Abstract
Transthyretin-related familial amyloid polyneuropathy (TTR-FAP) is a life-threatening disease caused by the accumulation of amyloidogenic transthyretin (TTR) protein in tissues. Mutations in TTR gene destabilize TTR protein to misfold from its native tetramer form to amyloidogenic monomer form. In endemic countries, TTR-FAP presents with length-dependent small fiber neuropathy, however in non-endemic countries clinical features can be highly variable. Genetic testing for TTR gene is mandatory for the diagnosis. Demonstrating amyloid deposits in tissues may be necessary for distinguishing symptomatic patients from asymptomatic carriers. Routine follow-up should include a wide range of tests to demonstrate systemic involvement. In recent years, treatment of TTR-FAP has significantly improved with new therapeutic approaches. TTR stabilizers and TTR-gene silencing drugs prevent the progression of the disease. Monoclonal antibodies that target amyloid deposits are currently under development. Early initiation of the treatment is important for better functional outcome.
Keywords: Transthyretin, genetic, hereditary, treatment, neuropathy
INTRODUCTION
More than 65 years ago, Andrade described the first case of transthyretin related familial amyloid polyneuropathy (TTR-FAP) in a 37-year-old Portuguese female and called this peculiar neuropathy “mal dos pesinhos” (1). It is an autosomal dominant disorder caused by mutations of the transthyretin (TTR) gene. Mutant TTR protein dissociates from its native tetramer form, gains a toxic function and aggregates in several tissues and organs. If left untreated, the disease can be fatal due to cachexia, infections or cardiac dysfunction. Mean survival time differs 7–11 years (2, 3).
Encoded by chromosome 18, TTR works as a transport protein for vitamin A and thyroxin. It is primarily synthesized from the liver, though little amounts of TTR are produced from choroid plexus, intestines and retinal epithelium. More than 150 mutations are described so far in the TTR gene. The first described Val30Met is still the most common mutation worldwide. Regional differences have an influence on the frequency of the mutation and causes genotypic and phenotypic heterogeneity (4, 5).
Thirty years ago, the disease was thought to be restricted to Portugal, Sweden and Japan which are called as endemic regions. However, with improved access to genetic testing, now, we know that TTR-FAP is observed worldwide. Prevalence of the disease is highly variable between the endemic and non-endemic countries. In some regions of Portugal, where the disease is highly prevalent, it can be as high as 1/1000 to 1/10,000. Yet, in Japan even in endemic regions like Kumamoto, Nagano and Ishikawa, the national prevalence estimate is 0.99/1,000,000. Data from non-endemic countries is not yet sufficient to estimate an exact prevalence (6, 7).
Clinical Features
In the last two decades our knowledge about the clinical features of TTR amyloidosis has notably changed. With the identification of new mutation types, we now know that the presenting symptom, age at onset, type of the neuropathy and additional systemic involvement can be highly variable. Some mutation types may manifest with polyneuropathy while others, like Val122Ile, can cause a pure cardiac phenotype. Age at onset is also affected by the geographic area. It is remarkably earlier in patients from Portugal and Japan carrying Val30Met mutation, compared to patients from Sweden with the same mutation (33 vs 56) (3, 8). Age at onset is typically later and usually in the sixth or seventh decades, in patients carrying Val30Met mutation from non-endemic regions. For example, in the Turkish cohort, the mean age of onset for patients with the Val30Met mutation was 54.5±10.4 years. (9). Late-onset cases have a more severe disease course and less autonomic involvement compared to early-onset cases. There is a marked male predominance in late-onset cases with a 1/10 ratio. Lack of a family history and low penetrance rates are other highlighted features of the disease in non-endemic regions (Table 1) (6).
Table 1.
Clinical features of TTR-FAP*
| Endemic | Non-endemic | |
|---|---|---|
| Mutation type | Homogenous (Val30Met) | Heterogeneous |
| Age at onset | Portugal →Early-onset | Generally late-onset |
| Japan →Bimodal distribution | ||
| Sweden →Late-onset | ||
| Gender distribution | Homogenous in early-onset cases | Marked male predominance |
| Male predominance in late onset cases | ||
| Family history | Common in early-onset cases | Rare |
| Rare in late-onset cases | ||
| Neuropathy type | Length-dependent small-fiber sensory-motor polyneuropathy in early-onset | Affecting all fibers. Occasionally upper limb onset, motor neuropathy, ataxic neuropathy. |
| Loss of all sensory modalities and early distal weakness in late-onset cases | ||
| Autonomic involvement | Common and severe in early-onset | Rare |
| Rare in late-onset cases |
TTR-FAP=transthyretin related familial amyloid polyneuropathy
Adapted from6.
Length dependent sensory-motor neuropathy with autonomic involvement is the hallmark of the disease. At stage 1 of the disease, small fibers are typically affected and neuropathic pain may be the presenting symptom. At stage 2, neurologic signs and symptoms continue to worsen with sensory loss extending up to the proximal and distal weakness appears in lower extremities. Eventually, patients start to use aids to walk. Sensory deficit gradually extends first to distal parts and then, proximal parts of upper extremities and anterior trunk, respectively (10). At stage 3, patients are wheel-chair bound or bed-ridden (11). Rarely, the disease can start with upper extremity symptoms which can mimic motor neuron disease (8, 12). Polyneuropathy disability score (PND) is another scoring system depending on patient’s walking capacity. It consists of four stages: stage 1 (sensory disturbances without a deficiency in walking capacity); stage 2 (impaired walking without a need for a stick); stage 3 (preserved walking with the help of one stick (3A) or two sticks (3B)); and stage 4 (wheelchair bound or bedridden) (11). Neuropathy Impairment Score (NIS), NIS+7 and Norfolk Quality of Life (Norfolk QoL) are other quantitative scoring systems which are primarily used in drug development studies (Table 2) (13).
Table 2.
Severity scoring systems in TTR-FAP*
| FAP stage | PND score | ||
|---|---|---|---|
| Stage | Symptom | Score | Symptom |
| 0 | Asymptomatic | 1 | Sensory neuropathy |
| 1 | Sensory neuropathy | 2 | Motor symptoms but preserved walking |
| 2 | Require assistance for walking | 3A | Require one stick for walking |
| 3 | Bedridden or wheelchair bound | 3B | Require two sticks for walking |
| 4 | Bedridden or wheelchair bound | ||
TTR-FAP=transthyretin related familial amyloid polyneuropathy, FAP=familial amyloid polyneuropathy, PND=polyneurapathy disability
Adapted from11.
Autonomic involvement causes serious problems in the entire disease period. Patients may complain of diarrhea, impotence or orthostatism. Clinicians especially should interrogate about erectile dysfunction which can precede sensorial symptoms. In late stages, autonomic symptoms become more evident and may be life threatening (14).
Focal amyloid deposits in the transverse carpal ligament increases the liability for carpal tunnel syndrome. Some specific mutations, like Glu89Gln, are associated with the increased risk of carpal tunnel syndrome (9, 15, 16). It can manifest uni or bilaterally. Clinicians must consider the possibility of TTR-FAP, in patients with a polyneuropathy of unknown cause, accompanied by carpal tunnel syndrome, especially in men. Amyloid deposits may also accumulate at nerve plexuses, nerve trunks, or cranial nerves and cause various focal neurologic presentations (3).
Central nervous system (CNS) involvement is caused by accumulation of amyloidogenic TTR in the meninges and perivascular regions. Stroke-like, focal neurologic deficits, also named “amyloid spells” are the most common features in CNS involvement. Other rare presentations include dementia, hydrocephalus and myelopathy (17). Several mutations, like Gly53Glu, Leu12Pro, Asp18Gly, are associated with CNS amyloidosis (9, 18–20).
Cardiac involvement is the most common extra-neurological manifestation and observed in 80% of the patients. Common clinical features in cardiac amyloidosis include arrhythmias, syncope attacks due to conduction problems and heart failure caused by restrictive cardiomyopathy (21). Val122Ile, Ile68Leu and Thr60Ala are well-known mutations that predominantly induce cardiomyopathy (22, 23). Wild-type TTR accumulation may cause senile systemic amyloidosis, an acquired disorder mainly affecting men after the age of 60 years, presents with the symptoms of hypertrophic cardiomyopathy (24). Low-voltage in electrocardiogram (ECG), granular pattern of myocardium and increased ventricular wall thickness in echocardiogram are the common red-flags for the suspicion of cardiac TTR amyloidosis (25).
Ocular manifestations of TTR amyloidosis include vitreous deposits, abnormal conjunctival vessels, keratoconjunctivitis sicca, glaucoma and pupillary abnormalities. TTR amyloid can be visualized as cotton wool inclusions in the vitreous body (26). Renal involvement is uncommon but it can cause microalbuminuria and may precede the onset of the neuropathy. End-stage renal failure requiring dialysis is rare in TTR-FAP (27).
Diagnostic Techniques
Diagnosis of TTR-FAP should be analyzed in two separate groups. First group involves the patients with a known family history and the second group with sporadic presentation. In the first group, diagnosis is more straightforward, if the patient complains about the typical symptoms of TTR amyloidosis. On the other hand, in the second group, diagnosis is usually delayed, due to many diagnostic pitfalls and variable inaugural symptoms.
After a detailed examination, patients should undergo for electrodiagnostic evaluation. In the earliest stage, when small fiber neuropathy is the only and predominant symptom, nerve conduction studies may be normal. Yet, with the progression of the disease, length-dependent axonal polyneuropathy will be the prominent feature. However, observation of demyelinating features is not uncommon, which causes diagnostic challenges. Patients may even fulfill the European Federation of Neurological Societies/PNS definite criteria for chronic inflammatory demyelinating polyneuropathy (CIDP), which is one of the most common diagnostic errors in TTR-FAP (28). Another feature that may mimic CIDP, is the presence of elevated protein level in cerebrospinal fluid (CSF). Lumbar spinal stenosis, AL amyloidosis, toxic peripheral neuropathy, motor neuron disease and vasculitic peripheral neuropathy are the other most common misdiagnoses (5).
Diagnosis of small fiber neuropathy is crucial to find out early disease manifestations. Sympathetic skin responses and heart rate variability (HRV) are non-invasive and easy tests to assess the autonomic neuropathy. Sudoscan® is another diagnostic tool to identify sudomotor disfunction (29). Other emerging diagnostic techniques for detecting small fiber neuropathy include confocal corneal microscopy that measures nerve fiber length in cornea, quantitative sensory testing (QST) and laser Doppler flowmetry (30, 31). But still, skin biopsy remains as the gold standard to assess the density of small fibers (32).
Genetic testing for TTR is essential for the diagnosis, absence of a pathologic mutation excludes TTR-FAP. In patients with a positive family history, targeting that certain mutation type would be logical. Yet, in sporadic cases, particularly from non-endemic regions, sequencing the TTR gene may be required. An online registry that contains pathologic mutation types and their clinical features is a helpful diagnostic tool for clinicians (33).
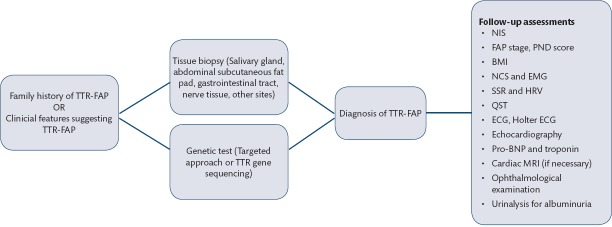
Even with the presence of a positive genetic test, demonstrating amyloid deposits in tissue is also important to distinguish symptomatic patients from carriers. Main sites for tissue biopsy are labial salivary gland, abdominal subcutaneous fat pad, gastrointestinal tract, nerve tissue, endo-myocardial tissue (Figure 1 and 2). Congo Red staining or polarized microscopic evaluation of the tissue reveals amyloid deposits (4). TTR immunolabeling of the amyloid deposits may be necessary to differentiate TTR amyloidosis from AL amyloidosis (34). One should keep in mind that negative biopsy does not exclude TTR-FAP.
Figure 1.

Sural nerve biopsy findings of a patient with Glu89Gln mutation. Note endoneural, perivascular amyloid deposits (arrow); there is a remarked reduction of myelinating fibres (semi-thin section ×40, Thionin staining). (Neuropathology Lab, Neuromuscular Unit of the Istanbul Faculty of Medicine)
Figure 2.

Sural nerve biopsy findings of a patient with Glu89Gln mutation. Note endoneural amyloid deposits (arrow) (longitudinal paraffin section ×10, modified Gomori trichrome staining). (Neuropathology Lab, Neuromuscular Unit of the Istanbul Faculty of Medicine)
ECG, Holter ECG, N-terminal of the prohormone brain natriuretic peptide (NT-proBNP), troponin, and echocardiography can be performed to assess cardiac involvement. Ophthalmological examination and detailed urinalysis are mandatory (Figure 3).
Figure 3.
Diagnostic techniques and diagnostic algorithm of TTR-FAP*
TTR-FAP: transthyretin-related familial amyloid polyneuropathy, TTR: transthyretin, NIS: neuropathy impaiment score, FAP: familial amyloid polyneuropathy, PND: polyneuropathy disability, BMI: body-mass intex, NCS: nerve conduction studies, EMG: electromyography, SSR: sympathetic skin response, HRV: heart rate variability, QST: quantitative sensory testing, ECG: electrocardiography, BNP: brain natriuretic peptide, MRI: magnetic resonance imaging. Adapted from5.
Scanning for asymptomatic carriers is very important for detecting patients in early stages of the disease. Asymptomatic carries should be followed up regularly with a frequency depending on the mutation type, age at onset of other affected family members and patient’s age (35). European network for TTR-FAP or amyloidosis (ATTReuNET), a group of experts on TTR amyloidosis suggest that, scanning of the disease should include detailed history and neurologic examination with the measurement of NIS, body-mass index (BMI), NCS and EMG, sympathetic skin response and HRV, urine for microalbuminuria and ECG (36). If these findings change compared to the baseline in the course of the follow-up, tissue biopsy should be performed to confirm the diagnosis.
After the definite diagnosis, follow-up examinations for systemic involvement should include wider spectrum of tests.
Current Disease Management and Emerging Therapeutic Options
Treatment of TTR-FAP has significantly improved in recent years with new therapeutic approaches. Liver transplantation (LT) loses its position as a standard treatment. Though, in some countries, like Japan, it is still the first line treatment option (5). Primary aim of LT is to remove the main source of amyloidogenic TTR. LT can halt the progression by knocking down 95% of circulating TTR (37). Positive effects of LT are significantly seen in patients with early-onset Val30Met mutation with a short disease course. Twenty-year survival rate is as high as 55.3% (38). However, LT is unable to halt the progression of the cardiac amyloidosis, as wild-type TTR continues to accumulate on cardiac tissue (39). Similarly, ocular and leptomeningeal amyloidosis may worsen after LT due to the local production of amyloidogenic TTR in choroid plexus and retinal epithelium (40, 41). Combined transplantation of liver with heart and liver with kidney are suggested treatment options for patients with severe cardiomyopathy and severe nephropathy, respectively (36).
Other than LT, recent treatment options cover two main concerns:
Disease modifying anti-amyloid therapy
Symptomatic treatment of polyneuropathy and other disease related symptoms.
Disease modifying drugs show their effects either by stabilizing TTR tetramer or by reducing the production of total TTR.
In 2011, tetramer stabilizer Tafamidis is approved by the European Medical Agency (EMA) for stage 1 TTR-FAP patients. In the pivotal trial, although Tafamidis 20 mg once daily failed to achieve primary endpoints, reduction in neurologic deterioration, preservation of nerve fiber function, maintenance of QOL, and TTR stabilization compared to placebo are demonstrated (42). Twelve-month open label, extension study showed similar results (43). There were no major adverse effects. Follow-up studies showed results consistent with the pivotal trial except a multicenter study held in Italy, in which Tafamidis was unable to slow neurologic and cardiac worsening (43–46). In late-onset cases and non-Val30Met mutations results are more conflictive. Older age at onset and lower body mass index (BMI) at the baseline were associated with poor outcome (47). The phase 2 study failed to demonstrate the efficiency of Tafamidis in TTR cardiomyopathy, however post-hoc analysis showed that it may be effective in earlier stage cardiac disease (48, 49). Phase 3, placebo-controlled, double-blind, randomized study that was designed for the efficacy of Tafamidis in patients with TTR cardiomyopathy has ended in February 2018 (NCT01994889). Patients with late stage cardiomyopathy (NYHA stage 4) were excluded in the study (50). The topline results showed a significant reduction of mortality and cardiovascular events requiring hospitalization. Hence, FDA grants Tafamidis for “Breakthrough Therapy” designation for the treatment of TTR cardiomyopathy.
Diflunisal, a non-steroid anti-inflammatory drug (NSAI), is also a tetramer stabilizer (51). In a randomized, double-blind, placebo-controlled study in a cohort of 130 patients, Diflunisal 250 mg administered twice daily showed better NIS+7 and quality of life (QOL) results compared to placebo in two years. However, safety profile is worrisome due to the common gastrointestinal and urinary side effects which caused high discontinuation rates. (52) Contraindication of NSAI drugs in end stage cardiac and renal diseases limits the use of Diflunisal in patients with cardiac and renal involvement (2).
Patisiran, a small interfering RNA (siRNA) encapsulated in lipid nanoparticle, demonstrated a significant knock-down of circulating TTR in healthy volunteers by silencing the TTR gene (53). Then, in phase 2 open-label study intravenous Patisiran 0.3 mg/kg once every three weeks showed very promising results by halting the disease progression (54). In phase 3 trial, primary end-point is designed to measure the change of mNIS+7, the scoring system that consists NIS, autonomic tests and QST, in 18 months. The results were significantly better in Patisiran group compared to placebo and nearly half of the patients in Patisiran group had a decrease in mNIS+7 scores suggesting an improvement. Mean knock-down of serum TTR was 81%. All other secondary end-points are matched in the study. The frequency of adverse events was similar in the Patisiran group compared to placebo (55). According to these positive results, Patisiran received the FDA approval for the treatment of TTR-FAP in August 2018.
Another promising drug, that recently completed the phase 3 trial, is Inotersen, an antisense oligonucleotide that selectively binds TTR mRNA and decreases its production. Recruited patients in this trial were in early stages (stage 1 or stage 2) and without any prominent cardiac dysfunction (less than NYHA class 3). Patients received three subcutaneous injections during the first week, followed by a one-weekly subcutaneous injection for the next 64 weeks. Primary end points, mNIS+7 and Norfolk QOL, were matched in the study. Mean decrease in serum TTR was 74%. The safety concerns associated with Inotersen treatment were thrombocytopenia and glomerulonephritis, which were observed in 6 out of 112 patients (56). The drug is approved by European Medicine Agency (EMA) for stage 1 and 2 of the disease and accepted for Priority Review by FDA.
In transgenic mouse models, combination of Doxycycline, an antibiotic, and Tauroursodeoxycholic acid (TUDCA), a bile acid, showed promising results by reducing the amyloid deposition in tissues (57). Results of open-label phase 2 study were consistent with stabilization of cardiac disease in 75% of the patients receiving Doxycycline and TUDCA combination (58). An 18-month open label study of the tolerability and efficiency of Doxycycline and TUDCA combination in patients with TTR cardiomyopathy was completed but the data has not been published so far (NCT01855360).
Antibodies against serum amyloid P (SAP) and TTR, which target to clear amyloid from the tissues, are currently under development (59, 60). A phase 2 study intended to evaluate the efficiency of the combination of Anti-SAP antibody (GSK2398852) and SAP inhibitor (GSK2315698) for the treatment of AL and TTR amyloidosis, is now recruiting patients (NCT03044353). Anti-TTR antibodies, that bind amyloidogenic form of TTR, is also another promising treatment option. These antibodies, by enhancing phagocytic uptake, promote clearance of amyloidogenic TTR from the tissues and may also help to diagnose the disease (60).
Symptomatic treatment options include neuropathic pain medication, treatment for orthostatic hypotension, incontinence and diarrhea. Walking aids and orthoses are required to preserve mobilization. Cardiovascular events are the main cause of death in TTR amyloidosis, therefore treating cardiac failure and conduction disorders is particularly important (Figure 4).
Figure 4.
Current suggested treatment algorithm of TTR-FAP*
TTR-FAP: transthyretin-related familial amyloid polyneuropathy, 1= recently approved or on the approval process of United States Food and Drug Administration (FDA) also for Stage 1 disease..Adapted from36.
CONCLUSION
TTR-FAP is a rare but life threatening disease. The disease can present in many different forms with variable signs and symptoms, thus it is important to raise the awareness among clinicians. Early initiation of anti-amyloid treatment is essential for a better outcome and functional activity. New treatment options are promising to improve the prognosis.
Footnotes
Peer-review: Externally peer-reviewed.
Author Contributions: Concept– AÇ, HDT, YP; Design– AÇ, HDT, YP; Supervision– AÇ, HDT, YP; Resource– AÇ, HDT, YP; Materials– AÇ, HDT, YP; Data Collection and/or Processing– AÇ, HDT, YP; Analysis and/or Interpretation– AÇ, HDT, YP; Literature Search– AÇ, HDT, YP; Writing– AÇ, HDT, YP; Critical Reviews– AÇ, HDT, YP.
Financial Disclosure: The authors have nothing to disclose.
Conflict of Interest: None
REFERENCES
- 1.Andrade C. A peculiar form of peripheral neuropathy;familiar atypical generalized amyloidosis with special involvement of the peripheral nerves. Brain. 1952;75:408–427. doi: 10.1093/brain/75.3.408. [DOI] [PubMed] [Google Scholar]
- 2.Hawkins PN, Ando Y, Dispenzeri A, Gonzalez-Duarte A, Adams D, Suhr OB. Evolving landscape in the management of transthyretin amyloidosis. Ann Med. 2015;47:625–638. doi: 10.3109/07853890.2015.1068949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Plante-Bordeneuve V. Update in the diagnosis and management of transthyretin familial amyloid polyneuropathy. J Neurol. 2014;261:1227–1233. doi: 10.1007/s00415-014-7373-0. [DOI] [PubMed] [Google Scholar]
- 4.Freeman R, Barroso F. Recent advances in familial amyloid polyneuropathy. Curr Opin Neurol. 2015;28:494–499. doi: 10.1097/WCO.0000000000000244. [DOI] [PubMed] [Google Scholar]
- 5.Sekijima Y, Ueda M, Koike H, Misawa S, Ishii T, Ando Y. Diagnosis and management of transthyretin familial amyloid polyneuropathy in Japan:red-flag symptom clusters and treatment algorithm. Orphanet J Rare Dis. 2018;13:6. doi: 10.1186/s13023-017-0726-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parman Y, Adams D, Obici L, Galan L, Guergueltcheva V, Suhr OB, Coelho T. Sixty years of transthyretin familial amyloid polyneuropathy (TTR-FAP) in Europe:where are we now?A European network approach to defining the epidemiology and management patterns for TTR-FAP. Curr Opin Neurol. 2016;29(Suppl 1):S3–S13. doi: 10.1097/WCO.0000000000000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamashita T, Ando Y, Okamoto S, Misumi Y, Hirahara T, Ueda M, Obayashi K, Nakamura M, Jono H, Shono M, Asonuma K, Inomata Y, Uchino M. Long-term survival after liver transplantation in patients with familial amyloid polyneuropathy. Neurology. 2012;78:637–643. doi: 10.1212/WNL.0b013e318248df18. [DOI] [PubMed] [Google Scholar]
- 8.Koike H, Tanaka F, Hashimoto R, Tomita M, Kawagashira Y, Iijima M, Fujitake J, Kawanami T, Kato T, Yamamoto M, Sobue G. Natural history of transthyretin Val30Met familial amyloid polyneuropathy:analysis of late-onset cases from non-endemic areas. J Neurol Neurosurg Psychiatry. 2012;83:152–158. doi: 10.1136/jnnp-2011-301299. [DOI] [PubMed] [Google Scholar]
- 9.Durmus-Tekce H, Matur Z, Mert Atmaca M, Poda M, Cakar A, Hidir Ulas U, Oflazer-Serdaroglu P, Deymeer F, Parman YG. Genotypic and phenotypic presentation of transthyretin-related familial amyloid polyneuropathy (TTR-FAP) in Turkey. Neuromuscul Disord. 2016;26:441–446. doi: 10.1016/j.nmd.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 10.Adams D. Hereditary and acquired amyloid neuropathies. J Neurol. 2001;248:647–657. doi: 10.1007/s004150170109. [DOI] [PubMed] [Google Scholar]
- 11.Adams D. Recent advances in the treatment of familial amyloid polyneuropathy. Ther Adv Neurol Disord. 2013;6:129–139. doi: 10.1177/1756285612470192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sahin E, Cakar A, Durmus-Tekce H, Parman Y. A Val30Met sporadic familial amyloid polyneuropathy case with atypical presentation:upper limb onset of symptoms. Acta Neurol Belg. 2018 doi: 10.1007/s13760-018-0959-z. [DOI] [PubMed] [Google Scholar]
- 13.Suanprasert N, Berk JL, Benson MD, Dyck PJ, Klein CJ, Gollob JA, Bettencourt BR, Karsten V, Dyck PJ. Retrospective study of a TTR FAP cohort to modify NIS+7 for therapeutic trials. J Neurol Sci. 2014;344:121–128. doi: 10.1016/j.jns.2014.06.041. [DOI] [PubMed] [Google Scholar]
- 14.Ando Y, Coelho T, Berk JL, Cruz MW, Ericzon BG, Ikeda S, Lewis WD, Obici L, Plante-Bordeneuve V, Rapezzi C, Said G, Salvi F. Guideline of transthyretin-related hereditary amyloidosis for clinicians. Orphanet J Rare Dis. 2013;8:31. doi: 10.1186/1750-1172-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mazzeo A, Russo M, Di Bella G, Minutoli F, Stancanelli C, Gentile L, Baldari S, Carerj S, Toscano A, Vita G. Transthyretin-Related Familial Amyloid Polyneuropathy (TTR-FAP):A Single-Center Experience in Sicily, an Italian Endemic Area. J Neuromuscul Dis. 2015;2(Suppl 2):S39–S48. doi: 10.3233/JND-150091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coelho T, Maurer MS, Suhr OB. THAOS - The Transthyretin Amyloidosis Outcomes Survey:initial report on clinical manifestations in patients with hereditary and wild-type transthyretin amyloidosis. Curr Med Res Opin. 2013;29:63–76. doi: 10.1185/03007995.2012.754348. [DOI] [PubMed] [Google Scholar]
- 17.Maia LF, Magalhaes R, Freitas J, Taipa R, Pires MM, Osorio H, Dias D, Pessegueiro H, Correia M, Coelho T. CNS involvement in V30M transthyretin amyloidosis:clinical, neuropathological and biochemical findings. J Neurol Neurosurg Psychiatry. 2015;86:159–167. doi: 10.1136/jnnp-2014-308107. [DOI] [PubMed] [Google Scholar]
- 18.Brett M, Persey MR, Reilly MM, Revesz T, Booth DR, Booth SE, Hawkins PN, Pepys MB, Morgan-Hughes JA. Transthyretin Leu12Pro is associated with systemic, neuropathic and leptomeningeal amyloidosis. Brain. 1999;122(Pt 2):183–190. doi: 10.1093/brain/122.2.183. [DOI] [PubMed] [Google Scholar]
- 19.Jin K, Sato S, Takahashi T, Nakazaki H, Date Y, Nakazato M, Tominaga T, Itoyama Y, Ikeda S. Familial leptomeningeal amyloidosis with a transthyretin variant Asp18Gly representing repeated subarachnoid haemorrhages with superficial siderosis. J Neurol Neurosurg Psychiatry. 2004;75:1463–1466. doi: 10.1136/jnnp.2003.029942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ellie E, Camou F, Vital A, Rummens C, Grateau G, Delpech M, Valleix S. Recurrent subarachnoid hemorrhage associated with a new transthyretin variant (Gly53Glu) Neurology. 2001;57:135–137. doi: 10.1212/wnl.57.1.135. [DOI] [PubMed] [Google Scholar]
- 21.Falk RH, Dubrey SW. Amyloid heart disease. Prog Cardiovasc Dis. 2010;52:347–361. doi: 10.1016/j.pcad.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 22.Gagliardi C, Perfetto F, Lorenzini M, Ferlini A, Salvi F, Milandri A, Quarta CC, Taborchi G, Bartolini S, Frusconi S, Martone R, Cinelli MM, Foffi S, Reggiani MLB, Fabbri G, Cataldo P, Cappelli F, Rapezzi C. Phenotypic profile of Il68Leu transthyretin amyloidosis:an underdiagnosed cause of heart failure. Eur J Heart Fail. 2018;20:1417–1425. doi: 10.1002/ejhf.1285. [DOI] [PubMed] [Google Scholar]
- 23.Maurer MS, Hanna M, Grogan M, Dispenzieri A, Witteles R, Drachman B, Judge DP, Lenihan DJ, Gottlieb SS, Shah SJ, Steidley DE, Ventura H, Murali S, Silver MA, Jacoby D, Fedson S, Hummel SL, Kristen AV, Damy T, Plante-Bordeneuve V, Coelho T, Mundayat R, Suhr OB, Waddington Cruz M, Rapezzi C T HAOS Investigators. Genotype and Phenotype of Transthyretin Cardiac Amyloidosis:THAOS (Transthyretin Amyloid Outcome Survey) J Am Coll Cardiol. 2016;68:161–172. doi: 10.1016/j.jacc.2016.03.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruberg FL, Berk JL. Transthyretin (TTR) cardiac amyloidosis. Circulation. 2012;126:1286–1300. doi: 10.1161/CIRCULATIONAHA.111.078915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nativi-Nicolau J, Maurer MS. Amyloidosis cardiomyopathy:update in the diagnosis and treatment of the most common types. Curr Opin Cardiol. 2018;33:571–579. doi: 10.1097/HCO.0000000000000547. [DOI] [PubMed] [Google Scholar]
- 26.Tripathy K, Chawla R, Selvan H, Venkatesh P. Ocular Manifestations of Familial Transthyretin Amyloidosis. Am J Ophthalmol. 2018;186:169–170. doi: 10.1016/j.ajo.2017.10.038. [DOI] [PubMed] [Google Scholar]
- 27.Lobato L, Beirao I, Silva M, Bravo F, Silvestre F, Guimaraes S, Sousa A, Noel LH, Sequeiros J. Familial ATTR amyloidosis:microalbuminuria as a predictor of symptomatic disease and clinical nephropathy. Nephrol Dial Transplant. 2003;18:532–538. doi: 10.1093/ndt/18.3.532. [DOI] [PubMed] [Google Scholar]
- 28.Mariani LL, Lozeron P, Theaudin M, Mincheva Z, Signate A, Ducot B, Algalarrondo V, Denier C, Adam C, Nicolas G, Samuel D, Slama MS, Lacroix C, Misrahi M, Adams D French Familial Amyloid Polyneuropathies Network (CORNAMYL) Study Group. Genotype-phenotype correlation and course of transthyretin familial amyloid polyneuropathies in France. Ann Neurol. 2015;78:901–916. doi: 10.1002/ana.24519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castro J, Miranda B, Castro I, de Carvalho M, Conceicao I. The diagnostic accuracy of Sudoscan in transthyretin familial amyloid polyneuropathy. Clin Neurophysiol. 2016;127:2222–2227. doi: 10.1016/j.clinph.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 30.Rousseau A, Cauquil C, Dupas B, Labbe A, Baudouin C, Barreau E, Theaudin M, Lacroix C, Guiochon-Mantel A, Benmalek A, Labetoulle M, Adams D. Potential Role of In Vivo Confocal Microscopy for Imaging Corneal Nerves in Transthyretin Familial Amyloid Polyneuropathy. JAMA Ophthalmol. 2016;134:983–989. doi: 10.1001/jamaophthalmol.2016.1889. [DOI] [PubMed] [Google Scholar]
- 31.Zouari HG, Ng Wing Tin S, Wahab A, Damy T, Lefaucheur JP. Assessment of autonomic innervation of the foot in familial amyloid polyneuropathy. EurJ Neurol. 2019;26(1):94–e10. doi: 10.1111/ene.13774. [DOI] [PubMed] [Google Scholar]
- 32.Adams D, Cauquil C, Labeyrie C. Familial amyloid polyneuropathy. Curr Opin Neurol. 2017;30:481–489. doi: 10.1097/WCO.0000000000000476. [DOI] [PubMed] [Google Scholar]
- 33.Rowczenio DM, Noor I, Gillmore JD, Lachmann HJ, Whelan C, Hawkins PN, Obici L, Westermark P, Grateau G, Wechalekar AD. Online registry for mutations in hereditary amyloidosis including nomenclature recommendations. Hum Mutat. 2014;35:E2403–E2412. doi: 10.1002/humu.22619. [DOI] [PubMed] [Google Scholar]
- 34.Picken MM, Westermark P. Amyloid detection and typing:summary of current practice and recommendations of the consensus group. Amyloid. 2011;18(Suppl 1):48–50. doi: 10.3109/13506129.2011.574354017. [DOI] [PubMed] [Google Scholar]
- 35.Lemos C, Coelho T, Alves-Ferreira M, Martins-da-Silva A, Sequeiros J, Mendonca D, Sousa A. Overcoming artefact:anticipation in 284 Portuguese kindreds with familial amyloid polyneuropathy (FAP) ATTRV30M. J Neurol Neurosurg Psychiatry. 2014;85:326–330. doi: 10.1136/jnnp-2013-305383. [DOI] [PubMed] [Google Scholar]
- 36.Adams D, Suhr OB, Hund E, Obici L, Tournev I, Campistol JM, Slama MS, Hazenberg BP, Coelho T. First European consensus for diagnosis, management, and treatment of transthyretin familial amyloid polyneuropathy. Curr Opin Neurol. 2016;29(Suppl 1):S14–S26. doi: 10.1097/WCO.0000000000000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holmgren G, Steen L, Ekstedt J, Groth CG, Ericzon BG, Eriksson S, Andersen O, Karlberg I, Norden G, Nakazato M, Hawkins P, Richardson S, Pepys M. Biochemical effect of liver transplantation in two Swedish patients with familial amyloidotic polyneuropathy (FAP-met30) Clin Genet. 1991;40:242–246. doi: 10.1111/j.1399-0004.1991.tb03085.x. [DOI] [PubMed] [Google Scholar]
- 38.Ericzon BG, Wilczek HE, Larsson M, Wijayatunga P, Stangou A, Pena JR, Furtado E, Barroso E, Daniel J, Samuel D, Adam R, Karam V, Poterucha J, Lewis D, Ferraz-Neto BH, Cruz MW, Munar-Ques M, Fabregat J, Ikeda S, Ando Y, Heaton N, Otto G, Suhr O. Liver Transplantation for Hereditary Transthyretin Amyloidosis:After 20 Years Still the Best Therapeutic Alternative? Transplantation. 2015;99:1847–1854. doi: 10.1097/TP.0000000000000574. [DOI] [PubMed] [Google Scholar]
- 39.Liepnieks JJ, Benson MD. Progression of cardiac amyloid deposition in hereditary transthyretin amyloidosis patients after liver transplantation. Amyloid. 2007;14:277–282. doi: 10.1080/13506120701614032. [DOI] [PubMed] [Google Scholar]
- 40.Hara R, Kawaji T, Ando E, Ohya Y, Ando Y, Tanihara H. Impact of liver transplantation on transthyretin-related ocular amyloidosis in Japanese patients. Arch Ophthalmol. 2010;128:206–210. doi: 10.1001/archophthalmol.2009.390. [DOI] [PubMed] [Google Scholar]
- 41.Ando Y, Terazaki H, Nakamura M, Ando E, Haraoka K, Yamashita T, Ueda M, Okabe H, Sasaki Y, Tanihara H, Uchino M, Inomata Y. A different amyloid formation mechanism:de novo oculoleptomeningeal amyloid deposits after liver transplantation. Transplantation. 2004;77:345–349. doi: 10.1097/01.TP.0000111516.60013.E6. [DOI] [PubMed] [Google Scholar]
- 42.Coelho T, Maia LF, Martins da Silva A, Waddington Cruz M, Plante-Bordeneuve V, Lozeron P, Suhr OB, Campistol JM, Conceicao IM, Schmidt HH, Trigo P, Kelly JW, Labaudiniere R, Chan J, Packman J, Wilson A, Grogan DR. Tafamidis for transthyretin familial amyloid polyneuropathy:a randomized, controlled trial. Neurology. 2012;79:785–792. doi: 10.1212/WNL.0b013e3182661eb1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coelho T, Maia LF, da Silva AM, Cruz MW, Plante-Bordeneuve V, Suhr OB, Conceicao I, Schmidt HH, Trigo P, Kelly JW, Labaudiniere R, Chan J, Packman J, Grogan DR. Long-term effects of tafamidis for the treatment of transthyretin familial amyloid polyneuropathy. J Neurol. 2013;260:2802–2814. doi: 10.1007/s00415-013-7051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Waddington Cruz M, Amass L, Keohane D, Schwartz J, Li H, Gundapaneni B. Early intervention with tafamidis provides long-term (5.5-year) delay of neurologic progression in transthyretin hereditary amyloid polyneuropathy. Amyloid. 2016;23:178–183. doi: 10.1080/13506129.2016.1207163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ando Y, Sekijima Y, Obayashi K, Yamashita T, Ueda M, Misumi Y, Morita H, Machii K, Ohta M, Takata A, Ikeda S. Effects of tafamidis treatment on transthyretin (TTR) stabilization, efficacy, and safety in Japanese patients with familial amyloid polyneuropathy (TTR-FAP) with Val30Met and non-Val30Met:A phase III, open-label study. J Neurol Sci. 2016;362:266–271. doi: 10.1016/j.jns.2016.01.046. [DOI] [PubMed] [Google Scholar]
- 46.Cortese A, Vita G, Luigetti M, Russo M, Bisogni G, Sabatelli M, Manganelli F, Santoro L, Cavallaro T, Fabrizi GM, Schenone A, Grandis M, Gemelli C, Mauro A, Pradotto LG, Gentile L, Stancanelli C, Lozza A, Perlini S, Piscosquito G, Calabrese D, Mazzeo A, Obici L, Pareyson D. Monitoring effectiveness and safety of Tafamidis in transthyretin amyloidosis in Italy:a longitudinal multicenter study in a non-endemic area. J Neurol. 2016;263:916–924. doi: 10.1007/s00415-016-8064-9. [DOI] [PubMed] [Google Scholar]
- 47.Plante-Bordeneuve V, Gorram F, Salhi H, Nordine T, Ayache SS, Le Corvoisier P, Azoulay D, Feray C, Damy T, Lefaucheur JP. Long-term treatment of transthyretin familial amyloid polyneuropathy with tafamidis:a clinical and neurophysiological study. J Neurol. 2017;264:268–276. doi: 10.1007/s00415-016-8337-3. [DOI] [PubMed] [Google Scholar]
- 48.Sultan MB, Gundapaneni B, Schumacher J, Schwartz JH. Treatment With Tafamidis Slows Disease Progression in Early-Stage Transthyretin Cardiomyopathy. Clin Med Insights Cardiol. 2017;11 doi: 10.1177/1179546817730322. 1179546817730322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maurer MS, Grogan DR, Judge DP, Mundayat R, Packman J, Lombardo I, Quyyumi AA, Aarts J, Falk RH. Tafamidis in transthyretin amyloid cardiomyopathy:effects on transthyretin stabilization and clinical outcomes. Circ Heart Fail. 2015;8:519–526. doi: 10.1161/CIRCHEARTFAILURE.113.000890. [DOI] [PubMed] [Google Scholar]
- 50.Maurer MS, Elliott P, Merlini G, Shah SJ, Cruz MW, Flynn A, Gundapaneni B, Hahn C, Riley S, Schwartz J, Sultan MB, Rapezzi C ATTR-ACT Study Investigators. Design and Rationale of the Phase 3 ATTR-ACT Clinical Trial (Tafamidis in Transthyretin Cardiomyopathy Clinical Trial) Circ Heart Fail. 2017:10. doi: 10.1161/CIRCHEARTFAILURE.116.003815. [DOI] [PubMed] [Google Scholar]
- 51.Sekijima Y, Dendle MA, Kelly JW. Orally administered diflunisal stabilizes transthyretin against dissociation required for amyloidogenesis. Amyloid. 2006;13:236–249. doi: 10.1080/13506120600960882. [DOI] [PubMed] [Google Scholar]
- 52.Berk JL, Suhr OB, Obici L, Sekijima Y, Zeldenrust SR, Yamashita T, Heneghan MA, Gorevic PD, Litchy WJ, Wiesman JF, Nordh E, Corato M, Lozza A, Cortese A, Robinson-Papp J, Colton T, Rybin DV, Bisbee AB, Ando Y, Ikeda S, Seldin DC, Merlini G, Skinner M, Kelly JW, Dyck PJ Diflunisal Trial Consortium. Repurposing diflunisal for familial amyloid polyneuropathy:a randomized clinical trial. JAMA. 2013;310:2658–2667. doi: 10.1001/jama.2013.283815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coelho T, Adams D, Silva A, Lozeron P, Hawkins PN, Mant T, Perez J, Chiesa J, Warrington S, Tranter E, Munisamy M, Falzone R, Harrop J, Cehelsky J, Bettencourt BR, Geissler M, Butler JS, Sehgal A, Meyers RE, Chen Q, Borland T, Hutabarat RM, Clausen VA, Alvarez R, Fitzgerald K, Gamba-Vitalo C, Nochur SV, Vaishnaw AK, Sah DW, Gollob JA, Suhr OB. Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N Engl J Med. 2013;369:819–829. doi: 10.1056/NEJMoa1208760. [DOI] [PubMed] [Google Scholar]
- 54.Suhr OB, Coelho T, Buades J, Pouget J, Conceicao I, Berk J, Schmidt H, Waddington-Cruz M, Campistol JM, Bettencourt BR, Vaishnaw A, Gollob J, Adams D. Efficacy and safety of patisiran for familial amyloidotic polyneuropathy:a phase II multi-dose study. Orphanet J Rare Dis. 2015;10:109. doi: 10.1186/s13023-015-0326-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Adams D, Gonzalez-Duarte A, O'Riordan WD, Yang CC, Ueda M, Kristen AV, Tournev I, Schmidt HH, Coelho T, Berk JL, Lin KP, Vita G, Attarian S, Plante-Bordeneuve V, Mezei MM, Campistol JM, Buades J, Brannagan TH, 3rd, Kim BJ, Oh J, Parman Y, Sekijima Y, Hawkins PN, Solomon SD, Polydefkis M, Dyck PJ, Gandhi PJ, Goyal S, Chen J, Strahs AL, Nochur SV, Sweetser MT, Garg PP, Vaishnaw AK, Gollob JA, Suhr OB. Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. N Engl J Med. 2018;379:11–21. doi: 10.1056/NEJMoa1716153. [DOI] [PubMed] [Google Scholar]
- 56.Benson MD, Waddington-Cruz M, Berk JL, Polydefkis M, Dyck PJ, Wang AK, Plante-Bordeneuve V, Barroso FA, Merlini G, Obici L, Scheinberg M, Brannagan TH, 3rd, Litchy WJ, Whelan C, Drachman BM, Adams D, Heitner SB, Conceicao I, Schmidt HH, Vita G, Campistol JM, Gamez J, Gorevic PD, Gane E, Shah AM, Solomon SD, Monia BP, Hughes SG, Kwoh TJ, McEvoy BW, Jung SW, Baker BF, Ackermann EJ, Gertz MA, Coelho T. Inotersen Treatment for Patients with Hereditary Transthyretin Amyloidosis. N Engl J Med. 2018;379:22–31. doi: 10.1056/NEJMoa1716793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cardoso I, Martins D, Ribeiro T, Merlini G, Saraiva MJ. Synergy of combined doxycycline/TUDCA treatment in lowering Transthyretin deposition and associated biomarkers:studies in FAP mouse models. J Transl Med. 2010;8:74. doi: 10.1186/1479-5876-8-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Obici L, Cortese A, Lozza A, Lucchetti J, Gobbi M, Palladini G, Perlini S, Saraiva MJ, Merlini G. Doxycycline plus tauroursodeoxycholic acid for transthyretin amyloidosis:a phase II study. Amyloid. 2012;19(Suppl 1):34–36. doi: 10.3109/13506129.2012.678508. [DOI] [PubMed] [Google Scholar]
- 59.Richards DB, Cookson LM, Berges AC, Barton SV, Lane T, Ritter JM, Fontana M, Moon JC, Pinzani M, Gillmore JD, Hawkins PN, Pepys MB. Therapeutic Clearance of Amyloid by Antibodies to Serum Amyloid P Component. N Engl J Med. 2015;373:1106–1114. doi: 10.1056/NEJMoa1504942. [DOI] [PubMed] [Google Scholar]
- 60.Higaki JN, Chakrabartty A, Galant NJ, Hadley KC, Hammerson B, Nijjar T, Torres R, Tapia JR, Salmans J, Barbour R, Tam SJ, Flanagan K, Zago W, Kinney GG. Novel conformation-specific monoclonal antibodies against amyloidogenic forms of transthyretin. Amyloid. 2016;23:86–97. doi: 10.3109/13506129.2016.1148025. [DOI] [PMC free article] [PubMed] [Google Scholar]