Abstract
BACKGROUND
Tuberculosis is the leading killer of patients with human immunodeficiency virus (HIV) infection. Preventive therapy is effective, but current regimens are limited by poor implementation and low completion rates.
METHODS
We conducted a randomized, open-label, phase 3 noninferiority trial comparing the efficacy and safety of a 1-month regimen of daily rifapentine plus isoniazid (1-month group) with 9 months of isoniazid alone (9-month group) in HIV-infected patients who were living in areas of high tuberculosis prevalence or who had evidence of latent tuberculosis infection. The primary end point was the first diagnosis of tuberculosis or death from tuberculosis or an unknown cause. Noninferiority would be shown if the upper limit of the 95% confidence interval for the between-group difference in the number of events per 100 person-years was less than 1.25.
RESULTS
A total of 3000 patients were enrolled and followed for a median of 3.3 years. Of these patients, 54% were women; the median CD4+ count was 470 cells per cubic millimeter, and half the patients were receiving antiretroviral therapy. The primary end point was reported in 32 of 1488 patients (2%) in the 1-month group and in 33 of 1498 (2%) in the 9-month group, for an incidence rate of 0.65 per 100 person-years and 0.67 per 100 person-years, respectively (rate difference in the 1-month group, −0.02 per 100 person years; upper limit of the 95% confidence interval, 0.30). Serious adverse events occurred in 6% of the patients in the 1-month group and in 7% of those in the 9-month group (P = 0.07). The percentage of treatment completion was significantly higher in the 1-month group than in the 9-month group (97% vs. 90%, P<0.001).
CONCLUSIONS
A 1-month regimen of rifapentine plus isoniazid was noninferior to 9 months of isoniazid alone for preventing tuberculosis in HIV-infected patients. The percentage of patients who completed treatment was significantly higher in the 1-month group. (Funded by the National Institute of Allergy and Infectious Diseases; BRIEF TB/A5279 ClinicalTrials.gov number, NCT01404312.)
Worldwide, approximately 1000 persons with human immunodeficiency virus (HIV) infection die from tuberculosis each day, including many who are receiving antiretroviral therapy.1 Preventive therapy with isoniazid substantially reduces the risk of tuberculosis and death among patients with HIV infection and is recommended by the World Health Organization, but globally only a small proportion of those who are eligible to receive this therapy are actually treated.1–6 Clinicians and HIV treatment programs have been reluctant to implement isoniazid preventive therapy owing in part to concern about adherence to 6 to 9 months of treatment, drug toxicity, and the emergence of drug resistance.7 Shorter courses of rifamycin-based preventive therapy are effective in HIV-infected patients, but the use of these regimens is also limited.8,9
In studies that used a murine model of latent tuberculosis, daily treatment with rifapen- tine plus isoniazid for 1 month was as effective as 3 months of weekly rifapentine plus isoniazid and at least as effective as 6 months of isoniazid alone.10,11 In the BRIEF TB/A5279 (Brief Rifa-pentine–Isoniazid Efficacy for TB [Tuberculosis] Prevention/A5279) trial, we hypothesized that a 1-month regimen of daily rifapentine plus isoniazid would be noninferior to 9 months of isoniazid alone for preventing tuberculosis in patients with HIV infection and that the shorter regimen would have better adherence and fewer adverse effects.
Methods
TRIAL DESIGN AND PATIENTS
From May 2012 through November 2014, we conducted this randomized, open-label, phase 3 noninferiority trial at 45 sites in 10 countries in Africa, Asia, South America, North America, and the Caribbean. Eligible patients had confirmed HIV infection, were 13 years of age or older, and either lived in an area with a tuberculosis prevalence of at least 60 cases per 100,000 population or had a positive test for latent tuberculosis. We excluded from the trial patients who had known or suspected active tuberculosis on the basis of clinical evaluation or who had recently received treatment for tuberculosis. Also excluded were pregnant or breast-feeding women and patients with elevated liver- enzyme levels or a body weight of less than 30 kg. Antiretroviral therapy with the use of efavirenz or nevirapine was permitted for the first month of trial participation, with any other regimen permitted after the first month.12 Patients were stratified according to antiretroviral-therapy status and CD4+ cell count at baseline.
Patients underwent randomization in a 1:1 ratio to receive either 4 weeks of rifapentine (at a dose of 300 mg daily for a weight of <35 kg, 450 mg daily for a weight of 35 to 45 kg, and 600 mg for a weight of >45 kg) plus isoniazid at a dose of 300 mg daily (1-month group) or 36 weeks of isoniazid alone at a dose of 300 mg daily (9-month group). All the patients received pyridoxine with each dose of a trial medication. Treatment was administered by the patients.
All the patients were followed until November 14, 2017, which was 3 years after the last patient had been enrolled. Trial visits were conducted at weeks 2, 4, 8, 12, 16, 20, 24, and 36 and every 12 weeks thereafter. Patients in the 1-month group were allowed 8 weeks to complete treatment, whereas those in the 9-month group were allowed 54 weeks to accommodate possible interruptions because of toxic effects. Treatment completion was defined as patientreported adherence to the trial regimen for the duration of the trial.
END POINTS
The primary end point in this time-to-event trial was the first diagnosis of active tuberculosis, death from tuberculosis, or death from an unknown cause. Secondary end points were safety, side-effect profile, death from any cause, and death from an unknown cause or causes unrelated to tuberculosis. Each end point was reviewed by one of two independent experts who were unaware of trial-group assignments. The criteria for the diagnosis of tuberculosis were defined in Appendix 100 of the Division of AIDS of the National Institute of Allergy and Infectious Diseases. Ascertainment of adverse events was conducted through week 156 in the two trial groups; additional pill counts and adherence assessments occurred from week 4 through week 36 in the 9-month group. All adverse events were graded according to the Division of AIDS table regardless of possible relationship to the trial medications. (Details are provided in the Supplementary Appendix, available with the full text of this article at NEJM.org.
TRIAL OVERSIGHT
Members of the protocol team from the AIDS Clinical Trials Group and the International Maternal–Pediatric–Adolescent AIDS Clinical Trials Network designed and implemented the trial and analyzed the data. (The protocol team included some of the authors as well as persons listed in the acknowledgment statement at the end of the article.) Sanofi provided rifapentine and financial support for the procurement of isoniazid, and a company representative participated on the protocol team.
All the authors vouch for the completeness and accuracy of the data and for the adherence of the trial to the protocol (available at NEJM.org). The protocol was approved by the institutional review board at each site; all the patients provided written informed consent. The trial was monitored by the African data and safety monitoring board of the National Institute of Allergy and Infectious Diseases.
STATISTICAL ANALYSIS
We used a noninferiority design to test the hypothesis that the efficacy of the 1-month regimen would be noninferior to that of the 9-month regimen in preventing the primary end point. We assumed that the incidence of tuberculosis in the 9-month group would be 2.00 cases per 100 person-years on the basis of the results of previous studies of tuberculosis preventive therapy in HIV-infected adults in southern Africa.8,13
Noninferiority would be shown if the upper limit of the 95% confidence interval for the between-group difference in the number of events per 100 person-years was less than 1.25. Kaplan–Meier estimates of the time until the primary end point in each group were computed, both overall and within each stratum of CD4+ count. For the primary end point, data from patients who had been lost to follow-up or had died from known causes unrelated to tuberculosis were censored at the time of the last assessment or death. We conducted one interim analysis using the Haybittle–Peto boundary We set the noninferiority margin at 1.25 events per 100 person-years as the boundary for the upper limit of the 95% confidence interval of the between-group difference, since it ensured the preservation of a reasonable amount of the treatment effect of the 9-month regimen over that of placebo, as determined from the results of earlier studies of isoniazid. (Details regarding this estimation are provided in the protocol.) The boundary represents the limits of a 95% confidence interval so that if noninferiority was confirmed, the observed rate in the 1-month group would be substantially less than the noninferiority margin.
On the basis of these calculations, we determined that the enrollment of 2452 patients would provide the trial with a power of 90% to confirm noninferiority at a one-sided significance level of 0.025 (i.e., a two-sided level of 0.05). We subsequently increased the enrollment to 3000 patients to permit the evaluation of essential subgroups with some precision. We also included upward adjustments for interim monitoring and for a combined loss to follow-up plus a death rate from nontuberculosis causes of 10% and a 2% increase for interim monitoring.
We used a modified intention-to-treat method for the primary analysis, which included all the patients who had received at least one dose of a trial medication. We used the Mantel– Haenszel method to estimate standardized incidence rates and incidence rate differences with 95% confidence intervals using the PROC STDRATE procedure in SAS software, version 9.4 (SAS Institute). Before the analyses were conducted, the lower two categories of CD4+ count were collapsed into one category (≤250 cells per cubic millimeter), since there were few patients in those categories.
We also conducted a per-protocol analysis, which included all the patients from the modified intention-to-treat population who had completed treatment or who had died or received a diagnosis of tuberculosis while they were receiving treatment. In additional sensitivity analyses, data regarding deaths from unknown causes were either censored at the time of death or treated as competing risks.
In analyses of safety and side-effect profiles, we considered the proportions of patients with serious adverse events, targeted safety events, and treatment discontinuations or modifications because of toxicity. For serious adverse events and targeted safety events, we estimated the difference in the proportions of patients who had at least one event and used Fisher’s exact test to calculate P values. We also estimated the incidence of grade 3 or 4 safety end points and compared them using a negative binomial model. For side-effect profiles, we used a proportional-odds model to estimate the odds of being in a better ordinal category within the hierarchy of no discontinuation, temporary treatment interruption, and permanent discontinuation because of toxicity. All the patients completed a medication-adherence questionnaire at weeks 2 and 4, and those in the 9-month group completed the questionnaire every 4 weeks thereafter until week 36.14
RESULTS
PATIENTS
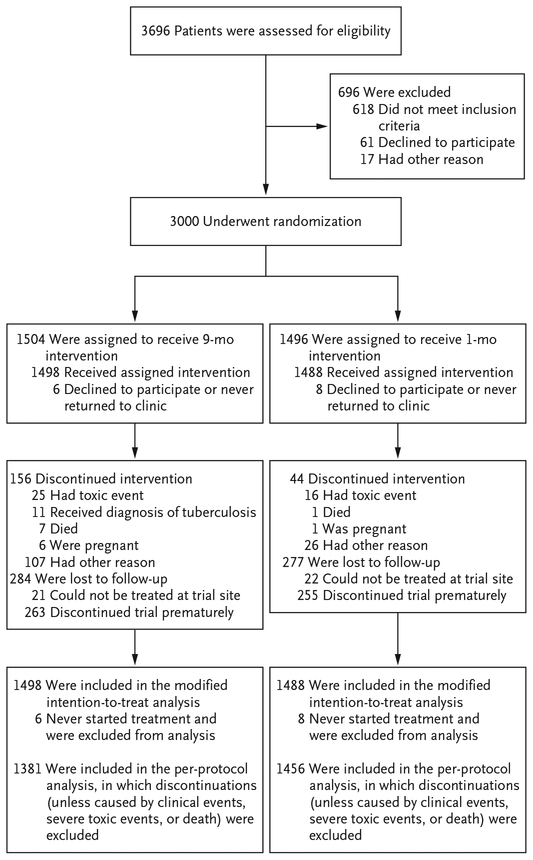
Of the 3000 patients who were enrolled in the trial, 14 never received a dose of trial medication and were excluded from the primary analysis (Fig. 1). The baseline characteristics of the patients were similar in the two groups (Table 1). A total of 1614 patients (54%) were women; the median CD4+ count was 470 cells per cubic millimeter (interquartile range, 346 to 635). Fifty percent of the patients were receiving antiretroviral therapy at entry, and 77% of these patients had an undetectable HIV viral load (HIV RNA, <40 copies per milliliter).
Figure 1.
Enrollment and Follow-up.
Table 1.
Characteristics of the Patients at Baseline.*
| Characteristic | 1-Month Group (N =1496) | 9-Month Group (N =1504) | All Patients (N = 3000) |
|---|---|---|---|
| Continent of residence — no. (%) | |||
| Africa | 772 (52) | 781 (52) | 1553 (52) |
| Asia | 121 (8) | 124 (8) | 245 (8) |
| South America | 360 (24) | 355 (24) | 715 (24) |
| North America | 243 (16) | 244 (16) | 487 (16) |
| Median age (IQR) — yr | 35 (28–43) | 35 (28–43) | 35 (28–43) |
| Sex — no. (%) | |||
| Male | 694 (46) | 692 (46) | 1386 (46) |
| Female | 802 (54) | 812 (54) | 1614 (54) |
| Race or ethnic group — no. (%)† | |||
| Black non-Hispanic | 992 (66) | 991 (66) | 1983 (66) |
| White non-Hispanic | 16 (1) | 12 (1) | 28 (1) |
| Asian or Pacific Islander | 122 (8) | 128 (9) | 250 (8) |
| Hispanic | 361 (24) | 369 (25) | 730 (24) |
| Unknown | 5 (<1) | 4 (<1) | 9 (<1) |
| Median body-mass index (IQR)‡ | 23.6 (20.9–27.1) | 23.5 (20.8–26.9) | 23.5 (20.9–27.1) |
| CD4+ count | |||
| Median (IQR) — no. of cells/mm3 | 473 (349–636) | 469 (341–634) | 470 (346–635) |
| Patients — no. (%) | |||
| >250 cells/mm3 | 1299 (87) | 1302 (87) | 2601 (87) |
| 100 to ≤250 cells/mm3 | 160 (11) | 165 (11) | 325 (11) |
| <100 cells/mm3 | 37 (2) | 37 (2) | 74 (2) |
| Receipt of antiretroviral therapy at entry — no. (%) | |||
| Efavirenz-based regimen | 650 (43) | 649 (43) | 1299 (43) |
| Nevirapine-based regimen | 97 (6) | 100 (7) | 197 (7) |
| Other | 3 (<1) | 6 (<1) | 9 (<1) |
| None | 746 (50) | 749 (50) | 1495 (50) |
| Viral load in patients receiving antiretroviral therapy —no./total no.(%) | |||
| Undetectable — <40 copies/ml | 569/750 (76) | 586/755 (78) | 1155/1505 (77) |
| Detectable — ≥40 copies/ml | 154/750 (21) | 143/755 (19) | 297/1505 (20) |
| Unavailable | 27/750 (4) | 26/755 (3) | 53/1505 (4) |
| Previous diagnosis of tuberculosis — no. (%) | 82 (5) | 89 (6) | 171 (6) |
| Tuberculin skin test — no. (%) | |||
| Positive | 311 (21) | 324 (22) | 635 (21) |
| Negative | 1033 (69) | 1021 (68) | 2054 (68) |
| Not done | 152 (10) | 159 (11) | 311 (10) |
| IGRA for tuberculosis — no. (%)§ | |||
| Positive | 36 (2) | 37 (2) | 73 (2) |
| Negative | 1 (<1) | 2 (<1) | 3 (<1) |
| Not done | 1459 (98) | 1465 (97) | 2924 (97) |
Patients in the 1-month group were assigned to receive a regimen of daily rifapentine plus isoniazid, and those in the 9-month group were assigned to receive daily isoniazid alone. Percentages may not total 100 because of rounding. IQR denotes interquartile range.
Race or ethnic group was reported by the patients.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
Results of the interferon-γ release assay (IGRA) were available only for patients at sites in the United States.
Among all the patients, 97% were from areas with a high prevalence of tuberculosis (≥60 cases per 100,000 population), and 692 (23%) had a positive tuberculin skin test, a positive result on the interferon-γ release assay, or both. Overall, patient-reported adherence to treatment was 90% or more in each group; treatment was completed in 97% of the patients in the 1-month group and in 90% of those in the 9-month group (P<0.001). A total of 18% of the patients in each group were lost to follow-up. In each group, the median time of participation in the trial was approximately 3.3 years, and adherence to scheduled visits averaged more than 95% among those who were still participating in the trial at a given week.
Among the patients who were not receiving antiretroviral therapy at entry, such treatment was initiated by 87% of those in the 1-month group and 85% in the 9-month group during follow-up, with a median uptake time of 12.0 months (interquartile range, 1.9 to 30.9) in the 1-month group and 11.7 months (interquartile range, 1.2 to 33.3) in the 9-month group. Among the patients in the two groups who did not initiate antiretroviral therapy, 75% had discontinued the trial early and 72% had fewer than 3 years of follow-up.
END POINTS
The primary end point occurred in 32 of 1488 patients (2%) in the 1-month group and in 33 of 1498 (2%) in the 9-month group, with incidence rates of 0.65 and 0.67 per 100 person years, respectively, for a between-group difference of −0.02 per 100 person-years (95% confidence interval [CI], −0.35 to 0.30) (Table 2 and Fig. 2). Active tuberculosis accounted for 29 of 32 events (91%), including 18 confirmed events and 11 probable events, in the 1-month group and for 26 of 33 events (79%), including 14 confirmed events, 10 probable events, and 2 tuberculosis-related deaths, in the 9-month group. There were 3 deaths from unknown causes in the 1-month group and 7 in the 9-month group. A total of 6 tuberculosis-related deaths (3 in each group, including 1 death attributed to tuberculosis immune reconstitution inflammatory syndrome) were reported.
Table 2.
Univariate Analysis of Risk Factors for the Primary End Point.*
| Variable | 1-Month Group | 9-Month Group | Difference in Incidence Rate (95% CI)† | ||
|---|---|---|---|---|---|
| no. of events/person-yr | incidence rate/100 person-yr | no. of events/person-yr | incidence rate/100 person-yr | ||
| All patients | 32/4926 | 0.65 | 33/896 | 0.67 | −0.02 (−0.35 to 0.30) |
| Per-protocol analysis | 31/876 | 0.64 | 29/4718 | 0.61 | 0.02 (−0.30 to 0.34) |
| Status on tuberculin skin test or IGRA | |||||
| Positive | 10/1110 | 0.90 | 11/1137 | 0.97 | −0.07 (−0.87 to 0.73) |
| Negative or unknown | 22/3815 | 0.58 | 22/3759 | 0.59 | −0.01 (−0.35 to 0.34) |
| Receipt of antiretroviral therapy at entry | |||||
| Yes | 13/2381 | 0.55 | 15/2387 | 0.63 | −0.08 (−0.52 to 0.35) |
| No | 19/2545 | 0.75 | 18/2508 | 0.72 | 0.03 (−0.44 to 0.50) |
| Screening CD4+ count | |||||
| ≤250 cells/mm3 | 12/621 | 1.93 | 8/628 | 1.28 | 0.66 (−0.75 to 2.06) |
| >250 cells/mm3 | 20/4304 | 0.47 | 25/4268 | 0.59 | −0.12 (−0.43 to 0.19) |
| Sex | |||||
| Male | 11/2303 | 0.48 | 15/2293 | 0.65 | −0.18 (−0.61 to 0.26) |
| Female | 21/623 | 0.80 | 18/2603 | 0.69 | 0.11 (−0.36 to 0.58) |
The primary end point was a diagnosis of tuberculosis or death from tuberculosis or an unknown cause.
This difference is the incidence rate in the 1-month group minus the rate in the 9-month group, so negative values indicate a lower risk in the 1-month group.
Figure 2. Kaplan–Meier Analysis of the Primary End Point.
Shown are the percentages of patients who were free from the primary end point — the diagnosis of tuberculosis or death from tuberculosis or an unknown cause — among all the patients (Panel A), among those with a CD4+ count of 250 cells per cubic millimeter or less (Panel B), and among those with a CD4+ count of more than 250 cells (Panel C), according to the receipt of either a 1-month regimen of daily rifapentine plus isoniazid (1-month group) or 9 months of isoniazid alone (9-month group).
The results of the per-protocol analysis were similar to those of the primary analysis, with incidence rates of 0.64 and 0.61 per 100 person- years in the 1-month group and the 9-month group, respectively, for a between-group difference of 0.02 per 100 person-years (95% CI, −0.30 to 0.34). The results were also similar in sensitivity analyses in which deaths from unknown causes were censored at the time of death or were treated as competing risks.
The percentage of patients with the primary end point was higher among those with evidence of latent tuberculosis infection and among those who were not receiving antiretroviral therapy at enrollment, although such differences were not significant (Table 2). The primary end point occurred in a higher proportion of women in the 1-month group than in the 9-month group, but the difference was not significant; conversely, the incidence of the primary end point was lower among men in the 1-month group than among those in the 9-month group (Table 2). Among patients with a CD4+ count of 250 cells per cubic millimeter or less at baseline, the incidence of the primary end point was slightly higher in the 1-month group than in the 9-month group, whereas among those with a CD4+ count of more than 250 cells per cubic millimeter, the incidence was slightly lower in the 1-month group, although neither of these differences was significant (Table 2 and Fig. 2B and 2C).
In one patient in each group, tuberculosis caused by a rifampin-resistant strain developed. In three patients (two in the 1-month group and one in the 9-month group), tuberculosis caused by an isoniazid-resistant strain was diagnosed.
ADVERSE EVENTS
The patients who discontinued treatment because of toxic effects included 16 (1%) in the 1-month group and 25 (2%) in the 9-month group; treatment was withheld for more than 7 days in 11 patients and 31 patients (1% vs. 2%), respectively. The proportional odds ratio for discontinuing or withholding a trial regimen was 2.09 (95% CI, 1.32 to 3.33) favoring the 1-month regimen.
During the entire follow-up period, serious adverse events of any grade occurred in 83 patients (6%) in the 1-month group and in 108 (7%) in the 9-month group (P = 0.07). (Serious adverse events of grades 3 through 5 are listed in Table 3.) Prespecified targeted safety events (including nausea and vomiting, rash, drug associated fever, elevated liver-enzyme levels, and peripheral neuropathy) of grade 3 or higher occurred in 44 patients (3%) in the 1-month group and in 52 (3%) in the 9-month group (P = 0.47). An analysis of the rates of combined grade 3 and 4 serious adverse events and targeted safety events over the entire follow-up period showed that fewer events occurred in the 1-month group than in the 9-month group (2.9 vs. 4.6 events per 100 person-years) (P = 0.01). Data from patients in the two groups were censored at the time they discontinued the trial or had a primary end point, regardless of the treatment duration. Elevations in liver-enzyme levels of grade 3 or greater and neurologic toxicity were less common in the 1-month group than in the 9-month group (2% vs. 3% and 1% vs. 2%, respectively), whereas neutropenia of grade 3 or higher was more common in the 1-month group (2% vs. 1%). (Details regarding adverse events are provided in the Supplementary Appendix.)
Table 3.
Adverse Events of Grade 3 or Greater.*
| Adverse Event | 1-Month Group (N =1488) | 9-Month Group (N =1498) | ||||||
|---|---|---|---|---|---|---|---|---|
| Grade 3 | Grade 4 | Grade 5 | Grades 3–5 | Grade 3 | Grade 4 | Grade 5 | Grades 3–5 | |
| number of patients (percent) | ||||||||
| Targeted adverse event† | 34 | 9 | 1 | 44 (3) | 32 | 20 | 0 | 52 (3) |
| Serious adverse event | 41 | 22 | 12 | 75 (5) | 49 | 25 | 19 | 93 (6) |
| Any systemic event | 101 | 9 | 1 | 111 (7) | 123 | 12 | 0 | 135 (9) |
| Any adverse event | 198 | 47 | 5 | 250 (17) | 213 | 59 | 2 | 274 (18) |
| Hematologic event | 41 | 22 | 0 | 63 (4) | 36 | 21 | 0 | 57 (4) |
| Thrombocytopenia | 0 | 3 | 0 | 3 (<1) | 4 | 1 | 0 | 5 (<1) |
| Anemia | 6 | 14 | 0 | 20 (1) | 8 | 18 | 0 | 26 (2) |
| Neutropenia | 28 | 8 | 0 | 36 (2) | 16 | 2 | 0 | 18 (1) |
| Hepatic event | 19 | 9 | 0 | 28 (2) | 24 | 18 | 0 | 42 (3) |
| Gastrointestinal event | 29 | 1 | 1 | 31 (2) | 22 | 2 | 0 | 24 (2) |
| Dermatologic event | 8 | 0 | 0 | 8 (1) | 11 | 0 | 0 | 11(1) |
| Neurologic event | 12 | 2 | 0 | 14 (1) | 25 | 4 | 1 | 30 (2) |
There was a significant between-group difference in neutropenia and in neurologic events (P = 0.02 for both comparisons) at an alpha level of 0.05 with no adjustment for multiple comparisons.
Targeted adverse events included nausea and vomiting, rash, drug-associated fever, elevated liver-enzyme levels, and peripheral neuropathy.
DISCUSSION
In this trial, we found that 1 month of daily rifapentine plus isoniazid was noninferior to daily isoniazid for 9 months for the prevention of tuberculosis in HIV-infected adults and adolescents. Patients in the 1-month group had a lower incidence of adverse events and were more likely to complete treatment than were those in the 9-month group. Because the trial was open-label, the outcomes that were measured reflect the pragmatic effectiveness of the 1-month regimen. However, the high degree of adherence in the two groups suggests that the trial design also captured the clinical efficacy of the shorter regimen.
Despite extensive high-quality evidence sup- porting the efficacy of preventive therapy for tuberculosis and recommendations from the World Health Organization and others, the use of such an intervention worldwide has been low.1,3,4,7,15 In 2017, fewer than 1 million HIV- infected patients received preventive treatment, with an estimated 30 million eligible.1 Implementation of tuberculosis preventive therapy in patients with HIV infection has been hampered by operational concerns, poor adherence to long- duration regimens, concern about drug resistance, drug–drug interactions with antiretroviral agents, and skepticism about the effect of this strategy during a time when antiretroviral therapy was being increasingly prescribed.15–17 Several studies have shown that isoniazid therapy reduces the risk of death regardless of the receipt of antiretroviral therapy among HIV- infected patients.18–20
In research settings, moderately good adherence was shown with longer durations of isoniazid preventive therapy, but only 50 to 70% of the patients at increased risk for tuberculosis completed 6 to 9 months of isoniazid therapy in programmatic settings.21,22 Shortening treatment to 3 months with supervised weekly administration of rifapentine and isoniazid increased completion rates to 87 to 96% in research settings and to 82% in clinical practice.8,9,21 In our trial, 97% of the patients who started 1 month of self-administered rifapentine plus isoniazid completed the regimen, and completion rates of 9 months of isoniazid therapy were also high.
Adverse drug reactions, including hepatotoxicity, are an important concern that influences the use of tuberculosis preventive therapy.22–27 There were significantly fewer treatment interruptions or discontinuations because of drug toxicity in the 1-month group than in the 9-month group. Cases of hepatotoxicity and peripheral neuropathy were unusual in the 1-month group (2% of recipients), and no hypersensitivity reactions were reported.28,29
Concern about the development of drugresistant tuberculosis is also a barrier to the implementation of preventive therapy, despite a lack of evidence, even in areas with high background rates of drug-resistant tuberculosis.8,26 We observed low rates of drug-resistant tuberculosis among patients in whom active tuberculosis disease developed, a finding that was consistent with the background prevalence of resistance in the participating countries. Finally, although rates of tuberculosis were higher among patients with evidence of latent infection, we found that the 1-month regimen was noninferior to the 9-month regimen in HIV- infected patients without evidence of latent infection. The TEMPRANO trial confirmed the superiority of isoniazid over placebo for pre- venting tuberculosis and death in HIV-infected patients who had a negative test for latent tuberculosis infection. Our results show the non- inferiority of a 1-month regimen of rifapentine plus isoniazid to 9 months of isoniazid alone in such patients.3,4
Our trial has several limitations. We enrolled only adults who were not pregnant or breast- feeding and adolescents (≥13 years of age) with HIV infection, so the effectiveness of the 1-month regimen in HIV-uninfected persons, younger children, and pregnant women is not known. The overall tuberculosis incidence that was observed in our trial was lower than expected at approximately 0.70 per 100 person-years, which limited our ability to evaluate differences in sub groups with precision. In particular, the number of patients with a CD4+ count of less than 250 cells per cubic millimeter was small, and neither inferiority nor noninferiority of the 1-month regimen was shown in this stratum. We had based our assumptions on the evidence that was available during the trial development, but this did not include more recent studies showing an independent benefit from antiretroviral therapy in preventing tuberculosis.3–5 Approximately half the patients in our trial were receiving antiretroviral therapy at trial entry, and more than 90% were receiving such therapy at trial completion. We previously had found that the coadministration of rifapentine and isoniazid with efavirenz did not adversely affect efavirenz concentrations, but future studies are likely to investigate more contemporary antiretroviral agents, such as integrase strand transfer inhibitors, including dolutegravir.12,29,30
In conclusion, our results showed that 1 month of daily rifapentine plus isoniazid was noninferior to 9 months of isoniazid in preventing tuberculosis in high-risk patients with HIV infection, as predicted by the murine model.10,11
Supplementary Material
Acknowledgments
Supported by grants (UM1 AI068634, UM1 AI068636, and UM1 AI106701) from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health. Sanofi provided rifapentine and financial support for the procurement of isoniazid, and company representatives participated on the protocol team.
Dr. Swindells reports receiving grant support, paid to her university, from Merck Laboratories and ViiV Healthcare; Dr. Mohapi, receiving grant support, paid to her institution, from Janssen Pharmaceuticals, Merck, ViiV Healthcare, Johnson & Johnson, Pfizer, and Bristol-Myers Squibb, and protocol support from Kowa Pharmaceuticals America and Sanofi Aventis; Dr. Hakim, receiving advisory board fees from Mylan Pharmaceuticals; and Dr. Chaisson, receiving consulting fees from Otsuka Pharmaceutical and Merck. No other potential conflict of interest relevant to this article was reported.
We thank the patients and other team members, including Katherine Shin, pharmacist; Anthony T. Podany, pharmacologist; Ian Sanne, Network Leadership representative; Janet Nico-tera, field representative; David L. Shugarts, laboratory technologist; Amina M. Shali, community representative; and Brigitte Demers, Sanofi representative; the members of the Division of AIDS African data and safety monitoring board for their oversight of the trial; and Timothy Sterling and Prudence Ive for performing the independent end-point reviews.
Appendix
The authors’ full names and academic degrees are as follows: Susan Swindells, M.B., B.S., Ritesh Ramchandani, Ph.D., Amita Gupta, M.D., Constance A. Benson, M.D., Jorge Leon-Cruz, M.S., Noluthando Mwelase, M.B., Ch.B., Marc A. Jean Juste, M.D., Javier R. Lama, M.D., M.P.H., Javier Valencia, M.D., Ayotunde Omoz-Oarhe, M.D., Khuanchai Supparatpinyo, M.D., Gaerolwe Masheto, M.D., Lerato Mohapi, M.D., Rodrigo O. da Silva Escada, M.D., Sajeeda Mawlana, M.B., Ch.B., Peter Banda, M.B., B.S., Patrice Severe, M.D., James Hakim, M.B., Ch.B., M.Med., M.Med.Sc., Cecilia Kanyama, M.B., B.S., Deborah Langat, M.B., Ch.B., Laura Moran, M.P.H., Janet Andersen, Sc.D., Courtney V. Fletcher, Pharm.D., Eric Nuermberger, M.D., and Richard E. Chaisson, M.D.
Footnotes
A complete list of the members of the BRIEF TB/A5279 Study Team is provided in the Supplementary Appendix, available at NEJM.org.
The views expressed in this article are those of the authors and do not necessarily represent the official views of the National Institutes of Health.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Global tuberculosis report 2018. Geneva: World Health Organization, 2018. (https://www.who.int/tb/publications/global_report/en/). [Google Scholar]
- 2.Akolo C, Adetifa I, Shepperd S, Volmink J. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database Syst Rev 2010; 1: CD000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The TEMPRANO ANRS 12136 Study Group. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med 2015; 373: 808–22. [DOI] [PubMed] [Google Scholar]
- 4.Rangaka MX, Wilkinson RJ, Boulle A, et al. Isoniazid plus antiretroviral therapy to prevent tuberculosis: a randomised double-blind, placebo-controlled trial. Lancet 2014; 384: 682–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Golub JE, Cohn S, Saraceni V, et al. Long-term protection from isoniazid preventive therapy for tuberculosis in HIV infected patients in a medium-burden tuberculosis setting: the TB/HIV in Rio (THRio) study. Clin Infect Dis 2015; 60: 639–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Golub JE, Saraceni V, Cavalcante SC, et al. The impact of antiretroviral therapy and isoniazid preventive therapy on tuberculosis incidence in HIV-infected patients in Rio de Janeiro, Brazil. AIDS 2007; 21: 1441–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eldred LJ, Churchyard G, Durovni B, et al. Isoniazid preventive therapy for HIV-infected people: evidence to support implementation. AIDS 2010; 24: Suppl 5: S1–S3. [DOI] [PubMed] [Google Scholar]
- 8.Martinson NA, Barnes GL, Moulton LH, et al. New regimens to prevent tuberculosis in adults with HIV infection. N Engl J Med 2011; 365: 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sterling TR, Scott NA, Miro JM, et al. Three months of weekly rifapentine and isoniazid for treatment of Mycobacterium tuberculosis infection in HIV-coinfected persons. AIDS 2016; 30: 1607–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang T, Zhang M, Rosenthal IM, Grosset JH, Nuermberger EL. Short-course therapy with daily rifapentine in a murine model of latent tuberculosis infection. Am J Respir Crit Care Med 2009; 180: 1151–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang T, Li SY, Williams KN, Andries K, Nuermberger EL. Short-course chemo therapy with TMC207 and rifapentine in a murine model of latent tuberculosis infection. Am J Respir Crit Care Med 2011; 184: 732–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Podany AT, Bao Y, Swindells S, et al. Efavirenz pharmacokinetics and pharmacodynamics in HIV-infected persons receiving rifapentine and isoniazid for tuberculosis prevention. Clin Infect Dis 2015; 61: 1322–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samandari T, Agizew TB, Nyirenda S, et al. Tuberculosis incidence after 36 months’ isoniazid prophylaxis in HIV- infected adults in Botswana: a posttrial observational analysis. AIDS 2015; 29: 351–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chesney MA, Ickovics JR, Chambers DB, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. AIDS Care 2000; 12: 255–66. [DOI] [PubMed] [Google Scholar]
- 15.Chaisson RE, Golub JE. Preventing tuberculosis in people with HIV — no more excuses. Lancet Glob Health 2017; 5(11): e1048–e1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawn SD, Wood R. Short-course un- targeted isoniazid preventive therapy in South Africa: time to rethink policy? Int J Tuberc Lung Dis 2012; 16: 995–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Getahun H, Granich R, Sculier D, et al. Implementation of isoniazid preventive therapy for people living with HIV worldwide: barriers and solutions. AIDS 2010; 24: Suppl 5: S57–S65. [DOI] [PubMed] [Google Scholar]
- 18.Badje A, Moh R, Gabillard D, et al. Effect of isoniazid preventive therapy on risk of death in west African, HIV-infected adults with high CD4 cell counts: long- term follow-up of the Temprano ANRS 12136 trial. Lancet Glob Health 2017; 5(11): e1080–e1089. [DOI] [PubMed] [Google Scholar]
- 19.Hakim J, Musiime V, Szubert AJ, et al. Enhanced prophylaxis plus antiretroviral therapy for advanced HIV infection in Africa. N Engl J Med 2017; 377: 233–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Durovni B, Saraceni V, Moulton LH, et al. Effect of improved tuberculosis screening and isoniazid preventive therapy on incidence of tuberculosis and death in patients with HIV in clinics in Rio de Janeiro, Brazil: a stepped wedge, cluster randomised trial. Lancet Infect Dis 2013; 13: 852–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fiske CT, Yan FX, Hirsch-Moverman Y, Sterling TR, Reichler MR. Risk factors for treatment default in close contacts with latent tuberculous infection. Int J Tuberc Lung Dis 2014; 18: 421–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sandul AL, Nwana N, Holcombe JM, et al. High rate of treatment completion in program settings with 12-dose weekly isoniazid and rifapentine for latent Mycobacterium tuberculosis infection. Clin Infect Dis 2017; 65: 1085–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kopanoff DE, Snider DE Jr, Caras GJ. Isoniazid-related hepatitis: a U.S. Public Health Service cooperative surveillance study. Am Rev Respir Dis 1978; 117: 991–1001. [DOI] [PubMed] [Google Scholar]
- 24.Fatal and severe hepatitis associated with rifampin and pyrazinamide for the treatment of latent tuberculosis infection — New York and Georgia, 2000. MMWR Morb Mortal Wkly Rep 2001; 50: 289–91. [PubMed] [Google Scholar]
- 25.Schechter M, Zajdenverg R, Falco G, et al. Weekly rifapentine/isoniazid or daily rifampin/pyrazinamide for latent tuberculosis in household contacts. Am J Respir Crit Care Med 2006; 173: 922–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halsey NA, Coberly JS, Desormeaux J, et al. Randomised trial of isoniazid versus rifampicin and pyrazinamide for prevention of tuberculosis in HIV-1 infection. Lancet 1998; 351: 786–92. [DOI] [PubMed] [Google Scholar]
- 27.Gordin F, Chaisson RE, Matts JP, et al. Rifampin and pyrazinamide vs isoniazid for prevention of tuberculosis in HIV- infected persons: an international randomized trial. JAMA 2000; 283: 1445–50. [DOI] [PubMed] [Google Scholar]
- 28.Sterling TR, Moro RN, Borisov AS, et al. Flu-like and other systemic drug reactions among persons receiving weekly rifapentine plus isoniazid or daily isoniazid for treatment of latent tuberculosis infection in the PREVENT Tuberculosis Study. Clin Infect Dis 2015; 61: 527–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brooks KM, George JM, Pau AK, et al. Cytokine-mediated systemic adverse drug reactions in a drug-drug interaction study of dolutegravir with once-weekly isoniazid and rifapentine. Clin Infect Dis 2018; 67: 193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dooley KE, Churchyard C, Savic R, et al. Safety and PK of weekly rifapentine/isoniazid (3HP) in adults with HIV on dolutegravir. Presented at the Conference on Retroviruses and Opportunistic Infections (CROI) 2019, Seattle, March 4–7, 2019: 4037 abstract. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.