In this study, we examine trajectories of adolescents’ diabetes-related emotional distress and assess predictors of adaptive and maladaptive patterns over a 16-month timespan.
Abstract
BACKGROUND AND OBJECTIVES:
Many adolescents with type 1 diabetes experience diabetes-related distress (DRD; the negative emotional reactions specific to managing diabetes), but most research on DRD among adolescents relies on cross-sectional data. We assess adolescents’ longitudinal DRD patterns and predictors of chronic DRD.
METHODS:
This secondary analysis of data from a depression prevention clinical trial included 264 adolescents with type 1 diabetes randomly assigned to a resilience or education intervention (mean age: 15.7 ± 1.1 years; 59.8% female). Youth reported their DRD at 5 assessments over 16 months. Using latent class growth analysis, we classified adolescents into trajectory groups according to baseline starting point (intercept) and rate of change (slope) of DRD. We examined bivariate associations between trajectory group membership and demographic and clinical factors. Baseline predictors of chronic DRD were assessed via multiple logistic regression.
RESULTS:
Participants were classified into 4 groups: stable high DRD (7.2%; high intercept, flat slope), stable moderate DRD (28.0%; above-average intercept, flat slope), improving DRD (33.7%; average intercept, downward slope), and low DRD (31.1%; below-average intercept, downward slope). Lower hemoglobin A1c, greater adherence, fewer socioemotional difficulties, and more adaptive coping distinguished the improving and low DRD trajectories. Chronic DRD patterns were associated with female sex and higher depressive symptoms and hemoglobin A1c.
CONCLUSIONS:
In this study of adolescents’ DRD trajectories during and after a psychoeducational intervention, one-third of youth were classified as having chronic, elevated DRD. Links with multiple clinical factors support efforts for routine DRD screening and comprehensive interventions for distressed youth.
What’s Known on This Subject:
Diabetes-related distress (DRD), the common negative emotional reactions specific to managing diabetes, is associated with adolescents' type 1 diabetes outcomes. Yet little is known about stability or change in DRD longitudinally and what predicts these patterns in this age group.
What This Study Adds:
Although this study specifically sampled youth without diagnosed depression, one-third of participants experienced chronic, elevated levels of DRD over time. A sizable subset of youth may need more intensive intervention to address DRD and concomitant risk factors.
Despite significant medical advances in the treatment of type 1 diabetes (T1D), fewer than one-quarter of adolescents achieve clinically recommended levels of glycemic control,1 putting many youth at risk for future kidney damage, cardiovascular disease, and other serious complications.2 A potential area of intervention focus concerns psychosocial vulnerabilities such as depression, anxiety, and diabetes-related family conflict,3–5 given their associations with problematic self-management for many adolescents with T1D.6–9 Diabetes-related distress (DRD) (the frustration, helplessness, and other negative emotional experiences of managing diabetes) is another common challenge and is related to suboptimal diabetes outcomes among adolescents10,11 even after accounting for the effects of depression.12
Change in DRD over time has been assessed in few studies. Longitudinal analysis is needed to examine stability, rate of change, and factors predicting DRD severity or improvement, guiding potential intervention strategies. Trajectory analysis, which is used to classify groups of individuals on the basis of their patterns of change on a given outcome,13 has not been used to study DRD among adolescents. In a study in which DRD trajectories were assessed among adults with type 2 diabetes, researchers found small subgroups whose DRD trajectories indicated potential clinical concern; these included 2.4% with persistently severe DRD and 6.5% with moderate but increasing DRD.14 In one analysis of a related construct (acute diabetes-specific stress) among 59 adolescents with newly diagnosed T1D, researchers found little change over 1 year and null associations with self-care behaviors and glycemic control, perhaps because of the small sample size and reliance on a single-item stress measure.15
Using a larger sample studied over a longer period of time, and using a validated DRD measure, we examined longitudinal patterns of DRD among youth without a history of depression participating in a comparative efficacy trial of 2 psychoeducational interventions. In this secondary analysis of the intervention trial data, there were 3 aims: (1) to characterize adolescents’ trajectories of DRD by starting point and rate of change over time, (2) to examine associations between trajectory group membership and demographic and clinical characteristics of youth, and (3) focusing on youth with elevated and persistent patterns of DRD, to identify baseline predictors of these chronic forms of DRD.
Methods
Study Population
The sample included 264 adolescents with T1D participating in the STePS depression prevention study.16,17 The study was conducted at 2 sites in metropolitan areas of the United States and recruited participants via mailings, diabetes clinic flyers, and hospital Web sites offering youth the opportunity to learn diabetes management strategies. Included youth were required to be age 14 to 18, at least 1 year postdiabetes diagnosis, to understand English, and to be prescribed at least 0.5 U per 1 kg daily insulin. Exclusion criteria were current antidepressant medication and previous diagnosis with major depression, a developmental disorder, other major mental disorder, or other chronic illness not including thyroid or celiac disease.
Study Procedures
Study procedures were approved by the study sites’ institutional review boards. Youth were randomly assigned to 1 of 2 interventions conducted in 9 group sessions every 2 weeks. The resilience intervention, delivered by a master’s-level clinician, taught cognitive behavioral skill building for handling general and diabetes-related stressors, whereas participants in the education intervention received advanced diabetes information from a certified diabetes educator. Participants completed a total of 5 assessments over 16 months: scheduled at baseline, immediately postintervention (baseline plus 4.5 months), and then at 4 months, 8 months, and 1 year postintervention. During assessment visits, participants completed surveys privately using an electronic survey. Participant retention at the end of 16 months was high; 92.4% of youth (n = 244) were actively participating, 16 (6.1%) were lost to follow-up, and 4 (1.5%) formally withdrew.
Measures
DRD
Youth completed the 26-item Problem Areas in Diabetes-Teen version,18 which is used to assess past-month emotional difficulties specific to living with diabetes (eg, worry about future complications, being overwhelmed by the diabetes regimen, lack of perceived control over blood glucose or eating). Scores can range from 26 to 156, with higher values signifying more DRD.
Demographics
We evaluated age, sex, minority race and ethnicity (minority or white), caregiver-reported mother’s education (college graduate or not), caregiver-reported family income (income <$50 000 vs ≥$50 000), and family composition (2-caregiver household or other).
Diabetes-Related Characteristics
Diabetes variables were diabetes duration in years and a dichotomous variable for insulin regimen (pump or injections).
Hemoglobin A1C
A capillary blood sample was obtained during assessment visits and processed at the central laboratory (Diabetes Diagnostic Laboratory at the University of Missouri; reference range = 4.0%–6.0%).
Adherence
We estimated blood glucose checks per day from the 14-day history recorded by participants’ glucometers and downloaded at baseline. Self-care behaviors were rated by youth on the Self-Care Inventory,19 a 15-item measure of how well over the last 1 to 2 months youth followed self-management recommendations such as checking blood glucose. Response options ranged from 1 (“never”) to 5 (“always”) on a 5-point scale.
Depressive and Anxious Symptoms
Youth completed the 27-item Children’s Depression Inventory20 and the 20-item State Trait Anxiety Inventory-Trait subscale.21
Diabetes-Related Family Conflict
Participants rated the amount they argue with family members about diabetes self-management tasks using the 20-item Diabetes Family Conflict Scale.4 Response options ranged from 1 (“almost never argue”) to 3 (“almost always argue”).
Coping Efficacy
Youth appraised their coping skills using the 8-item Coping Efficacy Questionnaire.22 Responses were made on a 4-point scale from 1 (eg, “not at all good”) to 4 (eg, “very good).
Problem-Solving
To capture problem-solving style and skill, we used the total score on the 25-item Social Problem-Solving Inventory–Revised Short Form.23 Higher scores signified more adaptive problem-solving ability.
Analytic Plan
To describe DRD trajectories (aim 1), we used latent class growth analysis (LCGA) to classify individuals on the basis of their starting point (intercept) and rate of change over time (slope).13,24 In LCGA, a series of models are estimated with a prespecified number of trajectory groups (classes). Model optimization attempts to form classes that vary maximally from each other while remaining homogeneous within class. LCGA differs from growth mixture modeling by forcing within-class variability (ie, intercept and slope variances) to be fixed to 0. In our preliminary analyses, models with more classes had convergence difficulties when variances were freed, a common issue with smaller samples, and therefore LCGA was pursued.
Models with increasing numbers of classes were tested and compared on the basis of conventional criteria.25,26 Improved fit was indicated by reduction in the Bayesian information criterion (BIC) and the approximate weight of evidence criterion (AWE). Significant results (P < .05) for the Lo-Mendell-Rubin likelihood ratio test (LRT) and the bootstrap likelihood ratio test (BLRT) indicated improvement in model fit compared with the solution with one fewer class. We also used entropy, an estimate of how well each model separated individuals into classes, as a criterion for model selection, with values closer to 1.0 preferred. Finally, we assessed each model solution for clinically relevant considerations, including the number of individuals in the smallest class. In LCGA, individuals are assigned a likelihood of membership to each class (posterior probability). However, for descriptive purposes, we assigned each individual a class membership on the basis of the highest posterior probability, assuming that average posterior probabilities were high, which suggests good separation by the model. Quadratic and cubic growth terms were added in a stepwise fashion to determine if these were significant and improved model fit. LCGA models were estimated in MPlus statistical analysis software, version 7.11, by using full-information maximum-likelihood estimation to handle missing data.27
To examine associations between trajectory group membership and demographic and clinical factors (aim 2), we primarily focused on baseline characteristics by comparing these variables across trajectory groups, using χ2 statistics and analysis of variance, as appropriate. We also examined depressive symptoms and hemoglobin A1c (HbA1C) within each trajectory group longitudinally over the study period. To assess differential growth in depressive symptoms and HbA1C by class, we estimated a mixed effects model for each outcome, with a random intercept and slope for individuals across time points (assuming an unstructured covariance matrix) and by using trajectory group membership, time (continuous), and group × time as predictors.
To identify predictors of persistent, elevated DRD (aim 3), we used multiple logistic regression in which the outcome criterion was a combination of trajectory groups exhibiting more chronic patterns of DRD, and all other groups were a single reference category. Predictors were baseline characteristics. We conducted analyses for aims 2 and 3 in SPSS statistical analysis software, version 24 (IBM SPSS Statistics, IBM Corporation).28
Results
Sample Characteristics
Participants were of mean age 15.7 ± 1.1 years at the start of the study (Table 1). More girls than boys enrolled (59.8% vs 40.2%). One-third of youth were nonwhite, and 16.7% came from families earning <$50 000 annual income. Mean diabetes duration was 6.9 ± 4.0 years. Most participants (70.1%) administered insulin using a pump rather than injections. Average HbA1C (9.1% ± 1.9%) was comparable to other adolescent samples1 but higher than the 7.5% clinical guideline for pediatrics.29 Adolescents checked blood glucose on average 3.7 ± 2.4 times per day.
TABLE 1.
Participants’ Baseline Characteristics (N = 264)
| Characteristics | Education Intervention (n = 131) | Resilience Intervention (n = 133) | All Participants (N = 264) |
|---|---|---|---|
| Age in y, mean ± SD | 15.7 ± 1.1 | 15.7 ± 1.1 | 15.7 ± 1.1 |
| Girls, % | 60.3 | 59.4 | 59.8 |
| Race and ethnicity, % | |||
| White, non-Hispanic | 66.4 | 64.7 | 65.5 |
| African American | 14.5 | 14.3 | 14.4 |
| Hispanic | 10.7 | 11.3 | 11.0 |
| Asian American or Pacific Islander | 2.3 | 2.3 | 2.3 |
| Indian American or Alaskan Native | 0 | 2.3 | 1.1 |
| Reported as “other” | 6.1 | 5.3 | 5.7 |
| Low family income, % <$50 000 | 14.9 | 18.5 | 16.7 |
| Mother’s education, % college graduate | 57.3 | 65.4 | 61.4 |
| Family composition, % 2-caregiver household | 84.0a | 73.7 | 78.8 |
| Diabetes duration in y, mean ± SD | 6.5 ± 3.9 | 7.3 ± 4.2 | 6.9 ± 4.0 |
| Insulin regimen, % | |||
| Injections | 32.1 | 27.8 | 29.9 |
| Insulin pump | 67.9 | 72.2 | 70.1 |
| DRD, mean ± SD | 74.2 ± 25.0 | 72.1 ± 28.3 | 73.1 ± 26.7 |
| HbA1C %, mean ± SD | 9.1 ± 2.0 | 9.1 ± 1.9 | 9.1 ± 1.9 |
| Blood glucose monitoring frequency, daily checks, mean ± SD | 3.5 ± 2.2 | 3.9 ± 2.5 | 3.7 ± 2.4 |
| Self-care behaviors, mean ± SD | 54.7 ± 7.1 | 53.4 ± 8.5 | 54.1 ± 7.8 |
| Depressive symptoms, mean ± SD | 7.9 ± 6.3 | 7.6 ± 6.1 | 7.7 ± 6.2 |
| Anxious symptoms, mean ± SD | 37.9 ± 11.0 | 37.2 ± 10.4 | 37.6 ± 10.7 |
| Diabetes-related family conflict, mean ± SD | 27.6 ± 6.0 | 27.7 ± 8.1 | 27.7 ± 7.1 |
| Coping efficacy, mean ± SD | 24.8 ± 4.0 | 24.6 ± 4.4 | 24.7 ± 4.2 |
| Problem-solving, mean ± SD | 12.8 ± 2.5 | 13.0 ± 2.8 | 12.9 ± 2.7 |
Participants in the education intervention were more likely to have 2 caregivers in the home (P = .04). Other variables did not significantly differ across intervention groups.
Aim 1: DRD Trajectories
LCGA models were estimated for 1 to 5 classes (Supplemental Table 4). Decreasing BIC and AWE, and significant BLRT tests, suggested improved fit with a greater number of classes, although the significant LRT results for 2-class and 4-class solutions suggested superiority of these to other models. The 3-, 4-, and 5-class models all contained a small trajectory group (<60 individuals) with high DRD levels over time. In comparing these 3 models, the 4-class solution offered greater differentiation than the 3-class solution of individuals with elevated DRD because it had 2 elevated DRD classes. We saw this differentiation of individuals with elevated DRD as more clinically informative. The 5-class solution contained 2 low-DRD classes, which we did not see as clinically informative compared with the 4-class solution, which contained 1 low-DRD class. Therefore, the 4-class solution was selected. Addition of quadratic and cubic terms did not significantly improve fit. Average posterior probabilities for most likely group membership were high (0.86–0.98), supporting subsequent analyses describing trajectory groups according to their most likely members.
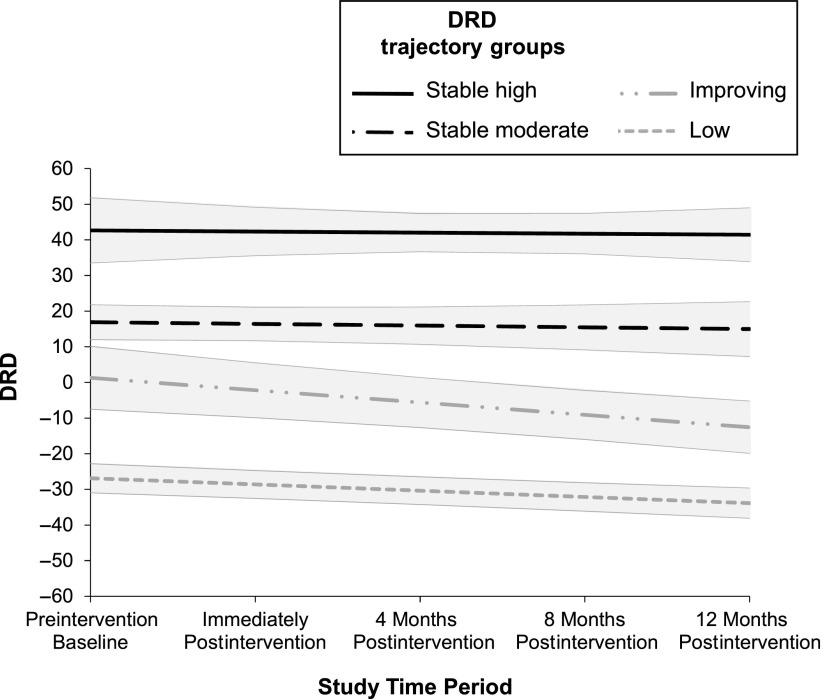
Plots of the 4 trajectory groups are shown in Fig 1. (Additionally, in Supplemental Table 5 we provide model parameter estimates and in Supplemental Fig 2 we present raw trajectory data for all participants.) To increase interpretability of the intercept, DRD values at all time points were centered at the baseline DRD grand mean. Therefore, an intercept close to 0 (ie, nonsignificant) denoted that the given trajectory group reported average levels of DRD at baseline, whereas a significantly positive (or negative) intercept indicated above-average (or below-average) DRD at baseline. Participant groupings were as follows: 7.2% stable high DRD with a high starting point (estimate 42.64, P < .001) and flat slope (estimate −0.31, P = .85); 28.0% stable moderate DRD with an above-average starting point (estimate 16.89, P < .001) and flat slope (estimate −0.48, P = .62); 33.7% improving DRD with an average starting point (estimate 1.29, P = .78) and downward slope (estimate −3.47, P < .01); and 31.1% low DRD with a below-average starting point (estimate −26.89, P < .001) and downward slope (estimate −1.75, P < .001).
FIGURE 1.
DRD estimates for the 4 distress trajectory groups with bands indicating 95% confidence intervals. The y-axis represents DRD scores as compared with the overall baseline average.
Aim 2: Associations Between Trajectory Group and Participant Characteristics
When assessed according to baseline demographic and clinical characteristics (Table 2), trajectory groups significantly differed from each other on sex, HbA1C, self-care behaviors, and all socioemotional variables but not on other characteristics.
TABLE 2.
Differences Among DRD Trajectory Groups
| Baseline Characteristic | Stable High DRD (n = 19) | Stable Moderate DRD (n = 74) | Improving DRD (n = 89) | Low DRD (n = 82) | P |
|---|---|---|---|---|---|
| Girl,a % | 89.5 | 68.9 | 64.0 | 40.2 | <.001b |
| Minority race or ethnicity, % | 31.6 | 37.8 | 29.2 | 29.3 | .62 |
| Low family income, % | 31.6 | 17.6 | 11.2 | 12.2 | .17 |
| College-graduate mother, % | 63.2 | 59.5 | 62.9 | 61.0 | .97 |
| Two-caregiver household, % | 63.2 | 77.0 | 77.5 | 85.4 | .16 |
| Injection insulin regimen, % | 21.1 | 39.2 | 29.2 | 24.4 | .18 |
| Age in y, mean ± SD | 15.4 ± 1.1 | 15.8 ± 1.0 | 15.8 ± 1.1 | 15.7 ± 1.1 | .60 |
| Diabetes duration in y, mean ± SD | 6.2 ± 3.9 | 6.8 ± 4.1 | 6.9 ± 4.0 | 7.2 ± 4.1 | .81 |
| HbA1C %,c,d mean ± SD | 10.0 ± 1.7 | 10.1 ± 2.0 | 9.0 ± 1.8 | 8.4 ± 1.5 | <.001b |
| Daily blood glucose checks, mean ± SD | 3.2 ± 1.8 | 3.3 ± 2.3 | 4.0 ± 2.6 | 3.9 ± 2.2 | .20 |
| Self-care behaviors,c,e mean ± SD | 48.8 ± 7.6 | 51.1 ± 6.9 | 53.9 ± 7.7 | 58.0 ± 7.0 | <.001b |
| Depressive symptoms,f mean ± SD | 16.5 ± 6.8 | 10.5 ± 5.6 | 7.2 ± 5.0 | 3.8 ± 4.0 | <.001b |
| Anxious symptoms,f mean ± SD | 51.3 ± 9.0 | 42.4 ± 9.2 | 37.1 ± 9.7 | 30.8 ± 7.8 | <.001b |
| Diabetes-related family conflict,c,g mean ± SD | 34.5 ± 8.2 | 29.3 ± 5.4 | 27.7 ± 6.1 | 24.7 ± 7.8 | <.001b |
| Coping efficacy,c,h mean ± SD | 21.3 ± 5.1 | 22.7 ± 3.6 | 24.7 ± 3.7 | 27.2 ± 3.4 | <.001b |
| Problem solving,f mean ± SD | 10.2 ± 2.7 | 12.0 ± 2.4 | 13.0 ± 2.6 | 14.2 ± 2.2 | <.001b |
Girls were more likely to be in the stable high DRD group and less likely to be in the low DRD group than boys.
Significant at P < .003 (Bonferroni correction for multiple comparisons).
Low DRD differed from all other groups.
Improving DRD differed from stable moderate DRD.
Improving DRD differed from stable high DRD.
All groups differed from each other.
Stable high DRD differed from all other groups.
Improving DRD differed from all other groups.
Youth in the stable high DRD group were nearly all girls (89.5%). At baseline, both the stable high and stable moderate DRD groups had the highest HbA1C and reported the lowest levels of self-care behaviors. The stable high and stable moderate DRD groups reported the highest levels of depressive and anxious symptoms and family conflict and the lowest levels of problem-solving ability, with the stable high DRD group reporting the most maladjustment. Coping efficacy was similar in both the stable high and stable moderate DRD groups and lower than that of the improving DRD and low DRD groups.
The improving DRD group had worse HbA1C, self-care behaviors, depressive and anxious symptoms, diabetes-related family conflict, coping efficacy, and problem-solving ability than the low DRD group but fared better on these variables compared with the stable high and stable moderate DRD groups. Adolescents classified as low DRD were more likely to be boys (59.8%) than girls. The low DRD group had the lowest baseline HbA1C, the lowest levels of depressive and anxious symptoms and diabetes-related family conflict, and the highest levels of self-care behaviors, coping efficacy, and problem-solving ability.
In longitudinal analyses, depressive symptoms significantly increased in the stable moderate DRD group compared with the low DRD reference group (from mixed effects model: group × time B = 0.98; 95% confidence interval: 0.11–1.8; P = .03), whereas no significant change was seen differentially in other groups. Except for the stable moderate DRD group, depressive symptoms were flat across time (time effect P = .97). HbA1C remained flat across the 16-month study period regardless of trajectory group (group × time effect P values > .10; time effect P = .99).
Aim 3: Baseline Predictors of Chronic DRD
For the multiple logistic regression model examining what variables distinguished more-severe DRD trajectory groups (Table 3), we combined the stable high and stable moderate DRD groups as the outcome criterion (called chronic DRD), leaving the 2 remaining groups (improving DRD and low DRD) as the combined reference category (lower risk).
TABLE 3.
Predicting Membership to Chronic DRD Trajectories
| Baseline Characteristic | Adjusted Odds Ratio | 95% Confidence Interval | P |
|---|---|---|---|
| Girl (ref: boy)a | 2.82** | 1.40–5.71 | .004 |
| Age in yb | 0.89 | 0.65–1.21 | .46 |
| Minority race or ethnicity (ref: white, non-Hispanic)a | 1.13 | 0.51–2.51 | .77 |
| Low family income (ref: ≥$50 000)a | 1.45 | 0.51–4.15 | .49 |
| College-graduate mother (ref: some college or less)a | 1.77 | 0.81–3.88 | .16 |
| Two-caregiver household (ref: other)a | 0.80 | 0.34–1.87 | .61 |
| Diabetes duration in yb | 1.04 | 0.95–1.14 | .42 |
| Injection insulin regimen (ref: insulin pump)a | 1.15 | 0.56–2.38 | .71 |
| HbA1C %b | 1.28* | 1.05–1.56 | .01 |
| Daily blood glucose checksb | 1.04 | 0.88–1.23 | .63 |
| Self-care behaviors | 0.73 | 0.49–1.08 | .12 |
| Depressive symptoms | 2.03* | 1.15–3.60 | .02 |
| Anxious symptoms | 1.29 | 0.73–2.29 | .38 |
| Diabetes-related family conflict | 1.22 | 0.82–1.81 | .33 |
| Coping efficacy | 0.83 | 0.50–1.39 | .47 |
| Problem-solving | 0.93 | 0.59–1.45 | .74 |
| Constant | 0.45** | 0.27–0.74 | .002 |
The outcome criterion of the multiple logistic regression model was membership to either the stable high DRD or stable moderate DRD trajectory groups (chronic DRD). The reference group was a combined “lower risk” group (improving DRD and low DRD trajectory groups). Continuous predictors were standardized unless otherwise indicated. The model was also adjusted by using an effect-coded variable for intervention (0.5 = yes, −0.5 = no).
Effect-coded dichotomous variable.
Grand mean centered.
P < .05; ** P < .01.
After adjusting for whether participants received the resilience or education intervention, 3 significant independent predictors emerged. Girls had nearly 3 times higher odds of being in the chronic DRD group (adjusted odds ratio: 2.82; 95% confidence interval: 1.40–5.71). A 1-point higher baseline HbA1C percentage was associated with 28% higher odds of being in the chronic DRD group (adjusted odds ratio: 1.28; 95% confidence interval: 1.05–1.56). Finally, a 1-SD higher score on depressive symptoms was associated with twice the odds of being in the chronic DRD group (adjusted odds ratio: 2.03; 95% confidence interval: 1.15–3.60).
Discussion
In this study of adolescents’ DRD trajectories, we classified two-thirds of youth as improving over time; these youth tended to start at low to average DRD levels. One-third of youth were classified as having significant, chronic DRD; on average, they reported moderate or high levels of DRD at baseline, their DRD did not significantly improve over time, and they were more likely to start the study with above-target glycemic control, less-frequent self-care behaviors, elevated psychological distress, and more maladaptive coping. There was also a small subset of these youth (stable high DRD, 7% of the whole sample), nearly all girls, who had particularly elevated symptoms of depression and anxiety, high diabetes-related family conflict, and poor problem-solving. Despite the small size of this group, their scores on these variables were significantly worse than those for another trajectory group with elevated DRD (stable moderate DRD), suggesting a clinically meaningful distinction between youth with high versus moderate levels. Youth in the stable moderate DRD trajectory group also had positive growth in depressive symptoms over time, suggesting that elevated DRD can be an early sign of later difficulties with depression.
By describing common longitudinal patterns of DRD and the characteristics of youth who have these patterns, in this study we provide a typology of adolescents with T1D that is clinically informative. Female sex, baseline HbA1C, and depressive symptoms independently predicted membership to a chronic DRD trajectory group. Girls’ vulnerability to DRD is consistent with broader sex differences in the prevalence of emotional difficulties in adolescence.30 Most girls in the current study were classified into one of the lower or improving DRD trajectory groups, demonstrating that elevated DRD is not an expected experience for youth of either sex. However, there may be a benefit to considering sex in interventions designed particularly for highly distressed youth with T1D. The robust positive associations found in the current study between chronic DRD and elevated HbA1C and depressive symptoms is consistent with evidence finding overlap among these 3 problem areas.31,32 Of note, youth with past major depression were excluded from the current study, which was originally devised to test a depression prevention intervention. In spite of this exclusion, 35% of youth in this study experienced persistent, problematic levels of DRD, which underscores the importance of specifically screening for and treating DRD.
It is possible that some DRD improvement in the current study was driven by youth participation in the STePS study interventions, particularly the resilience intervention, which in a previous analysis was associated with more reduction in DRD compared with the education intervention.17 In the absence of an intervention, DRD often persists and can worsen over time, according to studies with adults.14,33,34 The resilience intervention tested in the STePS study contained components that have been shown to help address DRD, including cognitive-behavioral skills.10,35 Targeted clinical screening of DRD with existing validated measures36,37 can efficiently identify adolescents needing intervention.
Study limitations should be considered. Fit and classification indices did not definitively indicate which of the trajectory models was the best solution for the data. Our clinical judgment in selecting the 4-class model may have introduced bias. The most severe DRD trajectory group had only 19 members, limiting the generalizability of this group’s attributes. In this study, we also could not address how DRD might unfold over time outside the context of an intervention because there was no nonintervention control group. Future investigation of DRD in a larger, nonintervention sample over a longer period of time would help clarify the nature of DRD trajectories, including the degree of longitudinal change or fluctuation.
Conclusions
In this study, we identify clinically meaningful subgroups of adolescents with T1D. Not only do we describe clinical profiles of importance to diabetes research and intervention, but we also provide an example of how to consider demographic, clinical, and psychosocial variables in relation to chronic disease-specific distress more generally, informing prevention and treatment across pediatric subspecialties. Investigation is needed of what distinguishes those who recover from elevated disease-specific distress earlier in life from those who do not. Adolescence may provide an opportune window to intervene, setting the stage for positive adjustment in the context of chronic disease management across the life span.
Glossary
- AWE
approximate weight of evidence criterion
- BIC
Bayesian information criterion
- BLRT
bootstrap likelihood ratio test
- DRD
diabetes-related distress
- HbA1C
hemoglobin A1c
- LCGA
latent class growth analysis
- LRT
Lo-Mendell-Rubin likelihood ratio test
- T1D
type 1 diabetes
Footnotes
Dr Iturralde developed the study concept, conducted the literature review, analyzed and interpreted the data, and wrote the manuscript; Drs Hood and Weissberg-Benchell served as site principal investigators, designed the parent study, obtained ethical approvals, oversaw data collection, and contributed to manuscript revision; Dr Rausch contributed to research design, data management, and manuscript revision; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
FINANCIAL DISCLOSURE: Dr Hood receives research support from Dexcom, Inc for an investigator-initiated study and consultant fees from Lilly Innovation Center and Roche. The other authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK090030). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Miller KM, Foster NC, Beck RW, et al. ; T1D Exchange Clinic Network . Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabetes Care. 2015;38(6):971–978 [DOI] [PubMed] [Google Scholar]
- 2.Nathan DM; DCCT/EDIC Research Group . The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care. 2014;37(1):9–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hood KK, Huestis S, Maher A, Butler D, Volkening L, Laffel LMB. Depressive symptoms in children and adolescents with type 1 diabetes: association with diabetes-specific characteristics. Diabetes Care. 2006;29(6):1389–1391 [DOI] [PubMed] [Google Scholar]
- 4.Hood KK, Butler DA, Anderson BJ, Laffel LM. Updated and revised diabetes family conflict scale. Diabetes Care. 2007;30(7):1764–1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reynolds KA, Helgeson VS. Children with diabetes compared to peers: depressed? Distressed? A meta-analytic review. Ann Behav Med. 2011;42(1):29–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herzer M, Hood KK. Anxiety symptoms in adolescents with type 1 diabetes: association with blood glucose monitoring and glycemic control. J Pediatr Psychol. 2010;35(4):415–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hilliard ME, Guilfoyle SM, Dolan LM, Hood KK. Prediction of adolescents’ glycemic control 1 year after diabetes-specific family conflict: the mediating role of blood glucose monitoring adherence. Arch Pediatr Adolesc Med. 2011;165(7):624–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGrady ME, Laffel L, Drotar D, Repaske D, Hood KK. Depressive symptoms and glycemic control in adolescents with type 1 diabetes: mediational role of blood glucose monitoring. Diabetes Care. 2009;32(5):804–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stewart SM, Rao U, Emslie GJ, Klein D, White PC. Depressive symptoms predict hospitalization for adolescents with type 1 diabetes mellitus. Pediatrics. 2005;115(5):1315–1319 [DOI] [PubMed] [Google Scholar]
- 10.Hagger V, Hendrieckx C, Sturt J, Skinner TC, Speight J. Diabetes distress among adolescents with type 1 diabetes: a systematic review. Curr Diab Rep. 2016;16(1):9. [DOI] [PubMed] [Google Scholar]
- 11.Iturralde E, Weissberg-Benchell J, Hood KK. Avoidant coping and diabetes-related distress: pathways to adolescents’ type 1 diabetes outcomes. Health Psychol. 2017;36(3):236–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hagger V, Hendrieckx C, Cameron F, Pouwer F, Skinner TC, Speight J. Diabetes distress is more strongly associated with HbA1c than depressive symptoms in adolescents with type 1 diabetes: results from Diabetes MILES Youth-Australia. Pediatr Diabetes. 2018;19(4):840–847 [DOI] [PubMed] [Google Scholar]
- 13.Nagin DS. Analyzing developmental trajectories: a semiparametric, group-based approach. Psychol Methods. 1999;4(2):139. [DOI] [PubMed] [Google Scholar]
- 14.Lipscombe C, Burns RJ, Schmitz N. Exploring trajectories of diabetes distress in adults with type 2 diabetes; a latent class growth modeling approach. J Affect Disord. 2015;188:160–166 [DOI] [PubMed] [Google Scholar]
- 15.Yi-Frazier JP, Cochrane K, Whitlock K, et al. Trajectories of acute diabetes-specific stress in adolescents with type 1 diabetes and their caregivers within the first year of diagnosis. J Pediatr Psychol. 2018;43(6):645–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weissberg-Benchell J, Rausch J, Iturralde E, Jedraszko A, Hood K. A randomized clinical trial aimed at preventing poor psychosocial and glycemic outcomes in teens with type 1 diabetes (T1D). Contemp Clin Trials. 2016;49:78–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hood KK, Iturralde E, Rausch J, Weissberg-Benchell J. Preventing diabetes distress in adolescents with type 1 diabetes: results 1 year after participation in the STePS program. Diabetes Care. 2018;41(8):1623–1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weissberg-Benchell J, Antisdel-Lomaglio J. Diabetes-specific emotional distress among adolescents: feasibility, reliability, and validity of the problem areas in diabetes-teen version. Pediatr Diabetes. 2011;12(4 pt 1):341–344 [DOI] [PubMed] [Google Scholar]
- 19.Weinger K, Butler HA, Welch GW, La Greca AM. Measuring diabetes self-care: a psychometric analysis of the Self-Care Inventory-Revised with adults. Diabetes Care. 2005;28(6):1346–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kovacs M, Staff M. Children’s Depression Inventory (CDI): Technical Manual Update. North Tonawanda, NY: Multi-Health Systems, Inc; 2003 [Google Scholar]
- 21.Spielberger C. Preliminary Manual for the State-Trait Anxiety Inventory for Children. Palo Alto, CA: Consulting Psychologist Press; 1973 [Google Scholar]
- 22.Sandler IN, Tein JY, Mehta P, Wolchik S, Ayers T. Coping efficacy and psychological problems of children of divorce. Child Dev. 2000;71(4):1099–1118 [DOI] [PubMed] [Google Scholar]
- 23.D’Zurilla TJ, Nezu AM, Maydeu-Olivares A. Social Problem-Solving Inventory—Revised: Technical Manual. North Tonawanda, NY: Multi-Health Systems; 2002 [Google Scholar]
- 24.Berlin KS, Parra GR, Williams NA. An introduction to latent variable mixture modeling (part 2): longitudinal latent class growth analysis and growth mixture models. J Pediatr Psychol. 2014;39(2):188–203 [DOI] [PubMed] [Google Scholar]
- 25.Jung T, Wickrama K. An introduction to latent class growth analysis and growth mixture modeling. Soc Personal Psychol Compass. 2008;2(1):302–317 [Google Scholar]
- 26.Masyn KE. Latent class analysis and finite mixture modeling In: Little TD, ed. The Oxford Handbook of Quantitative Methods in Psychology. Vol 2 New York, NY: Oxford University Press; 2013:551 [Google Scholar]
- 27.Muthén L, Muthén B. Mplus User’s Guide. 7th ed. Los Angeles, CA: Muthén & Muthén; 2012 [Google Scholar]
- 28.Corp IBM. IBM SPSS Statistics for Windows, Version 24. Armonk, NY: IBM Corp; 2016 [Google Scholar]
- 29.American Diabetes Association Standards of medical care in diabetes 2016. Diabetes Care. 2016;39(suppl 1):S1–S112 [DOI] [PubMed] [Google Scholar]
- 30.Nolen-Hoeksema S, Girgus JS. The emergence of gender differences in depression during adolescence. Psychol Bull. 1994;115(3):424–443 [DOI] [PubMed] [Google Scholar]
- 31.Baucom KJ, Queen TL, Wiebe DJ, et al. Depressive symptoms, daily stress, and adherence in late adolescents with type 1 diabetes. Health Psychol. 2015;34(5):522–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hilliard ME, Yi-Frazier JP, Hessler D, Butler AM, Anderson BJ, Jaser S. Stress and A1c among people with diabetes across the lifespan. Curr Diab Rep. 2016;16(8):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fisher L, Skaff MM, Mullan JT, Arean P, Glasgow R, Masharani U. A longitudinal study of affective and anxiety disorders, depressive affect and diabetes distress in adults with Type 2 diabetes. Diabet Med. 2008;25(9):1096–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karlsen B, Bru E. The relationship between diabetes-related distress and clinical variables and perceived support among adults with type 2 diabetes: a prospective study. Int J Nurs Stud. 2014;51(3):438–447 [DOI] [PubMed] [Google Scholar]
- 35.Hood K, Iturralde E, Rausch J, Weissberg-Benchell J. Preventing diabetes distress in adolescents with type 1 diabetes: results 1 year after participation in the STePS program. Diabetes Care. 2018;41(8):1623–1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shapiro JB, Vesco AT, Weil LE, Evans MA, Hood KK, Weissberg-Benchell J. Psychometric properties of the Problem Areas in Diabetes: teen and parent of teen versions. J Pediatr Psychol. 2018;43(5):561–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sturt J, Dennick K, Due-Christensen M, McCarthy K. The detection and management of diabetes distress in people with type 1 diabetes. Curr Diab Rep. 2015;15(11):101. [DOI] [PubMed] [Google Scholar]