Abstract
Introduction:
The aim of the present study was to predict paroxysmal atrial fibrillation (PAF) in acute ischemic stroke patients with presumed cryptogenic embolic etiology.
Methods:
In this retrospective cohort study, demographics, blood tests, data of neuroimaging studies such as non-contrast computed tomography (NCCT), magnetic resonance imaging (MRI), standard 12-lead electrocardigraphy (ECG), 24-hour Holter ECG, echocardiography was collected. The diagnostic work-up to detect atrial fibrillation (AF) was either medical history of the patient or 12-lead ECG or 24-hour Holter ECG or continuous ECG monitoring. Score for the targeting of atrial fibrillation (STAF) was calculated for all patients. Cryptogenic ischemic stroke (CS) patients with and without documented AF were recorded.
Results:
Between July 2014 and December 2015, a total of 133 of the 258 patients with CS were included in this study. Overall, 133 patients were enrolled and AF was detected in 30 (22.6%) patients. In univariate analysis gender (p<0.001), age (p=0.001), smoking habit (p=0.004), aortic and mitral valve insufficiency (p=0.014 and p=0.021), left ventricular systolic dysfunction (p=0.04), and left atrial dilatation (p=0.03) were predictors of AF but multivariate analysis showed that only gender and age were independent predictors of AF in patients with presumed cryptogenic ischemic stroke. According to ROC analysis, area under the curve was 70% and the sensitivity and specificity of STAF score of ≥5 was 86% and 71% respectively.
Conclusion:
STAF score predicted with fair accuracy, and has a limited use for the risk of PAF in stroke patients.
Keywords: STAF score, atrial fibrillation, cryptogenic stroke, stroke
INTRODUCTION
Atrial fibrillation (AF) –either paroxysmal or persistent– is an important risk factor for ischemic strokes (1). The timely diagnosis of AF and the institution of oral anticoagulation therapy in patients with ischemic strokes can reduce the number of subsequent adverse events. Paroxysmal AF (PAF) carries the same risk of ischemic stroke as persistent AF (2), and PAF is more prevalent than persistent AF in patients with ischemic strokes and transient ischemic attacks (TIA) (3). Although there are numerous diagnostic studies for the detection of PAF, electrocardiography (ECG) is often preferred due to its low sensitivity (4), while tests such as early-phase ECG monitoring, long term Holter ECG monitoring, cardiac event monitoring and implanted PAF monitoring devices are either highly time consuming, or do not facilitate the early predicting of those patients with PAF (1).
In 1993, the Trial of Org 10172 in Acute Stroke Treatment (TOAST) criterion was introduced, classifying strokes according to etiologies (5). The term ‘cryptogenic stroke’ (CS) refers to a stroke of undefined etiology (after adequate diagnostic studies have been conducted) (6). CS comprises approximately 25–40% of all strokes. CS is often hypothesized to have originated from distant embolization (7). AF is a notable risk factor for embolic ischemic strokes, with a prevalence of 4.9% to 9.2% in CS patients (8, 9). In 2014, the term called ESUS (embolic stroke of undetermined source) was defined by the CS/ESUS international working group (10-B) and defined as a subset of patients with cryptogenic stroke who have embolic strokes and determined as a non-lacunar brain infarct without proximal arterial stenosis or cardioembolic sources with sufficient diagnostic assessment (7). In literature, studies have mostly used the term CS which may fulfill the ESUS criteria as well (10). Thus, we preferred to use the term CS.
Approximately one third of all ischemic strokes are thought to have cryptogenic etiology. PAF has gained great attention as a potential etiology of cryptogenic strokes (2). The minimum diagnostic workup for detection of a cryptogenic embolic stroke includes: brain computed tomography (CT) or MRI; 12-lead ECG; transthoracic echocardiography; and cardiac monitoring for at least 24 hours with automated rhythm detection, and imaging of extra- and intracranial arteries (11).
Cardiologic tests are time-consuming, costly and may be inconvenient due to low sensitivity. Identifying ischemic stroke patients who have a particularly high risk of AF is important. Recently, a scoring system (STAF) was introduced to predict the probability of AF in this patient population (12).
The aim of the present study was to assess the Score for the Targeting of Atrial Fibrillation (STAF) in patients with cryptogenic stroke in determining the risk of PAF.
METHODS
This is a retrospective cohort study on patients with cryptogenic embolic acute ischemic stroke conducted between July 2014 and December 2015. The study was approved by the local ethics committee. During the study period, those patients thought to have CS were included in the study. Demographic data, risk factors, and the severity of stroke were collected. The minimum diagnostic work up during the hospitalization period included: CT and/or MRI; 12-lead ECG; transthoracic echocardiography; cardiac monitoring for at least 24 hours with automated rhythm detection; imaging of both extracranial and intracranial arteries, Holter monitoring within the previous 3 months. Special coagulation tests were only ordered for those patients who had a personal or family history of a prothrombotic process (11).
The diagnosis of cryptogenic embolic stroke was based on the following diagnostic criteria (11):
1-Non-lacunar stroke detected by CT or MRI. The largest dimension for CT and MRI diffusion images was <1.5 cm and ≤2 cm, respectively.
2-Absence of extracranial and intracranial atherosclerosis causing at least 50% luminal stenosis in arteries supplying the area of ischemia.
3-No major risk for cardioembolic source of embolism (persistent or paroxysmal atrial fibrillation, sick sinus syndrome/atrial asystole, intracardiac thrombus formation, prosthetic heart valve, atrial myxoma or other cardiac sources, mitral stenosis, myocardial infarction within 4 weeks, ejection fraction <30%, presence of valvular vegetation or infective endocarditis)
4-No other specific factors such as arteritis, dissection, migraine were identified.
Transthoracic echocardiography was performed on all patients (DMS software NV, USA-CardioScan 12.0 DM Software, NV, USA). The left atrial diameter was measured at the parasternal border in M mode. Left atrial dilatation was defined as a diameter >40 mm according to the published guidelines of The American Society of Echocardiography (13). Carotid ultrasound was performed according to the published criteria. Those patients who were admitted to the intensive care unit, or had lacunar infarcts or histories of TIA, were excluded from the study.
The STAF score was calculated for each individual patient and includes age, baseline NIHSS score, left atrial dilatation, vascular etiology. The STAF scoring system is shown in Table 1.
Table 1.
STAF criteria and scoring (13)
| Criteria | Points |
|---|---|
| Age | |
| >62 years | 2 |
| ≤62 years | 0 |
| Baseline NIHSS Score | |
| ≥8 | 1 |
| <8 | 0 |
| Left Atrial Dilatation | |
| Yes | 2 |
| No | 0 |
| Vascular Etiology | |
| Yes | 0 |
| No | 3 |
| Total |
Statistical Analysis
The study data were analyzed in SPSS 23.0 for Windows (SPSS Inc., Chicago, Illinois, USA). Demographic and baseline characteristics were summarized as a mean ± SD for continuous variables and as a percentage of the group for categorical variables. Non-normally distributed data are presented as medians (inter-quartile range). The normality analysis was performed with the Kolmogorov-Smirnov test. Univariate analysis was performed to determine the predictors for AF. The Chi-square test was used for dichotomous variables and Student’s t-test for continuous variables and Mann-Whitney U-test for non-normally distributed data or ordinal variables. Logistic regression analysis was performed to reveal a predictive model from univariate analysis. The capacity of STAF score in predicting PAF was analyzed using ROC curve analysis. All the hypotheses were constructed as two-tailed, and the α critical value was accepted as 0.05.
RESULTS
Between July 2014 and December 2015, 133 out of 258 patients with CS were included in this study. The excluded patients’ characteristic details were as follows: 54 patients due to lacunar infarct or large vessel disease, as well as 42 patients with TIA; 13 patients because of incomplete data, and 16 patients who had Holter monitoring beyond 3 months. The study population included 73 (54.9%) males and 60 (45.1%) females, the mean age was 63.1±15 years. AF was documented in 30 (22.6%) patients after 3 months of follow-up. The demographic properties of the patients are presented in Table 2.
Table 2.
Patients demographics
| All patients | AF | No AF | p value, OR (95% CI) | |
|---|---|---|---|---|
| Male, n | 73 (54.9%) | 7 (23.3%) | 66 (64.1%) | <0.001, OR 0.17 (95% CI 0.06–0.439 |
| Age (years), mean ± SD | 63.1±15 | 71.3±10 | 60±15 | p=0.001 |
| Age >62 year, n | 73 | 24 | 49 | p=0.002 OR 4.4 (95% CI 1.6–11.6) |
| Hypertension, n | 83 (62.4%) | 22 (73.3%) | 61 (59.2%) | p=0.16 OR 1.8 (95% CI 0.77–4.6) |
| DM, n | 51 (38.3%) | 11 (36.7%) | 40 (38.8%) | p=0.83 OR 0.91 (95% CI 0.39–2.16) |
| CAD, n | 22 (16.5%) | 2 (6.7%) | 20 (19.4) | p=0.16 OR 0.29 (95% CI 0.06–1.34) |
| Previous stroke/TIA, n | 33 (24.8%) | 6 (20%) | 27 (26.2%) | p=0.48 OR 0.70 (95% CI 0.26–1.90) |
| PAD, n | 1 (0.8%) 0 | 0 | 1 (1%) | p=1 |
| Hyperlipidemia, n | 49 (36.8%) | 10 (33.3%) | 39 (37.9%) | p=0.65 OR 0.82 (95% CI 0.34–1.93) |
| Smoking, n | 21 (15.8%) | 0 | 21 (20.4%) | p=0.004 |
| NIHSS baseline | 4 (IQR3–7) 5.1±3.1 |
4.5 (IQR3–8.25) 5.7±3.6 |
4 (IQR3–7) 4.9±2.9 |
p=0.35 |
| NIHSS ≥8, n | 26 | 8 | 18 | p=0.264 |
| mRs | 1 (IQR1–2) | 1 (IQR1–2) | 1 (IQR1–2) | p=0.48 |
| Mitral insufficiency, n | 73 (% 54.9) | 22 (73.3%) | 51 (49.5%) | p=0.023 OR 2.8 (95% CI 1.14–6.87) |
| Mitral stenosis, n | 1 (0.8%) | 0 | 1 (1%) | p=1 |
| Aortic insufficiency, n | 30 (23.3%) | 12 (40%) | 18 (18.4%) | p=0.014 OR 2.9 (95% CI 1.21–7.13) |
| Aortic stenosis, n | 1 (0.8%) | 0 | 1 (1%) | p=1 |
| LVH, n | 83 (62.4%) | 21 (70%) | 62 (60.2%) | p=0.32 OR 1.54 (95% CI 0.64–3.70) |
| Left ventricular systolic dysfunction, n | 13 (9.8%) | 0 | 13 (12.6%) | |
| Left atrial dilatation, n | 49 (36.8%) | 16 (53.3%) | 33 (32%) | p=0.033 OR 2.4 (95% CI 1.05–5.54) |
AF, atrial fibrillation; DM, diabetes mellitus; CAD, coronary artery disease; NIHSS, National Institute of Stroke Scale; LVH, left ventricular hypertrophy; PAD, peripheral artery disease; mRs, modified Rankin scale; NIHSS, National Institutes of Health Stroke Scale.
Univariate analysis revealed that male gender (OR 0.17, 95% CI 0.06–0.439), older age (OR 4.4 (95% CI 1.6–11.6), smoking, left atrial dilatation (OR 2.4, 95% CI 1.05–5.54), mitral insufficiency (OR 2.8, 95% CI 1.14–6.87), aortic insufficiency (OR 2.9, 95% CI 1.21–7.13) were the factors associated with AF. Multivariate analysis revealed that gender (OR 0.21 (95% CI 0.078–0.587) and age 1.04 (95% CI 1.007–1.08) was seen as independent predictors of AF in our study population. Area under the curve was found to be 0.70 for the STAF score (CI 0.59–0.80) (Figure 1). A STAF score of ≥5 identified the patients with AF with a sensitivity of 86% and specificity of 71%.
Figure 1.
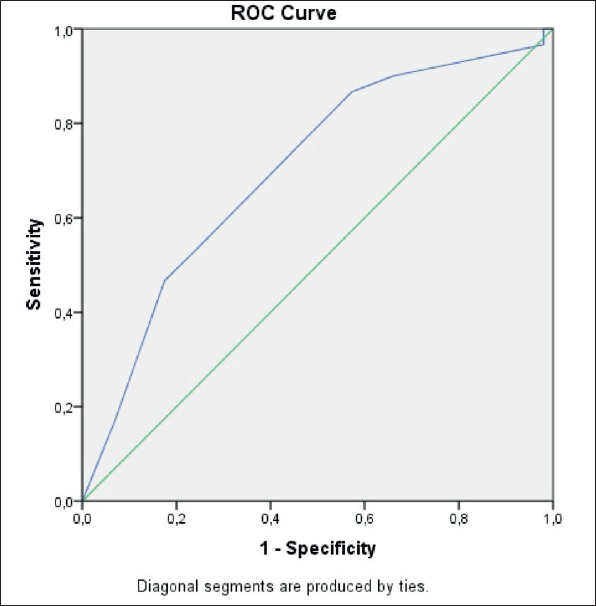
ROC analysis of STAF score.
DISCUSSION
According to this study, gender and older age can predict PAF. However, the STAF score has a limited utility for predicting PAF in patients with cryptogenic embolic strokes.
The prevalence of PAF in our study population was 22.6%. This is consistent with the previous studies published in the literature, which ranged from 5% to 21% (1, 3, 4, 14–17).
Our study revealed several important differences when compared with the original study of the STAF score published by Suissa et al. (18). In their study, all the patients presenting to their neurovascular unit for the treatment of ischemic stroke were included. They also included patients with AF by initial ECG. STAF ≥5 identified the AF patients with a sensitivity and specificity of 89% and 88%, respectively.
In a similar study to that of the original (14), Horstmann et al. enrolled patients with ischemic strokes for whom multivariate analysis, age, NIHSS, left atrial dilatation and absence of vascular etiology were independent predictors of AF, but ROC analysis revealed an area under the curve of 0.84 and a STAF score of ≥5. The sensitivity and specificity were 79% and 74%, respectively (12). The sensitivity and specificity of this study was much lower than the original study. According to explanation given by Horstmann et al., the incongruity between the two studies comes from the patients with stroke of undetermined etiology. In their cohort, 17.8% of the patients were classified as belonging in this undetermined group. Actually, the patient group with undetermined etiology defines our study population. In the study of Horstmann et al., a STAF score of ≥5 identified the AF patients-without a history of AF and without AF on the admission ECG –with a sensitivity of 58% and specificity of 74% (12). The sensitivity is much lower from our study: this possibly reflects the recruitment of a different patient population between our study and the previous study. However, the STAF score in the group of undetermined etiologies gave only poor sensitivity and specificity of 35% and 22%, respectively. In the Horstmann et al.’s study, patients with ‘two causes’ and ‘unknown etiology’ were classified in the ‘undetermined etiology’ group. 22.1% of the patients in their undetermined etiology group constituted at least two causes for their stroke, whereas patients with more than one cause were not included in our study.
In the study of Suissa et al., the mean NIHSS score was 8.05±7.33 and the mean difference between the patients with or without documented AF was statistically significant in multivariate analysis. Patients with NIHSS score ≥8 comprise 43% of all patients. The mean and median NIHSS scores in our study did not exhibit a statistically significant difference, while the mean NIHSS score was lower than the previous study, perhaps again reflecting the excluded patients in our study that had been admitted to ICU.
Our study has several limitations; retrospective single-center design makes the results of this study prone to selection bias and limits its generalizability.
In conclusion, the result of our study suggests that the STAF score is of limited use in patients with undetermined etiology for ischemic stroke without a history of AF and without AF on admission. A study in the future aiming to analyze risk scores in stroke subtypes may guide anticoagulation.
Footnotes
Ethics Committee Approval: This study was approved by the local ethics committee of Antalya Education Research Hospital Clinical Research.
Informed Consent: Not required.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – EÖG; Design – EÖG, BY; Supervision – EÖG; Resources – EK, ME; Materials – AG; Data Collection and/or Processing – AG, EK, ME; Analysis and/or Interpretation – AÜ, EÖG; Literature Search – BY, EÖG; Writing Manuscript – EÖG; Critical Review – AY, AÜ, EÖG.
Conflict of Interest: The authors declare that there is no conflict of interest.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Yoshioka K, Watanabe K, Zeniya S, Ito Y, Hizume M, Kanazawa T, Tomita M, Ishibashi S, Miake H, Tanaka H, Yokota T, Mizusawa H. A Score for Predicting Paroxysmal Atrial Fibrillation in Acute Stroke Patients:iPAB Score. J Stroke Cerebrovasc Dis. 2015;24:2263–2269. doi: 10.1016/j.jstrokecerebrovasdis.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 2.Zhang C, Kasner SE. Paroxysmal Atrial Fibrillation in Cryptogenic Stroke:an Overlooked Explanation? Curr Atheroscler Rep. 2015;17:66. doi: 10.1007/s11883-015-0547-0. [DOI] [PubMed] [Google Scholar]
- 3.Rizos T, Wagner A, Jenetzky E, Ringleb PA, Becker R, Hacke W, Veltkamp R. Paroxysmal atrial fibrillation is more prevalent than persistent atrial fibrillation in acute stroke and transient ischemic attack patients. Cerebrovasc Dis. 2011;32:276–282. doi: 10.1159/000330348. [DOI] [PubMed] [Google Scholar]
- 4.Liao J, Khalid Z, Scallan C, Morillo C, O'Donnell M. Noninvasive cardiac monitoring for detecting paroxysmal atrial fibrillation or flutter after acute ischemic stroke:a systematic review. Stroke. 2007;38:2935–2940. doi: 10.1161/STROKEAHA.106.478685. [DOI] [PubMed] [Google Scholar]
- 5.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE., 3rd Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 6.Akrawinthawong K, Venkatesh Prasad K, Mehdirad AA, Ferreira SW. Atrial Fibrillation Monitoring in Cryptogenic Stroke:the Gaps Between Evidence and Practice. Curr Cardiol Rep. 2015;17:118. doi: 10.1007/s11886-015-0674-9. [DOI] [PubMed] [Google Scholar]
- 7.Hart RG, Diener HC, Coutts SB, Easton JD, Granger CB, O'Donnell MJ, Sacco RL, Connolly SJ Cryptogenic Stroke/ESUS International Working Group. Embolic strokes of undetermined source:the case for a new clinical construct. Lancet Neurol. 2014;13:429–438. doi: 10.1016/S1474-4422(13)70310-7. [DOI] [PubMed] [Google Scholar]
- 8.Christensen LM, Krieger DW, Hojberg S, Pedersen OD, Karlsen FM, Jacobsen MD, Worck R, Nielsen H, Aegidius K, Jeppesen LL, Rosenbaum S, Marstrand J, Christensen H. Paroxysmal atrial fibrillation occurs often in cryptogenic ischaemic stroke. Final results from the SURPRISE study. Eur J Neurol. 2014;21:884–889. doi: 10.1111/ene.12400. [DOI] [PubMed] [Google Scholar]
- 9.Jabaudon D, Sztajzel J, Sievert K, Landis T, Sztajzel R. Usefulness of ambulatory 7-day ECG monitoring for the detection of atrial fibrillation and flutter after acute stroke and transient ischemic attack. Stroke. 2004;35:1647–1651. doi: 10.1161/01.STR.0000131269.69502.d9. [DOI] [PubMed] [Google Scholar]
- 10.Nouh A, Hussain M, Mehta T, Yaghi S. Embolic Strokes of Unknown Source and Cryptogenic Stroke:Implications in Clinical Practice. Front Neurol. 2016;7:37. doi: 10.3389/fneur.2016.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weimar C. Stroke of undetermined cause:workup and secondary prevention. Curr Opin Neurol. 2016;29:4–8. doi: 10.1097/WCO.0000000000000280. [DOI] [PubMed] [Google Scholar]
- 12.Horstmann S, Rizos T, Güntner J, Hug A, Jenetzky E, Krumsdorf U, Veltkamp R. Does the STAF score help detect paroxysmal atrial fibrillation in acute stroke patients? Eur J Neurol. 2013;20:147–152. doi: 10.1111/j.1468-1331.2012.03816.x. [DOI] [PubMed] [Google Scholar]
- 13.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ Chamber Quantification Writing Group;American Society of Echocardiography's Guidelines and Standards Committee;European Association of Echocardiography. Recommendations for chamber quantification:a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Rizos T, Güntner J, Jenetzky E, Marquardt L, Reichardt C, Becker R, Reinhardt R, Hepp T, Kirchhof P, Aleynichenko E, Ringleb P, Hacke W, Veltkamp R. Continuous stroke unit electrocardiographic monitoring versus 24-hour Holter electrocardiography for detection of paroxysmal atrial fibrillation after stroke. Stroke. 2012;43:2689–2694. doi: 10.1161/STROKEAHA.112.654954. [DOI] [PubMed] [Google Scholar]
- 15.Flint AC, Banki NM, Ren X, Rao VA, Go AS. Detection of paroxysmal atrial fibrillation by 30-day event monitoring in cryptogenic ischemic stroke:the Stroke and Monitoring for PAF in Real Time (SMART) Registry. Stroke. 2012;43:2788–2790. doi: 10.1161/STROKEAHA.112.665844. [DOI] [PubMed] [Google Scholar]
- 16.Stahrenberg R, Weber-Krüger M, Seegers J, Edelmann F, Lahno R, Haase B, Mende M, Wohlfahrt J, Kermer P, Vollmann D, Hasenfuss G, Gröschel K, Wachter R. Enhanced detection of paroxysmal atrial fibrillation by early and prolonged continuous holter monitoring in patients with cerebral ischemia presenting in sinus rhythm. Stroke. 2010;41:2884–2888. doi: 10.1161/STROKEAHA.110.591958. [DOI] [PubMed] [Google Scholar]
- 17.Suissa L, Lachaud S, Mahagne MH. Optimal timing and duration of continuous electrocardiographic monitoring for detecting atrial fibrillation in stroke patients. J Stroke Cerebrovasc Dis. 2013;7:991–995. doi: 10.1016/j.jstrokecerebrovasdis.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 18.Suissa L, Bertora D, Lachaud S, Mahagne MH. Score for the targeting of atrial fibrillation (STAF):a new approach to the detection of atrial fibrillation in the secondary prevention of ischemic stroke. Stroke. 2009;40:2866–2868. doi: 10.1161/STROKEAHA.109.552679. [DOI] [PubMed] [Google Scholar]


