Serum FFA concentrations were found to be a helpful biochemical test to identify the fasted versus fed state in children.
Abstract
BACKGROUND:
Ensuring children are fasting for blood draws is necessary to diagnose abnormalities in glucose homeostasis. We sought to determine if serum free fatty acid (FFA) concentrations might be a useful marker to differentiate the fed and fasted states among children.
METHODS:
A total of 442 inpatient (fasting) and 323 (postglucose load) oral glucose tolerance test samples of glucose, insulin, and FFA from children (age 5–18 years) who had healthy weight, overweight, or obesity were examined by receiver operating characteristic (ROC) curve analysis to identify a cut point for nonfasting. In a cross-sectional study, we compared mean FFA and percentage of FFA values below this cut point as a function of inpatient (n = 442) versus outpatient (n = 442) setting.
RESULTS:
The area under the curve of FFA was significantly better (P values < .001) than the area under the curve of glucose or insulin for identifying nonfasting. FFA <287 mEq/mL had 99.0% sensitivity and 98.0% specificity for nonfasting. Mean FFA was lower in outpatients than inpatients (P < .001); only 1.6% inpatient but 9.7% outpatient FFA values were consistent with nonfasting (P < .001).
CONCLUSIONS:
Clinicians cannot assume that pediatric patients are adequately fasted on arrival for fasting blood work. On the basis of having significantly lower outpatient than inpatient FFA values and more frequently suppressed FFA, children appeared less likely to be fasting at outpatient appointments. FFA value <287 mEq/mL was a sensitive and specific cutoff for nonfasting in children that may prove clinically useful.
What’s Known on This Subject:
There is evidence that patients may not always follow recommendations for clinically related fasting. This has been demonstrated in surveys of adults presenting for outpatient blood work and surveys of parents of children instructed to fast in a perioperative setting.
What This Study Adds:
A biochemical test to provide assurance of the fasted state could be valuable for interpretation of fasting glucose measurements in children. Serum free fatty acid concentrations were a useful marker to differentiate the fasted from the fed state.
The prevalence of diabetes among children and adolescents in the United States appears to be increasing.1 The high prevalence of type 2 diabetes has been attributed at least in part to the increasing prevalence of obesity (BMI ≥95th percentile for age and sex) and severe obesity (BMI ≥120% of the 95th percentile).2,3 The American Academy of Pediatrics and the Endocrine Society both have guidelines that recommend providers screen youth with overweight and obesity for impaired glucose metabolism.4,5 These screening tests are obtained by using fasting blood work because recent energy intake can increase glucose concentrations and lead to incorrect diagnosis. Although such results impact clinical decisions, there is no accepted method, other than patient report, that providers can use to discriminate those who are fasting versus those who are not when blood samples are collected.
There is evidence that some patients do not follow recommendations for pre–blood work fasting. In a survey of adults at the time of morning blood draws intended to be collected after a 12-hour fast, it was determined that only 60% had properly fasted for ≥12 hours and 6% were in an acute fed state.6 Similar data from children are lacking, although it was found in a survey administered to 104 parents after their children returned from elective surgeries that 13% did not adhere to fasting instructions and 7% were inadequately fasted.7 The low frequency with which carbohydrate metabolism abnormalities in children are confirmed at a second test8,9 might at least in part be due to initial sample collection in a nonfasted state. A method for identifying children who are not fasting could therefore be helpful to improve clinical decision-making.
When energy-containing foodstuffs are consumed, normal physiology predicts accompanying increases in circulating glucose and insulin; however, the presence of obesity-induced insulin resistance may also increase measured glucose and insulin10–14 in the absence of energy intake. A biochemical test that could be used to identify the nonfasted state among children regardless of obesity status would thus be valuable. We therefore sought a biochemical analyte whose concentrations would be expected to diverge under fasting and nonfasting conditions. Serum free fatty acid (FFA) concentrations rise in the fasted state but are decreased by food intake.15 Circulating FFA increases when there is insufficient peripheral insulin action to suppress adipocyte lipolysis.15,16 Ongoing lipolysis causes children’s serum FFA to increase as the length of time since food was ingested increases.17 Serum FFA also increase in states of insulin resistance, including obesity.10,14,17–20 However, when insulin concentrations rise after a meal, insulin stimulates adipose tissue fat storage and suppresses fat mobilization.18,20,21 Suppression of circulating FFA is observed even among those with significant obesity.19,22,23 Therefore, serum FFA might provide an indication of the fed versus fasted state, even in children with obesity.
We sought to determine if serum FFA is a useful analyte to differentiate the fed and fasted state among children of all weight strata. We conducted an analysis of inpatient (ie, high-confidence fasting) and outpatient (self-reported as fasting) oral glucose tolerance test (OGTT) serum FFA data to examine if testing FFA concentrations would be superior, compared with glucose or insulin concentrations, to distinguish between the fed and fasting state in children. We expected that children’s glucose and insulin would rise, and children’s FFA would be suppressed, at the 2-hour time point of an OGTT versus baseline. We hypothesized that an FFA cut point that discriminated inpatient fasting versus 2-hour OGTT (nonfasting) samples would have good sensitivity and specificity for the nonfasted state and that a receiver operating characteristic (ROC) curve analysis would identify serum FFA as superior to glucose or insulin for the identification of the nonfasting state. We also hypothesized that mean FFA concentrations would be significantly lower in children’s outpatient (reportedly fasting) samples in comparison with inpatient (high-confidence) fasting samples.
Methods
Participants and Variables
A convenience sample of children ages 5 to 18 years who were nondiabetic was assembled from multiple research protocol cohorts (registered at clinicaltrials.gov: NCT00001195, NCT00001522, NCT00001723, NCT00005669, NCT00315172, NCT00758108, NCT01237041, NCT01888939, NCT02390765, NCT03223649), each of which measured glucose, insulin, and FFA in the fasted state or during an OGTT (see Supplemental Table 4 for more information about each study). These measures were collected to determine if the children were healthy enough to participate in research as volunteers and to establish baseline values for interventional studies. In these observational and interventional research studies, researchers recruited generally healthy children (with the exception of obesity-related comorbidities such as hypertension) who used no medications expected to alter body weight. Participants were eligible for this analysis if they were of normal BMI or had overweight or obesity as determined by the United States Centers for Disease Control growth standards.24 The Institutional Review Board of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (NIH) approved each study; parents or guardians and children provided written consent and assent for participation. All participants were evaluated at the NIH Hatfield Clinical Research Center. Demographic and laboratory data were retrieved from participants’ electronic study records and medical records. If children had been recruited for an interventional study, data were used only from preintervention visits.
Three cohorts were developed: children who underwent a morning 2-hour OGTT (1.75 g/kg with a maximum dose of 75 g), whose 2-hour values were therefore assured to be nonfasting; children admitted overnight as inpatients whose orders specified they could not eat after 10 pm the previous night and who were therefore believed with high confidence to be in a fasted state for a morning blood draw; and children studied as outpatients, who reported to staff they had not eaten after 10 pm the previous night. Fasting samples were generally drawn between 8 and 10 am.
Using these cohorts, 3 analyses were conducted: (1) comparison of baseline and 2-hour OGTT values to confirm that oral glucose reduced FFA as it increased glucose and insulin in children with normal weight and with overweight or obesity; (2) ROC curve analysis to determine if inpatient (high-confidence fasting) samples could be reliably discriminated from 2-hour OGTT (assured nonfasting) samples by using glucose, insulin, or FFA concentrations and identify an appropriate cut point for the nonfasting state; and (3) comparisons of inpatient and outpatient samples by using the derived cut points for nonfasting that were determined by ROC curve analysis to examine the hypothesis that FFA would be significantly lower in outpatient samples and that there would be a higher frequency of FFA concentrations consistent with nonfasting than FFA from inpatient samples. For this last analysis, 2 groups were assembled: first, a cross-sectional analysis of children studied uniquely as either inpatients or outpatients (3A) and second, a paired analysis of children studied both as inpatients and outpatients on separate dates but no more than 150 days apart (3B).
Assays
All laboratory analyses were performed by the NIH Clinical Center Department of Laboratory Medicine. FFAs were measured by using the Wako enzymatic method with a Siemens Vista instrument (analytical sensitivity 0.01 mEq/L; intra- and interassay covariances 2.0% and 6.3%) before 2011 or a Roche Cobas 6000 (c501) instrument (analytical sensitivity 0.01 mEq/L; intra- and interassay covariances 1.6% and 5.1%). Insulin was measured on a Roche Cobas 6000 (e601) instrument (analytical sensitivity 0.2 µU/mL; intra- and interassay covariances 1.1% and 4.3%) from 2011 to 2018.
Statistical Methods
Analyses were performed by using IBM SPSS Statistics for Windows, version 25 (IBM Corporation, Armonk, NY). All data were screened for outliers and normality. Insulin concentrations were log transformed to achieve normality; back-transformed adjusted mean ± SEM are provided in graphs for interpretation. For analysis 1, 3 repeated measures analysis of covariance (ANCOVA) were conducted to analyze the differences between mean glucose, insulin, and FFA concentrations at baseline and 2-hour time points of the OGTT, accounting for age, sex, race, and BMIz. Linear regressions were also used to examine 2-hour glucose, insulin, and FFA values as a function of BMIz. For analysis 2, ROC curves25 were generated for glucose, insulin, and FFA by using high-confidence fasting data collected from inpatients and the nonfasting data from the 2-hour time point of OGTT data. For ROC, the area under the curve (AUC) for glucose, insulin, and FFA for identification of nonfasting was determined. AUCs of 0.5 to 0.7 are considered to indicate relatively low test accuracy, 0.7 to 0.9 indicates moderate accuracy, and >0.9 indicates high accuracy.26 The AUCs of glucose, insulin, and FFA were compared as previously described with the Mann-Whitney U test for correlated curves.27 Results from the ROC curve analysis were also used to identify the cut point for each variable with the lowest false-positive and highest true-positive rates (value with maximal Youden J index = sensitivity + specificity − 1).25 The sensitivity, specificity, positive and negative predictive values, and positive and negative likelihood ratios were calculated for the maximal Youden J index values of glucose, insulin, and FFA. For analysis 3A, an ANCOVA was used to compare fasting FFA cross-sectional data in unique participants, with inpatient versus outpatient status as the main independent variable, accounting for race and ethnicity, age, sex, and BMIz. For analysis 3B, a repeated measures ANCOVA was used to compare paired FFA values from inpatient and outpatient visits of each individual, accounting for race and ethnicity, age, sex, and BMIz between subjects. For both analyses 3A and 3B, we quantified the percentage of inpatient and outpatient concentrations that would be designated as nonfasting according to the FFA cut point identified by ROC curve analysis and compared these percentages using χ2 analysis for independent samples or McNemar’s test for correlated data, as appropriate.28
Results
A total of 442 samples were available from participants studied exclusively as inpatients, 442 samples were also available from participants studied exclusively as outpatients. A total of 323 samples were available from participants who underwent OGTT and had both baseline and 2-hour data available. Demographic characteristics of these samples are given in Table 1. Paired inpatient and outpatient samples reported as fasting were available from 293 participants (Table 2).
TABLE 1.
Sample Characteristics From the 3 Samples
| Characteristic | Inpatient, Expected to Be Fasting, n = 442 | Outpatient, Self-Reported as Fasting, n = 442 | Baseline and 2-h OGTT, n = 323 |
|---|---|---|---|
| Age in y, mean ± SD | 11.9 ± 2.9 | 12.2 ± 3.0 | 12.4 ± 2.8 |
| BMI, mean ± SD | 33.2 ± 11.0 | 23.6 ± 8.8 | 33.0 ± 12.6 |
| BMIz, mean ± SD | 2.13 ± 0.89 | 0.86 ± 1.21 | 1.89 ± 1.09 |
| Sex, n (%) | |||
| Male | 190 (43) | 203 (45.9) | 141 (43.7) |
| Female | 252 (57) | 239 (54.1) | 182 (56.3) |
| Race, n (%) | |||
| African American | 205 (46.4) | 153 (34.6) | 158 (48.9) |
| White | 222 (50.2) | 208 (47.1) | 141 (43.8) |
| Multiple races or other | 15 (3.4) | 81 (18.3) | 24 (7.3) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 12 (2.7) | 36 (8.1) | 9 (2.8) |
| Serum glucose reported as fasting, mg/dL, mean ± SD | 88.9 ± 12.5 (n = 436) | 88.9 ± 10.8 (n = 437) | 87.6 ± 7.9 |
| 2-h OGTT serum glucose, mg/dL, mean ± SD | — | — | 109.4 ± 20.0 (n = 322) |
| Serum insulin reported as fasting, µU/mL, mean ± SD | 20.1 ± 15.9 (n = 438) | 14.1 ± 15.6 (n = 441) | 22.5 ± 21.6 |
| 2-h OGTT serum insulin, µU/mL, mean ± SD | — | — | 111.3 ± 151.1 |
| Serum FFA reported as fasting, mEq/mL, mean ± SD | 767.5 ± 426.9 | 579.2 ± 248.9 | 602.1 ± 235.4 |
| 2-h OGTT serum FFA, mEq/mL, mean ± SD | — | — | 98.0 ± 59.0 |
Fasting was considered assured for participants with inpatient data collection after an overnight admission. —, not applicable.
TABLE 2.
Characteristics for Participants Studied as Inpatients and Outpatients for Paired Analysis (N = 293)
| Characteristic | Value, n (%) | Value for Participants at Inpatient Visit (Expected to Be Fasting), Mean ± SD | Value for Participants at Outpatient Visit (Self-Reported as Fasting), Mean ± SD |
|---|---|---|---|
| Sex | — | — | |
| Male | 123 (42.0) | — | — |
| Female | 170 (58.0) | — | — |
| Race | — | — | |
| African American | 148 (50.5) | — | — |
| White | 142 (48.5) | — | — |
| Multiple races or other | 3 (1.0) | — | — |
| Ethnicity | — | — | |
| Hispanic or Latino | 1 (0.3) | — | — |
| Age, y | — | 12.47 ± 2.97 | 12.37 ± 3.03 |
| BMI | — | 34.34 ± 11.87 | 34.57 ± 11.99 |
| BMIz | — | 2.13 ± 0.85 | 2.16 ± 0.84 |
| Serum glucose, mg/dL | — | 89.5 ± 15.6 | 88.1 ± 12.2 |
| Serum insulin, µU/mL | — | 21.2 ± 17.6 | 22.9 ± 18.6 |
| Serum FFA, mEq/mL | — | 787.5 ± 454.8 | 630.6 ± 241.0 |
Average days between visits: 38.1 ± 37.5. Serum glucose was available in 287 participants studied as inpatients and 293 participants studied as outpatients. Serum insulin was available in 288 participants studied as inpatients and 287 participants studied as outpatients. —, not applicable
Analysis 1: OGTT Data
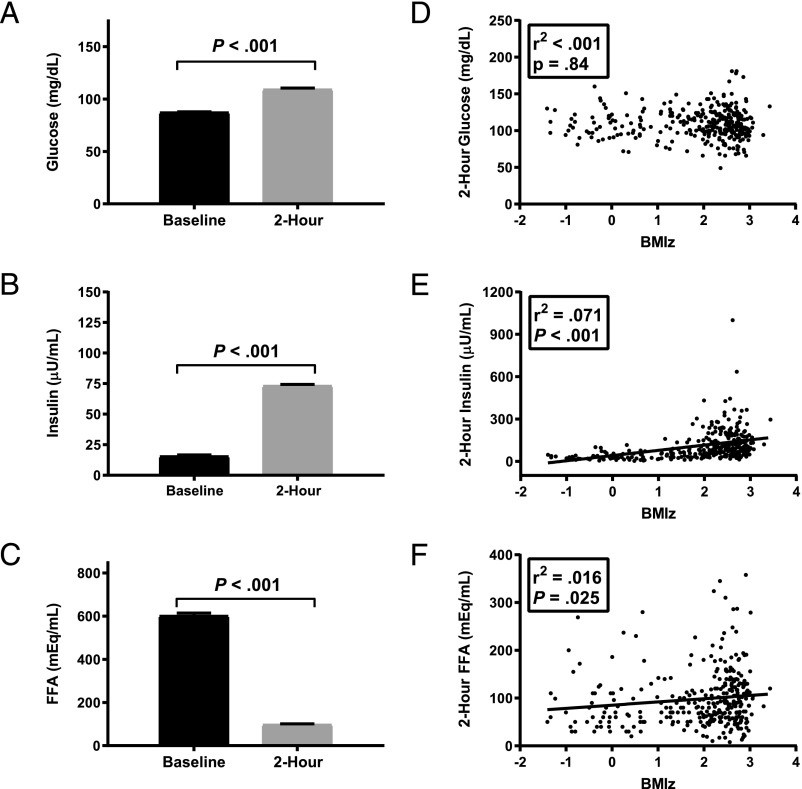
During the OGTT, mean serum glucose (Fig 1A, P < .001) and insulin (Fig 1B, P < .001) increased, whereas serum FFA (Fig 1C, P < .001) decreased. The relationship of 2-hour OGTT glucose with BMIz was not significant (Fig 1D, P = .84), whereas the relationships of 2-hour OGTT insulin and FFA with BMIz were each significant (Fig 1 E and F, P values < .05). BMIz accounted for relatively little of the variance in 2-hour OGTT glucose (r2 < 0.001; P = .84) or 2-hour OGTT FFA (r2 = 0.02, P = .03) and ∼7% of variance in 2-hour OGTT insulin (r2 = 0.07; P < .001). Age was significantly related to fasting FFA (B = −27.1; P < .001) but not 2-hour OGTT FFA (P = .937) and was not significantly related to fasting or 2-hour OGTT glucose and insulin (all P values > .10).
FIGURE 1.
Results of OGTT. Mean ± SE at baseline and 2-hour time points for (A) glucose, (B) insulin, and (C) FFAs. Associations between BMI z score for age and sex (BMIz) and (D) glucose, (E) insulin, and (F) FFAs.
Analysis 2: ROC Curve Analysis
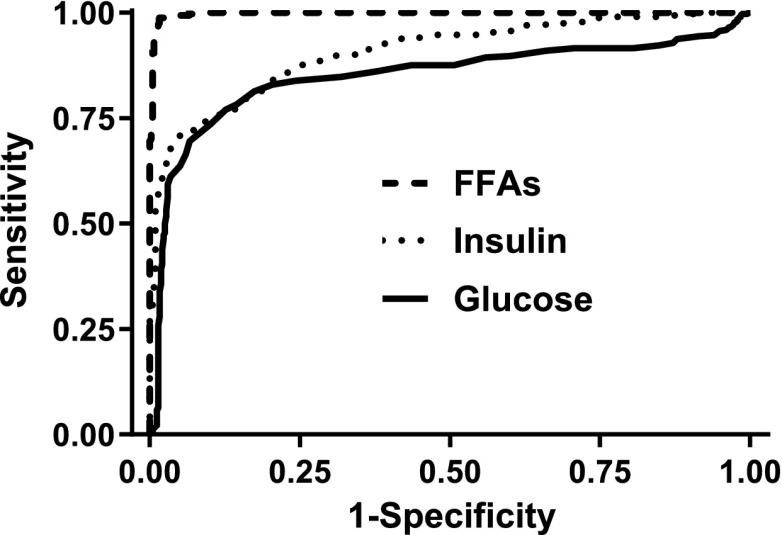
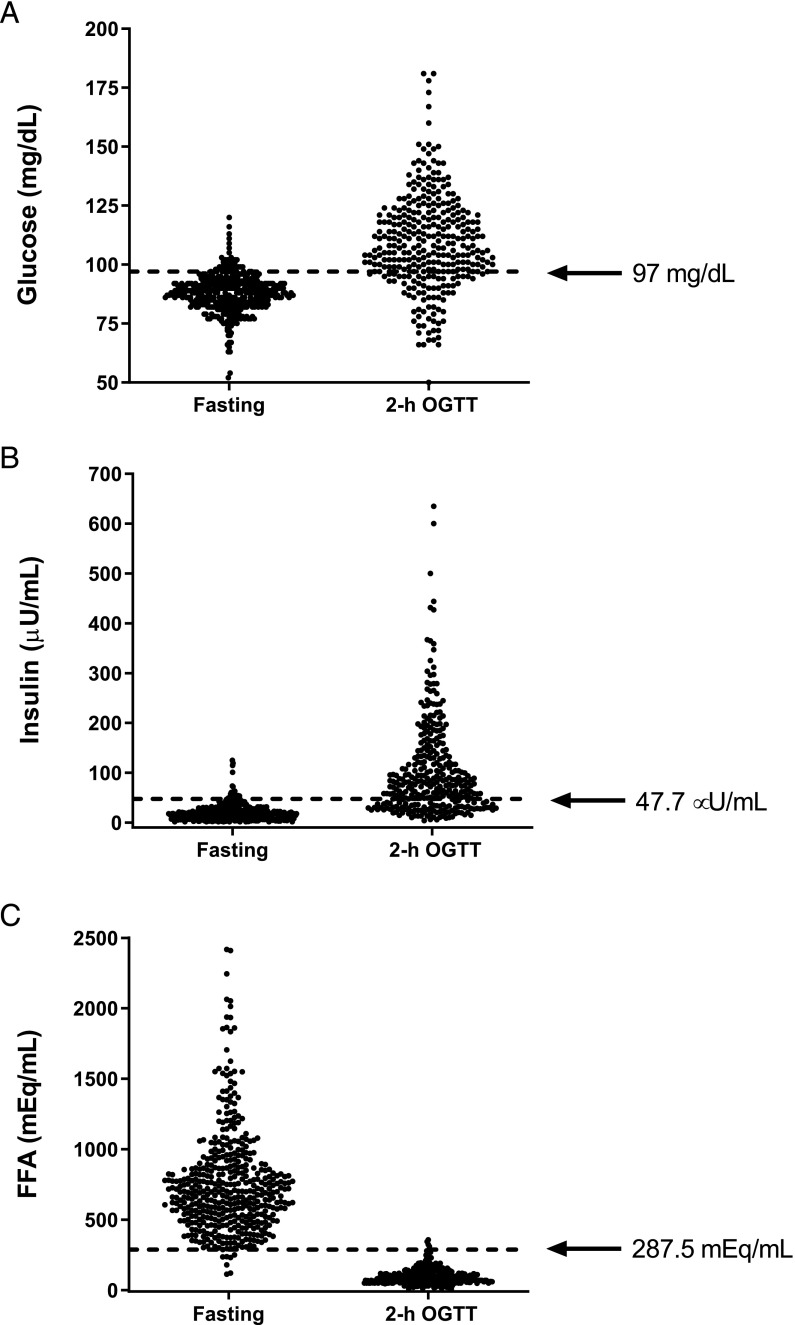
The AUC values for glucose, insulin, and FFA as tests to identify nonfasting are shown in Table 3, and the ROC curves are demonstrated in Fig 2. The AUCs for glucose (0.850) and insulin (0.906) suggested both were moderately accurate tests for nonfasting. The AUC for FFA was 0.998, consistent with high accuracy for nonfasting. The AUC for FFA was significantly greater than the AUC for glucose or insulin (P values < .001). The cut points identified by the maximal Youden J index for glucose (>97 mg/dL; Fig 3A) and insulin (>47.7 µU/mL; Fig 3B) had good sensitivity (0.77 and 0.71) and very good specificity (0.87 and 0.95) for nonfasting. The sensitivity (0.99) and specificity (0.98) of the cut point for FFA for nonfasting (<287 mEq/mL; Fig 3C) were both excellent. Correspondingly, the positive likelihood ratio for FFA to identify nonfasting (62.4) greatly exceeded the positive likelihood ratios for glucose (6.1) and insulin (14.8).
TABLE 3.
ROC Curve Analysis for Identifying the Nonfasted State
| FFA | Glucose | Insulin | |
|---|---|---|---|
| AUC, mean ± SE | 0.998 ± 0.001 | 0.850 ± 0.016 | 0.906 ± 0.011 |
| P value for comparison with FFA AUC | — | <.001 | <.001 |
| Maximal Youden cut point | 287 mEq/mL | 97 mg/dL | 47.7 μU/mL |
| Sensitivity | 0.99 | 0.77 | 0.71 |
| Specificity | 0.98 | 0.87 | 0.95 |
| Positive predictive value | 0.98 | 0.82 | 0.92 |
| Negative predictive value | 0.99 | 0.84 | 0.82 |
| Positive likelihood ratio | 62.36 | 6.11 | 14.8 |
| Negative likelihood ratio | 0.01 | 0.26 | 0.31 |
—, not applicable.
FIGURE 2.
ROC curves for detection of nonfasting.
FIGURE 3.
Individual data for (A) glucose, (B) insulin, and (C) FFAs. Cut points for fasting, determined as the maximal Youden index from ROC curve analyses, are shown.
Analysis 3A: Outpatient Versus Inpatient Cross-Sectional FFA Comparison
The mean (±SEM) FFA concentration of samples reported to be fasting, adjusted for covariates, was significantly greater among the 442 participants studied as inpatients (754.0 ± 17.7 mEq/mL) than among the 442 participants studied as outpatients (592.8 ± 17.7 mEq/mL; P < .001). By using the ROC curve–identified cut point for nonfasting of 287 mEq/mL, only 1.6% of inpatient, versus 9.7% of outpatient, samples were below the cut point (P < .001).
Analysis 3B: Outpatient Versus Inpatient FFA Paired Comparison
Among the 293 participants studied both as inpatients and outpatients, the mean (±SEM) fasting FFA concentration, adjusted for covariates, at inpatient visits was 787.5 ± 25.1 mEq/mL and at outpatient visits was 630.6 ± 13.1 mEq/mL (P = .001). The percentage of FFA values below the cut point of 287 mEq/mL was 2.0% for inpatient samples versus 5.1% for outpatient samples (P = .09).
Discussion
In this analysis of a large sample of volunteer children, we compared glucose, insulin, and FFA as markers for nonfasting (as modeled by values obtained at the 2-hour time point of an OGTT). ROC curve analyses of glucose and insulin as potential tests to discriminate the fasted state both revealed moderate accuracy but with significantly lower AUCs than FFA. The maximal Youden J index value for FFA identified a cut point of <287 mEq/mL that had excellent sensitivity (99%) and specificity (98%) for the nonfasted state. The cut points for glucose (>97 mg/dL) and insulin (>47.7 µU/mL) had both lower sensitivity (77% and 71%) and specificity (87% and 95%) than FFA, suggesting that serum FFA may be a superior test for the discrimination between fasting and fed states. Further, although BMIz and FFA were positively correlated, FFA suppression was observed among participants of all weight strata, consistent with previous studies.19,22,23
We then used the identified FFA cut point to examine whether a greater proportion of samples from pediatric subjects in the outpatient setting that were reported as obtained after an overnight fast were likely not to have been obtained in the fasted state. We found significant differences between fasting FFA values of children whose samples were obtained in an inpatient setting (where fasting is largely assured because of subjects’ medical orders and monitoring of foodstuffs delivered to the room by research nurses and staff) versus an outpatient setting, with lower FFA values in the outpatient setting for a large cross-sectional cohort and among a smaller paired-samples cohort. As determined by the FFA cut point to identify the fed state, children appeared less likely to be in the fasted state when they presented for an outpatient appointment, with a significantly greater percentage of suppressed FFA concentrations in the larger cohort studied cross-sectionally. On the basis of the calculated FFA cut point for these data, between 1 in 20 and 1 in 10 children in the outpatient setting had a suppressed FFA that suggested inadequate fasting. Although we do not know with absolute certainty that the children studied as outpatients who had suppressed FFA were not fasting, these data are consistent with past studies6,7 and provide additional evidence that a test to identify nonfasting individuals could be particularly useful in the outpatient setting, where most children are initially screened.
Standard clinical guidelines recommend that providers screen for obesity-related comorbidities, including type 2 diabetes, in children with obesity; fasting blood laboratory studies are often needed.4,5,29,30 There are limited data available for the extent of erroneous self-reports of the fasted state in children. However, in studies of adult and pediatric patients, it has been suggested that at least 6% of patients report for a fasting blood draw or other procedures in a nonfasting state.6,7 Because the prevalence of pediatric obesity in the United States was 18.5%,3 and there were ∼73.6 million children in the United States in 2016,31 there were ∼13 million children with obesity who, according to guidelines, would be recommended to be screened for abnormalities in glucose homeostasis. If even 5% were to undergo screening when not fasting, over 600 000 children would potentially have erroneous results from such screening and be required to return for additional testing. In the results of the current study, it is suggested that obtaining FFA values along with metabolic screening may help avoid unnecessary retesting and aid providers’ interpretations. For clinicians who are often overburdened with clinical and administrative tasks, a test that could reduce the frequency with which they need to explain to families what abnormal glucose measurements might mean, along with a lessening of the need to schedule repeated laboratory draws, could be of substantial benefit. The Medicare laboratory fee schedule for 2019 lists the reimbursement for FFAs as $18.77, similar to the reimbursement for measuring insulin ($14.39) and glycosylated hemoglobin ($10.79).
To our knowledge, this is the first study in which researchers have specifically examined FFA as a potential indicator of the fasting state in children. The convenience sample analyzed was large and diverse in age, race and ethnicity, and BMIz; however, because participants were recruited for research, their results may not necessarily be applicable to the general US pediatric population. Another strength is the large number of participants who were studied as inpatients, which allowed for the analysis of data from participants who were extremely likely to be fasting; such data are not usually available in typical laboratory settings. However, it remains possible, although unlikely, that some participants ate before their inpatient blood draws were completed. Such consumption would be anticipated to make it more difficult to identify a test with excellent sensitivity and specificity for the fed state. Data obtained from participants’ OGTTs allowed us to examine the variations in glucose, insulin, and FFA concentrations after a glucose load. However, we examined only 1 time point; other time points after energy consumption might yield different results.32 Because the OGTT contains only simple carbohydrate, but no lipid or protein, additional studies are also needed to assess if a similar criterion for FFA suppression is applicable in children who consume more-complex meals. Another limitation is that the exact time of blood draw was not available for most study participants; thus, although by protocol the fasting samples were all obtained between 8 and 10 am, it is theoretically possible that the samples of outpatients could have been drawn earlier or later than those of inpatients. Because children’s serum FFA varies according to time since the last meal, with elevations observed during the morning as length of time in the fasted state increases,17 earlier sampling could account for lower mean FFA in outpatients than inpatients. However, given the greater unpredictability of the timing of phlebotomy that typically occurs in the outpatient setting, consistent earlier sampling would seem unlikely to explain this study’s results; consistent later sampling in the outpatient setting, if true, would only further support the findings presented here. The effects of fasting on FFA are age dependent,33,34 and it is possible that the diagnostic accuracy of FFA for nonfasting may be reduced in older adolescents. We did not, however, find an association between age and FFA suppression by OGTT, suggesting age does not greatly affect the likelihood of FFA suppression by glucose consumption. We did not have a large enough sample to separately evaluate the impact of pubertal status on these findings. Finally, the value of a screening test depends on the prevalence of the outcome being sought. By creating nonfasting conditions in half of participants used to create the ROC curves (as opposed to the ∼<10% expected in the real world), we likely overestimated the positive predictive power of the criteria identified. These findings should be extended in similar studies using a mixed meal in place of OGTT to identify an appropriate FFA cut point for fasting, as well as testing the derived cut points in other cohorts.
Conclusions
Given the plethora of standard clinical guidelines for the evaluation of children with obesity that include recommendations for metabolic screening, providers in the community frequently screen for glucose abnormalities using fasting blood samples obtained in an outpatient setting. Clinicians cannot assume that all pediatric patients are adequately fasted on arrival for fasting blood work. A standardized method to distinguish between the fasting and fed states would thus be clinically useful. We propose that an FFA concentration <287 mEq/mL may be useful as a cut point for nonfasting in children. Fasting versus nonfasting is not, however, dichotomous but rather is continuous, and the suppression of FFA is expected to vary according to the time since the last meal. Clinicians should have a high index of suspicion that samples with low FFA and abnormally high glucose concentrations may not have been obtained after an adequate fast. Serum FFA values may provide helpful adjunctive data when assessing a child’s metabolic status and risk.
Glossary
- ANCOVA
analysis of covariance
- AUC
area under the curve
- FFA
free fatty acid
- NIH
National Institutes of Health
- OGTT
oral glucose tolerance test
- ROC
receiver operating characteristic
Footnotes
Ms Collins assisted with acquisition, statistical analysis, and interpretation of data, provided administrative, technical, or material support, and drafted the initial manuscript; Dr Broadney assisted with acquisition, statistical analysis, and interpretation of data, provided administrative, technical, or material support, and aided with study supervision; Ms Ghane, Ms Davis, Ms Jaramillo, and Ms Brady assisted with acquisition and interpretation of data and provided administrative, technical, or material support; Ms Shank assisted with acquisition, statistical analysis, and interpretation of data and provided administrative, technical, or material support; Dr Yanovski obtained funding, conceptualized and designed the study, assisted with acquisition, statistical analysis, and interpretation of data, drafted the initial manuscript, and supervised the entire study; and all authors were responsible for critical revision of the manuscript for important intellectual content, approved the final manuscript as submitted, and agree to be accountable for all aspects of the work.
J.A. Yanovski is a Commissioned Officer in the US Public Health Service. The opinions and assertions expressed herein are those of the authors and are not to be construed as reflecting the views of the US Public Health Service.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by grant 1ZIAHD000641 from the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development with supplemental funding from the National Institutes of Health (NIH) Clinical Center Bench to Bedside Program, the Office of Research on Women’s Health, and the Office of Behavioral and Social Sciences Research of the NIH (to J.A.Y.). Ms Collins is supported by the NIH Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from the Doris Duke Charitable Foundation (grant 2014194), Genentech, Elsevier, and other private donors. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the article; or decision to submit the article for publication. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Dabelea D, Mayer-Davis EJ, Saydah S, et al. ; SEARCH for Diabetes in Youth Study . Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA. 2014;311(17):1778–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Lawman HG, et al. Trends in obesity prevalence among children and adolescents in the United States, 1988-1994 through 2013-2014. JAMA. 2016;315(21):2292–2299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007-2008 to 2015-2016. JAMA. 2018;319(16):1723–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magge SN, Goodman E, Armstrong SC. Committee on Nutrition; Section on Endocrinology; Section on Obesity . The metabolic syndrome in children and adolescents: shifting the focus to cardiometabolic risk factor clustering. Pediatrics. 2017;140(2):e20171603. [DOI] [PubMed] [Google Scholar]
- 5.Styne DM, Arslanian SA, Connor EL, et al. Pediatric obesity-assessment, treatment, and prevention: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2017;102(3):709–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kackov S, Simundic AM, Gatti-Drnic A. Are patients well informed about the fasting requirements for laboratory blood testing? Biochem Med (Zagreb). 2013;23(3):326–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cantellow S, Lightfoot J, Bould H, Beringer R. Parents’ understanding of and compliance with fasting instruction for pediatric day case surgery. Paediatr Anaesth. 2012;22(9):897–900 [DOI] [PubMed] [Google Scholar]
- 8.Dolan LM, Bean J, D’Alessio D, et al. Frequency of abnormal carbohydrate metabolism and diabetes in a population-based screening of adolescents. J Pediatr. 2005;146(6):751–758 [DOI] [PubMed] [Google Scholar]
- 9.Gustafson JK, Yanoff LB, Easter BD, et al. The stability of metabolic syndrome in children and adolescents. J Clin Endocrinol Metab. 2009;94(12):4828–4834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hershkop K, Besor O, Santoro N, Pierpont B, Caprio S, Weiss R. Adipose insulin resistance in obese adolescents across the spectrum of glucose tolerance. J Clin Endocrinol Metab. 2016;101(6):2423–2431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sinha R, Fisch G, Teague B, et al. Prevalence of impaired glucose tolerance among children and adolescents with marked obesity [published correction appears in N Engl J Med. 2002;346(22):1756]. N Engl J Med. 2002;346(11):802–810 [DOI] [PubMed] [Google Scholar]
- 12.Kim G, Caprio S. Diabetes and insulin resistance in pediatric obesity. Pediatr Clin North Am. 2011;58(6):1355–1361, ix [DOI] [PubMed] [Google Scholar]
- 13.Weiss R, Dziura J, Burgert TS, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004;350(23):2362–2374 [DOI] [PubMed] [Google Scholar]
- 14.Sabin MA, De Hora M, Holly JM, et al. Fasting nonesterified fatty acid profiles in childhood and their relationship with adiposity, insulin sensitivity, and lipid levels. Pediatrics. 2007;120(6). Available at: www.pediatrics.org/cgi/content/full/120/6/e1426 [DOI] [PubMed] [Google Scholar]
- 15.Waterhouse C, Baker N, Rostami H. Effect of glucose ingestion on the metabolism of free fatty acids in human subjects. J Lipid Res. 1969;10(5):487–494 [PubMed] [Google Scholar]
- 16.Ferrannini E, Barrett EJ, Bevilacqua S, DeFronzo RA. Effect of fatty acids on glucose production and utilization in man. J Clin Invest. 1983;72(5):1737–1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toledo-Corral CM, Alderete TL, Richey J, Sequeira P, Goran MI, Weigensberg MJ. Fasting, post-OGTT challenge, and nocturnal free fatty acids in prediabetic versus normal glucose tolerant overweight and obese Latino adolescents. Acta Diabetol. 2015;52(2):277–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frayn KN, Arner P, Yki-Järvinen H. Fatty acid metabolism in adipose tissue, muscle and liver in health and disease. Essays Biochem. 2006;42:89–103 [DOI] [PubMed] [Google Scholar]
- 19.Heptulla R, Smitten A, Teague B, Tamborlane WV, Ma YZ, Caprio S. Temporal patterns of circulating leptin levels in lean and obese adolescents: relationships to insulin, growth hormone, and free fatty acids rhythmicity. J Clin Endocrinol Metab. 2001;86(1):90–96 [DOI] [PubMed] [Google Scholar]
- 20.Meek SE, Nair KS, Jensen MD. Insulin regulation of regional free fatty acid metabolism. Diabetes. 1999;48(1):10–14 [DOI] [PubMed] [Google Scholar]
- 21.Lestradet H, Deschamps I, Giron B. Insulin and free fatty acid levels during oral glucose tolerance tests and their relation to age in 70 healthy children. Diabetes. 1976;25(6):505–508 [DOI] [PubMed] [Google Scholar]
- 22.Deschamps I, Giron BJ, Lestradet H. Blood glucose, insulin, and free fatty acid levels during oral glucose tolerance tests in 158 obese children. Diabetes. 1977;26(2):89–93 [DOI] [PubMed] [Google Scholar]
- 23.Desjeux JF, Gernez-Lestradet C, Deschamps I, Machinot S, Rolland F, Lestradet H. Circadian metabolic rhythms in obese children. Ann Nutr Metab. 1982;26(2):106–110 [DOI] [PubMed] [Google Scholar]
- 24.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000;314(314):1–27 [PubMed] [Google Scholar]
- 25.Goksuluk D, Korkmaz S, Zararsiz G, Karaagaoglu AE. easyROC: an interactive web-tool for ROC curve analysis using R language environment. R J. 2016;8(2):213–230 [Google Scholar]
- 26.Swets JA. Measuring the accuracy of diagnostic systems. Science. 1988;240(4857):1285–1293 [DOI] [PubMed] [Google Scholar]
- 27.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143(1):29–36 [DOI] [PubMed] [Google Scholar]
- 28.Brownlee KA. Statistical Theory and Methodology in Science and Engineering. 2nd ed. New York, NY: Wiley; 1965 [Google Scholar]
- 29.Barlow SE; Expert Committee . Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(suppl 4):S164–S192 [DOI] [PubMed] [Google Scholar]
- 30.Benson LJ, Baer HJ, Kaelber DC. Screening for obesity-related complications among obese children and adolescents: 1999-2008. Obesity (Silver Spring). 2011;19(5):1077–1082 [DOI] [PubMed] [Google Scholar]
- 31.Federal Interagency Forum on Child and Family Statistics America’s Children: Key National Indicators of Well-Being. Washington, DC: U.S. Government Printing Office; 2017 [Google Scholar]
- 32.Evans K, Laker MF. Intra-individual factors affecting lipid, lipoprotein and apolipoprotein measurement: a review. Ann Clin Biochem. 1995;32(pt 3):261–280 [DOI] [PubMed] [Google Scholar]
- 33.Chaussain JL, Georges P, Calzada L, Job JC. Glycemic response to 24-hour fast in normal children: III. Influence of age. J Pediatr. 1977;91(5):711–714 [DOI] [PubMed] [Google Scholar]
- 34.Bonnefont JP, Specola NB, Vassault A, et al. The fasting test in paediatrics: application to the diagnosis of pathological hypo- and hyperketotic states. Eur J Pediatr. 1990;150(2):80–85 [DOI] [PubMed] [Google Scholar]