMSO–STZ groups.
There was a significant rise in systemic Nox levels in STZ-alone-treated diabetic rats compared to the control rats (p<0.05; Fig. 7B), while resveratrol treatment significantly diminished the elevation of Nox in STZ-induced diabetic rats (p<0.01; Fig. 7B). However, increased Nox levels in diabetic rats were not influenced by imipramine treatment and DMSO (p>0.05; Fig. 7B). DMSO, treatment with resveratrol and imipramine in control animals did not change the basal levels of Nox in control animals (p>0.05; Fig. 7B).
DISCUSSION
Because resveratrol has a variety of biological activities, its therapeutic properties were investigated. Here, we demonstrated that resveratrol has antidepressant-and anxiolytic-like effect in STZ-induced diabetic animals, as demonstrated by the decline in immobility behaviors in the FST and TST and, the reduction in anxious behaviors in the EPM. These effects were not observed in the normal control rats, indicating diabetes-specific effect of resveratrol. We have also used imipramine as a standard drug to compare the activity of resveratrol, and we observed similar antidepressant and anxiolytic-like effects displayed by imipramine in these rats.
Experimental STZ-induced diabetes is a widely used diabetic model that involved in elucidating the underlying mechanisms of diabetes and the associated central nervous system dysfunctions. In the current study, we induced diabetes by a single injection of STZ (i. p., 50 mg/kg) and we observed a marked increase in blood glucose levels of animals after 2 days. Induction of Type-I diabetes was associated with diminished body weight as reported earlier (21). Accordingly, we observed a significant decrease of body weight in all diabetic groups. Weight loss and enhanced plasma glucose levels that occurred in diabetic rats were attenuated by resveratrol treatment. These effects of resveratrol might be attributable either to increase in glucose uptake, utilization and storage or to restoration of abnormal insulin signaling pathways and increase in insulin secretion and/or sensitivity (12). However, imipramine treatment did not decrease weight gain or improve the high blood glucose levels observed in diabetic animals. This finding is consistent with other studies (6, 22).
Depressive-like behaviors have been demonstrated in STZ-induced diabetic animals (23). The TST and FST are well known rodent behavioral tests used routinely to evaluate antidepressant activity (24). This study indicated that STZ resulted in a significant increase in the immobility behavior of rats in the TST and FST. The immobility observed in the FST reflects the state of hopelessness or lowered mood (19). Our findings are parallel to previous reports which showed the development of behavioral despair in STZ-induced diabetes (25). Clinically useful antidepressant drugs are known to decrease the duration of immobility in the FST and TST (26). In the current study, FST results demonstrated that treatment with resveratrol reduced the duration of immobility in STZ-induced diabetic rats. Besides, latency to immobility, which is the duration elapsed from the first entry of the animal into the water until the first moment of immobility, is also an important parameter in the FST (27). Resveratrol also increased the latency to immobility in diabetic rats. This behavior, together with the immobility time indicates the antidepressant-like effect of resveratrol. Moreover, we confirmed the antidepressant activity of resveratrol in STZ-induced diabetes as showed by a marked decline in the immobility time in the TST.
Imipramine treatment also decreased the duration of immobility in diabetic rats in the TST and FST. These results of our study are in accordance with previous reports, which revealed the antidepressant properties of imipramine in STZ-induced diabetes (22). Additionally, neither resveratrol nor imipramine produced any significant alterations in the locomotor activity of animals. The ability of resveratrol and imipramine to diminish the duration of immobility in diabetic rats therefore suggests the possession of their antidepressant-like effects in animals. On the other hand, in control animals, resveratrol and imipramine had no effect in the FST and TST, showing that these drugs display antidepressant-like effect in only diabetic condition.
Anxiety-like behaviors have been reported in STZ-induced diabetic rats (28). The EPM is a well-known assay in which anxious behaviors are reflected in decreases in both the time spent in and the number of entries into the open arms (29). The anxiolytic-like effects of resveratrol in diabetic rats were assessed using EPM. We found that diabetic rats displayed anxiety-like behaviors as shown by a reduction in the percentage of entries into and the time spent in the open arms. In addition to its antidepressant-like effect, resveratrol also exerted anxiolytic-like effect in diabetic animals. Our findings are corroborated a previous report which demonstrated anxiolytic role shown by resveratrol in prediabetic rats (30). Moreover, imipramine enhanced the percentage of entries into and the time spent in the open arms in the EPM. This evidence demonstrated its anxiolytic-like effects in STZ-induced diabetic rats. However, in the EPM, neither resveratrol nor imipramine affected the percentage of entries into and the time spent in the open arms in control rats. These findings suggest that anxiolytic-like effects of these drugs occur when the animals were diabetic.
It has been well established that persistent hyperglycemia increases oxidative stress, which results from enhancing of reactive oxygen species generation and/or impairment of antioxidant defenses (8). Elevated oxidative stress has been suggested to play a crucial role on the pathophysiology of depression and anxiety (9). Resveratrol possesses antioxidant activities in the central nervous system, demonstrated by the ability to up-regulate antioxidant enzymes and scavenge free radicals (11). In the current report, STZ-induced diabetes displayed a marked decrease in SOD and a significant increment in Nox levels. However, all of these effects were reversed by resveratrol in diabetic rats. Thus, this evidence suggested that the anxiolytic-and antidepressant-like properties of resveratrol in STZ-induced diabetes might be due to its inhibitory effects on oxidative stress. On the other hand, when considering the weight loss and blood glucose levels were lower in resveratrol-treated diabetic rats, it can be speculated that these anxiolytic-and antidepressant-like effects may depend on these properties of resveratrol. However, clinically used antidepressant drug imipramine decreased neither weight loss nor blood glucose levels in diabetic rats. Thus, the mechanisms under the behavioral beneficial effects of resveratrol on diabetes need to be further investigated. Moreover, imipramine did not change SOD and Nox levels in diabetic rats.
In summary, the results of the present study revealed that resveratrol exhibited anxiolytic-and antidepressant-like effect in diabetic animals that may be due to its ability to suppress oxidative stress. However, our limitation of the study is not to show the antioxidant effect of resveratrol on brain. Further researches are needed to elucidate the mechanisms underlying diabetes-induced depression and anxiety.
Footnotes
Ethics Committee Approval: Ethical approval was granted by the Kocaeli University Animal Research Ethics Committee.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - TDŞ, SSG, FCE, TU; Design - TDŞ, SSG, FCE, TU; Supervision - TDŞ, SSG, FCE, TU; Resource - SSG, FCE; Materials - TU; Data Collection and/ or Processing - TDŞ, SSG, FCE; Analysis and/or Interpretation - TDŞ, SSG; Literature Search - TDŞ; Writing - TDŞ; Critical Reviews -TU.
Conflicts of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declare that this study has received no financial support.
REFERENCES
- 1.Semenkovich K, Brown ME, Svrakic DM, Lustman PJ. Depression in type 2 diabetes mellitus:prevalence, impact, and treatment. Drugs. 2015;75:577–587. doi: 10.1007/s40265-015-0347-4. [DOI] [PubMed] [Google Scholar]
- 2.Cherrington A, Wallston KA, Rothman RL. Exploring the relationship between diabetes self-efficacy, depressive symptoms, and glycemic control among men and women with type 2 diabetes. J Behav Med. 2010;33:81–89. doi: 10.1007/s10865-009-9233-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Wit LM, van Straten A, van Herten M, Penninx BW, Cuijpers P. Depression and body mass index, a u-shaped association. BMC Public Health. 2009;9:14. doi: 10.1186/1471-2458-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schram MT, Baan CA, Pouwer F. Depression and quality of life in patients with diabetes:a systematic review from the European depression in diabetes (EDID) research consortium. Curr Diabetes Rev. 2009;5:112–119. doi: 10.2174/157339909788166828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beauquis J, Homo-Delarche F, Giroix MH, Ehses J, Coulaud J, Roig P, Portha B, De Nicola AF, Saravia F. Hippocampal neurovascular and hypothalamic-pituitary-adrenal axis alterations in spontaneously type 2 diabetic GK rats. Exp Neurol. 2010;222:125–134. doi: 10.1016/j.expneurol.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 6.de Morais H, de Souza CP, da Silva LM, Ferreira DM, Werner MF, Andreatini R, da Cunha JM, Zanoveli JM. Increased oxidative stress in prefrontal cortex and hippocampus is related to depressive-like behavior in streptozotocin-diabetic rats. Behav Brain Res. 2014;258:52–64. doi: 10.1016/j.bbr.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 7.Prabhakar V, Gupta D, Kanade P, Radhakrishnan M. Diabetes-associated depression:the serotonergic system as a novel multifunctional target. Indian J Pharmacol. 2015;47:4–10. doi: 10.4103/0253-7613.150305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maritim AC, Sanders RA, Watkins JB., 3rd Diabetes, oxidative stress, and antioxidants:a review. J Biochem Mol Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 9.Palta P, Samuel LJ, Miller ER, 3rd, Szanton SL. Depression and oxidative stress:results from a meta-analysis of observational studies. Psychosom Med. 2014;76:12–19. doi: 10.1097/PSY.0000000000000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schiepers OJ, Wichers MC, Maes M. Cytokines and major depression. Prog Neuro-Psychopharmacol Biol Psychiatry. 2005;29:201–217. doi: 10.1016/j.pnpbp.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Orsu P, Murthy BV, Akula A. Cerebroprotective potential of resveratrol through anti-oxidant and anti-inflammatory mechanisms in rats. J Neural Transm. 2013;120:1217–1223. doi: 10.1007/s00702-013-0982-4. [DOI] [PubMed] [Google Scholar]
- 12.Oyenihi OR, Oyenihi AB, Adeyanju AA, Oguntibeju OO. Antidiabetic Effects of Resveratrol:The Way Forward in Its Clinical Utility. J Diabetes Res. 2016;2016:9737483. doi: 10.1155/2016/9737483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu S, Li T, Liu H, Wang X, Bo S, Xie Y, Bai X, Wu L, Wang Z, Liu D. Resveratrol exerts antidepressant properties in the chronic unpredictable mild stress model through the regulation of oxidative stress and mTOR pathway in the rat hippocampus and prefrontal cortex. Behav Brain Res. 2016;302:191–199. doi: 10.1016/j.bbr.2016.01.037. [DOI] [PubMed] [Google Scholar]
- 14.Finnell JE, Lombard CM, Melson MN, Singh NP, Nagarkatti M, Nagarkatti P, Fadel JR, Wood CS, Wood SK. The protective effects of resveratrol on social stress-induced cytokine release and depressive-like behavior. Brain Behav Immun. 2017;59:147–157. doi: 10.1016/j.bbi.2016.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu Y, Wang Z, You W, Zhang X, Li S, Barish PA, Vernon MM, Du X, Li G, Pan J, Ogle WO. Antidepressant-like effect of trans-resveratrol:involvement of serotonin and noradrenaline system. Eur Neuropsychopharmacol. 2010;20:405–413. doi: 10.1016/j.euroneuro.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Pang C, Cao L, Wu F, Wang L, Wang G, Yu Y, Zhang M, Chen L, Wang W, Lv W, Chen L, Zhu J, Pan J, Zhang H, Xu Y, Ding L. The effect of trans-resveratrol on post-stroke depression via regulation of hypothalamus-pituitary-adrenal axis. Neuropharmacology. 2015;97:447–456. doi: 10.1016/j.neuropharm.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 17.Davinelli S, Scapagnini G, Marzatico F, Nobile V, Ferrara N, Corbi G. Influence of equol and resveratrol supplementa- tion on health-related quality of life in menopausal women:a randomized, placebo-controlled study. Maturitas. 2017;96:77–83. doi: 10.1016/j.maturitas.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 18.Steru L, Chermat R, Thierry B, Simon P. The tail suspension test:a new method for screening antidepressants in mice. Psychopharmacology (Berl) 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- 19.Porsolt RD, Le Pichon M, Jalfre M. Depression:a new animal model sensitive to antidepressant treatment. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 20.Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- 21.Su HC, Hung LM, Chen JK. Resveratrol, a red wine antioxidant, possesses an insulin-like effect in streptozotocin-induced diabetic rats. Am J Physiol Endocrinol Metab. 2006;290:E1339–E1346. doi: 10.1152/ajpendo.00487.2005. [DOI] [PubMed] [Google Scholar]
- 22.Rahul S, Vinay K, Dilpesha J, Kumar SA. Antidepressant activity of karnim in diabetes associated depression in experimental animals. Pharmacologia. 2012;3:413–419. [Google Scholar]
- 23.Hirano S, Miyata S, Kamei J. Antidepressant-like effect of leptin in streptozotocin-induced diabetic mice. Pharmacol Biochem Behav. 2007;86:27–31. doi: 10.1016/j.pbb.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Fuchs E, Flïugge G. Experimental animal models for the simulation of depression and anxiety. Dialogues Clin Neurosci. 2006;8:323–333. doi: 10.31887/DCNS.2006.8.3/efuchs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caletti G, Olguins DB, Pedrollo EF, Barros HM, Gomez R. Antidepressant effect of taurine in diabetic rats. Amino Acids. 2012;43:1525–1533. doi: 10.1007/s00726-012-1226-x. [DOI] [PubMed] [Google Scholar]
- 26.Kang S, Kim HJ, Kim HJ, Shin SK, Choi SH, Lee MS, Shin KH. Effects of reboxetine and citalopram pretreatment on changes in cocaine and amphetamine regulated transcript (CART) expression in rat brain induced by the forced swimming test. Eur J Pharmacol. 2010;647:110–116. doi: 10.1016/j.ejphar.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 27.Contreras CM, Martínez-Mota L, Saavedra M. Desipramine restricts estral cycle oscillations in swimming. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22:1121–1128. doi: 10.1016/s0278-5846(98)00066-9. [DOI] [PubMed] [Google Scholar]
- 28.Tang ZJ, Zou W, Yuan J, Zhang P, Tian Y, Xiao ZF, Li MH, Wei HJ, Tang XQ. Antidepressant-like and anxiolytic-like effects of hydrogen sulfide in streptozotocin-induced diabetic rats through inhibition of hippocampal oxidative stress. Behav Pharmacol. 2015;26:427–435. doi: 10.1097/FBP.0000000000000143. [DOI] [PubMed] [Google Scholar]
- 29.Lee B, Sur B, Kwon S, Yeom M, Shim I, Lee H, Hahm DH. Chronic administration of catechin decreases depression and anxiety-like behaviors in a rat model using chronic corticosterone injections. Biomol Ther (Seoul) 2013;21:313–322. doi: 10.4062/biomolther.2013.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reddy BR, Maitra S, Jhelum P, Kumar KP, Bagul PK, Kaur G, Banerjee SK, Kumar A, Chakravarty S. Sirtuin 1 and 7 mediate resveratrol-induced recovery from hyper-anxiety in high fructose-fed prediabetic rats. J Biosci. 2016;41:407–417. doi: 10.1007/s12038-016-9627-8. [DOI] [PubMed] [Google Scholar]