Etanercept in a randomized clinical trial exhibited potential benefit as an adjunctive IVIg treatment of acute KD in select prespecified subgroups.
Abstract
Video Abstract
OBJECTIVES:
Patients with Kawasaki disease can develop life-altering coronary arterial abnormalities, particularly in those resistant to intravenous immunoglobulin (IVIg) therapy. We tested the tumor necrosis factor α receptor antagonist etanercept for reducing both IVIg resistance and coronary artery (CA) disease progression.
METHODS:
In a double-blind multicenter trial, patients with Kawasaki disease received either etanercept (0.8 mg/kg; n = 100) or placebo (n = 101) subcutaneously starting immediately after IVIg infusion. IVIg resistance was the primary outcome with prespecified subgroup analyses according to age, sex, and race. Secondary outcomes included echocardiographic CA measures within subgroups defined by coronary dilation (z score >2.5) at baseline. We used generalized estimating equations to analyze z score change and a prespecified algorithm for change in absolute diameters.
RESULTS:
IVIg resistance occurred in 22% (placebo) and 13% (etanercept) of patients (P = .10). Etanercept reduced IVIg resistance in patients >1 year of age (P = .03). In the entire population, 46 (23%) had a coronary z score >2.5 at baseline. Etanercept reduced coronary z score change in those with and without baseline dilation (P = .04 and P = .001); no improvement occurred in the analogous placebo groups. Etanercept (n = 22) reduced dilation progression compared with placebo (n = 24) by algorithm in those with baseline dilation (P = .03). No difference in the safety profile occurred between etanercept and placebo.
CONCLUSIONS:
Etanercept showed no significant benefit in IVIg resistance in the entire population. However, preplanned analyses showed benefit in patients >1 year. Importantly, etanercept appeared to ameliorate CA dilation, particularly in patients with baseline abnormalities.
What’s Known on This Subject:
Intravenous immunoglobulin is effective in most patients with Kawasaki disease, but some respond poorly with continuing fever and greater propensity for persistent coronary artery abnormalities. Randomized controlled trials are needed to validate intravenous immunoglobulin adjunct therapy for multiethnic populations.
What This Study Adds:
Etanercept in a controlled trial improved clinical response in select groups of children with Kawasaki disease, including those >1 year and those with early coronary dilation. Etanercept was also safe, suggesting a positive risk/benefit profile.
Primary therapy for acute Kawasaki disease (KD) according to recently published American Heart Association (AHA) guidelines1 includes only high-dose intravenous immunoglobulin (IVIg) and aspirin. In most children with KD, this treatment eradicates inflammation and fever and, more importantly, inhibits coronary dilation. Conversely, some patients exhibit IVIg resistance, imposing substantially higher risk for life-altering and debilitating coronary artery (CA) abnormalities.1,2 Thus, various therapy intensification strategies directed at reducing IVIg resistance and CA pathology have been proposed and tested in randomized clinical trials.1,3,4
A National Institutes of Health–funded Pediatric Heart Network trial revealed that a single methyl prednisolone pulse had no enhancing effect on IVIg response either in the entire cohort or in subgroups predetermined according to age, sex, and coronary dilation status at presentation.3 In contrast, the multicenter Randomized Controlled Trial to Assess Immunoglobulin Plus Steroid Efficacy for Kawasaki Disease (RAISE), performed in Japan, revealed that a more prolonged corticosteroid course initiated along with IVIg lowered the incidence of persistent CA abnormalities.5 The RAISE regimen included 5 days of intravenous prednisolone (2 mg/kg per day) in 3 divided doses followed by at least 15 days of tapering oral prednisolone. The trial researchers defined patient risk and eligibility using a Japanese-specific algorithm.6–9 Subsequently, an AHA expert panel deemed that more research is needed to develop reliable methods for determining risk in children outside of Japan and then to test the RAISE regimen efficacy in those multiethnic populations.1 The RAISE trial also excluded subjects displaying early coronary dilation or aneurysm. Results of a recent Japanese national survey suggest that early steroid therapy increases the risk of thrombosis or rupture in patients with giant coronary aneurysms.10 Accordingly, safety concerns persist over early steroid use in patients with preexisting coronary abnormalities. Thus, an unmet clinical need remains for an adjunctive IVIg therapy, which is applicable and safe for all patients with acute KD, regardless of early CA status.3
Tumor necrosis factor (TNF) α, a proinflammatory cytokine, exhibits dramatic rises in circulating levels during acute KD and is strongly implicated as a participant in the KD inflammatory process.11–16 Therefore, TNF-α antagonism represents a potential alternative to corticosteroids as a treatment mode. Following this logic, a 2-center randomized clinical trial was used to evaluate infliximab, a monoclonal antibody against TNF-α administered intravenously, but no treatment impact on the IVIg resistance rate was found.4 These trial results revealed that TNF-α antagonism is not an effective adjunct to IVIg during early KD. However, etanercept, a soluble TNF receptor fusion protein, also antagonizes endogenous TNF. Furthermore, etanercept is used globally as an option to infliximab or even as the preferred biological product for treatment of children and adults with certain chronic inflammatory diseases.17–20 Etanercept has a substantially shorter clearance period than infliximab and is administered subcutaneously weekly to maintain steady-state therapeutic levels.21,22 Additionally, whereas infliximab rapidly promotes the development of antidrug antibodies that can hinder drug distribution and efficacy, etanercept does not have this obstacle.23–26
We therefore investigated the potential for etanercept as an adjunct to IVIg for acute KD in an open-label single-center trial.27 The pilot trial results revealed that children with KD tolerated etanercept well, suggesting clinical benefit. Pharmacokinetics in children with KD approximated the profile displayed in other pediatric populations receiving etanercept; unlike infliximab, these properties were not altered by IVIg administration. These results prompted performance of a larger randomized controlled trial supported by funding through the US Food and Drug Administration.28
Methods
Trial Design
The Etanercept as Adjunctive Treatment for Acute Kawasaki Disease (EATAK)28 is a phase 3, multicenter, placebo-controlled, double-blind investigator-initiated randomized trial (NCT00841789). EATAK was approved by institutional review boards at each participating center. An independent clinical research organization assisted in monitoring performance across multiple sites to ensure data accuracy and fidelity to the protocol. An independent data monitoring committee (Supplemental Information) performed interim safety data reviews and provided recommendations. Written consent from a parent or guardian and, when appropriate, child assent, were obtained before enrollment. The trial protocol appears in the Supplemental Information.
Study Outcomes
The primary outcome was the proportion of participants exhibiting IVIg resistance defined according to published AHA guidelines: “fever persistence (≥38°C) or recurrence greater than 36 hours and up to 7 days following completion of the IVIG infusion.” We specified subgroup analyses for this end point during study design and in advance of initial patient enrollment. Subgroups were defined by age and sex, as well as ethnic and racial demographics (Hispanic or Latino, African American [AA], Asian, non-Hispanic white, and other). Although IVIg resistance represents an objective parameter for evaluating drug response, reducing development or progression of CA dilation or aneurysm remains the primary therapeutic goal. Accordingly, changes in CA parameters provided by echocardiography served as secondary clinically relevant outcomes.
Participants
Eight pediatric centers were initiated and enrolled participants aged 2 months to 18 years who met the diagnostic criteria for complete or incomplete KD defined by AHA and American Academy of Pediatrics 2004 guidelines (see section 4.1 in the Supplemental Information).29 Patients were eligible if their IVIg treatment initiated by day 10 after fever onset or to day 12 if their fever continued with C-reactive protein (CRP) >3.0. Patients were not eligible if they received corticosteroid therapy or any other TNF-α antagonist before IVIg.
Randomization and Treatment
Each site-investigational pharmacist received a unique randomization list using size 4 blocks with a 1:1 allocation and prepared study medication maintaining double blinding. Participants received etanercept (0.8 mg/kg) or a comparable placebo volume subcutaneously shortly after IVIg infusion and then 2 more weekly doses within the protocol-specified time windows. All patients received aspirin (80–100 mg/kg per day divided every 6 hours) orally until afebrile, and then the dose was reduced to 3 to 5 mg/kg per day until study end, or longer if needed. Patients continued treatment within the protocol even if they demonstrated IVIg resistance and received a second IVIg infusion. We used a modified intention-to-treat (mITT) design including only patients who received at least 1 dose of study drug (placebo or etanercept), which excluded randomly assigned patients withdrawn before dosing.
Trial Assessments
Participants were monitored for recurrent or persistent fever (>38°C) after IVIg by axillary, rectal, or oral temperature in hospital and then recorded by parents in diaries at least daily after discharge for 14 days. Echocardiograms were obtained within 12 hours of IVIg initiation (baseline) and then at 2 and 5 to 6 weeks after receiving IVIg per protocol window. All echocardiograms were reviewed for CA dimensions for the left main CA, left anterior descending coronary artery (LAD), and right CA, and for aneurysms. Digital images were forwarded to blinded core readers at Seattle Children’s Research Institute (B.D.S. and S.B.) with high interobserver agreement who confirmed or superseded echocardiogram interpretations and measures, assigned coronary z scores using Boston criteria resident in syngo Dynamics, and entered these into the database. Laboratory studies were obtained at baseline before IVIg, at 1 to 2 days after IVIg, and then at 1, 2, and 6 weeks of follow-up. Safety and side-effect profiles were evaluated by using reported adverse events (AEs) and serious adverse events (SAEs).
Statistical Analyses
The study principal hypothesis was that etanercept significantly reduces the IVIg resistance rate when compared with the placebo. On the basis of the apparent etanercept impact in the pilot trial, we assumed a 17.4% refractory rate in the control group and predicted a 4.3% refractory rate in the etanercept group. Using these rates, 200 participants would be required to provide 80% power at a 5% 2-sided type I error rate. The primary outcome was compared by using the χ2 test for the mITT population, and the odds ratio (OR) for resistance to IVIg was calculated by using logistic regression with treatment assignment included as a fixed covariate. The t test for independent samples or the Wilcoxon test was used to compare continuous baseline characteristics across treatment groups, and the χ2 test or Fisher’s exact test were used to compare categorical variables. All P values and 95% confidence intervals (CIs) are 2-sided, and P < .05 was used as the criterion for statistical significance. Analyses were conducted by using SAS version 9.4 (SAS Institute, Inc, Cary, NC) or R version 3.3.3. Because defined subgroups were prespecified and based on previous characterizations in KD, we did not perform any adjustments for multiple comparisons. Therefore, these subgroup analyses should be considered exploratory.
CA Analyses
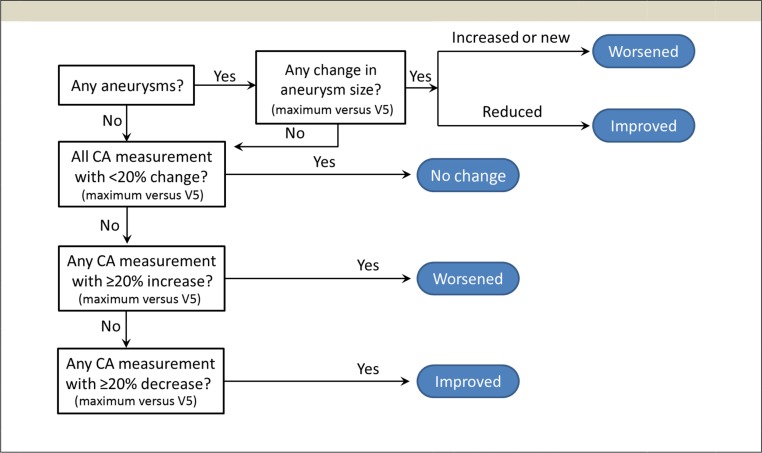
We posed a second hypothesis that etanercept reduces the progression of CA dilation or aneurysm between baseline and final echocardiogram. Coronary z scores are used clinically to evaluate baseline and change in individual arteries during KD.1 However, small increments in absolute diameter or body surface area can create large changes in the z scores.30 Also, the impact and temporal changes on each coronary in KD can differ; therefore, measures of individual coronaries will not provide an accurate estimate of overall treatment response. Furthermore, baseline z scores are linked to ultimate CA dilation. To assess changes in coronary diameters or aneurysm size, we used 2 different methods. Both included terms defining CA dilation by z score >2.5 consistent with AHA recommendations.1
We applied a generalized estimating equation (GEE) model to determine z score change values (see protocol page 36 in Supplemental Information). The GEE model is a standard approach that adjusts for the correlation between observations obtained on the same individual at multiple time points during therapy.31,32 GEE overview is provided at https://support.sas.com/rnd/app/stat/topics/gee/gee.pdf. Additionally, the GEE model accounts for interdependence among the 3 measured CAs: left main CA, LAD, and right CA. Because etanercept could have a differing magnitude of impact on change in artery z score depending on baseline diameter, terms for dilation status at baseline and the interaction between baseline dilation and treatment group were included in the model (as prespecified in the protocol in the Supplemental Information). In a separate analysis, we also applied a prespecified algorithm to define overall improvement or worsening based on at least a 20% change in absolute CA dimensions. Results of this classification were assessed by using logistic regression. For consistency, and because no definition of dilation based on absolute measurements is widely used, we also used the same definition for dilated (z >2.5) for subgroup analyses by algorithm and the GEE.
Laboratory Values
Resolution of the inflammatory parameter, CRP, and hemoglobin usually affected in KD were considered less important secondary outcomes because of their limited clinical impact. These were analyzed with the Wilcoxon rank-sum test.
Results
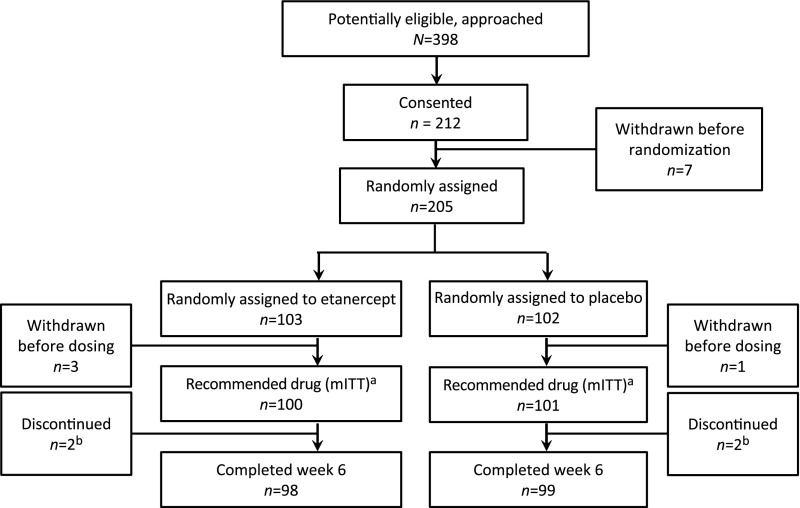
We approached 398 eligible participants and enrolled 212 from May 2009 to April 2016. Eleven were excluded before treatment before or after randomization. A total of 205 participants were randomly assigned to either placebo (102) or etanercept (103) (Fig 1). Four participants withdrew consent before receiving study medication and were not included in the mITT population. We therefore studied a mITT population with 100 participants randomly assigned to etanercept and 101 to placebo. One patient randomly assigned to etanercept through pharmacy error received the placebo. As per protocol, this patient was included in the etanercept group for mITT analyses but was included in the placebo group for safety analyses (for safety: etanercept, n = 99; placebo, n = 102). Demographics including age, sex, and ethnicity were well balanced between the treatment groups (Table 1). Twenty-two patients, 10 in the etanercept group and 12 in the placebo group, qualified as incomplete KD according to AHA criteria, and the remaining qualified with complete KD. Most participants (n = 186) received IVIg (2 g/kg) initiated within 10 days of fever onset. The remaining 15 participants demonstrated continuing fever and CRP elevation (etanercept, n = 7; placebo, n = 8), and IVIg treatment initiated between 10 and 12 days. Median days from fever onset were not significantly different (etanercept: 6.1; placebo: 5.6; P = .10).
FIGURE 1.
Approach, consent, randomization, and follow-up of participants. Four randomly assigned patients withdrew before dosing. A total of 201 patients received study medication; 4 received at least an initial dose but did not receive a second or third dose. They were included in the mITT analyses. Patients received “drug” either etanercept or placebo. a One subject was randomly assigned to etanercept but received the placebo. b In the etanercept arm, 1 subject was determined to have measles and not KD, and the parents of the other refused to attend the final follow-up visit. In the placebo arm, 2 subjects’ parents refused further dosing.
TABLE 1.
Baseline Demographic Characteristics (mITT Population)
| Characteristic | Etanercept (N = 100) | Placebo (N = 101) | Pa |
|---|---|---|---|
| Age, y (mean ± SD) | 3.77 (±2.67) | 3.66 (±2.75) | .77 |
| Age group, n (%) | |||
| Age ≥1 y | 85 (85) | 83 (82) | .59 |
| Age <1 y | 15 (15) | 18 (18) | |
| Sex, n (%) | |||
| Male | 66 (66) | 61 (60) | .41 |
| Female | 34 (34) | 40 (40) | |
| Race group, n (%) | |||
| Non-Hispanic white | 36 (36) | 43 (43) | .88 |
| AA | 12 (12) | 9 (9) | |
| Asianb | 15 (15) | 14 (14) | |
| Hispanic or Latinoc | 19 (19) | 17 (17) | |
| Other | 18 (18) | 18 (18) |
P values from the t test for continuous variables and the χ2 test for categorical variables.
Asian, overwhelmingly East Asian, not originating from the Indian subcontinent.
Hispanic, overwhelmingly of Mexican or Central American descent.
IVIg Resistance
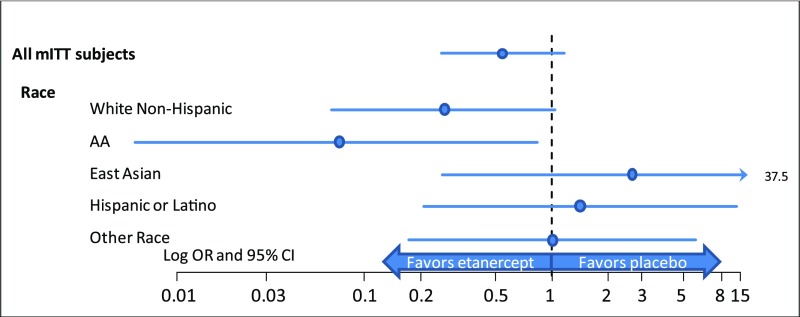
Thirty-five participants (17.4%) in the entire study cohort showed IVIg resistance, and all received a second IVIg dose. Participants receiving etanercept showed a slightly lower resistance rate (n = 13; 13%) than those receiving placebo (n = 22; 22%), although this difference was not statistically significant (P = .10; Table 2). The OR comparing IVIg resistance for etanercept and placebo in the entire study population was 0.54 (95% CI: 0.25 to 1.14; Fig 2).
TABLE 2.
Primary End Point, IVIg Response (mITT Population)
| Subgroup | Etanercept (N = 100) | Placebo (N = 101) | Pa | ||
|---|---|---|---|---|---|
| Refractory, n (%) | Responder, n (%) | Refractory, n (%) | Responder, n (%) | ||
| All mITT subjects | 13 (13) | 87 (87) | 22 (22) | 79 (78) | .10 |
| Age group | |||||
| Age <1 y | 4 (27) | 11 (73) | 3 (17) | 15 (83) | .67 |
| Age ≥1 y | 9 (11) | 76 (89) | 19 (23) | 64 (77) | .03 |
| Sex | |||||
| Male | 11 (17) | 55 (83) | 16 (26) | 45 (74) | .19 |
| Female | 2 (6) | 32 (94) | 6 (15) | 34 (85) | .28 |
| Race group | |||||
| Non-Hispanic white | 3 (8) | 33 (92) | 11 (26) | 32 (74) | .07 |
| AA | 1 (8) | 11 (92) | 5 (56) | 4 (44) | .05 |
| Asian | 3 (20) | 12 (80) | 1 (7) | 13 (93) | .60 |
| Hispanic or Latino | 3 (16) | 16 (84) | 2 (12) | 15 (88) | .99 |
| Other | 3 (17) | 15 (83) | 3 (17) | 15 (83) | .99 |
P value is based on the χ2 test or Fisher’s exact test as appropriate. All values for percent are rounded to the nearest whole decimal, and P values to the nearest hundredth. The unrounded P value for AA is .046, achieving significance by our protocol specifications.
FIGURE 2.
Primary end point ORs and 95% CIs for IVIg resistance in prespecified ethnic subgroups. A favorable and significant response to etanercept is shown for AAs.
Comparisons of proportions with IVIg resistance within prespecified subgroups appear in Table 2. No significant treatment differences occurred for sex. However, etanercept significantly reduced the IVIg resistance rate (P = .032) in subjects >1 year of age (OR: 0.40; 95% CI: 0.17–0.94), but not in the 33 participants (16%) <1 year of age (etanercept, n = 15; placebo, n = 18). Variability in fever response to IVIg therapy occurred according to race and ethnicity with resistance rates ranging from 7.1% in Asians to 56.6% in AAs (Table 2; Fig 2). We found that etanercept marginally reduced the refractory rate in AAs to 8% (P = .046; Table 2) (OR: 0.07; 95% CI: 0.01–0.83; Fig 2). We analyzed the number of days from fever onset to IVIg initiation as a potential reason for this variation. However, we found similar median times from fever onset to IVIg initiation among all ethnic and race subgroups.
CA Disease by Echocardiography
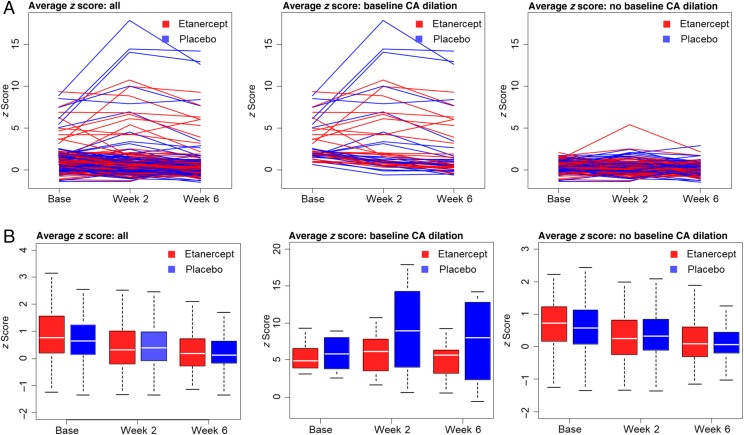
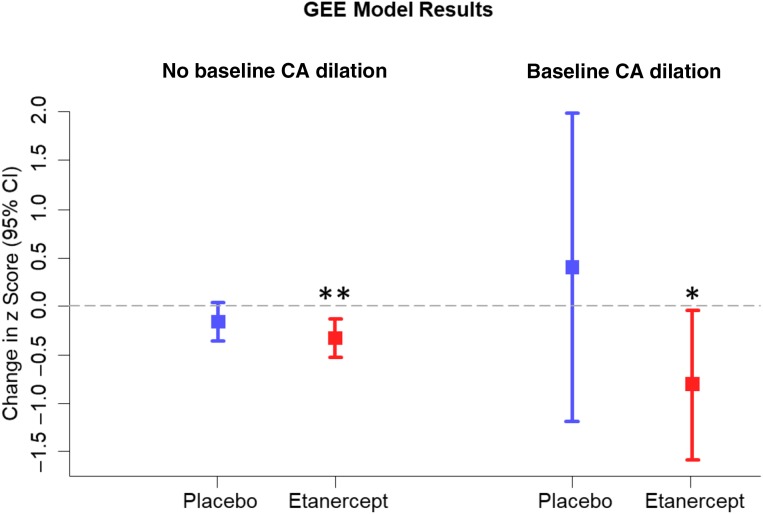
Within the entire mITT population, 45 participants had at least 1 dilated coronary on the baseline echocardiogram (etanercept n = 23; placebo, n = 22). Demographics including age, sex, and race were well balanced between treatment groups for both dilation status cohorts (Supplemental Tables 6 and 7). The baseline CA dilation rate was similar between the 2 mITT groups (Table 3). Both placebo and etanercept groups with a baseline dilation revealed substantially higher rates of coronary dilation defined as an individual z score >2.5 at the final study visit (36%) than those without initial dilation (4%). Figure 3A shows trajectories in a spaghetti diagram format for the mean of the 3 coronary z scores at each time point. The diagram reveals a clear dichotomy in the disease course, which depends on presence of early CA dilations. Note that the scatter and range of z score change are substantially greater in the placebo group than in the etanercept group. Figure 3B displays the same data as box plots showing cohort median with 25th and 75th percentiles for the average of the 3 coronary z scores, which illustrates the variation in placebo patients with baseline CA dilation. The GEE model is shown in Fig 4. The marked variability for z score changes in placebo reduces the statistical ability to assess treatment differences. Therefore, comparisons between the treatment groups were not significant (P = .18 for no baseline CA dilation; P = .23 for baseline CA dilation). However, analyses of within-patient change in each treatment group revealed that etanercept subjects experienced significant reductions in z scores across time among those with (P = .04) and without (P = .001) baseline dilation. In contrast, placebo patients showed no significant decrease or increase. The study-specific coronary outcome algorithm based on 20% change in absolute diameter (Fig 5) also indicated that etanercept reduced the proportion with progressive dilation compared with the placebo (8.3% with etanercept; 31.8% with the placebo; P = .03; Table 3) in patients with a dilated coronary at baseline.
TABLE 3.
Coronary Aneurysm Response (mITT Population)
| Change Description | Etanercept (N = 98),a n (%) | Placebo (N = 99),b n (%) | Pc |
|---|---|---|---|
| Change from maximum to visit 5 | |||
| CA change | |||
| Better | 56 (57) | 53 (54) | .86 |
| No change | 32 (33) | 36 (36) | |
| Worse | 10 (10) | 10 (10) | |
| CA improvement | |||
| Improved | 56 (57) | 53 (54) | .61 |
| Unchanged or worse | 42 (43) | 46 (46) | |
| Aneurysm improvement | |||
| Improved | 2 (50) | 3 (37.5) | 1.00 |
| Unchanged or worse | 2 (50) | 5 (62.5) | |
| Change from baseline to visit 5 | |||
| CA change, all | |||
| Better | 42 (43) | 41 (41) | .83 |
| No change | 36 (37) | 34 (34) | |
| Worse | 20 (20) | 24 (24) | |
| CA change, base CA dilation | |||
| Better | 15 (63) | 14 (64) | .03 |
| No change | 7 (29) | 1 (5) | |
| Worse | 2 (8) | 7 (32) | |
| CA change, no base CA dilation | |||
| Better | 27 (37) | 27 (36) | .91 |
| No change | 28 (38) | 32 (42) | |
| Worse | 18 (25) | 17 (22) |
Four subjects in the mITT population had no postbaseline echocardiographic measurements.
CA dilation is defined as a z score ≥2.5 in any artery or any aneurysm at baseline.
P values from the t test for continuous variables and the χ2 test for categorical variables.
FIGURE 3.
A, Trajectories of the average (mean) of all 3 CA z scores over time in spaghetti diagram format. The center panel indicates more variability in response in placebo subjects with baseline CA dilation (etanercept baseline dilated, n = 24; placebo baseline dilated, n = 22; etanercept baseline nondilated, n = 73; placebo baseline nondilated, n = 76). The z scores from individual coronaries were included in the GEE model, and averages are shown for descriptive purposes. B, Box plots revealing the 3 CA average z scores overall and within dilation subgroups over time. These data are provided for descriptive purposes.
FIGURE 4.
GEE model results for change in z scores over time including all 3 CAs. Etanercept revealed significant reductions in z scores from baseline; the entire 95% CI lies below 0 for patients without and patients with baseline CA dilation. For placebo, there was a numerical reduction among patients with no dilation and a numerical increase among patients with dilation, but neither reached significance. ** P = .001, * P = .04.
FIGURE 5.
Algorithm design for evaluating change in absolute CA dimensions, used to determine primary echocardiographic end point. Absolute echocardiographic measurements (diameter in millimeters) were used to determine this echocardiographic end point. Scores provided for worsened, improved, or no change were submitted for logistic regression. V5, visit 5.
CRP and Hemoglobin
CRP level serves as a general laboratory estimate for the degree of inflammation in KD. We used CRP and hemoglobin as index laboratory values. By week 1, 59.8% of the placebo group and 68.5% of the etanercept group had normal CRP values (<0.8 mg/dL), although this difference was not significant. Nearly all participants had normal CRP levels by week 6. At week 1, 27% of etanercept and 26% of placebo participants had normal hemoglobin levels. By 6 weeks, ∼82% in each group had hemoglobin levels above the lower limit of normal range for age and sex. No significant differences occurred in changes for these values between treatment groups (Table 4).
TABLE 4.
Laboratory Parameter Changes (mITT Population)
| Subgroup | Hemoglobin, g/dL | CRP, mg/dL | ||
|---|---|---|---|---|
| Etanercept | Placebo | Etanercept | Placebo | |
| Baseline value | ||||
| Median | 10.80 | 10.70 | 7.90 | 8.80 |
| Percentile, 25th, 75th | 9.60, 11.4 | 9.90, 11.2 | 5.02, 15.40 | 6.12, 17.70 |
| Change from baseline to wk 1 | ||||
| Median | 0.00 | −0.20 | −7.15 | −8.00 |
| Percentile, 25th, 75th | −1.10, 0.80 | −1.3, 0.70 | −13.69, −4.46 | −16.00, −4.50 |
| Within normal range at wk 1, n (%) | 25 (27) | 24 (26) | 63 (69) | 55 (60) |
| Change from baseline to wk 2 | ||||
| Median | 0.30 | 0.35 | −7.20 | −8.00 |
| Percentile, 25th, 75th | −0.50, 1.10 | −0.45, 0.90 | −14.75, −4.58 | −17.00, −4.80 |
| Within normal range at wk 2, n (%) | 36 (36) | 41 (42) | 80 (85) | 77 (82) |
| Change from baseline to wk 6 | ||||
| Median | 1.60 | 1.35 | −7.20 | −7.80 |
| Percentile, 25th, 75th | 0.60, 2.20 | 0.60, 1.90 | −14.60, −4.00 | −14.80, −5.60 |
| Within normal range at wk 6, n (%) | 79 (82) | 76 (82) | 90 (95) | 87 (95) |
No significant differences were found across treatment groups at any visit.
Safety
Safety estimates include summaries of serious and nonserious AE incidences (Table 5).
TABLE 5.
AEs (Safety Population)
| AEs | Etanercept (N = 99), n (%) | Placebo (N = 102), n (%) |
|---|---|---|
| Incidence summarya | ||
| Any AE | 53 (54) | 58 (57) |
| SAE | 9 (9) | 10 (10) |
| AE related to study druga | 15 (15) | 12 (12) |
| Unexpected AE | 22 (22) | 25 (25) |
| Unexpected SAE | 0 (0) | 2 (2) |
| SAE requiring or prolonging hospitalization | 7 (7) | 10 (10) |
| Most frequent AEsb | ||
| Abdominal pain | 4 (4) | 3 (3) |
| Anemia | 2 (2) | 5 (5) |
| Arthralgia | 4 (4) | 2 (2) |
| Cough | 5 (5) | 1 (1) |
| Diarrhea | 5 (5) | 1 (1) |
| Emesis | 7 (7) | 7 (7) |
| Epistaxis | 4 (4) | 6 (6) |
| Headache | 6 (6) | 2 (2) |
| Hematoma | 5 (5) | 1 (1) |
| Pyrexia | 13 (13) | 13 (13) |
| Rash | 10 (10) | 12 (11) |
| Urticaria | 2 (2) | 4 (4) |
Subjects may be counted at most once per row.
AEs in at least 3% of total safety population. Discrepancies in numbers per treatment group in mITT population from Table 1 are due to pharmacy error in a single patient as noted in the text.
Recurrent fevers at home, necessitating readmission and IVIg retreatment, represented all SAEs, except for 1 brief readmission for influenza in the etanercept arm. Gastrointestinal symptoms were the most common AEs, and there were no differences in the incidence of AEs or SAEs by treatment. Specifically, infections were uncommon and similar with 4 (4.0%) in etanercept and 7 (6.9%) in placebo groups. No patients experienced aneurysm rupture or thrombosis in this trial.
Discussion
EATAK identified etanercept clinical benefit in subgroup analyses, although the decrease in IVIg resistance, the primary outcome, did not achieve significance within the entire cohort. The marked variability in KD presentation and outcomes justifies performance of the important subgroup analyses. We prespecified these analyses on the basis of demographic characteristics, which impact KD susceptibility, severity, treatment response, and pharmacogenetic profile.1,33–35 Our population divisions also conform to predetermined subgroupings or cohort stratifications conducted in other North American KD clinical trials.3,4,36
Because of complexities involved in comparing coronary echocardiographic parameters, KD trials have used IVIg resistance as a primary outcome while reserving coronary measures as secondary end points.4 In EATAK, etanercept reduced the IVIg resistance rate by 8% when evaluating the entire mITT population. However, this effect did not achieve significance (P = .10) because we had powered the study to detect a substantially larger reduction for the primary end point. We based power estimates on the open-label pilot trial results, which revealed no IVIg resistance in 17 consecutively enrolled cases treated with etanercept.27 In context, Tremoulet et al,4 using infliximab, enrolled similar subject numbers and found identical IVIg resistance rates of 11% in treatment and placebo groups (P = .81). Despite the lack of supporting evidence for efficacy from a randomized controlled trial, infliximab has attained use as adjunctive therapy to IVIg in some centers.37
Etanercept reduced the IVIg resistance rate in patients >1 year of age (P = .03), representing 84% of our total population. Inclusion of the younger subgroup with substantially lower subject numbers adversely affected our ability to detect a significant reduction in IVIg resistance in the entire study population. Conceivably, important factors related to pharmacokinetics and drug distribution affected results in the younger age group. The potential of these influences underlies the reasoning for prespecifying these age subgroups for analyses in pediatric studies but also suggests that further assessment of the youngest patients is warranted in future studies.27,38,39
During protocol design, we also anticipated that the diverse ethnicity of the population with KD in this multicenter study could impact overall trial results. Ethnic differences in IVIg treatment response have been difficult to discern from the literature because of practice variation and unclear or divergent definitions for outcome parameters within both prospective and retrospective data sets.40–43 Authors of a recent single-center study in San Diego County suggested that no ethnic differences occurred in IVIg response.44 However, Clark et al,45 with a substantially larger AA cohort, showed race as a risk factor for nonresponse, as well as for more severe and prolonged coronary dilation. With our analyses in placebo subgroups, we found wide variations in IVIg response according to ethnicity, with an especially high resistance rate in AAs. Furthermore, etanercept significantly reduced IVIg resistance in this higher-risk AA population, although confirmation is needed in a larger cohort. Racial diversity in genotypes, which influence immune regulation and therapeutic reactions to vaccines, IVIg, and monoclonal antibodies, provides a plausible reason for these ethnic variations.33,35,46 Accordingly, authors conducting clinical KD trials should include adequate AA representation or guidance from genotyping.47,48
Previous KD clinical trials have used various arbitrary coronary outcome parameters, including Japanese Ministry of Health criteria, the maximum z score between the right and LAD coronaries, absolute and individual artery z scores, or late persistence of a coronary z score >2.5.4,5,49–51 No trials have been used to fully account for the variable responses or interdependence among the individual CAs, nor do authors using these methods optimize the use of longitudinal echocardiographic data typically obtained in KD clinical trials. For instance, the authors of the infliximab trial used change in z score for 2 CAs (each as individual secondary parameters) and found some improvement in LAD z score with treatment.4 The GEE is a standard method used frequently in both cardiovascular and pediatric studies to evaluate longitudinal changes in interdependent parameters including risk factors and CA graft patency.52–56 With our GEE model, we incorporated data from the individual 3 coronary z scores to determine overall progression of coronary dilation. During study design, we recognized the complexity and dependence on multiple factors including body surface area measurements in assignment of coronary z scores during a longitudinal study.30 To circumvent these issues and further test our GEE model, we also applied an algorithm using absolute CA diameters, which are not subjected to as many sources of error as the z scores. The algorithm results supported the GEE finding that potential etanercept advantage exists over placebo, particularly in patients presenting with dilated coronaries.
Conclusions
This randomized controlled clinical trial reveals no significant decrease in IVIg resistance in the overall study population. However, etanercept reduced IVIg resistance in a subset of patients >1 year of age and marginally in AAs. Importantly, and more relevant to long-term quality of life for children with KD, we also demonstrated a decrease in coronary dilation by etanercept, especially in those showing an early coronary abnormality. Finally, with this trial, we found safety in this brief course of etanercept. The study results could be considered along with the relative ease of the etanercept regimen administration. In comparison, the RAISE regimen requires accurate early identification of high-risk patients and prolonged intravenous drug administration with concomitant hospitalization. Furthermore, patients with early coronary dilation are not recommended to receive steroids as early IVIg adjunctive treatment because of safety concerns. With these considerations, EATAK results reveal a reasonable risk/benefit profile for etanercept. Future clinical trials, conducted in these subgroups or stratified according to patient demographics or genotypes, will be necessary to validate our findings before wide clinical adoption.
Acknowledgments
The following is the full list of EATAK Investigators: Michael A Portman, MD, Aaron K. Olson, MD, Brian Soriano, MD, Sujatha Buddhe, MD, MS, Kamill Del Toro, MD, Soultana Kourtidou, MD, Bethany Wisotzkey, MD, Margaret Bruce, BS, Jennifer Cox, MASc (Seattle, WA); Richard Williams, MD, Jamie Reeder (Salt Lake City, UT); Edward Kirkpatrick, MD, Dominic Co, MD, Marsha Malloy, RN, Mary Krolikowslki, RN (Milwaukee, WI); Nagib Dahdah, MD, Julie Briere, RN, Fabiola Breault, RN (Montreal, Quebec, Canada); Sujatha Rajan, MD, Deborah Mensch, MD, Nancy Stellato, RN, MSN, Lorry Rubin, MD, Sunil Sood, MD, Jill Leibowitz, MD, Rita Shah, MD, Vijaya Soma, MD, Rubin Cooper, MD, Christine DeMers, RN (Long Island, NY); Carolyn Altman, MD, Sara K. Sexson-Tejtel, MD, PhD, MPH, Debra Griffin, MBA, BSN, RN, Teniola Shittu, MSc, MPH (Houston, TX); Nadine Choueiter, MD, Iona Munjal, MD, Kelly Ann Balem, RN (Bronx, NY); Lisa Imundo, MD, Josephine Isgro, MD, Anne Ferris, MBBS (Manhattan, NY); April Slee (London, United Kingdom).
We thank the data monitoring committee for their work: Carlos Rose, MD (Wilmington, DE); Stanford Shulman (Chicago, IL); Mark Lewin (Seattle, WA); and Phillip Morgan, MD (Seattle, WA) as independent safety monitors. Deidentified individual participant data (including data dictionaries) will be made available in addition to study protocols, the statistical analysis plan, and the informed consent form. The data will be made available after publication to researchers who provide a methodologically sound proposal for use in achieving the goals of the approved proposal. Proposals should be submitted to michael.portman@seattlechildrens.org.
Glossary
- AA
African American
- AE
adverse event
- AHA
American Heart Association
- CA
coronary artery
- CI
confidence interval
- CRP
C-reactive protein
- EATAK
Etanercept as Adjunctive Treatment for Acute Kawasaki Disease
- GEE
generalized estimating equation
- IVIg
intravenous immunoglobulin
- KD
Kawasaki disease
- LAD
left anterior descending coronary artery
- mITT
modified intention-to-treat
- OR
odds ratio
- RAISE
Randomized Controlled Trial to Assess Immunoglobulin Plus Steroid Efficacy for Kawasaki Disease
- SAE
serious adverse event
- TNF
tumor necrosis factor
Footnotes
Dr Portman served as sponsor and principal investigator for the Food and Drug Administration grant, conceptualized and designed the study, conducted the study, drafted the initial manuscript, and reviewed and revised the manuscript; Drs Dahdah, Olson, and Choueiter conducted the study, acquired data, and reviewed and revised the manuscript; Ms Slee designed and performed statistical analyses and reviewed and revised the manuscript; Dr Altman conducted the study and acquired data; Drs Soriano and Buddhe interpreted data and conducted major portions of the study; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
This trial has been registered at www.clinicaltrials.gov (identifier NCT00841789).
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Funded by the US Food and Drug Administration Office of Orphan Product Development R01-FD-003526 to Dr Portman. Amgen supplied supplementary funding through an investigator-initiated grant. Amgen also supplied commercial-grade drugs and placebos for this trial. Amgen had no role in the trial conduct, data analyses, interpretation, or manuscript preparation. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.McCrindle BW, Rowley AH, Newburger JW, et al. ; American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Surgery and Anesthesia; Council on Epidemiology and Prevention . Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135(17):e927–e999 [DOI] [PubMed] [Google Scholar]
- 2.Miura M, Kobayashi T, Kaneko T, et al. ; and The Z-score Project 2nd Stage Study Group . Association of severity of coronary artery aneurysms in patients with Kawasaki disease and risk of later coronary events. JAMA Pediatr. 2018;172(5):e180030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newburger JW, Sleeper LA, McCrindle BW, et al. ; Pediatric Heart Network Investigators . Randomized trial of pulsed corticosteroid therapy for primary treatment of Kawasaki disease. N Engl J Med. 2007;356(7):663–675 [DOI] [PubMed] [Google Scholar]
- 4.Tremoulet AH, Jain S, Jaggi P, et al. Infliximab for intensification of primary therapy for Kawasaki disease: a phase 3 randomised, double-blind, placebo-controlled trial. Lancet. 2014;383(9930):1731–1738 [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi T, Saji T, Otani T, et al. ; RAISE Study Group Investigators . Efficacy of immunoglobulin plus prednisolone for prevention of coronary artery abnormalities in severe Kawasaki disease (RAISE study): a randomised, open-label, blinded-endpoints trial. Lancet. 2012;379(9826):1613–1620 [DOI] [PubMed] [Google Scholar]
- 6.Sleeper LA, Minich LL, McCrindle BM, et al. Evaluation of Kawasaki disease risk-scoring systems for intravenous immunoglobulin resistance. J Pediatr. 2011;158(5):831–835.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shin J, Lee H, Eun L. Verification of current risk scores for Kawasaki disease in Korean children. J Korean Med Sci. 2017;32(12):1991–1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song R, Yao W, Li X. Efficacy of four scoring systems in predicting intravenous immunoglobulin resistance in children with Kawasaki disease in a children’s hospital in Beijing, North China. J Pediatr. 2017;184:120–124 [DOI] [PubMed] [Google Scholar]
- 9.Arane K, Mendelsohn K, Mimouni M, et al. Japanese scoring systems to predict resistance to intravenous immunoglobulin in Kawasaki disease were unreliable for Caucasian Israeli children. Acta Paediatr. 2018;107(12):2179–2184 [DOI] [PubMed] [Google Scholar]
- 10.Fukazawa R, Kobayashi T, Mikami M, et al. Nationwide survey of patients with giant coronary aneurysm secondary to Kawasaki disease 1999-2010 in Japan. Circ J. 2017;82(1):239–246 [DOI] [PubMed] [Google Scholar]
- 11.Leung DY, Geha RS, Newburger JW, et al. Two monokines, interleukin 1 and tumor necrosis factor, render cultured vascular endothelial cells susceptible to lysis by antibodies circulating during Kawasaki syndrome. J Exp Med. 1986;164(6):1958–1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki H, Uemura S, Tone S, et al. Effects of immunoglobulin and gamma-interferon on the production of tumour necrosis factor-alpha and interleukin-1 beta by peripheral blood monocytes in the acute phase of Kawasaki disease. Eur J Pediatr. 1996;155(4):291–296 [DOI] [PubMed] [Google Scholar]
- 13.Gupta M, Noel GJ, Schaefer M, Friedman D, Bussel J, Johann-Liang R. Cytokine modulation with immune gamma-globulin in peripheral blood of normal children and its implications in Kawasaki disease treatment. J Clin Immunol. 2001;21(3):193–199 [DOI] [PubMed] [Google Scholar]
- 14.Ichiyama T, Ueno Y, Hasegawa M, Niimi A, Matsubara T, Furukawa S. Intravenous immunoglobulin inhibits NF-kappaB activation and affects Fcgamma receptor expression in monocytes/macrophages. Naunyn Schmiedebergs Arch Pharmacol. 2004;369(4):428–433 [DOI] [PubMed] [Google Scholar]
- 15.Hui-Yuen JS, Duong TT, Yeung RS. TNF-alpha is necessary for induction of coronary artery inflammation and aneurysm formation in an animal model of Kawasaki disease. J Immunol. 2006;176(10):6294–6301 [DOI] [PubMed] [Google Scholar]
- 16.Tracey D, Klareskog L, Sasso EH, Salfeld JG, Tak PP. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther. 2008;117(2):244–279 [DOI] [PubMed] [Google Scholar]
- 17.Lovell DJ, Reiff A, Jones OY, et al. ; Pediatric Rheumatology Collaborative Study Group . Long-term safety and efficacy of etanercept in children with polyarticular-course juvenile rheumatoid arthritis. Arthritis Rheum. 2006;54(6):1987–1994 [DOI] [PubMed] [Google Scholar]
- 18.Lovell DJ, Reiff A, Ilowite NT, et al. ; Pediatric Rheumatology Collaborative Study Group . Safety and efficacy of up to eight years of continuous etanercept therapy in patients with juvenile rheumatoid arthritis. Arthritis Rheum. 2008;58(5):1496–1504 [DOI] [PubMed] [Google Scholar]
- 19.Paller AS, Siegfried EC, Langley EC, et al. ; Etanercept Pediatric Psoriasis Study Group . Etanercept treatment for children and adolescents with plaque psoriasis. N Engl J Med. 2008;358(3):241–251 [DOI] [PubMed] [Google Scholar]
- 20.Paller AS, Siegfried EC, Pariser DM, et al. Long-term safety and efficacy of etanercept in children and adolescents with plaque psoriasis. J Am Acad Dermatol. 2016;74(2):280–287.e1–e3 [DOI] [PubMed] [Google Scholar]
- 21.Nestorov I. Clinical pharmacokinetics of TNF antagonists: how do they differ? Semin Arthritis Rheum. 2005;34(5 suppl 1):12–18 [DOI] [PubMed] [Google Scholar]
- 22.Nestorov I. Clinical pharmacokinetics of tumor necrosis factor antagonists. J Rheumatol Suppl. 2005;74:13–18 [PubMed] [Google Scholar]
- 23.Mok CC, van der Kleij D, Wolbink GJ. Drug levels, anti-drug antibodies, and clinical efficacy of the anti-TNFα biologics in rheumatic diseases. Clin Rheumatol. 2013;32(10):1429–1435 [DOI] [PubMed] [Google Scholar]
- 24.Moots RJ, Xavier RM, Mok CC, et al. The impact of anti-drug antibodies on drug concentrations and clinical outcomes in rheumatoid arthritis patients treated with adalimumab, etanercept, or infliximab: results from a multinational, real-world clinical practice, non-interventional study. PLoS One. 2017;12(4):e0175207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah CA. ‘Lower anti-drug antibodies with etanercept biosimilar: can Ctrough explain the differences?’. Ann Rheum Dis. 2016;75(9):e60. [DOI] [PubMed] [Google Scholar]
- 26.Kansen HM, van Rheenen PF, Houwen RHJ, et al. ; Kids with Crohnʼs, Colitis (KiCC) Working Group for Collaborative Paediatric IBD Research in the Netherlands . Less anti-infliximab antibody formation in paediatric crohn patients on concomitant immunomodulators. J Pediatr Gastroenterol Nutr. 2017;65(4):425–429 [DOI] [PubMed] [Google Scholar]
- 27.Choueiter NF, Olson AK, Shen DD, Portman MA. Prospective open-label trial of etanercept as adjunctive therapy for Kawasaki disease. J Pediatr. 2010;157(6):960–966.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Portman MA, Olson A, Soriano B, Dahdah N, Williams R, Kirkpatrick E. Etanercept as Adjunctive Treatment for Acute Kawasaki Disease: study design and rationale. Am Heart J. 2011;161(3):494–499 [DOI] [PubMed] [Google Scholar]
- 29.Newburger JW, Takahashi M, Gerber MA, et al. ; Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association . Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association [published correction appears in Pediatrics. 2005;115(4):1118]. Pediatrics. 2004;114(6):1708–1733 [DOI] [PubMed] [Google Scholar]
- 30.Ronai C, Hamaoka-Okamoto A, Baker AL, et al. Coronary artery aneurysm measurement and Z score variability in Kawasaki disease. J Am Soc Echocardiogr. 2016;29(2):150–157 [DOI] [PubMed] [Google Scholar]
- 31.Gardiner JC, Luo Z, Roman LA. Fixed effects, random effects and GEE: what are the differences? Stat Med. 2009;28(2):221–239 [DOI] [PubMed] [Google Scholar]
- 32.Glynn RJ, Rosner B. Regression methods when the eye is the unit of analysis. Ophthalmic Epidemiol. 2012;19(3):159–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Portman MA, Shrestha S. One size does not fit all: genetic prediction of Kawasaki disease treatment response in diverse populations. Circ Cardiovasc Genet. 2017;10(5):e001917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shrestha S, Wiener H, Shendre A, et al. Role of activating FcγR gene polymorphisms in Kawasaki disease susceptibility and intravenous immunoglobulin response. Circ Cardiovasc Genet. 2012;5(3):309–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shrestha S, Wiener HW, Olson AK, et al. Functional FCGR2B gene variants influence intravenous immunoglobulin response in patients with Kawasaki disease. J Allergy Clin Immunol. 2011;128(3):677–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tremoulet AH, Jain S, Kim S, et al. Rationale and study design for a phase I/IIa trial of anakinra in children with Kawasaki disease and early coronary artery abnormalities (the ANAKID trial). Contemp Clin Trials. 2016;48:70–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jone PN, Anderson MS, Mulvahill MJ, Heizer H, Glodé MP, Dominguez SR. Infliximab plus intravenous immunoglobulin (IVIG) versus IVIG alone as initial therapy in children with Kawasaki disease presenting with coronary artery lesions: is dual therapy more effective? Pediatr Infect Dis J. 2018;37(10):976–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu H, Rosenbaum S. Developmental pharmacokinetics in pediatric populations. J Pediatr Pharmacol Ther. 2014;19(4):262–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Portman MA, Slee A, Olson AK, et al. ; TRICC Investigators . Triiodothyronine supplementation in infants and children undergoing cardiopulmonary bypass (TRICC): a multicenter placebo-controlled randomized trial: age analysis. Circulation. 2010;122(suppl 11):S224–S233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uehara R, Belay ED, Maddox RA, et al. Analysis of potential risk factors associated with nonresponse to initial intravenous immunoglobulin treatment among Kawasaki disease patients in Japan. Pediatr Infect Dis J. 2008;27(2):155–160 [DOI] [PubMed] [Google Scholar]
- 41.Downie ML, Manlhiot C, Latino GA, et al. Variability in response to intravenous immunoglobulin in the treatment of Kawasaki disease. J Pediatr. 2016;179:124–130.e1 [DOI] [PubMed] [Google Scholar]
- 42.Porcalla AR, Sable CA, Patel KM, Martin GR, Singh N. The epidemiology of Kawasaki disease in an urban hospital: does African American race protect against coronary artery aneurysms? Pediatr Cardiol. 2005;26(6):775–781 [DOI] [PubMed] [Google Scholar]
- 43.Abuhammour WM, Hasan RA, Eljamal A, Asmar B. Kawasaki disease hospitalizations in a predominantly African-American population. Clin Pediatr (Phila). 2005;44(8):721–725 [DOI] [PubMed] [Google Scholar]
- 44.Skochko SM, Jain S, Sun X, et al. Kawasaki disease outcomes and response to therapy in a multiethnic community: a 10-year experience. J Pediatr. 2018;203:408–415.e3 [DOI] [PubMed] [Google Scholar]
- 45.Clark DE, Denby KJ, Kaufman LM, et al. Predictors of intravenous immunoglobulin nonresponse and racial disparities in Kawasaki disease. Pediatr Infect Dis J. 2018;37(12):1227–1234 [DOI] [PubMed] [Google Scholar]
- 46.Lassaunière R, Tiemessen CT. Variability at the FCGR locus: characterization in Black South Africans and evidence for ethnic variation in and out of Africa. Genes Immun. 2016;17(2):93–104 [DOI] [PubMed] [Google Scholar]
- 47.Mak ACY, White MJ, Eckalbar WL, et al. ; NHLBI Trans-Omics for Precision Medicine (TOPMed) Consortium . Whole-genome sequencing of pharmacogenetic drug response in racially diverse children with asthma. Am J Respir Crit Care Med. 2018;197(12):1552–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pereira NL, Sargent DJ, Farkouh ME, Rihal CS. Genotype-based clinical trials in cardiovascular disease. Nat Rev Cardiol. 2015;12(8):475–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Inoue Y, Okada Y, Shinohara M, et al. A multicenter prospective randomized trial of corticosteroids in primary therapy for Kawasaki disease: clinical course and coronary artery outcome. J Pediatr. 2006;149(3):336–341 [DOI] [PubMed] [Google Scholar]
- 50.Newburger JW, Takahashi M, Beiser AS, et al. A single intravenous infusion of gamma globulin as compared with four infusions in the treatment of acute Kawasaki syndrome. N Engl J Med. 1991;324(23):1633–1639 [DOI] [PubMed] [Google Scholar]
- 51.Newburger JW, Takahashi M, Burns JC, et al. The treatment of Kawasaki syndrome with intravenous gamma globulin. N Engl J Med. 1986;315(6):341–347 [DOI] [PubMed] [Google Scholar]
- 52.Brilakis ES, O’Donnell CI, Penny W, et al. Percutaneous coronary intervention in native coronary arteries versus bypass grafts in patients with prior coronary artery bypass graft surgery: insights from the Veterans Affairs Clinical Assessment, Reporting, and Tracking Program. JACC Cardiovasc Interv. 2016;9(9):884–893 [DOI] [PubMed] [Google Scholar]
- 53.Nakano J, Okabayashi H, Noma H, Sato T, Sakata R. Early angiographic evaluation after off-pump coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2013;146(5):1119–1125 [DOI] [PubMed] [Google Scholar]
- 54.Norby FL, Soliman EZ, Chen LY, et al. Trajectories of cardiovascular risk factors and incidence of atrial fibrillation over a 25-year follow-up: the ARIC study (Atherosclerosis Risk in Communities). Circulation. 2016;134(8):599–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kulik A, Levin R, Ruel M, Mesana TG, Solomon DH, Choudhry NK. Patterns and predictors of statin use after coronary artery bypass graft surgery. J Thorac Cardiovasc Surg. 2007;134(4):932–938 [DOI] [PubMed] [Google Scholar]
- 56.Gidding SS, Barton BA, Dorgan JA, et al. Higher self-reported physical activity is associated with lower systolic blood pressure: the Dietary Intervention Study in Childhood (DISC). Pediatrics. 2006;118(6):2388–2393 [DOI] [PubMed] [Google Scholar]