Abstract
Purpose
There is growing recognition that biologic features of the tumor microenvironment affect the response to cancer therapies and the outcome of cancer patients. In head and neck cancer (HNC) one such feature is hypoxia. Here we investigated the utility of 18F-fluoromisonidazole (FMISO) dynamic positron emission tomography (dPET) for monitoring the early microenvironmental response to chemoradiotherapy in HNC.
Experimental Design
72 HNC patients underwent FMISO dPET scans in a customized immobilization mask (0–30 min dynamic acquisition, followed by 10 min static acquisitions starting at ~95 min and ~160 min post-injection) at baseline and early into treatment where patients have already received 1 cycle of chemotherapy and anywhere from 5–10 fractions of 2 Gy per fraction radiation therapy. Voxelwise pharmacokinetic modeling was conducted using an irreversible one-plasma two-tissue compartment model to calculate surrogate biomarkers of tumor hypoxia (k3 and Tumor-to-Blood Ratio (TBR)), perfusion (K1) and FMISO distribution volume (DV). Additionally, Tumor-to-Muscle Ratios (TMR) were derived by visual inspection by an experienced nuclear medicine physician, with TMR>1.2 defining hypoxia.
Results
135 lesions in total were analyzed. TBR, k3 and DV decreased on early response scans, while no significant change was observed for K1. The k3-TBR correlation decreased substantially from baseline scans (Pearson’s r = 0.72 and 0.76 for mean intratumor and pooled voxelwise values, respectively) to early response scans (Pearson’s r = 0.39 and 0.40, respectively). Both concordant and discordant examples of changes in intratumor k3 and TBR were identified; the latter partially mediated by the change in DV. In 13 normoxic patients according to visual analysis (all however having lesions with TMR=1.2), subvolumes were identified where k3 indicated the presence of hypoxia.
Conclusion
Pharmacokinetic modeling of FMISO dynamic PET reveals a more detailed characterization of the tumor microenvironment and assessment of response to chemoradiotherapy in HNC patients than a single static image does. In a clinical trial where absence of hypoxia in primary tumor and lymph nodes would lead to de-escalation of therapy, the observed disagreement between visual analysis and pharmacokinetic modeling results would have affected patient management in <20% cases. While simple static PET imaging is easily implemented for clinical trials, the clinical applicability of pharmacokinetic modeling remains to be investigated.
Keywords: Treatment response, 18F-FMISO, hypoxia, dynamic PET, head and neck cancer
Introduction
There is growing interest in studying essential biologic features of cancer, such as metabolism, hypoxia, and expression of transmembrane receptors, noninvasively and repeatedly, during therapy. In parallel, efforts are underway to adapt therapies based on early treatment response reflecting cancer biology rather than simple structural changes (e.g., changes in tumor volume or shape). Such biologic changes may be a crucial component in the effective management of many cancer patients [1–5]. An improved understanding of biologic processes and their relationship to functional imaging data and tumor response may yield pertinent information that can be applied in a rational way such as adaptive radiotherapy [6], patient stratification in clinical trials (e.g., utilizing hypoxia-activated prodrugs; [7]), development of novel pharmaceutical drugs [8] or optimization of treatments utilizing combinations of targeted drugs with radiotherapy [9].
Hypoxia is a common feature of solid tumors and a major negative prognostic indicator regarding patient outcome after radiotherapy [10]. A meta-analysis of clinical trials in patients with head and neck cancer (HNC) undergoing radiotherapy with curative intent concluded that there is evidence in favor of targeting hypoxia through various treatment modifications [11], although discordant results have also been reported [12]. Hypoxic modification continues to have a limited impact on clinical practice, likely due to the non-selectivity of patients entered on clinical trials [13].
Positron emission tomography (PET) using hypoxia selective imaging probes has the ability to detect tumor hypoxia non-invasively. PET imaging with 18F-fluoromisonidazole (FMISO), the most extensively studied hypoxia PET radiotracer, has been shown to be clinically feasible [14, 15] and reproducible [16, 17]. However, Tumor-to-Blood Ratio (TBR) or Tumor-to-Muscle Ratio (TMR), metrics derived from static FMISO acquisitions that are commonly used as surrogate biomarkers of tumor hypoxia, depend on the exact time point at which images are obtained; in addition, TBR and TMR also depend on the FMISO distribution volume that is non-uniform both within and between tumors [18]. Therefore, presence and extent of hypoxia may be over- or underestimated with this approach. A method that allows for more accurate quantification of hypoxia may help to improve confidence in interpreting hypoxia PET imaging data, in particular if the presence of hypoxia, and changes in hypoxia under treatment, are to be used to guide patient management.
Pharmacokinetic modeling of FMISO dynamic PET (dPET) in principle enables the derivation of a surrogate biomarker that more directly reflects hypoxia-mediated entrapment, while simultaneously providing information on tumor perfusion that may be important in distinguishing different tumor subtypes [19]. Tumor perfusion is an important measure of drug delivery, and changes in perfusion may provide an insight into the response to either nonspecific or targeted (e.g. antiangiogenic) therapies [20]. We recently demonstrated that simultaneous, multiparametric and single modality imaging of tumor hypoxia and perfusion with FMISO dPET can be performed in HNC patients with acquisition protocols with overall scan times as low as 20 min, comparable in duration to diagnostic 18F-Fluorodeoxyglucose (FDG) PET [18].
Complex relationships between tumor hypoxia, perfusion and glucose metabolism support the use of composite metrics providing additional information on tumor biology that may help in designing adaptive therapies [8, 21] and may result in better prediction of patient survival [22, 23]. Of note, these characteristics of the tumor microenvironment may not change in a uniform manner under therapy [8].
In the present study, tumor hypoxia, perfusion and FMISO distribution volume were assessed simultaneously from FMISO dPET scans in 72 head and neck cancer patients at baseline and after 1 cycle of chemotherapy concurrent with anywhere from 5 to 10 fractions of 2 Gy radiation (early response). We hypothesized that pharmacokinetic modeling of FMISO dPET may enable a more comprehensive assessment of tumor response than can be obtained from single static images.
Materials and Methods
Patient selection
Patients with HNC were enrolled prospectively to Institutional Review Board approved multi-arm trial (Protocol #04-070Protocol, NCT00606294 on www.clinicaltrials.gov; inclusion criteria: 18+ age, histologically confirmed diagnosis of squamous cell carcinoma of the head and neck, Karnofsky performance status of ≥70; exclusion criteria: all nasopharyngeal, paranasal sinus, salivary cancer and thyroid malignancies, prior chemotherapy or radiotherapy within the last three years, previous surgical resection for the same disease, any prior radiotherapy to the head and neck region, and pregnancy). All patients signed a written informed consent regarding the examination and use of anonymous data for research and publication purposes. This article reports the results of a small sub-study of the currently ongoing trial (72 patients, aged 60±11 years (range, 25–86 years); Table 1). Flowchart of patient selection and inclusion is presented in Figure 1.
Table 1.
Subject demographics and clinical characteristics.
| N=72 (%) | |
|---|---|
| Gender | |
| Male | 66 (92) |
| Female | 6 (8) |
| Age at diagnosis, y | |
| <40 | 1 (1) |
| 40–49 | 10 (14) |
| 50–59 | 30 (42) |
| 60–69 | 20 (28) |
| 70–79 | 6 (8) |
| 80+ | 5 (7) |
| Clinical tumor classification | |
| T1 | 16 (22) |
| T2 | 36 (50) |
| T3 | 10 (14) |
| T4 | 8 (11) |
| Unknown | 2 (3) |
| Tumor subsite | |
| Base of tongue | 39 (54) |
| Tonsil | 28 (39) |
| Hypopharynx | 1 (1) |
| Supraglottic larynx | 2 (3) |
| Unknown primary | 2 (3) |
| Clinical lymph node classification | |
| N1 | 7 (10)* |
| N2a | 7 (10) |
| N2b | 31 (43) |
| N2c | 25 (35) |
| N3 | 2 ( 3) |
| HPV status | |
| Positive | 47 (85) |
| Negative | 8 (15) |
| Testing not performed | 17 |
| p16 status | |
| Positive | 5 (8) |
| Negative | 60 (92) |
| Testing not performed | 7 |
| Chemotherapy regimen | |
| Carboplatin/5-fluorouracil | 3 (4)* |
| Carboplatin/paclitaxel | 3 (4) |
| Cetuximab/cisplatin/bevacizumab/ | 1 (1) |
| Cetuximab | 7 (10) |
| Cisplatin | 55 (76) |
Numbers do not total 100% due to rounding.
Figure 1.
Flowchart of patient selection and inclusion.
*According to the visual analysis by nuclear medicine physician.
PET/CT image acquisition and analysis
All patients underwent baseline FDG PET/CT scans of the head and neck for radiotherapy planning purposes, followed after 8±6 days by a baseline FMISO dynamic PET/CT scans (FMISO1). A second FMISO scan (FMISO2) was acquired at 17±5 days after FMISO1, after patients have already received 1 cycle of chemotherapy and anywhere from 5–10 fractions of 2 Gy per fraction radiation therapy. For all scans, patients were positioned on a flat-top couch wearing a customized radiotherapy treatment immobilization mask that minimizes motion and allows for accurate repositioning. Intravenous lines inserted in antecubital veins were used for radiotracer injection. Patients were administered an intravenous bolus injection of 390±14 MBq of FMISO. Each FMISO dynamic PET acquisition consisted of 3 segments: (i) at time t=0, a 30 min acquisition binned into 6×5-sec, 3×10-sec, 4×60-sec, 2×150-sec, 2×300-sec and 1×600-sec frames, (ii) a 10 min static acquisition, starting at 93±6 min (FMISO1) and 96±9 min (FMISO2) and (iii) a 10 min static acquisition starting at 160±13 min (FMISO1) and 161±13 min (FMISO2) post-injection.
For both FMISO1 and FMISO2, all three FMISO segments were spatially co-registered using the rigid-body transformation calculated with General Co-Registration™ tool (General Electric Advantage Workstation v4.7) applied to their corresponding CT scans, and subsequently merged to form a concatenated FMISO dPET scan. These two scan series were also co-registered with the FDG PET scan. Lesions were delineated on the FDG PET images, using the adaptive threshold algorithm in the PET VCAR™ (Volume Computer Assisted Reading; General Electric Advantage Workstation v4.7).
Additionally, Tumor-to-Muscle Ratios (TMR) for both FMISO1 and FMISO2 were derived by visual inspection of the three FMISO segments by an experienced nuclear medicine physician (H.S.) as reported previously [6], independently of the medical physicist’s (M.G.) pharmacokinetic modeling. Spherical regions of interest were placed in tumors and metastatic lymph nodes, and activity concentration was measured using max SUV in the tumor volume of interest (VOI). Reference regions were placed in contralateral neck muscles showing low background activity and measured using mean SUV. Hypoxia-positive was defined as FMISO uptake in known sites of disease that was increasing or stable from scan time point i) to time point iii) of each scan series, with a TMR>1.2. Since TBRmax was defined similarly (max SUV in the tumor VOI divided by mean SUV in the jugular vein VOI), synchronization of delineation methods was not required.
Pharmacokinetic modeling
Voxelwise pharmacokinetic modeling of FMISO dPET images was performed in PMOD v3.604 (PMOD Technologies GmbH), utilizing an irreversible one-plasma two-tissue compartment model [18]. The output of the pharmacokinetic modeling are three kinetic rate constants: K1, the kinetic rate constant describing transport from the vascular compartment to the extravascular tissue compartment and a surrogate biomarker of tumor perfusion, k2, the kinetic rate constant describing the transport of FMISO from the extravascular tissue compartment back to blood and k3, the kinetic rate constant approximating the rate of irreversible binding of FMISO and a surrogate for hypoxia-mediated entrapment. An important composite parameter DV (distribution volume) is given by
| (1) |
where vB is the fractional vascular volume also calculated by the model. DV represents the total FMISO distribution volume i.e., overall concentration of unbound FMISO relative to blood, and sets the threshold above which FMISO accumulation is due to hypoxia specific binding. DV was previously shown to be non-uniform across a tumor upon tracer equilibration [18]. The input function (IF) was delineated on ipsilateral (with respect to the lesion) jugular vein on the early dynamic frame with the highest image intensity, by selecting ~100 hottest voxels. TBR was calculated from the 3rd FMISO acquisition, using the measured activity concentration in the IF volume of interest, Cp(t) as the reference,
| (2) |
The parameters vB and DV are unitless, whereas K1 (assuming unit density tissue), k2 and k3 are expressed in units of min−1.
Pharmacokinetic modeling was also performed for ipsilateral (with respect to the primary lesion) sternocleidomastoid muscle, adipose tissue posterior to the cervical vertebrae and ipsilateral submandibular and parotid glands, on both baseline and early response scans.
Statistical analysis
All correlations were performed using Pearson’s correlation coefficient r (measure of correlation strength). Linear regression was performed to model the relationship between TBR (dependent variable) and k3 (explanatory variable), and to calculate the slope coefficient (measure of correlation direction) and intercept coefficient. Comparisons of investigated metrics as calculated from (i) baseline and early response scans or (ii) primary lesions and lymph nodes were performed with unpaired 2-tailed Student t test. p<0.05 was assumed to represent statistical significance.
Results
In total, 135 lesions were analyzed (54 primaries and 81 lymph nodes) by both pharmacokinetic modeling and, independently, by visual analysis. The results of these analyses were subsequently compared side by side by nuclear medicine physician, nuclear medicine physicists and the radiation oncologists. The lesions ranged from 2 to 54 cm3 (median volume: 10 cm3). HPV (by in situ hybridization) and p16 testing (by immunohistochemistry) was performed in 56 and 64 patients, respectively, with 84% and 92% of tested patients harboring HPV positive and p16 positive tumors, respectively. 4 out of 72 patients were deceased at the time of writing, while additional 2 patients experienced local disease relapse (median follow-up period: 26 months, range: 5–73 months).
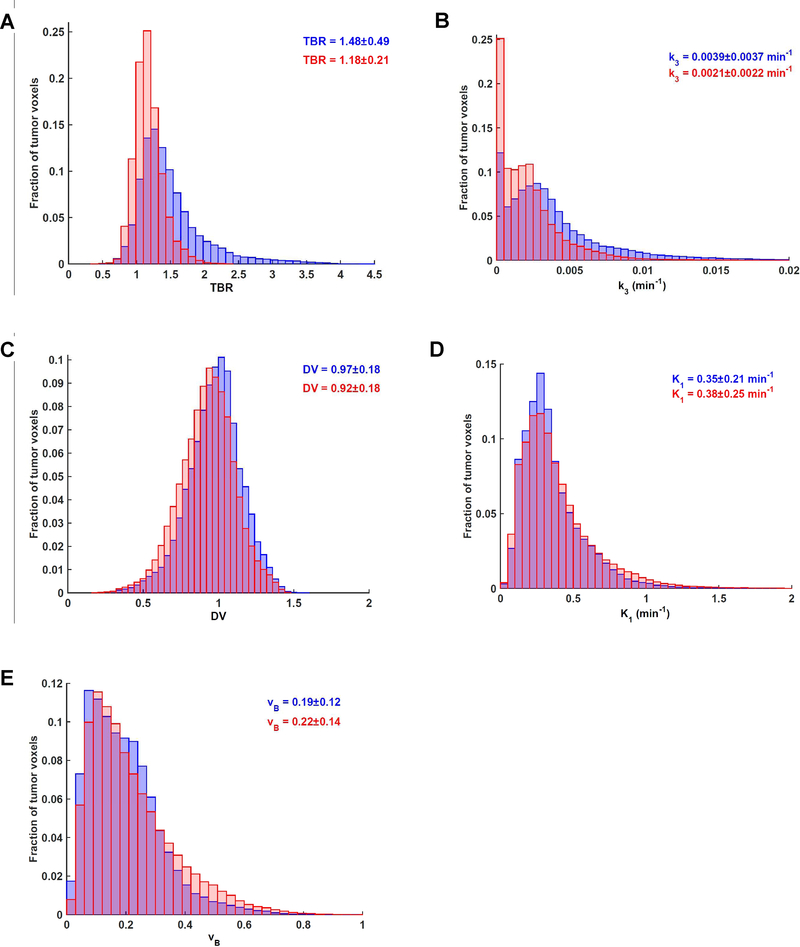
Mean values determined for all investigated parameters (i.e., TBR, k3, DV, K1 and vB) as well as means from voxelwise analysis of pooled data are summarized in Table 2. Changes between early response and baseline FMISO scan data are displayed in Figure 2 for TBR, k3, DV, K1 and vB. There is a visible shift to reduced values for both TBR and k3, indicative of a decrease in hypoxia early during treatment. Baseline k3 was the only metric that was significantly different between primaries and lymph nodes (Supplementary Table S1).
Table 2.
Summary of investigated metrics for the baseline and early response FMISO dynamic PET scans in tumors (n=135).
| Tumor-to-blood Ratio | k3 (min−1) | Distribution volume | K1 (min−1) | vB | |
|---|---|---|---|---|---|
| Baseline (mean intratumor) | 1.35±0.31 | 0.0029± 0.0022 | 0.97± 0.14 | 0.38± 0.17 | 0.20± 0.10 |
| Early response (mean intratumor) | 1.12±0.15 | 0.0020± 0.0016 | 0.91± 0.14 | 0.41± 0.21 | 0.21± 0.11 |
| p-value* | <0.01 | <0.01 | <0.01 | 0.20 | 0.28 |
| Mean difference | −0.22±0.26 | −0.0009± 0.0021 | −0.06± 0.14 | 0.03± 0.21 | 0.01± 0.12 |
| Baseline (voxelwise) | 1.48±0.49 | 0.0039± 0.0037 | 0.97± 0.18 | 0.35± 0.21 | 0.19± 0.12 |
| Early response (voxelwise) | 1.18±0.21 | 0.0021± 0.0022 | 0.92± 0.18 | 0.38± 0.25 | 0.22± 0.14 |
Unpaired 2-tailed Student t test.
Figure 2. Voxelwise distributions of investigated parameters derived from baseline and early response FMISO dynamic PET scans.
(A) Normalized distribution histogram of voxelwise TBR values at baseline (blue) and after 1 cycle of chemoradiotherapy (red), derived from pooled data.
(B) Equivalent histogram for k3.
(C) Equivalent histogram for DV.
(D) Equivalent histogram for K1.
(E) Equivalent histogram for vB.
Corresponding values for normal tissues in the head and neck region are presented in Table 3. Apart from a small but significant decrease in TBR and DV values in submandibular gland, none of the investigated parameters were significantly different between baseline and early response scans for any of analyzed normal tissues.
Table 3.
Summary of investigated metrics for the baseline and early response FMISO dynamic PET scans in normal tissues (n=72 patients).
| Target-to-blood Ratio | k3 (min−1) | Distribution volume | K1 (min−1) | vB | ||
|---|---|---|---|---|---|---|
| Sternocleidomastoid muscle | Baseline | 1.01±0.08 | 0.0007± 0.0006 | 0.89± 0.10 | 0.08± 0.04 | 0.05± 0.03 |
| Early response | 1.00±0.09 | 0.0008± 0.0007 | 0.89± 0.12 | 0.09± 0.04 | 0.06± 0.04 | |
| p-value* | 0.46 | 0.78 | 0.98 | 0.24 | 0.13 | |
| Mean difference | −0.01±0.09 | 0.0000± 0.0007 | 0.00± 0.07 | 0.01± 0.05 | 0.01± 0.04 | |
| Adipose tissue | Baseline | 0.27±0.07 | 0.0004± 0.0004 | 0.24± 0.06 | 0.02± 0.01 | 0.01± 0.01 |
| Early response | 0.25±0.07 | 0.0004± 0.0005 | 0.23± 0.06 | 0.02± 0.01 0.02 0.02 | 0.01± 0.01 0.01± 0.01 | |
| p-value* | 0.11 | 0.69 | 0.40 | 0.85 | 0.84 | |
| Mean difference | −0.02±0.05 | 0.0000± 0.0006 | −0.01± 0.05 | 0.00± 0.02 | 0.00± 0.01 | |
| Submandibular gland | Baseline | 1.02±0.08 | 0.0002± 0.0003 | 1.04± 0.11 | 0.74± 0.53 | 0.16± 0.10 |
| Early response | 1.00±0.09 | 0.0003± 0.0004 | 0.98±0.10 | 0.76± 0.40 | 0.18± 0.11 | |
| p-value* | 0.03 | 0.19 | <0.01 | 0.86 | 0.30 | |
| Mean difference | −0.03±0.08 | 0.0001± 0.0005 | −0.06± 0.10 | 0.01± 0.55 | 0.02± 0.13 | |
| Parotid gland | Baseline | 0.90±0.11 | 0.0002± 0.0004 | 0.88± 0.15 | 0.50± 0.32 | 0.10± 0.08 |
| Early response | 0.87±0.11 | 0.0003± 0.0005 | 0.84± 0.11 | 0.56± 0.32 | 0.12± 0.09 | |
| p-value* | 0.13 | 0.17 | 0.09 | 0.35 | 0.37 | |
| Mean difference | −0.03± 0.09 | 0.0001± 0.0006 | −0.04± 0.10 | 0.05± 0.32 | 0.01± 0.10 |
Unpaired 2-tailed Student t test.
Pearson’s r between mean voxelwise k3 and TBR in baseline scans was 0.72 (95% CI: 0.63–0.79; Fig. 3A). Each point in Figure 3A is color coded according to the mean DV for that tumor, illustrating that nonuniformity in the tumor DV reduces the k3-TBR correlation over the patient population as a whole. These analyses were also performed on a voxel by voxel basis for the entire voxel data ensemble from all tumors. The results are displayed as an intensity histogram for the pooled voxelwise k3-TBR data with the regression line (r = 0.76; Fig. 3B). Lower correlation between k3 and TBR was observed in the results from early response scans, both on intratumor (r = 0.39, 95% CI: 0.24–0.53; Fig. 3C) and voxelwise (r = 0.40; Fig. 3D) levels. The change in mean intratumor k3 (Δk3 = k3,FMISO2 - k3,FMISO1) positively correlated with the change in mean intratumor TBR (ΔTBR = TBRFMISO2 - TBRFMISO1; r = 0.70, p<0.01; Supplementary Figure S1A). Additionally, Δk3 negatively correlated with the change in mean intratumor DV (ΔDV = DVFMISO2 - DVFMISO1; r = −0.56, p<0.01; Supplementary Figure S1B). The correlations between metrics as calculated from baseline and early response scans were: r(TBRFMISO1,TBRFMISO2) = 0.58, r(k3,FMISO1,k3,FMISO2) = 0.41, r(DVFMISO1,DVFMISO2) = 0.53, r(K1,FMISO1,K1,FMISO2) = 0.41 and r(vB,FMISO1,vB,FMISO2) = 0.39 (p<0.01 in all cases). The correlations between metrics as calculated from baseline scans and their change after early response scans were r(TBRFMISO1, ΔTBR) = −0.90, r(k3,FMISO1, Δk3) = −0.75, r(DVFMISO1, ΔDV) = −0.53, r(K1,FMISO1, ΔK1) = −0.43 and r(vB,FMISO1, ΔvB) = −0.48 (p<0.01 in all cases).
Figure 3. Correlation between k3 and TBR as derived from baseline and early response FMISO dynamic PET scans.
(A) Scatterplot (one point per patient tumor) of the mean intratumor k3 versus TBR at baseline, with linear regression fit (solid line). The points are color-coded according to the magnitude of the mean intratumor DV at baseline.
(B) k3-TBR intensity histogram consisting of the pooled voxels from all tumors from baseline data, with the linear regression fit (solid line).
(C) Scatterplot of mean intratumor k3 and TBR from early response scans, with included linear regression fit (dashed line). Superimposed is the linear regression fit from baseline data (A; solid line).
(D) Corresponding intensity histogram from early response scans, with included linear regression fit (dashed line). Superimposed is the linear regression fit from baseline data (B; solid line).
Pharmacokinetic modeling facilitated a more detailed early response assessment to chemoradiotherapy. This is best revealed by investigating individual patient cases that highlight the diversity of individual responses. The parametric maps for two patients reveal a concordance (Fig. 4A) and discordance (Fig. 4B) in changes in TBR and k3, two surrogate metrics of tumor hypoxia derived from static and dynamic PET scans, respectively. For patient #1, the decrease in k3 (Δk3 = −0.0066 min−1) was reflected in the decrease in TBR (ΔTBR = −1.26), with no discernible change in DV (Fig. 4A). However, for patient #2 (Fig. 4B), the decrease in TBR (ΔTBR = −0.17) and DV (ΔDV = −0.24) was observed whereas k3 increased (Δk3 = 0.0026 min−1).
Figure 4. Example of concordant and discordant changes in k3 and TBR.
(A) Patient #1 with 37 cm3 T4aN2c, HPV-positive, p16-positive tumor originating in the tonsil. Results from baseline and early response scans are presented in top and bottom rows, respectively. From left to right: Sagittal view of the late 10-min FMISO PET/CT scan, tumor-to-blood ratio (TBR) map of intratumor voxels, k3 map representing hypoxia-mediated entrapment of FMISO and FMISO distribution volume (DV), representing overall concentration of unbound FMISO relative to blood. For this patient, both TBR and k3 decreased after 1 cycle of chemoradiotherapy, while DV did not change substantially. ΔTBR = −1.26 (ΔTBRmax = −2.82), Δk3 = −0.0066 min−1 (Δk3,max = −0.0160 min−1), ΔDV =−0.01.
(B) Corresponding images for Patient #2 with 44 cm3 T1N2b, HPV-negative, p16-negative tumor originating in the supraglottic larynx. For this patient, TBR decreased, while k3 increased. Decreased DV further contributed to underestimation of TBR. ΔTBR = −0.17 (ΔTBRmax = −0.71), Δk3 = 0.0026 min−1 (Δk3,max = 0.0059 min−1), ΔDV =−0.24.
TMR readings for both baseline and early response FMISO scans were available for 129 out of 135 lesions (for the remaining 6 lesions, TMR readings were not made on early response scans due to indiscernible FMISO uptake, thus assuming the resolution of hypoxia). Pearson’s r between TBRmax (derived as part of pharmacokinetic modeling) and TMR (derived independently by a nuclear medicine physician) was 0.94 and 0.84 on baseline and early response scans, respectively. However, Pearson’s r between k3,max and TMR was 0.66 and 0.33 on baseline and early response scans, respectively.
On baseline scans, TMR was ≤1.2 in 22 lesions, suggesting absence of hypoxia, with 18 out of these 22 lesions having TMR≤1.2 also on early response scans (in the remaining 4 lesions, TMR increased to 1.3). TMR≤1.2 was observed in 56 lesions on early response scans (included are lesions for which FMISO uptake was indiscernible). In 21 out of these 56 lesions (8 primaries and 13 lymph nodes), 4+ consecutive voxels with k3 ≥0.0026 min−1 were identified on early response scans, suggesting the presence of some hypoxia by pharmacokinetic modeling. The k3 threshold was derived as mean k3 + 3 standard deviations of the value as measured in non-diseased sternocleidomastoid muscle on both baseline and early response scans. In all cases, the tumor region with highest k3 also exhibited the lowest DV, typically between 0.4 and 0.6 (thus causing underestimation of total FMISO uptake), possibly indicating a mixture of severely hypoxic, cystic and/or partially necrotic tissue. In 14 lesions, TMR>1.2 was observed on early response scans whereas k3 was <0.0026 min−1 throughout the lesion.
Patient-based results are summarized in Table 4. The majority of patients (69 out of 72) were hypoxia- positive (i.e., having at least 1 lesion with TMR>1.2) on baseline scans. The normoxic three patients remained hypoxia-negative on early response scans, while hypoxia resolution was observed in 20 out of the previously hypoxic 69 patients. In 13 patients without TMR>1.2 lesions on early response scans, pharmacokinetic modeling indicated the presence of hypoxic regions. However, all 13 of these patients had at least one lesion with TMR=1.2. In 5 patients, TMR>1.2 was observed on early response scans whereas k3 was <0.0026 min−1 throughout all patients’ lesions.
Table 4.
Discordance between visual analysis and pharmacokinetic modeling for identification of patients with hypoxia (n=72 patients).
| Baseline | Early response | |
|---|---|---|
| Patients with no hypoxic lesions according to TMR>1.2 (VA-) | 3 | 23 |
| Patients with no hypoxic lesions according to k3>0.0026 min−1 (PM-) | 4 | 15 |
| VA−, PM− | 1 | 10 |
| VA−, PM+ | 2 | 13 |
| VA+, PM+ | 66 | 44 |
| VA+, PM− | 3 | 5 |
TMR - Tumor-to-Muscle Ratio, VA - visual analysis, PM - pharmacokinetic modeling
Discussion
Monitoring early treatment response by imaging studies may provide information that can be used to design response-adapted therapy strategies. However, it is imperative that the therapy-induced changes be interpreted correctly. Establishing a non-invasive and quantitative measurement of the tumor hypoxic burden is an important first step in testing the clinical relevance of hypoxia and its utility as a tumor response biomarker. While the association between pre-treatment tumor hypoxia and poor overall survival after radiotherapy has been reviewed previously [10], recent reports also suggest that FMISO imaging during the initial phase of treatment carries prognostic value for identifying HNC patients at risk from local recurrence [24, 25]. In the current study, we performed baseline and early response FMISO dynamic PET scans in 72 HNC patients to assess the added value of pharmacokinetic modeling for elucidating changes in tumor hypoxia after initiation of chemoradiotherapy.
Static metrics of FMISO images rely on the implicit assumption that FMISO distribution volume (DV) is close to unity in all tissues with dispersion not greater than what can be attributed to image noise. While we have found that DV is indeed close to 1.0 in normal non-adipose tissues in the head and neck region, including sternocleidomastoid muscle and large salivary glands, DV was observed to be non-uniform with a relatively wide spread around 1 within tumor lesions, resulting in an over- or underestimation of the tumor hypoxic fraction [18]. In adipose tissue, the DV was found to be ~0.25 (reflecting the octanol-water partition coefficient of ~0.4). The dependence of DV on tissue composition may potentially explain low intratumor DV values resulting from heterogeneous stroma that contains adipose-like tissue and partially necrotic areas with dysfunctional vasculature. Lesions with DV values greater than 1.0 were also identified (Fig. 4B), suggesting a non-specific mechanism of radiotracer accretion.
Calculation of k3 (a time independent kinetic rate constant) from pharmacokinetic modeling of dynamic PET data results in a more direct surrogate for hypoxia-mediated entrapment than the FMISO uptake from a static scan, since the estimation of k3 is independent of DV, tumor perfusion and imaging time post-injection. In general, changes in TBR and k3 on a whole tumor level were well correlated. However, in 18 out of 148 lesions the total intratumor FMISO uptake decreased on the early response scan (ΔTBR ≤ −0.10) while the hypoxia parameter k3 remained unchanged or increased (Δk3 ≥ 0.0000 min−1), in part because of a decrease in DV (ΔDV = −0.22±0.12). The correlation between k3 and TBR was lower on early response scans compared to baseline scans, indicating the importance of also accounting for the changes in DV when interpreting FMISO static uptake. The changes in DV might be secondary to radiation-induced alteration of the soluble and insoluble components of the tumor microenvironment that affects tissue composition (e.g., leading to the development of cystic structures or necrosis; [26]). The early response scans also result in a convergence of TBR values to values around 1.0 and an increase in the number of operationally zero k3 values, a possible and expected consequence of radiation-induced reoxygenation [27]. FMISO2 scans were performed relatively early into treatment, after patients received 1 cycle of chemotherapy and 5–10 fractions of 2Gy/fraction radiotherapy. We observed that in general, tumor shrinkage lagged behind functional change. In a recent study in a same cohort of HNC patients, Paudyal et al. reported an increase in the apparent diffusion coefficient on diffusion-weighted MRI, indicating a decrease in cellularity in the tumors weeks before any observed volume reduction [28].
The patient examples highlight the potential of FMISO pharmacokinetic modeling to elucidate microenvironmental changes that might be helpful in identifying appropriate therapeutic options. However, the added benefit of performing FMISO dPET depends on the clinical task. For example, in a clinical trial where absence of hypoxia in both primaries and lymph nodes would lead to de-escalation of therapy [6], the disagreement regarding hypoxic status of lesions between visual analysis (TMR>1.2) and pharmacokinetic modeling results (k3≥0.0026 min−1) would have affected 13 out of 72 patients, however all of these patients exhibited lesions with TMR=1.2 (i.e., if TMR≥1.2 were adopted as hypoxia threshold, there would be no disagreement between results from pharmacokinetic modeling and visual analysis for the purpose of dose de-escalation). Low DV values in these cases indicate the onset of therapy-induced necrosis, making such discordance possibly clinically inconsequential. The consequence of false positive hypoxia readings, i.e., when the nuclear medicine physician reports a TMR>1.2 but the k3 value is near zero, is less egregious (5 out of 72 patients, Table 4). In such cases, dose de-escalation would not be recommended and conventional radiation therapy doses delivered. If hypoxia would have been targeted (e.g. via dose escalation), a consensus among the investigators would have to be reached on whether to classify patients as having hypoxic or normoxic lesions. However, the encouraging outcome results suggest that dose escalation, with the intent to improve outcome, is of lower priority in this cohort of patients. 18F-FMISO pharmacokinetic modeling might however be more useful for characterization of the response to treatments that have a direct effect on tumor vascularization and perfusion [29].
Further work is necessary to understand the clinical relevance of the disagreement between visual analysis and pharmacokinetic modeling results and to establish the reproducibility of pharmacokinetic modeling. As only 4 out of 72 patients were deceased at the time of writing (1 metastatic recurrence, 1 local and metastatic recurrence and 2 deaths from other causes; median follow-up period: 26 months), and additional 2 patients experienced local recurrence, survival analysis is presently underpowered due to a paucity of events.
Conclusion
Pharmacokinetic modeling of FMISO dynamic PET reveals a more detailed characterization of the tumor microenvironment and assessment of response to chemoradiotherapy in HNC patients than a single static image does. In a clinical trial where absence of hypoxia in primary tumor and lymph nodes would lead to de-escalation of therapy, the observed disagreement between visual analysis and pharmacokinetic modeling results would have affected patient management in <20% cases. While simple static PET imaging is easily implemented for clinical trials, the clinical applicability of pharmacokinetic modeling remains to be investigated.
Supplementary Material
Supplementary Figure S1. Changes in k3 are correlated with changes in TBR.
(A) Scatterplot of changes in mean intratumor values of k3 and TBR after 1 cycle of chemoradiotherapy, color-coded according to changes in mean intratumor DV.
(B) Scatterplot of changes in mean intratumor values of k3 and DV after 1 cycle of chemoradiotherapy, color-coded according to changes in mean intratumor TBR.
Acknowledgments
Funding: This study was supported by NIH grants 5R01CA157770-04 (P.I. Nancy Y. Lee), U01 CA157442-3 (P.I. Sadek A. Nehmeh) and the cancer center grant P30 CA008748 (P.I. Craig B. Thompson).
Footnotes
Compliance with Ethical Standards:
Conflict of Interest: The authors declare no potential conflicts of interest.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
References
- 1.Schöder H, Fury M, Lee N, Kraus D. PET monitoring of therapy response in head and neck squamous cell carcinoma. J Nucl Med. 2009;50 Suppl 1:74S–88S. [DOI] [PubMed] [Google Scholar]
- 2.Moding EJ, Kastan MB, Kirsch DG. Strategies for optimizing the response of cancer and normal tissue to radiation. Nat Rev Drug Discov. 2013;12:526–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dubois LJ, Niemans R, van Kuijk SK, et al. New ways to image and target tumour hypoxia and its molecular responses. Radiother Oncol. 2015;116:352–357. [DOI] [PubMed] [Google Scholar]
- 4.van Dijk LK, Boerman OC, Kaanders JH, Bussink J. PET Imaging in Head and Neck Cancer Patients to Monitor Treatment Response: A Future Role for EGFR-Targeted Imaging. Clin Cancer Res. 2015;21:3602–3609. [DOI] [PubMed] [Google Scholar]
- 5.Yankeelov TE, Mankoff DA, Schwartz LH, et al. Quantitative Imaging in Cancer Clinical Trials. Clin Cancer Res. 2016;22:284–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee NY, Schöder H, Beattie B, et al. Strategy of Using Intratreatment Hypoxia Imaging to Selectively and Safely Guide Radiation Dose De-escalation Concurrent With Chemotherapy for Locoregionally Advanced Human Papillomavirus-Related Oropharyngeal Carcinoma. Int J Radiat Oncol Biol Phys. 2016;96:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yeh JJ, Kim WY. Targeting tumor hypoxia with hypoxia-activated prodrugs. J Clin Oncol. 2015;33:1505–1508. [DOI] [PubMed] [Google Scholar]
- 8.Padhani AR, Miles KA. Multiparametric imaging of tumor response to therapy. Radiology 2010;2:348–364. [DOI] [PubMed] [Google Scholar]
- 9.Sharma RA, Plummer R, Stock JK, et al. Clinical development of new drug-radiotherapy combinations. Nat Rev Clin Oncol. 2016;13:627–642. [DOI] [PubMed] [Google Scholar]
- 10.Horsman MR, Mortensen LS, Petersen JB, Busk M, Overgaard J. Imaging hypoxia to improve radiotherapy outcome. Nat Rev Clin Oncol. 2012;9:674–687. [DOI] [PubMed] [Google Scholar]
- 11.Overgaard J Hypoxic modification of radiotherapy in squamous cell carcinoma of the head and neck--a systematic review and meta-analysis. Radiother Oncol. 2011;100:22–32. [DOI] [PubMed] [Google Scholar]
- 12.Lim AM, Rischin D, Fisher R, et al. Prognostic significance of plasma osteopontin in patients with locoregionally advanced head and neck squamous cell carcinoma treated on TROG 02.02 phase III trial. Clin Cancer Res. 2012;18:301–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DiSilvestro PA, Ali S, Craighead PS, et al. Phase III randomized trial of weekly cisplatin and irradiation versus cisplatin and tirapazamine and irradiation in stages IB2, IIA, IIB, IIIB, and IVA cervical carcinoma limited to the pelvis: a Gynecologic Oncology Group study. J Clin Oncol. 2014;32:458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koh WJ, Rasey JS, Evans ML, et al. Imaging of hypoxia in human tumors with [F-18]fluoromisonidazole. Int J Radiat Oncol Biol Phys. 1992;22:199–212. [DOI] [PubMed] [Google Scholar]
- 15.Rasey JS, Koh WJ, Evans ML, et al. Quantifying regional hypoxia in human tumors with positron emission tomography of [18F]fluoromisonidazole: a pretherapy study of 37 patients. Int J Radiat Oncol Biol Phys. 1996;36:417–428. [DOI] [PubMed] [Google Scholar]
- 16.Okamoto S, Shiga T, Yasuda K, et al. High reproducibility of tumor hypoxia evaluated by 18Ffluoromisonidazole PET for head and neck cancer. J Nucl Med. 2013;54:201–207. [DOI] [PubMed] [Google Scholar]
- 17.Grkovski M, Schwartz J, Rimner A, et al. Reproducibility of 18F-fluoromisonidazole intratumour distribution in non-small cell lung cancer. EJNMMI Res. 2016;6:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grkovski M, Schöder H, Lee NY, et al. Multiparametric imaging of tumor hypoxia and perfusion with 18F-fluoromisonidazole dynamic PET in head and neck cancer. J Nucl Med. February 9, 2017. doi: 10.2967/jnumed.116.188649 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koch CJ, Evans SM. Optimizing hypoxia detection and treatment strategies. Semin Nucl Med. 2015;45:163–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rajendran JG, Mankoff DA. Positron Emission Tomography Imaging of Blood Flow and Hypoxia in Tumors In Shields AF, Price P In vivo imaging of cancer therapy. Springer Science & Business Media, 2007. [Google Scholar]
- 21.Kadrmas DJ, Hoffman JM. Methodology for quantitative rapid multi-tracer PET tumor characterizations. Theranostics. 2013;3:757–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dunnwald LK, Gralow JR, Ellis GK, Livingston RB, Linden HM, Specht JM, et al. Tumor metabolism and blood flow changes by positron emission tomography: relation to survival in patients treated with neoadjuvant chemotherapy for locally advanced breast cancer. J Clin Oncol. 2008;26:4449–4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Komar G, Kauhanen S, Liukko K, Seppänen M, Kajander S, Ovaska J, et al. Decreased blood flow with increased metabolic activity: a novel sign of pancreatic tumor aggressiveness. Clin Cancer Res. 2009;15:5511–5517. [DOI] [PubMed] [Google Scholar]
- 24.Zips D, Zöphel K, Abolmaali N, et al. Exploratory prospective trial of hypoxia-specific PET imaging during radiochemotherapy in patients with locally advanced head-and-neck cancer. Radiother Oncol. 2012;105:21–28. [DOI] [PubMed] [Google Scholar]
- 25.Wiedenmann NE, Bucher S, Hentschel M, et al. Serial [18F]-fluoromisonidazole PET during radiochemotherapy for locally advanced head and neck cancer and its correlation with outcome. Radiother Oncol. 2015;117:113–117. [DOI] [PubMed] [Google Scholar]
- 26.Barcellos-Hoff MH, Park C, Wright EG. Radiation and the microenvironment - tumorigenesis and therapy. Nat Rev Cancer 2005;5:867–875. [DOI] [PubMed] [Google Scholar]
- 27.Dewhirst MW, Cao Y, Moeller B. Cycling hypoxia and free radicals regulate angiogenesis and radiotherapy response. Nat Rev Cancer 2008;8:425–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paudyal R, Oh JH, Riaz N, et al. Intravoxel incoherent motion diffusion-weighted MRI during chemoradiation therapy to characterize and monitor treatment response in human papillomavirus head and neck squamous cell carcinoma. J Mang Reson Imaging 2017;45:1013–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grkovski M, Emmas SA, Carlin SD. 18F-fluoromisonidazole kinetic modeling for characterization of tumor perfusion and hypoxia in response to antiangiogenic therapy. J Nucl Med. March 30, 2017. doi: 10.2967/jnumed.117.190892. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1. Changes in k3 are correlated with changes in TBR.
(A) Scatterplot of changes in mean intratumor values of k3 and TBR after 1 cycle of chemoradiotherapy, color-coded according to changes in mean intratumor DV.
(B) Scatterplot of changes in mean intratumor values of k3 and DV after 1 cycle of chemoradiotherapy, color-coded according to changes in mean intratumor TBR.