Abstract
Activation of stretch-sensitive baroreceptor neurons exerts acute control over heart rate and blood pressure. Although this homeostatic baroreflex has been described for over 80 years, the molecular identity of baroreceptor mechanosensitivity remains unknown. We discovered that mechanically activated ion channels PIEZO1 and PIEZO2 are together required for baroreception. Genetic ablation of both Piezo1 and Piezo2 in the nodose and petrosal sensory ganglia abolished drug-induced baroreflex and aortic depressor nerve activity. Awake, behaving animals that lack Piezos had labile hypertension and increased blood pressure variability, consistent with phenotypes in baroreceptor-denervated animals and humans with baroreflex failure. Optogenetic activation of Piezo2+ sensory afferents was sufficient to initiate baroreflex in mice. These findings suggest that PIEZO1 and PIEZO2 are the long-sought baroreceptor mechanosensors critical for acute blood-pressure control.
Blood pressure (BP) is tightly regulated to ensure that the body is prepared to meet varied daily activity demands. Mechanisms that change blood volume control long-term BP regulation. Within seconds and minutes, BP regulation is initiated primarily by baroreceptors, a class of stretch sensitive neurons within the nodose and petrosal ganglia with peripheral projections in the walls of the aorta and carotid sinus (1, 2). An increase in BP stretches baroreceptor nerve endings to trigger afferent signals that are transmitted to the central nervous system (CNS). The consequences of baroreceptor activation are a decrease in heart rate (HR), cardiac output, and vascular resistance that counteract the initial increase in BP (1, 2). Compromised baroreceptor function predicts arrhythmias and premature death in humans with post-myocardial infarction and heart failure (3, 4).
Several ion channels (5–9) have been suggested to contribute to baroreception. However, substantial residual baroreceptor sensitivity is observed when these channels are ablated, implicating the involvement of other sensory systems. None of the candidate ion channels have been directly activated by mechanical stimuli in heterologous systems, which may lack accessory tethering molecules to form a mechanosensory complex. Furthermore, whether these channels are acting as sensors or play a role downstream of mechanotransduction is not clear. PIEZO1 and PIEZO2 are mechanically activated ion channels that play crucial roles in several mechanotransduction processes (10). PIEZO1 is prominently expressed in the cardiovascular system (11, 12), and PIEZO2 is abundant in various populations of sensory neurons (13–15).
We assessed Piezo1 and Piezo2 transcript expression in nodose and petrosal ganglia, where baroreceptor cell bodies are located (1). These ganglia are fused with each other and with the jugular ganglion in mice. Piezo1 and Piezo2 were highly expressed in the nodose-petrosal-jugular ganglion complex (NPJc) (Fig. 1A). Comparable numbers of cells were identified that highly expressed either Piezo1 or Piezo2 exclusively (123 and 124 cells with each transcript, respectively, Fig. 1B). A small population of neurons expressed both (43 double Piezo-positive cells, or 14.8% of all Piezo-expressing cells, n=6 mice, Fig.1B).
Fig. 1. Expression of Piezo1 and Piezo2 transcript in NPJc.
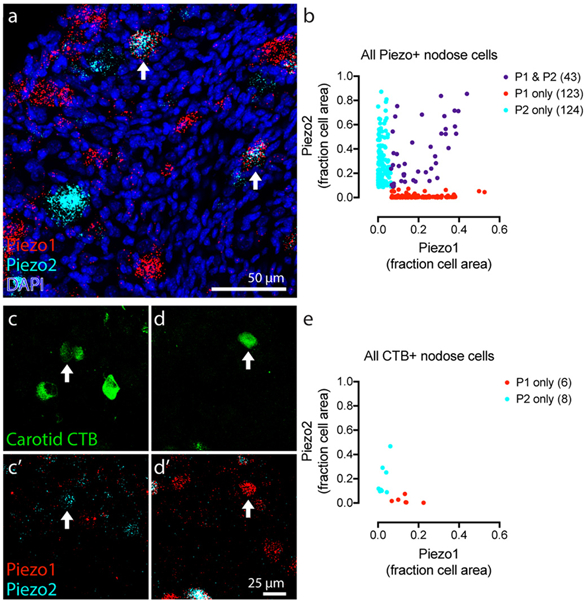
A, Z-projection of NPJc tissue after fluorescent in-situ hybridization with probes targeting Piezo1 (red) and Piezo2 (cyan). Nuclei labeled with DAPI (blue). Arrows mark double-positive cells. B, Quantification of transcript labeling area as a fraction of total cell area (n = 290 cells, 6 mice). Each dot represents one cell. C-F, NPJc cell bodies back-labeled by carotid sinus CTB injections (green) and Piezo transcript (below) shows a Piezo2+ (C, E) and Piezo1+ (D, F) cell (arrows). G, Quantification of Piezo transcript labeling area in CTB+ cells (n = 95 cells, 8 mice). Piezo-negative cells not shown.
To test whether Piezo1 and Piezo2 are expressed in baroreceptors, we performed retrograde labeling of carotid sensory neurons. We injected fluorescent Cholera Toxin B (CTB) (16) into the carotid sinus beneath the serosal vessel covering. All CTB+ neurons detected in the NPJc from eight mice were quantified for the presence of Piezo1 or Piezo2 transcript (Fig. 1C–F). Six out of 95 of retrogradely labeled cells were Piezo1-positive, and eight were Piezo2-positive (Fig. 1B). Piezo-negative cells were likely chemoreceptors, which abundantly innervate the carotid sinus but do not require mechanosensitivity. None of the 95 CTB-labeled cells were double-positive. These data suggest that a subset of neurons that innervate the carotid sinus (which include mechanoreceptors and chemoreceptors) express either Piezo1 or Piezo2 (Fig. 1G). We hypothesized that these cells could function as baroreceptors.
We therefore crossed Piezo floxed mice to the Phox2bCre line, which express Cre recombinase in epibranchial placode-derived ganglia (e.g. nodose and petrosal), but not neural crest-derived ganglia (jugular, trigeminal, and dorsal root) (17). We first analyzed the baroreflex in anesthetized mice in response to phenylephrine (PE). PE induces rapid vasoconstriction (6), which elevates BP. Increased BP then triggers baroreceptor activity and induces a reflex decrease in HR. PE-induced baroreflex changes were compared in conditional double knockout mice (dKO; Phox2bCre+;Piezo1f/fPiezo2f/f) and Cre-negative wild-type littermates (WT). Infusion of PE into the jugular vein produced a dose-dependent and transient increase in systolic BP and a consequent fall in HR, reflecting baroreflex control (6) (Fig. 2A). The PE-induced HR reduction (−29 ± 20 versus −234 ± 24 bpm, P<0.001) and decreased baroreflex sensitivity (−0.6 ± 0.4 versus −5.0 ± 0.5 Δbpm/ΔmmHg, P<0.001) were essentially abolished in the dKO mice (Fig. 2A–D). PE-induced systolic BP elevation in dKO mice was significantly higher than in WT littermates (55.7 ± 3 versus 45.7 ± 6 mmHg, P<0.05) (Fig. 2A, B). HR response to sodium nitroprusside-induced acute baroreceptor unloading was also absent in dKO mice (Fig. S1A–C). In contrast, Phox2bCre+;Piezo1f/f (P1cKO) and Phox2bCre+;Piezo2f/f (P2cKO) single knockout mice showed no difference in PE-induced change of baroreflex compared to WT littermates (Fig. 2B–D). We focused remaining analyses primarily on dKO mice.
Fig. 2. Baroreflex is abolished in nodose/petrosal ganglia-specific dKO mice.

A, Cardiovascular recordings show PE-induced baroreflex in WT mice, but no baroreflex in dKO littermates. BP, raw blood pressure signal. SYS, systolic blood pressure derived from raw BP. HR, heart rate. Changes of B, systolic blood pressure C, heart rate and D, baroreflex (10 s after i.v. injection of PE) in knock-out mice. Number of animals shown in bars (B) also apply for C-D. Piezo1: Phox2bCre+;Piezo1f/f (KO) mice. Piezo2: Phox2bCre+;Piezo2f/f (KO) mice. Piezo1 Piezo2: Phox2bCre+;Piezo1f/fPiezo2f/f (KO) mice. All WT are littermates. E, Traces show BP and ADN activity induced by PE and sodium nitroprusside injection in a WT and a dKO mouse. F, Statistical analysis of drug-induced ADN activity in WT and dKO mice. G, Raw BP and ADN activity example before and after PE injection. Expanded time scale showed bursts of ADN activity in phase with individual arterial pulses in WT. No integrated activity is observed in dKO mice. *p < 0.05, ***p < 0.001 and n.s., statistically not significant, unpaired Student’s t-test, means ± s.e.m.
We next measured aortic depressor nerve (ADN) activity during a rise of BP induced by PE. We observed a lack of drug-induced ADN activity in dKO mice compared to WT (−131.9 ± 163.9 versus 5558 ± 1234 normalized area under curve of integrated ADN activity, P<0.001, Fig. 2E–G). The dKO mice had no appreciable responses during both phasic and tonic phases of PE-induced ADN activity (Fig. S1D–E). This is not due to gross anatomical deficits, because we observed comparable baroreceptor endings within the aortic arch of dKO and WT mice (Fig. S2).
Impaired baroreceptor function leads to dysregulation of BP, including volatile hypertension and increased BP variability in humans (18–20). We examined daily BP variability in freely moving, conscious mice using a telemetric sensor (6). The dKO mice showed significantly elevated mean arterial pressure during their active time (gray shading, 6pm-6am) compared to WT littermates (112 ± 0.4 versus 95 ± 0.5 mmHg, P<0.001) (Fig. 3A, B, Fig. S3A, B, and Fig. S4). HR of dKO mice was slightly increased during active times compared to WT (583 ± 3 versus 566 ± 3 bpm, P<0.001), while the HR remained unchanged during inactive times (6am-6pm, 532 ± 3 versus 536 ± 3 bpm, not significant) (Fig. 3B). No difference in locomotor activity was observed between dKO and WT mice (Fig. S3C), ruling out the possibility that activity caused the increased BP and HR in dKO mice.
Fig. 3. Increased BP variability in conscious nodose/petrosal ganglia-specific dKO mice.

A, Continuous measurements of mean arterial pressure (MAP) and HR over 72 h, binned by hour. The differences between groups were significant during night (gray shading, two-way ANOVA, means ± s.e.m). B, Average MAP and HR during day (6am-6pm) and night (6pm-6am). C, sBRS, expressed as change in PI (ms) per change in systolic BP (mmHg), was significantly reduced in dKO mice (n = 17) compared to WT (n = 15). D, Frequency distribution histogram of the systolic blood pressure from 72 h. Red arrows indicate wider distribution of BP in dKO mice. E, BP variability reported as standard deviation from 72-h period. F, Maximum and minimum BP values. P values are indicated in the bars. *p < 0.05, **p < 0.01 and ***p < 0.001, n.s. is not significant. Unpaired Student’s t-test unless indicated otherwise, means ± s.e.m. n = 7–17.
We scanned telemetry data for spontaneous changes in systolic BP and pulse interval (PI) consistent with a baroreflex relationship. This method (sequence technique) is used to noninvasively assess baroreflex function (21, 22). The spontaneous baroreflex sensitivity (sBRS) is defined as the slope of changes in systolic BP versus PI from 1-hour of recording. sBRS was severely reduced in dKO mice (2.0 ± 0.1 versus 3.8 ± 0.2 ms/mmHg for WT, P<0.001, Fig. 3C). Sinoaortic baroreceptor denervated mice also show residual sBRS (22), and this may be due to compensation from other sensory systems.
We compared the BP variability of WT and dKO mice. The systolic BP values of dKO mice were distributed in a broader range than those of WT littermates (Fig. 3D). Variability was greatly enhanced in dKO mice (7.9 ± 0.3 versus 6.1 ± 0.3 mmHg in WT, P<0.001) (Fig. 3E). We quantified the range of BP variability of mean, systolic, and diastolic BP between each group in a 72-hour period. Maximum values of BP from dKO mice were significantly higher than WT littermates, while minimum values were significantly lower (Fig. 3F). Lastly, homovanillic acid levels from dKO mouse urine were significantly higher than in WT (13.9 ± 0.05 versus 12.1 ± 0.06 μg/mL, Fig. S3D), suggesting an increase of hormone norepinephrine, as in human baroreflex failure patients (18). There were no significant BP variability and sBRS differences in P1cKO (Fig. S5) and P2cKO (Fig. S6) single knockout mice compared to WT littermates. P2cKO mice showed a subtle hypotensive BP distribution (Fig. S6).
We next investigated whether stimulating Piezo2-positive neurons can induce the baroreflex in adult mice. We crossed Piezo2GFP-IRES-Cre (Piezo2Cre) knock-in mice with Cre-dependent channelrhodopsin-2 (Ai32) reporter to generate Piezo2Cre+;ChR2-eYFP mice (13), and recorded the cardiovascular response of activating different regions of Piezo2+ vagal sensory nerves by optogenetics (Fig. 4A). We did not observe cardiovascular changes during long optogenetic stimulation (5 ms pulses, 50 Hz, 10 s) of the vagal nerve trunk (area 1, BP: −5.3 ± 1.0%, HR: −2.0 ± 1.0%, not significant) (Fig. 4A–C). Next, we focused on specifically activating baroreceptor afferents. For aortic baroreceptors, we stimulated the superior laryngeal nerve branch, which carries afferent inputs from the ADN (Fig. 4A, area 2). For carotid baroreceptors, we exposed the carotid sinus region and directly stimulated the local nerve terminals (Fig. 4A, area 3). Light stimulations at both locations caused an immediate decrease in both BP and HR (area 2: BP: −55.6 ± 2.0%, HR: −50.5 ± 2.0%; area 3: BP: −37.5 ± 3.5%, HR: −32.3 ± 3.7%, P<0.001) compared to the unstimulated baseline (Fig. 4B, C). A prominent consequence of baroreceptor activation is rapid inhibition of efferent sympathetic activity (1). We found that light-induced decrease in HR was markedly attenuated after administration of the beta-adrenergic receptor blocker propranolol, indicating that the reflex bradycardia was mediated primarily by inhibition of cardiac sympathetic nerve activity (Fig. S7). Piezo2Cre-;ChR2-eYFP mice (WT) did not show any changes during optogenetic stimulation in all three regions (BP: −0.6 ± 0.6%, HR: 0.4 ± 0.8%, not significant) (Fig. 4B, C).
Fig. 4. Piezo2-positive sensory neurons acutely control blood pressure.

A, Schematic depiction of the optogenetic strategy. The carotid sinus and vagus nerve is illuminated to activate ChR2-expressing Piezo2-sensory neurons. The optical fiber was placed on area 1) vagus nerve trunk, 2) superior laryngeal branch and 3) carotid sinus. B, Traces of cardiovascular effects following focal vagus nerve illumination (blue shading) in anesthetized Piezo2Cre-;ChR2-eYFP (WT, black trace) and Piezo2Cre+;ChR2-eYFP mice (Piezo2Cre+, grey, blue, and pink traces). Numbers on left (1–3) correspond to locations in A. Blood pressure was measured by a pressure transducer cannulated in the left carotid artery. BP, carotid arterial pressure. HR, heart rate. C, Light-induced changes in BP and HR were calculated over the 10 s (n = 7–18 as indicated. ***p < 0.001, n.s., statistically not significant, unpaired Student’s t-test, means ± s.e.m.).
In summary, this study demonstrates that the mechanosensitive ion channels PIEZO1 and PIEZO2 are together required for arterial baroreceptor activity and function. Baroreflex is critical to maintain short term BP homeostasis in mammals. The long-term changes observed in heart rate and blood pressure that accompany baroreflex failure are complex. Acute elimination of baroreceptor function (e.g., sino-aortic denervation) causes immediate, large increases in BP and HR (23, 24). Over time, the mean BP decreases but remains labile hypertensive, and BP variability is markedly increased and persists (18–20, 24, 25). We observed a significant increase in MAP during the Piezo dKO mice’s active period that falls just under the designation for hypertension (26), and dKO mice also developed increased blood pressure variability. These data show that losing PIEZO1 and PIEZO2 function recapitulates the phenotype observed in animal models (24, 25) and humans with baroreflex failure (18–20). However, we cannot exclude the possibility that sensory mechanisms beyond the baroreceptors within the vagus contribute to the observed elevated blood pressure.
Supplementary Material
Acknowledgements:
We thank Donald Morgan, Shang Ma, and Keiko Nonomura for assistance.
Funding: This work was supported by NIH grants R01 DE022358 and R35 NS105067 to A.P. W.-Z.Z. was supported by a postdoctoral fellowship from the George Hewitt Foundation for Medical Research. S.D.L. was supported by NIH grants DP1 AT009497 and OT2 OD023848. M.W.C and F.M.A were supported by NIH grant P01 HL14388. A.P. and S.D.L. are investigators of the Howard Hughes Medical Institute.
Footnotes
Competing interests: Authors declare no competing interests.
Data and materials availability: All data is available in the main text or the supplementary materials.
References and Notes
- 1.Wehrwein EA, Joyner MJ, Handb Clin Neurol 117, 89–102 (2013). [DOI] [PubMed] [Google Scholar]
- 2.Kirchheim HR, Physiol Rev 56, 100–177 (1976). [DOI] [PubMed] [Google Scholar]
- 3.Mortara A et al. , Circulation 96, 3450–3458 (1997). [DOI] [PubMed] [Google Scholar]
- 4.La Rovere MT, Bigger JT Jr., Marcus FI, Mortara A, Schwartz PJ, Lancet 351, 478–484 (1998). [DOI] [PubMed] [Google Scholar]
- 5.Drummond HA, Price MP, Welsh MJ, Abboud FM, Neuron 21, 1435–1441 (1998). [DOI] [PubMed] [Google Scholar]
- 6.Lu YJ et al. , Neuron 64, 885–897 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chesler AT et al. , New Engl J Med 375, 1355–1364 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lau OC, Shen B, Wong CO, Yao X, Nat Commun 9, 1244 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thakore P, Brain SD, Beech DJ, Nat Commun 9, 1245 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murthy SE, Dubin AE, Patapoutian A, Nat Rev Mol Cell Biol 18, 771–783 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Wang SP et al. , J Clin Invest 126, 4527–4536 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Retailleau K et al. , Cell Rep 13, 1161–1171 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Nonomura K et al. , Nature 541, 176–181 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woo SH et al. , Nat Neurosci 18, 1756–1762 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ranade SS et al. , Nature 516, 121–U330 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marshall KL et al. , Cell Rep 11, 851–858 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pattyn A, Morin X, Cremer H, Goridis C, Brunet JF, Development 124, 4065–4075 (1997). [DOI] [PubMed] [Google Scholar]
- 18.Robertson D et al. , N Engl J Med 329, 1449–1455 (1993). [DOI] [PubMed] [Google Scholar]
- 19.Heusser K, Tank J, Luft FC, Jordan J, Hypertension 45, 834–839 (2005). [DOI] [PubMed] [Google Scholar]
- 20.Ketch T, Biaggioni I, Robertson R, Robertson D, Circulation 105, 2518–2523 (2002). [DOI] [PubMed] [Google Scholar]
- 21.Oosting J, Struijker-Boudier HA, Janssen BJ, J Hypertens 15, 391–399 (1997). [DOI] [PubMed] [Google Scholar]
- 22.Martinka P et al. , Am J Physiol Regul Integr Comp Physiol 288, R767–776 (2005). [DOI] [PubMed] [Google Scholar]
- 23.Wenker IC et al. , J Neurosci 37, 4565–4583 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodrigues FL, de Oliveira M, Salgado HC, Fazan R Jr., Exp Physiol 96, 853–862 (2011). [DOI] [PubMed] [Google Scholar]
- 25.Ito CS, Scher AM, Circ Res 48, 576–591 (1981). [DOI] [PubMed] [Google Scholar]
- 26.James PA et al. , JAMA 311, 507–520 (2014). [DOI] [PubMed] [Google Scholar]
- 27.Shehab SAS, Spike RC, Todd AJ, Brain Res 964, 218–227 (2003). [DOI] [PubMed] [Google Scholar]
- 28.Lai BQ et al. , Plos One 10, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


