Abstract
Background
Inhaled corticosteroids (ICS) are the most effective treatment for children with persistent asthma. Although treatment with ICS is generally considered to be safe in children, the potential adverse effects of these drugs on growth remains a matter of concern for parents and physicians.
Objectives
To assess the impact of different inhaled corticosteroid drugs and delivery devices on the linear growth of children with persistent asthma.
Search methods
We searched the Cochrane Airways Trials Register, which is derived from systematic searches of bibliographic databases including CENTRAL, MEDLINE, Embase, CINAHL, AMED and PsycINFO. We handsearched respiratory journals and meeting abstracts. We also conducted a search of ClinicalTrials.gov and manufacturers' clinical trial databases, or contacted the manufacturer, to search for potential relevant unpublished studies. The literature search was initially conducted in September 2014, and updated in November 2015, September 2018, and April 2019.
Selection criteria
We selected parallel‐group randomized controlled trials of at least three months' duration. To be included, trials had to compare linear growth between different inhaled corticosteroid molecules at equivalent doses, delivered by the same type of device, or between different devices used to deliver the same inhaled corticosteroid molecule at the same dose, in children up to 18 years of age with persistent asthma.
Data collection and analysis
At least two review authors independently selected studies and assessed risk of bias in included studies. The data were extracted by one author and checked by another. The primary outcome was linear growth velocity. We conducted meta‐analyses using Review Manager 5.3 software. We used mean differences (MDs) and 95% confidence intervals (CIs ) as the metrics for treatment effects, and the random‐effects model for meta‐analyses. We did not perform planned subgroup analyses due to there being too few included trials.
Main results
We included six randomized trials involving 1199 children aged from 4 to 12 years (per‐protocol population: 1008), with mild‐to‐moderate persistent asthma. Two trials were from single hospitals, and the remaining four trials were multicentre studies. The duration of trials varied from six to 20 months.
One trial with 23 participants compared fluticasone with beclomethasone, and showed that fluticasone given at an equivalent dose was associated with a significant greater linear growth velocity (MD 0.81 cm/year, 95% CI 0.46 to 1.16, low certainty evidence). Three trials compared fluticasone with budesonide. Fluticasone given at an equivalent dose had a less suppressive effect than budesonide on growth, as measured by change in height over a period from 20 weeks to 12 months (MD 0.97 cm, 95% CI 0.62 to 1.32; 2 trials, 359 participants; moderate certainty evidence). However, we observed no significant difference in linear growth velocity between fluticasone and budesonide at equivalent doses (MD 0.39 cm/year, 95% CI ‐0.94 to 1.73; 2 trials, 236 participants; very low certainty evidence).
Two trials compared inhalation devices. One trial with 212 participants revealed a comparable linear growth velocity between beclomethasone administered via hydrofluoroalkane‐metered dose inhaler (HFA‐MDI) and beclomethasone administered via chlorofluorocarbon‐metered dose inhaler (CFC‐MDI) at an equivalent dose (MD ‐0.44 cm/year, 95% CI ‐1.00 to 0.12; low certainty evidence). Another trial with 229 participants showed a small but statistically significant greater increase in height over a period of six months in favour of budesonide via Easyhaler, compared to budesonide given at the same dose via Turbuhaler (MD 0.37 cm, 95% CI 0.12 to 0.62; low certainty evidence).
Authors' conclusions
This review suggests that the drug molecule and delivery device may impact the effect size of ICS on growth in children with persistent asthma. Fluticasone at an equivalent dose seems to inhibit growth less than beclomethasone and budesonide. Easyhaler is likely to have less adverse effect on growth than Turbuhaler when used for delivery of budesonide. However, the evidence from this systematic review of head‐to‐head trials is not certain enough to inform the selection of inhaled corticosteroid or inhalation device for the treatment of children with persistent asthma. Further studies are needed, and pragmatic trials and real‐life observational studies seem more attractive and feasible.
Plain language summary
Which inhaled corticosteroid and inhalation device has least impact on growth in children with asthma?
Review question
We reviewed the evidence about which inhaled corticosteroid and inhalation device has least impact on growth in children with asthma.
Background
Inhaled corticosteroids (ICS) are the most effective treatment for children with persistent asthma. Persistent asthma is a more severe asthma that requires daily use of medications for controlling symptoms. Although treatment with ICS is generally considered safe in children, daily use of these drugs over a long period of time may cause reduction of growth. The effect on growth may depend on type of steroid and delivery device.
Study characteristics
In this review, we included trials that compared either different inhaled corticosteroid drugs or inhalation devices, for at least three months in children aged from 4 to 12 years with mild‐to‐moderate persistent asthma. We found six trials involving 1199 people, and we included information from 1008 people in our analysis. Four trials compared the drug fluticasone with either beclomethasone or budesonide. Two trials compared different inhalation devices. Four trials were conducted in more than two different centres (multicentre studies). The multicentre studies were financially supported by industry companies that manufacture the drugs or devices.
This systematic review did not include children with persistent asthma treated with other ICS besides beclomethasone, budesonide and fluticasone, or ICS combined with medications called long acting beta2‐agonists (LABA). Thus, evidence derived from this review does not apply for these people.
Key results
One trial with 23 people showed that fluticasone had less negative effects on children's growth compared to beclomethasone (low certainty evidence). Three trials compared fluticasone and budesonide, and showed some different results. The combined results of two trials with 359 people suggested that fluticasone had less negative effects on children's height compared to budesonide (moderate certainty evidence), while the combined results of another two trials with 236 people revealed similar growth velocity (average increase in height per year) between fluticasone and budesonide (very low certainty evidence).
Two trials compared inhalation devices. One trial with 212 people showed a similar growth velocity between beclomethasone delivered by hydrofluoroalkane‐metered dose inhaler (HFA‐MDI) at half the dose, and beclomethasone delivered by chlorofluorocarbon‐metered dose inhaler (CFC‐MDI) (low certainty evidence). Another trial with 229 people showed that budesonide delivered by Easyhaler had less negative effects on children's height over a period of six months, compared to budesonide given at the same dose through Turbuhaler (low certainty evidence).
Certainty of the evidence
We judged the certainty of the evidence in this review to range from very low to moderate, mainly because of small numbers of trials and people, low quality of some included trials, and the possible influence of industry funding on reporting of trial results. 'Very low certainty' means that we are very uncertain about the results, while 'moderate certainty' means that further research is likely to have an important impact on the results and may change the current conclusions.
Conclusions
The type of drug and inhalation device may affect the size of negative effects of ICS on growth in children with persistent asthma. Fluticasone seems to inhibit growth less than beclomethasone and budesonide. Easyhaler is likely to have less a negative effect on growth than Turbuhaler when used for delivery of budesonide. However, the evidence from this review is not certain enough to help people select which inhaled corticosteroid or inhalation device to use for the treatment of children with asthma. Further studies are needed.
The well‐established benefits of ICS in controlling asthma outweigh the potential risk of a relatively small suppression in growth. Fear of drug side effects means some children do not take their steroid inhalers as prescribed, leading to poor asthma control. Uncontrolled asthma can also impair children's growth, and can cause significant morbidity and mortality. Good communication between healthcare professionals and parents is essential to reduce people's concerns about using steroids and to improve treatment adherence.
This review is current to April 2019.
Summary of findings
Background
This review is the last in a series of three Cochrane Reviews about the effects of inhaled corticosteroids (ICS) on growth of children with persistent asthma. The first review (Zhang 2014) provides an overview regarding the effects of ICS on growth, and the second (Pruteanu 2014) examines the dose‐response effect of ICS on growth. This third review aimed to compare the effects of different ICS drug molecules and delivery devices on growth.
Description of the condition
Asthma is the most common chronic disease of childhood, affecting around 14% of children globally, i.e. probably over 100 million children have self‐reported asthma symptoms during the last 12 months (FIRS 2017; The Global Asthma Network 2014). It is a chronic inflammatory disorder of the airways, in which different types of cells and inflammatory mediators play a role (GINA 2018). The inflammatory process leads to vascular leakage, bronchoconstriction, mucus hypersecretion, inflammatory cell infiltration, airway hyper‐responsiveness and ultimately airway remodelling (GINA 2018; NHLBI 2007). Chronic inflammation involves bronchi and small airways, and leads to recurrent episodes of wheezing due to airflow obstruction within the lung.
In high‐income countries, the prevalence of asthma in children has increased markedly over the past 40 to 50 years, but this increase has now levelled off (Asher 2014). In contrast, asthma prevalence is increasing in some low‐ and middle‐income countries (Asher 2014; Ferrante 2018). The overall global burden of childhood asthma is thus rising, and global disparities in asthma prevalence are decreasing.
Description of the intervention
Inhaled corticosteroids are the first‐line treatment for persistent asthma (BTS 2016; GINA 2018; NHLBI 2007). Numerous studies have demonstrated that ICS can improve pulmonary function and quality of life, and reduce asthma symptoms, exacerbations, hospitalizations, airway hyper‐responsiveness, airway inflammation, and asthma‐related deaths (Zhang 2014). Eight ICS are available for clinical use worldwide: beclomethasone dipropionate, budesonide, fluticasone propionate, fluticasone furoate, mometasone furoate, ciclesonide, flunisolide and triamcinolone acetonide. ICS have different pharmacokinetic and pharmacodynamic properties and biologic characteristics, but all ICS can achieve similar therapeutic benefits when given at equipotent doses (BTS 2016; GINA 2018).
The optimal doses of ICS for persistent childhood asthma remain unclear. Recent asthma guidelines recommend doses of up to 400 µg/day beclomethasone‐ hydrofluoroalkane (HFA) equivalent for children with mild‐to‐moderate persistent asthma (BTS 2016; GINA 2018; NHLBI 2007). When asthma in children is not controlled by low‐dose ICS, the global guideline from the Global Initiative for Asthma (GINA) suggests increased‐dose ICS, or the addition of leukotriene receptor antagonist (LTRA) (GINA 2018). The British guidelines are more cautious and suggest the addition of LTRA and long‐acting beta‐agonists (LABA) before increasing the dose of ICS (NICE 2017).
One systematic review (Zhang 2011) showed no clinically relevant therapeutic advantage of moderate doses (300 µg/day to 400 µg/day) over low doses (200 µg/day or less) of ICS in children with mild‐to‐moderate persistent asthma. However, higher doses of ICS should be considered in selected children with more severe asthma, or those with poor response to low to moderate doses of ICS (BTS 2016; GINA 2018; NHLBI 2007).
Although ICS are generally considered safe and highly effective treatments for children with asthma, the potential systemic adverse effects of long‐term use of ICS, especially the effects on growth, continue to be a matter of concern (Pruteanu 2014; Zhang 2014). Other possible adverse effects related to adrenal suppression or neurotoxic effects may be more important, but retardation of growth could be used as a surrogate marker for these effects. Recently, a systematic review of adverse effects of asthma medications was published by Leung 2017. It showed that alternatives to ICS are not free from adverse effects: monotherapy with LABA may have caused deaths in adults and non‐fatal severe adverse events in children, and LTRA may lead to increased risk for neuropsychiatric disorders in children and adolescents. Our goal must therefore be to find the optimal balance between benefit and harms of different asthma medicines.
How the intervention might work
Inhaled corticosteroids are the most potent anti‐inflammatory drugs available for the long‐term treatment of persistent asthma. The therapeutic benefits of ICS have been directly related to a decrease in airway inflammation (Djukanovic 1992). The molecular mechanisms by which ICS exert anti‐inflammatory effects and the mechanism of corticosteroid‐ induced growth impairment are briefly reviewed by Zhang 2014. Systemically absorbed ICS can suppress linear growth through interruption of the growth hormone (GH) axis. Centrally, glucocorticoids diminish pulsatile GH secretion. Peripherally, ICS reduce GH receptor expression in growth plates and liver, and reduce the production and bioactivity of its secondary messenger, insulin‐like growth factor 1. Without a normal GH/insulin‐like growth factor 1 effect, growth plate cell proliferation is diminished and chondrocyte apoptosis is increased (Allen 2015; Kapadia 2016; Wolfgram 2015). If the growth plate cells proliferate more slowly, the longitudinal bones (and the stature of the child) grow more slowly. Inhaled corticosteroid compounds are extraordinarily potent glucocorticoids with eight to 23 times the potency of dexamethasone. As a result, small amounts of systemically absorbed ICS can produce a significant glucocorticoid effect capable of suppressing childhood growth (Allen 2015).
The therapeutic index (i.e. the ratio of therapeutic effect to systemic effect) of available ICS varies, mainly based on their pharmacodynamic and pharmacokinetic properties, such as glucocorticoid receptor binding affinity (potency), oral bioavailability and lung bioavailability (Colice 2000). Bioavailability refers to the amount of an administered dose of unchanged drug that reaches the systemic circulation.The systemic inhaled corticosteroid burden represents the sum of absorption of the drug delivered to the lung (therapeutic fraction) plus the intestinal absorption of the swallowed drug that escapes hepatic first‐pass metabolism (oral bioavailability). For all available ICS, the most efficient path to the systemic circulation is via the lungs, so that a highly efficient formulation or inhalation device (or both) for an inhaled corticosteroid that improves lung delivery may also increase systemic bioavailability and the risk of systemic effects unless commensurate dosage reductions are made (Allen 2015).
Why it is important to do this review
In the first of a series of three Cochrane Reviews (Zhang 2014), the post hoc subgroup analysis of trials that used similar doses of ICS (equivalence of 200 μg/day HFA‐beclomethasone) showed a significant difference between five molecules regarding the effect size on linear growth velocity during a treatment time of one year (P = 0.004), with mean reductions of: ‐1.0 cm/year with beclomethasone, ‐0.61 cm/year with budesonide, ‐0.15 cm/year with ciclesonide, ‐0.42 cm/year with fluticasone, and ‐0.67 cm/year with mometasone. However, the effects of inhalation devices were not taken into account in both reviews.
Another post hoc subgroup analysis for inhalation devices within the molecule fluticasone propionate (200 μg/day) did not show a statistically significant difference between chlorofluorocarbon‐metered dose inhaler (CFC‐MDI), HFA‐MDI and dry powder inhaler (DPI) regarding the effect size of fluticasone on linear growth velocity during a one‐year treatment period.
The certainty of evidence from such post hoc subgroup analyses is very low due to indirect comparisons and low statistical power. Moreover, given the complex interaction between inhaled corticosteroid molecules, inhalation devices and inhaled corticosteroid doses, any definitive conclusions regarding the influence of each factor on the magnitude of growth‐suppressive effects of ICS should be derived from head‐to‐head trials comparing the same molecule at the same dose delivered by different inhalers, or comparing different drug molecules at equivalent doses delivered by the same type of inhaler.
The second of a series of three Cochrane Reviews (Pruteanu 2014) aimed to assess whether increasing the dose of ICS is associated with slower linear growth, weight gain and skeletal maturation in children with asthma. In the four comparisons reporting linear growth over 12 months, a significant group difference was observed, with lower growth velocity in the group receiving higher doses of ICS, compared to the group receiving lower doses (5.74 cm/year and 5.94 cm/year, respectively). The ICS molecules (ciclesonide, fluticasone, mometasone) used in these four comparisons did not significantly influence the magnitude of effect. The effects of inhalation devices were not assessed.
We therefore decided to conduct this systematic review of head‐to‐head randomized trials (trials that directly compare one intervention to another) to assess the influence of drug molecules and delivery devices on the size of adverse effects of ICS on growth in children with persistent asthma.
Objectives
To assess the impact of different inhaled corticosteroid drugs and delivery devices on the linear growth of children with persistent asthma.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) which used a parallel‐group design.
Types of participants
We included children up to 18 years of age with the diagnosis of persistent asthma, or who were taking continuous medication for the prevention and treatment of episodic wheezing.
Types of interventions
To be included, trials had to assess the daily use of inhaled corticosteroids (ICS) for at least three months, delivered by any type of delivery device, compared with either another type of inhaled corticosteroid or another delivery device. Therefore, the possible comparisons were:
comparison of different inhaled corticosteroid molecules at equivalent doses delivered by the same type of device; or
comparison of the same inhaled corticosteroid molecule at the same dose delivered by different devices.
We considered Diskus (GlaxoWellcome) and Turbuhaler (Astra) as the same type of device, i.e. dry powder inhaler (DPI).
Types of outcome measures
Primary outcomes
Linear growth velocity (cm/year), obtained by measuring height with a stadiometer at a number of time points during the study, and performing linear regression of height over time (Price 2002).
Secondary outcomes
Change in height standard deviation (SD) score, defined as the difference between the individual's growth velocity and predicted normal growth velocity divided by the predicted normal growth velocity SD for individuals of the same age, sex, and ethnicity (if available) (Pedersen 2001).
Change in absolute height (cm) over a period of time.
Change in height z‐score over a period of time.
Change in weight (kg).
We did not include lower leg length measured by knemometry as an outcome, because this measurement correlates poorly with statural height and tends to overestimate any potential effects of ICS on growth (Allen 1999; Efthimiou 1998).
Search methods for identification of studies
Electronic searches
We identified studies from the Cochrane Airways Trials Register, which is maintained by the Information Specialist for the Group. The Cochrane Airways Trials Register contains studies identified from several sources:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL), through the Cochrane Register of Studies Online (crso.cochrane.org);
weekly searches of MEDLINE Ovid SP;
weekly searches of Embase Ovid SP;
monthly searches of PsycINFO Ovid SP;
monthly searches of CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature);
monthly searches of AMED EBSCO (Allied and Complementary Medicine);
handsearches of the proceedings of major respiratory conferences.
Studies contained in the Trials Register are identified through search strategies based on the scope of Cochrane Airways. Details of these strategies, as well as a list of handsearched conference proceedings, are in Appendix 1. See Appendix 2 for search terms used to identify studies for this review.
We searched the following trials registries.
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov/)
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch/)
We searched the Cochrane Airways Trials Register and additional sources from inception with no restriction on language of publication. The literature search was initially conducted in September 2014, and was updated in November 2015, September 2018, and April 2019.
Searching other resources
We checked reference lists of all included studies and review articles for additional references. In order to find any newer studies, we used Web of Science to review papers which in their list of references referred to the included studies. This search was conducted in September 2018.
We contacted lead authors of identified trials and experts in the field to identify other published and unpublished studies.
AstraZeneca and GlaxoSmithKline (GSK) held the patents for the medicines compared in the retrieved studies: budesonide (Pulmicort, AstraZeneca), fluticasone (Flutide, GSK) and beclomethasone (Becotide, GSK). We searched the GSK Clinical Study Register for fluticasone and beclomethasone on 17 August 2017.
AstraZeneca has no clinical study register available on the website. We therefore contacted AstraZeneca R&D, Mölndal, Sweden, in April 2018, and were informed that AstraZeneca had no relevant trials in addition to those that we had retrieved.
Data collection and analysis
Selection of studies
Two review authors (IA, SOP) independently assessed the titles and abstracts of all the studies identified by the search strategy. Full‐text articles were retrieved when the papers appeared to meet the inclusion criteria, or when there were insufficient data in the title and abstract to make a clear decision for their inclusion. We resolved any disagreement through discussion.
Data extraction and management
The data were extracted by one review author (IA) using specially designed and pilot‐tested data extraction forms. The extracted data were checked by another author (EN) for accuracy and completeness. We resolved any disagreement by discussion. We entered the extracted data into Review Manager version 5.3 (Review Manager 2014).
We extracted the following data.
Study characteristics: year of publication, name of the first author, country of origin, setting and source/sponsorship.
Methods: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, completeness of outcome data, selective reporting and other sources of bias.
Participants: sample size, demographics, inclusion and exclusion criteria, age, pubertal status (pre‐pubertal or not).
Intervention: type of inhaled corticosteroid, dosage, frequency of administration, inhalation device and treatment duration.
Comparator: another inhaled corticosteroid or another delivery device (the same details as for intervention).
Cointerventions, i.e. other steroids: nasal or oral.
Results: mean value of the outcome measures in each group, SD or other metrics for uncertainty (standard errors (SE), confidence intervals (CIs), t values or P values for difference in means) of the outcome measurements in each group, number of participants who underwent randomization (intention‐to‐treat population) and number of participants completing the study without major protocol deviations (per‐protocol population) in each group.
We converted SEs or 95% CIs to SDs using the calculator of Review Manager (Review Manager 2014).
Assessment of risk of bias in included studies
Three review authors (IA, EN, LZ) independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreements were resolved by discussion. We assessed the risk of bias according to the following domains.
Allocation sequence generation
Concealment of allocation
Blinding of participants and personnel
Blinding of outcome assessment
Incomplete outcome data
Selective outcome reporting
We noted other sources of bias. For each study, we judged each potential source of bias as either low risk, high risk or unclear.
Measures of treatment effect
The measurements of growth are continuous outcomes, so we used mean difference (MD) and 95% CIs as the metrics for treatment effects.
Unit of analysis issues
We considered each individual trial using a parallel‐group design as a unit of analysis. ICS dose‐equivalence used for this review is based on the British Thoracic Society (BTS) guideline: 1 µg fluticasone = 1 µg mometasone = 1 µg ciclesonide = 1 µg HFA‐beclomethasone dipropionate (extrafine HFA‐BDP) = 2 µg budesonide = 2 µg chlorofluorocarbon (CFC)‐BDP (BTS 2016).
Dealing with missing data
We did not contact investigators or study sponsors to verify key study characteristics and obtain missing numerical outcome data because all included trials were conducted more than ten years ago and there were no missing outcome data. In Ferguson 1999, only 154 of 333 children were measured with stadiometer and included in the study.
Assessment of heterogeneity
We used the I2 statistic to measure heterogeneity among the trials in each analysis (Higgins 2011, Box 11.3.a). The I² statistic ranges from 0% to 100% and measures the degree of inconsistency across studies. We used the following thresholds for the interpretation of the I² statistic: 0% to 40%: might not be important; 30% to 60%: may represent moderate heterogeneity; 50% to 90%: may represent substantial heterogeneity; 75% to 100%: considerable heterogeneity.
Assessment of reporting biases
The number of included trials was too small to warrant generation of funnel plots for detecting possible publication bias. To minimize publication bias, we performed a comprehensive search to identify both published and unpublished studies in any language. We planned to conduct a sensitivity analysis excluding the trials which had high risk of reporting biases, but such analysis was not done due to the small number of trials. For industry‐funded trials, we considered high risk of selective reporting bias if the study conclusion was favourable to the sponsor’s product.
Data synthesis
We performed the meta‐analyses using the Cochrane statistical package Review Manager 2014, based on the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We used the random‐effects model for meta‐analysis because it is more appropriate than the fixed‐effect model and provides more conservative estimates with wider CIs when heterogeneity across studies is significant. Otherwise, the two models generate similar results. We used data from the per‐protocol population rather than the intention‐to‐treat population because the main aim of this review was to answer the question of whether there is any difference between different inhaled corticosteroid molecules and inhalation devices regarding growth suppression in ICS‐treated children with persistent asthma. We have presented data in forest plots with mean differences and 95% CIs.
We evaluated the certainty of the evidence using GRADE methodology and prepared 'Summary of findings' tables using the following outcomes: linear growth velocity (cm/year), and change in absolute height (cm) over a period of time.
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses; however, the number of included trials was too small to conduct these.
Dosage of ICS (HFA‐beclomethasone equivalent): low daily dose (200 µg or less), medium daily dose (more than 200 µg to 400 µg), high daily dose (more than 400 µg).
Duration of exposure: < 3 months, > 3 to 6 months, > 6 to 12 months, > 12 months. Catch‐up growth after cessation of ICS treatment.
Asthma severity: mild persistent asthma, moderate‐to‐severe persistent asthma (NHLBI 2007).
Participant age: preschoolers (two to five years), prepubertal children (> 5 to 12 years), adolescents (> 12 to 18 years).
Concomitant use of non‐steroidal anti‐asthmatic drugs: ICS alone, ICS combined with steroidal or non‐steroidal drugs.
We planned to use the following outcomes in the subgroup analyses.
Linear growth velocity.
Change in height SD score.
Change in weight.
We analyzed heterogeneity as described in Assessment of heterogeneity.
If there had been 10 or more trials, we planned to perform meta‐regression analysis to assess the potential influence of the above‐mentioned treatment and child characteristics on adverse effects of ICS. This method allows the effects of multiple factors to be investigated simultaneously (Higgins 2008).
Sensitivity analysis
We planned to conduct the following sensitivity analyses to assess the potential impact of particular decisions or missing information on the findings of the review (Higgins 2008). However, we did not perform these analyses due to the small number of trials.
Excluding from the analysis the trials with high or unclear risk of allocation concealment, or bias due to missing data or lack of blinding, or both.
Excluding from the analysis the trials in which adherence rate to ICS lower than 80%, or no data were available regarding adherence to treatment.
Excluding from the analysis trials sponsored by the pharmaceutical industry.
Results
Description of studies
Results of the search
The initial search of electronic databases in September 2014 yielded 421 citations, and the update searches in November 2015 and in September 2018 found eight and 20 citations, respectively. A further search update in April 2019 did not retrieve any additional citations to consider. After screening the titles and abstracts of the retrieved records, we identified 18 papers as potentially relevant, each of which we reviewed in full text. We excluded a total of 12 articles after full review, leaving six articles reporting six trials for inclusion in the present review. We found no additional trials by checking the reference lists of primary studies and review articles, or by screening 220 papers identified from Web of Science which had cited eight included trials. Thus, six trials were finally included in this review (Figure 1).
1.
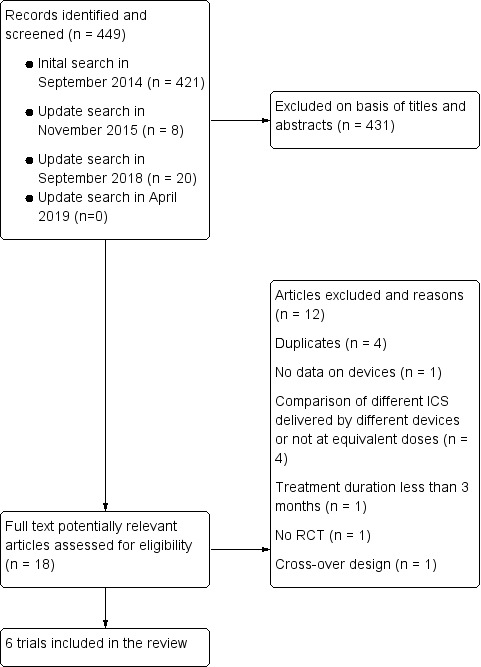
Flow diagram.
Included studies
We included six RCTs involving 1199 participants (per protocol population: 1008). See Characteristics of included studies and Table 5.
1. Study and study sponsorship.
| Study | Sample size (N randomised (PP)) | Comparisions | Sponsor | Study conclusions and sponsorship |
| Acun 2005 | 100 (100) | MDI‐FP + spacer (250 μg/day) vs MDI‐BUD + spacer (400 μg/day) | None mentioned | No significant difference between FP (GSK) and BUD (AstraZeneca) in linear growth velocity |
| Ferguson 1999 | 333 (154 underwent stadiometry and were analyzed) | Diskus‐FP (400 μg/day) vs Turbuhaler‐BUD (800 μg/day) |
Study co‐authored by researchers from GSK | FP (GSK) has less suppressive effect on linear growth velocity than BUD (AstraZeneca) |
| Ferguson 2007 | 233 (136) | Diskus‐FP (200 μg/day) vs Turbuhaler‐BUD (400 μg/day) |
Study funded by GSK | FP (GSK) has less suppressive effect on linear growth velocity than BUD (AstraZeneca) |
| Pedersen 2002 | 256 (212) | HFA‐BDP via Autohaler (100‐200 μg/day) vs CFC‐BDP + spacer (200‐400 μg/day) | Study funded by 3M Pharmaceuticals | No significant difference between HFA‐BDP via Autohaler (3M Pharmaceuticals) and conventional CFC‐BDP (GSK)* in linear growth velocity |
| Rao 1999 | 23 (23) | MDI‐FP + volumatic spacer (200 μg/day) vs MDI‐BDP + volumatic spacer (400 μg/day) |
None mentioned | FP (GSK) has less suppressive effect on linear growth velocity than BDP (GSK) |
| Vanto 2004 | 254 (229) | Easyhaler‐BUD (200 μg/day) vs Turbuhaler‐BUD (200 μg/day) | Study co‐authored by researchers from Orion Pharma | Easyhaler‐BUD (Orion Pharma) has less suppressive effect on linear growth velocity than Turbuhaler‐BUD (AstraZeneca) |
BDP: beclomethasone dipropionate BUD: budesonide CFC: chlorofluorocarbon DPI: dry powder inhaler FP: fluticasone propionate GSK: GlaxoSmithKline HFA: hydrofluoroalkane MDI: metered dose inhaler PP: per protocol population
* CFC‐metered dose inhaler is no longer available because of ozone depletion related to use of chlorofluorocarbon.
All studies were randomized, parallel‐group, controlled trials. Two trials were funded by pharmaceutical companies (Ferguson 2007; Pedersen 2002), and another two trials were co‐authored by researchers affiliated with pharmaceutical companies that manufacture tested ICS or devices (Ferguson 1999; Vanto 2004). Two studies were from single hospitals: one in Turkey (Acun 2005) and one in England (Rao 1999). The remaining four studies were multinational studies (Ferguson 1999; Ferguson 2007; Pedersen 2002; Vanto 2004).
Interventions
Four studies compared different inhaled corticosteroid molecules (Acun 2005; Rao 1999; Ferguson 1999; Ferguson 2007) (Table 5). One trial with 23 participants compared fluticasone (200 μg/day) with beclomethasone (400 μg/day), delivered via MDI with volumaticTM spacers (Rao 1999). Three trials compared fluticasone with budesonide (Acun 2005; Ferguson 1999; Ferguson 2007). Two trials compared different inhalation devices, of which one compared HFA‐MDI with CFC‐MDI for delivery of beclomethasone (Pedersen 2002), and another compared Easyhaler with Turbohaler for delivery of budesonide (Vanto 2004).
Outcomes
Four trials were designed to assess the effects of ICS on growth (Acun 2005; Ferguson 2007; Pedersen 2002; Rao 1999), and three of them reported efficacy data (Ferguson 2007; Pedersen 2002; Rao 1999). Two trials assessed both the efficacy and safety of ICS (Ferguson 1999; Vanto 2004). In all but two trials (Acun 2005; Ferguson 1999), height was measured with a stadiometer using a standardized method.
Participants
All trials included children with persistent asthma requiring inhaled corticosteroid therapy. The average age of the participants was 7 to 10 years, and 51% to 72% of the children in the studies were boys.
The number of intention‐to‐treat and per‐protocol participants, comparisons, sponsors, and tested ICS or devices of eight included trials are shown in the Table 5.
Excluded studies
Twelve studies were excluded (Characteristics of excluded studies): four were duplicates, two compared different ICS delivered by different devices, two compared different ICS not at equivalent doses, one did not provide the data on devices, one had a treatment duration of less than three months, one used cross‐over study design, and one was pragmatic study.
Risk of bias in included studies
Full details of the risk of bias for each trial can be found under Characteristics of included studies. A graphical summary of our 'Risk of bias' judgements can be found in Figure 2.
2.
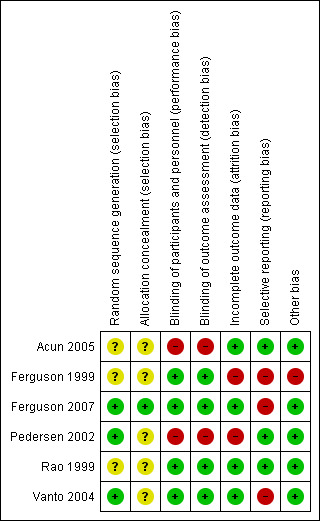
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
One trial used adequate methods of random sequence generation and allocation concealment (Ferguson 2007). Another two trials used adequate methods of random sequence generation, but failed to report the method of allocation concealment (Pedersen 2002; Vanto 2004). The remaining three trials did not provide information about random sequence generation and allocation concealment (Acun 2005; Ferguson 1999; Rao 1999).
Blinding
Four trials were double‐blind (Ferguson 1999; Ferguson 2007; Rao 1999; Vanto 2004), and two trials were open‐label (Acun 2005; Pedersen 2002).
Incomplete outcome data
There were substantial differences between intention‐to‐treat and per‐protocol populations in two trials (Ferguson 2007; Pedersen 2002), and the analyses by intention‐to‐treat and per‐protocol populations yielded similar results in one trial (Ferguson 2007). One trial had high risk of attrition bias because growth data from half of the participants were used (Ferguson 1999).
Selective reporting
The number of included trials was too small to assess the possibility of publication bias through funnel plots. Study protocols were not available for the six trials, but published reports included all expected outcomes. There was a risk of reporting bias in three trials since the study conclusion was favourable to the sponsor's product (Ferguson 1999; Ferguson 2007; Vanto 2004). Table 5 displays the study conclusions and sponsorship.
Other potential sources of bias
In all but one trial (Ferguson 1999), no other sources of bias were identified. Ferguson 1999 was not designed to assess growth effects of ICS, and did not use a standardized method for measuring height, which was used primarily to calculate predicted values for spirometry.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Summary of findings for the main comparison. Effects of different inhaled corticosteroids on growth in children with persistent asthma: fluticasone versus beclomethasone.
| Effects of different inhaled corticosteroids on growth in children with persistent asthma: fluticasone versus beclomethasone | ||||||
|
Patient or population: children up to 18 years of age with persistent asthma Setting: outpatient Intervention: fluticasone Comparison: beclomethasone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with beclomethasone | Risk with fluticasone | |||||
| Linear growth velocity (cm/year) Follow‐up: 20 months |
The mean linear growth velocity in the control group was 4.94 cm/year | The mean linear growth velocity in the intervention groups was on average 0.81 cm/yeargreater (95% CI: 0.46 greater to 1.16 greater) |
MD 0.81 (0.46 to 1.16) | 23 (1 RCT) | ⊕⊕⊝⊝ LOW1 |
|
| *The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomized controlled trial | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
1 Downgraded because of risk of bias (unclear risk of selection bias) and small sample size
Summary of findings 2. Effects of different inhaled corticosteroids on growth in children with persistent asthma: fluticasone versus budesonide.
| Effects of different inhaled corticosteroids on growth in children with persistent asthma: fluticasone versus budesonide | ||||||
|
Patient or population: children up to 18 years of age with persistent asthma Setting: outpatient Intervention: fluticasone Comparison: budesonide | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with budesonide | Risk with fluticasone | |||||
| Linear growth velocity (cm/year) Follow‐up: 12 months |
The mean linear growth velocity across control groups ranged from 4.3 to 6.3 cm/year | The mean linear growth velocity in the intervention groups was on average 0.39 cm/yeargreater (95% CI: 0.94 less to 1.73 more) |
MD 0.39 (‐0.94 to 1.73) | 236 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW1 |
|
| Change in height (cm) over a period of time Follow‐up: 20 weeks to 12 months | The mean change in height over a period from 20‐week to 12 months across control groups ranged from 1.99 to 4.7 cm | The mean change in height over a period from 20‐week to 12 months in the intervention group was on average 0.97 cm greater (95% CI 0.62 greater to 1.32 greater) | MD 0.97 (0.62 to 1.32) | 359 (2 RCTs) | ⊕⊕⊕⊝ MODERATE2 |
|
| *The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomized controlled trial | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
1 Downgraded because of risk of bias (unblinding in one trial and risk of selective reporting bias in another industry‐sponsored trial), inconsistency (I2 = 87%) and imprecision (CI includes both appreciable benefit and harm)
2 Downgraded because of risk of bias (high risk of attrition bias in one trial, and risk of selective reporting bias due to industry sponsorship in both trials)
Summary of findings 3. Effects of different delivery devices on growth in children with persistent asthma: hydrofluoroalkane‐metered dose inhaler beclomethasone versus chlorofluorocarbon‐metered dose inhaler beclomethasone.
| Effects of different devices on growth in children with persistent asthma: HFA‐MDI beclomethasone versus CFC‐MDI beclomethasone | ||||||
|
Patient or population: children up to 18 years of age with persistent asthma Setting: outpatient Intervention: HFA‐MDI beclomethasone Comparison: CFC‐MDI beclomethasone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with CFC‐MDI beclomethasone | Risk with HFA‐MDI beclomethasone | |||||
| Linear growth velocity (cm/year) Follow‐up: 12 months |
The mean linear growth velocity in the control group was 5.71 cm/year | The mean linear growth velocity in the intervention group was on average 0.44 cm/yearsmaller (95% CI: 1.00 smaller to 0.12 greater) | MD ‐0.44 (‐1.00 to 0.12) | 212 (1 RCT) | ⊕⊕⊝⊝ LOW1 |
|
| *The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CFC‐MDI: chlorofluorocarbon‐metered dose inhaler; CI: confidence interval; HFA‐MDI: hydrofluoroalkane‐metered dose inhaler; MD: mean difference; RCT: randomized controlled trial | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
1 Downgraded because of risk of bias (unblinding and substantial differences between intention‐to‐treat and per‐protocol populations), small sample size and imprecision (CI includes both appreciable benefit and harm)
Summary of findings 4. Effects of different delivery devices on growth in children with persistent asthma: Easyhaler‐budesonide versus Turbuhaler‐budesonide.
| Effects of different devices on growth in children with persistent asthma: Easyhaler‐budesonide versus Turbuhaler‐budesonide | ||||||
|
Patient or population: children up to 18 years of age with persistent asthma Setting: outpatient Intervention: Easyhaler‐budesonide Comparison: Turbuhaler‐budesonide | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with Turbuhaler‐Budesonide | Risk with Easyhaler‐Budesonide | |||||
| Change in height (cm) over a period of time Follow‐up: 6 months |
The mean change in height over a 6‐month period in the control group was 2.17 cm | The mean change in height over a 6‐month period in the intervention group was on average 0.37 cm greater (95% CI: 0.12 greater to 0.62 greater) | MD 0.37 (0.12 to 0.62) | 229 (1 RCT) | ⊕⊕⊝⊝ LOW1 |
|
| * The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomized controlled trial | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
1 Downgraded because of risk of bias (risk of selective reporting bias due to industry sponsorship), small effect size and small sample size
See: Table 1; Table 2; Table 3; and Table 4.
Comparison of different inhaled corticosteroids
Four trials compared fluticasone with other ICS. None of these studies contained data on the change in height z‐score and the change of children's weight.
Rao 1999 compared fluticasone (200 μg/day) with beclomethasone (400 μg/day) delivered via MDI with volumaticTM spacers; fluticasone given at an equivalent dose was associated with a significant greater linear growth velocity (MD 0.81 cm/year, 95% CI 0.46 to 1.16; P < 0.00001; 23 participants; low certainty evidence; Analysis 1.1) (Figure 3).
1.1. Analysis.

Comparison 1 Comparison of fluticasone and beclomethasone, Outcome 1 Linear growth velocity (cm/year).
3.

Forest plot of comparison: 1 Comparison of fluticasone and beclomethasone, outcome: 1.1 Linear growth velocity (cm/year).
Three trials compared fluticasone with budesonide (Acun 2005; Ferguson 1999; Ferguson 2007). Two trials used linear growth velocity (cm/year) as the growth outcome (Acun 2005; Ferguson 2007). The pooled results of these two trials did not reveal a significant difference in linear growth velocity between fluticasone and budesonide at equivalent doses (MD 0.39 cm/year, 95% CI ‐0.94 to 1.73; P = 0.57, I2 = 87%; 236 participants; very low certainty evidence; Analysis 2.1) (Figure 4).
2.1. Analysis.
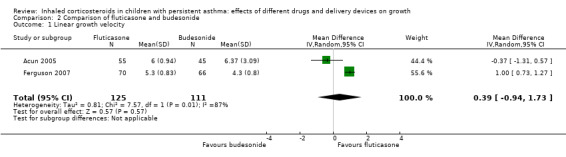
Comparison 2 Comparison of fluticasone and budesonide, Outcome 1 Linear growth velocity.
4.

Forest plot of comparison: 2 Comparison of fluticasone and budesonide, outcome: 2.1 Linear growth velocity.
Ferguson 1999 and Ferguson 2007 used change in height (cm) over a period of time as the growth outcome. Compared to budesonide delivered via Turbuhaler (400 μg/day), fluticasone via Diskus (200 μg/day) was associated with a greater increase in height over a period from 20 weeks to 12 months (MD 0.97 cm, 95% CI 0.62 to 1.32; P < 0.0001, I2 = 0%; 2 trials, 359 participants; moderate certainty evidence; Analysis 2.2) (Figure 5).
2.2. Analysis.
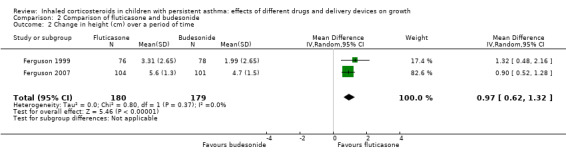
Comparison 2 Comparison of fluticasone and budesonide, Outcome 2 Change in height (cm) over a period of time.
5.
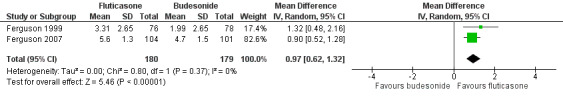
Forest plot of comparison: 2 Comparison of fluticasone and budesonide, outcome: 2.2 Change in height (cm) over a period of time.
Comparison of different inhalation devices
Two trials compared different inhalation devices, of which one compared HFA‐MDI with CFC‐MDI for the delivery of beclomethasone (Pedersen 2002), and another compared Easyhaler with Turbohaler for the delivery of budesonide (Vanto 2004). Pedersen 2002 used linear growth velocity (cm/year), and Vanto 2004 used change in height (cm) during a six‐month period as the growth outcome.
There was no significant difference between HFA‐BDP (100 μg/day to 200 μg/day) and CFC‐BDP (200 μg/day to 400 μg/day) in linear growth velocity (MD ‐0.44 cm/year, 95% CI ‐1.00 to 0.12; P = 0.12; 212 participants; low certainty evidence, Analysis 3.1). In contrast, budesonide delivered via Easyhaler (200 μg/day) resulted in a greater increase in height during a six‐month period, compared to budesonide delivered by Turbuhaler (200 μg/day) (MD 0.37 cm, 95% CI 0.12 to 0.62; P = 0.003; 229 participants; low certainty evidence; Analysis 3.2).
3.1. Analysis.

Comparison 3 Comparison of different devices, Outcome 1 Linear growth velocity (cm/yr).
3.2. Analysis.

Comparison 3 Comparison of different devices, Outcome 2 Change in height (cm) during a period of time.
Discussion
Summary of main results
We included six RCTs involving 1199 children with asthma (per‐protocol population: 1008) in this review. One trial with 23 participants showed that fluticasone given at an equivalent dose was associated with a significant greater linear growth velocity (MD 0.81 cm/year, 95% CI 0.46 to 1.16; low certainty evidence). Three trials compared fluticasone with budesonide. Fluticasone given at an equivalent dose had less suppressive effect than budesonide on growth, as measured by change in height over a period from 20 weeks to 12 months (MD 0.97 cm, 95% CI 0.62 to 1.32; 2 trials, 359 participants; moderate certainty evidence). However, no significant difference in linear growth velocity was observed between fluticasone and budesonide at equivalent doses (MD 0.39 cm/year, 95% CI ‐0.94 to 1.73; 2 trials, 236 participants; very low certainty evidence).
Two trials compared inhalation devices. One trial with 212 participants revealed a comparable linear growth velocity between beclomethasone via hydrofluoroalkane‐metered dose inhaler (HFA‐MDI) and beclomethasone via chlorofluorocarbon‐metered dose inhaler (CFC‐MDI) at an equivalent dose (MD ‐0.44 cm/year, 95% CI ‐1.00 to 0.12; low certainty evidence). Another trial with 229 participants showed a small but statistically significant greater increase in height over a period of six months with budesonide via Easyhaler, compared to budesonide given at the same dose via Turbuhaler (MD 0.37 cm, 95% CI 0.12 to 0.62; low certainty evidence).
Overall completeness and applicability of evidence
Head‐to‐head RCTs are of the optimal design to compare the growth effects of different ICS in children with asthma. However, the number of such studies is small, and the comparison was available only for fluticasone versus beclomethasone or budesonide at low to moderate doses. The currently available evidence from head‐to‐head comparisons showed that fluticasone had a less suppressive effect than beclomethasone and budesonide on growth in children with asthma, but the applicability of the evidence is limited. The growth effect of ICS is only one of the safety outcomes that needs to be taken into account in selecting an ICS for treating children with asthma. The selection of inhaled corticosteroid should be based on the efficacy, overall safety profile, ease of use, availability, cost of the drug, and preference of the child.
Chlorofluorocarbon‐propelled MDIs are no longer available on the market due to environmental concerns. Switching from CFC to HFA‐propelled inhalers has different impact on the therapeutic index of ICS (Allen 2015). For fluticasone, such switching does not lead to a change in particle size, and thus does not significantly impact lung or systemic circulation delivery. In contrast, a HFA‐propelled MDI delivers more than 50% of a dispensed dose of beclomethasone to the lung using a solution with extrafine particles, compared with only 4% to 8% delivered by a CFC‐MDI. An improved lung delivery of beclomethasone via HFA‐MDI also increases systemic bioavailability and risk of systemic effects when given at doses equal to those delivered via a CFC‐MDI. One trial included in this review showed that beclomethasone via HFA‐MDI at half the dose had a comparable linear growth velocity compared to beclomethasone via a CFC‐MDI.
Budesonide delivered via Easyhaler had a statistically significant greater increase in height (0.37 cm) over a six‐month period in children with asthma, compared to budesonide delivered via Turbuhaler. However, the clinical relevance of such a small difference between two dry powder inhalers is limited.
This systematic review did not include children with persistent asthma treated with other ICS besides beclomethasone, budesonide and fluticasone, or ICS combined with long acting beta2‐agonists (LABA). Thus, evidence derived from this review does not apply for these patients.
Certainty of the evidence
The certainty of evidence for the outcomes ranged from very low to moderate, as shown in Table 1; Table 2; Table 3; and Table 4. The most common reasons for downgrading the certainty of evidence were risk of bias in individual trials (unblinding, lack of information about methods of random sequence generation and allocation concealment, and risk of selective reporting bias due to industry sponsorship), and small number of trials and participants.
Two GlaxoSmithKline‐sponsored trials, from the same research group, reported results favourable to the sponsor's product (fluticasone), compared to another inhaled corticosteroid (budesonide) (Ferguson 1999; Ferguson 2007). This may raise the concern about the risk of reporting bias. In contrast, of two non‐industry‐sponsored trials, one showed that fluticasone had less adverse effect than beclomethasone on children's growth (Rao 1999), but another revealed no significant difference between fluticasone and budesonide on growth (Acun 2005).
In this systematic review, we selected trials which compared different inhaled corticosteroid molecules at equivalent doses. However, it cannot be assumed that nominal "equivalent doses" have the same degree of effect in all participants, given the variety of delivery systems, formulations and metabolism. Both therapeutic and adverse effects of ICS are related to the quantity of drugs being effectively delivered to the lungs, and should be presented together in growth trials. Three of five trials which compared fluticasone with other ICS reported efficacy data, and revealed that fluticasone at half the dose was equal or superior to budesonide and beclomethasone in controlling asthma (Ferguson 1999; Ferguson 2007; Rao 1999).
Potential biases in the review process
The main strength of this review is the inclusion of head‐to‐head trials that allows for direct comparison of different ICS and different delivery devices regarding the effects of ICS on growth in children with asthma. We are confident that we have identified all relevant studies; however, given the relatively restrictive literature search strategy used for this review, there is a small possibility that we failed to identify efficacy trials in which adverse effects of ICS on growth have been collected as secondary outcomes but not reported. Moreover, when screening results by titles and abstracts for eligibility, we cannot rule out the possibility that we missed efficacy trials that might have included growth data in the main text (tables and figures) but did not report them in the abstract.
Agreements and disagreements with other studies or reviews
We are not aware of other systematic reviews of similar scope. The first of a series of three Cochrane Reviews (Zhang 2014) performed the subgroup analyses to assess the impact of drug molecules and delivery devices on the effect size of ICS on growth in children with asthma. The subgroup analysis on ICS molecules showed that some first‐generation drugs had a slightly larger suppressive effect on growth than newer drugs, with a mean reduction in linear growth velocity of ‐1.0 cm/year (95% CI ‐1.45 to ‐0.45), ‐0.61 cm/year (95% CI ‐0.84 to ‐0.38), ‐0.15 cm/year (95% CI ‐0.37 to 0.07), ‐0.42 cm/year (95% CI ‐0.66 to ‐0.18) and ‐0.67 cm/year (95% CI ‐1.19 to ‐0.15) for beclomethasone (three trials), budesonide (two trials), ciclesonide (one trial), fluticasone (five trials) and mometasone (one trial), respectively.The evidence from indirect comparisons through subgroup analyses is partly consistent with the findings of this review of head‐to‐head trials, showing less suppressive effects of fluticasone than beclomethasone and budesonide in children with asthma. However, the implications of these findings for clinical practice remain uncertain.
The second of a series of three Cochrane Reviews (Pruteanu 2014) showed that the type of inhaled corticosteroid among newer molecules (ciclesonide, fluticasone, mometasone) did not seem to influence the impact on growth over one year. The effects of inhalation devices were not assessed.
In Zhang 2014, the subgroup analysis for inhalation devices within the molecule fluticasone (200 μg/day) did not show a statistically significant difference between CFC‐MDI, HFA‐MDI and DPI regarding the effects of ICS on linear growth velocity during a one‐year treatment period. The present review included only two trials that compared different devices for delivery of beclomethasone and budesonide, and one of them revealed a comparable linear growth velocity between beclomethasone via HFA‐MDI and beclomethasone via CFC‐MDI at an equivalent dose. Another trial showed that Easyhaler has less adverse effect on growth than Turbuhaler when used for delivery of budesonide, but these results may not be able to be extrapolated to other ICS.
The series of three Cochrane Reviews give a comprehensive picture of adverse effects of ICS on growth in children with persistent asthma. Regular use of ICS is associated with an average reduction of ‐0.48 cm/year in linear growth rate in the first year of treatment in prepubertal children with mild‐to‐moderate persistent asthma, and the effects tend to be less pronounced in the subsequent years of treatment (moderate certainty evidence). The initial decrease in attained height related to ICS in prepubertal age may persist as a reduction in adult height that is neither progressive nor cumulative. Inhaled‐corticosteroid‐induced growth suppression appears to be dose dependent (moderate certainty evidence), and ICS molecule and delivery device may impact the effect size of ICS on growth (very low to moderate certainty evidence).
The well‐established benefits of ICS in controlling asthma outweigh the potential risk of a relatively small and non‐cumulative suppression in linear growth in children with persistent asthma. The unjustified fear of drug side effects has been recognized as one of the reasons for under use and non‐adherence to treatment with ICS, and consequently poor asthma control in children (Gazala 2005). Uncontrolled asthma not only adversely impairs children’s growth, but also causes significant morbidity and mortality (Zhang 2019). Good communication between healthcare professionals and parents is essential to reduce steroid phobia and improve treatment adherence.
Authors' conclusions
Implications for practice.
This review suggests that drug molecule and delivery device may impact the effect size of inhaled corticosteroids (ICS) on growth in children with persistent asthma. Fluticasone at an equivalent dose seems to inhibit growth less than beclomethasone and budesonide. Easyhaler is likely to have less adverse effect on growth than Turbuhaler when used for delivery of budesonide. However, the evidence from this systematic review of head‐to‐head trials is not certain enough to definitively inform the selection of inhaled corticosteroid or inhalation device for the treatment of these patients. The selection of inhaled corticosteroid and delivery device should be based on the efficacy, overall safety profile, ease of use, availability, cost of treatment, and preference of the child. No matter the type of drug and inhalation device, use of the lowest effective dose of ICS along with regular monitoring of growth are recommended for all asthmatic children who receive long‐term treatment with ICS.
Implications for research.
There is a limited number of head‐to‐head randomized trials that compare different inhaled corticosteroid molecules and delivery devices regarding the effects on growth in children with asthma. Both patient factors (age, asthma severity, and adherence to treatment) and drug factors (inhaled corticosteroid molecules, doses, formulation, and delivery devices) can affect the effect size of ICS on growth. These factors should be taken into account in study design, and this is one of the main methodological challenges of such head‐to‐head trials. We did not find any ongoing trials by searching the database ClinicalTrials.gov. There is probably little impetus for both pharmaceutical industries and researchers to conduct further head‐to‐head randomized trials to compare inhaled corticosteroid molecules and devices because of complexities in study design, limited clinical relevance, and concerns for conflict of interests. Pragmatic randomized trials have the same methodological challenges as mentioned above. However, such a study design enables the inclusion of a broader spectrum of patients and interventions, as seen in real‐life practice, and therefore the results from pragmatic trials have greater applicability. Real‐life observational studies are another feasible option to assess the effects of ICS on growth in children with asthma. Confounding is one of the main shortcomings of observational studies, and should be identified and addressed appropriately.
Acknowledgements
Thanks to Elizabeth Stovold for help in developing the search strategy and conducting searches. Thanks also to Chris Cates for useful comments, to Emma Dennett and Emma Jackson for commenting and ongoing assistance, and to Prof Francine Ducharme for providing helpful editorial feedback on our protocol.
The authors and Cochrane Airways editorial team are grateful to the following peer reviewers for their time and comments: Alexander Ferguson, Emeritus Professor of Pediatrics, University of British Columbia; Professor Hilary Pinnock, Asthma UK Centre for Applied Research, Usher Institute of Population Health Sciences and Informatics, The University of Edinburgh; and our consumer reviewer, Aireen Wingert, Canada.
The Background and Methods sections of this review are based on a standard template used by Cochrane Airways.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Airways Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Trials Register
Electronic searches: core databases
| Database | Dates searched | Frequency of search |
| CENTRAL (via the Cochrane Register of Studies (CRS)) | From inception | Monthly |
| MEDLINE (Ovid) | 1946 onwards | Weekly |
| Embase (Ovid) | 1974 onwards | Weekly |
| PsycINFO (Ovid) | 1967 onwards | Monthly |
| CINAHL (EBSCO) | 1937 onwards | Monthly |
| AMED (EBSCO) | From inception | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the Cochrane Airways Trials Register
Asthma search
1. exp Asthma/
2. asthma$.mp.
3. (antiasthma$ or anti‐asthma$).mp.
4. Respiratory Sounds/
5. wheez$.mp.
6. Bronchial Spasm/
7. bronchospas$.mp.
8. (bronch$ adj3 spasm$).mp.
9. bronchoconstrict$.mp.
10. exp Bronchoconstriction/
11. (bronch$ adj3 constrict$).mp.
12. Bronchial Hyperreactivity/
13. Respiratory Hypersensitivity/
14. ((bronchial$ or respiratory or airway$ or lung$) adj3 (hypersensitiv$ or hyperreactiv$ or allerg$ or insufficiency)).mp.
15. ((dust or mite$) adj3 (allerg$ or hypersensitiv$)).mp.
16. or/1‐15
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomised or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify trials in other electronic database.
Appendix 2. Search strategy to identify references from the Cochrane Airways Trials Register
#1 AST:MISC1 #2 MeSH DESCRIPTOR Asthma Explode All #3 asthma*:ti,ab #4 #1 or #2 or #3 #5 steroid* #6 corticosteroid* #7 glucocorticoid* #8 inhal* #9 (#5 or #6 or #7) and #8 #10 budesonide #11 Pulmicort #12 fluticasone #13 Flixotide #14 Flovent #15 ciclesonide #16 Alvesco #17 triamcinolone #18 Kenalog #19 beclomethasone #20 Becotide #21 Becotide #22 Becloforte #23 Becodisk #24 QVAR #25 Flunisolide #26 AeroBid #27 mometasone #28 Asmanex #29 #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 #30 grow* #31 knemomet* #32 height* #33 leg* #34 SDS #35 #30 or #31 or #32 or #33 or #34 #36 child* #37 paediat* #38 pediat* #39 adolesc* #40 teen* #41 prepubertal* #42 pre‐pubertal* #43 puberty #44 pubertal* #45 infant* #46 toddler* #47 baby or babies #48 young* #49 #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 or #44 or #45 or #46 or #47 or #48 #50#4 and #29 and #35 and #49
[In search line #1, MISC1 denotes the field in the record where the reference has been coded for condition, in this case, asthma]
Data and analyses
Comparison 1. Comparison of fluticasone and beclomethasone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Linear growth velocity (cm/year) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 2. Comparison of fluticasone and budesonide.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Linear growth velocity | 2 | 236 | Mean Difference (IV, Random, 95% CI) | 0.39 [‐0.94, 1.73] |
| 2 Change in height (cm) over a period of time | 2 | 359 | Mean Difference (IV, Random, 95% CI) | 0.97 [0.62, 1.32] |
Comparison 3. Comparison of different devices.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Linear growth velocity (cm/yr) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 HFA‐BDP versus CFC‐BDP | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Change in height (cm) during a period of time | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Easyhaler‐BUD versus Turbuhaler‐BUD | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Acun 2005.
| Methods | Design: randomized, open‐label, parallel‐group trial Sponsorship: not stated |
|
| Participants | Setting: one university in Turkey Eligible: children with moderate persisting asthma Intention‐to‐treat population: 100 (FP 55, BUD 45) Per‐protocol population: 100 (FP 55, BUD 45) Gender (male): 51% Age, yr (mean; range): FP 7.1, BUD 6.6; 4‐11.5 Inclusion criteria: recent diagnosis of moderate, persistent asthma with exacerbation of asthma twice weekly, or symptoms every day, or night symptoms more than once a week, or a combination of these criteria. Treatment with only inhaled salbutamol Exclusion criteria: symptoms were only seasonal or exercise‐induced, regular therapy with ICS, and other chronic diseases |
|
| Interventions | First drug: fluticasone propionate (GlaxoSmithKline, UK) Dose: 250 µg/day (125 µg twice daily) Inhaler: medium‐size volume‐spacer MDI Duration: 12 months Second drug: budesonide (Astra Zeneca, UK) Dose: 400 µg/day (200 µg twice daily) Inhaler: medium‐size volume‐spacer MDI Duration: 12 months |
|
| Outcomes | Linear growth velocity (cm/year, mean ± SD): FP 6.0 ± 0.94, BUD 6.37 ± 3.09 (P > 0.05) | |
| Notes | No data on ethical approval Efficacy data not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No data available |
| Allocation concealment (selection bias) | Unclear risk | No data available |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals reported |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but published reports include all expected outcomes |
| Other bias | Low risk | No other bias identified |
Ferguson 1999.
| Methods | Design: randomized, double‐blind, double‐dummy, parallel‐group, multinational trial Sponsorship: at least one author worked for GlaxoWellcome | |
| Participants | Setting: 7 countries (Canada, Denmark, Finland, Netherlands, Indonesia, Poland and South Africa) Eligible: 442 Intention‐to‐treat population: 333 (FP 166, BUD 167) Per‐protocol population: 308 (FP 151, BUD 157); 154 (FP 76, BUD 78) underwent stadiometry and were included for analysis Gender (male): 67% Age, yr (mean; range): FP 8.2, BUD 7.9; 4‐12 Inclusion criteria: moderate to severe asthma which required moderate to high doses of ICS to control symptoms for at least 1 month preceding the start. Prepubertal (Tanner stage 1) Exclusion criteria: children who had received combination bronchodilators or systemic corticosteroids, had any sign of serious disease other than asthma, or had been admitted to hospital in the month preceding the study. Seasonal or exercise‐induced asthma | |
| Interventions | First drug: fluticasone propionate (GlaxoWellcome) Dose: 400 μg/day (200 μg twice daily) Inhaler: Diskus Duration: 20 weeks Second drug: budesonide (Astra Pharma) Dose: 800 μg/day (400 μg twice daily) Inhaler: Turbuhaler Duration: 20 weeks | |
| Outcomes | Mean increase in height over a period of 20 weeks: FP 3.31 cm, BUD 1.99 cm. The difference was 1.32 cm (95% CI 0.48 to 2.17, P = 0.002) | |
| Notes | Ethical approval: yes FP at half the dose was superior to BUD in improving PEF, but there were no significant differences between FP and BUD in symptom scores, need for rescue medication, FEV1, FVC, and FEF25‐75% Only 46% (154/333) of the participants underwent stadiometry. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization was not described |
| Allocation concealment (selection bias) | Unclear risk | No data available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind and double‐dummy |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind and double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data from half of the patients were used. |
| Selective reporting (reporting bias) | High risk | Reporting bias was possible since the study conclusion was favourable to the sponsor’s product |
| Other bias | High risk | The study was not designed to assess growth, and measurement of height was done primarily to calculate predicted values for spirometry |
Ferguson 2007.
| Methods | Design: randomized, double‐blind, double‐dummy, parallel‐group, multi‐centred trial Sponsorship: funded by GlaxoSmithKline [Study FMS40001] | |
| Participants | Setting: 35 centres (mainly secondary care) in 11 countries Eligible: 233 Intention‐to‐treat population: 233 (FP 114, BUD 119) Per‐protocol population: 136 (FP 70, BUD 66) Gender (male): 69 % Age, yr (mean; range): FP 7.2, BUD 7.4; 6‐9 Inclusion criteria: persistent asthma for ≥ 6 months, a FEV1 of ≥ 60% predicted for height and age, an improvement in PEF of ≥ 15% after inhalation of 200 μg of salbutamol, ability to use the Diskus and the Turbuhaler correctly, no changes in asthma medication and no treatment with ICS in the previous 4 weeks. Patients had to be prepubescent (Tanner sexual maturity rating of 1) and considered by the investigator to require ICS. Height between the 5th and 95th centiles for their age and run‐in growth velocity (rate of change in height over time) between the 20th and 95th percentiles Exclusion criteria: received OCS on > 2 occasions or > 12 days or > 210 mg prednisone (or equivalent) during the previous 6 months. Known growth disorder, or history of glaucoma or cataracts | |
| Interventions | First drug: fluticasone propionate Dose: 200 μg/day (100 μg twice daily) Inhaler: Diskus Duration: 12 months Second drug: budesonide Dose: 400 μg/day (200 μg twice daily) Inhaler: Turbuhaler Duration: 12 months | |
| Outcomes | Adjusted linear growth velocity (cm/year, mean ± SE): FP: 5.3 ± 0.1, BUD 4.3 ± 0.1 (P < 0.001) Unadjusted change in height (cm) from baseline to week 52 (ITT; mean ± SD): 5.6 ± 1.3 (FP, n = 104), 4.7 ± 1.5 (BUD, n = 101) | |
| Notes | Ethical approval: yes There were no significant differences between FP at half the dose and BUD in the mean change over the treatment period for PEF, FVC and FEV1 at each visit, with the exception of a smaller increase in FVC at weeks 16 and 52, and a smaller increase in FEV1 at week 16 in FP treated participants. There were also no significant differences between two groups in asthma symptoms, need for rescue medication, and acute exacerbation. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization schedule generated by the Glaxo‐Wellcome computer program ‘Patient Allocation for Clinical Trials’ (block size of 4) |
| Allocation concealment (selection bias) | Low risk | Treatment packs were numbered and the allocations were not revealed to investigators or other study participants until all analyses were complete. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind and double dummy |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind and double dummy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Substantial differences between ITT and PP populations, but the analyses by ITT and PP populations yielded the similar results |
| Selective reporting (reporting bias) | High risk | Reporting bias was possible since the study conclusion was favourable to the sponsor’s product. |
| Other bias | Low risk | No other bias identified |
Pedersen 2002.
| Methods | Design: randomized, open‐label, multicentre study Sponsorship: funded by 3M Pharmaceuticals, St Paul, Minnesota (produces the Autohaler) |
|
| Participants | Setting: 56 sites in USA (26), UK (10), Germany (7), Belgium and the Netherlands (3), Australia (3), Scandinavia (3) and France (4) Eligible: 300 children 5‐11 years old. Boys: 5‐11, girls: 5‐10 Intention‐to‐treat population: 256 (HFA‐BDP 189, CFC‐BDP 67) Per‐protocol population: 212 (HFA‐BDP 156, CFC‐BDP 56) Gender (male): 72% Age, yr (mean; range): HFA‐BDP 8.7, CFC‐BDP 8.4; 5‐12 Inclusion criteria: a clinical diagnosis of asthma extending back for ≥ 6 months; asthma symptoms that remained stable during the previous month on 200 to 800 µg/day (ex‐valve) beclomethasone dipropionate or budesonide from a pMDI (+spacer) or a dry powder inhaler; FEV1 of ≥ 60% of predicted normal, eligible symptoms Exclusion criteria: significant chronic disease other than asthma; acute upper or lower respiratory tract infection within 2 weeks; immobilized; injectable steroids within 8 weeks or OCS within 4 weeks; current use of > 200 µg/day beclomethasone dipropionate or equivalent of nasal steroids; antibiotic treatment for respiratory disorders within 2 weeks; visible oropharyngeal candidiasis; or obesity. Patients who missed > 2 complete days of diary data or > 10 diary card entries during the last 2 weeks of the run‐in period |
|
| Interventions | First drug: hydrofluoroalkane 134a (HFA)‐beclomethasone dipropionate extrafine aerosol Dose: half dose compared with pre‐study dose; 100‐200 μg twice daily Inhaler: Autohaler [QVAR Autohaler, 3M Pharmaceuticals] Duration: 12 months Second drug: chlorofluorocarbon beclomethasone dipropionate (CFC‐BDP) Dose: same as pre‐study dose; 200‐400 μg twice daily. Inhaler: a press‐and‐breathe MDI and spacer [Volumatic, Glaxo Wellcome] Duration: 12 months |
|
| Outcomes | Linear growth velocity (cm/year, mean ± SE): HFA‐BDP: 5.27 ± 0.14; CFC‐BDP: 5.71 ± 0.25 (P = 0.12) Change in height over a period of 12 months: 5.23 cm ± 0.14; 5.66 cm ± 0.26 (P = 0.15) |
|
| Notes | Ethical approval: yes CFC‐BDP is no longer available in the market. There were no significant differences between HFA‐BDP at half dose and CFC‐BDP in PEF, FEV1, and asthma symptoms. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization generated by computer |
| Allocation concealment (selection bias) | Unclear risk | No data available |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Substantial differences between ITT and PP populations |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but published reports include all expected outcomes. No significant difference was reported between the sponsor's product and the product from the other manufacturer. |
| Other bias | Low risk | No other bias identified |
Rao 1999.
| Methods | Design: randomized, double‐blind, parallel‐group trial Sponsorship: not stated |
|
| Participants | Setting: a single hospital in England Eligible: 23 Intention‐to‐treat population: 23 (FP 15, BDP 8) Per‐protocol population: 23 (FP 15, BDP 8) Gender (male): no data Age, yr (mean; range): 6.77; 5‐10 Inclusion criteria: moderately severe asthma with unsatisfactory control, eligible for prophylactic therapy according to international guidelines and at least one of the following features: 1) asthma symptoms of at least a score of 1 (out of a maximum of 6) on at least 2 separate days out of 7 consecutive run‐in days; 2) diurnal variation in peak flows ≥ 20% on at least 2 out of 7 consecutive run‐in days; and 3) one acute exacerbation of asthma during the previous month Exclusion criteria: no data |
|
| Interventions | First drug: beclomethasone dipropionate Dose: 400 µg/day (200 μg twice daily) Inhaler: a metered‐dose inhaler with a VolumaticTM spacer (GlaxoWellcome, Middlesex, UK) Duration: 20 months Second drug: fluticasone propionate Dose: 200 µg/day (100 μg twice daily) Inhaler: a metered‐dose inhaler with a VolumaticTM spacer (GlaxoWellcome, Middlesex, UK) Duration: 20 months |
|
| Outcomes | Linear growth velocity (cm/year): BDP: 4.94, FP: 5.75; difference 0.81 (95% CI: 0.45 to 1.16) | |
| Notes | Ethical approval: yes There were no significant differences between FP at half the dose and BDP in FEV1, FEF25‐75%, and symptom score. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No data |
| Allocation concealment (selection bias) | Unclear risk | No data |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded with placebo control |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blinded with placebo control |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals reported |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but published reports include all expected outcomes |
| Other bias | Low risk | No other bias identified |
Vanto 2004.
| Methods | Design: randomized, double‐blind, double‐dummy, parallel‐group study Sponsorship: two authors were from Orion Pharma, Kuopio, Finland |
|
| Participants | Setting: 32 centres in Scandinavia and Finland Eligible: 254 Intention‐to‐treat population: 254 (Easyhaler 123, Turbuhaler 131) Per‐protocol population: 229 (Easyhaler 111, Turbuhaler 118) Gender (male): 62% Age, yr (mean; range): Easyhaler 7.2, Turbuhaler 7.7; 5‐10 Inclusion criteria: (1) age 5–10 years, (2) symptomatic bronchial asthma requiring ICS, (3) PEF or FEV1 ≥ 60% of the predicted value, (4) asthma diagnosis meeting at least one of the following criteria: either a 15% increase in FEV1 or PEF after sympathomimetic inhalation or a 15% decrease in FEV1 or PEF after an exercise test or a 20% diurnal variability in PEF on at least 4 days during 1 week, and able to use device Exclusion criteria: ICS within 1 month before the screening period, or OCS within the month before the first visit |
|
| Interventions | First drug: budesonide (Easyhaler) + placebo (Turbuhaler) Dose: 800 µg/day (400 μg twice daily) for 2 months, then 200 µg/day (100 μg twice daily) for 4 months Inhaler: Easyhaler dry powder inhaler (Orion) Duration: 6 months Second drug: budesonide (Turbuhaler) + placebo (Easyhaler) Dose: 800 µg/day for 2 months, then 200 µg/day for 4 months Inhaler: Turbuhaler (AstraZeneca) Duration: 6 months |
|
| Outcomes | Change in height over a period (cm/6 months, mean ± SD): Easyhaler: 2.54 ± 1.0, Turbuhaler: 2.17 ± 0.9 (P = 0.002) | |
| Notes | Ethical approval: not stated There were no significant differences between BUD 800 µg/day via Easyhaler and BUD 800 µg/day via Turbuhaler in PEF, FEV1, FVC, and rescue use of beta‐2 agonists. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was performed centrally and stratified by centre. |
| Allocation concealment (selection bias) | Unclear risk | No data available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind and double‐dummy |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind and double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐out 10% |
| Selective reporting (reporting bias) | High risk | Reporting bias was possible since the study conclusion was favourable to the sponsor's product. |
| Other bias | Low risk | No other bias identified |
BDP: beclomethasone dipropionate BUD: budesonide CFC: chlorofluorocarbon DPI: dry powder inhaler FEF25‐75%: forced expiratory flow between 25% and 75% of forced vital capacity FEV1: forced expiratory volume in the first second FVC: forced vital capacity FP: fluticasone propionate ICS: inhaled corticosteroids ITT: intention‐to‐treat HFA: hydrofluoroalkane MDI: metered dose inhaler OCS: oral corticosteroids PEF: peak expiratory flow PP: per protocol SD: standard deviation SDS: standard deviation score SE: standard error
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Altintas 2005 | No data on devices |
| de Benedictis 2000 | Comparison of two different ICS (fluticasone propionate vs beclomethasone dipropionate), and not at equivalent doses |
| Delacourt 2003 | Treatment < 3 months; no growth data. |
| Ferguson 2001 | Congress abstract; data published in Ferguson 2007 |
| Ferguson 2002 | Congress abstract; data published in Ferguson 2007 |
| Ferguson 2003 | Congress abstract; data published in Ferguson 2007 |
| Fitzgerald 1998 | Cross‐over study design |
| Gillman 2002 | Comparison of two different ICS (fluticasone propionate vs beclomethasone dipropionate), delivered by different devices (HFA‐MDI vs CFC‐MDI) |
| GSK 2005 | The same study as Ferguson 2007 |
| Kannisto 2000 | Comparison of two different ICS (fluticasone propionate vs budesonide) not at exactly equivalent doses due to available formulation during the first two months of treatment. 62% (37/60) of participants randomized to ICS groups were allocated to receive cromones after four months of ICS treatment. |
| Kannisto 2002 | Pragmatic study design |
| Salvatoni 2000 | Comparison of two different ICS (fluticasone propionate vs budesonide), delivered by different devices (MDI + spacer vs Turbohaler) |
Differences between protocol and review
Subgroup analyses and sensitivity analyses were not performed due to the small number of included studies.
Funnel plot was not generated due to the small number of included studies.
A search of Web of Science was added to the other searches.
'Summary of findings tables' were added.
Differences in thresholds for the interpretation of the I² statistic: in the protocol, we defined the cut‐off values of 25%, 50% and 75% for low, moderate and high heterogeneity, respectively. In the review, we used the following thresholds: 0% to 40%, not important; 30% to 60%, moderate heterogeneity; 50% to 90%, substantial heterogeneity; and 75% to 100%, considerable heterogeneity.
Contributions of authors
Inge Axelsson (IA) and Linjie Zhang (LZ) wrote the review protocol and full review text. Sílvio OM Prietsch (SOP) provided input to the drafting of the protocol. IA and SOP selected studies. IA extracted data from the selected studies and Estelle Naumburg (EN) checked the extracted data for accuracy and completeness. IA, EN, SOP and LZ participated in either study selection, data extraction or assessment of risk of bias in included studies. LZ conducted all statistical analyses. All authors have approved the final version of the review.
Contributions of editorial team
Rebecca Fortescue (Co‐ordinating Editor): edited the review; advised on methodology, interpretation and content; approved the final review prior to publication. Chris Cates (Co‐ordinating Editor): checked the data entry prior to the full write‐up of the review. Emma Dennett (Managing Editor): co‐ordinated the editorial process; advised on interpretation and content; edited the review. Emma Jackson (Assistant Managing Editor): conducted peer review; edited the plain language summary and reference sections of the protocol and the review. Elizabeth Stovold (Information Specialist): designed the search strategy; ran the searches; edited the search methods section.
Sources of support
Internal sources
The authors declare that no such funding was received for this systematic review, Other.
External sources
-
Brazilian National Council for Scientific and Technological Development (CNPq), Brazil.
Dr. Linjie Zhang is currently a Productivity Research Fellow (level 2) of the Brazilian National Council for Scientific and Technological Development (CNPq).
Declarations of interest
IA: none known. EN: none known. SOP: none known. LZ: none known.
New
References
References to studies included in this review
Acun 2005 {published data only}
- Acun C, Tomac N, Ermis B, Onk G. Effects of inhaled corticosteroids on growth in asthmatic children: a comparison of fluticasone propionate with budesonide. Allergy and Asthma Proceedings 2005;26(3):204‐6. [PubMed] [Google Scholar]
Ferguson 1999 {published data only}
- Ferguson AC, Spier S, Manjra A, Versteegh FG, Mark S, Zhang P. Efficacy and safety of high‐dose inhaled steroids in children with asthma: a comparison of fluticasone propionate with budesonide. Journal of Pediatrics 1999;134(4):422‐7. [DOI] [PubMed] [Google Scholar]
Ferguson 2007 {published data only}
- Ferguson AC, Bever HP, Teper AM, Lasytsya O, Goldfrad CH, Whitehead PJ. A comparison of the relative growth velocities with budesonide and fluticasone propionate in children with asthma. Respiratory Medicine 2007;101(1):118‐29. [DOI] [PubMed] [Google Scholar]
Pedersen 2002 {published data only}
- Pedersen S, Warner J, Wahn U, Staab D, Bourgeois M, Essen‐Zandvliet E, et al. Growth, systemic safety, and efficacy during 1 year of asthma treatment with different beclomethasone dipropionate formulations: an open‐label, randomized comparison of extrafine and conventional aerosols in children. Pediatrics 2002;109(6):e92. [DOI] [PubMed] [Google Scholar]
Rao 1999 {published data only}
- Rao R, Gregson RK, Jones AC, Miles EA, Campbell MJ, Warner JO. Systemic effects of inhaled corticosteroids on growth and bone turnover in childhood asthma: a comparison of fluticasone with beclomethasone. European Respiratory Journal 1999;13(1):87‐94. [DOI] [PubMed] [Google Scholar]
Vanto 2004 {published data only}
- Vanto T, Hämäläinen KM, Vahteristo M, Wille S, Njå F, Hyldebrandt N, et al. Comparison of two budesonide dry powder inhalers in the treatment of asthma in children. Journal of Aerosol Medicine 2004;17(1):15‐24. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Altintas 2005 {published data only}
- Altintas DU, Karakoc GB, Can S, Yilmaz M, Kendirli SG. The effects of long term use of inhaled corticosteroids on linear growth, adrenal function and bone mineral density in children. Allergologia et Immunopathologia 2005;33(4):204‐9. [DOI] [PubMed] [Google Scholar]
de Benedictis 2000 {published data only}
- Benedictis FM, Teper A, Green RJ, Boner AL, Williams L, Medley H, International Study Group. Effects of 2 inhaled corticosteroids on growth: results of a randomized controlled trial. Archives of Pediatrics and Adolescent Medicine 2001;155(11):1248‐54. [DOI] [PubMed] [Google Scholar]
Delacourt 2003 {published data only}
- Delacourt C, Dutau G, Lefrançois G, Clerson P, Beclospin Clinical Development Group. Comparison of the efficacy and safety of nebulized beclometasone dipropionate and budesonide in severe persistent childhood asthma. Respiratory Medicine 2003;97(Suppl B):S27‐33. [PubMed] [Google Scholar]
Ferguson 2001 {published data only}
- Ferguson AC. Randomised, double‐blind, double‐dummy, parallel‐group comparison of the effect of fluticasone propionate (FP) 100 μg bd and budesonide (BUD) 200 μg bd on childhood growth: protocol and baseline characteristics. European Respiratory Journal 2001;18:289s. [Google Scholar]
Ferguson 2002 {published data only}
- Ferguson AC. Fluticasone propionate 100 μg bd (FP100) has significantly less effect than budesonide 200 μg bd (BUD200) on childhood growth over 1 year of treatment in asthmatics. European Respiratory Journal 2001;20:219s. [Google Scholar]
Ferguson 2003 {published data only}
- Ferguson AC. Significantly reduced growth velocity over 1 year with budesonide 200 μg bd (BUD200) compared to fluticasone propionate 100 μg bd (FP100) in children with asthma. American Thoracic Society 99th International Conference 2003:A117 Poster D63. [Google Scholar]
Fitzgerald 1998 {published data only}
- Fitzgerald D, Asperen P, Mellis C, Honner M, Smith L, Ambler G. Fluticasone propionate 750 micrograms/day versus beclomethasone dipropionate 1500micrograms/day: comparison of efficacy and adrenal function in paediatric asthma. Thorax 1998;53(8):656‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gillman 2002 {published data only}
- Gillman SA, Anolik R, Schenkel E, Newman K. One‐year trial on safety and normal linear growth with flunisolide HFA in children with asthma. Clinical Pediatrics 2002;41(5):333‐40. [DOI] [PubMed] [Google Scholar]
GSK 2005 {published data only}
- GSK. A randomised, double‐blind, double‐dummy, parallel group, multicentre study to compare the effect on growth measured by stadiometry of fluticasone propionate (100μg bd) administered via DISKUS™ with budesonide (200μg bd) administered via Turbuhaler in prepubescent children. GSK 2005:study no. FMS40001. [Google Scholar]
Kannisto 2000 {published data only}
- Kannisto S, Korppi M, Remes K, Voutilainen R. Adrenal suppression, evaluated by a low dose adrenocorticotropin test, and growth in asthmatic children treated with inhaled steroids. Journal of Clinical Endocrinology and Metabolism 2000;85(2):652‐7. [DOI] [PubMed] [Google Scholar]
Kannisto 2002 {published data only}
- Kannisto S, Voutilainen R, Remes K, Korppi M. Efficacy and safety of inhaled steroid and cromone treatment in school‐age children: a randomized pragmatic pilot study. Pediatric Allergy and Immunology 2002;13(1):24‐30. [DOI] [PubMed] [Google Scholar]
Salvatoni 2000 {published data only}
- Salvatoni A, Nosetti L, Broggini M, Nespoli L. Body composition and growth in asthmatic children treated with inhaled steroids. Annals of Allergy, Asthma and Immunology 2000;85(3):221‐6. [DOI] [PubMed] [Google Scholar]
Additional references
Allen 1999
- Allen DB. Limitations of short‐term studies in predicting long‐term adverse effects of inhaled corticosteroids. Allergy 1999;54(Suppl 49):29‐34. [DOI] [PubMed] [Google Scholar]
Allen 2015
- Allen DB. Inhaled corticosteroids and growth: still an issue after all these years. Journal of Pediatrics 2015;166(2):463‐9. [DOI] [PubMed] [Google Scholar]
Asher 2014
- Asher I, Pearce N. Global burden of asthma among children. International Journal of Tuberculosis and Lung Disease 2014;18(11):1269‐78. [DOI] [PubMed] [Google Scholar]
BTS 2016
- British Thoracic Society. Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma. A national clinical guideline. www.brit‐thoracic.org.uk/document‐library/clinical‐information/asthma/btssign‐asthma‐guideline‐2016 (accessed prior to 28 November 2018).
Colice 2000
- Colice GL. Comparing inhaled corticosteroids. Respiratory Care 2000;45(7):846‐53. [PubMed] [Google Scholar]
Djukanovic 1992
- Djukanovic R, Wilson JW, Britten KM, Wilson SJ, Walls AF, Roche WR, et al. Effect of an inhaled corticosteroid on airway inflammation and symptoms in asthma. American Review of Respiratory Disease 1992;145(3):669‐74. [DOI] [PubMed] [Google Scholar]
Efthimiou 1998
- Efthimiou J, Barnes PJ. Effect of inhaled corticosteroids on bones and growth. European Respiratory Journal 1998;11(5):1167‐77. [DOI] [PubMed] [Google Scholar]
Ferrante 2018
- Ferrante G, Grutta S. The burden of pediatric asthma. Frontiers in Pediatrics 2018;6:186. [DOI: 10.3389/fped.2018.00186] [DOI] [PMC free article] [PubMed] [Google Scholar]
FIRS 2017
- Forum of International Respiratory Societies. The Global Impact of Respiratory Disease. 2nd Edition. Sheffield: European Respiratory Society, 2017. [Google Scholar]
Gazala 2005
- Gazala E, Sadka R, Bilenko N. Parents’ fears and concerns toward inhaled corticosteroid treatment for their asthmatic children. Pediatric Allergy and Immunology 2005;18(2):82‐7. [Google Scholar]
GINA 2018
- Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention 2018. ginasthma.org/2018‐gina‐report‐global‐strategy‐for‐asthma‐management‐and‐prevention/ (accessed 5 April 2018).
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Kapadia 2016
- Kapadia CR, Nebesio TD, Myers SE, Willi S, Miller BS, Allen DB, et al. Endocrine effects of inhaled corticosteroids in children. JAMA Pediatrics 2016;170(2):163‐70. [DOI] [PubMed] [Google Scholar]
Leung 2017
- Leung JS, Johnson DW, Sperou AJ, Crotts J, Saude E, Hartling L, et al. A systematic review of adverse drug events associated with administration of common asthma medications in children. PLoS One 2017;12(8):e0182738. [DOI] [PMC free article] [PubMed] [Google Scholar]
NHLBI 2007
- National Heart, Lung and Blood Institute (NHLBI). Guidelines for the diagnosis and management of asthma (EPR‐3). www.nhlbi.nih.gov/health‐topics/guidelines‐for‐diagnosis‐management‐of‐asthma Vol. (accessed 5 April 2018).
NICE 2017
- NICE. Asthma: diagnosis, monitoring and chronic asthma management. www.nice.org.uk/guidance/ng80 (accessed prior to 28 November 2018).
Pedersen 2001
- Pedersen S. Do inhaled corticosteroids inhibit growth in children?. Americian Journal of Respiratory and Critical CareMedicine 2001;164(4):521‐35. [DOI] [PubMed] [Google Scholar]
Price 2002
- Price J, Hindmarsh P, Hughes S, Efthimiou J. Evaluating the effects of asthma therapy on childhood growth: what can be learnt from the published literature?. European Respiratory Journal 2002;19:1179–93. [DOI] [PubMed] [Google Scholar]
Pruteanu 2014
- Pruteanu AI, Chauhan BF, Zhang L, Prietsch SOM, Ducharme FM. Inhaled corticosteroids in children with persistent asthma: dose‐response effects on growth. Cochrane Database of Systematic Reviews 2014, Issue 7. [DOI: 10.1002/14651858.CD009878.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
The Global Asthma Network 2014
- Global Asthma Network. The Global Asthma Report 2014. www.globalasthmareport.org/2014/index.php (accessed prior to 28 November 2018).
Wolfgram 2015
- Wolfgram PM, Allen DB. Factors influencing growth effects of inhaled corticosteroids in children. Journal of Allergy and Clinical Immunology 2015;136(6):1711‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2011
- Zhang L, Axelsson I, Chung M, Lau J. Dose response of inhaled corticosteroids in children with persistent asthma: a systematic review. Pediatrics 2011;127(1):129‐38. [DOI] [PubMed] [Google Scholar]
Zhang 2014
- Zhang L, Prietsch SOM, Ducharme FM. Inhaled corticosteroids in children with persistent asthma: effects on growth. Cochrane Database of Systematic Reviews 2014, Issue 7. [DOI: 10.1002/14651858.CD009471.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2019
- Zhang L, Lasmar LB, Castro‐Rodriguez JA. The impact of asthma and its treatment on growth:an evidence‐based review. Jornal de Pediatria 2019;95(Suppl 1):10‐22. [DOI] [PubMed] [Google Scholar]


