Abstract
A detrimental consequence of hypermethylation is hyperhomocysteinemia (HHcy), that causes oxidative stress, inflammation, and matrix degradation, which leads to multi-pathology in different organs. Although, it is well known that hypermethylation leads to overall gene silencing and hypomethylation leads to overall gene activation, the role of such process in skeletal muscle dysfunction during HHcy condition is unclear. In this study, we emphasized the multiple mechanisms including epigenetic alteration by which HHcy causes skeletal muscle myopathy. This review also highlights possible role of methylation, histone modification, and RNA interference in skeletal muscle dysfunction during HHcy condition and potential therapeutic molecules, putative challenges, and methodologies to deal with HHcy mediated skeletal muscle dysfunction. We also highlighted that B vitamins (mainly B12 and B6), with folic acid supplementation, could be useful as an adjuvant therapy to reverse these consequences associated with this HHcy conditions in skeletal muscle. However, we would recommend to further study involving long-term trials could help to assess efficacy of the use of these therapeutic agents.
Keywords: HYPERMETHYLATION, HOMOCYSTEINE, OXIDATIVE STRESS, MUSCLE DYSFUNCTION, ECM REMODELING
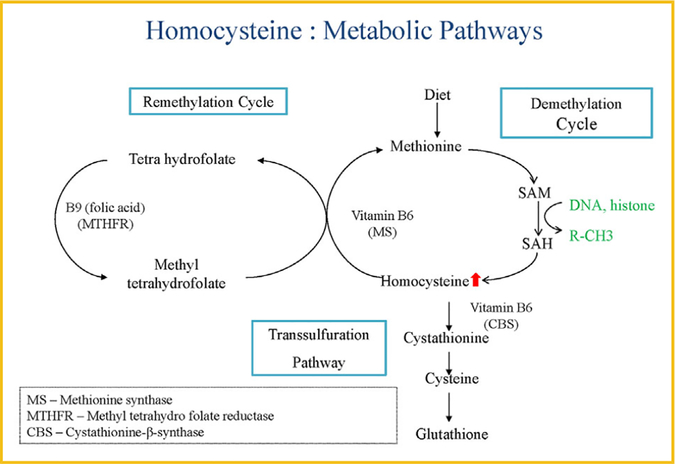
Hyperhomocysteinemia (HHcy/HHCY) is a complex metabolic multifactorial disorder with defects in Hcy (thiol-containing amino acid) metabolism with high prevalence in the general population (~5%) [Brustolin et al., 2010]. HHcy is typically caused either by genetic defects in the enzymes involved in Hcy metabolism (e.g., 677C>T and 1298A>C polymorphism in methylene tetrahydrofolate reductase MTHFR gene) or by nutritional deficiencies in vitamin B12, B6, and folate [Brustolin et al., 2010; Picker and Levy, 2014]. There are also several factors such as age, sex, physical activity, alcohol intake, certain medications, and different disease conditions that can modulate the Hcy level in blood [Ames et al., 2002; Van Guldener, 2006; Signorello et al., 2007; Neuman et al., 2013; Choi et al., 2015]. Depending upon severity of this HHcy conditions it can be classified as follows: (1) moderate HHcy: 15 and 30 μmol/L; (2) intermediate HHcy: 30−100 μmol/L; and (3) severe HHcy: >100 μmol/L [Tiahou et al., 2009]. Hcy is produced during the methionine (MET) cycle, where dietary MET is first converts to S-adenosylmethionine (SAM), then SAM converts to S-adenosylhomocysteine(SAH), which then, finally converts to Hcy [Veeranki and Tyagi, 2013]. The plasma level of Hcy is controlled by two processes: either Hcy is degraded to cysteine via transsulfuration reaction using cystathionine beta synthase (CBS), cystathionine γ-lyase (CSE) enzyme, and B6 as a cofactor or Hcy can be re-methylated to MET by MET-synthase using vitamin B12 as a cofactor and MTHF as the methyl donor through folate cycle [Veeranki and Tyagi, 2013]. In normal conditions, the synthesis and elimination of Hcy are in balance (~50% being re-methylated and ~50% eliminated) [Cascella et al., 2015], however, in diseased state the rate of cellular Hcy synthesis is faster than the elimination, which leads to excess release of Hcy into the blood circulation, and this condition is known as HHcy (Fig. 1) [Cascella et al., 2015].
Fig. 1.
Molecular mechanism of Hyperhomocysteinemia.
HHcy is well accepted risk factor for vascular diseases [Markand et al., 2015], however, several studies also reported that HHcy conditions leads to skeletal muscle weakness and functional impairment [Veeranki and Tyagi, 2013; Picker and Levy, 2014]. Children born with severe homocystinuria due to CBS deficiency exhibit poor body weight, skeletal muscle myopathy, and die in teenage [Picker and Levy, 2014], whereas HHcy condition displays decreased bodyweight and skeletal muscle mass, which ultimately leads to muscle myopathy [Veeranki and Tyagi, 2013]. Results from our laboratory in this field, previously, showed that the expression of CBS and CSE were very less in skeletal muscles, which increases the risk for HHcy mediated injury [Veeranki and Tyagi, 2015]. In the same study, Veeranki et al. [2015] showed that HHcy mice (CBS+/−gene) has lower body weight and skeletal muscle weight of these mice are less fatigue resistant, produces less contractile force, have lower muscle ATP levels, low dystrophin, and mitochondrial transcription factor A (mtTFA) compared to control mice (C57BL/6J) [Veeranki and Tyagi, 2015]. In a study, Kanwar et al. [1976] found that HHcy can cause focal fragmentation, disruption, and smearing of the Z-discs and disorganization of the myofilaments in the skeletal muscles. Its showed that Hcy can reduce cellular metabolic activity and induce energy imbalance in gastrocnemius rat skeletal muscle by decreasing the activity of pyruvate kinase, creatine kinase, and increasing the activity of succinate dehydrogenase [Kolling et al., 2013]. Similarly, Veeranki et al. [2015] showed in a study that Hcy affects fatty acid oxidation of energy metabolism in skeletal muscle via PGC-1α specific protein nitrotyrosylation and reduce its association with PPARγ [Veeranki and Tyagi, 2015]. On the other hand many neurological disorders like amyotrophic lateral sclerosis (ALS) and multiple sclerosis which affect muscle degeneration are also connected to HHcy [Zoccolella et al., 2008; Valentino et al., 2010; Zoccolella et al., 2012]. HHcy also leads to significantly lower physical functions by deteriorating skeletal muscle functions in older people comparing to age matched healthy subjects [Kado et al., 2002], which might suggests that ageing is one of the potential factor to be consider in this process [Westerblad et al., 2010].
HHcy is known to produce oxidative stress, which interfere with different signaling pathway and also inhibits methylation reactions [Hayden and Tyagi, 2004; DiBello et al., 2010; Koz et al., 2010; Mishra et al., 2010], but how ROS affect skeletal muscles during HHcy is unclear. Whereas, various reports also suggested that HHcy develops immune activation via releasing of inflammatory cytokines [Craven and Derubertis, 1984; Wang et al., 2000; Delerive et al., 2001; Au-Yeung et al., 2003; Durga et al., 2005; Gori et al., 2005; Holven et al., 2006; Lazzerini et al., 2007; Thompson et al., 2007; Brustolin etal., 2010; Oudi et al., 2010], the mechanismof such processin skeletal muscle is undetermined. Nevertheless, different studies also showed that HHcy causes proliferative fibrous intimal plaque formation, disorganization, and fibrosis of the media and extracellular matrix (ECM) remodeling [Vermeulen et al., 2001; Mujumdar et al., 2002; Kundu et al., 2009; Tyagi et al., 2009; Mann et al., 2011; Steed and Tyagi, 2011; Lee et al., 2012a; Dick, 2013; Veeranki et al., 2015; Winchester et al., 2015], however, the mechanism of such process is poorly understoodin skeletalmuscle. Due to high MET dietSAMlevelis increased, which leads to global hyper-methylation that causes gene silencing and produces high level of Hcy in this process (HHcy), which affect multiple signaling pathways via oxidative stress, inflammation, and ECM remodeling, but how such conditions cause skeletal muscle myopathy is poorly characterized. In this article we described Hcy mediated skeletal muscle dysfunction via oxidative stress, inflammation, and matrix remodeling and cross talk between different cytokines inthis process. We alsoemphasized the role Hcy in epigenetic alteration through DNA methylation, histone modification, and RNA interference that can be potential risk factors for skeletal muscle dysfunction and summarized the ideas of reversal of HHcy effect in skeletal myopathy.
HHCY MEDIATES OXIDATIVE STRESS AND SKELETAL MUSCLE DYSFUNCTION
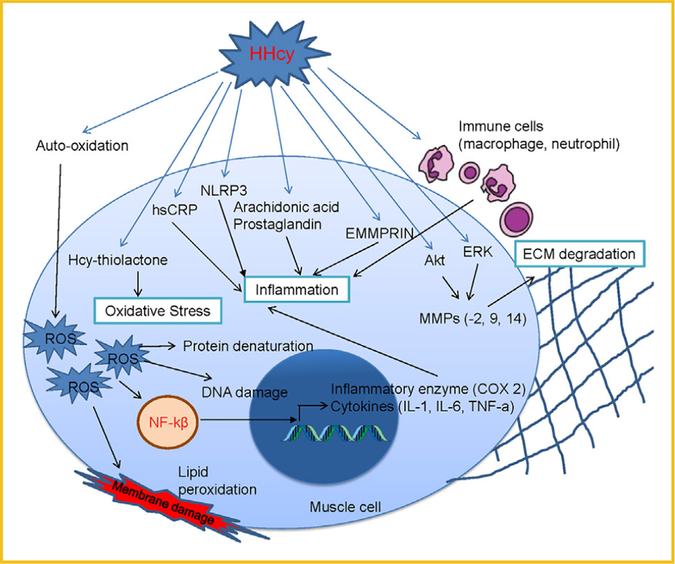
Oxidative stress is known as over production of free radicals than its detoxification that affects many cellular signaling pathways in different organs. Several studies have been reported that HHcy creates oxidative stress that promotes apoptosis, inflammation, insulin resistance, and dysregulation of lipid metabolism in different organs [DiBello et al., 2010]. Although many reports suggested that oxidative stress during HHcy affect different organs, there is no study to support that on skeletal muscle. HHcy typically generates oxidative stress via auto-oxidation of the sulfhydryl group of Hcy that produces oxidized disulfide, two H+, two electrons (e−), promotes the production of reactive oxygen species (ROS) such as superoxide, hydrogen peroxide, and hydroxide radicals [Hayden and Tyagi, 2004]. This increased level of ROS further can cause lipid peroxidation, protein denaturation, and DNA damage (Fig. 2), which ultimately damage cellular components; modulates gene expression, alters cell signaling pathways, and energy imbalance [Veeranki and Tyagi, 2013]. In addition, Hcy can also form Hcy-thiolactone and acetylates free amino groups in proteins, which further intensify the exaggerated oxidative stress condition [Hayden and Tyagi, 2004]. DiBello et al. [2010] showed that proteins (PRDX1, PRDX2, and HSP90AA) which are produce as a response to oxidative stress was significantly upregulated in liver during HHcy [Mishra et al., 2010]. One study showed that production of ROS was ameliorated by PPAR-γ activation in ECs [Hayden and Tyagi, 2004], as previously reported from our laboratory showing that PPAR γ was reduced in HHcy [Mishra et al., 2010], this suggests that HHcy can also induce ROS levels via PPAR- γ mediated pathway. Furthermore, during HHcy, Hcy competitively inhibit cysteine entrance inside the cells. As cysteine is indispensable for the synthesis of glutathione (GSH), lack of cysteine inside the cells in HHcy conditions can compromise the cellular antioxidant potential in skeletal muscle [Brustolin et al., 2010]. HHcy lead to oxidative stress and that interferes with different cellular signaling pathways in different organs, so more studies are required to see such effect on skeletal muscle myopathy in HHcy.
Fig. 2.
Hyperhomocysteinemia mediates skeletal muscle dysfunction.
HHCY MEDIATES INFLAMMATION AND SKELETAL MUSCLE DYSFUNCTION
Inflammation is a biological response of body tissue by external factors (like ROS), which helps to heal that damaged tissue via releasing different inflammatory cytokines, but chronic inflammation can promote skeletal muscle dysfunction. Several studies have been reported that HHcy induces chronic inflammation, which leads to different pathology in different organs; however, the molecular mechanism of this process is still unclear [Brustolin et al., 2010]. During inflammatory condition (myositis) immune cells (CD8+ T lymphocytes) infiltrate through the membrane and attack skeletal muscle cells (auto immune responses), that causes degeneration, muscle weakness, and fibrosis (Fig. 2) [Brustolin et al., 2010]. High concentration Hcy known to activate NF-κB via oxidative stress [Delerive et al., 2001], leads to release of inflammatory cytokine (Fig. 2). Consistent with this HHcy can also activate Caspase-1 through nod-like receptor protein 3 (NLRP3), leading to IL-1β secretion [Brustolin et al., 2010]. Previous studies from our research group showed that HHcy causes reduction of PPAR-g level in muscle and that PPAR- γ shown to have anti-inflammatory activities by inhibiting the expression of pro-inflammatory genes, this suggest that one of the factor for inflammation in HHcy is reduction of PPAR- γ level [Delerive et al., 2001; Mishra et al., 2010]. In another study, Thompson et al. [2007] suggested the anti-inflammatory activity of PPAR-γ by showing the presence of IL-10 promoter under PPAR responsive element (PPRE). Oudi et al. [2010] also found that tHcy correlated with IL-6, TNFα, and high-sensitivity C-reactive protein (hsCRP) levels, which initiate inflammation. In addition to these findings, Holven et al. [2006] suggested that HHcy causes cardiovascular disease via inflammation through increasing IL-1ra, IL-6 levels [Gori et al., 2005; Oudi et al., 2010]. A high blood level of Hcy promotes higher blood levels of arachidonic acid and prostaglandin E2 (PGE2) which can lead to inflammation [Durga et al., 2005]. Various studies also showed that lowering of Hcy concentrations via folic acid supplementation ameliorates these effect and inflammation [Durga et al., 2005]. Finally, as HHcy impair the blood flow through vascular dysfunction, it is very plausible that HHcy could promote or exacerbate pathological remodeling of skeletal muscle as well.
HHCY MEDIATES ECM REMODELING AND SKELETAL MUSCLE DYSFUNCTION
The extracellular matrix (ECM) is present outside of cells surrounding the muscle tissue ECM provides a very rigid structure that helps muscle to overcome high levels of mechanical stress that is placed on it [Au-Yeung et al., 2003]. Hence, muscle remodeling (degradation and regeneration of fibrous ECM proteins like collagen, elastin and fibronectin) is very important to understanding the pathology of myopathic diseases [Au-Yeung et al., 2003]. During chronic inflammation, it comes to excessive collagen deposition and elastin depletion occur, which makes muscle fibrotic, unstable, and rigid (pathological remodeling) [Mann et al., 2011]. Matrix metalloproteinases (MMP’s) are largely responsible for the proper degradation of the ECM in healthy individuals, however study from our laboratory showed that HHcy causes pathogenic vascular remodeling by inducing the levels of MMPs (−2, 9, 14) and reducing elastin, TIMP-4 (Fig. 2) [Kundu et al.,2009]. In another study, it is suggested that Hcy may enhance vascular constrictive remodeling by inactivating PPAR-g in endothelial cells (ECs) and smooth muscle cells (SMCs) but the molecular mechanism is still unknown [Mujumdar et al., 2002]. It has been suggested that HHcy can activate the MMP-9, which leads to degradation of ECM components such as collagen type IV and elastin in vascular system [Mujumdar et al., 2002]. Oxidative stress can also activate MMP-9 via an extracellular signal-regulated kinase (ERK) signaling pathway, that leads to degradation of the gap junction protein connexin-43 (Cx-43) in the myocardium, which causes fibrosis and ventricular dysfunction (Fig. 2) [Tyagi et al., 2009]. Similarly, in another study, Lee et al. [2012a] suggested that Hcy can enhances the production of MMP-9 via ERK signaling pathways, and as well through the Akt pathway in murine macrophages. In addition to activation of MMP-9, Hcy can also induce ECM metalloproteinase inducer (EMMPRIN) that can be induced through inflammatory factors such as interleukin IL-18 [Winchester et al., 2015]. Macrophage are required for the growth and repair of damaged muscle tissue, but during HHcy condition macrophage gets activated due to chronic inflammation which leads to adverse skeletal muscle remodeling [Winchester et al., 2015]. It, also, has been shown that Hcy acts as an antagonist of the gamma aminobutyric acid–A(GABA-A) receptor, which leads to increased ROS production, induced MMP9 activity, and decreased NO production through the uncoupling of eNOS [Winchester et al., 2015]. Furthermore, HHcy can causes skeletal muscle cytoskeletal network degradation including dystrophin and cause the muscle wasting via matrix remodeling [Veeranki et al., 2015]. Regarding to all studies that we described here, it is very clear that HHcy condition leads to skeletal muscle dysfunction via inducing oxidative stress, chronic inflammation, and ECM degradation.
EPIGENETIC REGULATION OF HHCY MEDIATES SKELETAL MUSCLE DYSFUNCTION
Epigenetics is defined as change in gene expression (phenotype) without alterations of DNA sequence, which mostly heritable but can also be occurred during life-time (gene-environment interactions) [Dick, 2013]. There are three major epigenetic modifications which can either silence or activate gene expression: (1) DNA methylation; (2) histone modification; and (3) RNA interference. As Hcy levels are regulated by re-methylation process (converting into methionine) or transsulfuration (converting into cysteine) [Veeranki et al., 2015], due to high MET diet or genetic mutation SAM level is increased, which leads to highpermethylation of DNA and histone [Veeranki et al., 2015]. Global DNA hyper-methylation is known to silent gene expression during HHcy condition, which leads to multi factorial pathology in skeletal muscle [Veeranki et al., 2015]. In addition to DNA methylation, SAM level also serves as the methyl donor for more than one hundred different cellular methyltransferase reactions (histone, RNA etc.). Gene activation or deactivation depends upon methylation pattern of N-terminal tail of histones [Martin and Zhang, 2005]. Moreover, crosstalk between these histone tail modification (methylation, acetylation, and homocysteinylation) may have mechanistic linkages with different disease pathology [Xu L et al., 2015]. Though, there are plenty of evidences which suggested that HHcy is associated with epigenetic alteration that can affect gene expression and cause multifactorial pathology [Handy et al., 2011], but very limited studies have showed the role of these modifications in skeletal muscle dysfunction. Some researchers also reported that the dissimilar detrimental effects of Hcy in various concentrations may be altering gene silencing and activation in different pattern. Mild to moderate HHcy may influence gene expression mainly through the interference of transferring methyl-group metabolism but severe HHcy may induce more injurious effects [Zhou et al., 2014], may be by increasing oxidative stress, promoting apoptosis, inflammation, and matrix remodeling in skeletal muscle.
HHCY MEDIATES SKELETAL MUSCLE DYSFUNCTION THROUGH DNA METHYLATION
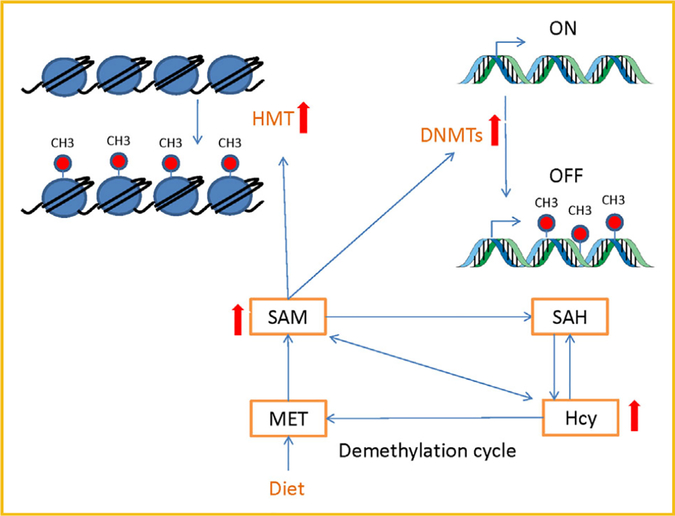
Intake of high MET diet induces SAM (methyl donor) level, which creates hypermethylation as well as increase Hcy level, however, elevated Hcy level may induce SAH synthesis [Zhou et al., 2014]. However, increase in SAH can inhibit SAM-dependent methyltransferases, such as DNMTs via negative feedback mechanism. [Zhou et al., 2014] As DNMTs transfer methyl groups from SAM to cytosine residues in DNA, hence dysfunction of Hcy metabolic pathways may result in DNA hyper/hypomethylation [Zhou et al., 2014]. Several studies in human and animal model have been suggested that HHcy leads to hyper/hypomethylation in tissue specific manner (Fig. 3) [Devlin et al., 2004; Lund et al., 2004; Chaturvedi et al., 2014]. Yideng et al. showed hypomethylation of LINE-1 and Alu after treating of vascular smooth muscle cells (VSMCs) with high Hcy concentration [Zhou et al., 2014], they also indicated that high Hcy may increase SAH and decrease SAM concentrations by changing SAH hydrolase expression and enhancing activity of DNA methyltransferase [Zhou et al., 2014], this suggested that Hcy may inhibit SAH hydrolase and DNA methyltransferase by negative feedback mechanism as we described previously. In contrast, Su et al. [2009] showed that HHcy can induce hypermethylation of CpG islands located in promoter of ERα gene and facilitate the initiation and development of atherosclerosis. Moreover, in a study, Zhou et al. [2014] suggested that HHcy caused hypermethylation in DDAH2, led to apoptosis of ECs, which suggest higher activity of DNMT during HHcy. However, Zhang et al. [2007] showed that 10 and 30 μmol/L Hcy concentration may induce hypomethylation, 100 and 300 μmol/L may induce hypermethylation in the promoter CpG island of DDAH2 gene. Our previous work revealed that HHcy increase global methylation through increasing DNMT −3a, −3b, and H3K18ac expression in CBS−/+ mouse model [Chaturvedi et al., 2014]. Some studies suggested that expression of PPAR-g gene is regulated by DNA methylation of its promoter region and we also described previously that HHcy reduce PPAR level in different tissue [Fujiki et al., 2009], that causes oxidative stress, inflammation, and ECM degradation, but whether HHcy causes myopathy through epigenetic modification of PPAR-γ promoter is still unknown. Although, we have seen that hypo/hyper methylation of DNA depends on duration and degree of the hyperhomocysteinemic state and tissue types [Stern et al., 2000; Yi et al., 2000; Friso et al., 2002; Lund et al., 2004; Castro et al., 2006], but no study has been done to show these effect on skeletal muscle. There are also many other factors have been identified that can modify DNA methylation patterns including the rate of cell growth and DNA replication, chromatin accessibility, local availability of SAM, nutritional factors (folate supplementation), inflammation, dyslipidemias, oxidative stress, and aging [Kouzarides, 2007], which further open up the idea that HHcy can cause muscle dysfunction by changing the expression of specific genes through epigenetic mechanism. So, further studies are required to resolve possible Hcy’s effects on methylation, gene expression, and myopathy.
Fig. 3.
Hyperhomocysteinemia mediates hypermethylation and alteration of gene expression.
HHCY MEDIATES SKELETAL MUSCLE DYSFUNCTION THROUGH HISTONE MODIFICATION
DNA is generally wrapped around the histones protein (two subunit of each H2A, H2B, H3, and H4) to form nucleosome in specific interval of DNA [Mariño-Ramírez et al., 2005]. Histone can be post-translationally modified (acetylation, methylation, phosphorylation, ubiquitination, and sumoylation) by different sets of enzymes which leads to gene activation and repression [Kouzarides, 2007]. This modification is dynamic in nature that means these activation and repressive marks can be reverse by specific sets of enzyme [Lister et al., 2009]. For example, histone methyltransferase (HMTs) transfer a methyl group from SAM to a lysine residue mainly on H3 or H4, while histone demethylases eliminate methyl groups [Lister et al., 2009]. Although alteration of histone modifications can causes upregulation or downregulation of specific gene expression, but very limited studies have been on HHcy mediated histone modification and its associated pathology [Martin and Zhang, 2005]. Since, HHcy can inhibit SAM-dependent methyltransferases via negative feedback mechanism; it can be concluded that HHcy can also alter histone methylation pattern that might influence cellular signaling. In a recent study it was showed that diet induced HHcy can reduce the H3R8me2a marks in brain tissue of wistar rats [Esse et al., 2013]. Same way in CBS mouse model it is showed that global protein arginine methylation status was decreased (10−35%) in liver and brain tissue due to HHcy condition [Esse et al., 2014]. After treatment when Hcy level is reduced, it has shown that causes reduction of repressive mark H3K9me2 on the promoter of COL1A1 human liver cells via decreasing the expression of histone methyltransferase (G9a) [Lei et al., 2015]. Histone (H3 and H4) acetylation also found to be increased in monocytes after treatment with Hcy (100 μmol/L) via suppression of HDAC activity [Wang et al., 2002].
HHCY MEDIATED SKELETAL MUSCLE DYSFUNCTION THROUGH RNA INTERFERENCE
Previously, researchers believed that RNA has only housekeeping function (tRNAs and rRNAs) and messenger (mRNA) between the genes encoded on the DNA [Wang et al., 2002]. In recent years, many new classes of regulatory non-coding RNAs have been identified, including micro-RNA, endogenous small interfering RNAs (endo-siRNAs), PIWI-associated RNAs (piRNAs), and long non-coding RNAs [Wang et al., 2002; Yang et al., 2016]. Different types of RNA interfere with gene expression in different way. In a study by Li et al. [2015a] showed that Hcy (1 mmol/L) regulates miR-30b level, that is involved in cell apoptosis by regulating the expression of caspase-3. In addition to this, Hcy also showed to down-regulate miR-18a that also involves in regulating cell apoptosis [Li et al., 2015a]. Significant decrease in NRF-1 (transcriptional regulator of TFAM A expression) in HHcy mice enhanced mir-494, which cause enhanced fatigability of muscle [Veeranki et al., 2015]. In another study, Mishra et al. [2010] showed that HHcy induces cardiac hypertrophy via suppression of anti-hypertrophy miR-133a in cardiomyocytes [Kesherwani et al., 2015]. Hcy regulates miRNA expression in different tissue types that leads to multiple pathologies, but there were no studies done to show miRNA regulation in skeletal muscle during HHcy, so studies are necessary to determine all predicted miRNA (depending upon the predicted binding site) in skeletal muscle cells to check their regulation during HHcy.
REVERSAL OF HHCY MEDIATES SKELETAL MUSCLE DYSFUNCTION
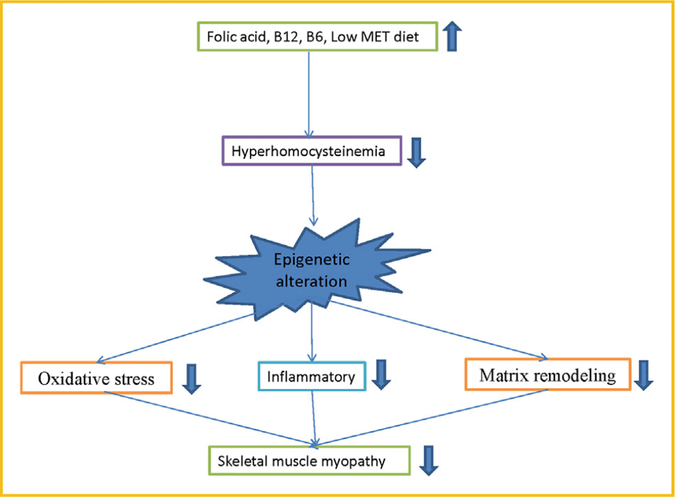
As we already know that Hcy metabolize in two pathways: re-methylation and transsulfuration, which require folate, vitamins B6 and B12 as a cofactors. HHcy is caused due to dietary factors, which mainly include intake of high methionine and deficiency of folate, vitamins B6 and B12 in diet, however, dietary supplementation of these vitamins significantly reduce Hcy levels (Fig. 4) [Kraus et al., 1999; Potena et al., 2000; Riddell et al., 2000; Assanelli et al., 2004; Linnebank et al., 2004; Chanson et al., 2005; Sultan et al., 2007; Stover, 2009; Partearroyo et al., 2010; Sibal et al., 2010; Pizzolo et al., 2011; Lee et al., 2012a; Stamm and Houghton, 2013; Xia et al., 2014; Amini et al., 2015; Dehkordi et al., 2016; Ibrahimagic et al., 2016; Office of Dietary Supplements, 2016]. Several studies have been reported that low vitamin B12 and/or folate are associated with high Hcy and increase the risk for cardiovascular disease, pregnancy complications and neural tube defects [Kraus et al., 1999; Stamm and Houghton, 2013; Ibrahimagic et al., 2016], but very limited study has been done in regards to skeletal muscle dysfunction in HHcy. Although clinical trials suggested that folate therapy is the most effective Hcy-lowering treatment [Monfared et al., 2016], however, some studies have found progression of cardiovascular complications after controlling Hcy level due to may be “memory” effect [Brude et al., 1999]. Whereas, dietary choline and betaine are also important co-factors of the one-carbon pathway, act as an indirect methyl donor [Petr et al., 2013]. In the first part of this article, we have described how HHcy can alters gene expression via DNA methylation (hypo/hyper), histone modification (acetylation/methylation), and RNA interference (mainly microRNAs) in different cell lines, however HHcy mediated epigenetic changes and muscle myopathy yet to be studied. Moreover, all these modifications can be reversible via specific sets of enzymes. Specific environmental factors can induce skeletal muscle dysfunction via modulating Hcy level, to that there should be some factors which can reverse these pathological consequences. Here we predicted various future prospective treatments like supplementation of vitamin B6, B9, B12, choline, betaine, creatinine, exercise, yoga, H2S, and lifestyle modification to ameliorate HHcy mediated skeletal muscle dysfunction.
Fig. 4.
Reversal of Hyperhomocysteinemia mediates skeletal muscle dysfunction.
VITAMIN B6 SUPPLEMENTATION
Vitamin B6 is a cofactor of CBS and CSE in trassulfuration reaction, which eliminate Hcy by converting it to cysteine, hence one of the primary treatment for HHcy is vitamin B6 supplementation. so it is a critical factor for Hcy metabolism. Different study showed that vitamin B6 supplementation prevents the oxidative stress and decrease of prostacyclin generation in HHcy rats [Stamm and Houghton, 2013]. Although many studies showed that vitamin B6 supplementation ameliorate the pathological effect of HHcy, unfortunately not all patients respond same way to vitamin B6 and the exact reason for this is not known yet. Preliminary studies have shown that patients with I278T or R266 K polymorphism in CBS gene respond to vitamin B6 treatment very well [Kraus et al., 1999], whereas individual with G307S and c.1224–2A>C polymorphism doesnot respond to vitamin B6 treatment [Stover, 2009], therefore, vitamin B6 should be given to individual based on measurement of total serum Hcy level [Office of Dietary Supplements, 2016]. Hence, in most cases, B6 responsive individuals also require protein-restricted diet (or MET free formulated supplement) to reverse these pathology associated with HHcy.
B12 SUPPLEMENTATION
Vitamin B12 is most important in MET cycle and act as a cofactor of methionine synthase, which transfer methyl group from folate to homocysteine during remethylation reaction. In a case-control11 study Tayebi et al. [2016], showed that vitamin B12 supplementation reduce serum Hcy levels in patients undergoing hemodialysis [Monfared et al., 2016]; they also found that combined treatment with folate (10 mg/day) and vitamin B12 (1 mg/day) can be considered as a favorable treatment for HHcy. Another study by Menon et al. [2016] suggested that combined supplementation with folic acid, vitamin B6 and vitaminB12 is very effective treatment for HHcy patients [Petr et al., 2013]. It has been shown that supplementation with B vitamins (B9 and B12) correlated to the MTHFR genotypes and have been shown to lower significantly tHcy in hemodialysis patients [Achour et al., 2016]. From these above studies, we might say that intervention with B12 combined with other B vitamins can be possible treatment for skeletal muscle dysfunction in HHcy condition. More study has to be done to confirm this association.
FOLATE (VITAMIN B9) SUPPLEMENTATION
Folate helps in optimizing the conversion of Hcy into MET (in remethylation reaction) by enhancing MET-synthase activity and, thus, lower serum Hcy level. HHcy occurs due to disruption of folate metabolism, which provides one-carbon donor to Hcy for re-methylation reaction and therefore to SAM for methylation reactions [Sibal et al., 2010]. Folic acid is the most effective B vitamin to reduce elevated concentrations of Hcy ~25%; in combination with vitamin B6 and vitamin B12 can lower Hcy concentrations an additional 7% [Liu et al., 2014]. Many studies showed folates are required for cellular proliferation, methylation reactions, and maintenance of Hcy activation/repressive marks at non-toxic levels. That is why a demand of folate increase during embryonic development (due to globalremethylation and demethylation) is necessary and inadequate supply of folate leads neural tube defect [Stamm and Houghton, 2013]. Iamopas [2015] showed that folic acid supplementation can also reduce Hcy level in obese children [Dehkordi et al., 2016]. Folate supplementation can modulate biochemical markers in one-carbon metabolism such as tHcy and the AdoMet/AdoHcy ratio in HHcy subjects [Pizzolo et al., 2011], hence low-folate intake and a polymorphism in MTHFR have been associated with increased risk for several cancers, likely through dysregulated DNA and histone methylation patterns [Lee et al., 2012b]. However, some studies also found that folic acid supplementation does not exert a protective effect for HHcy and global DNA methylation [Partearroyo et al., 2010] which may be due to the involvement of some other confounding factors like age, sex, and ethnicity. One report has suggested that simple non-toxic folic acid intervention might be useful in primary cardiovascular prevention in this high risk group because Hcy is a stronger risk factor for [Sultan et al., 2007]. In another study, it is showed that combined treatment with folic acid, vitamins B12 and B6 for 2 years normalize high Hcy level in regular extracorporeal dialysis patients [Amini et al., 2015]. Folic acid is also a supplement of choice in treatment of HHcy coexisting with other different diseases [Potena et al., 2000; Assanelli et al., 2004; Sibal et al., 2010; Xia et al., 2014; Ibrahimagic et al., 2016].
CHOLINE SUPPLEMENTATION
Choline can also provide carbon units to remethylate Hcy (predominantly in the liver and kidney) through its metabolite betaine [Jadavji, 2015], this pathway is increased when folate- dependent remethylation is disturbed [Schwahn et al., 2004]. The requirement for choline is also increased during pregnancy for remethylation of Hcy, synthesis of acetylcholine, neurogenesis, and myelination [Zeisel, 2006; Meck et al., 2008]. Maternal choline stores can be depleted during pregnancy and lactation due supply to the fetus, which suggest choline supplementation is as important as other B vitamins to reversal of HHcy effect [Zeisel, 2006].
BETAINE SUPPLEMENTATION
Betaine is an important co-factor of the one-carbon pathway of Hcy metabolism, hence it has Hcy-lowering effects and act as a methyl donor. Sometimes betaine along with folic acid is given to vitamin B6 non-responsive patients [Office of Dietary Supplements, 2016]. Study showed that betaine or spinach could completely suppress the HHcy induced by choline deficiency resulting from stimulating the Hcy removal by both remethylation and cystathionine formation [Liu et al., 2014]. Substances like folic acid, vitamins B12 and B6, or betaine can influence the methionine-homocysteine cycle and thus lower the concentrations of Hcy [Rajaie and Esmaillzadeh, 2011; Rajdl et al., 2016], which suggest that betaine supplementation can also reduce skeletal muscle myopathy during HHcy condition.
CREATININE SUPPLEMENTATION
Creatinine supplementation has been known to enhance muscle fiber size, strength and increased lean body mass [Greenhaff et al., 1994]. In addition to energy production via phosphorylated creatinine (PCr), it is also connects to methionine cycle, where guanidinoacetate (GAA) gets methylated from SAM to produce creatinine [Petr et al., 2013]. Creatinine supplementation stimulates production of sarcosine which can serve to regenerate tetrahydrofolate (THF) to 5,10-methylene-THF, which could increase MTHFR enzyme activity and subsequently Hcy remethylation [Petr et al., 2013]. So, creatinine supplementation reduces the body’s demands for methyl group, which could be beneficial treatment for HHcy condition in skeletal muscle.
EXERCISE AND YOGA
Interestingly, elevated levels of Hcy can also affect beta-2 adrenergic receptors (2AR), gamma amino butyric acid (GABA), and peroxisome [Winchester et al., 2014]. During excercise contracting skeletal muscle releases interleukin (IL)-6, which increases the production of anti-inflammatory cytokines, antigenic factors, and follistatin, which improves muscle growth and possibly insulin sensitivity through the inhibition of myostatin [Greenhaff et al., 1994]. It has also been revealed that exercise induce cellular anti-oxidative capacity, which reduces levels of free radicals and mitigation of pathologies induced by ROS [Winchester et al., 2014]. Different studies suggested that exercise lowers Hcy levels, which reduces the adverse effect associated with HHcy. Barre’s et al. [2012] showed that exercise also changes gene expression through alteration of DNA methylation that triggers structural and metabolic adaptations in skeletal muscle [Winchester et al., 2014]. Neuman et al. [2013] stated that exercise lower plasma Hcy concentration in normal rangein folate-restricted diet mouse models [Winchester et al., 2014]. Another study showed that exercise can prevent Hcy-induced increases in lipid peroxidation and decreases in superoxide dismutase and catalase activity in mice [Lee et al., 2012a], suggesting that oxidative stress caused by HHcy can be reversed by exercise. Studies in humans have also revealed that exercise lowers Hcy levels in overweight and obese adults as well as in women with polycystic ovarian syndrome [Sam, 2007]. In a study, Gaume et al. [2005] suggested that demonstrates that the combined effects of a chronic physical exercise and a high folate and vitamin B intake could be responsible for the reduction of plasma tHcy and total cysteine (tCys) concentrations that might be a key for the prevention of many diseases [Gaume et al., 2005]. In addition to exercise, yoga also was found to be associated with reductions in severity of dysmenorrhea and may be effective in lowering serum Hcy levels after an intervention period of 8 weeks [Chien et al., 2013]. It is ideal that exercise and yoga can reduce skeletal muscle myopathy via normalizing the effect of HHcy.
H2S TREATMENT
Hydrogen sulfide (H2S) has been shown to have an important role in different physiological functions, [Weber et al., 2016] it has shown that GYY4137 (H2S-releasing compound) reduced the TNF-α and IL-1β levels in the plasma of HHcy mice [Li et al., 2015b]. Hcy inhibited CSE expression and H2S production in macrophages, accompanied by the increases of DNA methyltransferase (DNMT) expression and DNA hypermethylation in CSE promoter region [Li et al., 2015b]. Hence, H2S can be used as a potential treatment to reduce HHcy related pathology in skeletal muscle myopathy.
LIFE STYLE CHANGES
Smoking habits are related with the increase of basal and after methionine load HHcy (probably via decrease in vitamin B6 levels) which suggest that vitamin B6 supplements for smokers could decrease the vascular risk related with smoking habit [Reis et al., 2016]. Other risk factors modification efforts, often not highlighted, include managing HHcy, and sedentary behavior. These factors are presented as equally important for vascular disease. Our findings indicated that cigarette smoking was associated with renal function deterioration in hypertensive patients, and the association between cigarette smoking and renal function deterioration was probably mediated by elevated Hcy. Therefore, Hcy-lowering therapy may be beneficial for renal function deterioration in hypertensive smoking patients. In life style modification, diet is an important factor; a study suggested that low methionine diet (reduction of the daily intake of methionine to 15−20 mg/kg of body weight) reduce the concentrations of methionine, tHcy, AdoMet, and AdoHcy in plasma [Dawsona et al., 2016], which may be the first step to reduce the Hcy level in our body during HHcy.
OMEGA-3 FATTY ACIDS
Animal and in-vitro studies reveal that omega-3 PUFAs enhance lipid metabolism and decrease Hcy concentration by up-regulating metabolic enzymes and improving substrate availability for Hcy degradation [Monfared et al., 2016]. This study provides more evidence to support of omega-3 PUFAs for reducing Hcy.
COMMERCIAL DRUGS
Statins improve prognosis in patients with coronary heart diseases by decreasing the incidence of vascular events. These data support the association between lower tHcy levels and atorvastatin administration in renal transplant recipients [Palomba et al., 2010]. Further clinical trials are recommended to clarify Hcy lowering effect of atorvastatin. Metformin is a drug which has been used to treat type II diabetes patients. However administration of this drug shown to increase Hcy level, so it is suggested that with this therapy patients use vitamin B12 and folic acid supplementation for balancing the Hcy [Palomba et al., 2010]. As plasma Hcy and the mononuclear cell mRNA levels of PPARδ) level is negatively correlated [Brude et al., 1999], therefore, Thiazolidinedione (TZD) group drugs (PPAR-γ agonist) will also be beneficial to counteract with Hcy mediated effects, more studies need to prove the positive effect of TZD during HHcy via reducing the pathological effect of Hcy like skeletal muscle dysfunction.
CONCLUSION
In the above studies, we have noticed that HHcy induce oxidative stress, inflammation, and matrix remodeling in different organs via multiple mechanisms. There are very limited studies which showed this similar effect in skeletal muscle HHcy condition. Whereas, during HHcy condition, we have seen DNA and histone hyper/hypo methylation occurs, that is, leading to alteration of cellular signaling in different organs. These hyper/hypo methylation are tissue specific, so effect of such process may vary in skeletal muscle during HHcy condition. There are various factors also shown alter epigenetic pattern which includes the rate of cell growth and DNA replication, chromatin accessibility, local availability of SAM, nutritional factors including folate supplementation, duration, and degree of the hyperhomocysteinemic state, inflammation, dyslipidemias, oxidative stress, and aging. Thus, the relation between increased Hcy and DNA global hypomethylation might be masked in the clinical setting due to the presence of these confounders. Another important aspect to consider is that DNA methylation is unequally distributed throughout chromosomes of differentiated cells. Thus, hyper/hypomethylated regions can coexist in the genome and global DNA methylation status need not correspond to the methylation status of specific genomic regions. We have also found supplementation with different external factors such as vitamins B6, B9, B12, choline, betaine, creatinine, exercise, yoga, H2S, and lifestyle modification, affect the methylation reaction can reverse these pathological consequences via reducing Hcy level. In this paper, we identified that folic acid combined with other B vitamins can significantly reduce Hcy level as well as pathological consequence of HHcy, which suggests that B vitamins and folic acid is most potential treatments to reversal of skeletal muscle myopathy due to HHcy condition, although long-term trials are necessary to assess efficacy of the use of these therapeutic agents.
We also suggest further studies in the area of hypermethylation and homocysteine mediated skeletal muscle dysfunction.
Grant sponsor:
National Institutes of Health grants; Grant number: HL-74185.
REFERENCES
- Achour O, Elmtaoua S, Zellama D, Omezzine A, Moussa A, Rejeb J, Boumaiza I, Bouacida L, Rejeb NB, Achour A, Bouslama A. 2016. The C677T MTHFR genotypes influence the efficacy of B9 and B12 vitamins supplementation to lowering plasma total homocysteine in hemodialysis. J Nephrol 29(5):691–698. [DOI] [PubMed] [Google Scholar]
- Ames BN, Elson-Schwab I, Silver EA. 2002. High-dose vitamin therapy stimulates variant enzymes with decreased coenzyme binding affinity (increased Km): Relevance to genetic disease and polymorphisms1,2,3. Am J Clin Nutr 75:616–658. [DOI] [PubMed] [Google Scholar]
- Amini M, Khosravi LM, Baradaran HR. 2015. Vitamin B12 supplementation in end stage renal diseases: A systematic review. Med J Islam Repub Iran 29:167. [PMC free article] [PubMed] [Google Scholar]
- Assanelli DL, Bonanome A, Pezzini A. 2004. Folic acid and vitamin E supplementation effects on homocysteinemia, endothelial function and plasma antioxidant capacity in young myocardial-infarction patients. Pharmacol Res 49(1):79–84. [DOI] [PubMed] [Google Scholar]
- Au-Yeung KK, Woo CW, Sung FL, Yip JC, Siow YL, O K. 2003. Hyperhomocysteinemia activates nuclear factor-kappaB in endothelial cells via oxidative stress. Circ Res 94(1):28–36. [DOI] [PubMed] [Google Scholar]
- Barre’s R, Yan J, Egan B. 2012. Acute exercise remodels promoter methylation in human skeletal muscle. Cell Metab 15:405–411. [DOI] [PubMed] [Google Scholar]
- Brude IR, Finstad HS, Seljeflot I. 1999. Plasma homocysteine concentration related to diet, endothelial function and mononuclear cell gene expression among male hyperlipidaemic smokers. Eur J Clin Investig 29:100–108. [DOI] [PubMed] [Google Scholar]
- Brustolin S, Giugliani R, Félix TM. 2010. Genetics of homocysteine metabolism and associated disorders. Braz J Med Biol Res 43(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascella MMC, Arcamone MMA, Morelli EEM. 2015. Multidisciplinary approach and anesthetic management of a surgical cancer patient with methylene tetrahydrofolate reductase deficiency: A case report and review of the literature. J Med Case Rep 9:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro R, Rivera I, Blom HJ, Jakobs C, Tavaresde Almeida I. 2006. Homocysteine metabolism, hyperhomocysteinaemia and vascular disease: An overview. J Inherit Metab Dis 29:3–20. [DOI] [PubMed] [Google Scholar]
- Chanson A, Sayd T, Rock E. 2005. Proteomic analysis reveals changes in the liver protein pattern of rats exposed to dietary folate deficiency. J Nutr 135(11):2524–2529. [DOI] [PubMed] [Google Scholar]
- Chaturvedi P, Kalani A, Givvimani S. 2014. Differential regulation of DNA methylation versus histone acetylation in cardiomyocytes during HHcy in vitro and in vivo: An epigenetic mechanism. Physiol Genomics 46(7): 245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien LW, Chang HC, Liu CF. 2013. Effect of yoga on serum homocysteine and nitric oxide levels in adolescent women with and without dysmenorrhea. J Altern Complement Med 19(1):20–23. [DOI] [PubMed] [Google Scholar]
- Choi S-H, Choi-Kwon S, Kim M-S. 2015. Poor nutrition and alcohol consumption are related to high serum homocysteine level at post-stroke. Nutr Res Pract 5:503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven PA, Derubertis FR. 1984. Phospholipid methylation in the calcium- dependent release of arachidonate for prostaglandin synthesis in renal medulla. J Lab Clin Med 104(4):480–493. [PubMed] [Google Scholar]
- Dawsona SL, Bowe SJ, Crowe TC. 2016. A combination of omega −3 fatty acids, folic acid and B-group vitamins is superior at lowering homocysteine than omega −3 alone: A meta-analysis. Nutr Res 36(6):499–508. [DOI] [PubMed] [Google Scholar]
- Dehkordi EH, Sattari F, Khoshdel A. 2016. Effect of folic acid and metformin on insulin resistance and inflammatory factors of obese children and adolescents. J Res Med Sci 21:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pl Delerive, Fruchart JC, Staels B. 2001. Peroxisome proliferator-activated receptors in inflammation control. J Endocrinol 169(3):453–459. [DOI] [PubMed] [Google Scholar]
- Devlin AM, Arning E, Bottiglieri T, Faraci FM, Rozen R, Lentz SR. 2004. Effect of Mthfr genotype on diet-induced hyperhomocysteinemia and vascular function in mice. Blood 103:2624–2629. [DOI] [PubMed] [Google Scholar]
- DiBello PM, Dayal S, Kaveti S. 2010. The nutrigenetics of hyperhomocysteinemia. Mol Cell Proteomics 9:471–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM. 2013. Gene-environment interaction in psychological traits and disorders. Annu Rev Clin Psychol 7:383–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durga J, van Tit LJ, Schouten EG. 2005. Effect of lowering of homocysteine levels on inflammatory markers. Arch Intern Med 165:1388–1394. [DOI] [PubMed] [Google Scholar]
- Esse R, Florindo C, Imbard A, Rocha MS, de Vriese AS, Smulders YM, Teerlink T, Tavares de Almeida I, Castro R, Blom HJ. 2013. Global protein and histone arginine methylation are affected in a tissue-specific manner in a rat model of diet-induced hyperhomocysteinemia. Biochim Biophys Acta 1832:1708–1714. [DOI] [PubMed] [Google Scholar]
- Esse R, Imbard A, Florindo C, Gupta S, Quinlivan EP, Davids M, Teerlink T, Tavares de Almeida I, Kruger WD, Blom HJ, Castro R. 2014. Protein arginine hypomethylation in a mouse model of cystathionine β-synthase deficiency. FASEB J 28:2686–2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friso S, Choi SW, Girelli D, Mason JB, Dolnikowski GG, Bagley PJ, Olivieri O, Jacques PF, Rosenberg IH, Corrocher R, Selhub J. 2002. A common mutation in the 5,10-methylenetetrahydrofolate reductase gene affects genomic DNA methylation through an interaction with folate status. Proc Natl Acad Sci USA 99:5606–5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki K, Kano F, Shiota K. 2009. Expression of the peroxisome proliferator activated receptor gamma gene is repressed by DNA methylation in visceral adipose tissue of mouse models of diabetes. BMC Biol 7:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaume V, Mougin F, Figard H. 2005. Physical training decreases total plasma homocysteine and cysteine in middle-aged subjects. Ann Nutr Metab 49: 125–131. [DOI] [PubMed] [Google Scholar]
- Gori AM, Corsi AM, Fedi S. 2005. A proinflammatory state is associated with hyperhomocysteinemia in the elderly. Am J Clin Nutr 82(2):335–341. [DOI] [PubMed] [Google Scholar]
- Greenhaff PI, Bodin K, Soderlund K, Hultman E. 1994. Effect of oral creatine supplementation on skeletal muscle phosphocreatine resynthesis. Am J Physiol 266:E725–E730. [DOI] [PubMed] [Google Scholar]
- Handy DE, Castro R, Loscalzo J. 2011. Epigenetic modifications: Basic mechanisms and role in cardiovascular disease. Circulation 123(19): 2145–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden MR, Tyagi SC. 2004. Homocysteine and reactive oxygen species in metabolic syndrome, type 2 diabetes mellitus, and atheroscleropathy: The pleiotropic effects of folate supplementation. Nutr J 3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holven KB, Aukrust P, Retterstol K. 2006. Increased levels of C-reactive protein and interleukin-6 in hyperhomocysteinemic subjects. Scand J Clin Lab Invest 66(1):45–54. [DOI] [PubMed] [Google Scholar]
- Iamopas O 2015. Effect of folic acid supplementation on plasma homocysteine in obese children: A randomized, double-blind, placebo-controlled trial. J Med Assoc Thai 97(S-6):195–204. [PubMed] [Google Scholar]
- Ibrahimagic OC, Smajlovic D, Dostovic Z. 2016. Hyperhomocysteinemia and its treatment in patients with parkinson’s disease. Mater Sociomed 28(4):303–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadavji N 2015. MTHFR deficiency or reduced intake of folate or choline in pregnant mice results in impaired short-term memory and increased apoptosis in hippocampus of wild-type offspring. Neuroscience 300:1–9. [DOI] [PubMed] [Google Scholar]
- Kado DM, Bucur A, Selhub J. 2002. Homocysteine levels and decline in physical function: MacArthur studies of successful aging. Am J Med 113: 537–542. [DOI] [PubMed] [Google Scholar]
- Kanwar YS, Manaligod JR, Wong PW. 1976. Morphologic studies in a patient with homocystinuria due to 5, 10-methylenetetrahydrofolate reductase deficiency. Pediatr Res 10(6):598–609. [DOI] [PubMed] [Google Scholar]
- Kesherwani V, Nandi SS, Sharawat SK. 2015. Hydrogen sulfide mitigates homocysteine mediated pathological remodeling by inducing miR-133a in cardiomyocytes. Mol Cell Biochem 404:241–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolling J, Scherer EBS, Siebert C. 2013. Homocysteine induces energy imbalance in rat skeletal muscle:Iscreatine a protector? Cell Biochem Funct 31:575–584. [DOI] [PubMed] [Google Scholar]
- Kouzarides T 2007. Chromatin modifications and their function. Cell 128:693–705. [DOI] [PubMed] [Google Scholar]
- Koz ST, Gouwy NT, Demir N. 2010. Effects of maternal hyperhomocysteinemia induced by methionine intake on oxidative stress and apoptosis in pup rat brain. Int J Dev Neurosci 28:325–329. [DOI] [PubMed] [Google Scholar]
- Kraus JP, Janosik M, Kozich V. 1999. Cystathionine? synthase mutations in homocystinuria. Hum Mutat 13(5):362–375. [DOI] [PubMed] [Google Scholar]
- Kundu S, Tyagi N, Sen U. 2009. Matrix imbalance by inducing expression of metalloproteinase and oxidative stress in cochlea of hyperhomocysteinemic mice. Mol Cell Biochem 332(1–2):215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzerini PE, Capecchi PL, Selvi E. 2007. Hyperhomocysteinemia, inflammation and autoimmunity. Autoimmun Rev 6:503–509. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Lee YS, Seo KW. 2012a. Homocysteine enhances MMP-9 production in murine macrophages via ERK and Akt signaling pathways. Toxicol Appl Pharmacol 260(1):89–94. [DOI] [PubMed] [Google Scholar]
- Lee M-S, Asomaning K, Su L, Wain JC. 2012b. MTHFR Polymorphisms, folate intake, and carcinogen DNA adducts in the lung. Int J Cancer 131(5): 1203–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei W, Long Y, Li S. 2015. Homocysteine induces collagen I expression by downregulatin histone methyltransferase G9a. PLoS ONE 10(7):e0130421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Chen Q, Song X. 2015. MiR-30b is involved in the homocysteine-induced apoptosis in human coronary artery endothelial cells by regulating the expression of caspase 3. Int J Mol Sci 16(8):17682–17695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JJ, Li Q, Du HP, Wang YL. 2015. Homocysteine triggers inflammatory responses in macrophages through inhibiting CSE-H2S signaling via DNA hypermethylation of CSE promoter. Int J Mol Sci 16(6):12560–12577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnebank M, Janosik M, Kozich V. 2004. The cystathionine-synthase (CBS) mutation c.1224–2A>C in Central Europe: Vitamin B6 nonresponsiveness and a common ancestral haplotype. Hum Mutat 24(4):352–353. [DOI] [PubMed] [Google Scholar]
- Lister R, Pelizzola M, Dowen RH, Hawkins RD, Hon G, Tonti-Filippini J, Nery JR, Lee L, Ye Z, Ngo QM, Edsall L, Antosiewicz-Bourget J, Stewart R, Ruotti V, Millar AH, Thomson JA, Ren B, Ecker JR. 2009. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature 462:315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YQ, Jia Z, Han F. 2014. Suppression effects of betaine-enriched spinach on hyperhomocysteinemia induced by guanidinoacetic acid and choline deficiency in rats. Sci World J 2014:904501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund G, Andersson L, Lauria M, Lindholm M, Fraga MF, Villar-Garea A, Ballestar E, Esteller M, Zaina S. 2004. DNA methylation polymorphisms precede any histological sign of atherosclerosis in mice lacking apolipoprotein E. J Biol Chem 279:29147–29154. [DOI] [PubMed] [Google Scholar]
- Mann CJ, Perdiguero E, Kharraz Y. 2011. Aberrant repair and fibrosis development in skeletal muscle. Skelet Muscle 1:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariño-Ramírez L, Kann MG, Shoemaker BA. 2005. Histone structure and nucleosome stability. Expert Rev Proteomics 2(5):719–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markand S, Saul A, Roon P. 2015. Retinal ganglion cell loss and mild vasculopathy in methylene tetrahydrofolate reductase (Mthfr)-Deficient mice: A model of mild hyperhomocysteinemia. Invest Ophthalmol Vis Sci 56(4):2684–2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C, Zhang Y. 2005. The diverse functions of histone lysine methylation. Nat Rev Mol Cell Biol 6:838–849. [DOI] [PubMed] [Google Scholar]
- Meck WH, Williams CL, Cermak JM. 2008. Developmental periods of choline sensitivity provide an ontogenetic mechanism for regulating memory capacity and age-related dementia. Logo Front Integneuro 1(7):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon S, Nasir B, Avgan N. 2016. The effect of 1 mg folic acid supplementation on clinical outcomes in female migraine with aura patients. J Headache Pain 17(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra PK, Tyagi N, Sen U. 2010. Synergism in hyperhomocysteinemia and diabetes: Role of PPAR gamma and tempol. Cardiovasc Diabetol 9:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monfared A, Azimi SZ, Kazemnezhad E. 2016. The association between atorvastatin administration and plasma total homocysteine levels in renal transplant recipients. J Nephropathol 5(3):98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujumdar VS, Tummalapalli CM, Aru GM. 2002. Mechanism of constrictive vascular remodeling by homocysteine: Role of PPAR. Am J Physiol Cell Physiol 282(5):C1009–C1015. [DOI] [PubMed] [Google Scholar]
- Neuman JC, Albright KA, Schalinske KL. 2013. Exercise prevents hyperhomocysteinemia in a dietary folate-restricted mouse model. Nutr Res 33(6): 487–493. [DOI] [PubMed] [Google Scholar]
- Office of Dietary Supplements. 2016. Dietary supplement Fact Sheet: Vitamin B6.
- Oudi ME, Aouni Z, Mazigh C. 2010. Homocysteine and markers of inflammation in acute coronary syndrome. Exp Clin Cardiol 15(2):e25–e28. [PMC free article] [PubMed] [Google Scholar]
- Petr M, Šteffl M, Kohlíková E. 2013. Effect of the MTHFR 677C/T polymorphism on homocysteinemia in response to creatine supplementation: A case study. Physiol Res 62:721–729. [DOI] [PubMed] [Google Scholar]
- Palomba S, Falbo A, Giallauria F. 2010. Effects of metformin with or without supplementation with folate on homocysteine levels and vascular endothelium of women with polycystic ovary syndrome. Diabetes Care 33(2): 246–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partearroyo T, Úbeda N, Alonso-Aperte E, Varela-Moreiras G. 2010. Moderate or supranormal folic acid supplementation does not exert a protective effect for homocysteinemia and methylation markers in growing rats. Ann Nutr Metab 56:143–151. [DOI] [PubMed] [Google Scholar]
- Picker JD, Levy HL. 2014. Homocystinuria caused by cystathionine beta-synthase deficiency: NCBI bookshelf A service of the National Library of Medicine, National Institutes of Health. [Google Scholar]
- Pizzolo F, Blom HJ, Choi SW, Girelli D. 2011. Folic acid effects on s-adenosylmethionine, s-adenosylhomocysteine, and DNA methylation in patients with intermediate hyperhomocysteinemia. J Am Coll Nutr 30(1):11–18. [DOI] [PubMed] [Google Scholar]
- Potena LL, Grigioni F, Magnani G. 2000. Increasing plasma homocysteine during follow-up in heart transplant recipients: Effects of folate and renal function. Ital Heart J 1(5):344–348. [PubMed] [Google Scholar]
- Rajaie S, Esmaillzadeh A. 2011. Dietary choline and betaine intakes and risk of cardiovascular diseases: Review of epidemiological evidence. ARYA Atheroscler 7(2):78–86. [PMC free article] [PubMed] [Google Scholar]
- Rajdl D, Racek J, Trefil L. 2016. Effect of folic acid, betaine, vitamin B6, and vitamin B12 on homocysteine and dimethylglycine levels in middle-aged men drinking white wine. Nutrients 8(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis RP, Azinheira J, Reis HP. 2016. Influence of smoking on homocysteinemia at baseline and after methionine load 5:2. [PubMed] [Google Scholar]
- Riddell LJ, Chisholm A, Williams S. 2000. Dietary strategies for lowering homocysteine concentrations. Am J Clin Nutr 71(6):1448–1454. [DOI] [PubMed] [Google Scholar]
- Sam S 2007. Obesity and polycystic ovary syndrome. Obes Manag 3(2):69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwahn B, Laryea M, Chen Z, Melnyk S, Pogribny I, Garrow T, Rozen R. 2004. Betaine rescue of an animal model with methylenetetrahydrofolate reductase deficiency 382(Pt3):831–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibal L, Agarwal SC, Home PD. 2010. The role of asymmetric dimethylarginine (ADMA) in endothelial dysfunction and cardiovascular disease. Curr Cardiol Rev 6(2):82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signorello MG, Viviani GL, Armani U, Cerone R, Minniti G, Piana A, Leoncini G. 2007. Homocysteine, reactive oxygen species and nitric oxide in type 2 diabetes mellitus. Thromb Res 120(4):607–613. [DOI] [PubMed] [Google Scholar]
- Stamm RA, Houghton LA. 2013. Nutrient intake values for folate during pregnancy and lactation vary widely around the world. Nutrients 5(10):3920–3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steed MM, Tyagi SC. 2011. Mechanisms of cardiovascular remodeling in hyperhomocysteinemia. Antioxid Redox Signal 15(7):1927–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern LL, Mason JB, Selhub J, Choi SW. 2000. Genomic DNA hypomethylation, a characteristic of most cancers, is present in peripheral leukocytes of individuals who are homozygous for the C677T polymorphism in the methylenetetrahydrofolate reductase gene. Cancer Epidemiol Biomarkers Prev 9:849–853. [PubMed] [Google Scholar]
- Stover PJ. 2009. One-carbon metabolism–genome interactions in folate- associated pathologies. J Nutr 139(12):2402–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J, Wang S, Hunag Y, Jinag Y. 2009. A comparative study on pathogenic effects of homocysteine and cysteine on atherosclerosis. Wei Sheng Yan Jiu 38(1):43–46. [PubMed] [Google Scholar]
- Sultan N, Khan MA, Malik S. 2007. Effect of folic acid supplementation on homocysteine level in postmenopausal women. J Ayub Med Coll Abbottabad 19(4):78–81. [PubMed] [Google Scholar]
- Tayebi A, Biniaz V, Savari S. 2016. Effect of vitamin B12 supplementation on serum homocysteine in patients undergoing hemodialysis: A randomized controlled trial. Saudi J Kidney Dis Transpl 27(2):256–262. [DOI] [PubMed] [Google Scholar]
- Thompson PW, Bayliffe AI, Warren AP. 2007. Interleukin-10 is upregulated by nanomolar rosiglitazone treatment of mature dendritic cells and human CD4þ T cells. Cytokine 39:184–191. [DOI] [PubMed] [Google Scholar]
- Tiahou G, Dupuy A-M, Jaussent I. 2009. Determinants of homocysteine levels in ivorian rural population. Int J Vitam Nutr Res 79(5–6):319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi N, Gillespie W, Vacek JC, Sen U, Tyagi SC, Lominadze D. 2009. Activation of GABA-A receptor ameliorates homocysteine-Induced MMP −9 activation by ERK pathway. J Cell Physiol 220(1):257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino F, Bivona G, Butera D, Paladino P, Fazzari M, Piccoli T, Ciaccio M, La Bella V. 2010. Elevated cerebrospinal fluid and plasma homocysteine levels in ALS. Eur J Neurol 17:84–89. [DOI] [PubMed] [Google Scholar]
- Van Guldener C 2006. Why is homocysteine elevated in renal failure and what can be expected from homocysteine-lowering? Nephrol Dial Transplant 21(5):1161–1166. [DOI] [PubMed] [Google Scholar]
- Veeranki S, Tyagi SC. 2013. Defective homocysteine metabolism: Potential implications for skeletal muscle malfunction. Int J Mol Sci 14(7):15074–15091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeranki S, Tyagi SC. 2015. Mechanisms of hyperhomocysteinemia induced skeletal muscle myopathy after ischemia in the CBS−/+ mouse model. Int J Mol Sci 16:1252–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeranki S, Winchester LJ, Tyagi SC. 2015. Hyperhomocysteinemia associated skeletal muscle weakness involves mitochondrial dysfunction and epigenetic modifications. Biochim Biophys Acta 5:732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen EGJ, Niessen HWM, Bogels M. 2001. Decreased smooth muscle cell/extracellular matrix ratio of media of femoral artery in patients with atherosclerosis and hyperhomocysteinemia. Arterioscler Thromb Vasc Biol 21:573–577. [DOI] [PubMed] [Google Scholar]
- Wang G, Siow YL, Karmin O. 2000. Homocysteine stimulates nuclear factor κB activity and monocyte chemoattractant protein-1 expression in vascular smooth-muscle cells: A possible role for protein kinase C. Biochem J 352: 817–826. [PMC free article] [PubMed] [Google Scholar]
- Wang H, Jiang XH, Yang F. 2002. Cyclin A transcriptional suppression is the major mechanism mediating homocysteine-induced endothelial cell growth inhibition. Blood 99:939–945. [PMC free article] [PubMed] [Google Scholar]
- Weber GJ, Pushpakumar S, Tyagi SC. 2016. Homocysteine and hydrogen sulfide in epigenetic, metabolic and microbiota related renovascular hypertension. Pharmacol Res 113(PtA):300–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Place N, Yamada T. 2010. Mechanisms of skeletal muscle weakness. Adv Exp Med Biol 682:279–296. [DOI] [PubMed] [Google Scholar]
- Winchester L, Veeranki S, Givvimani S. 2014. Exercise mitigates the adverse effects of hyperhomocysteinemia on macrophages, MMP-9, skeletal muscle, and white adipocytes. Can J Physiol Pharmacol 92(7): 575–582. [DOI] [PubMed] [Google Scholar]
- Winchester LJ, Veeranki Sr, Givvimani S. 2015. Homocysteine elicits an M1 phenotype in murine macrophages through an EMMPRIN-mediated pathway. Can J Physiol Pharmacol 93(7):577–584. [DOI] [PubMed] [Google Scholar]
- Xia XS, Li X, Wang L. 2014. Supplementation of folic acid and vitamin B12 reduces plasma levels of asymmetric dimethylarginine in patients with acute ischemic stroke. J Clin Neurosci 21(9):1586–1590. [DOI] [PubMed] [Google Scholar]
- Xu L, Chen J, Gao J, Yu H, Yang P. 2015. Crosstalk of homocysteinylation, methylation and acetylation on histone H3. Analyst 140(9):3057–3063. [DOI] [PubMed] [Google Scholar]
- Yang JX, Rastetter RH, Wilhelm D. 2016. Non-coding RNAs: An introduction. Adv Exp Med Biol 886:13–32. [DOI] [PubMed] [Google Scholar]
- Yi P, Melnyk S, Pogribna M, Pogribny IP, Hine RJ, James SJ. 2000. Increase in plasma homocysteine associated with parallel increases in plasma S-adenosylhomocysteine and lymphocyte DNA hypomethylation. J Biol Chem 275:29318–29323. [DOI] [PubMed] [Google Scholar]
- Zeisel SH. 2006. Choline: Critical role during fetal development and dietary requirements in adults. Annu Rev Nutr 26:229–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JG, Liu JX, Li ZH. 2007. Dysfunction of endothelial NO system originated from homocysteine-induced aberrant methylation pattern in promoter region of DDAH2 gene. Chin Med J (Engl) 120(23):2132–2137. [PubMed] [Google Scholar]
- Zhou S, Zhang Z, Xu G. 2014. Notable epigenetic role of hyperhomocysteinemia in atherogenesis. Lipids Health Dis 13:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoccolella S, Simone IL, Lamberti P. 2008. Elevated plasma homocysteine levels in patients with amyotrophic lateral sclerosis. Neurology 70: 222–225. [DOI] [PubMed] [Google Scholar]
- Zoccolella S, Tortorella C, Iaffaldano P. 2012. Elevated plasma homocysteine levels in patients with multiple sclerosis are associated with male gender. J Neurol 259:2105–2110. [DOI] [PubMed] [Google Scholar]