Abstract
Sulfonates are frequently used to endow water solubility on hydrophobic molecules, but the repertoire of sulfonate protecting groups remains limited. Here we describe the first sulfonate esters that can be unmasked by the mild reducing conditions found in live mammalian cells. Self-immolative cleavage releases the sulfonate and the two-electron reduction product of a thioquinone methide.
The presence of an anionic sulfonate can dominate the physical properties of small molecules and polymers. When sulfonates are appended to hydrophobic molecules such as fluorescent dyes, they become water soluble. The high water solubility of sulfonated dyes is useful for maximizing optical performance in aqueous solutions and in extracellular applications, but limits diffusion across cell membranes. We previously developed esterase-labile protecting groups to deliver sulfonated molecules into live cells.1,2 However, unmasking of the anionic sulfonate within cells requires the activity of one or more unknown esterases, and the presence of an esterase-labile group limits stability in the presence of extracellular esterases. Additional strategies to protect, utilize, regulate, and deliver sulfonated molecules are therefore desirable.
The interior of the mammalian cell is a reducing environment, where a millimolar concentration of reduced glutathione is maintained by the electron donor NADPH and the enzyme glutathione reductase.3 Reduced glutathione can be directly attached to drug metabolites via the catalytic action of glutathione S-transferases, but also undergoes uncatalyzed reactions with redox-active molecules. We therefore hypothesized that reductively-labile sulfonate esters would allow the delivery and unmasking of sulfonated molecules within the cell (Fig. 1). Although many drugs, fluorophores, and functional groups have been masked with reductively-labile groups to take advantage of the intracellular reducing environment, none have been applied to sulfonates.4
Figure 1:
Sulfonated molecules cannot cross the cellular membrane. Protection with a reductively-labile sulfonate ester allows the molecule to cross the membrane and be unmasked by intracellular glutathione (GSH).
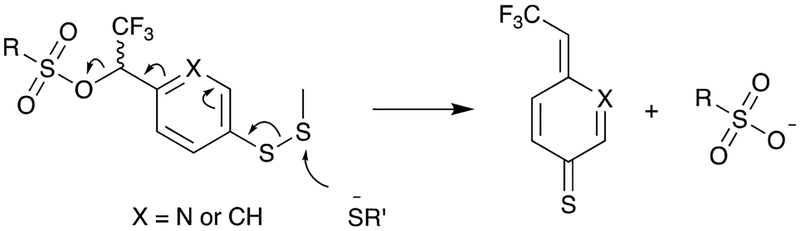
The α-trifluoromethyl benzyl (TFMB) scaffold is a particularly well-suited structure upon which to design selectively-cleavable sulfonate esters. TFMB sulfonates are stable to nucleophilic attack and solvolysis, yet their stability can be tuned by judicious modification of the aryl scaffold.1,5–7 Although TFMB and other sulfonate esters are generally stable to reductants,5,8 we hypothesized that the requisite reductive lability could be introduced into the TFMB scaffold by placing a disulfide in the para position (Fig. 2). Reduction of the disulfide was anticipated to result in release of the free sulfonate by 1,6-elimination (Fig. 2).
Figure 2:
Proposed cleavage mechanism. Reduction of the disulfide triggers 1,6 elimination, releasing a sulfonate and a p-thioquinone methide.
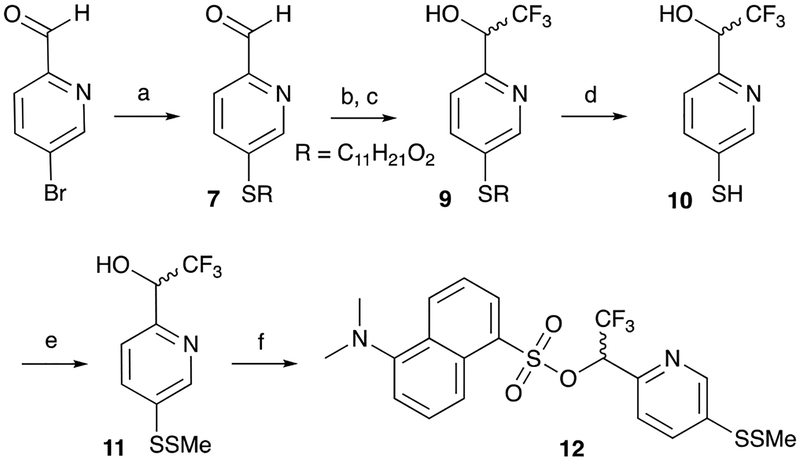
To access the disulfide, we first needed to synthesize the corresponding thiophenol. A common method to synthesize thiophenols is the Newman-Kwart rearrangement of thiocarbamates.9,10 However, this three-step procedure from phenols requires very high temperatures (and often high pressure) to promote the rearrangement. We therefore attempted a novel route to synthesize the desired thiophenol from a readily available thioanisole starting material (Scheme 1). Starting from 4-methylthio-benzaldehyde, the Ruppert-Prakash reaction was performed and the resulting trimethylsilyl (TMS) ether 1 purified and isolated.6 Demethylation of the thioanisole was achieved with N-bromosuccinimide (NBS) to form the N-(thio)succinimide,11 which was followed by HCl cleavage of the TMS ether to afford alcohol 3. To our delight, we were then able to directly access the desired thiophenol 4 by borohydride reduction of the N-(thio)succinimide. Although N-(thio)succinimides are finding increasing use as electrophilic reagents for sulfenylation,12–14 surprisingly they do not appear to have been used to synthesize thiophenols. This nominal two-step conversion of a thioanisole to a thiophenol should find broader synthetic use, as the conditions were milder (room temperature or below) and easier to perform than the classic Newman-Kwart route from phenols. Thus formed, the thiophenol was readily converted to the methyl disulfide 5 with MeSSO2Me, and the dansyl sulfonate ester MeSSTFMB-Dan 6 was formed using DABCO.1,5
Scheme 1:
Synthesis of MeSSTFMB-Dan: a) Me3SiCF3, TBAF, THF, 0°C to RT; b) NBS, CH2Cl2, RT; c) 1:1 aq. HCl/THF, RT; d) NaBH4, MeOH, −40°C to RT; e) MeSSO2Me, MeOH, pH 8 buffer, RT; f) dansyl chloride, DABCO, CH2Cl2, RT.
We next tested the stability of 6 and other sulfonate esters to reducing conditions using a fluorescence assay to monitor the formation of free dansyl sulfonate (Fig. 3, S2). Since alkyl sulfonate esters are prone to SN2 displacement by thiols, one might assume that millimolar glutathione would rapidly cleave even simple alkyl sulfonate esters. This would imply that no special design is needed to create sulfonate esters that are labile to intracellular glutathione. However, simple alkyl dansyl esters are not rapidly cleaved by 5 mM glutathione in PBS (Fig. S2). HPLC analysis revealed very little cleavage by either glutathione or the more powerful reductant TCEP after 15 minutes (Fig. S3). On the other hand, 6 exhibited a substantial increase in dansyl sulfonate fluorescence after treatment with glutathione, BME, DTT, and most notably TCEP (Fig. 3). After 15 minutes in 5 mM glutathione, ~30% cleavage was observed by HPLC, whereas ~60% cleavage was achieved in the presence of 1 mM TCEP (Fig. S3). The ester 6 is stable to aqueous conditions without reductant, as no cleavage occurred in PBS alone (Fig. S2, S3). Thus, introducing a disulfide trigger into the TFMB scaffold resulted in a reductively-labile sulfonate protecting group.
Figure 3:
Fluorescence monitoring of sulfonate release. Reductive cleavage of a) MeSSTFMB-Dan and b) MeSSTFMP-Dan under different reducing conditions in PBS. Cleavage was followed by monitoring the fluorescence emission of dansyl sulfonate (498 nm).
To further improve the rate of reductive cleavage, we revisited our design, incorporating a pyridyl nitrogen into the TFMB scaffold (Figs. 1, 2). This modification was expected to retain the core stability properties of TFMB, yet increase its lability to reduction due to the presence of the inductively-withdrawing pyridyl nitrogen. Starting from commercially-available 5-bromo-2-pyridinecarboxaldehyde, palladium-catalyzed coupling with 2-ethylhexyl 3-mercaptopropionate as reported by Itoh and Mase afforded the thioether 7 (Scheme 2).15,16 Subsequent Ruppert-Prakash reaction and hydrolysis of the TMS ether yielded alcohol 9. Base-mediated elimination with sodium methoxide yielded thiophenol 10, which was converted to the disulfide MeSSTFMP 11 with MeSSO2Me, followed by dansylation to yield MeSSTFMP-Dan 12.
Scheme 2:
Synthesis of MeSSTFMP-Dan. Reagents and conditions: a) 2-Ethylhexyl 3-Mercaptopropionate, Pd2(dba)3, Xantphos, toluene, reflux; b) Me3SiCF3, TBAF, THF, 0°C to RT; c) 1:1 aq. HCl/THF, RT; d) Toluene, 25% NaOMe in MeOH, RT; e) MeSSO2Me, MeOH, pH 8 buffer, RT; f) dansyl chloride, DABCO, CH2Cl2, RT.
As expected, reductive cleavage of sulfonate ester 12 was much more rapid than that of 6 (Fig. 3). Based on fluorescence monitoring of dansyl sulfonate formation, near complete cleavage occurred within 2–3 minutes under all of the tested reducing conditions (Fig. 3b). HPLC analysis after 15 minutes of exposure to 5 mM glutathione, 1 mM BME, 1 mM DTT, or 1 mM TCEP confirmed that 12 is fully cleaved to the free dansyl sulfonate, while no cleavage occurred in PBS without reductant (Fig. S3). An MeSSTFMP-protected alkyl sulfonate could be similarly cleaved under reducing conditions (Fig. S6).
Reductive cleavage of the protecting group is presumed to form a reactive thioquinone methide intermediate, a class of molecules which have been rarely if ever isolated (Fig. 4).17–19 Interestingly, HPLC and MS analysis of the cleavage products detected a peak consistent with the two-electron reduction product of the thioquinone methide, in strong preference to the corresponding nucleophilic addition product typically formed from quinone methides (Figs. S4–5).20–22 NMR, UV absorbance, and HRMS analysis of the products of MeSSTFMP-Dan reduction confirmed formation of the two-electron reduction product (ESI; Fig S7–8), highlighting a fundamental difference in reactivity between quinone methides and thioquinone methides.
Figure 4:
Reduction of MeSSTFMP sulfonate esters yields the two-electron reduction product of a thioquinone methide (major) in strong preference to a reductant adduct (minor).
Although self-immolative linkers that generate quinone methides have been widely used to release drugs and reporters,23 these reactive intermediates are potentially cytotoxic. We therefore evaluated the protected dansylates in HeLa cells using an XTT assay. TFMB-Dan cannot form a quinone methide, and was found to be nontoxic (Fig. S9). Reductively-labile dansyl esters 6 and 12 are capable of forming thioquinone methides, but exhibited negligible toxicity at doses < 10 μM. On the other hand, AcOTFMB-Dan,1 which liberates a quinone methide rather than a thioquinone methide, had an IC50 value of ~8 μM (Fig. S9). These differences are presumably related to the relative toxicity of thioquinone and quinone methides, as well as the rate of their formation. However, we cannot rule out the possibility that rapid release of the sulfonate itself is contributory. Regardless, the protected dansylates were well tolerated at low micromolar concentrations (Fig. S9).
Finally, we assessed the ability of these reductively-labile sulfonate esters to deliver dansyl sulfonate into live cells. HeLa cells were treated with 1 μM dansyl dye and imaged by fluorescence microscopy (Figs. 5 and S10). Free (anionic) dansyl sulfonate cannot enter the cells (Fig. S10), while stable dansyl sulfonate esters, such as TFMB-Dan, yield a punctate yellow-green fluorescence within the cell (Fig. 5). The esterase-labile dye AcOTFMB-Dan yields diffuse blue fluorescence throughout the cytoplasm and nucleus (Fig. S10).1 Here we find that reductively-labile sulfonate esters 6 and 12 are also able to deliver dansyl sulfonate into the cell, and that the more rapidly cleaving dye 12 is superior to 6 (Fig. 5e–f).
Figure 5:
Delivery of dansyl sulfonate into live HeLa cells. Fluorescence (a-c) and differential interference contrast (DIC) images (d-f) of cells treated with TFMB-Dan (a and d); MeSSTFMB-Dan (b and e); or MeSSTFMP-Dan (c and f). Images are shown at 50x magnification. Composite pictures g-i are pseudocolored. Scale bars = 10 μm.
Conclusions
We designed sulfonate esters that are cleaved by mild reducing conditions. The construction of these new protecting groups benefitted greatly from the palladium-catalyzed thiolation chemistry developed by Itoh and Mase, as well as the novel mild transformation of a thioanisole to a thiophenol described herein. In complement to esterase-labile sulfonate esters, we find that reductively-labile sulfonate esters allow the delivery of a sulfonated dye into live mammalian cells. In particular, the MeSSTFMP sulfonate protecting group is rapidly cleaved by physiological levels of glutathione to yield the free sulfonate and the two-electron reduction product of a thioquinone methide. We envision that further modifications could refine the reductive lability and solubility properties of the protected sulfonates and allow their attachment to other materials. This general strategy thus holds promise for creating a variety of novel molecules and materials where the selective release of a free sulfonate in a reducing environment is desired.
Supplementary Material
Acknowledgements
We thank the US National Institutes of Health (GM087460) for funding, and members of the McCollum, Kelch, and Thompson labs for the use of equipment.
Footnotes
Electronic Supplementary Information (ESI) available: See DOI: 10.1039/x0xx00000x
Notes and references
- 1.Rusha L and Miller SC, Chem. Commun, 2011, 47, 2038–2040. [DOI] [PubMed] [Google Scholar]
- 2.Pauff SM and Miller SC, Org. Lett, 2011, 13, 6196–6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lushchak VI, J. Amino Acids, 2012, 2012, e736837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee MH, Yang Z, Lim CW, Lee YH, Dongbang S, Kang C and Kim JS, Chem. Rev, 2013, 113, 5071–5109. [DOI] [PubMed] [Google Scholar]
- 5.Miller SC, J Org Chem, 2010, 75, 4632–4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pauff SM and Miller SC, J. Org. Chem, 2013, 78, 711–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma F, Fischer M, Han Y, Withers SG, Feng Y and Yang G-Y, Anal. Chem, 2016, 88, 8587–8595. [DOI] [PubMed] [Google Scholar]
- 8.Ali AM, Hill B and Taylor SD, J. Org. Chem, 2009, 74, 3583–3586. [DOI] [PubMed] [Google Scholar]
- 9.Newman MS and Karnes HA, J. Org. Chem, 1966, 31, 3980–3984. [Google Scholar]
- 10.Kwart H and Evans ER, J. Org. Chem, 1966, 31, 410–413. [Google Scholar]
- 11.Groebel W, Chem. Ber, 1959, 92, 2887–2892. [Google Scholar]
- 12.Qiao B, Liu X, Duan S, Yan L and Jiang Z, Org. Lett, 2014, 16, 672–675. [DOI] [PubMed] [Google Scholar]
- 13.Hostier T, Ferey V, Ricci G, Gomez Pardo D and Cossy J, Org. Lett, 2015, 17, 3898–3901. [DOI] [PubMed] [Google Scholar]
- 14.Hostier T, Ferey V, Ricci G, Gomez Pardo D and Cossy J, Chem. Commun, 2015, 51, 13898–13901. [DOI] [PubMed] [Google Scholar]
- 15.Itoh T and Mase T, Org. Lett, 2004, 6, 4587–4590. [DOI] [PubMed] [Google Scholar]
- 16.Itoh T and Mase T, J. Org. Chem, 2006, 71, 2203–2206. [DOI] [PubMed] [Google Scholar]
- 17.Itoh T, Fujikawa K and Kubo M, J. Org. Chem, 1996, 61, 8329–8331. [DOI] [PubMed] [Google Scholar]
- 18.Vigalok A and Milstein D, J. Am. Chem. Soc, 1997, 119, 7873–7874. [Google Scholar]
- 19.Senter PD, Pearce WE and Greenfield RS, J. Org. Chem, 1990, 55, 2975–2978. [Google Scholar]
- 20.Toteva MM and Richard JP, Adv. Phys. Org. Chem, 2011, 45, 39–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holmgren A, Brunow G, Henriksson G, Zhang L and Ralph J, Org. Biomol. Chem, 2006, 4, 3456–3461. [DOI] [PubMed] [Google Scholar]
- 22.Richard JP, Tetrahedron Lett, 1989, 30, 23–26. [Google Scholar]
- 23.Alouane A, Labruère R, Le Saux T, Schmidt F and Jullien L, Angew. Chem. Int. Ed, 2015, 54, 7492–7509. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.