Abstract
We show that in Dictyostelium discoideum an endogenous gene as well as a transgene can be silenced by introduction of a gene construct that is transcribed into a hairpin RNA. Gene silencing was accompanied by the appearance of sequence-specific RNA ∼23mers and seemed to have a limited capacity. The three Dictyostelium homologues of the RNA-directed RNA polymerase (RrpA, RrpB, and DosA) all contain an N-terminal helicase domain homologous to the one in the dicer nuclease, suggesting exon shuffling between RNA-directed RNA polymerase and the dicer homologue. Only the knock-out of rrpA resulted in a loss of the hairpin RNA effect and simultaneously in a loss of detectable ∼23mers. However, ∼23mers were still generated by the Dictyostelium dsRNase in vitro with extracts from rrpA−, rrpB−, and DosA− cells. Both RrpA and a target gene were required for production of detectable amounts of ∼23mers, suggesting that target sequences are involved in ∼23mer amplification.
INTRODUCTION
RNA interference by double-stranded RNA (dsRNA) has been applied efficiently to silence genes in various organisms ranging from fungi and nematodes to plants. dsRNA has been introduced into organisms by microinjection (Fire et al., 1998), by transformation with gene constructs generating complementary RNAs or fold-back RNA (Waterhouse et al., 1998; Wang et al., 2000), or by feeding an organism with Escherichia coli-expressing dsRNA (Kamath et al., 2000). There is increasing evidence that the antisense strand in the duplex determines the sequence-specific degradation of the target mRNA but only in regions of sequence homology to the dsRNA (Hammond et al., 2000; Yang et al., 2000). In silenced Drosophila and plants, ∼23mers have been identified as products of hairpin RNA (RNAi), which hybridize to both the sense and the antisense strand. It has been shown that in vitro the target mRNA is degraded to ∼23mers (Zamore et al., 2000), but also the initial dsRNA or an amplification product of it apparently serves as a precursor for ∼23mers (Fleenor et al., 2000). A nuclease containing a ∼23mer guide RNA has been proposed to mediate sequence-specific mRNA degradation (Yang et al. 2000) and appears to be part of a complex termed RNA-induced silencing complex (RISC) (Hammond et al., 2000). However, this nuclease activity is not responsible for the generation of ∼23mers from dsRNA. Bernstein et al. (2001) have shown in Drosophila that a complex containing the RNase “dicer” and RISC are distinct entities and can be separated by high-speed centrifugation.
Only recently, the small RNAs have been unambiguously characterized as 21 and 22mers (Elbashir et al., 2001b), and it has been demonstrated that synthetic double-stranded 21mers can confer gene silencing in mammalian cells (Elbashir et al., 2001a).
We have previously identified a dsRNase that processes dsRNA to ∼23mers in vitro but does not by itself display single-stranded RNase activity (Novotny et al. 2001). This large (∼450 kDa) complex may be the Dictyostelium equivalent of the Drosophila dicer complex.
In a search of genes required for the RNAi mechanism, several RNA helicases have been identified (e.g., Wu-Scharf et al., 2000). In addition, the RNA-directed RNA polymerase (RdRP) has been found to be necessary for RNAi in Caenorhabditis elegans (Smardon et al., 2000) and Arabidopsis (Dalmay et al., 2000) and for quelling in Neurospora (Cogoni and Macino, 1999).
Here we show that RNAi mediates posttranscriptional gene silencing (PTGS) in Dictyostelium and that the knock-out of one of three RdRP homologues, RrpA, is sufficient to impair the mechanism. Sequence-specific ∼23mers are found in silenced Dictyostelium cells in vivo, and these products are similar in size to the ∼23mers generated in vitro by the partially purified Dictyostelium dsRNase.
MATERIALS AND METHODS
Dictyostelium AX2 cells and transformants were grown in association with Klebsiella aerogenes in suspension culture or on plates or in AX2 medium. Development was done in phosphate buffer suspension (Spudich, 1987). Dictyostelium transformation, was carried out as described previously (Nellen et al. 1987; Howard et al., 1988). Transformation with vectors containing the G418 resistance cassette resulted in multicopy tandem integration into the genome, whereas transformation with the blasticidin resistance cassette gave single- or low-copy integration. Cotransformation was done as described by Nellen and Firtel (1985).
Transformants were subcloned on a lawn of K. aerogenes and then grown in plates (Costar, Cambridge, MA). For RNAi analysis, populations of primary transformants and several individual clones were assayed.
Total cellular RNA was prepared as detailed by Maniak et al. (1989); enrichment for small RNA, RNA PAGE, blotting, and hybridization was done according to the method of Hamilton and Baulcombe (1999). For Northern blots and slot blots on total cellular RNA, 10 μg were either separated on a 1.2% agarose gel containing 20 mM guanidiniumthiocyanate and blotted to a nylon membrane or directly applied to the membrane using a vacuum slot blot device. For slot blotting, RNA was dissolved in 20 mM 3-(N-morpholino)propanesulfonic acid, 8 mM sodium acetate, 1 mM EDTA, 7% (vol/vol) formaldehyde, 50% (vol/vol) deionized formamide, and bromophenol blue. Prehybridization and hybridization were carried out as described by Crowley et al. (1985). Radioactively labeled in vitro transcripts were used as probes. In vitro transcription was carried out with T7 and SP6 RNA polymerase as described by Weber and Gross (1997). Reverse transcription (RT)-PCR on rrpA and rrpB was done with 3 μg of total RNA from wild-type AX2, RrpA−, and RrpB− cells. The RNA was treated with DNase before cDNA synthesis to eliminate genomic DNA contaminations. For first-strand synthesis the oligonucleotide (GAAATCACCAATATAAACCAACTGATC) that binds 3′ in both Rrp genes was used. The final amplification was done with primer pair B (5′- primer: GAAGACGAGGAAGCAGAGTTCATTATAAC, 3′ primer: GAAATCACCAATATAAACCAACTGATC). Amplification products of the two genes could be distinguished by ClaI cleavage. For semiquantitative RT-PCR on rrpA, equal amounts of total RNA from the RrpB− strain were used either in a multiplex PCR with additional primers for thioredoxin 1 (see below) or in parallel PCRs. 16 RT-PCR on β-galactosidase (β-gal) was done with 1 μg of total RNA. The RNA was treated with DNase to eliminate genomic DNA contaminations. For the first-strand synthesis the oligo (CCGCTCGAGATCTATAGCTGAATTGTTGGCTATACG) that binds to a 3′ sequence tag in the β-gal reporter construct was used. The final amplification was done with primer pair C (5′ primer: TAACGAGCTCCTGCACTGGATGG, 3′ primer: CCGCTCGAGATCTATAGCTGAATTGTTGGCTATACG). As a control we performed RT-PCR on thioredoxin 1 mRNA with oligo (CGCGGATCCTTATTT-GTTTGCTTCTAGAGTACTTC) for first-strand synthesis and primer pair D (5′ primer GAACGAGCTCCATGGCCAATAGAGTAATTCATG, 3′ primer CGCGGATCCTTATTTGTTTGCTTCTAGAGTACTTC) for the PCR reaction.
Western blotting and detection of discoidin was done as described by Wetterauer et al. (1993) using the monoclonal antibody 80–52-13 and a phosphatase-coupled secondary antibody.
Vector Constructs
Fragments of β-gal and discoidin genes for RNAi and antisense constructs were obtained by PCR including suitable restriction sites. β-Gal was expressed from the actin 6 promoter either in the pGem 7z vector for cotransformation experiments or in vectors containing a BSR cassette (Sutoh, 1993) when selection for blasticidin resistance was possible.
The pV18gal-i vector was generated by replacing the discoidin promoter in pVEII (Blusch et al., 1992) for the V18 promoter (Ken and Singleton, 1994). The first β-gal fragment of 815 bp was fused in sense orientation to the V18 promoter; the second fragment of 1326 bp was ligated tail to tail to the first fragment. The additional 511 bp constitute the predicted hairpin loop in the fold-back transcript.
The discoidin RNAi construct was introduced into the pDneo2 vector (Witke et al., 1987) with loop and dsRNA sizes of 259 and 509 bp, respectively.
DosA replacement and rrpA and rrpB disruptions were done by homologous recombination in AX3 (for DosA) and AX2 (for rrpA and rrpB), essentially as described by Witke et al. (1987). The BSR selection cassette was flanked by arms of 1.8 and 2.5 kbp for DosA, 798 and 982 bp for rrpA, and 586 and 1191 bp for rrpB. Clones were picked and analyzed by PCR.
PCR was performed on genomic DNA of potential rrpA and rrpB disruption clones using primer pair A (5′-primer: CGCTACTTCTACTAATTCTAGA, 3′-primer: GAAATCACCAATATAAACCAACTGATC) in which one primer binds within the coding sequence of the BSR cassette and one in identical sequence regions of the rrpA and rrpB genes outside the recombinogenic arm. Positive clones were further analyzed with primer pair B (5′- primer: GAAGACGAGGAAGCAGAGTTCATTATAAC, 3′ primer: GAAATCACCAATATAAACCAACTGATC), which bind within identical coding sequences of the rrpa and rrpB genes that flank the BSR cassette. Under the conditions used, PCR did not proceed across the inserted BSR cassette. Therefore, only products of nondisrupted genes were obtained. Amplification products of rrpA and rrpB could be distinguished by cutting with ClaI and EcoRV. ClaI cleaved the rrpA but not the rrpB PCR product and EcoRV cleaved the rrpA PCR product twice and rrpB product once.
β-Gal Assays
β-Gal assays were done essentially as described by Dingermann et al. (1990). Briefly, cells were harvested, washed with phosphate buffer, and lysed in assay buffer (60 mM Na2HPO4, 40 mM NaH2PO4, 10 mM KCl, 1 mM MgSO4, and 7 ml/l mercaptoethanol) by freezing in liquid nitrogen and thawing at 37°C. Cell debris was removed by centrifugation at 10,000 × g. The supernatant was incubated with o-nitrophenyl-d-galactoside at 37°C. The reaction was stopped with 0.5 volume of 1 M Na2CO3. β-Gal activity was measured photometrically at 420 nm and standardized to the protein concentration of the sample. Activities are given in units per milligram of total protein. One unit is the amount of enzyme that produces 1 nmol o-nitrophenol/min at 37°C. U/mg = (E420 × 1.7 × D)/(0.0045 × t × c), where t is time in minutes, D is dilution of protein sample in the assay, and c is concentration of protein sample in mg/ml.
dsRNase Preparation and In Vitro Assay
dsRNase was prepared as detailed by Novotny et al. (2001). For Figure 5B, a preparation purified by three column steps was used, whereas for Figure 6 crude extracts were used. For the experiment shown in Figure 6, 350 μg of protein cell extract were incubated for 3 h at room temperature with 13 ng of 32P-labeled 260-bp PSV-A dsRNA (131 kBq/μg) or with 40 ng of 32P-labeled 400-bp β-gal dsRNA (13 kBq/μg) in 1× assay buffer (50 mM Tris-HCl, pH 8.0, 25 mM KCl, 5 mM MgCl2, 2 mM dithiothreitol, 250 μg/ml tRNA, and 15% glycerol). Because of lower specific activity, a higher amount of the β-gal substrate was used. After phenol/chloroform extraction, the assay was precipitated with 3 volumes of 100% ethanol and washed with 70% ethanol. The products were separated on an 8 M urea PAGE and analyzed with a Fuji X BAS 1500 (Raytest, Straubenhardt, Germany) bioimaging analyzer after 4 h of exposure. The amount of ∼23mers was quantified with the TINA software (Raytest).
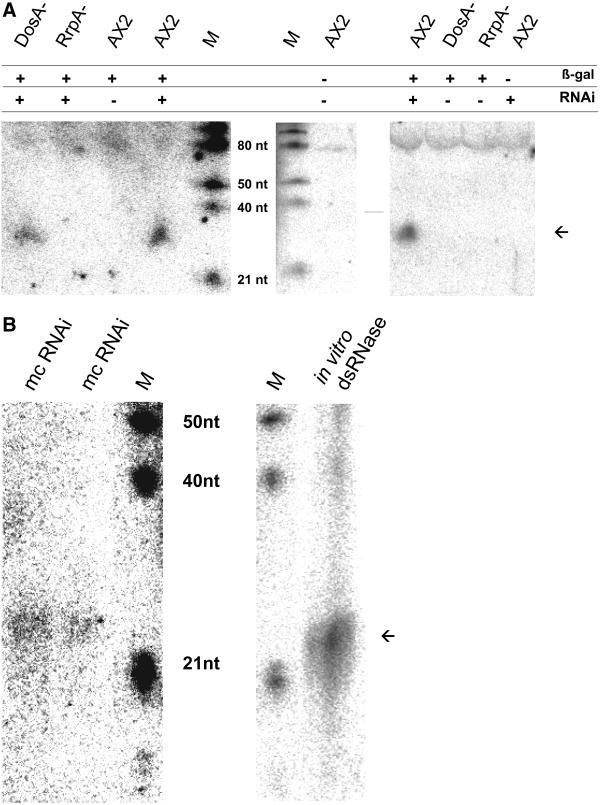
Figure 5.
(A) Low molecular weight RNAs from silenced and nonsilenced strains. Low molecular weight RNA was isolated from the strains indicated, blotted, and hybridized with a 32P-labeled β-gal sense probe. The position of ∼23mers is indicated by an arrowhead. (B) 32P-Labeled dsRNA was generated by in vitro transcription of a PSV-A gene fragment and incubated with a partially purified dsRNase preparation from Dictyostelium. Reaction products were separated by PAGE in parallel with small RNA isolated from RNAi-silenced β-gal cells. RNA was blotted and hybridized with a 32P-labeled β-gal probe. The blot was exposed for 1 wk (left) and over night (right).
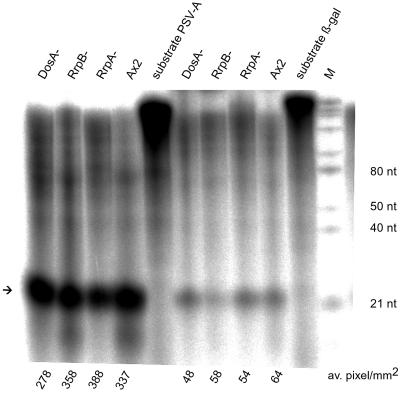
Figure 6.
In vitro assays with dsRNase preparations from various strains on a 32P-labeled in vitro prepared PSV-A dsRNA gene fragment and a 32P-labeled β-gal dsRNA fragment. Crude extract (350 μg) was incubated with 13 ng of PSV-A or 40 ng of β-gal dsRNA. ∼23mers are indicated by an arrow. ∼23mers were quantified from four independent assays by the TINA program (Raytest,). Averages are given in pixels per square millimeter below the lanes.
Sequence Alignments
Multiple alignments were done with the MultAlign interface (Corpet, 1988) and the LALIGN program (accessed at: http://www.expasy.ch/tools/).
Sequence data for D. discoideum chromosome 6 were obtained from The Sanger Center website at http://www.sanger.ac.uk/Projects/D.discoideum/. Sequencing of D. discoideum chromosome 6 was accomplished as part of the EUDICT consortium with support by The European Union.
Further sequence data were obtained from the Genome Sequencing Center, Jena, website at http://genome.imb-jena.de/dictyostelium. The German part of the D. discoideum Genome Project is carried out by the Institute of Biochemistry I, Cologne, and the Genome Sequencing Center, Jena, with support by the Deutsche Forschungsgemeinschaft. (grant 113/10-1 and 10-2).
The gene sequences are derived from unfinished contigs. They have been mapped by restriction analysis but may still contain sequencing errors.
Accession Numbers
QDE-1 (Neurospora crassa): CAB42634, DosA (D. discoideum): AAD29638.1, CAF (A. thaliana): AAF03534, Ego1 (C. elegans): AAF80367, Dicer (Drosophila melanogaster): AAF56056, Dicer (C. elegans): P34529, RrpA (D. discoideum): AJ314909, RrpB (D. discoideum): AJ314910, DrnA (D. discoideum): AJ314911.
RESULTS
PTGS by RNAi
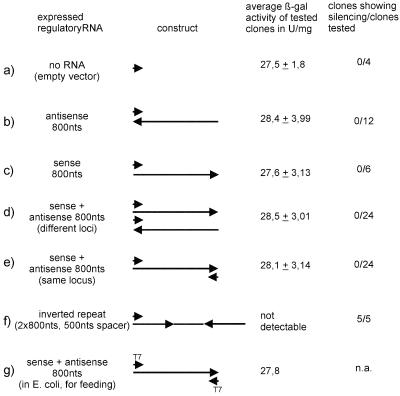
To test whether PTGS by RNA interference is functional in Dictyostelium, we examined gene silencing by dsRNA using different methods and constructs, starting with a transgenic strain expressing β-gal from the actin 6 promoter with an average reporter activity of 26.6 ± 3.5 U/mg protein. As shown in Figure 1, introduction of a 807-bp-long antisense construct targeted against the first 800 nucleotide-coding region of the β-gal mRNA did not result in efficient silencing (Figure 1b), even though antisense RNA is functional in many cases (e.g., Crowley et al., 1985). Transformation of the same fragment in sense orientation and cotransformation of the sense and antisense construct were not successful either (Figure 1, c and d). Similarly, the insertion of a 827-bp β-gal fragment (same sequence as in the sense and antisense constructs) between two promoters (actin 15 and V18) was not effective (Figure 1e). We then tried a construct containing inverted repeats of the same β-gal sequences that we used in the two-promoter construct separated by an ∼500-bp spacer (also consisting of β-gal sequences) under the control of the V18 promoter (Figure 1f). The transcript was expected to fold into a stem-loop structure consisting of 802 bp of dsRNA and a 538-base hairpin loop. With this, expression of β-gal could be reduced to undetectable levels. RT-PCR on β-gal mRNA in the silenced strain yielded no detectable product, whereas the unsilenced strain showed the expected PCR product (Martens, Novotny, Oberstrass, Postlethwait, and Nellen, unpublished results). Using a 100-bp inverted repeat—hairpin loop—construct resulted in some reduction (∼50%) of total β-gal activity (Martens, Novotny, Oberstrass, Postlethwait, and Nellen, unpublished results). Feeding Dictyostelium cells with E. coli cells expressing β-gal dsRNA from a two-promoter construct (both T7) also targeted against the first 800 nucleotides of the β-gal mRNA was not successful (Figure 1g).
Figure 1.
Constructs used for β-gal gene silencing and their efficiency. In the first two lanes, the construct introduced into the cells is described. In the third lane, the average β-gal activity and SD of randomly chosen tested clones are given in units per milligram of total protein. The last lane shows how many clones have been tested and how many of them exhibited gene silencing. As (a) a control an empty vector was introduced; (b) an antisense copy (800-bp fragment) of the transgene was introduced (V18 promoter), (c) an additional copy (800-bp fragment) of the transgene controlled by the V18 promoter was introduced (equivalent to cosuppression approaches in plants), (d) sense and antisense RNA both under the control of the V18 promoter were expressed from different gene constructs in the same vector, (e) the 800-bp gene fragment was inserted between two opposing promoters (V18 and actin15), (f) an 800-bp inverted repeat with a 500-bp linker, corresponding to β-gal gene sequences, was fused to the V18 promoter, and (g) an 800-bp fragment was inserted between two T7 promoters and transformed bacteria (strain BL21) were used to feed Dictyostelium cells. Only in e was silencing of the transgene observed.
RNAi-mediated Silencing of Endogenous Genes
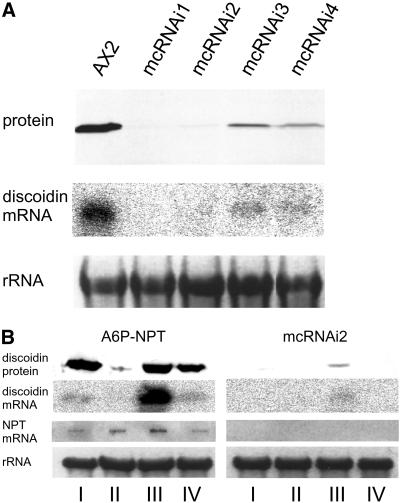
To demonstrate that endogenous genes can be silenced by RNAi, we made a similar construct expressing a stem-loop RNA from the actin 6 promoter directed against the discoidin gene family. Nine clones were randomly chosen and assayed for discoidin expression. Five of them exhibited complete and four exhibited partial discoidin silencing. Similar to antisense experiments (Crowley et al., 1985), it appeared that the entire discoidin gene family was affected. On the RNA level, discoidin transcripts were not detectable in cells exhibiting complete silencing and were strongly reduced in cell lines with partial silencing (Figure 2A). Surprisingly, the RNAi effect appeared to be reduced on the protein level in developing cells (Figure 2B). When these cells were transferred back to axenic medium, the RNAi effect was again observed to the same extent (Figure 2B). This demonstrated that cells were not reprogrammed and that the RNAi machinery as well as the RNAi construct was still functional. Examination of steady-state levels revealed that discoidin mRNA was 35-fold enhanced in developing cells compared with axenic growth, whereas the protein levels were similar (Figure 2B). This suggested that the RNAi machinery was saturated and possibly not capable of eliminating the high amounts of discoidin RNA in development. Alternatively, the RNAi mechanism could be developmentally regulated. To rule out that incomplete silencing was due to different expression levels of dsRNA, we tested for expression of a transgene (neomycine phosphotransferase, NPT) by the actin 6 promoter. NTP mRNA levels varied slightly under the different growth or developmental conditions and were rather higher (2.5-fold) in developing cells compared with axenic growth (Figure 2B). In colony blots, discoidin expression levels in developing cells were almost indistinguishable from the wild type (Martens, Novotny, Oberstrass, Postlethwait, and Nellen, unpublished results).
Figure 2.
(A) Western blot with a discoidin-specific antibody on protein isolated from wild-type AX2 cells (control) and from four of nine randomly chosen clones silenced by discoidin RNAi (top). mcRNAi1 and 2 display complete silencing, and mcRNAi 3 and 4 display partial silencing. A Northern blot with RNA from the same cell lines, hybridized with a discoidin-specific probe, is shown in the middle. As a control for equal loading, ethidium bromide-stained large rRNA is shown in the bottom. The RNAi construct was introduced into cells via a multicopy vector (mc). (B) Protein and RNA were isolated from an AX2 strain carrying the G418 resistance gene under control of the actin 6 promoter (A6P-NPT) and from the discoidin RNAi strain (mcRNAi2) expressing the RNAi from the same promoter grown in axenic medium to a density of 2 × 106 cells/ml (I), grown in bacterial suspension to a density of 3 × 106 cells/ml (II), grown in bacterial suspension to a density of 3 × 106 cells/ml and then developed in shaking culture for 5 h (III), and grown in axenic culture to a density of 2 × 106 cells/ml after passage through bacterial growth and development for 5 h (IV). First line, cellular protein separated by SDS-PAGE, blotted, and probed for discoidin. Second line, Northern blot probed for discoidin mRNA. Third line, slot blot probed for NPT mRNA. Fourth line, ethidium bromide-stained large rRNA as a loading control.
RrpA Is Required for RNAi in Dictyostelium
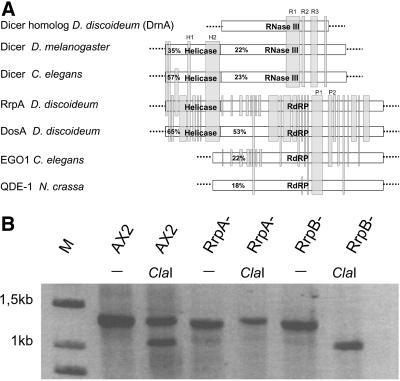
It has been shown that the RdRP is required for PTGS in Neurospora (Cogoni and Macino, 1999), Arabidopsis (Dalmay et al., 2000), and C. elegans (Tabara et al., 1999; Catalanotto et al., 2000; Smardon et al., 2000). A search in the Dictyostelium genome data base revealed three RdRP-related genes. RrpA and RrpB are closely related and differ by only 49 amino acids (<3%) in the available sequence, whereas DosA is less conserved. Nevertheless, the gene product is clearly identified as an RdRP homologue and shows similarity to all RdRPs from other organisms (Figure 3A). Interestingly, all three RdRPs have an N-terminal extension that shows good homology to the helicase domains in the Drosophila and C. elegans dicer nucleases and the plant CAF protein (see DISCUSSION). This N-terminal part is separated by an intron from the RdRP homology domain. The intron in rrpA and/or rrpB has been identified by cDNA sequencing (Martens, Novotny, Oberstrass, Postlethwait, and Nellen, unpublished results).
Figure 3.
(A) Schematic comparison of the Dictyostelium RrpA and DosA genes with RdRP genes from C. elegans and N. crassa and of a Dictyostelium dicer homologue gene with dicer from Drosophila and C. elegans. Shaded boxes exhibit at least 65% similarity to the Dictyostelium genes. Overall similarities to the corresponding Dictyostelium genes are given in percentages. The alignments are not exactly drawn to scale. Complete alignments are available as supplementary material on the web. Characteristic homology boxes in the RNaseIII domain (R), the helicase domain (H), and the RdRP domain (P) are marked; amino acid positions refer to the Dictyostelium proteins RrpA and dicer homologue: R1, LGDS domain, AA 907–969; R2, EALIG domain, AA 975–994; R3, DAVL domain, AA 1063–1103; H1, PRIL domain, AA 187–197; H2, VLEE domain, AA 441–500; P1, SGSD domain, AA 1306–1370; P2: SDQY domain, AA 1415–1454. (B) RT-PCR reactions with total RNA from AX2 wild type, RrpA−, and RrpB− cells. The resulting PCR products were separated on a 0.8% agarose gel before and after ClaI cleavage.
rrpA, rrpB, and DosA were disrupted by homologous recombination, and the knock-outs were confirmed by PCR and restriction analysis. Transcription of rrpA and rrpB was demonstrated by RT-PCR in the wild-type, the RrpA−, and the RrpB− strains. Figure 3B shows that both genes were transcribed in the wild-type strain, whereas in the knock-out strains only the undisrupted gene was expressed. RT-PCR products from rrpA and rrpB transcripts were distinguished by ClaI cleavage. Pretreatment of RNA with DNase confirmed that the RT-PCR products were derived from RNA and not from contaminating DNA (Martens, Novotny, Oberstrass, Postlethwait, and Nellen, unpublished results). Expression levels of both genes were too low to be detected by Northern blots.
The disruption strains and the parent AX2 strain were examined for RNAi function using the β-gal gene (Table 1) and the discoidin gene family as targets (Figure 4). β-Gal activity was measured by o-nitrophenyl-d-galactoside hydrolysis (Dingermann et al., 1990). Discoidin expression was quantified by Western blotting.
Table 1.
Silencing efficiency in wild type and RdRP mutant cells
| Host strain transformation | AX2
|
RrpA−
|
DosA−
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β-Gal + control
|
β-Gal +
mcRNAi
|
β-Gal + control
|
β-Gal +
mcRNAi
|
β-Gal + control
|
β-Gal +
mcRNAi
|
|||||||
| Clone 1A | 128 | Clone 2A | 6,1 | Clone 3A | 378 | Clone 4A | 10,2 | Clone 5A | 1257 | Clone 6A | 4,4 | |
| Clone 1B | 383 | Clone 2B | 8,9 | Clone 3B | 255 | Clone 4B | 74 | Clone 5B | 90 | Clone 6B | 8,8 | |
| Clone 1C | 457 | Clone 2C | 1,0 | Clone 3C | 23,2 | Clone 4C | 24,3 | Clone 5C | 1024 | Clone 6C | 14,9 | |
| Clone 1D | 155 | Clone 2D | 0,6 | Clone 3D | 246 | Clone 4D | 343 | Clone 5D | 233 | Clone 6D | 3,6 | |
| Clone 1E | 293 | Clone 3E | 26 | Clone 4E | 89 | Clone 5E | 838 | Clone 6E | 1,8 | |||
| Clone 3F | 334 | Clone 4F | 163 | Clone 5F | 413 | Clone 6F | 22,6 | |||||
| Clone 3G | 83 | Clone 4G | 656 | Clone 5G | 1184 | Clone 6G | 5,3 | |||||
| Clone 3H | 319 | Clone 4H | 16,4 | Clone 5H | 1271 | Clone 6H | 1,11 | |||||
| Clone 3I | 24 | Clone 4I | 140 | Clone 5I | 1063 | Clone 6I | 23,0 | |||||
| Clone 3J | 200 | Clone 4J | 509 | Clone 5J | 663 | Clone 6J | 4,6 | |||||
| Population | 316 | Population | 8,2 | Population | 210 | Population | 253 | Population | 419 | Population | 15,1 | |
| Average β-gal activity (U/mg) clones + pop. | 289 ± 128 | 5 ± 4 | 191 ± 132 | 207 ± 214 | 769 ± 428 | 9, 6 ± 8 | ||||||
| Unpaired t test p value | 0,0008 | 0,83 | 0,0001 | |||||||||
| Average reduction by RNAi | 98% | 0% | 99% | |||||||||
Figure 4.
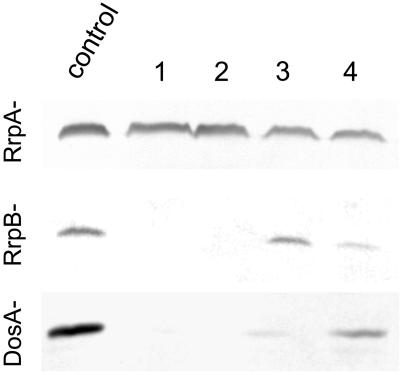
A discoidin Western blot is shown from four of six independent clones, each of the RrpA−, RrpB−, and DosA− transformants carrying the discoidin RNAi construct. Clones in lane 1 and 2 display complete silencing in RrpB− and DosA−, whereas clones in lanes 3 and 4 show only partial silencing. No significant reduction of discoidin levels was observed in RrpA− transformants.
Gene silencing by RNAi was assayed in the following ways: The β-gal vector was cotransformed together with the RNAi construct into the knock-out strains (RrpA−, DosA−) and, for comparison, also into the AX2 wild type. As shown in Table 1, an average of 98–99% RNAi mediated gene silencing of total β-gal activity was observed in the DosA−, and the wild-type strain, whereas no silencing was found for the RrpA− strain. The high variability in β-gal expression is due to the cotransformation method, which may result in different copy number integrations of both vectors at different sites in the genome of the tested clones. However the p values calculated from an unpaired t test show the significance of the data (p values: 0.0008 for AX2, 0.0001 for DosA−, and 0.83 for RrpA−; see also Table 1).
As another example, the wild-type strain AX2 and the knock-out strains (RrpA−, RrpB−, DosA−) were transformed with the discoidin RNAi construct and assayed for discoidin expression. RNAi-mediated silencing of the discoidin gene family is shown in Figure 4. In the RrpA− strain, none of six independent clones exhibited any gene silencing, whereas in both the RrpB− and the DosA− strain three of six clones each showed complete and the others partial gene silencing. Thus, RrpB− and DosA− cells were susceptible to RNAi to the same extent as wild-type cells (see also Figure 2A).
To address the question whether differential expression of RrpA was responsible for incomplete silencing of discoidin in development, the RrpB− strain was assayed for transcription of rrpA by semiquantitative RT-PCR. No significant changes were detectable under the different growth and developmental conditions, which were used for the experiment in Figure 2B (Martens, Novotny, Oberstrass, Postlethwait, and Nellen, unpublished results).
Gene Silencing Is Accompanied by the Production of ∼23mers
In several organisms, it has been shown that RNA interference is accompanied by the production of ∼23mers of the RNAi and/or the target gene. To test this, we hybridized a β-gal sense probe to enriched small RNA isolated from various cell lines. As shown in Figure 5A, β-gal–specific antisense ∼23mers were found in strains with, but not in the strains without, RNAi. Hybridization with a β-gal antisense probe yielded similar amounts of sense ∼23mers, whereas no ∼23mers were detectable with a β-gal probe that was not covered by the RNAi construct (Martens, Novotny, Oberstrass, Postlethwait, and Nellen, unpublished results). In the rrpA knock-out mutant, ∼23mers were not found.
No ∼23mers were observed in a strain containing only the RNAi construct but not the β-gal target gene. When this cell line was subsequently transformed with the β-gal reporter construct, no reporter activity was detected in the transformed population or in six randomly chosen clones (background activity below 2 U/mg). The strain was thus predisposed to silence the newly introduced transgene.
Extracts from the rrpA, the rrpB, and the DosA Knock-Out Strains Generate ∼23mers In Vitro
We have previously described a dsRNase from Dictyostelium that specifically digests any dsRNA to fragments of ∼23 nucleotides or base pairs (Novotny et al. 2001). It was of interest to examine whether these products could be related to the ∼23mers observed in RNAi silenced strains. Figure 5B shows an in vitro assay of partially purified dsRNase on a 260-bp dsRNA substrate from the PSV-A gene (Sadiq et al., 1994). The digestion products of the dsRNase are very similar in size to the ∼23mers found in vivo in RNAi silenced cells, which are shown for comparison in the adjacent lane of the gel. We therefore suggest that the Dictyostelium dsRNase is involved in the generation of RNAi-mediated ∼23mers. The lack of in vivo ∼23mers in the RrpA− strain raised the question whether extracts from these cells were able to produce ∼23mers from dsRNA in vitro. Figure 6 shows that crude dsRNase extracts from all mutant cell lines had similar activities on both PSV-A and β-gal dsRNA, thus demonstrating that none of the three RdRP homologues per se was required for the production of ∼23mers in vitro.
DISCUSSION
RNA interference proved to be functional in Dictyostelium when constructs transcribing inverted repeats separated by an unpaired loop were stably transformed into the cells. Double promoters and feeding of bacteria expressing sense and antisense RNA from a target gene did not result in silencing in the experiments performed here.
The rrpA gene, one of three homologues to RdRP, was strictly required for RNAi, whereas knock-outs of rrpB and dosA had no obvious effect on the mechanism. This was surprising because rrpB differed from rrpA in only 49 of 1780 amino acids within the known sequence. The possibility that rrpB was a nontranscribed pseudogene could be ruled out since RT-PCR products were detected. Both genes are transcribed at very low levels and could not be shown by Northern blotting.
As in other organisms, RNA interference resulted in the production of sequence-specific siRNAs (small interfering RNAs).
The RNAi construct alone (without the target gene) did not show any detectable ∼23mers in the wild type in vivo and the same was true for RNAi plus target in RrpA− cells. In both cases, however, ∼23mers were found in the in vitro assay. Because we assume that the dsRNase generates RNAi ∼23mers in vivo, we have to conclude that detection of ∼23mers requires amplification by RrpA. The RNAi supplied in vivo is therefore not sufficient to generate detectable ∼23mers and most likely not a target for RdRP. We propose that interaction between RNAi and the target mRNA is necessary to initiate the amplification process by RrpA and that amplification is required for efficient gene silencing. This hypothesis is supported by the following observation: Large quantities of antisense ∼23mers detected in silencing strains can obviously not be degradation products of mRNA, and they can also not be (exclusively) derived from the RNAi because they are not seen in strains with only the RNAi construct. Small amounts of ∼23mers produced by the dsRNase may serve as “primers” for RrpA, which synthesizes the antisense strand using the mRNA as a template. The resulting dsRNA could then again be degraded by the dsRNase into ∼23mers. These could reinitiate the amplification cycle or mediate mRNA degradation by a putative RISC homolog. This finding appears to contrast with experiments done in Drosophila, in which degradation of mRNA and coinjected dsRNA were readily observed. In these experiments, the appearance and persistence of ∼23mers correlated precisely with gene silencing (Yang et al., 2000). However, the authors could not exclude an amplification process in which the products mediated gene silencing. ∼23mers generated directly or indirectly (e.g., by RdRP) would not be labeled and would have thus escaped detection in their assay. In the wild-type background without β-gal reporter gene, the “silent” β-gal RNAi construct becomes an active interference agent when a target gene is subsequently introduced. It is therefore likely that ∼23mers are produced from the inverted repeat but are amplified to detectable levels only when the target is present.
Because RrpA− cells still generate ∼23mers in vitro, it is likely that the dsRNA is also degraded in the knock-out strain but that the products are below the level of detection. dsRNA should be rather stable and others have shown that only a fraction of it is processed in vivo (Yang et al., 2000) and in vitro (Zamore et al., 2000). However, we did not see any residual dsRNA in Northern blots (Martens, Novotny, Oberstrass, Postlethwait, and Nellen, unpublished results).
The substrate specificity of the partially purified Dictyostelium dsRNase and the size of the products strongly suggest that this enzyme complex generates the RNAi ∼23mers
Although the sequence similarity between Dictyostelium RrpA and other RdRPs is significant (22% similarity to EGO1 from C. elegans) the Dictyostelium enzyme contains an N-terminal extension not found in other RdRPs. Surprisingly, this domain, which is separated by an intron from the rest of the coding sequence, shows similarity to various RNA helicases and gives the best match to the helicase domain of K12H4.8 from C. elegans, a member of the dicer gene family (see Figure 3A; Bass, 2000). Dicer is the recently identified bidentate RNase that cleaves dsRNA to ∼23mers (Bernstein et al., 2001). Furthermore, it is a homologue of the Arabidopsis CAF gene (Jacobsen et al., 1999) mutations of which cause a floral phenotype. Members of this family consist of an N-terminal helicase domain, a C-terminal RNase III homology domain and a dsRNA binding domain. We have recently identified two dicer/CAF homologues in Dictyostelium that do not contain the helicase motif but show high similarity to the RNaseIII domain of dicer and CAF (Martens, Novotny, Oberstrass, Postlethwait, and Nellen, unpublished results). Assuming that RdRP and dicer/CAF are both components of the same RNAi machinery, it is intriguing to speculate that domain swapping has occurred between the nuclease and the polymerase. If the Dictyostelium dicer homologue and RdRP are really components of the same complex, this may suggest that cleavage of dsRNA and amplification of the signal/guide RNA are spatially linked.
DosA displays 63% similarity to RrpA and 22% similarity to EGO1 from C. elegans. Good matches in highly conserved regions support our conclusion that the gene encodes a genuine RdRP. The observation that DosA is not required for RNAi makes this an “orphan RdRP” with no known function. This is similar to the situation in plants in which several RdRP-related genes were found but only specific ones appear to be involved in gene silencing. More surprising is the fact that RrpB cannot compensate for a knock out of the closely related RrpA gene. A detailed analysis of these two genes may help to specify the features of an RNAi RdRP.
The feasibility of gene silencing was shown with the endogenous Dictyostelium discoidin gene family. Both mRNA and protein were clearly reduced, in many cases to nondetectable levels. The observation that more discoidin expression was found in developing cells and that almost no RNAi effect was observed in cells grown on a bacterial lawn indicated that RNAi mechanisms are either under developmental control or that the limited capacity of the RNAi machinery cannot completely abolish the high amounts of discoidin mRNA transcribed during development. Experiments described previously (Novotny et al., 2001), and here, rule out that reduced activity of dsRNase or reduced transcription of rrpA during development caused the residual expression levels of discoidin in silenced developing cells. A similar reduced silencing effect of the mybB gene in Dictyostelium has also been observed by others (H. Otsuka, R. Dottin, and J. Gross, personal communication). This is reminiscent of the situation in C. elegans in which silencing in specific cell types was found to work poorly (Tavernarakis et al., 2000).
ACKNOWLEDGMENTS
We thank Sonja Apel, Sonja Diegel and Sandra Wille for excellent technical support. This work was supported, in part, by a grant from the Deutsche Forschungsgemeinschaft to W.N., a grant from the National Science Foundation to T.L.S., and by the Zentrale Forschungsförderung of Kassel University. H.M. is a Boehringer-Ingelheim fellow.
Note added in proof.
While this paper was under revision, Lipardi et al. (Cell 2001; 107, 297–307) and Sijen et al. (Cell 2001; 107, 465–479) submitted, revised, and published data that confirmed our conclusions, that siRNAs serve as primers for RdRP to amplify the RNAi effect.
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.01–04-0211. Article and publication date are at www.molbiolcell.org/cgi/10.1091/mbc.01–04-0211.
REFERENCES
- Bass BL. Double-stranded RNA as a template for gene silencing. Cell. 2000;101:235–238. doi: 10.1016/s0092-8674(02)71133-1. [DOI] [PubMed] [Google Scholar]
- Bernstein E, Caudy AA, Hammond SM, Hannon GJ. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409:363–366. doi: 10.1038/35053110. [DOI] [PubMed] [Google Scholar]
- Blusch J, Morandini P, Nellen W. Transcriptional regulation by folate: inducible gene expression in Dictyosteliumtransformants during growth and early development. Nucleic Acids Res. 1992;20:6235–6238. doi: 10.1093/nar/20.23.6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalanotto C, Azzalin G, Macino G, Cogoni C. Gene silencing in worms, and fungi. Nature. 2000;404:245. doi: 10.1038/35005169. [DOI] [PubMed] [Google Scholar]
- Cogoni C, Macino G. Gene silencing in Neurospora crassarequires a protein homologous to RNA-directed RNA polymerase. Nature. 1999;399:166–169. doi: 10.1038/20215. [DOI] [PubMed] [Google Scholar]
- Corpet F. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res, 1988;16:10881–10890. doi: 10.1093/nar/16.22.10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley TE, Nellen W, Gomer RH, Firtel RA. Phenocopy of discoidin I-minus mutants by antisense transformation in Dictyostelium. Cell. 1985;43:633–641. doi: 10.1016/0092-8674(85)90235-1. [DOI] [PubMed] [Google Scholar]
- Dalmay T, Hamilton A, Rudd S, Angell S, Baulcombe DC. An RNA-directed RNA polymerase gene in Arabidopsisis required for posttranscriptional gene silencing mediated by a transgene but not by a virus. Cell. 2000;101:543–553. doi: 10.1016/s0092-8674(00)80864-8. [DOI] [PubMed] [Google Scholar]
- Dingermann T, Reindl N, Brechner T, Werner H, Nerke K, Parrish S. Nonsense suppression in Dictyostelium discoideum. Dev Genet. 1990;11:410–417. doi: 10.1002/dvg.1020110514. [DOI] [PubMed] [Google Scholar]
- Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001a;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- Elbashir SM, Lendeckel W, Tuschl T. RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev. 2001b;15:188–200. doi: 10.1101/gad.862301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- Fleenor J, Xu S, Mello C, Fire A. Functional anatomy of a dsRNA trigger: differential requirement for the two trigger strands in RNA interference. Mol Cell. 2000;6:1077–1087. doi: 10.1016/s1097-2765(00)00106-4. [DOI] [PubMed] [Google Scholar]
- Hamilton AJ, Baulcombe DC. A species of small antisense RNA in posttranscriptional gene silencing in plants. Science. 1999;286:950–952. doi: 10.1126/science.286.5441.950. [DOI] [PubMed] [Google Scholar]
- Hammond SM, Bernstein E, Beach D, Hannon GJ. An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophilacells. Nature. 2000;404:293–296. doi: 10.1038/35005107. [DOI] [PubMed] [Google Scholar]
- Howard PK, Ahern KG, Firtel RA. Establishment of a transient expression system for Dictyostelium discoideum. Nucleic Acids Res. 1988;16:2613–2623. doi: 10.1093/nar/16.6.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter CP. Gene silencing: shrinking the black box of RNAi. Curr Biol. 2000;10:137–140. doi: 10.1016/s0960-9822(00)00325-0. [DOI] [PubMed] [Google Scholar]
- Jacobsen SE, Running MP, Meyerowitz EM. Disruption of an RNA helicase/RNAse III gene in Arabidopsiscauses unregulated cell division in floral meristems. Development. 1999;126:5231–5243. doi: 10.1242/dev.126.23.5231. [DOI] [PubMed] [Google Scholar]
- Kamath RS, Martinez-Campos M, Zipperlen P, Fraser AG, Ahringer J. Effectiveness of specific RNA-mediated interference through ingested double-stranded RNA in Caenorhabditis elegans. Genome Biol. 2000;2:1–10. doi: 10.1186/gb-2000-2-1-research0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ken R, Singleton CK. Redundant regulatory elements account for the developmental control of a ribosomal protein gene of Dictyosteliumdiscoideum. Differentiation. 1994;55:97–103. doi: 10.1046/j.1432-0436.1994.5520097.x. [DOI] [PubMed] [Google Scholar]
- Maniak M, Saur U, Nellen W. A colony-blot technique for the detection of specific transcripts in eukaryotes. Anal Biochem. 1989;176:78–81. doi: 10.1016/0003-2697(89)90275-3. [DOI] [PubMed] [Google Scholar]
- Nellen W, Datta S, Reymond C, Sivertsen A, Mann S, Crowley T, Firtel RA. Molecular biology in Dictyostelium: tools and applications. Methods Cell Biol. 1987;28:67–100. doi: 10.1016/s0091-679x(08)61637-4. [DOI] [PubMed] [Google Scholar]
- Nellen W, Firtel RA. High-copy-number transformants and co-transformation in Dictyostelium. Gene. 1985;39:155–163. doi: 10.1016/0378-1119(85)90309-9. [DOI] [PubMed] [Google Scholar]
- Novotny J, Diegel S, Schirmacher H, Möhrle A, Hildebrandt M, Oberstrass J, Nellen W. DictyosteliumdsRNase. Methods Enzymol. 2001;342:193–212. doi: 10.1016/s0076-6879(01)42545-6. [DOI] [PubMed] [Google Scholar]
- Sadiq M, Hildebrandt M, Maniak M, Nellen W. Developmental regulation of antisense-mediated gene silencing in Dictyostelium. Antisense Res Dev. 1994;4:263–267. doi: 10.1089/ard.1994.4.263. [DOI] [PubMed] [Google Scholar]
- Smardon A, Spoerke JM, Stacey SC, Klein ME, Mackin N, Maine EM. EGO-1 is related to RNA-directed RNA polymerase, and functions in germ-line development, and RNA interference in C. elegans. Curr Biol. 2000;10:169–178. doi: 10.1016/s0960-9822(00)00323-7. [DOI] [PubMed] [Google Scholar]
- Spudich J. Dictyostelium discoideum: molecular approaches to cell biology. Methods Cell Biol. 1987;28:3–8. [PubMed] [Google Scholar]
- Sutoh K. A transformation vector for Dictyostelium discoideumwith a new selectable marker bsr. Plasmid. 1993;30:150–154. doi: 10.1006/plas.1993.1042. [DOI] [PubMed] [Google Scholar]
- Tabara H, Sarkissian M, Kelly WG, Fleenor J, Grishok A, Timmons L, Fire A, Mello CC. The rde-1 gene, RNA interference, and transposon silencing in C. elegans. Cell. 1999;99:123–132. doi: 10.1016/s0092-8674(00)81644-x. [DOI] [PubMed] [Google Scholar]
- Tavernarakis N, Wang S, L. Dorovkov M, Ryazanov A, Driscoll M. Heritable, and inducible genetic interference by double-stranded RNA encoded by transgenes. Nat Genet. 2000;24:180–183. doi: 10.1038/72850. [DOI] [PubMed] [Google Scholar]
- Wang Z, Morris JC, Drew ME, Englund PT. Inhibition of Trypanosoma bruceigene expression by RNA interference using an integratable vector with opposing T7 promoters. J Biol Chem. 2000;275:40174–40179. doi: 10.1074/jbc.M008405200. [DOI] [PubMed] [Google Scholar]
- Waterhouse PM, Graham MW, Wang MB. Virus resistance and gene silencing in plants induced by simultaneous expression of sense and antisense RNA. Proc Natl Acad Sci USA. 1998;95:13959–13964. doi: 10.1073/pnas.95.23.13959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber U, Gross HJ. In vitroRNAs. In: Lichtenstein, Nellen, editors. Antisense Technology: A Practical Approach. Oxford, UK: Oxford University Press; 1997. [Google Scholar]
- Wetterauer B, Jacobsen G, Morandini P, MacWilliams H. Mutants of Dictyostelium discoideumwith defects in the regulation of discoidin I expression. Dev Biol. 1993;159:184–195. doi: 10.1006/dbio.1993.1232. [DOI] [PubMed] [Google Scholar]
- Witke W, Nellen W, Noegel A. Homologous recombination in the Dictyosteliumalpha-actinin gene leads to an altered mRNA and lack of the protein. EMBO J. 1987;6:4143–4148. doi: 10.1002/j.1460-2075.1987.tb02760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu-Scharf D, Jeong B, Zhang C, Cerutti H. Transgene, and transposon silencing in Chlamydomonas reinhardtiiby a DEAH-box RNA helicase. Science. 2000;290:1159–1162. doi: 10.1126/science.290.5494.1159. [DOI] [PubMed] [Google Scholar]
- Yang D, Lu H, Erickson JW. Evidence that processed small dsRNAs may mediate sequence-specific mRNA degradation during RNAi in Drosophilaembryos. Curr Biol. 2000;10:1191–1200. doi: 10.1016/s0960-9822(00)00732-6. [DOI] [PubMed] [Google Scholar]
- Zamore PD, Tuschl T, Sharp PA, Bartel DP. RNAi. double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell. 2000;101:25–33. doi: 10.1016/S0092-8674(00)80620-0. [DOI] [PubMed] [Google Scholar]