Abstract
The past two decades in research has revealed the importance of leucine-rich repeat kinase 2 (LRRK2) in both monogenic and sporadic forms of Parkinson’s disease (PD). In families, mutations in LRRK2 can cause PD with age-dependent but variable penetrance and genome-wide association studies have found variants of the gene that are risk factors for sporadic PD. Functional studies have suggested that the common mechanism that links all disease-associated variants is that they increase LRRK2 kinase activity, albeit in different ways. Here, we will discuss the roles of LRRK2 in areas of inflammation and vesicular trafficking in the context of monogenic and sporadic PD. We will also provide a hypothetical model that links inflammation and vesicular trafficking together in an effort to outline how these pathways might interact and eventually lead to neuronal cell death. We will also highlight the translational potential of LRRK2-specific kinase inhibitors for the treatment of PD.
Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder affecting over 4 million people over the age of 50 within the world’s top 15 most populated countries, a number that is expected to double by 2030 [1]. Historically, PD had been considered a purely sporadic disorder. It is now understood that many forms of PD have a genetic component as well as an environmental component of varying degrees [2–4]. Although ~90% of PD cases are sporadic (sPD), i.e. having no clearly defined single cause, the remaining 10% show a clear family history and are thus considered monogenic PD [5].
A central question in PD research is whether genetic PD is distinguishable mechanistically from the sporadic disease as this has implications for whether treatments for one group of patients might be useful for the other. Many studies have addressed a possible divergence in the clinical and pathological characteristics of patients harboring PD-linked mutations compared with sporadic cases. Patients carrying mutations in the Leucine-rich repeat kinase 2 (LRRK2) gene, one of the most common genetic contributors of PD [6–8], manifest clinical features which are almost indistinguishable from the sporadic form. Similar patterns of motor symptoms between both forms, including the hallmark bradykinesia, tremor, rigidity, and postural instability have been reported. Nonmotor symptoms which include hallucinations, depression, anxiety, cognitive impairment, and pain also appear in both familial and sporadic PD [9,10]. Interestingly, LRRK2-PD has been associated with a spectrum of neuropathological features, including α-synuclein positive Lewy bodies, accumulation of phosphory-lated tau as well as TDP-43 aggregates [11]. Nonetheless, neuronal loss and gliosis in the substantia nigra is the common pathological feature amongst all of the LRRK2 mutation cases, with the majority of G2019S LRRK2-PD cases displaying Lewy body pathology comparable with that of sporadic PD [11–13]. This suggests that there are convergent pathways that drive neuropathology in genetic and sporadic disease and close examination of the genes and risk factors involved in the two forms can highlight common cellular pathways of dysfunction.
In this review, we outline evidence from genetics and functional data that support a role for LRRK2 in the pathogenesis of both familial and sporadic forms of PD. This review aims to serve three purposes: first, to highlight major findings regarding LRRK2 as a common factor between genetic and sporadic PD via genome-wide association studies (GWAS), as well as new in vitro and in vivo studies that have been published in the last few years supporting this association. Second, to explore potential implications of LRRK2 genetics and function on disease etiology through a comprehensive model of cellular pathways. Finally, to investigate LRRK2 as a druggable target and highlight the current efforts focusing on the development of future therapeutics.
Genetics
LRRK2 mutations cause autosomal dominant PD and can be a risk factor for sPD
Nearly 16 years ago, the PARK8 locus was identified in a large Japanese family exhibiting autosomal dominant parkinsonism [14]. Linkage analysis identified 116 genes within the locus located on chromosome 12, and the specific gene responsible was discovered independently by two other groups [15,16]. The original family was then shown to have a mutation in the same gene, LRRK2 [17]. Mutations in LRRK2 were subsequently shown to be a relatively common genetic cause of PD worldwide [18]. To date, out of the ~100 mutations identified within this gene [19–21], only six of these have been convincingly segregated as disease-causing: G2019S, R1441C/G/H, Y1699C, and I2020T [22,23]. The two most common mutations, G2019S and R1441C, are each responsible for up to ~30% of inherited PD cases in certain populations, and up to 10 and 2.5% of sporadic PD cases, respectively [24–27]. The presence of mutations in what appears to be sporadic cases is likely due to incomplete but age-dependent penetrance. For example, G2019S has an age range of penetrance increasing from 17% at 50 years old to 85% at 70 years old; additionally, there are some carriers who never develop PD [28–30]. Similarly, R1441C has been shown to have reduced penetrance, suggesting that although these monogenic mutations significantly increase disease risk, they do not always lead to disease [31,32].
The LRRK2 gene is made up of 51 exons and encodes a 2527 amino-acid protein with a predicted molecular mass of ~286 kDa. LRRK2 is a multidomain protein where the Ras of complex proteins (Roc), C-terminal of ROC (COR) and kinase domains constitute a catalytic core that is flanked by protein-protein interaction domains: N-terminal armadillo, ankyrin, leucine-rich repeats and a C-terminal WD40 domain. Interestingly, most disease-linked LRRK2 mutations are found within the core enzymatic domains and alter enzyme activity in vitro [33]. The G2019S mutation is located within the kinase domain and increases Vmax for kinase activity while R1441C/G/H and Y1699C mutations decrease the GTPase activity of the Roc domain [34–38]. It is thought that these two events are related to each other in that kinase and GTPase activities being encoded on the same protein may regulate each other. Supporting this idea, measurements of Rab substrates in cells show that all mutations support increased phosphorylation [39,40].
In recent years, GWAS have been used to identify risk loci for many disorders, including PD. These genomic surveys have shown that the LRRK2 locus contains common variation that increases risk of developing sporadic PD [41,42]. The GWAS signal is explained by non-coding variability, which imparts only a moderate risk for disease. Additionally, LRRK2 contains some protein-coding changes that increase risk for disease. Sequencing LRRK2 from the Taiwanese population identified G2385R variant as a common variant among healthy controls, present in ~5% of people. However, the variant was found to be twice as common in patients with PD [18]. The two-fold increase in risk was replicated in many other studies, confirming G2385R as a risk factor in Asian populations [43–49]. Located in the C-terminal WD40 domain, this mutation likely alters the protein structure and modulates LRRK2 binding to interactors associated with vesicular trafficking [50]. Our laboratory has shown that the G2385R variant promotes LRRK2 protein turnover by increasing binding affinity to Hsc70 and CHIP resulting in lower steady-state levels [51,52] and recent data suggest that this variant compromises LRRK2 dimerization [53]. In terms of effects on the kinase activity, purified protein shows decreased autophosphorylation in in vitro kinase assays and can rescue the hyperactivation effect of the G2019S mutation [51]. In a cellular context, however, G2385R autophosphorylation at S1292 is retained [54]. This highlights how the context of the assay, in vitro purified protein versus cellular context, is important when assaying LRRK2 function. As outlined below, LRRK2 is sequestered to the TGN (trans-Golgi network) in co-expression with Rab29 and phosphorylates membrane-bound Rab GTPases, while the R1441C, Y1699C and G2019S mutations enhance this activation [40,55]. In this assay, the G2385R variant is sequestered to the TGN much like the WT protein [56]. In co-expression experiments in cells, however, G2385R shows increased kinase activity towards Rab10 compared with WT protein similar to what is reported for the other genetic variants [53]. These results suggest that certain molecular mechanisms that mediate G2385R disease risk may be shared with familial mutations, however, the effects of this variant result in G2385R being a risk factor rather than a penetrant mutation.
Similarly, the rarer R1628P variant has also been found to increase risk of PD two-fold in Asian populations [57]. This variant has been found to increase LRRK2 kinase activity indirectly and cause cell death in vitro [58]. Intriguingly, R1398H has been identified as protective against PD in several cohorts by lowering activity of the protein [59–61]. Rare coding variants in LRRK2 have been associated with an earlier age of onset of disease [28,62–65].
Collectively, these findings show that both coding and non-coding variants at the LRRK2 locus have a strong link to PD and influence penetrance, age of onset, and cause both vulnerability towards and protection against developing PD. Importantly, all of these cases are ‘sporadic’ PD, suggesting that LRRK2 plays a role in this more common form of the disease. Another way to think about the role of LRRK2 in PD is that because the various alleles have differing penetrance, some will occur in a recognizable pattern in families while others will be found in isolated cases without apparent family history.
GWAS suggest modified expression of LRRK2 is associated with sporadic PD
There has been speculation that non-coding LRRK2 variants affect risk of disease through modulation of LRRK2 protein expression. For example, it has been shown that a common risk variant at the LRRK2 locus is associated with higher LRRK2 expression in microglia-like cells derived from human monocytes [66]. In 94 healthy participants, 94 genes from loci associated with Alzheimer’s, Parkinson’s, and multiple sclerosis diseases were examined in both monocytes and their monocyte-derived microglia-like counterparts. Comparing these data with a GWAS-derived list of disease-associated single nucleotide polymorphisms (SNPs) [67], results suggest the T allele at rs76904798 LRRK2 increased in expression. This finding suggests that LRRK2 gene expression can be altered by specific alleles or haplotypes. It also leads to the hypothesis that LRRK2 may have a specific role within microglia. As microglia are influenced by myriad of conditions (inflammation, neuronal apoptosis, etc.) this suggests that the role of LRRK2 risk variants may be more prominent under situations where microglia are stimulated. We speculate that this concept might be related to penetrance of variants in LRRK2, if there is a requirement for immune stimulation to express disease processes. This will be discussed in more depth in the next section.
Higher LRRK2 activity is found in both genetic and sporadic PD patients and is reflected in in vitro and in vivo models
LRRK2 is a large multidomain protein harboring two enzymatic cores that has a large spectrum of interacting factors linking it to diverse cellular pathways. LRRK2 expression is ubiquitous, with varying degrees of expression in peripheral tissues as well as the brain [15,68–70]. At the cellular level, LRRK2 expression has been reported in astrocytes, microglia, neurons, endothelial cells and peripheral immune cells [71–74]. Thus, it is likely that LRRK2 plays distinct signaling roles in different cell types, specifically involving its kinase activity and autophosphorylation (Figure 1). In this section, we will discuss recent findings that support the relationship of LRRK2 in monogenic and sporadic PD to inflammation and vesicular trafficking.
Figure 1. Physiological pathways of LRRK2 activity.
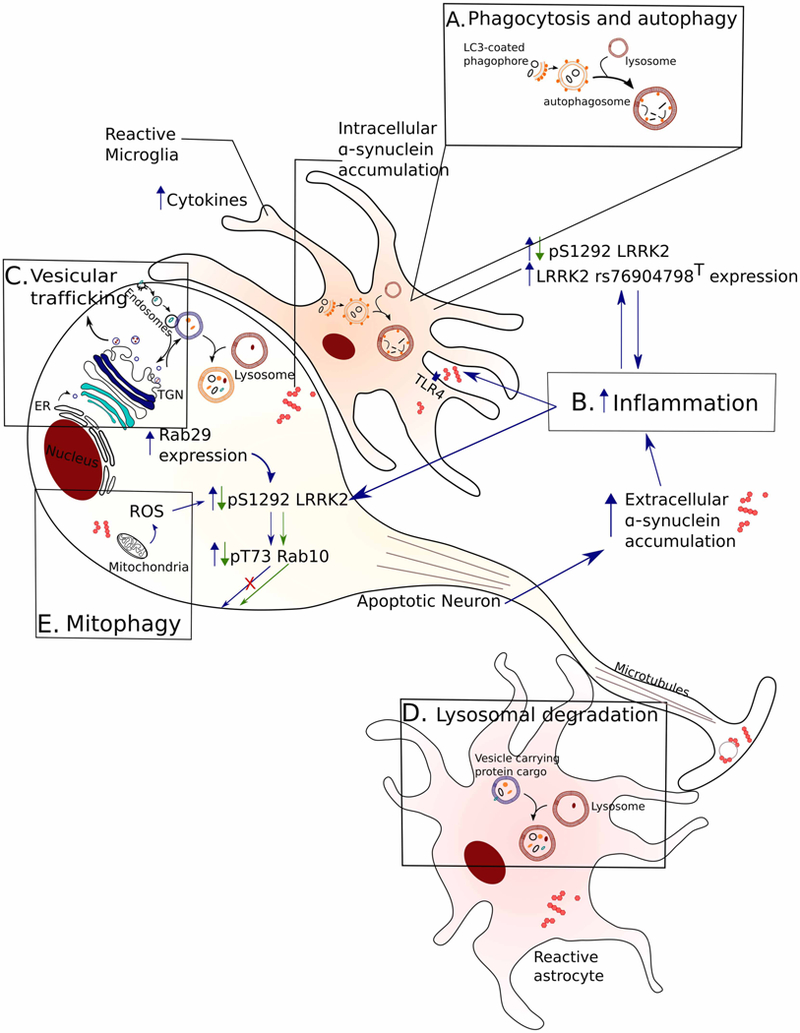
A common function of LRRK2 in genetic and sPD can be modeled on physiological signaling pathways important for neuronal survival. A generic apoptotic neuron (without dendrites for simplicity) and reactive glial cells are depicted and highlighted pathways are shown along with downstream effects of increased LRRK2 activity. Blue arrows indicate a gain-of-function pathway where increased LRRK2 kinase activity may drive dysfunction while green arrows highlight the molecular events of pharmacological LRRK2 inhibition. (A) LRRK2 has been linked to autophagosome maturation and impaired phagocytosis nominating these processes as possible culprits in the clearance of aggregated synuclein. (B) LRRK2 is activated in systems of induced inflammation while higher LRRK2 levels are seen in immune cells from idiopathic PD patients compared with healthy controls. Accumulation of extracellular synuclein can further induce an inflammatory response and this, in turn, can lead to LRRK2 activation. (C) LRRK2 interacts with Rab29 and mediates TGN dynamics while Rab29 expression can drive LRRK2 activation. Active LRRK2 phosphorylates Rab GTPases with downstream effects on vesicular trafficking as well as impaired autophagy/lysosomal pathways (D) that can impair clearance of aggregated proteins. (E) Progressive increase in ROS with ageing can activate LRRK2 that in turn can affect mitochondrial function and impair mitochondrial clearance through mitophagy.
The role of LRRK2 in PD-related inflammation
It is increasingly appreciated that microglia can contribute to disease pathogenesis as they mediate the immune responses in the central nervous system and inflammation is a key factor in neurodegeneration. Many studies have nominated LRRK2 as an integral part of inflammatory response downstream of various proinflammatory signals. Recent studies propose a role of LRRK2 in phagocytosis and highlight how an increase in protein levels or activity may impair an inflammatory response [75,76]. In vitro studies using mouse cultures report increased LRRK2 expression in microglia as well as bone marrow-derived macrophages after introducing proinflamma-tory agents such as lipopolysaccharide. Additionally, LRRK2 knockdown ameliorates this inflammatory signaling, suggesting LRRK2 and inflammation have a complex, modulatory relationship that still needs mechanistic clarification [72,77]. A similar relationship has been found to occur in peripheral immune cells in sPD patients [78]. For example, LRRK2 expression is higher in T cells, B cells, and CD16+ monocytes from sporadic PD patients compared with controls and this is correlated with higher cytokine levels. This supports the idea that the increase in LRRK2 expression is relevant to sporadic PD pathology and may contribute to inflammation associated with PD.
LRRK2 is autophosphorylated at serine 1292 and this is a robust physiological readout of its kinase activity in different systems [79–81]. In a recent study, a novel approach that allows pS1292 visualization within cells was developed using proximity-ligation amplification to examine endogenous LRRK2 autophosphorylation [82]. The authors used in vivo administration of rotenone as a model of reactive oxygen species (ROS)-induction and PD-related pathology, based on previous research that linked ROS and mitochondrial impairment to LRRK2 activation in cells [83–86]. They reported an increase in pS1292 LRRK2 in nigrostriatal neurons of rotenone-treated rats compared with controls, which was ameliorated with LRRK2-specific kinase inhibitors. In the same study, pS1292 LRRK2 autophosphorylation was six-fold higher in surviving nigrostriatal neurons from sporadic PD patients compared with healthy controls. There was also a four-fold increase in phosphorylated Rab10 (pT73), a LRRK2 substrate that is known to play a role in vesicular trafficking. This study supports a concomitant activation of LRRK2 across familial and sporadic PD and links the oxidative stress response to LRRK2-mediated pathways of neurodegeneration.
The kinase activity of LRRK2 was found to be increased in microglia in sporadic PD postmortem tissue, through monitoring its autophosphorylation state and also downstream substrates [82] suggesting that inflammation may be a trigger of LRRK2 activity in PD patients. Microglial inflammation in PD could be triggered by an interaction of extracellular forms of the neuronal protein a-synuclein with TLR4 receptors [87]. Such species of α-synuclein could either be actively secreted from neurons or might accumulate due to neuronal cell death. Such events could then perpetuate neuroinflammation by releasing additional toxic materials such as ROS [88]. Alpha-synuclein accumulation in neurons can also induce mitophagy, a process linked to LRRK2 activity [89,90], resulting in increased ROS production [91]. Taken together, these data highlight how ROS accumulation and inflammation may activate wild type LRRK2 in sporadic PD within nigrostriatal dopamine neurons and microglia, and that amplification of damage by LRRK2 in both glial and neuronal cells is a plausible mechanism by which disease may progress (Figure 1).
The role of LRRK2 mutations in vesicular trafficking
The above considerations suggest that there are multiple cell types that may mediate the involvement of LRRK2 in monogenic and sporadic PD. However, it is important to also discuss the subcellular events that LRRK2 can influence and whether these are relevant to neurodegeneration. LRRK2 has been linked to processes of vesicular trafficking through interactions with multiple proteins associated with cellular membranes [92]. For example, LRRK2 can phosphorylate a subset of Rab GTPases, including Rab8a and Rab10, both of which play roles in vesicular trafficking to the plasma membrane [39,93]. Importantly, all pathogenic variants in LRRK2 that cause disease have been shown to enhance Rab phosphorylation in cells [39,40]. Another Rab upstream of LRRK2 is Rab29 which has been shown to bind LRRK2 at its ankyrin domain and recruits it to the TGN [55]. Interestingly, Rab29 is found in the PARK16 locus and is reported to be a PD risk factor [94]. Overexpression of Rab29 results in an increase in pS1292 LRRK2 and pT73 Rab10 in vitro, which were ameliorated followed by Rab29 knockdown [54]. Similar results were seen in studies that also demonstrated a requirement for Rab29 to be associated with membranes due to prenylation at the C-terminus of the protein [40]. These results are consistent with prior observations that LRRK2 mutants, which are activated by Rab29 to a greater extent that the wild type protein, enhance association of LRRK2 with the TGN [55].
Once activated, LRRK2 can affect multiple aspects of vesicular trafficking, one of which is the autophagy/lysosomal pathway (Figure 1). Autophagy is a maintenance process for degrading damaged organelles and proteins within the cell and is an integral part of the inflammatory response. During the initiation of autophagy, the protein LC3 is lipidated and associates with the membrane of nascent autophagic vesicles. Inhibition of LRRK2 kinase activity has been shown to increase the lipidation of LC3 in astrocytes [95]. This may be indicative of either induction of autophagosome formation or inhibition of autophagosome/autolysosome degradation. Expression of GFP-tagged, PD-causing LRRK2 mutants results in an increase in lysosome size which is dependent on kinase activity and is associated with a reduction in lysosomal pH [96]. In vivo, loss of LRRK2 led to accumulation of autophagic markers LC3-II and p62 [97]. Interestingly, comparing these markers in postmortem tissue from G2019S LRRK2 PD and sPD patients showed decreased LC3-II levels in the basal ganglia of both sporadic and genetic PD forms compared with controls [98]. This suggests LRRK2 plays a crucial role in trafficking within the autophagy/lysosomal pathway and is necessary for normal lysosomal function. In turn, autophagy influences inflammation through the transport of degradable material to the lysosome via phagophores. Thus, although we have not yet identified all the intracellular pathways involved in LRRK2-related PD, one possibility is that inflammatory reactions and intracellular trafficking that affects the autophagy-lysosome system are mechanistically related to each other.
Kinase activity: a push towards new LRRK2-targeting drug therapies
The results discussed above strongly indicate that LRRK2 mutations lead to a gain of function of kinase-dependent activity. Consistent with this idea, rare loss of function LRRK2 alleles can be found within the human population but are not associated with PD [99]. Furthermore, kinase-dead versions of mutant LRRK2 are less toxic than their kinase active counterparts in many cellular and animal models. Therefore, it is reasonable to expect that lowering LRRK2 kinase activity would be therapeutically useful. This hypothesis has led to the development of small molecule LRRK2 kinase inhibitors.
Pharmacological inhibition of LRRK2 has been shown to be neuroprotective in human cell lines and PD-relevant animal models [100,101]. In parallel to the development of these tools, discovery of robust and reliable biomarkers of kinase activity has been critical to be able to monitor the effectiveness of such compounds. A recent study from our laboratory found that pS1292 is a reliable readout of LRRK2 kinase activity in vivo [79] while others have shown the same for downstream Rab proteins [102]. A recent study suggested that peripheral blood neutrophils may be useful to monitor LRRK2 activity in the clinic as they are abundant, homogenous and express relatively high levels of LRRK2 and the substrate Rab10 [103].
Therefore, there are potential therapeutic agents and biomarkers of target engagement that would allow the hypothesis that LRRK2 activity is pathogenic in PD to be tested in a clinical setting. However, it remains unclear as to which patients would benefit from such a therapy. Importantly, if monogenic and sporadic PD share not just some clinical features but are also mechanistically linked by LRRK2 kinase activity, then LRRK2 inhibitors may be beneficial not only for mutation carriers but for the broader sporadic PD population. Recently, one small-molecule LRRK2 inhibitor, DNL201, reached clinical testing in 2017 and showed inhibition of LRRK2 kinase activity in a healthy volunteer phase I study [104]. Another LRRK2 inhibitor, DNL151, is currently being assessed in healthy volunteers in the Netherlands in order to select the most promising molecule to be assessed in patients with PD carrying an LRRK2 mutation.
Although these inhibitors are promising, it is worth mentioning that since normal LRRK2 function is not fully elucidated, the downstream effects of inhibiting this enzymatic activity in humans are currently unknown. Polymorphisms around LRRK2 have been identified as risk variants for Chron’s disease and leprosy [105,106] while LRRK2 phosphorylation and protein levels are modulated in response to different proinflammatory stimuli [73,107]. In turn, LRRK2 expression enhances transcriptional activation of inflammatory responses [73] and PD-linked mutations induce cytokine production in activated microglia [108]. Inhibiting LRRK2 pharmacologically can impair microglial inflammatory responses [77] and LRRK2 deficiency impairs pathogen clearance in vivo [109].
In the context of vesicular trafficking, LRRK2 has a spectrum of interacting factors and pathways that it is involved in, therefore nominating possible side effects of targeting its activity is challenging. In vivo models suggest that LRRK2 deficiency induces lysosomal defects [ 110] and compromises the ability of lysosomes to degrade autophagic cargo by impairing trans-Golgi to lysosome trafficking [111]. Compromised lysosomal function can alter the capacity of lysosomes to degrade phagocytosed material and modulate the inflammatory response and cytokine production. These studies highlight a role of LRRK2 in lysosomal processing and inflammatory signaling and suggest that pharmacological inhibition of its kinase activity may compromise activation of inflammatory responses. A decline in immune function is an established hallmark of aging, and, in the setting of chronic LRRK2 kinase inhibition that is relevant to the clinic, it will be important to monitor the integrity of the patients’ immune system. Studies on the safety implications of targeting LRRK2 kinase activity have reported macroscopic changes in in vivo model organisms. Morphological changes in lung from nonhuman primates, and kidney tissue from rats treated with specific LRRK2 inhibitors have been reported [112–114]. The mechanism by which the lung and kidney are affected is not completely clear but may be mediated by changes in epithelial integrity, as the lung phenotype is associated with infiltrating type-II pneumocytes into the alveolar space and the kidney phenotype is driven in part by accumulation of hemoglobin in the kidney parenchyma. The key question for drug development aimed at LRRK2 kinase activity is whether such events induce clinically significant problems in persons with PD. Reassuringly, effects are reversible after cessation of dosing in animal models, suggesting that there are unlikely to be long-term adverse events, but human safety data will be critical to whether LRRK2 kinase inhibitors can be tolerated clinically in PD patients.
Perspectives
Convergent results from genetic and functional assays support the idea that LRRK2 is a viable drug target in both monogenic and sporadic PD. While there are important additional mechanistic data that are required for a full understanding of LRRK2-associated pathways, current models suggest that intracellular trafficking may be affected by LRRK2 mutations and that this may be related to events in both neurons and non-neuronal cells that mediate neuroinflammation. With these events in mind, it can be speculated that the complex role of LRRK2 within the cell may be one of a master orchestrator, interacting within various cellular pathways in order to maintain homeostasis. A gain-of-function mutation may shift this delicate balancing act, disrupting normal degradative processes and eventually leading to PD pathogenesis.
Roughly 1 million people are living with PD in the US alone which is predicted to increase [115]. As of yet, there continues to be a significant unmet medical need in the field of neurodegeneration for effective, long-lasting treatments that either halt or slow disease progression. Currently, there are very few options in drug treatment, all of which target symptoms of the disease rather than cause, for many reasons — insufficient drug exposure in the brain, failure to provide evidence of target engagement using biomarkers, lack of a causative candidate that is targetable, etc. — however, this is starting to change. With the improvement of clinically applicable technologies, laboratory explorations of LRRK2 substrates and functions have identified potential biomarkers for clinical use in blood and urinary exosomes [81] providing direct assessments of LRRK2-specific drug inhibitors. These assessments can now provide data that can be used to predict potential areas of concern for on-target side effects of chronic LRRK2 inhibition. Therefore, while the hypothesis that LRRK2 kinase activity can be inhibited to benefit people with PD remains untested, the important tools that would be needed to address this idea are now available.
Acknowledgments
Funding
This research was supported by the Intramural Research Program of the NIH, National Institute on Aging.
Abbreviations
- COR
C-terminus of Roc
- GWAS
genome-wide association studies
- LBs
Lewy Bodies
- LRRK2
leucine-rich repeat kinase 2
- PBMCs
peripheral blood mononuclear cells
- PD
Parkinson’s disease
- Roc
Ras of complex
- ROS
reactive oxygen species
- SNCA
Alpha-synuclein
- SNPs
single nucleotide polymorphisms
- sPD
sporadic Parkinson’s disease
- TGN
trans-Golgi network
Footnotes
Competing Interests
The Authors declare that there are no competing interests associated with the manuscript.
References
- 1.Dorsey ER, Constantinescu R, Thompson JP, Biglan KM, Holloway RG, Kieburtz K et al. (2007) Projected number of people with Parkinson disease in the most populous nation, 2005 through 2030. Neurology 68, 384–386 10.1212/01.wnl.0000247740.47667.03 [DOI] [PubMed] [Google Scholar]
- 2.Farrer MJ, Williams LN, Algom AA, Kachergus J, Hulihan MM, Ross OA et al. (2009) Glucosidase-beta variations and Lewy body disorders. Parkinsonism Relat. Disord. 15, 414–416 https://doi.org/10.1016Zj.parkreldis.2008.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singleton A and Hardy J (2011) A generalizable hypothesis for the genetic architecture of disease: pleomorphic risk loci. Hum. Mol. Genet. 20, R158–R162 10.1093/hmg/ddr358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singleton AB, Farrer MJ and Bonifati V (2013) The genetics of Parkinson’s disease: progress and therapeutic implications. Mov. Disord. 28, 14–23 10.1002/mds.25249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corti O, Lesage S and Brice A (2011) What genetics tells us about the causes and mechanisms of Parkinson’s disease. Physiol. Rev. 91, 1161–1218 10.1152/physrev.00022.2010 [DOI] [PubMed] [Google Scholar]
- 6.Brice A (2005) Genetics of Parkinson’s disease: LRRK2 on the rise. Brain 128, 2760–2762 10.1093/brain/awh676 [DOI] [PubMed] [Google Scholar]
- 7.Lesage S, Dürr A, Tazir M, Lohmann E, Leutenegger A-L, Janin S et al. (2006) LRRK2 g2019s as a cause of Parkinson’s disease in North African Arabs. N. Engl. J. Med. 354, 422–423 10.1056/NEJMc055540 [DOI] [PubMed] [Google Scholar]
- 8.Ozelius LJ, Senthil G, Saunders-Pullman R, Ohmann E, Deligtisch A, Tagliati M et al. (2006) LRRK2 g2019s as a cause of Parkinson’s disease in Ashkenazi Jews. N. Engl. J. Med. 354, 424–425 10.1056/NEJMc055509 [DOI] [PubMed] [Google Scholar]
- 9.Haugarvoll K, Rademakers R, Kachergus JM, Nuytemans K, Ross OA, Gibson JM et al. (2008) LRRK2 r1441c parkinsonism is clinically similar to sporadic Parkinson disease. Neurology 70, 1456–1460 10.1212/01.wnl.0000304044.22253.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Healy DG, Wood NW and Schapira AHV (2008) Test for LRRK2 mutations in patients with Parkinson’s disease. Pract. Neurol. 8, 381–385 10.1136/jnnp.2008.162420 [DOI] [PubMed] [Google Scholar]
- 11.Wider C, Dickson DW and Wszolek ZK (2010) Leucine-rich repeat kinase 2 gene-associated disease: redefining genotype-phenotype correlation. Neurodegener. Dis. 7, 175–179 10.1159/000289232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poulopoulos M, Cortes E, Vonsattel J-PG, Fahn S, Waters C, Cote LJ et al. (2012) Clinical and pathological characteristics of LRRK2 G2019S patients with PD. J. Mol. Neurosci. 47, 139–143 10.1007/s12031-011-9696-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalia LV, Lang AE, Hazrati L-N, Fujioka S, Wszolek ZK, Dickson DW et al. (2015) Clinical correlations with Lewy body pathology in LRRK2-related Parkinson disease. JAMA Neurol. 72, 100–105 10.1001/jamaneurol.2014.2704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Funayama M, Hasegawa K, Kowa H, Saito M, Tsuji S and Obata F (2002) A new locus for Parkinson’s disease (PARK8) maps to chromosome 12p11.2-q13.1. Ann. Neurol. 51, 296–301 10.1002/ana.10113 [DOI] [PubMed] [Google Scholar]
- 15.Paisan-Ruiz C, Jain S, Evans EW, Gilks WP, Simon J, van der Brug M et al. (2004) Cloning of the gene containing mutations that cause PARK8-linked Parkinson’s disease. Neuron 44, 595–600 https://doi.org/10.1016Zj.neuron.2004.10.023 [DOI] [PubMed] [Google Scholar]
- 16.Zimprich A, Müller-Myhsok B, Farrer M, Leitner P, Sharma M, Hulihan M et al. (2004) The PARK8 locus in autosomal dominant parkinsonism: confirmation of linkage and further delineation of the disease-containing interval. Am. J. Hum. Genet. 74, 11–19 10.1086/380647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Funayama M, Hasegawa K, Ohta E, Kawashima N, Komiyama M, Kowa H et al. (2005) An LRRK2 mutation as a cause for the parkinsonism in the original PARK8 family. Ann. Neurol. 57, 918–921 10.1002/ana.20484 [DOI] [PubMed] [Google Scholar]
- 18.Di Fonzo A, Tassorelli C, De Mari M, Chien HF, Ferreira J, Rohe CF et al. (2006) Comprehensive analysis of the LRRK2 gene in sixty families with Parkinson’s disease. Eur. J. Hum. Genet. 14, 322–331 10.1038/sj.ejhg.5201539 [DOI] [PubMed] [Google Scholar]
- 19.Cruts M, Theuns J and Van Broeckhoven C (2012) Locus-specific mutation databases for neurodegenerative brain diseases. Hum. Mutat. 33, 1340–1344 10.1002/humu.22117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nuytemans K, Theuns J, Cruts M and Van Broeckhoven C (2010) Genetic etiology of Parkinson disease associated with mutations in the SNCA, PARK2, PINK1, PARK7, and LRRK2 genes: a mutation update. Hum. Mutat. 31, 763–780 10.1002/humu.21277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paisan-Ruiz C, Nath P, Washecka N, Gibbs JR and Singleton AB (2008) Comprehensive analysis of LRRK2 in publicly available Parkinson’s disease cases and neurologically normal controls. Hum. Mutat. 29, 485–490 10.1002/humu.20668 [DOI] [PubMed] [Google Scholar]
- 22.Gasser T (2009) Molecular pathogenesis of Parkinson disease: insights from genetic studies. Expert. Rev. Mol. Med. 11, e22 10.1017/S1462399409001148 [DOI] [PubMed] [Google Scholar]
- 23.Aasly JO, Vilarino-Güell C, Dachsel JC, Webber PJ, West AB, Haugarvoll K et al. (2010) Novel pathogenic LRRK2 p.Asn1437His substitution in familial Parkinson’s disease. Mov. Disord. 25, 2156–2163 10.1002/mds.23265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paisan-Ruiz C, Lewis PA and Singleton AB (2013) LRRK2: cause, risk, and mechanism. J. Parkinsons Dis. 3, 85–103 10.3233/JPD-130192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bardien S, Lesage S, Brice A and Carr J (2011) Genetic characteristics of leucine-rich repeat kinase 2 (LRRK2) associated Parkinson’s disease. Parkinsonism Relat. Disord. 17, 501–508 10.1016/j.parkreldis.2010.11.008 [DOI] [PubMed] [Google Scholar]
- 26.Guedes L C, Ferreira JJ, Rosa MM, Coelho M, Bonifati V and Sampaio C (2010) Worldwide frequency of G2019S LRRK2 mutation in Parkinson’s disease: a systematic review. Parkinsonism Relat. Disord. 16, 237–242 10.1016/j.parkreldis.2009.11.004 [DOI] [PubMed] [Google Scholar]
- 27.Deng H, Le W, Guo Y, Hunter CB, Xie W, Huang M et al. (2006) Genetic analysis of LRRK2 mutations in patients with Parkinson disease. J. Neurol. Sei. 251, 102–106 10.1016/jjns.2006.09.017 [DOI] [PubMed] [Google Scholar]
- 28.Lee AJ, Wang Y, Alcalay RN, Mejia-Santana H, Saunders-Pullman R, Bressman S et al. (2017) Penetrance estimate of LRRK2 p.G2019S mutation in individuals of non-Ashkenazi Jewish ancestry. Mov. Disord. 32, 1432–1438 10.1002/mds.27059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kachergus J, Mata IF, Hulihan M, Taylor JP, Lincoln S, Aasly J et al. (2005) Identification of a novel LRRK2 mutation linked to autosomal dominant parkinsonism: evidence of a common founder across European populations. Am. J. Hum. Genet. 76, 672–680 10.1086/429256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.San Luciano M, Lipton RB, Wang C, Katz M, Zimmerman ME, Sanders AE et al. (2010) Clinical expression of LRRK2 G2019S mutations in the elderly. Mov. Disord. 25, 2571–2576 10.1002/mds.23330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trinh J, Guella I and Farrer MJ (2014) Disease penetrance of late-onset parkinsonism: a meta-analysis. JAMA Neurol. 71, 1535–1539 10.1001/jamaneurol.2014.1909 [DOI] [PubMed] [Google Scholar]
- 32.Reed X, Bandres-Ciga S, Blauwendraat C and Cookson MR (2019) The role of monogenic genes in idiopathic Parkinson’s disease. Neurobiol. Dis. 124, 230–239 10.1016/j.nbd.2018.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rudenko IN and Cookson MR (2014) Heterogeneity of leucine-rich repeat kinase 2 mutations: genetics, mechanisms and therapeutic implications. Neurotherapeutics 11, 738–750 10.1007/s13311-014-0284-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cookson MR (2015) LRRK2 pathways leading to neurodegeneration. Curr. Neurol. Neurosci. Rep. 15, 42 10.1007/s11910-015-0564-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lewis PA, Greggio E, Beilina A, Jain S, Baker A and Cookson MR (2007) The R1441C mutation of LRRK2 disrupts GTP hydrolysis. Biochem. Biophys. Res. Commun. 357, 668–671 10.1016/j.bbrc.2007.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daniels V, Vancraenenbroeck R, Law BMH, Greggio E, Lobbestael E, Gao F et al. (2011) Insight into the mode of action of the LRRK2 Y1699C pathogenic mutant. J. Neurochem. 116, 304–315 10.1111/j.1471-4159.2010.07105.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liao J, Wu C-X, Burlak C, Zhang S, Sahm H, Wang M et al. (2014) Parkinson disease-associated mutation R1441H in LRRK2 prolongs the ‘active state’ of its GTPase domain. Proc. Natl Acad. Sci. U.S.A. 111, 4055–4060 10.1073/pnas.1323285111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo L, Gandhi PN, Wang W, Petersen RB, Wilson-Delfosse AL and Chen SG (2007) The Parkinson’s disease-associated protein, leucine-rich repeat kinase 2 (LRRK2), is an authentic GTPase that stimulates kinase activity. Exp. Cell Res. 313, 3658–3670 10.1016/j.yexcr.2007.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steger M, Tonelli F, Ito G, Davies P, Trost M, Vetter M et al. (2016) Phosphoproteomics reveals that Parkinson’s disease kinase LRRK2 regulates a subset of Rab GTPases. eLife 5, e12813 10.7554/eLife.12813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Z, Bryant N, Kumaran R, Beilina A, Abeliovich A, Cookson MR et al. (2018) LRRK2 phosphorylates membrane-bound Rabs and is activated by GTP-bound Rab7L1 to promote recruitment to the trans-Golgi network. Hum. Mol. Genet. 27, 385–395 10.1093/hmg/ddx410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang D, Nalls MA, Hallgrfmsdottir IB, Hunkapiller J, van der Brug M, Cai F et al. (2017) A meta-analysis of genome-wide association studies identifies 17 new Parkinson’s disease risk loci. Nat. Genet. 49, 1511–1516 10.1038/ng.3955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.International Parkinson Disease Genomics Consortium, Nalls MA, Plagnol V, Hernandez DG, Sharma M, Sheerin U-M et al. (2011) Imputation of sequence variants for identification of genetic risks for Parkinson’s disease: a meta-analysis of genome-wide association studies. Lancet 377, 641–649 10.1016/S0140-6736(10)62345-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li C, Ting Z, Qin X, Ying W, Li B, Guo Qiang L et al. (2007) The prevalence of LRRK2 Gly2385Arg variant in Chinese Han population with Parkinson’s disease. Mov. Disord. 22, 2439–2443 10.1002/mds.21763 [DOI] [PubMed] [Google Scholar]
- 44.Tan E-K (2007) The role of common genetic risk variants in Parkinson disease. Clin. Genet. 72, 387–393 https://doi.org/10.1111Zj.1399-0004.2007.00890.x [DOI] [PubMed] [Google Scholar]
- 45.Funayama M, Li Y, Tomiyama H, Yoshino H, Imamichi Y, Yamamoto M et al. (2007) Leucine-rich repeat kinase 2 G2385R variant is a risk factor for Parkinson disease in Asian population. NeuroReport 18, 273–275 10.1097/WNR.0b013e32801254b6 [DOI] [PubMed] [Google Scholar]
- 46.Chan DKY, Ng PW, Mok V, Yeung J, Fang ZM, Clarke R et al. (2008) LRRK2 gly2385arg mutation and clinical features in a Chinese population with early-onset Parkinson’s disease compared to late-onset patients. J. Neural Transm. 115, 1275–1277 10.1007/s00702-008-0065-0 [DOI] [PubMed] [Google Scholar]
- 47.Choi JM, Woo MS, Ma H-I, Kang SY, Sung Y-H, Yong SW et al. (2008) Analysis of PARK genes in a Korean cohort of early-onset Parkinson disease. Neurogenetics 9, 263–269 10.1007/s10048-008-0138-0 [DOI] [PubMed] [Google Scholar]
- 48.Zabetian CP, Yamamoto M, Lopez AN, Ujike H, Mata IF, Izumi Y et al. (2009) LRRK2 mutations and risk variants in Japanese patients with Parkinson’s disease. Mov. Disord. 24, 1034–1041 10.1002/mds.22514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miyake Y, Tsuboi Y, Koyanagi M, Fujimoto T, Shirasawa S, Kiyohara C et al. (2010) LRRK2 gly2385arg polymorphism, cigarette smoking, and risk of sporadic Parkinson’s disease: a case-control study in Japan. J. Neurol. Sci. 297, 15–18 10.1016/jjns.2010.07.002 [DOI] [PubMed] [Google Scholar]
- 50.Carrion MDP, Marsicano S, Daniele F, Marte A, Pischedda F, Cairano ED et al. (2017) The LRRK2 G2385R variant is a partial loss-of-function mutation that affects synaptic vesicle trafficking through altered protein interactions. Sci. Rep. 7, 5377 10.1038/s41598-017-05760-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rudenko IN, Kaganovich A, Hauser DN, Beylina A, Chia R, Ding J et al. (2012) The G2385R variant of leucine-rich repeat kinase 2 associated with Parkinson’s disease is a partial loss-of-function mutation. Biochem. J. 446, 99–111 10.1042/BJ20120637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rudenko IN, Kaganovich A, Langston RG, Beilina A, Ndukwe K, Kumaran R et al. (2017) The G2385R risk factor for Parkinson’s disease enhances CHIP-dependent intracellular degradation of LRRK2. Biochem J. 474, 1547–1558 10.1042/BCJ20160909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang P, Fan Y, Ru H, Wang L, Magupalli VG, Taylor SS et al. (2019) Crystal structure of the WD40 domain dimer of LRRK2. Proc. Natl Acad. Sci. U.S.A. 116, 1579–1584 10.1073/pnas.1817889116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Purlyte E, Dhekne HS, Sarhan AR, Gomez R, Lis P, Wightman M et al. (2018) Rab29 activation of the Parkinson’s disease-associated LRRK2 kinase. EMBO J. 37, 1–18 10.15252/embj.201798099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beilina A, Rudenko IN, Kaganovich A, Civiero L, Chau H, Kalia SK et al. (2014) Unbiased screen for interactors of leucine-rich repeat kinase 2 supports a common pathway for sporadic and familial Parkinson disease. Proc. Natl Acad. Sci. U.S.A. 111, 2626–2631 10.1073/pnas.1318306111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Langston RG, Rudenko IN, Kumaran R, Hauser DN, Kaganovich A, Ponce LB et al. (2018) Differences in stability, activity and mutation effects between human and mouse leucine-rich repeat kinase 2. Neurochem. Res. 10.1007/s11064-018-2650-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ross OA, Wu Y-R, Lee M-C, Funayama M, Chen M-L, Soto AI et al. (2008) Analysis of Lrrk2 R1628P as a risk factor for Parkinson’s disease. Ann. Neurol. 64, 88–92 10.1002/ana.21405 [DOI] [PubMed] [Google Scholar]
- 58.Shu Y, Ming J, Zhang P, Wang Q, Jiao F and Tian B (2016) Parkinson-related LRRK2 mutation R1628P enables Cdk5 phosphorylation of LRRK2 and upregulates its kinase activity. PLoS ONE 11, e0149739 10.1371/journal.pone.0149739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ross OA, Soto-Ortolaza AI, Heckman MG, Aasly JO, Abahuni N, Annesi G et al. (2011) Association of LRRK2 exonic variants with susceptibility to Parkinson’s disease: a case-control study. Lancet Neurol. 10, 898–908 10.1016/S1474-4422(11)70175-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tan E-K, Peng R, Teo Y-Y, Tan LC, Angeles D, Ho P et al. (2010) Multiple LRRK2 variants modulate risk of Parkinson disease: a Chinese multicenter study. Hum. Mutat. 31, 561–568 10.1002/humu.21225 [DOI] [PubMed] [Google Scholar]
- 61.Nixon-Abell J, Berwick DC, Granno S, Spain VA, Blackstone C and Harvey K (2016) Protective LRRK2 R1398H variant enhances GTPase and Wnt signaling activity. Front. Mol. Neurosci. 9, 18 10.3389/fnmol.2016.00018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Malek N, Weil R, Bresner C, Lawton M, Grosset K, Tan M et al. (2018) Features of GBA-associated Parkinson’s disease at presentation in the United Kingdom tracking Parkinson’s study. J. Neurol. Neurosurg. Psychiatry 89, 702–709 10.1136/jnnp-2017-317348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marder K, Wang Y, Alcalay RN, Mejia-Santana H, Tang M-X, Lee A et al. (2015) Age-specific penetrance of LRRK2 G2019S in the Michael J. Fox Ashkenazi Jewish LRRK2 Consortium. Neurology 85, 89–95 10.1212/WNL.0000000000001708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xiao B, Deng X, Ng EY-L, Allen JC, Lim S-Y, Ahmad-Annuar A et al. (2018) Association of LRRK2 haplotype with age at onset in Parkinson disease. JAMA Neurol. 75, 127–128 10.1001/jamaneurol.2017.3363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Blauwendraat C, Heilbron K, Vallerga CL, Bandres-Ciga S, von Coelln R, Pihlstrom L et al. (2018) Parkinson disease age of onset GWAS: defining heritability, genetic loci and a-synuclein mechanisms. bioRxiv https://doi.org/10.1101/424010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ryan KJ, White CC, Patel K, Xu J, Olah M, Replogle JM et al. (2017) A human microglia-like cellular model for assessing the effects of neurodegenerative disease gene variants. Sci. Transl. Med. 9, eaai7635 10.1126/scitranslmed.aai7635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nalls MA, Pankratz N, Lill CM, Do CB, Hernandez DG, Saad M et al. (2014) Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson’s disease. Nat. Genet. 46, 989–993 10.1038/ng.3043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S et al. (2004) Mutations In LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron 44, 601–607 10.1016/j.neuron.2004.11.005 [DOI] [PubMed] [Google Scholar]
- 69.Miklossy J, Arai T, Guo J-P, Klegeris A, Yu S, McGeer EG et al. (2006) LRRK2 expression in normal and pathologic human brain and in human cell lines. J. Neuropathol. Exp. Neurol. 65, 953–963 10.1097/01.jnen.0000235121.98052.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Westerlund M, Ran C, Borgkvist A, Sterky FH, Lindqvist E, Lundströmer K et al. (2008) Lrrk2 and a-synuclein are co-regulated in rodent striatum. Mol. Cell. Neurosci. 39, 586–591 https://doi.org/10.1016Zj.mcn.2008.08.001 [DOI] [PubMed] [Google Scholar]
- 71.Cook DA and Tansey MG (2017) LRRK2 In Neuroimmune Pharmacology (Ikezu T, Gendelman HE, eds), pp. 107–116, Springer International Publishing, Cham [Google Scholar]
- 72.Hakimi M, Selvanantham T, Swinton E, Padmore RF, Tong Y, Kabbach G et al. (2011) Parkinson’s disease-linked LRRK2 is expressed in circulating and tissue immune cells and upregulated following recognition of microbial structures. J. Neural. Transm. 118, 795–808 10.1007/s00702-011-0653-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gardet A, Benita Y, Li C, Sands BE, Ballester I, Stevens C et al. (2010) LRRK2 is involved in the IFN-y response and host response to pathogens. J. Immunol. 185, 5577–5585 10.4049/jimmunol.1000548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hongge L, Kexin G, Xiaojie M, Nian X and Jinsha H (2015) The role of LRRK2 in the regulation of monocyte adhesion to endothelial cells. J. Mol. Neurosci. 55, 233–239 10.1007/s12031-014-0312-9 [DOI] [PubMed] [Google Scholar]
- 75.Härtlova A, Herbst S, Peltier J, Rodgers A, Bilkei-Gorzo O, Fearns A et al. (2018) LRRK2 is a negative regulator of Mycobacterium tuberculosis phagosome maturation in macrophages. EMBO J. 37, e98694 10.15252/embj.201798694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim KS, Marcogliese PC, Yang J, Callaghan SM, Resende V, Abdel-Messih E et al. (2018) Regulation of myeloid cell phagocytosis by LRRK2 via WAVE2 complex stabilization is altered in Parkinson’s disease. Proc. Natl Acad. Sci. U.S.A. 115, E5164–E5173 10.1073/pnas.1718946115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Moehle MS, Webber PJ, Tse T, Sukar N, Standaert DG, DeSilva TM et al. (2012) LRRK2 inhibition attenuates microglial inflammatory responses. J. Neurosci. 32, 1602–1611 10.1523/JNEUR0SCI.5601-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cook DA, Kannarkat GT, Cintron AF, Butkovich LM, Fraser KB, Chang J et al. (2017) LRRK2 levels in immune cells are increased in Parkinson’s disease. NPJ Parkinsons Dis. 3, 11 10.1038/s41531-017-0010-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kluss JH, Conti MM, Kaganovich A, Beilina A, Melrose HL, Cookson MR et al. (2018) Detection of endogenous S1292 LRRK2 autophosphorylation in mouse tissue as a readout for kinase activity. NPJ Parkinsons Dis. 4, 13 10.1038/s41531-018-0049-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sheng Z, Zhang S, Bustos D, Kleinheinz T, Pichon CEL, Dominguez SL et al. (2012) Ser1292 autophosphorylation is an indicator of LRRK2 kinase activity and contributes to the cellular effects of PD mutations. Sci. Transl. Med. 4, 164ra161 10.1126/scitranslmed.3004485 [DOI] [PubMed] [Google Scholar]
- 81.Fraser KB, Rawlins AB, Clark RG, Alcalay RN, Standaert DG, Liu N et al. (2016) Ser(P)-1292 LRRK2 in urinary exosomes is elevated in idiopathic Parkinson’s disease. Mov. Disord. 31, 1543–1550 10.1002/mds.26686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maio RD, Hoffman EK, Rocha EM, Keeney MT, Sanders LH, Miranda BRD et al. (2018) LRRK2 activation in idiopathic Parkinson’s disease. Sci. Transi Med. 10, eaar5429 10.1126/scitranslmed.aar5429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mamais A, Chia R, Beilina A, Hauser DN, Hall C, Lewis PA et al. (2014) Arsenite stress down-regulates phosphorylation and 14–3-3 binding of leucine-rich repeat kinase 2 (LRRK2), promoting self-association and cellular redistribution. J. Biol. Chem. 289, 21386–21400 10.1074/jbc.M113.528463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mortiboys H, Johansen KK, Aasly JO and Bandmann 0 (2010) Mitochondrial impairment in patients with Parkinson disease with the G2019S mutation in LRRK2. Neurology 75, 2017–2020 10.1212/WNL.0b013e3181ff9685 [DOI] [PubMed] [Google Scholar]
- 85.Sanders LH, Laganière J, Cooper 0, Mak SK, Vu BJ, Huang YA et al. (2014) LRRK2 mutations cause mitochondrial DNA damage in iPSC-derived neural cells from Parkinson’s disease patients: reversal by gene correction. Neurobiol. Dis. 62, 381–386 10.1016/jnbd.2013.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Papkovskaia TD, Chau K-Y, Inesta-Vaquera F, Papkovsky DB, Healy DG, Nishio K et al. (2012) G2019s leucine-rich repeat kinase 2 causes uncoupling protein-mediated mitochondrial depolarization. Hum. Mol. Genet. 21, 4201–4213 10.1093/hmg/dds244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hoenen C, Gustin A, Birck C, Kirchmeyer M, Beaume N, Felten P et al. (2016) a-Synuclein proteins promote pro-inflammatory cascades in microglia: stronger effects of the A53T mutant. PLoS ONE 11, e0162717 10.1371/journal.pone.0162717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lull ME and Block ML (2010) Microglial activation and chronic neurodegeneration. Neurotherapeutics 7, 354–365 10.1016/jnurt.2010.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Saez-Atienzar S, Bonet-Ponce L, Blesa JR, Romero FJ, Murphy MP, Jordan J et al. (2014) The LRRK2 inhibitor GSK2578215A induces protective autophagy in SH-SY5Y cells: involvement of Drp-1-mediated mitochondrial fission and mitochondrial-derived ROS signaling. Cell Death Dis. 5, e1368 10.1038/cddis.2014.320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Su Y-C, Guo X and Qi X (2015) Threonine 56 phosphorylation of Bcl-2 is required for LRRK2 G2019S-induced mitochondrial depolarization and autophagy. Biochim. Biophys. Acta, Mol. Basis Dis. 1852, 12–21 10.1016/jbbadis.2014.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Choubey V, Safiulina D, Vaarmann A, Cagalinec M, Wareski P, Kuum M et al. (2011) Mutant A53T a-synuclein induces neuronal death by increasing mitochondrial autophagy. J. Biol. Chem. 286, 10814–10824 10.1074/jbc.M110.132514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cookson MR (2016) Cellular functions of LRRK2 implicate vesicular trafficking pathways in Parkinson’s disease. Biochem. Soc. Trans. 44, 1603–1610 10.1042/BST20160228 [DOI] [PubMed] [Google Scholar]
- 93.Ito G, Katsemonova K, Tonelli F, Lis P, Baptista MAS, Shpiro N et al. (2016) Phos-tag analysis of Rab10 phosphorylation by LRRK2: a powerful assay for assessing kinase function and inhibitors. Biochem. J. 473, 2671–2685 10.1042/BCJ20160557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Satake W, Nakabayashi Y, Mizuta I, Hirota Y, Ito C, Kubo M et al. (2009) Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson’s disease. Nat. Genet. 41, 1303–1307 10.1038/ng.485 [DOI] [PubMed] [Google Scholar]
- 95.Manzoni C, Mamais A, Dihanich S, Abeti R, Soutar MPM, Plun-Favreau H et al. (2013) Inhibition of LRRK2 kinase activity stimulates macroautophagy. Biochim. Biophys. Acta, Mol. Cell Res. 1833, 2900–2910 10.1016/j.bbamcr.2013.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Henry AG, Aghamohammadzadeh S, Samaroo H, Chen Y, Mou K, Needle E et al. (2015) Pathogenic LRRK2 mutations, through increased kinase activity, produce enlarged lysosomes with reduced degradative capacity and increase ATP13A2 expression. Hum. Mol. Genet. 24, 6013–6028 10.1093/hmg/ddv314 [DOI] [PubMed] [Google Scholar]
- 97.Tong Y, Yamaguchi H, Giaime E, Boyle S, Kopan R, Kelleher RJ et al. (2010) Loss of leucine-rich repeat kinase 2 causes impairment of protein degradation pathways, accumulation of a-synuclein, and apoptotic cell death in aged mice. Proc. Natl Acad. Sci. U.S.A. 107, 9879–9884 10.1073/pnas.1004676107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mamais A, Raja M, Manzoni C, Dihanich S, Lees A, Moore D et al. (2013) Divergent a-synuclein solubility and aggregation properties in G2019S LRRK2 Parkinson’s disease brains with Lewy Body pathology compared to idiopathic cases. Neurobiol. Dis. 58, 183–190 10.1016/j.nbd.2013.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Blauwendraat C, Reed X, Kia DA, Gan-Or Z, Lesage S, Pihlstr0m L et al. (2018) Frequency of loss of function variants in LRRK2 in Parkinson disease. JAMA Neurol. 75, 1416–1422 10.1001/jamaneurol.2018.1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Daher JPL, Abdelmotilib HA, Hu X, Volpicelli-Daley LA, Moehle MS, Faser KB et al. (2015) LRRK2 pharmacological inhibition abates α-synuclein induced neurodegeneration. J. Biol. Chem. 290, 19433–19444 10.1074/jbc.M115.660001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee BD, Shin J-H, VanKampen J, Petrucelli L, West AB, Ko HS et al. (2010) Inhibitors of leucine-rich repeat kinase-2 protect against models of Parkinson’s disease. Nat. Med. 16, 998–1000 10.1038/nm.2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Steger M, Diez F, Dhekne HS, Lis P, Nirujogi RS, Karayel O et al. (2017) Systematic proteomic analysis of LRRK2-mediated Rab GTPase phosphorylation establishes a connection to ciliogenesis. eLife 6, e31012 10.7554/eLife.31012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fan Y, Howden AJM, Sarhan AR, Lis P, Ito G, Martinez TN et al. (2018) Interrogating Parkinson’s disease LRRK2 kinase pathway activity by assessing Rab10 phosphorylation in human neutrophils. Biochem. J. 475, 23–44 10.1042/BCJ20170803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Denali Therapeutics Announces First Patient Dosed in Phase 1B Study. [Internet]. Denali. [cited 2019 Jan 8]. Available from: https://denalitherapeutics.com/investors/press-release/denali-therapeutics-announces-first-patient-dosed-in-phase-1b-study-of-dnl201-for-parkinsons-disease-1
- 105.Barrett JC, Hansoul S, Nicolae DL, Cho JH, Duerr RH, Rioux JD et al. (2008) Genome-wide association defines more than 30 distinct susceptibility loci for Crohn’s disease. Nat. Genet. 40, 955–962 10.1038/ng.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang F-R, Huang W, Chen S-M, Sun L-D, Liu H, Li Y et al. (2009) Genomewide association study of leprosy. N. Engl. J. Med. 361, 2609–2618 10.1056/NEJMoa0903753 [DOI] [PubMed] [Google Scholar]
- 107.Dzamko N, Inesta-Vaquera F, Zhang J, Xie C, Cai H, Arthur S et al. (2012) The IkappaB kinase family phosphorylates the Parkinson’s disease kinase LRRK2 at Ser935 and Ser910 during Toll-like receptor signaling. PLoS ONE 7, e39132 10.1371/journal.pone.0039132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gillardon F, Schmid R and Draheim H (2012) Parkinson’s disease-linked leucine-rich repeat kinase 2(R1441G) mutation increases proinflammatory cytokine release from activated primary microglial cells and resultant neurotoxicity. Neuroscience 208, 41–48 https://doi.org/10.1016Zj.neuroscience.2012.02.001 [DOI] [PubMed] [Google Scholar]
- 109.Liu W, Liu X, Li Y, Zhao J, Liu Z, Hu Z et al. (2017) LRRK2 promotes the activation of NLRC4 inflammasome during Salmonella Typhimurium infection. J. Exp. Med. 214, 3051–3066 10.1084/jem.20170014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pellegrini L, Hauser DN, Li Y, Mamais A, Beilina A, Kumaran R et al. (2018) Proteomic analysis reveals co-ordinated alterations in protein synthesis and degradation pathways in LRRK2 knockout mice. Hum. Mol. Genet. 27, 3257–3271 10.1093/hmg/ddy232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lanning NJ, VanOpstall C, Goodall ML, MacKeigan JP and Looyenga BD (2018) LRRK2 deficiency impairs irans-golgi to lysosome trafficking and endocytic cargo degradation in human renal proximal tubule epithelial cells. Am. J. Physiol. Renal. Physiol. 315, F1465–F1477 10.1152/ajprenal.00009.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fuji RN, Flagella M, Baca M, Baptista MAS, Brodbeck J, Chan BK et al. (2015) Effect of selective LRRK2 kinase inhibition on nonhuman primate lung. Sci. Transl. Med. 7, 273ra15 10.1126/scitranslmed.aaa3634 [DOI] [PubMed] [Google Scholar]
- 113.Andersen MA, Wegener KM, Larsen S, Badolo L, Smith GP, Jeggo R et al. (2018) PFE-360-induced LRRK2 inhibition induces reversible, non-adverse renal changes in rats. Toxicology 395, 15–22 https://doi.org/10.10167i.tox.2018.01.003 [DOI] [PubMed] [Google Scholar]
- 114.Baptista M, Merchant K, Barrett T, Bryce D, Ellis M, Estrada A et al. (2018) LRRK2 kinase inhibitors induce a reversible effect in the lungs of non-human primates with no measurable pulmonary deficits. bioRxiv 10.1101/390815 [DOI] [Google Scholar]
- 115.Marras C, Beck JC, Bower JH, Roberts E, Ritz B, Ross GW et al. (2018) Prevalence of Parkinson’s disease across North America. NPJ Parkinsons Dis. 4, 21 10.1038/s41531-018-0058-0 [DOI] [PMC free article] [PubMed] [Google Scholar]


