Abstract
One-day-old broilers were randomly allocated to five treatment groups: basal diet and orally administered sterile saline (negative control, n-control); basal diet challenged with E. coli O78 (positive control, p-control); basal diet supplemented with 1×108 CFU/kg L. plantarum 15–1 and challenged with E. coli O78 (LP); basal diet supplemented with 5 g/kg fructooligosaccharides (FOS) and challenged with E. coli O78 (FOS); and basal diet supplemented with both L. plantarum 15–1 and FOS and challenged with E. coli O78 (LP+FOS). The broilers in the LP, FOS, and LP+FOS groups displayed a decrease of crypt depth at day 14 compared with the control groups. Furthermore, at days 14 and 21, the broilers in the LP group exhibited reduced serum levels of diamine oxidase (DAO) compared with the p-control group (p<0.05), and the broilers in the LP+FOS group showed increased serum concentrations of IgA and IgG relative to both control groups and decreased DAO levels compared with the p-control group (p<0.05). Moreover, the LP group displayed higher levels of acetic acid and total short-chain fatty acids (SCFAs) compared with the p-control group at day 14 (p<0.05), and the FOS group showed higher levels of valeric acid and total SCFAs at day 21 (p<0.05). The LP+FOS group also displayed a higher level of butyric acid at day 14 (p<0.05). In conclusion, dietary supplementation with FOS improved the growth performance, while supplementation with L. plantarum 15–1 and FOS improved intestinal health by increasing the levels of SCFAs and mitigating the damage caused by E. coli O78, thus preventing intestinal damage and enhancing the immune response.
Introduction
Escherichia coli-induced diarrhea has become a global public health problem in both developed and developing countries. At present, the prevention and treatment of this disease is predominantly based on vaccines and drugs. Dietary intervention has also become an important approach [1]. In addition, certain E. coli strains produce the enterotoxin responsible for colibacillosis, which is a major problem in poultry production [2, 3]. In particular, E. coli serotypes O1, O2, O78, O15, and O55 have been associated with colibacillosis in chickens [4, 5]. Among these serotypes, O78 is often associated with E. coli strains causing avian septicemia and was previously demonstrated to contain colonization factors CFA/I [6], which may undermine the immune function to predispose host animals to colonization by the pathogens, representing a threat to health and food safety. Although antibiotic therapy is effective against colibacillosis, the use of antibiotics in poultry is increasingly being limited by restrictions and bans [7]. Possible candidates to replace antibiotics include prebiotics and probiotics, which can prevent and control colibacillosis and thus protect livestock animals. Short-chain fatty acids (SCFAs) produced by the intestinal microbiota are one of the important determinants of the interaction between intestinal microorganisms and pathogenic bacteria [8]. A previous study demonstrated that dietary supplementation with lactulose improved the body weight gain and feed conversion efficiency of 21-day-old broilers but had no effect on the growth performance of 42-day-old broilers. Furthermore, lactulose treatment increased the number of colonies of Lactobacillus in the cecum and the levels of acetic acid, propionic acid, butyric acid, and total SCFAs in the cecum contents of 7-day-old and 42-day-old broilers [9].
Probiotics are defined as live microbial feed supplements that exert a positive influence on the host animal by improving the intestinal microecology [10]. Probiotics help maintain a healthy intestinal microflora and stimulate the immune response of the host animal to suppress the pathogenic microbiota of the gut [11]. An increasing number of well-characterized probiotic strains have been investigated to inhibit pathogenic bacteria and thus maintain a healthy avian intestinal microbiota. In particular, numerous studies have examined the influence of feeding Lactobacillus spp. to broilers on immune function, performance, and pathogen shedding. For example, in vitro experiments using human intestinal Caco-2/TC7 cells and intestinal explants demonstrated that lactobacilli inhibited the TLR4 inflammatory signaling induced by enterotoxigenic E. coli via modulating inflammation and the involvement of TLR2 [12]. Lactobacillus plantarum was also found to inhibit the growth of E. coli O157:H7 in vitro [13] and improve the growth performance, reduce the number of Enterobacteriaceae, and increase the Lactobacillus population, small intestinal villus height, and fecal volatile fatty acid concentration in broilers [14].
Prebiotics are indigestible foods or feed ingredients that positively affect the host by selectively stimulating the growth and activity of one or a limited number of bacteria in the colon [15]. Common prebiotics include fructooligosaccharides (FOS), inulin, galactooligosaccharides (GOS), transgalactooligosaccharides (TOS), and lactulose. The intake of prebiotics can regulate the intestinal microbiota by increasing the population of particular probiotic bacteria, such as Lactobacillus and Bifidobacterium [16], or competing with pathogenic bacteria for attachment sites, thereby reducing the number of pathogenic bacteria in the intestinal tract [17]. Kim et al. investigated the influence of FOS on the growth performance and immune response in broiler chickens [18]. The results revealed that dietary supplementation with 0.25% FOS had a comparable effect to avilamycin, reducing the population of E. coli and increasing the population of lactobacilli. The aim of this study was to investigate whether dietary supplementation with L. plantarum 15–1 and FOS alone or in combination reduces the negative effect on the intestinal morphology and the decline of the immune response induced by E. coli O78.
Materials and methods
Broilers, diets, and experimental design
All animal procedures were approved by the Animal Ethics Committee of the Academy of National Food and Strategic Reserves Administration, Beijing, China (20170052), and performed according to the guidelines recommended in the Guide for the Laboratory Animal Ethical Commission of National Food and Strategic Reserves Administration. In this study, 150 one-day-old male Arbor Acres (AA) broiler chickens with an average body weight of 46.38 ± 0.13 g were used. The broilers were obtained from a commercial hatchery (Huadu Broiler Farms, Beijing, China) and randomly allocated to one of five treatments (five broilers per pen across six pens). The negative control (n-control) broilers were intragastrically administered with sterile saline solution and separated with the rest broiler which challenged with E. coli O78. The broilers were kept in cages with a wire mesh floor and a density of 550 cm2/broiler. Room temperature was set to 32±2°C for the first week and gradually reduced to 24°C by the end of the third week.
The diets fed to the five groups were as follows: 1) the n-control and p-control groups were fed a basal diet without any additives; 2) the LP group was fed a diet supplemented with L. plantarum 15–1 (1×108 CFU/kg of feed); 3) the FOS group was fed a diet supplemented with FOS (5 g/kg of feed); and 4) the LP+FOS group was fed a diet supplemented with both L. plantarum 15–1 and FOS (1×108 CFU/kg and 5 g/kg of feed, respectively). Powdered L. plantarum 15–1 was added to the basal diet to a final colony count of 1×108 CFU/kg of feed. FOS was purchased from BaoLing Bao Biology (Shandong, China, purity>95%). The components of the basal diet are summarized in Table 1. The basal diet was based on the Chinese Feeding Standard for chickens [19], was free of antibiotics, and met the nutritional requirements for starter feed (1–21 days) for chickens.
Table 1. Composition of basal diet.
| Item | Content |
|---|---|
| Ingredient (%) | |
| Corn | 55.75 |
| Soybean meal | 36.75 |
| Soybean oil | 2.96 |
| Calcium phosphate | 1.86 |
| Limestone | 1.2 |
| Sodium chloride | 0.35 |
| Lysine | 0.309 |
| Solid methionine | 0.287 |
| Threonine | 0.05 |
| Choline chloride(50%) | 0.26 |
| Minerals premix1 | 0.2 |
| Vitamin premix2 | 0.02 |
| Total | 100 |
| Calculated nutrient composition | |
| ME, kcal/kg | 3200 |
| Crude protein % | 22.16 |
| Ca % | 1.07 |
| Available P % | 0.68 |
| Lys % | 1.32 |
| Methionine | 0.48 |
1The vitamin premix provided the following per kilogram of diet: vitamin A, 9,500 IU; vitamin D3, 62.50 μg; vitamin K3, 2.65 mg; vitamin B12, 0.025 mg; vitamin B2, 6 mg; vitamin E, 30 IU; biotin, 0.0325 mg; folic acid, 1.25 mg; pantothenic acid, 12 mg; nicotinic acid, 50 mg.
2The mineral premix provided the following per kilogram of diet: Cu, 8 mg; Zn, 75 mg; Fe, 80 mg; Mn, 100 mg; Se, 0.15 mg; I, 0.35 mg.
Bacterial preparation and oral challenge
The strain of L. plantarum 15–1 used in this study was obtained from the Academy of National Food and Strategic Reserves Administration, Beijing, China. L. plantarum 15–1 was added to the basal diet as a freeze-dried powder to a final concentration of 1×108 CFU/kg. The number of colonies in the freeze-dried powder was determined by counting the number of colonies grown on plates. Briefly, 10 g of freeze-dried powder was added to 90 mL of sterile water and mixed thoroughly, the resulting mixture was diluted 1:10, and 100 μL aliquots of the dilution were plated evenly onto MRS agar (De Man, Rogosa, Sharpe). The plates were then incubated at 37°C for 10–12 h and the colonies on the plates were counted. The number of colony-forming units per gram was calculated based on the sample dilution.
E. coli O78 was obtained from the College of Animal Science and Technology, China Agricultural University, Beijing, China. The serotype of E. coli O78 was confirmed by the China Institute of Veterinary Drug Control, Beijing, China. The strain was aerobically cultured in Luria–Bertani (LB) broth for 18–24 h at 37°C with shaking at 160 rpm. A gradient dilution series (1:100) was plated evenly on LB solid media in sterile plates under sterile conditions. The plates were incubated at 35–37°C for 18–24 h and the colonies of E. coli O78 were counted [20]. The concentration after overnight culture was 3×108 CFU/mL. After performing a 1:3 dilution, 1×108 CFU of E. coli O78 was orally administered to the back of the oral cavity of each challenged chicken at seven days old using a sterile syringe once a day for three days.
Growth performance
Broiler chickens were casually selected and sacrificed at days 14 and 21 after fasting for 12 h, and the body weight and feed intake of each broiler were recorded on a cage-by-cage basis. The average daily gain (ADG) and average daily feed intake (ADFI) during days 1–14, 14–21, and 1–21 and the bursal index at days 14 and 21 were calculated. The mortality was calculated over the course of the experiment.
Sample collection
At days 14 and 21, blood samples were collected from the wing veins after fasting for 7–8 h. For each treatment, six broilers were randomly chosen from each cage and slaughtered by jugular bleeding. The bursa of Fabricius above the cloaca was weighed. A 1-cm section was cut from the center of the jejunum and fixed with formaldehyde solution for morphological examination. The cecal content was collected aseptically in sterile plastic tubes, quickly frozen in liquid nitrogen, and stored at −80°C until use.
Jejunal morphology
The jejunal tissue samples were gently rinsed with 0.9% NaCl and then fixed in 10% buffered formalin. After fixation, the samples were embedded in paraffin, cut into 2–5 μm slices, mounted on slides, and stained using hematoxylin and eosin [21]. Complete intestinal villi were selected and the villus height and crypt depth were measured. The villus height was measured from the villus basal lamina to the villus apex, and the crypt depth was measured between the base (which is by the villus height end) and the crypt/villus transition zone [22].
IgA, IgG, and Diamine Oxidase (DAO)
Blood samples were collected from the wing vein for the quantification of IgA, IgG, and DAO. After serum separation and centrifugation at 10000 ×g for 4 min, the samples were stored at −20°C. The serum concentrations of IgA, IgG, and DAO were determined by enzyme-linked immunosorbent assay (ELISA) using the Standard Chicken Kit (Nanjing Jiancheng Institute of Biological Engineering, Nanjing, China) following the manufacturer’s instructions. Briefly, the standard substance and samples were diluted to 100 μL and added into the wells and the plate was incubated at 37°C for 1 h. Next, the plate was washed three times. Next, the biotin–antibody conjugate was added into each well, the plate was incubated at 37°C for 60 min then washed three times, anti-chicken horseradish peroxidase (HRPO) was added into the wells, and the plate was incubated at 37°C for 30 min. 3,3ʹ,5,5ʹ-Tetramethylbenzidine substrate solution was added into every well and incubated at 37°C for no more than 30 min. The product concentration was then measured spectrophotometrically at 450 nm. The regression equation of the standard curve was determined from the standard concentrations and OD values, and the OD values measured for the samples were substituted into this equation to calculate the sample concentrations. All of the measurements were performed under the same conditions to minimize inter-assay variation.
Cecal short-chain fatty acids
The SCFA concentrations were determined using the method of Schäfer [23] with some modifications. Frozen cecal digesta samples were thawed at 4°C and diluted five-fold with double-distilled water in sterile screw-cap tubes, homogenized, and centrifuged (Centrifuge 5810R, Eppendorf, Hamburg, Germany) at 10000 rpm for 10 min. 2-Ethylbutyric acid (17.01 mmol/L) was used as an internal standard. The concentrations of acetate, propionate, butyrate, valerate, isovalerate, and isobutyrate in the samples were determined using a gas chromatography (GC) system (7890B, Agilent) equipped with a flame ionization detector (FID) and a DB-FF column (30 m × 0.25 mm, 0.5 μm particle diameter, Agilent Technologies, USA). Nitrogen was supplied at a flow rate of 40 mL/min as a carrier gas. The initial oven temperature was 80°C, which was maintained for 0.5 min, and the temperature was then increased to 130°C at 5°C/min and held for 2 min, then increased to 240°C at 20°C/min and held for 1 min. The temperatures of the FID and injection port were 270 and 200°C, respectively. The flow rates of hydrogen and air as the fuel gas and oxidant gas were 40 and 450 mL/min, respectively. The GC analysis was performed using an injection volume of 1 μL and a detection time of 19 min per sample. The SCFA concentration was calculated by multiplying the raw data by the dilution factor.
Statistical analysis
Statistical analyses were performed using SPSS for Windows. Data were analyzed using multivariate one-way ANOVA for the following parameters: ADG, ADFI, bursal index, jejunal villus height, crypt depth, immune response, and SCFA concentrations were statistical analysis, n = 6. Differences in the effects of L. plantarum 15–1 and FOS were analyzed using single degree of freedom contrast statements comparing the broilers challenged with E. coli O78 (p-control) with the unchallenged group (n-control) from 0 to 21 d. Differences were considered significant at p<0.05.
Results
Growth performance and survival
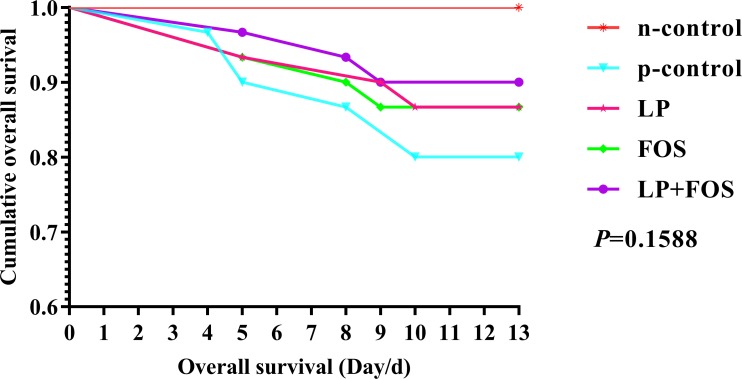
The growth performance of the broilers is shown in Table 2. Challenge with E. coli O78 lowered the average weight at day 21 and the ADFI was decreased (p<0.001). Moreover, the ADG of broiler of FOS group showed no difference compared with n-control in 14–21 days and the whole period, which may demonstrated that FOS could alleviate the negative effect of E. coli O78 on the ADG to some extent. Moreover, FOS improved the bursal index compared with the p-control (p<0.05) and no difference was observed relative to the n-control. These data demonstrate that challenge with E. coli O78 caused the broilers to lose weight and damaged the bursa of Fabricius. Furthermore, Kaplan–Meier curves were plotted to examine the survival of the broilers during the period of challenging with E. coli O78; the results demonstrated that L. plantarum 15–1 and the combination of L. plantarum 15–1 and FOS reduced the mortality relative to the p-control group but did not result in zero mortality as with the n-control group (Fig 1).
Table 2. Influence of dietary supplementation on performance and mortality of broilers.
| Parameter | Treatment (E. coli O78 challenge) | SEM | P-value | ||||
|---|---|---|---|---|---|---|---|
| n-control | p-control | LP | FOS | LP+FOS | |||
| Average weight (kg) | |||||||
| 14 days | 0.32 | 0.30 | 0.30 | 0.30 | 0.30 | 0.01 | 0.65 |
| 21 days | 0.78a | 0.59b | 0.61b | 0.68ab | 0.59b | 0.05 | 0.03 |
| ADG (g/d) | |||||||
| 1–14 days | 23.75 | 22.37 | 22.30 | 21.99 | 22.12 | 0.96 | 0.70 |
| 14–21 days | 91.65a | 68.32b | 70.80b | 79.20ab | 67.28b | 5.61 | 0.03 |
| 1–21 days | 49.62a | 39.87b | 40.78b | 43.79ab | 39.32b | 2.58 | 0.05 |
| ADFI (g/d) | |||||||
| 1–14 days | 30.87 | 28.57 | 28.00 | 27.76 | 28.79 | 0.99 | 0.99 |
| 14–21 days | 102.92A | 56.43B | 61.20B | 66.20B | 58.77B | 6.84 | 0.0003 |
| 1–21 days | 95.94A | 63.79B | 67.14B | 74.2B | 64.04B | 5.73 | 0.003 |
| Bursal index (g.kg-1) | |||||||
| 14 days | 2.86 | 2.41 | 1.98 | 2.02 | 2.28 | 0.27 | 0.25 |
| 21 days | 2.40a | 1.51c | 1.76bc | 2.32ab | 2.07abc | 0.20 | 0.20 |
| Mortality (%) | |||||||
| 1–21 days | 0.00 | 13.33 | 10.00 | 13.33 | 10.00 | NA | NA |
ADFI: average daily feed intake. ADG: average daily gain. NA: not available. Mortality: mortality after challenging with E. coli O78.
a, b, c means p<0.05, n = 6.
A, B means p<0.001, n-control (broilers fed with basal diet and orally administered sterile saline); p-control (broilers fed with basal diet and orally administered E. coli O78); LP (broilers fed with basal diet supplemented with 1×108 CFU/kg L. plantarum 15–1 and orally administered E. coli O78); FOS (broilers fed with basal diet supplemented with 5 g/kg FOS and orally administered E. coli O78); LP+FOS (broilers fed with basal diet supplemented with 1×108 CFU/kg L. plantarum 15–1 and 5 g/kg FOS and orally administered E. coli O78).
Fig 1. Kaplan–Meier curves showing survival of the broilers following E. coli O78 challenge for 13 days, n = 30.
Jejunal morphology
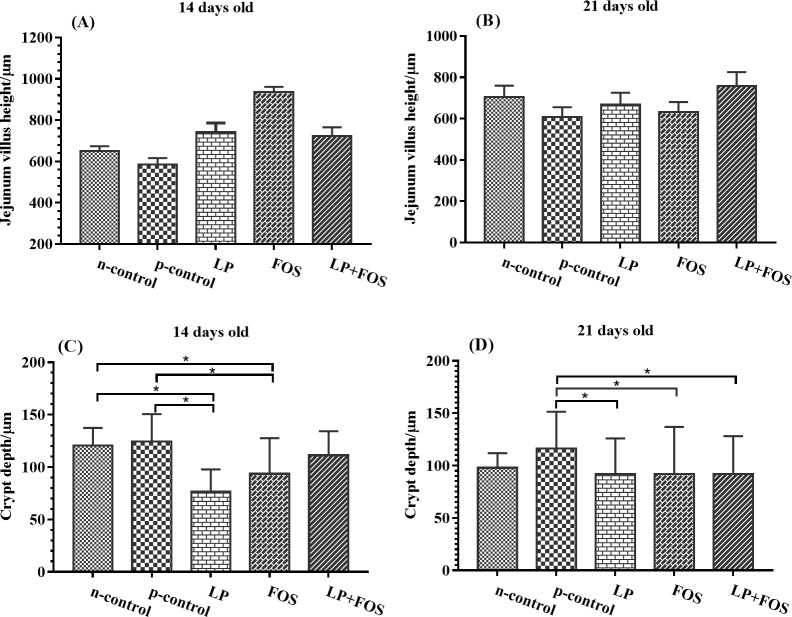
Supplementation with L. plantarum 15–1 or FOS decreased the crypt depth at day 14 relative to the n-control and p-control groups (Fig 2C, p<0.05). At day 21, a reduced crypt depth displayed in LP, FOS and LP+FOS group in comparison with the p-control group (p<0.05) but exhibited no difference relative to the n-control group (Fig 2D). No other significant differences were observed.
Fig 2. Influence of dietary supplementation on jejunal morphology of broilers after E. coli O78 challenge.
(A) The villus height of broiler in 14 days old. (B) The villus height of broiler in 14 days old. (C) The crypt depth of broiler in 14 days old. (D) The crypt depth of broiler in 21 days old. * indicates p<0.05.
Immune responses
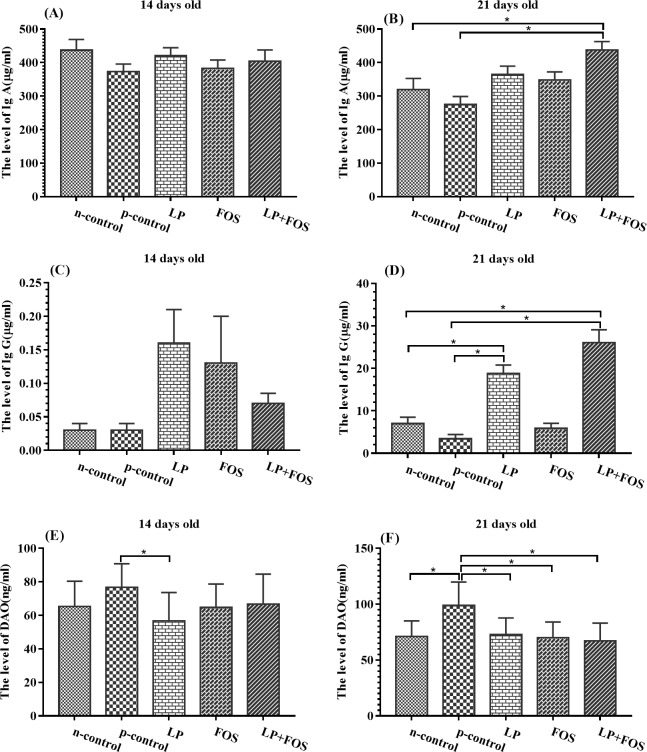
The effects of L. plantarum 15–1 and FOS on the sero-immunity levels are presented in Fig 3. The level of DAO was reduced in LP group compared with the p-control group, whereas the other groups exhibited no differences at day 14 (Fig 3E, p<0.005). Moreover, at 21 day, the level of IgA and IgG was increased in LP+FOS group in relative with n-control and p-control (Fig 3B, p<0.005), and IgG was reduced in LP compared with n-control and p-control (Fig 3D, p<0.005). In addition, the level of DAO was decreased in p-control in relative with other four groups at day 21 (Fig 3F, p<0.005).
Fig 3. Influence of dietary supplementation on serum concentrations of IgA, IgG, and DAO in broilers.
(A) The level of IgA in 14 days old. (B) The level of IgA in 21 days old. (C) The level of IgG in 14 days old. (D) The level of IgG in 21 days old. (E) The level of DAO in 14 days old. (F) The level of DAO in 21 days old. * indicates p<0.05.
Cecal short-chain fatty acids
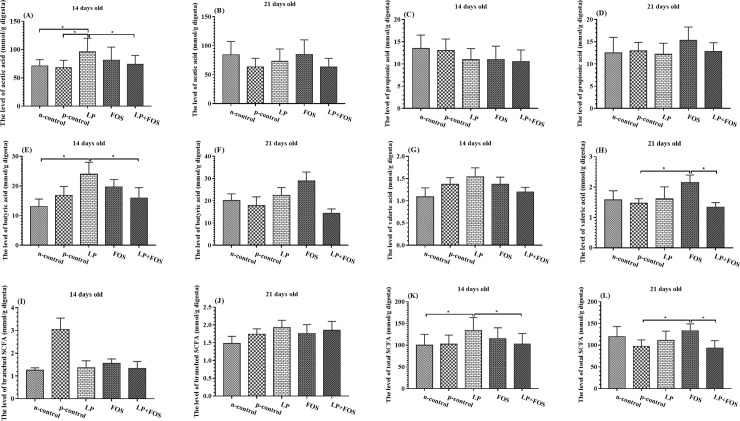
The cecal SCFA content was analyzed using gas chromatography after thawing the samples at 4°C. The results demonstrated that the level of caecal acetic acid/butyric acid (Fig 4A and 4E) was increased in LP group in relative to n-control and p-control/n-control respectively at 14 days old (p<0.05). Moreover, the concentration of valeric acid and the total SCFA was increased in FOS group in comparison with p-control at 21 days old (Fig 4H and 4L, p<0.05).
Fig 4. Influence of dietary supplementation on caecal SCFA levels of broilers at 14 and 21 days of age.
(A) The level of acetic acid in 14 days old. (B) The level of acetic acid in 21 days old. (C) The level of propionic acid in 14 days old. (D) The level of propionic acid in 21 days old. (E) The level of butyric acid in 14 days old. (F) The level of butyric acid in 21 days old. (G) The level of valeric acid in 14 days old. (H) The level of valeric acid in 21 days old. (I) The level of branched SCFA in 14 days old. (J) The level of branched SCFA in 21 days old. (K) The level of total SCFA in 14 days old. (L) The level of total SCFA in 21 days old. * indicates p<0.05.
Discussion
Reducing the immune and intestinal damage caused by pathogenic E. coli is of great significance for improving poultry health. FOS are conducive to animal growth and provide resistance to pathogenic bacteria. FOS are a preferred substrate for improving the growth of Bifidobacteria and promoting its association with the mucous membranes of the host, which hinders the binding of pathogenic bacteria to the intestinal tract [24]. The current results suggest that L. plantarum 15–1 and FOS can ameliorate the intestinal damage induced by E. coli O78 and increase the immune response, such as by increasing the levels of IgA and IgG and reducing the serum concentration of DAO. Moreover, L. plantarum 15–1 and FOS increased the SCFA levels in the cecal contents, which may help resist the invasion of pathogenic bacteria. These results indicate that L. plantarum 15–1 and FOS could help maintain the health status of broilers.
E. coli is associated with the deterioration of animal health, including weight loss, diarrhea, mortality, and necrotizing enteritis [25]. Research by Porcheron has demonstrated that FOS may be capable of regulating an extra-intestinal pathogenic E. coli strain, and this property is associated with a gene cluster called the fos locus, which plays a major role in intestinal colonization [26]. These results support the idea that the probiotic bacteria of the microbiota can metabolize in the intestine and decrease the population of pathogenic bacteria. However, no significant differences were observed in terms of the growth performance. The same findings have also been reported in other studies; dietary supplementation with 0.5% FOS did not influence on the growth performance of broilers [18]. In contrast, some studies have reported that FOS enhanced the feed efficiency (FCR) but reduced the feed intake and daily live weight gain in the absence of challenge with E. coli [27]. G.-B. Kim et al. reported that supplementation with 0.25% FOS improved the growth performance of broilers at 28 days [18]. Furthermore, Xu et al. found that FOS increased the body weight and feed conversion ratio, and this effect was considerably greater in three-week-old animals than in one-week-old animals [28]. These findings may indicate that it takes time for the beneficial microorganisms to utilize the FOS and become the dominant microflora to maintain the balance of the intestinal microbial environment and improve the growth of broilers challenged with E. coli. The results of the present study were in agreement with those of Xu et al. Numerous bacteria have been used as probiotics, including Lactobacillus, Leuconostoc, Pediococcus, Bifidobacterium and Enterococcus, which promote the growth of animals [29, 30]. Peng et al. reported that the use of Lactobacillus plantarum B1 led to higher ADG during the finisher period [14]. Saffar et al. examined the use of probiotics to reduce ascites in broilers in high-altitude areas, and the results revealed that probiotics reduce the ascites mortality has a role in promoting, but not to compensate for the growth of a strong impact [31]. This study indicated that L. plantarum and FOS improved the performance of broilers and reduced the mortality after challenging with E. coli O78. Moreover, L. plantarum 15–1 and FOS treatment decreased the crypt depth at days 14 and 21. However, there are no differences in body weight compared with the p-control group.
In poultry, a healthy intestinal tract is essential for absorbing nutrients and providing a barrier against pathogenic bacteria. Awad et al. reported that Lactobacilli exert a positive effect on the gastrointestinal tract as they increase feed consumption and nutrient absorption from the intestines [32]. The intestines are an important site of nutrient absorption, and the absorption efficiency is associated with the surface area due to an increasing villus and mucosa thickness [33]. Saffar et al. examined the use of probiotics to reduce ascites in broilers in high-altitude areas, and the results revealed that probiotics reduce the ascites mortality has a role in promoting, but not to compensate for the growth of a strong impact [34]. Pan et al. reported that challenge with enterotoxigenic E. coli K88 led to atrophy of the villi and destruction of the intestinal morphology, whereas supplementation with the probiotic (Bacillus licheniformis and Saccharomyces cerevisiae) protected the intestinal barrier function from the E. coli K88, indicating that probiotics could potentially replace antibiotics in the treatment of diarrhea [35]. Awad et al. reported that dietary supplementation with Lactobacillus increased the villus height and villus height:crypt depth ratio in broilers [36], and Song et al. reported that supplementation with a mixed probiotic consisting of Lactobacillus plantarum, Bacillus licheniformis, and Bacillus subtilis increased the jejunal villus height and decreased small intestinal coliforms [37]. The present study demonstrates that the addition of L. plantarum 15–1 to a basal diet improves the jejunal tissue morphology.
The serum level of DAO reflects the structure, function, and integrity of the small intestinal mucosa. Under normal circumstances, the serum level of DAO is very low, but it increases after damage to the intestinal mucosa [38]. Several studies have demonstrated that the consumption of probiotics and prebiotics improves host immunity by increasing the concentration of IgA. C.H. Kim et al. found that dietary supplementation with FOS increased the IgA levels in laying hens [39]. In contrast, G.-B. Kim et al. reported no difference in the plasma concentrations of IgA and IgG between broilers fed a diet supplemented with FOS and other treatment groups [18]. Maragkoudakis et al. also reported no differences in the plasmas levels of IgA, IgM, and IgG in goats upon dietary supplementation with FOS [40], and Gürbüz et al. reported no differences in the concentrations of IgA, IgM, and IgG in horses [41]. In this study, dietary supplementation with L. plantarum 15–1, FOS, and their combination increased the serum levels of IgA and IgG at day 21 and decreased the DAO levels.
SCFAs are the pivotal metabolite during the microbial fermentation of indigestible carbohydrates in the large intestine and are used by the colonic mucosa to enhance the epithelial barrier [14, 42]. Lactic acid bacteria enhance the physical barrier of the host intestine by increasing the concentration of SCFAs, as high levels of SCFAs inhibit the growth and reproduction of pathogenic bacteria. SCFAs are the final products of microbial fermentation and are absorbed through the colonic mucosa [43]. SCFAs are ideal biorenewable chemicals for inhibiting E. coli that function by chemically damaging the cell membrane of the pathogen [44]. The application of Lactobacillus reuteri DPC16 enhances the level of SCFAs, thus increasing the intestinal acidity, which is conducive to reducing the pathogen population in the gut of chickens [45]. Butyrate is also a direct source of energy for the colonic epithelium, possesses anti-inflammatory properties, and can enhance the colonic defense barrier [46]. Peng et al. reported that L. plantarum B1 increased the SCFA levels in the cecum [47]. In this study, dietary supplementation with L. plantarum 15–1, FOS, or both led to increased levels of total SCFAs, especially acetic acid and butyric acid at day 14 and valeric acid at day 21. This may partially account for the mortality reduction upon administration of probiotics. However, it is notable that one study reported that synbiotics containing two or more substances did not induce an additive effect with respect to growth performance, intestinal microbial population, or SCFA levels [48]. This discrepancy may originate from the different properties of prebiotics and probiotics.
In conclusion, dietary supplementation with FOS and L. plantarum 15–1 improved the intestinal morphology, enhanced the immune response, and increased the SCFA concentration in the cecum in broilers challenged with E. coli O78. Moreover, supplementation with L. plantarum 15–1 and FOS had no effect on growth performance but decreased the mortality of the challenged broilers. These results indicate that dietary supplementation with FOS and L. plantarum 15–1 may ameliorate the negative effects of E. coli O78.
Supporting information
(XLSX)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This study was supported by National Natural Science Foundation of China (No. 31772642, 31672457), Beijing Agriculture Innovation Consortium 04-2018 (BAIC04-2018), National Key Research and Development Program of China (2016YFD0500504, 2016YFD0501201), Ministry of Agricultural of the People's Republic of China (2015-Z64, 2016-X47), and Hunan Provincial Science and Technology Department (2017NK2322, 2016NK2101, 2016WK2008, 2016TP2005) to SD. The authors acknowledge the academy of science and technology of state administration of grain for their support to the study.
References
- 1.Zhang W, Sack DA. Current Progress in Developing Subunit Vaccines against Enterotoxigenic Escherichia coli-Associated Diarrhea. Clinical and vaccine immunology: CVI. 2015;22(9):983–91. Epub 2015/07/03. 10.1128/CVI.00224-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lau GL, Sieo CC, Tan WS, Hair-Bejo M, Jalila A, Ho YW. Efficacy of a bacteriophage isolated from chickens as a therapeutic agent for colibacillosis in broiler chickens. Poultry science. 2010;89(12):2589–96. Epub 2010/11/16. 10.3382/ps.2010-00904 . [DOI] [PubMed] [Google Scholar]
- 3.Cao GT, Zeng XF, Chen AG, Zhou L, Zhang L, Xiao YP, et al. Effects of a probiotic, Enterococcus faecium, on growth performance, intestinal morphology, immune response, and cecal microflora in broiler chickens challenged with Escherichia coli K88. Poultry science. 2013;92(11):2949–55. Epub 2013/10/19. 10.3382/ps.2013-03366 . [DOI] [PubMed] [Google Scholar]
- 4.Han Y, Liu Q, Willias S, Liang K, Li P, Cheng A, et al. A bivalent vaccine derived from attenuated Salmonella expressing O-antigen polysaccharide provides protection against avian pathogenic Escherichia coli O1 and O2 infection. Vaccine. 2018. [DOI] [PubMed] [Google Scholar]
- 5.Ghunaim H, Abu-Madi MA, Kariyawasam S. Advances in vaccination against avian pathogenic Escherichia coli respiratory disease: potentials and limitations. Veterinary microbiology. 2014;172(1–2):13–22. Epub 2014/06/01. 10.1016/j.vetmic.2014.04.019 . [DOI] [PubMed] [Google Scholar]
- 6.Lundgren A, Bourgeois L, Carlin N, Clements J, Gustafsson B, Hartford M, et al. Safety and immunogenicity of an improved oral inactivated multivalent enterotoxigenic Escherichia coli (ETEC) vaccine administered alone and together with dmLT adjuvant in a double-blind, randomized, placebo-controlled Phase I study. Vaccine. 2014;32(52):7077–84. Epub 2014/12/03. 10.1016/j.vaccine.2014.10.069 . [DOI] [PubMed] [Google Scholar]
- 7.Maron DF, Smith TJ, Nachman KE. Restrictions on antimicrobial use in food animal production: an international regulatory and economic survey. Globalization and health. 2013;9:48 Epub 2013/10/18. 10.1186/1744-8603-9-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Budden KF, Gellatly SL, Wood DL, Cooper MA, Morrison M, Hugenholtz P, et al. Emerging pathogenic links between microbiota and the gut-lung axis. Nature reviews Microbiology. 2017;15(1):55–63. Epub 2016/11/01. 10.1038/nrmicro.2016.142 . [DOI] [PubMed] [Google Scholar]
- 9.Calik A, Ergun A. Effect of lactulose supplementation on growth performance, intestinal histomorphology, cecal microbial population, and short-chain fatty acid composition of broiler chickens. Poultry science. 2015;94(9):2173–82. Epub 2015/07/19. 10.3382/ps/pev182 . [DOI] [PubMed] [Google Scholar]
- 10.Patel S, Goyal A. Functional oligosaccharides: production, properties and applications. World Journal of Microbiology and Biotechnology. 2011;27(5):1119–28. [Google Scholar]
- 11.Lee YK, Mazmanian SK. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science (New York, NY). 2010;330(6012):1768–73. Epub 2011/01/06. 10.1126/science.1195568 ; PubMed Central PMCID: PMCPmc3159383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finamore A, Roselli M, Imbinto A, Seeboth J, Oswald IP, Mengheri E. Lactobacillus amylovorus inhibits the TLR4 inflammatory signaling triggered by enterotoxigenic Escherichia coli via modulation of the negative regulators and involvement of TLR2 in intestinal Caco-2 cells and pig explants. PLoS One. 2014;9(4):e94891 10.1371/journal.pone.0094891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tshabalala PA, de Kock HL, Buys EM. Survival of Escherichia coli o157:h7 co-cultured with different levels of pseudomonas fluorescens and lactobacillus plantarum on fresh beef. Brazilian journal of microbiology: [publication of the Brazilian Society for Microbiology]. 2012;43(4):1406–13. Epub 2013/09/14. 10.1590/s1517-838220120004000023 ; PubMed Central PMCID: PMCPmc3769013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peng Q, Zeng XF, Zhu JL, Wang S, Liu XT, Hou CL, et al. Effects of dietary Lactobacillus plantarum B1 on growth performance, intestinal microbiota, and short chain fatty acid profiles in broiler chickens. Poultry science. 2016;95(4):893–900. Epub 2016/01/17. 10.3382/ps/pev435 . [DOI] [PubMed] [Google Scholar]
- 15.Rehman H, Vahjen W, Kohl-Parisini A, Ijaz A, Zentek J. Influence of fermentable carbohydrates on the intestinal bacteria and enteropathogens in broilers. World's poultry science journal. 2009;65(1):75–90. [Google Scholar]
- 16.Gourbeyre P, Denery S, Bodinier M. Probiotics, prebiotics, and synbiotics: impact on the gut immune system and allergic reactions. Journal of leukocyte biology. 2011;89(5):685–95. 10.1189/jlb.1109753 [DOI] [PubMed] [Google Scholar]
- 17.Song J, Jiao L, Xiao K, Luan Z, Hu C, Shi B, et al. Cello-oligosaccharide ameliorates heat stress-induced impairment of intestinal microflora, morphology and barrier integrity in broilers. Animal feed science and technology. 2013;185(3):175–81. [Google Scholar]
- 18.Kim GB, Seo YM, Kim CH, Paik IK. Effect of dietary prebiotic supplementation on the performance, intestinal microflora, and immune response of broilers. Poultry science. 2011;90(1):75–82. Epub 2010/12/24. 10.3382/ps.2010-00732 . [DOI] [PubMed] [Google Scholar]
- 19.NY/T 33-. (2004). Nutrient requirements of Chinese feeding standard of chicken. Beijing: The Ministry of Agriculture of the People’s Republic of China.
- 20.Wiegand I, Hilpert K, Hancock RE. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nature protocols. 2008;3(2):163 10.1038/nprot.2007.521 [DOI] [PubMed] [Google Scholar]
- 21.Luna LG. Manual of histologic staining methods of the Armed Forces Institute of Pathology. 1968.
- 22.Liu Y, Huang J, Hou Y, Zhu H, Zhao S, Ding B, et al. Dietary arginine supplementation alleviates intestinal mucosal disruption induced by Escherichia coli lipopolysaccharide in weaned pigs. British Journal of Nutrition. 2008;100(3):552–60. 10.1017/S0007114508911612 [DOI] [PubMed] [Google Scholar]
- 23.Shang HM, Song H, Shen SJ, Yao X, Wu B, Wang LN, et al. Effects of dietary polysaccharides from the submerged fermentation concentrate of Hericium caput-medusae (Bull.:Fr.) Pers. on fat deposition in broilers. Journal of the science of food and agriculture. 2015;95(2):267–74. Epub 2014/04/29. 10.1002/jsfa.6711 . [DOI] [PubMed] [Google Scholar]
- 24.Sugiharto S. Role of nutraceuticals in gut health and growth performance of poultry. Journal of the Saudi Society of Agricultural Sciences. 2016;15(2):99–111. [Google Scholar]
- 25.Wejryd E, Marti M, Marchini G, Werme A, Jonsson B, Landberg E, et al. Low Diversity of Human Milk Oligosaccharides is Associated with Necrotising Enterocolitis in Extremely Low Birth Weight Infants. Nutrients. 2018;10(10). Epub 2018/10/24. 10.3390/nu10101556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Porcheron G, Kut E, Canepa S, Maurel MC, Schouler C. Regulation of fructooligosaccharide metabolism in an extra‐intestinal pathogenic Escherichia coli strain. Molecular microbiology. 2011;81(3):717–33. 10.1111/j.1365-2958.2011.07725.x [DOI] [PubMed] [Google Scholar]
- 27.Williams J, Mallet S, Leconte M, Lessire M, Gabriel I. The effects of fructo-oligosaccharides or whole wheat on the performance and digestive tract of broiler chickens. British poultry science. 2008;49(3):329–39. Epub 2008/06/24. 10.1080/00071660802123351 . [DOI] [PubMed] [Google Scholar]
- 28.Xu Z, Hu C, Xia M, Zhan X, Wang M. Effects of dietary fructooligosaccharide on digestive enzyme activities, intestinal microflora and morphology of male broilers. Poultry science. 2003;82(6):1030–6. 10.1093/ps/82.6.1030 [DOI] [PubMed] [Google Scholar]
- 29.Allen HK, Levine UY, Looft T, Bandrick M, Casey TA. Treatment, promotion, commotion: antibiotic alternatives in food-producing animals. Trends in microbiology. 2013;21(3):114–9. Epub 2013/03/12. 10.1016/j.tim.2012.11.001 . [DOI] [PubMed] [Google Scholar]
- 30.Awad WA, Ghareeb K, Abdel-Raheem S, Bohm J. Effects of dietary inclusion of probiotic and synbiotic on growth performance, organ weights, and intestinal histomorphology of broiler chickens. Poultry science. 2009;88(1):49–56. Epub 2008/12/20. 10.3382/ps.2008-00244 . [DOI] [PubMed] [Google Scholar]
- 31.Khajali F, Saffar A. Application of Meal Feeding and Skip-A-Day Feeding With or Without Probiotics for Broiler Chickens Grown at High-Altitude to Prevent Ascites Mortality. American Journal of Animal and Veterinary Sciences. 2010;5(1). [Google Scholar]
- 32.Awad W, Ghareeb K, Böhm J. Effect of addition of a probiotic micro‐organism to broiler diet on intestinal mucosal architecture and electrophysiological parameters. Journal of animal physiology and animal nutrition. 2010;94(4):486–94. 10.1111/j.1439-0396.2009.00933.x [DOI] [PubMed] [Google Scholar]
- 33.Koropatkin NM, Cameron EA, Martens EC. How glycan metabolism shapes the human gut microbiota. Nature reviews Microbiology. 2012;10(5):323–35. Epub 2012/04/12. 10.1038/nrmicro2746 ; PubMed Central PMCID: PMCPmc4005082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saffar. Application of Meal Feeding and Skip-A-Day Feeding With or Without Probiotics for Broiler Chickens Grown at High-Altitude to Prevent Ascites Mortality. American Journal of Animal and Veterinary Sciences. 2010;5(1):13–9. 10.3844/ajavsp.2010.13.19 [DOI] [Google Scholar]
- 35.Pan L, Zhao P, Ma X, Shang Q, Xu Y, Long S, et al. Probiotic supplementation protects weaned pigs against enterotoxigenic K88 challenge and improves performance similar to antibiotics. Journal of Animal Science. 2017;95(6):2627–39. 10.2527/jas.2016.1243 [DOI] [PubMed] [Google Scholar]
- 36.Awad W, Ghareeb K, Abdel-Raheem S, Böhm J. Effects of dietary inclusion of probiotic and synbiotic on growth performance, organ weights, and intestinal histomorphology of broiler chickens. Poultry science. 2009;88(1):49–56. 10.3382/ps.2008-00244 [DOI] [PubMed] [Google Scholar]
- 37.Song J, Xiao K, Ke Y, Jiao L, Hu C, Diao Q, et al. Effect of a probiotic mixture on intestinal microflora, morphology, and barrier integrity of broilers subjected to heat stress. Poultry science. 2014;93(3):581–8. 10.3382/ps.2013-03455 [DOI] [PubMed] [Google Scholar]
- 38.Çakmaz R, Büyükaşık O, Kahramansoy N, Erkol H, Çöl C, Boran Ç, et al. A combination of plasma DAO and citrulline levels as a potential marker for acute mesenteric ischemia. Libyan Journal of Medicine. 2013;8(1):20596 10.3402/ljm.v8i0.20596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim C, Shin K, Woo K, Paik I. Effect of dietary oligosaccharides on the performance, intestinal microflora and serum immunoglobulin contents in laying hens. Korean Journal of Poultry Science. 2009;36(2):125–31. [Google Scholar]
- 40.Maragkoudakis PA, Mountzouris KC, Rosu C, Zoumpopoulou G, Papadimitriou K, Dalaka E, et al. Feed supplementation of Lactobacillusplantarum PCA 236 modulates gut microbiota and milk fatty acid composition in dairy goats—a preliminary study. International journal of food microbiology. 2010;141:S109–S16. 10.1016/j.ijfoodmicro.2010.03.007 [DOI] [PubMed] [Google Scholar]
- 41.Gürbüz E, Inal F, Ata SU, ÇİTİL ÖB, KAV K, Küçükkaya F. Effects of supplemental fructo-oligosaccharide and mannan-oligosaccharide on nutrient digestibilities, volatile fatty acid concentrations, and immune function in horses. Turkish Journal of Veterinary and Animal Sciences. 2010;34(1):39–44. [Google Scholar]
- 42.Reigstad CS, Salmonson CE, Rainey JF 3rd, Szurszewski JH, Linden DR, Sonnenburg JL, et al. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2015;29(4):1395–403. Epub 2015/01/01. 10.1096/fj.14-259598 ; PubMed Central PMCID: PMCPmc4396604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tremaroli V, Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489(7415):242–9. 10.1038/nature11552 . [DOI] [PubMed] [Google Scholar]
- 44.Royce LA, Liu P, Stebbins MJ, Hanson BC, Jarboe LR. The damaging effects of short chain fatty acids on Escherichia coli membranes. Applied microbiology and biotechnology. 2013;97(18):8317–27. 10.1007/s00253-013-5113-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bian L, Molan A-L, Maddox I, Shu Q. Antimicrobial activity of Lactobacillus reuteri DPC16 supernatants against selected food borne pathogens. World Journal of Microbiology and Biotechnology. 2010;27(4):991–8. 10.1007/s11274-010-0543-z [DOI] [Google Scholar]
- 46.Eeckhaut V, Van Immerseel F, Croubels S, De Baere S, Haesebrouck F, Ducatelle R, et al. Butyrate production in phylogenetically diverse Firmicutes isolated from the chicken caecum. Microbial biotechnology. 2011;4(4):503–12. 10.1111/j.1751-7915.2010.00244.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peng Q, Zeng X, Zhu J, Wang S, Liu X, Hou C, et al. Effects of dietary Lactobacillus plantarum B1 on growth performance, intestinal microbiota, and short chain fatty acid profiles in broiler chickens. Poultry science. 2016;95(4):893–900. 10.3382/ps/pev435 [DOI] [PubMed] [Google Scholar]
- 48.Mookiah S, Sieo CC, Ramasamy K, Abdullah N, Ho YW. Effects of dietary prebiotics, probiotic and synbiotics on performance, caecal bacterial populations and caecal fermentation concentrations of broiler chickens. Journal of the science of food and agriculture. 2014;94(2):341–8. Epub 2013/09/17. 10.1002/jsfa.6365 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.