Abstract
Background:
Few studies have investigated metabolic complications in HIV-infected African children and their relation with inflammation.
Methods:
We compared baseline and changes in insulin resistance [homeostatic model assessment of insulin resistance (HOMA-IR)] and in markers of inflammation over 48 weeks, in a subset of antiretroviral therapy (ART)–naive Ugandan children from the Children with HIV in Africa-Pharmacokinetics and Adherence/Acceptability of Simple Antiretroviral Regimens trial randomized to zidovudine-, stavudine or abacavir (ABC)–based regimen. Non-parametric methods were used to explore between-group and within-group differences, and multivariable analysis to assess associations of HOMA-IR.
Results:
One-hundred eighteen children were enrolled, and median age (interquartile range) was 2.8 years (1.7–4.3). Baseline median HOMA-IR (interquartile range) was 0.49 (0.38–1.07) and similar between the arms. At week 48, median relative changes in HOMA-IR were 14% (−29% to 97%) in the zidovudine arm, −1% (−30% to 69%) in the stavudine arm and 6% (−34% to 124%) in the ABC arm (P ≤ 0.03 for all the arms compared with baseline, but P = 0.90 for between-group differences). Several inflammation markers significantly decreased in all study arms; soluble CD14 increased on ABC and did not change in the other 2 arms. In multivariate analysis, only changes in soluble CD163 were positively associated with HOMA-IR changes.
Conclusions:
In ART-naive Ugandan children, HOMA-IR changed significantly after 48 weeks of ART and correlated with monocyte activation.
Keywords: insulin resistance, pediatric HIV, Uganda, treatment-naive, inflammatory markers, monocyte activation
Cardiovascular and metabolic diseases have become the leading cause of death in HIV-infected individuals,2 and specifically, disorders of glucose metabolism and insulin resistance have increasingly been reported in HIV-infected patients.3–7 HIV-infected children also have a higher prevalence of metabolic disorders compared with the general population.8–10 Limited longitudinal data also indicate an increased prevalence of insulin resistance in HIV-infected children over time.11,12 Persistent insulin resistance may increase the lifetime risk of developing type 2 diabetes mellitus which can lead to cardiovascular disease and other end organ dysfunctions.13–16 The etiology is likely multifactorial including HIV,18 specific antiretrovral therapy (ART) regimen19,20 and systemic inflammation.4
Markers of systemic inflammation have been associated with the development of diabetes and cardiovascular disease in the general population.21–23 Although ART decreases inflammation and coagulation markers,31,32 levels of inflammatory markers may remain elevated despite virologic suppression. In HIV-infected individuals on ART with good HIV virologic control, markers of systemic inflammation remain higher than in HIV-uninfected individuals.33
The available limited research evaluating the impact of inflammation in virally suppressed patients has been focusing in resourcerich settings, and it remains unclear how this relates to pediatric patients in resource-limited settings where the majority of pediatric HIV-infected patients live.1 Participants from the Children with HIV in Africa-Pharmacokinetics and Adherence/Acceptability of Simple Antiretroviral Regimens (CHAPAS)-3 trial who enrolled in Kampala at the Joint Clinical Research Centre (JCRC) present a unique opportunity to examine changes in insulin resistance after ART initiation.
MATERIALS AND METHODS
This was a substudy of the CHAPAS-3 clinical trial (ISRCTN69078957), which was an open, randomized phase II/III trial comparing toxicity and efficacy of stavudine (D4T)-, zidovudine (AZT)and abacavir (ABC)–based ART among children from Zambia and Uganda.17 There were 4 participating sites: University Teaching Hospital, Lusaka, Zambia; Baylor-Uganda Centre of Excellence, Kampala, Uganda; JCRC, Kampala, Uganda and JCRC, Gulu, Uganda. Caregivers gave written informed consent, and older children aware of their HIV status also gave informed assent following national guidelines. The trial was approved by Research Ethics Committees in Zambia, Uganda and United Kingdom. The sub study protocol was also approved by the JCRC Research Ethics Committee and the Uganda National Council of Science and Technology.
The primary objective of CHAPAS-3 was to determine toxicity and pharmacokinetics of the 3 treatment regimens in the pediatric population (Mulenga, 2016, number: 147). The substudy presented in this article focused on the 119 ART-naive children from 3 months to 4 years of age who were recruited at JCRC, Kampala.
Study Evaluations
At entry and week 48, fasting (6 hours) blood was obtained for real-time measurements of lipid profiles and CD4 count. Blood was processed, and plasma stored for batched measurement of HIV-RNA levels. A Material Transfer Agreement, approval from the Uganda National Council of Science and Technology as well as a permit from the Center for Disease Control were approved. The remainder of the frozen, never previously thawed plasma from the ART-naive children, was then shipped to Case Medical Center, Cleveland, OH. The plasma samples were used for measurement of glucose, insulin, soluble and cellular markers of monocyte immune activation and markers of systemic inflammation and coagulation. Measurements were performed by the Dahms Research Clinical Unit, which is part of the Case Clinical and Translational Science Collaborative of Cleveland, OH. Insulin was measured by enzyme linked immunosorbent assay sandwich immunoassay (ALPCO, Salem, NH), and the derived homeostatic model assessment of insulin resistance (HOMA-IR) was calculated as described,34 where insulin resistance is defined categorically as levels of HOMA-IR greater than 3.16.34,35
Inflammation, Coagulation and Soluble Immune Activation Markers
Plasma markers of monocyte activation [soluble CD14 (sCD14) and soluble CD163 (sCD163)], systemic inflammation [soluble tumor necrosis factor alpha receptor 1 and 2 (sTNFR1 and 2)], oxidized low-density lipoproteins (LDLs) and fibrinogen were measured. All markers were measured by enzyme-linked immunosorbent assay (R & D Systems, Minneapolis, MN and ALPCO, Salem, NH).
Statistical Analysis
The primary objectives of this analysis were to determine the changes in HOMA-IR and markers of inflammation 48 weeks after initiating the randomized treatments and to compare the changes between the groups. Secondary objectives were to determine the association between HOMA-IR and markers of systemic inflammation at baseline with clinically relevant factors and to explore the predictors of change in HOMA-IR and in markers of inflammation.
Weight- and height-for-age Z scores were obtained from World Health Organization growth chart standards. Baseline demographics, HIV-related factors, metabolic risk factors and HOMA-IR were described overall and by randomization group using median and interquartile range (IQR) for continuous variables and frequency and percent for categorical variables. Absolute and relative changes from baseline to week 48 were determined. To highlight the magnitude of the observed difference in HOMA-IR after ART initiation, baseline variables and absolute and relative changes were compared in the 3 groups by the Kruskal–Wallis test for continuous variables and by the χ2 test or Fisher exact test, as appropriate for categorical variables. Within-group changes were tested using the Wilcoxon matched-pairs signed-ranks test, and Spearman correlations were used to assess associations with HOMA-IR.
Multivariable linear regression was used to model the relative changes in HOMA-IR over 48 weeks, with variables with P < 0.1 in the correlation analysis and variables known to affect insulin resistance including age, sex, body mass index (BMI) and family history of diabetes being candidates for inclusion in the model. Mathematical transformations were used to achieve normality of distribution, as needed. The variance inflation factor statistic was used to gauge possible collinearity. Possible departures from normality of residuals and homoscedasticity were evaluated using graphical methods. All analyses were initially performed including all participants and all available data. The results of the analyses that included all participants did not differ from the results of the sensitivity analyses that included only participants with undetectable viral load at week 48; therefore, only the former data are presented. Analyses were performed with SAS9.4 (SAS Institute,Cary, NC).
RESULTS
Baseline Characteristics
Overall, 118 of 119 ART-naive CHAPAS-3 participants at JCRC, Kampala, had stored plasma samples available for HOMA- IR and inflammatory marker measurements and were included in the present analysis. Demographic information and baseline characteristics of the 118 participants are displayed in Table 1, except for viral load, which was higher in the D4T arm compared with the ABC arm, and all other indices were similar between the groups (P > 0.05). Median age (IQR) was 2.8 (1.5, 4.3) years, and 49% were male. Median weight-for-age Z score was −2 (−3.4, −0.5), and median HOMA-IR was 0.49 (0.38, 1.07). A total of 5 participants had HOMA-IR values ≥3.16. Median absolute and percent CD4+ T cell counts were 867 (648, 1544) cells/mm3 and 20% (15, 25), respectively. Median viral load was 405,755 (122,250 to 1,107,900) copies/mL. Levels of markers of systemic inflammation and monocyte activation were also similar between the groups.
TABLE 1.
Baseline Demographic, Clinical, HIV-related Factors and Inflammatory Biomarkers by Arms
| Baseline Variables | AZT Arm N = 35, Median (IQR) |
D4T Arm N = 42, Median (IQR) |
ABC Arm N = 41, Median (IQR) |
|---|---|---|---|
| Age (yr) | 3.3 (1.7–4.3) | 2.9 (1.8–3.9) | 2.3 (1.5–3.9) |
| Male sex, n (%) | 18 (51) | 19 (45) | 21 (51) |
| Weight (kg) | 10 (8–14) | 11.7 (9–14) | 11 (8–13) |
| Height (cm) | 82 (73.2–93.6) | 85.8 (76.0–95.4) | 80 (72.5–92.4) |
| BMI (kg/m2) | 15.6 (14.50–16.53) | 15.08 (14.46–17.04) | 16.04 (15.56–16.79) |
| Weight-for-age Z score | −2 (−3.4 to −1.0) | −1.8 (−3.2 to −0.7) | −2.1 (−2.6 to-0.5) |
| Height-for-age Z score | −2.4 (−3.5 to −1.1) | −2.6 (−3.3 to −1.3) | −2.4 (−3.6 to −1.4) |
| Family history of diabetes, n (%) | 3 (9) | 3 (7) | 2 (5) |
| Glucose level (mg/dL) | 62 (50–72) | 63.3 (57–68) | 59.5 (53–69) |
| Insulin (μIU/mL) | 3 (3–3.2) | 3 (3–3) | 3 (3–6.8) |
| HOMA-IR | 0.48 (0.38–0.57) | 0.49 (0.43–0.57) | 0.49 (0.41–1.07) |
| HOMA-IR ≥3.16, n(%) | 1 (3) | 2 (5) | 2 (5) |
| Total cholesterol level (mg/dL) | 116.6 (99.8–140.5) | 116 (99.4–146.0) | 128.1 (97.7–152.4) |
| High-density lipoprotein (mg/dL) | 26.3 (18.2–30.1) | 25.9 (19.1–32.4) | 24 (19.8–34.2) |
| Low-density lipoprotein (mg/dL) | 66 (42.5–85.8) | 66.9 (48.4–87.7) | 67.2 (50.7–95.5) |
| Triglycerides (mg/dL) | 111 (87.6–159.9) | 102.6 (83.6–177.2) | 114.5 (89.7–144.1) |
| Viral load (copies/mL) | 401,200 (171,650–1,079,645) | 500,975 (224,805–1,107,900)* | 315,090 (122, 250–646,190)* |
| CD4+ T cell counts (cells/mm3) | 925.5 (676–1505) | 864.7 (648–1236) | 810.5 (672–1544) |
| CD4+ T cell percent | 21 (16–25) | 19.5 (15–22) | 21 (16.5–24) |
| sTNFRl (pg/mL) | 1221 (926–1429) | 1249 (993–1480) | 1188 (995–1572) |
| STNFR2 (pg/mL) | 5739.5 (4848–7263) | 5851 (4210–7272) | 5478 (4482–8805) |
| sCD14 (ng/mL) | 1750.5 (1394–2260) | 1778.8 (1578–2298) | 2007.5 (1615–2308) |
| sCD163 (ng/mL) | 1417.5 (1154–1588) | 1345 (1118–1533) | 1340.3 (970–1680) |
| Oxidized LDL (p/L) | 140 (64–263) | 122.5 (62–239) | 80 (60–278) |
| Fibrinogen (mg/dL) | 2008.5 (1783–2305) | 1966 (1664–2149) | 1913 (1647–2260) |
Data are median value (interquartile range) or number (%) of patients.
P < 0.05 for between-group differences.
Changes in HOMA-IR After ART Initiation
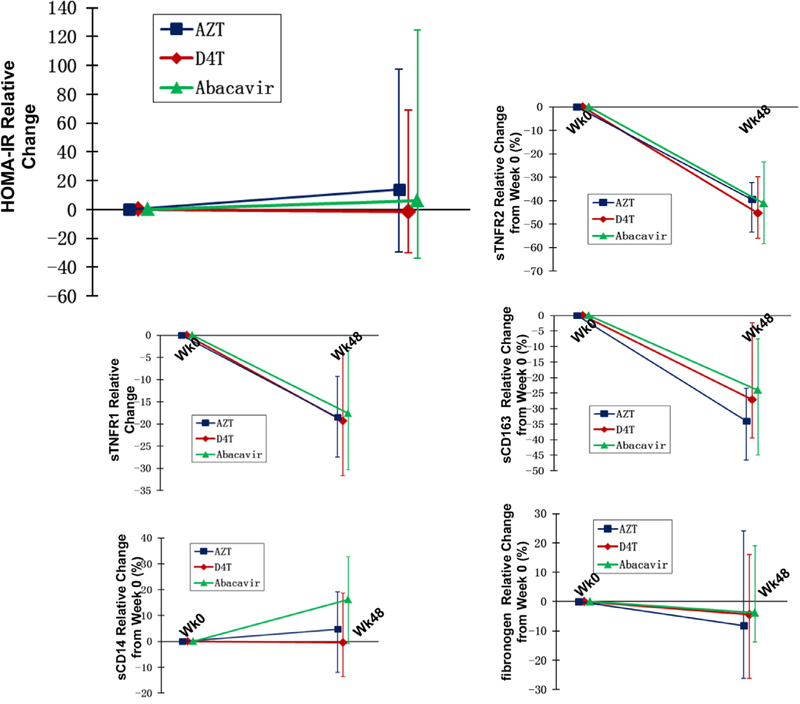
The relative changes from baseline to week 48 in HOMA-IR are shown in Figure 1. The relative changes in HOMA-IR did not differ between the arms, and were a median (IQR) of 14% (−29%, 97%) in the AZT arm (P = 0.03), −1% (−30%, 69%) in the D4T arm (P = 0.02) and 6% (−34%, 124%) in the ABC arm (P = 0.02). Four additional participants had HOMA-IR values ≥3.16 compared with baseline, with no differences between the arms. Within-group percentage changes in HOMA-IR were different from the absolute changes (Table 2). The absolute changes in HOMA-IR were not significant in any of the arms.
FIGURE 1.
Relative change in HOMA-IR and inflammatory markers.
TABLE 2.
Absolute Change Between Week 0 and 48 in HOMA-IR and Inflammatory Biomarkers
| Variables Measured | AZT Arm N = 35, Median (IQR) |
P* | D4T Arm N = 42, Median (IQR) |
P* | ABC Arm N = 41, Median (IQR) |
P* |
|---|---|---|---|---|---|---|
| HOMA-IR | 0.04 (−0.15 to 0.32) | 0.65 | −0.01 (−0.15 to 0.3) | 0.56 | 0.03 (−0.25 to 0.59) | 0.69 |
| sTNFRl (pg/mL) | −189.5 (−413.5 to −93.5) | 0.003 | −176.5 (−469 to −44) | 0.002 | −223 (−499 to −32) | <0.001 |
| sTNFR2 (pg/mL) | −2190 (−3762 to −1409) | <0.001 | −2335 (−4203 to −1228) | <0.001 | −2067 (−4358 to −1139) | <0.001 |
| sCD14 (ng/mL) | 61 (−328.5 to 368.3)† | 0.75 | −8 (−280 to 490.5) † | 0.29 | 284.5 (−17.5 to 638.5)† | <0.001 |
| sCD163 (ng/mL) | −490 (−665.5 to −260.5) | <0.001 | −378 (−553.7 to −20) | <0.001 | −372.7 (−720.5 to −103.5) | <0.001 |
| Oxidized LDL (μ/L) | −18.5 (−109.5 to 18) | 0.51 | −26 (−98 to 54) | 0.18 | −6 (−154 to 113) | 0.24 |
| Fibrinogen (mg/dL) | −171 (−553.5 to 408.5) | 0.20 | −82 (−553 to 227) | 0.65 | −75 (−290 to 300) | 0.96 |
Data are median value (interquartile range).
For within-group differences.
P < 0.05 for between-group differences.
Changes in Inflammatory Biomarkers After ART Initiation
sTNFR1 and 2 and sCD163 decreased significantly (P < 0.05 compared with baseline) within each of the 3 groups. sTNFR1 decreased by a median (IQR) of 18% (9%, 27%) in the AZT arm (P < 0.001), 19% (4%, 32%) in the D4T arm (P = 0.05) and 18% (3%, 30%) in the ABC arm (P < 0.001); sTNFR2 decreased by 39% (32%, 53%) in the AZT arm (P < 0.0001), 32% (30%, 56%) in the D4T arm (P < 0.0001) and 41% (23%, 58%) in the ABC arm (P < 0.0001) and sCD163 decreased by 31% (23%, 47%) in the AZT arm (P < 0.0001), 27% (2%, 40%) in the D4T arm (P < 0.0001) and 20% (7%, 45%) in the ABC arm (P = 0.02). The changes were not significantly different between the groups (P ≥ 0.09). sCD14 did not change in the AZT or D4T arm, with median changes of 5% (−12%, 19%) in the AZT arm (P = 0.14) and median decrease of 0.4% (14%, 19%) in the D4T arm (P = 0.12). sCD14 increased significantly in the ABC arm by a median of 16% (−0.09%, 33%; P = 0.0003). There was no difference in sCD14 between the AZT and D4T arms (P = 0.72); however, the changes in sCD14 were significantly different between AZT, D4T and ABC arms (P < 0.05). Oxidized LDL and fibrinogen did not change significantly within any of the groups and did not differ between the groups (P ≥ 0.2). Within-group percentage changes in levels of the inflammatory biomarkers were similar to the absolute changes (Fig. 1 and Table 2).
Changes in Other Clinically Relevant Factors After ART Initiation
There were significant increase in weight and height in all the arms with absolute median changes of weight-for-age Z score of 0.7 (IQR: 0.02–1.72, P < 0.01 for all arms) and absolute changes of height-for-age Z score of 0.4 (IQR: −0.03 to 0.94, P < 0.01 for all arms). The absolute median change in BMI significantly increased in the AZT arm (P = 0.02), but not in the D4T or ABC arm (P ≥ 0.44). Total cholesterol, high-density lipoprotein (HDL) and LDL increased within each of the arms (P ≤ 0.03).
After 48 weeks of ART, 66% of patients had undetectable viral load in the AZT arm, 57% in the D4T arm and 63% in the ABC arm; percent CD4 increased significantly in all arms with absolute median increase of 14% (IQR: 8.5–19.5, P < 0.01) for all arms. Insulin did not change at week 48 in any of the arms (P > 0.05). Except for median glucose that increased significantly only in the AZT arm [62 mg/dL (IQR: 50–72) at baseline and 69 mg/dL (IQR: 58–80); P = 0.03 at week 48], there were no significant changes between the arms for any of the other clinically relevant factors.
Baseline Associations With HOMA-IR and Inflammatory Markers
At baseline (pre-ART), the only inflammatory marker associated with HOMA-IR was oxidized LDL (α = −0.20, P = 0.04). Oxidized LDL was also negatively associated with weight and positively associated with CD4 count. sTNFR1 and 2 and sCD14 were negatively associated with weight, age, weight-for-age Z score and total and LDL cholesterol. sTNFR1 and 2 were positively associated with absolute CD4 count and viral load, whereas sCD14 was only negatively associated with percent CD4 (Table 3).
TABLE 3.
Factors Univariately Associated With HOMA-IR and Inflammatory Markers at Baseline
| sTNFRl | sTNFR32 | sCD14 | SCD163 | Oxidized LDL | Fibrinogen | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline variables | ρ | P | ρ | P | ρ | P | ρ | P | ρ | P | ρ | P |
| HOMA-IR | −0.02 | 0.82 | −0.03 | 0.73 | −0.04 | 0.64 | −0.03 | 0.72 | 0.2 | 0.04 | −0.02 | 0.87 |
| Age (yr) | −0.62 | <0.001 | −0.66 | <0.001 | −0.17 | 0.07 | −0.11 | 0.23 | −0.22 | 0.02 | 0.05 | 0.60 |
| Weight-for-age Z score | −0.55 | <0.001 | −0.49 | <0.001 | −0.35 | <0.001 | −0.15 | 0.10 | −0.22 | 0.02 | 0.05 | 0.60 |
| Weight (kg) | −0.69 | <0.001 | −0.68 | <0.001 | −0.29 | 0.002 | −0.13 | 0.16 | −0.22 | 0.02 | 0.04 | 0.7 |
| Cholesterol (mg/dL) | −0.26 | 0.007 | −0.26 | 0.009 | −0.21 | 0.03 | −0.03 | 0.72 | −0.02 | 0.84 | 0.14 | 0.15 |
| LDL (mg/dL) | −0.38 | <0.001 | −0.35 | 0.003 | −0.22 | 0.02 | −0.11 | 0.26 | −0.06 | 0.55 | 0.15 | 0.14 |
| CD4 αbs (cells/mm3) | 0.29 | 0.002 | 0.26 | 0.004 | 0.03 | 0.72 | 0.06 | 0.55 | 0.38 | <0.001 | −0.08 | 0.40 |
| CD4 % | −0.1 | 0.28 | −0.09 | 0.35 | −0.22 | 0.02 | −0.03 | 0.75 | 0.27 | 0.005 | −0.07 | 0.47 |
| Viral load (copies/mL) | 0.248 | 0.009 | 0.23 | 0.01 | 0.06 | 0.50 | −0.03 | 0.76 | −0.01 | 0.91 | −0.08 | 0.38 |
ρ = Spearman correlation coefficient.
Associations Between Changes in HOMA-IR and Changes in Biomarkers
Changes in sCD163 levels were positively associated with changes in HOMA-IR (Table 4). After adjusting for parameters known to affect insulin resistance including age, sex, BMI and family history of diabetes, only changes in sCD163 remained independently associated with changes in HOMA-IR (β coefficient = 0.635, P = 0.03).
TABLE 4.
Factors Associated With Relative Change From Baseline to Week 48 in HOMA-IR
| Univariable Analysis | Multivariable Analysis | |||
|---|---|---|---|---|
| Variables | Spearman Correlation | P | Parameter Estimate | P |
| Age (yr) | 0.003 | 0.969 | ||
| Male sex | −0.375 | 0.077 | ||
| Relative change in BMI (kg/m2) | 0.482 | 0.649 | ||
| Family history of diabetes | 0.585 | 0.161 | ||
| Relative change in sTNFRl | −0.11 | 0.28 | ||
| Relative change in sTNFR2 | −0.15 | 0.13 | ||
| Relative change in sCD14 | −0.07 | 0.50 | ||
| Relative change in sCD163 | 0.20 | 0.04 | 0.635 | 0.030 |
After 48 weeks of ART, changes in percent CD4 count, in weight and in LDL cholesterol were negatively associated with changes in sTNFR1 and 2 and in sCD14 (P < 0.05). After adjusting for age, sex and LDL, increase in weight remained independently associated with reductions in sTNFR1 and 2 (β coefficient = −0.62, P < 0.01 and β coefficient = −0.42, P = 0.05, respectively). Weight also remained independently associated with sCD14 after adjusting for age, sex, viral load and CD4 (β coefficient = −0.63, P = 0.02). All variance inflation factors were <2.0.
DISCUSSION
We investigated the effects of 48 weeks of ART in treatment-naive Ugandan children on insulin resistance and markers of inflammation. Data are lacking on the effects of ART on metabolic and inflammatory parameters in African children where the bulk of HIV-infected children reside. We found that in the setting of an non-nucleoside reserve transcriptase inhibitor (NNRTI)-based regimen, AZT and ABC increased HOMA-IR and that HOMA-IR is associated with the marker of monocyte activation sCD163.
Insulin resistance is a state in which insulin is associated with an abnormal glucose response and correlates with sequelae such as the development of diabetes,13 cardiovascular disease and malignancies. Although there are no reference values for HOMA-IR in healthy Ugandan children, the baseline HOMA-IR values in our population of underweight HIV-positive Ugandan children were similar to those reported in normal-weight HIV-negative European children of similar age36 and below 3.16, which has been defined as the cut off for defining insulin resistance in children.35 The clinical significance of the increase seen in HOMA-IR in the AZT and ABC arms after only 48 weeks is unclear. In addition, it is unknown whether this increase in insulin resistance would translate to important long-term consequences in this prepubertal cohort. Other studies have also demonstrated increased insulin resistance in HIV-infected children.9,11,37 In the study by Chantry et al,11 participants were either initiating or switching ART regimen. In this particular study, 25% of their patients were 3 years of age and under, and the baseline HOMA-IR value was 0.8, which is similar to our study; however, the change seen after 48 weeks was between 0.2 and 0.8, comparatively larger than the absolute change in our study of −0.01 and 0.04. However, unlike the participants in our study, these children were on protease inhibitor–based therapy, which has been linked to insulin resistance in HIV-infected adults38 and children.39 Median HOMA-IR values reported by Innes et al9 in a cross-sectional study of HIV-infected children in South Africa were also 0.8, but not different between children on lopinavir- and efavirenz-based regimens.
A novel finding is the relationship between HOMA-IR and the marker of monocyte activation sCD163 in HIV-infected children. sCD163 has been associated with HOMA-IR in healthy adults and obese children,22,40,41 but to our knowledge no similar correlation has been reported in HIV-infected subjects. sCD163 in HIV-infected adults has been linked with other prevalent and clinically significant comorbidities in HIV infection including noncalcified plaque42 and neurocognitive impairment.43 Unlike what we have found in our previous study in HIV-infected adults, sTNFα receptors were not significantly correlated with insulin resistance.4 We may not have been able to detect an association secondary to the small sample size of our study. Another hypothesis that has been proposed by Zanni et al22 is that sTNFα, because of its short half-life, may not reflect the inflammation in adipose tissues, unlike CD163 expressing macrophages, which can gain access to fat cells. Microbial translocation and lipopolysaccharides may play a role in the correlation seen between sCD163 and insulin resistance. Microbial translocation has been well documented in HIV-infected adults26,44 and more recently in ART-naive children in resource-limited settings.45 Markers of microbial translocation have been closely associated with several cardiovascular risk factors including insulin resistance,46,47 and they are potent stimulants for release of sCD163 from adipose tissue.48 We found that after controlling for known demographic characteristics, the relationship between sCD163 and HOMA-IR remained statistically significant. These findings suggest that insulin resistance in HIV-infected children may be mediated by immune activation, particularly monocyte activation.
Several markers of inflammation (sTNFR1 and 2 and sCD163) decreased significantly after 48 weeks of ART; however, sCD14 did not decrease, and it even increased in the ABC arm despite virologic suppression. In addition, oxidized LDL did not significantly change after 48 weeks of ART. Oxidized LDL is a marker of oxidative stress; in uninfected adults, it has been associated with obesity,27 insulin resistance28 and cardiovascular disease,29 and in HIV, it has been associated with markers of immune activation.30 The lack of change seen in oxidized LDL after 48 weeks of ART may be linked to the lack of change seen in sCD14 as our group has previously shown that plasma levels of oxidized LDL and sCD14 in HIV-infected patients are closely related, and oxidized LDL may play a role in monocyte activation.49
sCD14 contributes to the long-term complications seen in HIV and has been linked to subclinical atherosclerosis25,50 and over-all mortality in HIV.24 One possible explanation is that Ugandan children are exposed to different pathogens that could impact their intestinal micro biome. In addition, NNRTI-based regimens may not fully suppress viral replication in the gastrointestinal tract to control bacterial translocation. However, similar findings were seen in HIV-infected youth from the United States who were initiated on a protease inhibitor–based regimen with tenofovir and lamivudine.51 In this study, higher levels of sCD14 compared with baseline levels persisted despite 48 weeks of ART and viral suppression. Our group has previously reported on ART-naive adults randomized to different ART regimens and found that sCD14 decreased only in the integrase inhibitor arms, but not in protease inhibitor or NNRTI-based regimens.50,52,53 One of the mechanisms hypothesized is a higher concentration of integrase inhibitor in the enterocytes leading to better control over bacterial translocation.52 These data suggest HIV-infected Ugandan children even at a young age have increased immune activation and possibly bacterial translocation as measured by sCD14 that do not improve with early viral suppression with NNRTI-based regimens.
From a metabolic standpoint, we found that cholesterol, HDL and LDL increased in all arms. Unlike what was seen in ART-naive Ugandan children of similar age enrolled in the ARROW trial, total and HDL cholesterol were not lower in the AZT arm compared with the other 2 arms.54 After adjusting for parameters known to affect inflammatory markers, an increase in weight remained associated with reductions in sTNFR1 and 2 and sCD14. This is consistent with data recently presented in HIV-infected adults from the Prospective Evaluation of Antiretrovirals in Resource-Limited Settings trial that found that among HIV-infected persons initiating ART in resource-diverse settings, weight gain among underweight persons had a trend toward lower levels of TNFα and sCD14, whereas weight gain among obese persons was found to heighten inflammation/immune activation.55 Our findings suggest that weight gain after ART initiation among HIV-infected children in resource-limited settings, may reduce systemic inflammation and immune activation. Another possibility is that inflammation is reduced because of better virologic control, and the weight increase is a reflection of return to health.
Strengths of our study include randomized treatment allocation and evaluation of metabolic and inflammatory parameters in a young Ugandan cohort before and after ART initiation. We did not have a comparison group of HIV-uninfected children to compare the natural changes seen in insulin resistance in Ugandan children. In addition, we cannot prove causal relationships or exclude the possibility of residual confounding. We focused on a specific population of young HIV-infected Ugandan children who were under-weight at baseline; therefore, our findings may not be applicable to other HIV-infected populations.
ACKNOWLEDGMENTS
The authors would like to thank the study participants and the HIV-uninfected controls with their carers. We would also like to thank the members of the CHAPAS-3 trial team.
The work in this analysis was supported by an internal grant from Rainbow Babies and Children’s Hospital, the Clinical & Translational Science Collaborative of Cleveland NIH grant (UL1TR000439) and by the Infectious Diseases and Immunology Institute, Case Western Reserve University (to S.D-F.). CHAPAS-3 was funded by the European Developing Countries Clinical Trials Partnership (EDCTP) (IP.2007.33011.006), the Medical Research Council (MRC), United Kingdom, the Department for International Development (DfID), United Kingdom and the Ministerio de Sanidad y Consumo Spain. Cipla Ltd. donated first-line antiretroviral drugs.
Footnotes
S.D. sits on the Data Safety Monitoring Board (DSMB) for clinical trials of Johnson and Johnson. G.A.M. served as a consultant for Bristol Meyer Squibb (BMS), Gilead, VIIV, GlaxoSmithKline (GSK), ICON, Pfizer and has received research funding from Bristol-Myers Squibb, VIIV and Gilead. The other authors have no conflicts of interest to disclose.
REFERENCES
- 1.World Health Organization. The Use of Antiretroviral Drugs for Treatment and Prevention of HIV Infection. 2013. Available at: http://apps.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf. Accessed January 8, 2016. [Google Scholar]
- 2.Paula AA, Schechter M, Tuboi SH, et al. Continuous increase of cardiovascular diseases, diabetes, and non-HIV related cancers as causes of death in HIV-infected individuals in Brazil: an analysis of nationwide data. PLoS One. 2014;9:e94636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Domingos H, Cunha RV, Paniago AM, et al. Metabolic effects associated to the highly active antiretroviral therapy (HAART) in AIDS patients. Braz J Infect Dis. 2009;13:130–136. [DOI] [PubMed] [Google Scholar]
- 4.Brown TT, Tassiopoulos K, Bosch RJ, et al. Association between systemic inflammation and incident diabetes in HIV-infected patients after initiation of antiretroviral therapy. Diabetes Care. 2010;33:2244–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calza L, Masetti G, Piergentili B, et al. Prevalence of diabetes mellitus, hyperinsulinaemia and metabolic syndrome among 755 adult patients with HIV-1 infection. Int J STD AIDS. 2011;22:43–45. [DOI] [PubMed] [Google Scholar]
- 6.Capeau J, Bouteloup V, Katlama C, et al. ; ANRS CO8 APROCO- COPILOTE Cohort Study Group. Ten-year diabetes incidence in 1046 HIV- infected patients started on a combination antiretroviral treatment. AIDS. 2012;26:303–314. [DOI] [PubMed] [Google Scholar]
- 7.Galli L, Salpietro S, Pellicciotta G, et al. Risk of type 2 diabetes among HIV- infected and healthy subjects in Italy. Eur J Epidemiol. 2012;27:657–665. [DOI] [PubMed] [Google Scholar]
- 8.Dapena M, Jiménez B, Noguera-Julian A, et al. Metabolic disorders in vertically HIV-infected children: future adults at risk for cardiovascular disease. J Pediatr Endocrinol Metab. 2012;25:529–535. [DOI] [PubMed] [Google Scholar]
- 9.Innes S, Abdullah KL, Haubrich R, et al. High prevalence of dyslipidemia and insulin resistance in HIV-infected prepubertal African children on antiretroviral therapy. Pediatr Infect Dis J. 2016;35:e1–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arpadi S, Shiau S, Strehlau R, et al. Metabolic abnormalities and body composition of HIV-infected children on lopinavir or nevirapine-based antiretroviral therapy. Arch Dis Child. 2013;98:258–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chantry CJ, Hughes MD, Alvero C, et al. ; PACTG 1010 Team. Lipid and glucose alterations in HIV-infected children beginning or changing antiretroviral therapy. Pediatrics. 2008;122:e129–e138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Viganò A, Brambilla P, Pattarino G, et al. Long-term evaluation of glucose homeostasis in a cohort of HAART-treated HIV-infected children: a longitudinal, observational cohort study. Clin Drug Investig. 2009;29:101–109. [DOI] [PubMed] [Google Scholar]
- 13.Zaccardi F, Webb DR, Yates T, et al. Pathophysiology of type 1 and type 2 diabetes mellitus: a 90-year perspective. Postgrad Med J. 2016;92:63–69. [DOI] [PubMed] [Google Scholar]
- 14.Calvo-Sánchez M, Perelló R, Pérez I, et al. Differences between HIV- infected and uninfected adults in the contributions of smoking, diabetes and hypertension to acute coronary syndrome: two parallel case-control studies. HIV Med. 2013;14:40–48. [DOI] [PubMed] [Google Scholar]
- 15.Worm SW, De Wit S, Weber R, et al. Diabetes mellitus, preexisting coronary heart disease, and the risk of subsequent coronary heart disease events in patients infected with human immunodeficiency virus: the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D Study). Circulation. 2009;119:805–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Medapalli RK, Parikh CR, Gordon K, et al. Comorbid diabetes and the risk of progressive chronic kidney disease in HIV-infected adults: data from the Veterans Aging Cohort Study. J Acquir Immune Defic Syndr. 2012;60:393–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCutchan JA, Marquie-Beck JA, Fitzsimons CA, et al. ; CHARTER Group. Role of obesity, metabolic variables, and diabetes in HIV-associated neurocognitive disorder. Neurology. 2012;78:485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown TT, Cole SR, Li X, et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med. 2005;165:1179–1184. [DOI] [PubMed] [Google Scholar]
- 19.De Wit S, Sabin CA, Weber R, et al. ; Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study. Incidence and risk factors for new-onset diabetes in HIV-infected patients: the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study. Diabetes Care. 2008;31:1224–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tien PC, Schneider MF, Cole SR, et al. Antiretroviral therapy exposure and insulin resistance in the Women’s Interagency HIV study. J Acquir Immune Defic Syndr. 2008;49:369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crook MA, Tutt P, Pickup JC. Elevated serum sialic acid concentration in NIDDM and its relationship to blood pressure and retinopathy. Diabetes Care. 1993;16:57–60. [DOI] [PubMed] [Google Scholar]
- 22.Zanni MV, Burdo TH, Makimura H, et al. Relationship between monocyte/macrophage activation marker soluble CD163 and insulin resistance in obese and normal-weight subjects. Clin Endocrinol (Oxf). 2012;77:385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pickup JC, Mattock MB, Chusney GD, et al. NIDDM as a disease of the innate immune system: association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia. 1997;40:1286–1292. [DOI] [PubMed] [Google Scholar]
- 24.Sandler NG, Wand H, Roque A, et al. ; INSIGHT SMART Study Group. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011;203:780–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Longenecker CT, Jiang Y, Orringer CE, et al. Soluble CD14 is independently associated with coronary calcification and extent of subclinical vascular disease in treated HIV infection. AIDS. 2014;28:969–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Timmons T, Shen C, Aldrovandi G, et al. Microbial translocation and metabolic and body composition measures in treated and untreated HIV infection. AIDS Res Hum Retroviruses. 2014;30:272–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Couillard C, Ruel G, Archer WR, et al. Circulating levels of oxidative stress markers and endothelial adhesion molecules in men with abdominal obesity. J Clin Endocrinol Metab. 2005;90:6454–6459. [DOI] [PubMed] [Google Scholar]
- 28.Ho RC, Davy K, Davy B, et al. Whole-body insulin sensitivity, low-density lipoprotein (LDL) particle size, and oxidized LDL in overweight, nondiabetic men. Metabolism. 2002;51:1478–1483. [DOI] [PubMed] [Google Scholar]
- 29.Holvoet P, Vanhaecke J, Janssens S, et al. Oxidized LDL and malondialdehyde-modified LDL in patients with acute coronary syndromes and stable coronary artery disease. Circulation. 1998;98:1487–1494. [DOI] [PubMed] [Google Scholar]
- 30.Hileman CO, Turner R, Funderburg NT, et al. Changes in oxidized lipids drive the improvement in monocyte activation and vascular disease after statin therapy in HIV. AIDS. 2016;30:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McComsey GA, Kitch D, Daar ES, et al. Inflammation markers after randomization to abacavir/lamivudine or tenofovir/emtricitabine with efavirenz or atazanavir/ritonavir. AIDS. 2012;26:1371–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Funderburg NT. Markers of coagulation and inflammation often remain elevated in ART-treated HIV-infected patients. Curr Opin HIV AIDS. 2014;9:80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neuhaus J, Jacobs DR Jr, Baker JV, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010;201:1788–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. [DOI] [PubMed] [Google Scholar]
- 35.Keskin M, Kurtoglu S, Kendirci M, et al. Homeostasis model assessment is more reliable than the fasting glucose/insulin ratio and quantitative insulin sensitivity check index for assessing insulin resistance among obese children and adolescents. Pediatrics. 2005;115:e500–e503. [DOI] [PubMed] [Google Scholar]
- 36.Peplies J, Jiménez-Pavón D, Savva SC, et al. ; IDEFICS consortium. Percentiles of fasting serum insulin, glucose, HbA1c and HOMA-IR in pre-pubertal normal weight European children from the IDEFICS cohort. Int J Obes (Lond). 2014;38(suppl 2):S39–S47. [DOI] [PubMed] [Google Scholar]
- 37.dos Reis LC, de Carvalho Rondó PH, de Sousa Marques HH, et al. Dyslipidaemia and insulin resistance in vertically HIV-infected children and adolescents. Trans R Soc Trop Med Hyg. 2011;105:197–203. [DOI] [PubMed] [Google Scholar]
- 38.da Cunha J, Maselli LM, Stern AC, et al. Impact of antiretroviral therapy on lipid metabolism of human immunodeficiency virus-infected patients: old and new drugs. World J Virol. 2015;4:56–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bitnun A, Sochett E, Dick PT, et al. Insulin sensitivity and beta-cell function in protease inhibitor-treated and -naive human immunodeficiency virus-infected children. J Clin Endocrinol Metab. 2005;90:168–174. [DOI] [PubMed] [Google Scholar]
- 40.Parkner T, Sørensen LP, Nielsen AR, et al. Soluble CD163: a biomarker linking macrophages and insulin resistance. Diabetologia. 2012;55:1856–1862. [DOI] [PubMed] [Google Scholar]
- 41.Kazankov K, Møller HJ, Lange A, et al. The macrophage activation marker sCD163 is associated with changes in NAFLD and metabolic profile during lifestyle intervention in obese children. Pediatr Obes. 2015;10:226–233. [DOI] [PubMed] [Google Scholar]
- 42.Burdo TH, Lo J, Abbara S, et al. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis. 2011;204:1227–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burdo TH, Weiffenbach A, Woods SP, et al. Elevated sCD163 in plasma but not cerebrospinal fluid is a marker of neurocognitive impairment in HIV infection. AIDS. 2013;27:1387–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. [DOI] [PubMed] [Google Scholar]
- 45.Pilakka-Kanthikeel S, Kris A, Selvaraj A, et al. Immune activation is associated with increased gut microbial translocation in treatment-naive, HIV- infected children in a resource-limited setting. J Acquir Immune Defic Syndr. 2014;66:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pedersen KK, Pedersen M, Trøseid M, et al. Microbial translocation in HIV infection is associated with dyslipidemia, insulin resistance, and risk of myocardial infarction. J Acquir Immune Defic Syndr. 2013;64:425–433. [DOI] [PubMed] [Google Scholar]
- 47.Trøseid M, Manner IW, Pedersen KK, et al. Microbial translocation and cardiometabolic risk factors in HIV infection. AIDS Res Hum Retroviruses. 2014;30:514–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fjeldborg K, Møller HJ, Richelsen B, et al. Regulation of CD163 mRNA and soluble CD163 protein in human adipose tissue in vitro. J Mol Endocrinol. 2014;53:227–235. [DOI] [PubMed] [Google Scholar]
- 49.Zidar DA, Juchnowski S, Ferrari B, et al. Oxidized LDL levels are increased in HIV infection and may drive monocyte activation. J Acquir Immune Defic Syndr. 2015;69:154–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kelesidis T, Kendall MA, Yang OO, et al. Biomarkers of microbial translocation and macrophage activation: association with progression of subclinical atherosclerosis in HIV-1 infection. J Infect Dis. 2012;206:1558–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rudy BJ, Kapogiannis BG, Worrell C, et al. ; Adolescent Trials Network for HIVAIDS Interventions. Immune reconstitution but persistent activation after 48 weeks of antiretroviral therapy in youth with pre-therapy CD4 >350 in ATN 061. J Acquir Immune Defic Syndr. 2015;69:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hileman CO, Kinley B, Scharen-Guivel V, et al. Differential reduction in monocyte activation and vascular inflammation with integrase inhibitor based initial antiretroviral therapy among HIV-infected individuals. J Infect Dis. 2015;212:345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kelesidis T, Tran TT, Stein JH, et al. Changes in inflammation and immune activation with atazanavir-, raltegravir-, darunavir-based initial antiviral therapy: ACTG 5260s. Clin Infect Dis. 2015;61:651–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bwakura-Dangarembizi M, Musiime V, Szubert AJ, et al. ; ARROW Trial Team. Prevalence of lipodystrophy and metabolic abnormalities in HIV- infected African children after 3 years on first-line antiretroviral therapy. Pediatr Infect Dis J. 2015;34:e23–e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Erlandson KM, Gupte N, Lama JR, et al. Obesity and inflammation in resource-diversion settings of antiretroviral therapy initiation. In: Conference on Retroviruses and Opportunistic Infections; February 23–26, 2015; Seattle, WA. [Google Scholar]