Abstract
Temporal analysis of Acinetobacter calcoaceticus-baumannii complex isolates in a large, US healthcare system demonstrated decreased occurrence of antibiotic-susceptible isolates between November and May, while resistant isolate occurrence was temporally stable. This resulted in 50%–100% seasonal increases of resistance rates. This work offers insight into the phenomenon of Gram-negative pathogen seasonality.
Keywords: Acinetobacter baumannii, multidrug resistance, seasonality
Introduction
Acinetobacter calcoaceticus-baumannii complex (Abc) is a major global health threat due to its ability to survive in multiple environments and its association with high levels of antibiotic resistance. Though most notorious for their role in hospital-acquired infections, multiple studies report that 25%–65% of Abc clinical isolates are obtained in ambulatory settings or within 48–72 hours of hospital admission [1–3]. Further understanding Abc epidemiology is essential for developing interventions to curb the growing impact of multidrug-resistant (MDR) Abc infections.
Many Gram-negative pathogens, including Abc, increase in incidence during warmer, summer months, a phenomenon commonly referred as seasonality [4]. Accurate characterization of these seasonal trends is important for effective surveillance and infection control efforts. However, reports from single center studies conflict on Abc seasonality. For example, a 9-year Korean study of 3520 unique Abc isolates demonstrated that community-acquired isolates (n = 922), but not hospital-acquired isolates (n = 2598), had higher rates of incidence during warmer months [2]. An 11-year Pennsylvania study of 1476 isolates reported that non-MDR isolates (n = 692) exhibited seasonality, but contemporaneous MDR Abc isolates (n = 784) lacked seasonality [5]. In contrast, a 7-year study performed in Baltimore reported that all Abc isolates (n = 1444) exhibited seasonality independent of duration of hospital admission and antibiotic resistance [1]. We performed a retrospective analysis of Abc clinical isolates identified in and around St. Louis, Missouri, over a decade. Our aim was to analyze this cohort, of which over 45% of isolates were MDR, to investigate Abc seasonality in different epidemiological subgroups.
METHODS
Our retrospective analysis will be described elsewhere (unpublished data). Briefly, we compiled clinical and microbiology data on 1948 Abc isolation events from January 1, 2007, through December 31, 2016, in 11 BJC HealthCare System (BJC) hospitals located in and around Saint Louis. Only the first isolation event (index culture) per patient age >18 years was included. Similar to prior studies [1, 2], isolates were labelled as hospital-acquired (HA) if the index culture was obtained ≥48 hours after hospital admission, and all other isolates were labelled as nonhospital-acquired (nHA). Isolates were also grouped according to specimen source as respiratory, skin and soft tissue or musculoskeletal (SST/MSK), urinary, endovascular, or other. For antibiotic susceptibility analysis, isolates were classified as resistant if they were reported as resistant or intermediate per the Clinical and Laboratory Standards Institute’s guidelines [6]. The following antibiotics were grouped into classes: meropenem and imipenem (MEM/IPM) as carbapenems; ciprofloxacin and levofloxacin (CIP/LVX) as fluoroquinolones; piperacillin-tazobactam and ticarcillin-clavulanic acid (TZP/TIM) as antipseudomonal penicillins plus β-lactamase inhibitor; and tetracycline and doxycycline (TET/DOX) as tetracyclines. If an isolate was nonsusceptible to any antibiotic in a class, it was labelled resistant for that class.
Seasonality Analysis
According to the month an index culture was obtained, isolates were grouped into quarters as follows: December (from prior year) through February as Quarter 1 (Q1); March through May as Quarter 2 (Q2); June through August as Quarter 3 (Q3); and September through November as Quarter 4 (Q4). The number of isolates per quarter was plotted, starting with Q2 in 2007 (“07Q2”). To normalize isolate occurrence across multiple years for comparative analysis, we converted quarterly occurrence to a percentage of annual isolates (ie, [# of isolates in a quarter]/[# of isolates in all 4 quarters in the corresponding year] × 100 = “normalized occurrence”). Using this method we expect 25% of annual isolates, on average, to occur in each quarter, in the absence of seasonal variation. We also determined the resistance rates exhibited by isolates in each quarter. Normalized occurrence and resistance rates were averaged for all Q1–Q4 in the study period. Pairwise comparisons between quarters (eg, Q1 vs Q2, Q1 vs Q3, etc.) were performed using 2-sample independent t test analysis with SPSS v25 (IBM, USA). To compare our cohort to those from prior analyses [1, 2, 5], we performed subgroup seasonality analysis according to whether isolates were HA or nHA, whether they were susceptible or resistant to an antibiotic, and according to isolate tissue source.
RESULTS
We plotted the 10-year cumulative total of isolates obtained in each month, grouped according to susceptibility to gentamicin (GM), carbapenems (MEM/IPM), fluoroquinolones (CIP/LVX), or trimethoprim-sulfamethoxazole (SMX). As seen in Figure 1A, there was a peak in susceptible isolates between June and November without a corresponding peak for resistant isolates. These diverging epidemiological behaviors resulted in net antibiotic resistance rates being lowest in September or October and highest in February. Similar observations were made when examining isolate occurrence and resistance rates by quarter: there were annual peaks of Abc in Q3 and Q4, while resistance rates peaked during Q1 and Q2 (Supplementary Figure S1).
Figure 1.
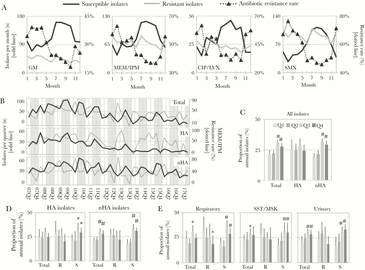
Seasonality among antibiotic-susceptible, but not -resistant, Abc isolates. A, The cumulative total (solid lines, left axis) and antibiotic resistance rates (dotted lines, right axis) of isolates obtained in each month (1–12, January through December) over the study period. Graphs depict numbers of isolates that are susceptible (gray line) or resistant (black line) to each antibiotic or antibiotic class (GM, gentamicin; MEM/IPM, meropenem/imipenem; CIP/LVX, ciprofloxacin/levofloxacin; SMX, trimethoprim/sulfamethoxazole); B, Total, hospital-acquired (HA), and nonhospital-acquired (nHA) Abc cases per quarter (YY–QQ) are plotted with solid lines. Corresponding quarterly MEM/IPM resistance rates are plotted with dotted lines. Shaded areas highlight peaks in seasonal occurrence in Q3 and Q4. C,D,E, Average proportion of annual isolates occurring in each quarter, according to Abc subgroups. In panel C, isolates were grouped into total, HA, and nHA cases. In panels D and E, HA and nHA isolates (panel D) and isolates from each anatomical source (panel E), were grouped into total (all isolates with susceptibility data), MEM/IPM resistant (R) and susceptible (S) isolates. Color key for panels C–E is located in panel C. Error bars represent standard deviations. Multi-year averages for each quarter were compared to corresponding Q1 average by independent t test. *, P < 0 .05; #, P ≤ 0 .001.
We next tested whether Abc seasonality was exhibited in different populations. Both total isolates (n = 1948) and nHA isolates (n = 1202, 61.7%) exhibited annual peaks during Q3 and Q4 (Figure 1B), resulting in a higher proportion of isolates during Q3 and Q4 compared to Q1 and Q2 (Figure 1C). Though the occurrence of HA isolates (n = 746, 38.3%) was indistinguishable between quarters (Figure 1C), MEM/IPM resistance rates among both nHA and HA isolates repeatedly peaked during Q1 and Q2 (Figure 1B). When HA and nHA isolates were grouped according to carbapenem susceptibility, a higher proportion of susceptible HA and nHA isolates occurred in Q3 and Q4, while carbapenem-resistant HA and nHA isolates lacked seasonal variation (Figure 1D). Similarly, when Abc cases were grouped according to anatomic site of isolation, only susceptible isolates exhibited increased occurrence during Q3 and Q4 (Figure 1E). The association between antibiotic susceptibility and increased Q3 and Q4 occurrence was observed regardless of antibiotic class (Supplementary Figure S2). The only exception was ceftriaxone (CRO), where both CRO-resistant and CRO-susceptible isolates displayed higher incidence during Q3 and Q4 compared to Q1 and Q2, though seasonal changes were of lower magnitude among CRO-resistant isolates (Supplementary Figure S2). Lastly, antibiotic resistance rates were lower during Q3 and Q4 compared to Q1 and Q2, regardless of tested antibiotic (Supplementary Figure S3A), classification as HA or nHA, and tissue source (Supplementary Figure S3B).
Discussion
Consistent with prior observations [5], we found that Abc seasonality was restricted to antibiotic-susceptible isolates. Seasonal cycling of antibiotic-susceptible, but not antibiotic-resistant, Abc resulted in seasonal fluctuations in antibiotic resistance rates. This phenomenon held true for all tested antibiotics and among isolates from different epidemiologic compartments. As reported by others [2], no seasonality was detected among HA isolates. Subgroup analysis, however, revealed higher Q3 and Q4 occurrence of antibiotic-susceptible, but not resistant, HA isolates. It is possible that the high proportion of HA resistant isolates masked the seasonality of susceptible HA isolates in this and prior studies.
Seasonal variation was first described in Acinetobacter in the 1970s, and since then has been described in many other Gram-negative and nosocomial infections [4]. The mechanisms mediating seasonality remains unclear. It has been argued that warmer, humid conditions or summer-associated host behaviors lead to a bloom of pathogenic bacteria [1, 2]. However, seasonality has been observed in tropical environments where temperature remains relatively stable throughout the year [7]. Furthermore, climate-dependent models do not explain the lack of seasonality among resistant Abc isolates. Notably, seasonality analyses typically have not controlled for seasonal antibiotic prescribing behaviors. A winter-associated increase in community antibiotic prescriptions has been repeatedly demonstrated [8, 9] and temporally-linked to seasonal increases in resistance rates among common human pathogens [8, 10]. It is possible that the higher use of community antibiotics (eg, tetracyclines, macrolides, sulfonamides, etc.) during winter months indirectly suppresses drug-susceptible organisms, mediating Gram-negative seasonality. This would explain the observation of decreased overall Gram-negative pathogen incidence and increased resistance rates during colder months (and vice versa). Furthermore, in Abc, where resistance to various antibiotics is frequently genetically linked through chromosomal and, to a lesser extent, plasmid-encoded resistance islands [11], pressure from antibiotic use in the community has the potential to select for resistance to multiple other drugs (eg, aminoglycosides, carbapenems, etc.).
Our findings have potentially large clinical and research implications. First, predictable seasonal changes in antibiotic resistance rates can help inform antibiotic choice for Abc infections while awaiting susceptibility testing results. Furthermore, our findings highlight why surveillance studies must account for annual fluctuations in resistance rates. For example, a study on Abc resistance rates analyzing isolates only obtained from June to October may mistakenly underestimate annual resistance rates. Finally, the potential link between community antibiotic practices and multidrug resistance must be further investigated, as establishing this association would emphasize the urgency to restrict unnecessary antibiotic usage, especially during late fall and winter months.
Our study is limited by its retrospective, single-regional healthcare system design and by examination of a single group of Gram-negative pathogens. However, the putative link between antibiotic resistance and seasonality is consistent with prior observations of Abc [2, 5] and other Gram-negative pathogens [8, 10]. In this unadjusted analysis, we cannot exclude unmeasured confounders that could influence the seasonality of drug-resistant phenotypes. Future studies should directly address whether seasonality in other Gram-negative pathogens also is correlated to drug susceptibility and whether selective pressure arising from seasonal antibiotic usage is the principle factor contributing to seasonal trends in incidence and resistance rate. Regardless, our findings suggest that future investigations on pathogen seasonality should control for fluctuations in local antibiotic usage and susceptibility rates.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
The authors would like to thank Dorothy Sinclair and Cherie Hill for their essential and expert contributions in data retrieval for this study.
Financial support. This work was supported by the National Institutes of Health (NIH; grant number T32 AI007172 to J.J.C. and R21 144220 to M.F.F.); and National Center for Advancing Translational Sciences and NIH Roadmap for Medical Research (grant number UL1 TR002345, Sub-Award KL2 TR002346 to J.P.B.). This manuscript’s contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH or NCATS.
Potential conflicts of interest. M.F.F. has been a consultant for Entasis Therapeutics. All other authors: No reported conflict of interests. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Perencevich EN, McGregor JC, Shardell M, et al. Summer peaks in the incidences of Gram-negative bacterial infection among hospitalized patients. Infect Control Hosp Epidemiol 2008; 29:1124–31. [DOI] [PubMed] [Google Scholar]
- 2. Kim YA, Kim JJ, Won DJ, Lee K. Seasonal and temperature-associated increase in community-onset acinetobacter baumannii complex colonization or infection. Ann Lab Med 2018; 38:266–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hoffman-Roberts H, Scoble P, Tabak YP, et al. National prevalence of multidrug-resistant Acinetobacter baumannii infections in the ambulatory and acute care settings, including carbapenem-resistant acinetobacter infections, in the United States in 2015. Open Forum Infect Dis 2016; 3(Suppl 1):1488. [Google Scholar]
- 4. Richet H. Seasonality in Gram-negative and healthcare-associated infections. Clin Microbiol Infect 2012; 18:934–40. [DOI] [PubMed] [Google Scholar]
- 5. Fukuta Y, Clarke LG, Shields RK, et al. Lack of seasonality in the occurrence of multidrug-resistant Acinectobacter baumannii complex. Infect Control Hosp Epidemiol 2012; 33:1051–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Institute CaLS. Performance standards for antimicrobial susceptibility testing. In: CLSI Document M100-S27. 27th ed Wayne, PA: Clinical and Laboratory Standards Institute; 2017. [Google Scholar]
- 7. Gales AC, Jones RN, Forward KR, Liñares J, Sader HS, Verhoef J. Emerging importance of multidrug-resistant Acinetobacter species and Stenotrophomonas maltophilia as pathogens in seriously ill patients: geographic patterns, epidemiological features, and trends in the SENTRY Antimicrobial Surveillance Program (1997–1999). Clin Infect Dis 2001; 32(Suppl 2):S104–13. [DOI] [PubMed] [Google Scholar]
- 8. Sun L, Klein EY, Laxminarayan R. Seasonality and temporal correlation between community antibiotic use and resistance in the United States. Clin Infect Dis 2012; 55:687–94. [DOI] [PubMed] [Google Scholar]
- 9. Durkin MJ, Jafarzadeh SR, Hsueh K, et al. Outpatient antibiotic prescription trends in the United States: a National Cohort Study. Infect Control Hosp Epidemiol 2018; 39:584–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jaffe HW, Zaidi AA, Thornsberry C, Reynolds GH, Wiesner PJ. Trends and seasonality of antibiotic resistance of Neisseria gonorrhoeae. J Infect Dis 1977; 136:684–8. [DOI] [PubMed] [Google Scholar]
- 11. Di Venanzio G, Moon KH, Weber BS, et al. Multidrug-resistant plasmids repress chromosomally encoded T6SS to enable their dissemination. Proc Natl Acad Sci U S A 2019; 116:1378–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


