Abstract
Aims/hypothesis
The metabolic syndrome is a cluster of risk correlates that can progress to type 2 diabetes. The present study aims to evaluate a novel molecule with a dual action against the metabolic syndrome and type 2 diabetes.
Methods
We developed and tested a novel dual modulator, RB394, which acts as a soluble epoxide hydrolase (sEH) inhibitor and a peroxisome proliferator-activated receptor-γ (PPAR-γ) agonist in rat models of the metabolic syndrome—the obese spontaneously hypertensive (SHROB) rat and the obese diabetic Zucker fatty/spontaneously hypertensive heart failure F1 hybrid (ZSF1) rat. In SHROB rats we studied the ability of RB394 to prevent metabolic syndrome phenotypes, while in ZSF1 obese diabetic rats we compared RB394 with the ACE inhibitor enalapril in the treatment of type 2 diabetes and associated comorbid conditions. RB394 (10 mg/kg daily) and enalapril (10 mg/kg daily) were administered orally for 8 weeks.
Results
RB394 blunted the development of hypertension, insulin resistance, hyperlipidaemia and kidney injury in SHROB rats and reduced fasting blood glucose and HbA1c, improved glucose tolerance, reduced blood pressure and improved lipid profiles in obese ZSF1 rats. A reduction in liver fibrosis and hepatosteatosis was evident in RB394-treated obese ZSF1 rats. Unlike RB394, enalapril did not demonstrate any positive effects in relation to diabetes, hyperlipidaemia or liver dysfunction in obese ZSF1 rats. RB394 ameliorated diabetic nephropathy by reducing renal interstitial fibrosis and renal tubular and glomerular injury in obese diabetic ZSF1 rats. Intriguingly, enalapril demonstrated a weaker action against diabetic nephropathy in obese ZSF1 rats.
Conclusions/interpretation
These findings demonstrate that a novel sHE inhibitor/PPAR-γ agonist molecule targets multiple risk factors of the metabolic syndrome and is a glucose-lowering agent with a strong ability to treat diabetic complications.
Keywords: Diabetic nephropathy, Drug development, Liver steatosis, Metabolic syndrome, Soluble epoxide hydrolase
Introduction
Metabolic complications due to obesity, hypertension, hyperlipidaemia and insulin resistance are considered a major health concern and are collectively known as the metabolic syndrome [1]. The metabolic syndrome has been recognised as a major risk factor for diseases in recent years as its prevalence has steadily increased. Indeed, the metabolic syndrome per se has a great impact on cardiovascular morbidity and mortality. Epidemiological studies have revealed an increased risk of developing type 2 diabetes as well as diabetic and cardiovascular complications in individuals with the metabolic syndrome [1, 2].
Developing adequate therapeutic and preventive measures for this multifactorial syndrome has been challenging. The complex pathophysiology of the metabolic syndrome and type 2 diabetes mean that treatment needs to involve multiple therapies aimed at regulation of lipid and glucose homeostasis as well as blood pressure. However, a multitherapy approach to treatment can result in decreased efficacy and enhanced toxicity of each drug and result in drug interactions and compromised patient compliance [3]. Drugs that can simultaneously target various metabolic syndrome components could avoid these limitations.
We developed a novel multi-target molecule that modulates soluble epoxide hydrolase (sEH) and peroxisome proliferatoractivated receptor-γ (PPAR-γ). Small-molecule sEH inhibitors are antihypertensive, organ protective and have beneficial actions on glucose metabolism [4–7]. Our novel molecule combined sEH inhibitor activity with PPAR-γ agonism, the latter being widely known to regulate glucose and lipid metabolism. The efficacy of this novel dual modulator in treating the metabolic syndrome-related pathologies was determined using two different pre-clinical rat models, obese spontaneously hypertensive (SHROB) rats and obese diabetic Zucker diabetic fatty/spontaneously hypertensive heart failure F1 hybrid (ZSF1) rats [8–11]. We hypothesised that the novel small dual modulator molecule RB394 would treat the metabolic syndrome, type 2 diabetes and associated complications.
Methods
Chemicals
The chemistry and synthesis processes for the dual PPAR-γ agonist/sEH inhibitor RB394 (see electronic supplementary material [ESM] Fig. 1) are as described previously [12]. Enalapril was purchased from Lab Express International (Fairfield, NJ, USA). All other chemicals used were purchased from Sigma Aldrich (St Louis, MO, USA) unless otherwise mentioned.
Animals and experimental design
The Medical College of Wisconsin Institutional Animal Care and Use Committee, which abides by the National Institutes of Health Guidelines, approved animal studies. Rat strains were purchased from Charles River Laboratories (Wilmington, MA, USA) and were housed at the Medical College of Wisconsin with a 12 h light–dark cycle and free access to water and rat chow. Mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA) and were housed at the Medical College of Wisconsin under conditions similar to rats.
Rat experiments were carried out using two different protocols (Protocols 1 and 2). In Protocol 1, SHROB (SHROB/ KolGmiCrl-Leprcp/Crl) rats were used as a pre-clinical model of the metabolic syndrome and Wistar–Kyoto (WKY) rats were used as control. Rats were treated with vehicle or RB394 (10 mg/kg daily) orally for 8 weeks (56 days) (ESM Fig. 2). Throughout the 8 week treatment protocol, glucose tolerance tests and tail-cuff blood pressure measurements were performed. Urine samples were collected and the rats were euthanised for blood and kidney tissue collection at the end of the 8 week treatment. See ESM Methods for details of Protocol 1. In Protocol 2, 16-week-old male obese ZSF1 (ZSF1 LeprfaLeprcp/Crl, Obese Strain code 378) rats were used as a model of type 2 diabetes and diabetic nephropathy. At this age ZSF1 obese rats develop hyperglycaemia, hypertension and proteinuria (ESM Fig. 3). These rats, along with control ZSF1 lean (ZSF1-LeprfaLeprcp/Crl, Lean Strain Code 379) rats, were treated with vehicle, RB394 (10 mg/kg daily) or enalapril (10 mg/kg daily) in drinking water for 8 weeks. During the (56 days) 8 week treatment period, glucose tolerance tests, blood pressure measurements and urine collections were performed (ESM Fig. 2). After the final urine collection, rats were euthanised for plasma and tissue collection. See ESM Methods for Protocol 2 details.
Mouse experiments were carried out in Protocol 3 wherein we used a renal fibrosis unilateral ureteric obstruction (UUO) model in C57Bl/6J mice. Mice with UUO or sham surgery were treated with vehicle or RB394 (10 mg/kg daily) for 10 days followed by kidney tissue collection. See ESM Methods for further details of Protocols 1, 2 and 3.
Glucose tolerance test
An intraperitoneal glucose tolerance test was carried out in rats at different time points, depending on the experimental protocol. See ESM Methods for details.
Biochemical analysis
Biochemical assays were carried out to assess glycaemic status, lipid profile, liver function, and renal injury in rats. See ESM Methods for details.
Real-time PCR analysis
Renal mRNA expression of fibronectin (Fn1) was carried out in mouse renal tissues using realtime PCR. The primer sequence of Fn1 is provided in ESM Table 1. See ESM Methods for details.
Histopathological analysis
Formalin-fixed and paraffinembedded kidney (rat and mouse) and liver (rat) were cut into 4 μm sections for histological analysis. Frozen liver sections (10 μm) were stained with Oil Red O dye to assess liver steatosis in ZSF1 rats. See ESM Methods for details.
Immunohistopathological analysis
Immune cell infiltration in the kidney for the different experimental groups rat was determined using immunohistopathological analysis. Kidney sections were immunostained with anti-CD68 (1:100; Serotec, Raleigh, NC, USA) to determine macrophage/ monocyte infiltration. Biotinylated rat anti-mouse secondary antibody (1:200) was used for development with avidin-biotinylated horseradish peroxidase complex (Vectastain ABC Elite kit; Vector Laboratories, Burlingame, CA, USA) followed by counterstaining with haematoxylin. See ESM Methods for details.
Immunofluorescence analysis
Formalin-fixed and paraffinembedded ZSF1 rat kidney sections were immunostained with anti-nephrin (1:100; Santa Cruz Biotechnology, Dallas, TX, USA) to determine nephrin expression. Donkey anti-rabbit IgG H&L (Alexa Fluor 488) secondary antibody (1:200; Abcam, Cambridge, MA, USA) was used for development with fluorescence quenching liquid (Vector Laboratories). See ESM Methods for details.
Immunoblotting
Western immunoblotting was done to determine the renal expression of TGF-β1 and fibronectin. Primary antibodies against TGF-β1 (1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA), fibronectin (1:2000; Cell Signaling Technology, Danvers, MA, USA) and β-tubulin (1:1000; Cell Signaling Technology) were used. Antibodies were checked against a species-specific positive control tissue and speciesspecific recombinant protein and tested by quenching with a competing peptide. See ESM Methods for details.
Statistical analysis
Data are reported as mean ± SEM. Statistical significance between groups was determined by a two-tailed unpaired Student’s t test (and among more than two groups determined by repeated measure one-way ANOVA followed by Tukey’s post hoc test) using GraphPad Prism Version 4.0 software (GraphPad Software, La Jolla, CA, USA). Difference between two groups was considered significant when p ≤ 0.05.
Results
Metabolic abnormalities are ameliorated in RB394-treated SHROB rats
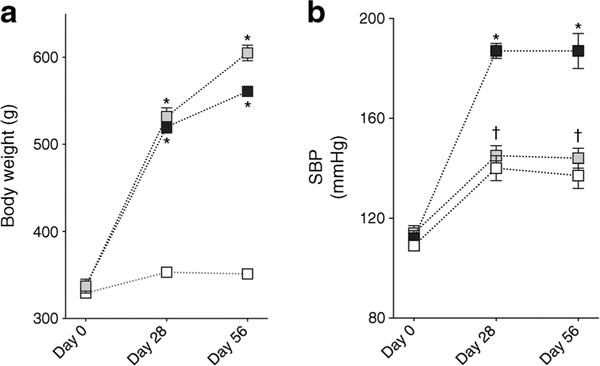
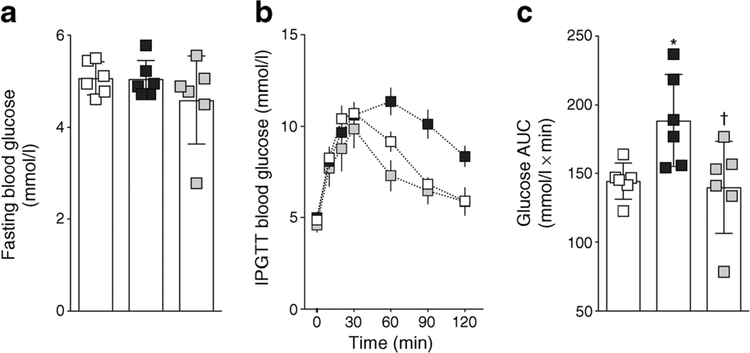
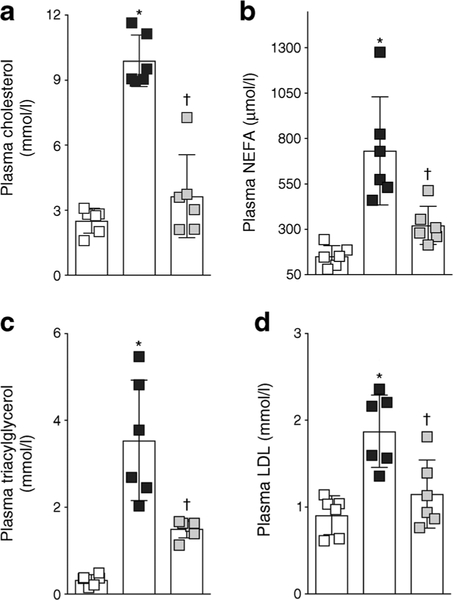
SHROB rats had a higher body weight and were hypertensive when compared with age-matched WKY rats (Fig. 1). RB394 treatment did not alter body weight but blunted the blood pressure elevation in SHROB rats. SHROB rats were not diabetic but demonstrated insulin resistance when compared with WKY rats (Fig. 2). RB394 treatment of SHROB rats prevented development of insulin resistance, with better glucose disposal. SHROB rats were hyperlipidaemic (Fig. 3), demonstrating raised plasma cholesterol, NEFA, triacylglycerol and LDL-cholesterol levels. RB394 treatment markedly attenuated these variables in SHROB rats.
Fig. 1.
Body weight (a) and SBP (b) of rats at baseline and at weeks 4 (day 28) and 8 (day 56) of the experimental protocol. Data are expressed as mean ± SEM, n = 6/group. *p < 0.05 vs vehicle-treated WKY rats;†p< 0.05 vs vehicle-treated SHROB rats. White squares, vehicle-treated WKY rats; black squares, vehicle-treated SHROB rats; grey squares, RB394-treated SHROB rats
Fig. 2.
Fasting blood glucose (a), IPGTT blood glucose (b) and glucose AUC calculated from IPGTT (c) in rats at the end of the 8 week (56 day) experimental protocol. Data are expressed as mean ± SEM, n = 6/group.*p < 0.05 vs vehicle-treated WKY rats;†p < 0.05 vs vehicle-treated SHROB rats. White squares, vehicle-treated WKY rats; black squares, vehicle-treated SHROB rats; grey squares, RB394-treated SHROB rats
Fig. 3.
Plasma levels of cholesterol (a), NEFA (b), triacylglycerol (c) and LDL-cholesterol (d) in rats at the end of the 8 week (56 day) experimental protocol. Data are expressed as mean ± SEM, n = 6/group. *p < 0.05 vs vehicle-treated WKY rats;†p < 0.05 vs vehicle-treated SHROB rats. White squares, vehicle-treated WKY rats; black squares, vehicle-treated SHROB rats; grey squares, RB394-treated SHROB rats
RB394 treatment decreases kidney injury in SHROB rats
Renal injury with fibrosis, inter-tubular proteinaceous cast formation, albuminuria and marked inflammation was evident in SHROB rats (Fig. 4). SHROB rats had greater kidney tubular cast area than WKY rats (Fig. 4a,c) and RB394 treatment of SHROB rats reduced the tubular cast area. We noted a higher collagen-positive renal fibrotic area in SHROB rats than in WKY rats (Fig. 4b,c) and RB394 treatment of the SHROB rats reduced this fibrosis. SHROB rats had higher degrees of albuminuria (Fig. 4d,f) and glomerular injury (Fig. 4e,f) when compared with WKY rats and RB394 treatment reduced the severity of these events in SHROB rats. SHROB rats also exhibited renal inflammation when compared with WKY rats (Fig. 4g–i). Urinary excretion of monocyte chemoattractant protein-1 (MCP-1) was increased (Fig. 4g) and renal CD68- positive inflammatory cells were more abundant (Fig. 4h,i) in SHROB rats when compared with WKY rats. RB394 treatment of SHROB rats reduced the number of CD68-positive cells and lowered the amount of MCP-1 excreted in the urine.
Fig. 4.
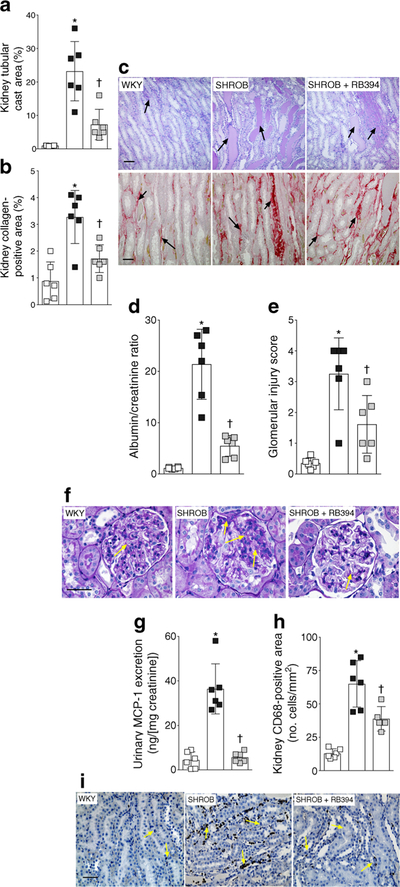
(a–c) Kidney tubular cast area (a), collagen-positive fibrotic area (b) and representative photomicrographs of renal tubular cast (stained blue, arrows) and collagen-positive areas (stained red, arrows) (c) in rats at the end of the 8 week (56 day) experimental protocol. (d–f) Albumin/ creatinine ratio (d), glomerular injury score (e) and representative photomicrographs depicting glomerular injury (arrows) (f) in rats at the end of the 8 week (56 day) experimental protocol. (g–i) Urinary excretion of MCP-1 (g), CD68-positive inflammatory cells in the kidney (h) and representative photomicrographs depicting CD68-positive cells in the kidney (arrows) (i) of rats at the end of the 8 week (56 day) experimental protocol. Data are expressed as mean ± SEM, n = 6/group. *p < 0.05 vs vehicle-treated WKY rats;†p < 0.05 vs vehicle-treated SHROB rats. White squares, vehicle-treated WKY rats; black squares, vehicle-treated SHROB rats; grey squares, RB394-treated SHROB rats. Scale bar, 50 μm in all photomicrographs
RB394 effectively reduces diabetes and hypertension in ZSF1 obese rats
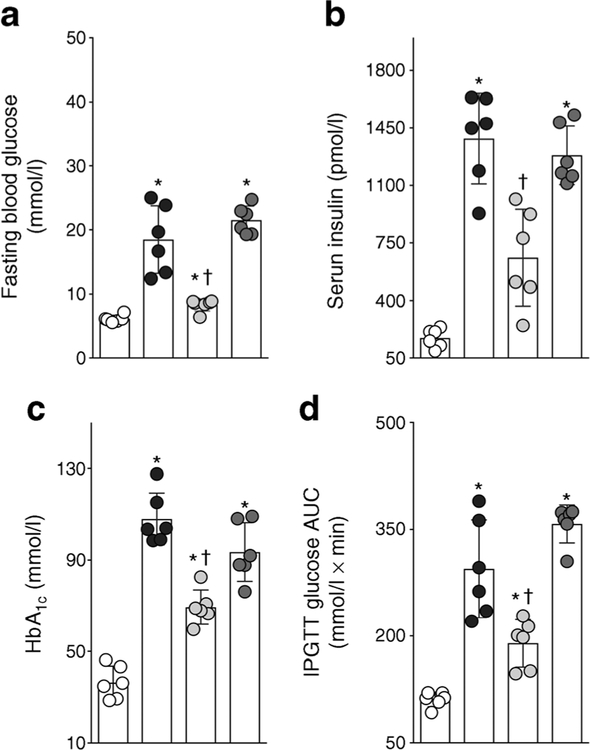
ZSF1 obese rats demonstrated overt diabetes, with a higher HbA1c and fasting blood glucose compared with ZSF1 lean rats (Fig. 5a,c). Eight weeks of treatment with RB394 reduced HbA1c and fasting blood glucose in the ZSF1 obese rats. Enalapril treatment did not reduce the hyperglycaemia in ZSF1 obese rats (Fig. 5a). ZSF1 obese rats demonstrated insulin resistance with impaired glucose tolerance (Fig. 5d). RB394 improved insulin sensitivity in ZSF1 obese rats while enalapril had no effect. ZSF1 obese rats demonstrated marked hyperinsulinaemia (Fig. 5b). Treatment with RB394 but not enalapril noticeably reduced serum insulin levels. The obese ZSF1 rats were hypertensive (systolic blood pressure [SBP] 177 ± 4 mmHg). An equipotent antihypertensive effect was demonstrated by RB394 (SBP 148 ± 5 mmHg) and enalapril (SBP 143 ± 4 mmHg) in obese ZSF1 rats.
Fig. 5.
Fasting blood glucose (a), serum insulin (b), HbA1c (c) and glucose AUC calculated from IPGTT (d) in rats at the end of the 8 week (56 day) experimental protocol. Data are expressed as mean ± SEM, n = 6/group. *p < 0.05 vs ZSF1 lean rats;†p < 0.05 vs vehicle-treated ZSF1 obese rats. White circles, vehicle-treated ZSF1 lean rats; black circles, vehicle-treated ZSF1 obese rats; light grey circles, RB394-treated ZSF1 obese rats; dark grey circles, enalapril-treated ZSF1 obese rats
Hyperlipidaemia is reduced by RB394 in diabetic ZSF1 obese rats
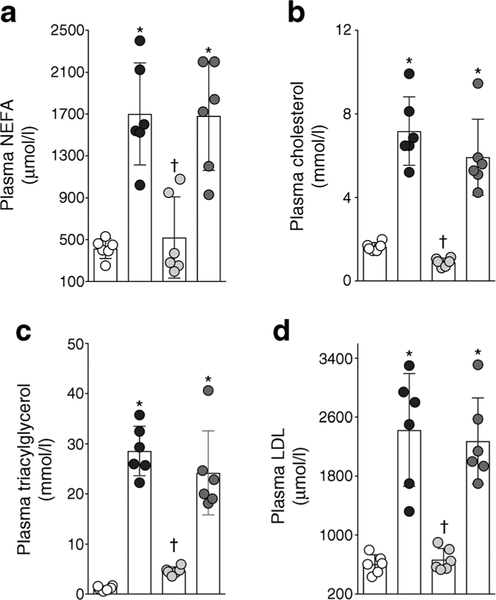
ZSF1 obese rats were hyperlipidaemic with an abnormally high lipid profile: ZSF1 obese rats had a higher plasma levels of NEFA, cholesterol, triacylglycerol and LDL- cholesterol compared with ZSF1 lean rats (Fig. 6). RB394 treatment effectively reduced plasma hyperlipidaemia in ZSF1 obese rats. In contrast to RB394, enalapril treatment did not improve the lipid profile in the obese ZSF1 rats.
Fig. 6.
Plasma levels of NEFA (a), cholesterol (b), triacylglycerol (c) and LDL-cholesterol (d) in rats at the end of the 8 week (56 day) experimental protocol. Data are expressed as mean ± SEM, n = 6/group. *p < 0.05 vs ZSF1 lean rats;†p < 0.05 vs vehicle-treated ZSF1 obese rats. White circles, vehicle-treated ZSF1 lean rats; black circles, vehicle-treated ZSF1 obese rats; light grey circles, RB394-treated ZSF1 obese rats; dark grey circles, enalapril-treated ZSF1 obese rats
RB394 ameliorates liver complications and steatosis in diabetic ZSF1 obese rats
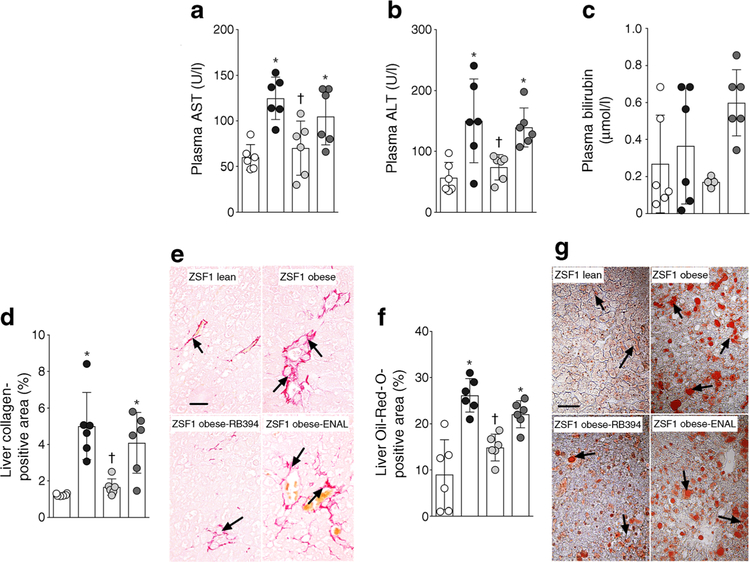
We assessed liver function, liver fibrosis and liver steatosis in ZSF1 obese rats. ZSF1 obese rats had higher plasma levels of alanine aminotransferase, aspartate aminotransferase and bilirubin than ZSF1 lean rats (Fig. 7a–c). RB394 treatment ameliorated these liver function abnormalities in ZSF1 obese rats. However, changes in bilirubin levels among the experimental groups did not reach statistical significance (p > 0.05). ZSF1 obese rats exhibited liver fibrosis, with a greater collagen-positive area compared with ZSF1 lean rats (Fig. 7d,e); this area was decreased following treatment with RB394. Enalapril treatment did not improve liver function or liver fibrosis in obese ZSF1 rats (Fig. 7a–e). ZSF1 obese rats demonstrated liver steatosis, with a greater Oil-Red- O-positive liver area compared with ZSF1 lean rats (Fig. 7f,g); treatment with RB394 but not enalapril reduced this area in ZSF1 obese rats.
Fig. 7.
(a–c) Plasma levels of aspartate aminotransferase (AST, a), alanine aminotransferase (ALT, b) and bilirubin (c) in rats at the end of the 8 week (56 day) experimental protocol. (d–g) Calculated value of liver collagen-positive area (d), representative photomicrographs depicting collagen-positive areas (arrows) in the liver (e), calculated values of Oil-Red-O-positive (steatosis) liver area (f) and representative photomicrographs of liver steatosis (arrows) (g) in rats at the end of the 8 week (56 days) experimental protocol. Data are expressed as mean ± SEM, n = 6/group. *p < 0.05 vs ZSF1 lean rats;†p < 0.05 vs vehicle-treated ZSF1 obese rats. White circles, vehicle-treated ZSF1 lean rats; black circles, vehicle-treated ZSF1 obese rats; light grey circles, RB394-treated ZSF1 obese rats; dark grey circles, enalapril-treated ZSF1 obese rats. Scale bar, 20 μm in all photomicrographs. ENAL, enalapril
RB394 reduces kidney injury in diabetic ZSF1 obese rats
ZSF1 obese rats exhibited kidney injury, with higher levels of albuminuria and greater renal tubular cast area than ZSF1 lean rats (Fig. 8a–d). RB394 treatment reduced the degree of kidney injury. Enalapril reduced the albuminuria and tubular cast formation but not to a statistically significant extent (p > 0.05). An increase in glomerular injury score associated with reduced glomerular nephrin expression was observed in ZSF1 obese rats (Fig. 8e–h). RB394 decreased the extent of the glomerular injury and increased the glomerular nephrin expression in ZSF1 obese rats. Enalapril decreased the glomerular injury score to a similar extent as RB394 in obese ZSF1 rats.
Fig. 8.
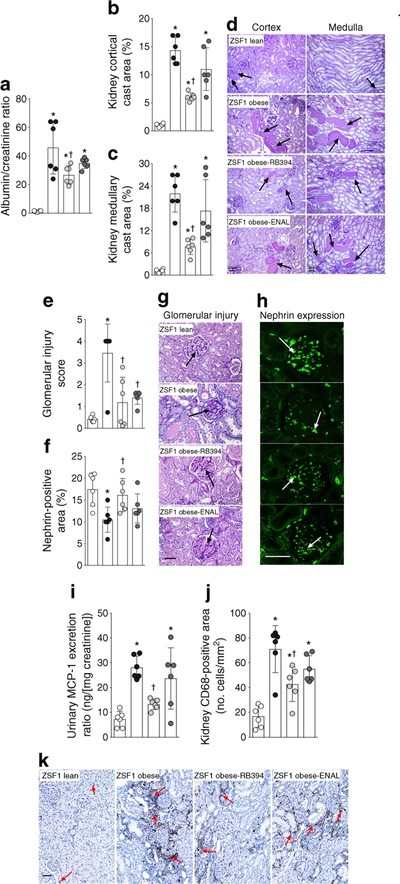
(a–d) Albuminuria (a), calculated values of renal cortical (b) and medullary (c) cast area, and representative photomicrographs showing tubular cast (arrows) in the renal cortex and medulla (d) in rats at the end of the 8 week (56 day) experimental protocol. (e–h) Glomerular injury score (e), renal nephrin expression (f) and representative photomicrographs showing glomerular damage (arrows) (g) and nephrin expression (arrows) (h) in rats at the end of the 8 week (56 day) experimental protocol. (i–k) Urinary excretion of MCP-1 (i), CD68-positive inflammatory cells in the kidney (j) and representative photomicrographs depicting CD68-positive cells in the kidney (k) of rats at the end of the 8 week (56 day) experimental protocol. Data are expressed as mean ± SEM, n = 6/group. *p < 0.05 vs ZSF1 lean rats;†p < 0.05 vs vehicle-treated ZSF1 obese rats. White circles, vehicle-treated ZSF1 lean rats; black circles, vehicle-treated ZSF1 obese rats; light grey circles, RB394-treated ZSF1 obese rats; dark grey circles, enalapriltreated ZSF1 obese rats. Scale bar, 50 μm in all photomicrographs. ENAL, enalapril
ZSF1 obese rats exhibited renal inflammation associated with higher urinary MCP-1 excretion and a greater number of CD68-positive cells in the kidney (Fig. 8i–k). RB394 reduced renal inflammation in ZSF1 obese rats. Interestingly, enalapril had a smaller effect on renal inflammation than RB394 in obese ZSF1 rats: enalapril reduced urinary MCP-1 excretion and renal infiltration of CD68 cells by only 13–20% (p > 0.05).
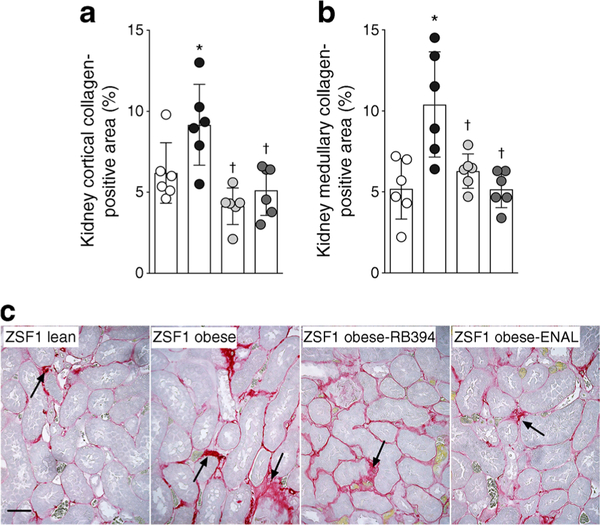
Next, we examined renal fibrosis and the associated elevated renal expression of profibrotic cytokine (TGF-β1) and fibrotic marker (fibronectin). ZSF1 obese rats exhibited a greater collagen-positive fibrotic area in the renal cortex and medulla compared with ZSF1 lean rats (Fig. 9). Accordingly, renal TGF-β1 expression levels were higher in obese ZSF1 rats than in ZSF1 lean rats (1.23 ± 0.06 vs 0.52 ± 0.07 arbitrary units), as were levels of fibronectin (3.00 ± 0.6 vs 0.75 ± 0.07 arbitrary units). Interestingly, treatment of obese ZSF1 rats with RB394 reduced renal fibrosis in the renal cortex and medulla (Fig. 9). Enalapril also reduced renal cortical and medullary collagen-positive areas in obese ZSF1 rats (Fig. 9). RB394 treatment of ZSF1 obese rats resulted in a reduction in expression levels of TGF-β1 and fibronectin in the kidney(0.47 ± 0.06 and 1.10 ± 0.09 arbitrary units, respectively). Enalapril reduced renal TGF-β1 expression (0.90 ± 0.06 arbitrary units) but not renal fibronectin expression (2.40 ± 0.30 arbitrary units).
Fig. 9.
Calculated values of the collagen-positive fibrotic area in the renal cortex (a) and medulla (b) and representative photomicrographs showing renal fibrosis (arrows) (c) in rats at the end of the 8 week (56 day) experimental protocol. Data are expressed as mean ± SEM, n = 6/group. *p <0.05 vs ZSF1 lean rats;†p < 0.05 vs vehicle-treated ZSF1 obese rats. White circles, vehicle-treated ZSF1 lean rats; black circles, vehicle-treated ZSF1 obese rats; light grey circles, RB394-treated ZSF1 obese rats; dark grey circles, enalapril-treated ZSF1 obese rats. Scale bar, 20 μm. ENAL, enalapril
RB394 reduces kidney injury in UUO in mice
The ability of RB394 to reduce kidney injury independent of concomitant changes in the metabolic syndrome variables was tested in a mouse model of UUO renal injury. RB394 treatment reduced kidney fibrosis and kidney fibronectin levels (ESM Fig. 4). These data demonstrate that RB394 can decrease kidney injury independent of changes in metabolic variables.
Discussion
We developed a novel dual-acting molecule, RB394, which simultaneously acts as an inhibitor of sEH and as a PPAR-γ agonist. Using two distinct approaches, we examined the efficacy of RB394 in pre-clinical models of the metabolic syndrome and type 2 diabetes. In one model (SHROB rats, which exhibit features of the metabolic syndrome) we investigated the ability of RB394 to prevent pathophysiological features of the metabolic syndrome and renal injury [13]. In another model (obese ZSF1 rat model of type 2 diabetes), we compared RB394 with an ACE inhibitor, enalapril, in treating diabetic complications since ACE inhibitors are widely used for blood pressure control and are particularly beneficial for treating diabetic nephropathy in hypertensive individuals with type 2 diabetes [14–17].
In SHROB rats, we demonstrated that RB394 blunts the development of hypertension, hyperlipidaemia and insulin resistance. Interestingly, in the diabetic obese ZSF1 rats, we demonstrated that RB394 but not enalapril effectively reduces hyperglycaemia, hyperlipidaemia and insulin resistance. We suggest that this ability of RB394 to provide multifaceted actions in the metabolic syndrome and type 2 diabetes can be attributed to its biological actions associated with sEH inhibition and PPAR-γ agonism. Indeed, it has been reported that sEH inhibitors reduce hypertension [13] as well as improve lipid profiles [6, 13] and insulin sensitivity [6] in the metabolic syndrome. Likewise, in the metabolic syndrome PPAR-γ agonists exert similar effects to reduce hypertension, hyperlipidaemia and insulin resistance [18, 19].
RB394 treatment did not alter body weight in SHROB and obese ZSF1 rats. This is an interesting finding as according to current views obesity and insulin resistance are closely and reciprocally interrelated [20]. It is, however, reported that sEH inhibitors do not affect body weight gain while improving insulin sensitivity in the metabolic syndrome [6]. PPAR-γ agonists do not reduce body weight and can even increase body weight in animals and humans [21]. It is important to note that the increase in body weight caused by PPAR-γ agonists can be a result of oedema, a serious side effect observed in certain populations [22]. It is reported that PPAR-γ agonists stimulate kidney epithelial sodium channels (ENaCs), which play a critical role in body water and electrolyte balance [23]. Interestingly, sEH inhibition can block ENaC activity by elevating endogenous epoxyeicosatrienoic acids, which are potent ENaC inhibitors [24–26]. These findings support the notion that RB394 can oppose the PPAR-γ agonistic activation of ENaC and subsequent oedema.
Our findings collectively demonstrate that unlike the current option of treatment with an ACE inhibitor, the novel dualacting molecule can act on multiple cardinal metabolic events in the metabolic syndrome. We demonstrate that RB394 as a single entity prevents the development of the metabolic syndrome and reduces the severity of type 2 diabetes and associated comorbid conditions. This is an important finding as type 2 diabetes is associated with high morbidity and mortality, largely due to the inability of currently available drugs to control comorbid conditions, such as hypertension and hyperlipidaemia, and to prevent the development of diabetic complications [27]. We suggest that RB394 would be beneficial in reducing diabetic complications including diabetic nephropathy.
Indeed, the increasing population with type 2 diabetes has created a major impact on the prevalence of diabetic nephropathy, which occurs in 20–40% of individuals with type 2 diabetes and is the leading cause of end-stage renal disease [28, 29]. Few new drugs aimed at reducing kidney complications have been successfully developed over the past 16 years [30]. This could be due to the complexity of aetiological factors associated with diabetic nephropathy. The prevention and treatment of diabetic nephropathy requires a multiple risk factor approach, the goal being to achieve glycaemic control along with reduction of hypertension and dyslipidaemia [31]. This medical need is, as yet, unmet. In the current study SHROB and ZSF1 rats exhibited marked renal fibrosis and glomerular injury. We demonstrated that RB394 prevented nephropathy development in SHROB rats and, more importantly, it effectively ameliorated diabetic nephropathy and was comparable or better than enalapril in obese diabetic ZSF1 rats (model of type 2 diabetes).
The marked beneficial renal actions of RB394 can be attributed to its ability to improve insulin sensitivity, lower blood pressure and decrease plasma lipids in the metabolic syndrome. Impaired glucose homeostasis, hypertension and hyperlipidaemia are known to contribute to the progression of diabetic nephropathy [32]. One prominent metabolic abnormality in the metabolic syndrome is insulin resistance, which leads to hyperinsulinaemia followed by glomerular hyperfiltration, endothelial dysfunction and eventual albuminuria [33]. Impaired insulin sensitivity is also associated with altered renal cellular metabolism, mesangial hyperplasia, increased endothelial cell proliferation and lipid and hyaluronate deposition, which contributes to kidney injury [34]. In SHROB and ZSF1 rats we demonstrated marked insulin resistance and in the obese ZSF1 rats insulin resistance was accompanied by hyperinsulinaemia. RB394 prevented and reduced insulin resistance and hyperinsulinaemia in SHROB and ZSF1 rats. These actions of RB394 could be related to the biological actions of the two pharmacophores that comprise RB394. Indeed, genetic deletion or pharmacological inhibition of sEH improves insulin sensitivity and glucose tolerance in mice [5, 6] and PPAR-γ agonists improve insulin sensitivity and glucose homeostasis [35].
In the present study enalapril had no effect on insulin sensitivity in obese ZSF1 rats. It should be noted that ACE inhibitors have been shown to improve insulin sensitivity by increasing nitric oxide levels [15]. However, this action of ACE inhibitors on insulin sensitivity is controversial and there are multiple studies demonstrating no such action. Indeed, no increases in insulin sensitivity were found in healthy individuals, hypertensive individuals with essentially normal insulin sensitivity or hypertensive insulin-resistant individuals with type 2 diabetes following treatment with trandolapril [36] or enalapril [37]. Animal studies have shown similar results— enalapril did not cause positive effects on glycaemic control in a rat model of type 2 diabetes/obesity [38]—and support our current findings.
The renal actions of RB394 demonstrated in the current study are also related to its antihypertensive and lipidlowering effects. In clinical studies, blood pressure reduction has been identified as a potent cardiovascular disease risk reducer in the metabolic syndrome and type 2 diabetes. Better blood pressure control in type 2 diabetes has been shown to decrease the onset or degree of albuminuria and vascular complications [39]. In the current study, RB394 prevented and reduced hypertension in rat models of the metabolic syndrome and type 2 diabetes. It should be noted that sEH inhibition and PPAR-γ agonism are known to produce an antihypertensive effect [13, 40]. As expected, the ACE inhibitor enalapril reduced hypertension in the obese ZSF1 rats and, interestingly, the antihypertensive actions of RB394 and enalapril were equipotent.
Akin to hypertension, there is increasing evidence for an association between dyslipidaemia and renal disease progression in individuals with type 2 diabetes [41]. Lipid-lowering agents demonstrate beneficial renal outcomes besides their beneficial effects on cardiovascular disease in type 2 diabetes [42]. In the present study, the lipid-lowering effect of RB394 can be attributed to the concerted action of its two pharmacophores [43, 44]. Unlike RB394, enalapril did not improve the lipid profile in obese ZSF1 rats. It is important to note that an earlier study suggested that enalapril may prevent the development of elevated cholesterol levels in obese ZSF1 rats after 12 and 24 weeks of treatment [38]. However, in this study enalapril was used in a preventive manner and it is not known whether enalapril or any other ACE inhibitor can reduce established hyperlipidaemia in a pre-clinical diabetic nephropathy model.
Apart from metabolic abnormalities per se, inflammation associated with metabolic abnormalities plays a crucial role in metabolic syndrome kidney injury. Chronic inflammation is a hallmark of the metabolic syndrome and its severity depends on the presence of different metabolic syndrome pathophysiologies [45]. It is proposed that metabolic syndrome-induced disruption in physiological regulatory systems due to excessive energy intake provokes stressor stimuli that trigger inflammation [45]. The substantial inflammatory macrophage infiltration and cytokines in the abdominal and peri-renal fat seen in animal models of the metabolic syndrome could serve as a channel for cytokines to access the kidney [46]. We earlier demonstrated renal inflammation and injury associated with increased MCP-1 excretion and elevated renal immune cell infiltration in animal models of the metabolic syndrome [8, 13]. The present study demonstrates that SHROB and obese diabetic ZSF1 rats exhibit renal inflammation and injury. Interestingly, we demonstrate that RB394 reduces renal inflammation by reducing MCP-1 excretion, renal immune cell infiltration and TGF-β expression. Conversely, the ACE inhibitor enalapril did not reduce renal inflammation in diabetic obese ZSF1 rats.
The anti-inflammatory and renal actions of RB394 can be attributed to sEH inhibition and/or PPAR-γ agonism. Antiinflammatory activity has been demonstrated for sEH inhibitors in the metabolic syndrome, type 2 diabetes and hypertension [7, 13]. Similarly, PPAR-γ agonists demonstrate renal anti-inflammatory activity in SHROB and ZSF1 obese rats [13, 38]. It is possible that the anti-inflammatory and renal actions of sEH inhibitors and PPAR-γ agonist in the metabolic syndrome are due to their ability to reduce metabolic abnormalities and that they do not have any true and independent anti-inflammatory or renal protective actions. Contrary to this notion, anti-inflammatory and renal protective actions for sEH inhibitors and PPAR-γ agonists have been demonstrated in pathological conditions that do not depend on antihypertensive or glucose-lowering effects [47–50]. These findings support the notion that RB394 possesses direct renal protective activity. Experiments conducted in obstructive nephropathy demonstrated that RB394 possesses kidney protective effects independent of its actions on metabolic pathologies. Overall, we demonstrate that RB394 reduces diabetic nephropathy by exerting a multifaceted action in the metabolic syndrome and in other renal pathologies not associated with the metabolic syndrome, hypertension or diabetes.
Individuals with type 2 diabetes have an increased risk of developing chronic liver disease, especially non-alcoholic fatty liver disease (NAFLD) [51]. The prevalence of NAFLD in diabetic individuals is higher than in those without diabetes, possibly as high as 75%, and the NAFLD can progress to liver fibrosis [52–54]. We examined whether RB394 or enalapril could treat liver dysfunction, fibrosis and steatosis in obese ZSF1 rats. Enalapril had no effect on liver dysfunction or hepatosteatosis, supporting earlier findings that enalapril did not reduce hepatosteatosis in obese ZSF1 rats [38]. Indeed, the hepatic action of ACE inhibitors is controversial and there are reports of ACE-inhibitor-associated hepatotoxicity in individuals with diabetes [55]. Ramipril, a well-acclaimed ACE inhibitor used in diabetic hypertensive patients, has been reported to cause liver dysfunction [56, 57]. In contrast, we demonstrated striking effects for RB394 in ameliorating liver dysfunction, fibrosis and steatosis in obese ZSF1 rats. This is an interesting finding as sEH and PPAR-γ differ in their actions in fatty liver and steatohepatitis. The role of PPAR-γ in hepatic steatosis is complex. Mouse models of the metabolic syndrome and diabetes, including ob/ob and KKAy mice, demonstrate the development of fatty livers in which expression of PPAR-γ is enhanced. These results suggest that PPAR-γ might be implicated in fatty liver pathophysiology in mice [58]. Nevertheless, PPAR-γ agonists have beneficial actions in non-alcoholic steatohepatitis as they reduce liver collagen levels [59]. sEH inhibitors reduced hepatic steatosis in a highfat-diet-induced model of the metabolic syndrome [60]. Moreover, sEH inhibitors demonstrate strong antifibrotic activity in carbon tetrachloride-induced cirrhotic hepatitis [61]. Although there is an apparent difference in the actions of the two pharmacophores of RB394, we suggest that the common biological actions of sEH and PPAR-γ on hyperglycaemia and hyperlipidaemia are associated with the liver protection. Indeed, there are roles for hyperglycaemia and hyperlipidaemia in NAFLD pathogenesis and consequent liver dysfunction and fibrosis [53]. In addition, hyperglycaemia is suggested to contribute to the pathophysiology of liver fibrosis in the metabolic syndrome and type 2 diabetes [62, 63]. Overall, we demonstrated that apart from its ability to treat diabetic nephropathy, RB394 can treat fatty liver and steatohepatitis in type 2 diabetes.
In summary, we demonstrate the therapeutic potential for a dual modulator, RB394, which acts as an sEH inhibitor and PPAR-γ agonist in rat models of the metabolic syndrome and type 2 diabetes. This novel molecule prevented the development of metabolic abnormalities and kidney injury in a model of the metabolic syndrome. Most importantly, we demonstrate the ability of RB394 to ameliorate type 2 diabetes and its comorbid conditions, such as insulin resistance, hypertension and hyperlipidaemia, and to reduce multiple diabetic complications such as diabetic nephropathy and liver injury. Overall, we demonstrate a promising dual-ligand small molecule, RB394, which can be developed as a therapeutic agent for the metabolic syndrome, type 2 diabetes and associated complications.
Supplementary Material
Research in context.
What is already known about this subject?
Obesity, hypertension, hyperlipidaemia and insulin resistance lead to a widely prevalent human condition, collectively known as the metabolic syndrome
The metabolic syndrome has a great impact on cardiovascular morbidity and mortality worldwide
Epidemiological studies demonstrate that individuals with the metabolic syndrome are at increased risk of developing type 2 diabetes and kidney, liver and cardiovascular complications
What is the key question?
Can the novel dual-acting molecule RB394 treat the metabolic syndrome and renal and hepatic complications of type 2 diabetes in pre-clinical animal models?
What are the new findings?
RB394, which simultaneously modulates soluble epoxide hydrolase and PPAR-γ, prevented hypertension, hyperlipidaemia, insulin resistance and kidney injury in a spontaneously hypertensive obese rat model of the metabolic syndrome
RB394, given in an interventional manner, improved glucose homeostasis, and decreased hyperlipidaemia, blood pressure, hepatosteatosis and kidney injury in an obese ZSF1 rat model of type 2 diabetes
How might this impact on clinical practice in the foreseeable future?
We provide strong evidence that a promising dual-acting molecule, RB394, could be an effective therapeutic agent to treat the metabolic syndrome, type 2 diabetes and associated complications
Funding
A National Institute of Health (NIH) grant (DK103616) and Dr. Ralph and Marian Falk Medical Research Trust Bank of America, N.A., Trustee grant to JDI supported this study. Research was supported by the Deutsche Forschungsgemeinschaft (Sachbeihilfe PR 1405/2–2, Heisenberg-Professur PR 1405/4–1 and SFB 1039 Teilprojekt A07) to EP. RB thanks Else-Kröner-Fresenius Foundation graduate college for Translational Research Innovation–Pharma (TRIP) for a PhD scholarship. MH thanks German Cancer Consortium (DKTK) for a PhD scholarship. The funding sources were not involved in the design of the study, the collection, analysis and interpretation of data, writing the manuscript or the decision to submit the manuscript for publication.
Abbreviations
- ENaC
Epithelial sodium channel
- MCP-1
Monocyte chemoattractant protein-1
- NAFLD
Non-alcoholic fatty liver disease
- PPAR-γ
Peroxisome proliferator-activated receptor-γ
- SBP
Systolic blood pressure
- sEH
Soluble epoxide hydrolase
- SHROB
Obese spontaneously hypertensive
- UUO
Unilateral ureteral obstruction
- WKY
Wistar–Kyoto
- ZSF1
Zucker diabetic fatty/spontaneously hypertensive heart failure F1 hybrid
Footnotes
Duality of interest JDI, EP, MAHK and RB have a patent application that covers the composition of matter for RB394. All other authors declare that there is no duality of interest associated with their contribution to this article.
Data availability The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s00125-018-4685-0) contains peer-reviewed but unedited supplementary material, which is available to authorised users.
References
- 1.Potenza MV, Mechanick JI (2009) The metabolic syndrome: definition, global impact, and pathophysiology. Nutr Clin Pract 24: 560–577 [DOI] [PubMed] [Google Scholar]
- 2.Isomaa B, Almgren P, Tuomi T et al. (2001) Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care 24:683–689 [DOI] [PubMed] [Google Scholar]
- 3.Grundy SM (2006) Drug therapy of the metabolic syndrome: minimizing the emerging crisis in polypharmacy. Nat Rev Drug Discov 5:295–309 [DOI] [PubMed] [Google Scholar]
- 4.Imig JD (2010) Targeting epoxides for organ damage in hypertension. J Cardiovasc Pharmacol 56:329–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luria A, Bettaieb A, Xi Y et al. (2011) Soluble epoxide hydrolase deficiency alters pancreatic islet size and improves glucose homeostasis in a model of insulin resistance. Proc Natl Acad Sci U S A 108:9038–9043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iyer A, Kauter K, Alam MA et al. (2012) Pharmacological inhibition of soluble epoxide hydrolase ameliorates diet-induced metabolic syndrome in rats. Exp Diabetes Res 2012:758614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roche C, Guerrot D, Harouki N et al. (2015) Impact of soluble epoxide hydrolase inhibition on early kidney damage in hyperglycemic overweight mice. Prostaglandins Other Lipid Mediat 120: 148–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hye Khan MA, Neckář J, Cummens B, Wahl GM, Imig JD (2014) Azilsartan decreases renal and cardiovascular injury in the spontaneously hypertensive obese rat. Cardiovasc Drugs Ther 28:313–322 [DOI] [PubMed] [Google Scholar]
- 9.Molinar-Toribio E, Pérez-Jiménez J, Ramos-Romero S et al. (2015) Effect of n-3 PUFA supplementation at different EPA:DHA ratios on the spontaneously hypertensive obese rat model of the metabolic syndrome. Br J Nutr 113:878–887 [DOI] [PubMed] [Google Scholar]
- 10.Tofovic SP, Kost CK Jr, Jackson EK, Bastacky SI (2002) Longterm caffeine consumption exacerbates renal failure in obese, diabetic, ZSF1 (fa-facp) rats. Kidney Int 61:1433–1444 [DOI] [PubMed] [Google Scholar]
- 11.Prabhakar S, Starnes J, Shi S, Lonis B, Tran R (2007) Diabetic nephropathy is associated with oxidative stress and decreased renal nitric oxide production. J Am Soc Nephrol 18:2945–2952 [DOI] [PubMed] [Google Scholar]
- 12.Blöcher R, Lamers C, Wittmann SK et al. (2016) NBenzylbenzamides: a novel merged scaffold for orally available dual soluble epoxide hydrolase/peroxisome proliferator-activated receptor γ modulators. J Med Chem 59:61–81 [DOI] [PubMed] [Google Scholar]
- 13.Imig JD, Walsh KA, Hye Khan MA et al. (2012) Soluble epoxide hydrolase inhibition and peroxisome proliferator activated receptor γ agonist improve vascular function and decrease renal injury in hypertensive obese rats. Exp Biol Med (Maywood) 237:1402–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rollason V, Vogt N (2003) Reduction of polypharmacy in the elderly: a systematic review of the role of the pharmacist. Drugs Aging 20:817–832 [DOI] [PubMed] [Google Scholar]
- 15.Henriksen EJ, Jacob S (2003) Modulation of metabolic control by angiotensin converting enzyme (ACE) inhibition. J Cell Physiol 196:171–179 [DOI] [PubMed] [Google Scholar]
- 16.Hunsicker LG (2004) Emerging trends for prevention and treatment of diabetic nephropathy: blockade of the RAAS and BP control. J Manag Care Pharm (5 Suppl A):S12–S17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Batlle D, Wysocki J, Soler MJ (2012) Angiotensin-converting enzyme 2: enhancing the degradation of angiotensin II as a potential therapy for diabetic nephropathy. Kidney Int 81:520–528 [DOI] [PubMed] [Google Scholar]
- 18.Sarafidis PA, Lasaridis AN (2006) Actions of peroxisome proliferator-activated receptors-gamma agonists explaining a possible blood pressure-lowering effect. Am J Hypertens 19:646–653 [DOI] [PubMed] [Google Scholar]
- 19.Fogo AB (2008) Potential for peroxisome proliferator-activated receptor-gamma agonists in progression: beyond metabolism. Curr Opin Nephrol Hypertens 17:282–285 [DOI] [PubMed] [Google Scholar]
- 20.Eckel RH, Grundy SM, Zimmet PZ (2005) The metabolic syndrome: epidemiology, mechanisms, and therapy. Lancet 365: 1415–1428 [DOI] [PubMed] [Google Scholar]
- 21.Carmona MC, Louche K, Nibbelink M et al. (2005) Fenofibrate prevents rosiglitazone-induced body weight gain in ob/ob mice. Int J Obes 29:864–871 [DOI] [PubMed] [Google Scholar]
- 22.Shah P, Mudaliar S (2010) Pioglitazone: side effect and safety profile. Expert Opin Drug Saf 9:347–354 [DOI] [PubMed] [Google Scholar]
- 23.Guan Y, Hao C, Cha DR et al. (2005) Thiazolidinediones expand body fluid volume through PPARγ stimulation of ENaC-mediated renal salt absorption. Nat Med 11:861–866 [DOI] [PubMed] [Google Scholar]
- 24.Hye Khan MA, Pavlov TS, Christain SV et al. (2014) Epoxyeicosatrienoic acid analogue lowers blood pressure through vasodilation and sodium channel inhibition. Clin Sci (Lond) 127: 463–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pavlov TS, Ilatovskaya DV, Levchenko V (2011) Effects of cytochrome P-450 metabolites of arachidonic acid on the epithelial sodium channel (ENaC). Am J Physiol Ren Physiol 301:F672–F681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pidkovka N, Rao R, Mei S et al. (2013) Epoxyeicosatrienoic acids (EETs) regulate epithelial sodium channel activity by extracellular signal-regulated kinase 1/2 (ERK1/2)-mediated phosphorylation. J Biol Chem 288:5223–5231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stark Casagrande S, Fradkin JE, Saydah SH, Rust KF, Cowie CC (2013) The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988–2010. Diabetes Care 36: 2271–2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J (2011) Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA 305:2532–2539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tuttle KR, Bakris GL, Bilous RW et al. (2014) Diabetic kidney disease: a report from an ADA Consensus Conference. Diabetes Care 37:2864–2883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brenneman J, Hill J, Pullen S (2016) Emerging therapeutics for the treatment of diabetic nephropathy. Bioorg Med Chem Lett 26: 4394–4402 [DOI] [PubMed] [Google Scholar]
- 31.John S (2016) Complication in diabetic nephropathy. Diabetes Metab Syndr 10:247–249 [DOI] [PubMed] [Google Scholar]
- 32.Singh DK, Winocour P, Farrington K (2011) Oxidative stress in early diabetic nephropathy: fueling the fire. Nat Rev Endocrinol 7:176–184 [DOI] [PubMed] [Google Scholar]
- 33.Dronavalli S, Duka I, Bakris GL (2008) The pathogenesis of diabetic nephropathy. Nat Clin Pract Endocrinol Metab 4:444–452 [DOI] [PubMed] [Google Scholar]
- 34.Groop PH, Forsblom C, Thomas MC (2005) Mechanisms of disease: pathway-selective insulin resistance and microvascular complications of diabetes. Nat Clin Pract Endocrinol Metab 1:100–110 [DOI] [PubMed] [Google Scholar]
- 35.Soccio RE, Chen ER, Lazar MA (2014) Thiazolidinediones and the promise of insulin sensitization in type 2 diabetes. Cell Metab 20: 573–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petrie JR, Morris AD, Ueda S et al. (2000) Trandolapril does not improve insulin sensitivity in patients with hypertension and type 2 diabetes: a double-blind, placebo-controlled crossover trial. J Clin Endocrinol Metab 85:1882–1889 [DOI] [PubMed] [Google Scholar]
- 37.Heise T, Heinemann L, Kristahn K et al. (1999) Insulin sensitivity in patients with essential hypertension: no influence of the ACE inhibitor enalapril. Horm Metab Res 31:418–423 [DOI] [PubMed] [Google Scholar]
- 38.Bilan VP, Salah EM, Bastacky S et al. (2011) Diabetic nephropathy and long-term treatment effects of rosiglitazone and enalapril in obese ZSF1 rats. J Endocrinol 210:293–308 [DOI] [PubMed] [Google Scholar]
- 39.Steigerwalt S (2008) Management of hypertension in diabetic patients with chronic kidney disease. Diabetes Spectrum 21:30–36 [Google Scholar]
- 40.Stump M, Mukohda M, Hu C, Sigmund CD (2015) PPARγ regulation in hypertension and metabolic syndrome. Curr Hypertens Rep 17:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muntner P, Coresh J, Smith JC, Eckfeldt J, Klag MJ (2000) Plasma lipids and risk of developing renal dysfunction: the atherosclerosis risk in communities study. Kidney Int 58:293–301 [DOI] [PubMed] [Google Scholar]
- 42.Cases A, Coll E (2005) Dyslipidemia and the progression of renal disease in chronic renal failure patients. Kidney Int Suppl 99:S87–S93 [DOI] [PubMed] [Google Scholar]
- 43.Zhang LN, Vincelette J, Cheng Y et al. (2009) Inhibition of soluble epoxide hydrolase attenuated atherosclerosis, abdominal aortic aneurysm formation, and dyslipidemia. Arterioscler Thromb Vasc Biol 29:1265–1270 [DOI] [PubMed] [Google Scholar]
- 44.He J, Wang C, Zhu Y, Ai D (2016) Soluble epoxide hydrolase: a potential target for metabolic diseases. J Diabetes 8:305–313 [DOI] [PubMed] [Google Scholar]
- 45.Hotamisligil GS (2006) Inflammation and metabolic disorders. Nature 444:860–867 [DOI] [PubMed] [Google Scholar]
- 46.Ma S, Zhu XY, Eirin A et al. (2016) Perirenal fat promotes renal arterial endothelial dysfunction in obese swine 508 through tumor necrosis factor-alpha. J Urol 195:1152–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu Y, Webb HK, Fukushima H et al. (2012) Attenuation of cisplatin-induced renal injury by inhibition of soluble epoxide hydrolase involves nuclear factor κB signaling. J Pharmacol Exp Ther 341:725–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim J, Yoon SP, Toews ML et al. (2015) Pharmacological inhibition of soluble epoxide hydrolase prevents renal interstitial fibrogenesis in obstructive nephropathy. Am J Physiol Ren Physiol 308:F131–F139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee S, Kim W, Moon SO et al. (2006) Rosiglitazone ameliorates cisplatin-induced renal injury in mice. Nephrol Dial Transplant 21: 2096–2105 [DOI] [PubMed] [Google Scholar]
- 50.Kawai T, Masaki T, Doi S et al. (2009) PPAR-γ agonist attenuates renal interstitial fibrosis and inflammation through reduction of TGF-β. Lab Investig 89:47–58 [DOI] [PubMed] [Google Scholar]
- 51.Rafiq N, Younossi ZM (2008) Effects of weight loss on nonalcoholic fatty liver disease. Semin. Liver Dis 28:427–433 [DOI] [PubMed] [Google Scholar]
- 52.Wong RJ, Aguilar M, Cheung R et al. (2015) Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 148:547–555 [DOI] [PubMed] [Google Scholar]
- 53.Tilg H, Moschen AR, Roden M (2017) NAFLD and diabetes mellitus. Nat Rev Gastroenterol Hepatol 14:32–42 [DOI] [PubMed] [Google Scholar]
- 54.Younossi ZM, Blissett D, Blissett R et al. (2016) The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology 64:1577–1586 [DOI] [PubMed] [Google Scholar]
- 55.Schattner A, Kozak N, Friedman J (2001) Captopril-induced jaundice: report of 2 cases and a review of 13 additional reports in the literature. Am J Med Sci 322:236–240 [DOI] [PubMed] [Google Scholar]
- 56.Douros A, Kauffmann W, Bronder E, Klimpel A, Garbe E (2013) Kreutz R (2013) Ramipril-induced liver injury: case report and review of the literature. Am J Hypertens 26:1070–1075 [DOI] [PubMed] [Google Scholar]
- 57.Yeung E, Wong FS, Wanless IR et al. (2003) Ramipril-associated hepatotoxicity. Arch Pathol Lab Med 127:1493–1497 [DOI] [PubMed] [Google Scholar]
- 58.Inoue M, Ohtake T, Motomura W et al. (2005) Increased expression of PPARγ in high fat diet-induced liver steatosis in mice. Biochem Biophys Res Commun 336:215–222 [DOI] [PubMed] [Google Scholar]
- 59.Pettinelli P, Videla LA (2011) Up-regulation of PPAR-γ mRNA expression in the liver of obese patients: an additional reinforcing lipogenic mechanism to SREBP-1c induction. J Clin Endocrinol Metab 96:1424–1430 [DOI] [PubMed] [Google Scholar]
- 60.Liu Y, Dang H, Li D, Pang W, Hammock BD, Zhu Y (2012) Inhibition of soluble epoxide hydrolase attenuates high-fat-diet–induced hepatic steatosis by reduced systemic inflammatory status in mice. PLoS One 7:e39165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harris TR, Bettaieb A, Kodani S et al. (2015) Inhibition of soluble epoxide hydrolase attenuates hepatic fibrosis and endoplasmic reticulum stress induced by carbon tetrachloride in mice. Toxicol Appl Pharmacol 286:102–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sumida Y, Seko Y, Yoneda M (2016) Japan Study Group of NAFLD (JSG-NAFLD). Novel antidiabetic medications for nonalcoholic fatty liver disease with type 2 diabetes. Hepatol Res 47: 266–280 [DOI] [PubMed] [Google Scholar]
- 63.Qiang G, Zhang L, Yang X et al. (2012) Effect of valsartan on the pathological progression of hepatic fibrosis in rats with type 2 diabetes. Eur J Pharmacol 685:156–164 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.