Abstract
Background
Standard androgen suppression therapy (AST) using surgical or medical castration is considered a mainstay of advanced hormone‐sensitive prostate cancer treatment. AST can be initiated early when disease is asymptomatic or deferred when patients suffer symptoms of disseminated prostate cancer.
Objectives
To assess the effects of early versus deferred standard AST for advanced hormone‐sensitive prostate cancer.
Search methods
For this Cochrane Review update, we performed a comprehensive search of multiple databases (CENTRAL, MEDLINE, Embase, Web of Science; last searched November 2018) and two clinical trial registers, with no restrictions on the language of publication or publication status. We also searched bibliographies of included studies and conference proceedings (last searched January 2019).
Selection criteria
We included all randomised controlled trials (RCTs) with a direct comparison of early versus deferred standard AST. We excluded all other study designs. Participants included had advanced hormone‐sensitive prostate cancer receiving surgical or medical castration.
Data collection and analysis
Two review authors independently classified studies and abstracted data. The primary outcomes were time to death of any cause and serious adverse events. Secondary outcomes were time to disease progression, time to death from prostate cancer, adverse events and quality of life. We performed statistical analyses using a random‐effects model and assessed the certainty of evidence according to GRADE. We performed subgroup analyses for advanced but non‐metastatic disease (T2‐4/N+ M0), metastatic disease (M1), and prostate‐specific antigen (PSA) relapse.
Main results
We identified seven new RCTs since publication of the original review in 2002. In total, we included 10 RCTs.
Primary outcomes Early AST probably reduces the risk of death from any cause over time (hazard ratio (HR) 0.82, 95% confidence interval (CI) 0.75 to 0.90; moderate‐certainty evidence; 4767 participants). This corresponds to 57 fewer deaths (95% CI 80 fewer to 31 fewer) per 1000 participants at 5 years for the moderate risk group and 23 fewer deaths (95% CI 32 fewer to 13 fewer) per 1000 participants at 5 years in the low risk group. We downgraded for study limitations. Early versus deferred AST may have little or no effect on serious adverse events (risk ratio (RR) 1.05, 95% CI 0.95 to 1.16; low‐certainty evidence; 10,575 participants) which corresponds to 6 more serious adverse events (6 fewer to 18 more) per 1000 participants. We downgraded the certainty of evidence for study limitations and selective reporting.
Secondary outcomes Early AST probably reduces the risk of death from prostate cancer over time (HR 0.69, 95% CI 0.57 to 0.84; moderate‐certainty evidence). This corresponds to 62 fewer prostate cancer deaths per 1000 (95% CI 87 fewer to 31 fewer) at 5 years for the moderate risk group and 24 fewer death from prostate cancer (95% CI 34 fewer to 12 fewer) per 1000 men at 5 years in the low risk group. We downgraded the certainty of evidence for study limitations.
Early AST may decrease the rate of skeletal events (RR 0.37, 95% CI 0.17 to 0.80; low‐certainty evidence) corresponding to 23 fewer skeletal events per 1000 (95% CI 31 fewer to 7 fewer). We downgraded for study limitations and imprecision. It may also increase fatigue (RR 1.41, 95% CI 1.23 to 1.62; low‐certainty evidence), corresponding to 31 more men with this complaint per 1000 (95% CI 18 more to 48 more). We downgraded for study limitations and imprecision. It may increase the risk of heart failure (RR 1.90, 95% CI 1.09 to 3.33; low‐certainty evidence) corresponding to 27 more events per 1000 (95% CI 3 more to 69 more). We downgraded the certainty of evidence for study limitations and imprecision.
Global quality of life is probably similar after two years as assessed with the EORTC QLQ‐C30 (version 3.0) questionnaire (mean difference −1.56, 95% CI −4.50 to 1.38; moderate‐certainty evidence) with higher scores reflecting better quality of life. We downgraded the certainty of evidence for study limitations.
Authors' conclusions
Early AST probably extends time to death of any cause and time to death from prostate cancer. It may slightly decrease the rate of skeletal events. Rates of serious adverse events and quality of life may be similar. It may increase fatigue and may increase the risk of heart failure. Better quality trials would be particularly important to better understand the outcomes related to possible treatment‐related harm, for which we only found low‐certainty evidence.
Plain language summary
Early versus late hormonal treatment for advanced prostate cancer
Review question Men with advanced prostate cancer get hormonal treatment that lowers the level of the male sex hormones. This does not cure men from cancer but can stop the cancer from growing and help men live longer. However, it is not clear whether it is better to start these hormone treatments early on or later, when there are x‐ray or laboratory findings showing that the cancer is growing or when men start having symptoms from the prostate cancer. We did this study to compare starting treatment early versus late.
Background Prostate cancer can be cured if the disease is only in the prostate gland. These men can have radiation or surgery to remove their prostate. If the cancer has spread outside the prostate, for example to the lymph nodes or the bones, there is no cure. Hormonal treatment that lowers the level of the male sex hormones can slow down cancer growth and prevent it from causing problems. This treatment can be started straight after the diagnosis is made (early) or when the cancer has been shown to grow (late) based on x‐ray or laboratory findings or when it has started causing problems (also late).
Study characteristics
We considered only studies in which chance decided whether men with prostate cancer got early or late hormonal treatment.
Key results We found 10 studies that matched our question. We found that early hormonal treatment probably lowers the risk of dying from any cause. The risk of serious unwanted effects may be similar to that of late treatment.
Early hormonal treatment probably lowers the risk of dying from prostate cancer and slightly lowers the risk of problems related to cancer spreading to the bones.
Men getting early treatment may be more likely to feel tired and develop heart weakness.
Overall quality of life is probably unaffected (or only slightly affected) by early treatment.
The certainty of evidence was either moderate, which means that the true results are likely close to what we found; or low, in which case our concern is that the true results could be quite different to what we found.
Summary of findings
Summary of findings for the main comparison. Early compared to deferred AST for advanced hormone‐sensitive prostate cancer.
| Early compared to deferred androgen suppression therapy (AST) for advanced hormone‐sensitive prostate cancer | |||||
| Patient or population: advanced hormone‐sensitive prostate cancer Setting: North America, Europe, Australia, Israel, Scandinavia, Mexico, South Africa Intervention: Early AST Comparison: deferred AST | |||||
| Outcomes | № of participants (studies) Follow up | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with deferred ADT | Risk difference with Early | ||||
| Time to death of any cause (here: all‐cause mortality at 5 years) follow‐up: range 5 years to 13 years | 4767 (10 RCTs) 2 | ⊕⊕⊕⊝ MODERATE 1 | HR 0.82 (0.75 to 0.90) | Lowa | |
| 136 per 1000 | 23 fewer per 1000 (32 fewer to 13 fewer) | ||||
| Moderateb | |||||
| 390 per 1000 | 57 fewer per 1000 (80 fewer to 31 fewer) | ||||
| Serious adverse events follow‐up: range 5 years to 13 years | 10575 (5 RCTs) | ⊕⊕⊝⊝ LOW 2 3 | RR 1.05 (0.95 to 1.16) | Study population | |
| 110 per 1000 | 6 more per 1000 (6 fewer to 18 more) | ||||
| Time to death from prostate cancer (here: prostate cancer mortality at 5 years) follow‐up: range 5 years to 13 years | 3677 (7 RCTs) 6 | ⊕⊕⊕⊝ MODERATE 2 | HR 0.69 (0.57 to 0.84) | Lowa | |
| 80 per 1000 | 24 fewer per 1000 (34 fewer to 12 fewer) | ||||
| Moderateb | |||||
| 218 per 1000 | 62 fewer per 1000 (87 fewer to 31 fewer) | ||||
| Skeletal events follow‐up: range 5 years to unclear years | 2209 (3 RCTs) | ⊕⊕⊝⊝ LOW 2 4 | RR 0.37 (0.17 to 0.80) | Study population | |
| 37 per 1000 | 23 fewer per 1000 (31 fewer to 7 fewer) | ||||
| Fatigue follow‐up: median 9.7 to 11.9 years | 8209 (2 RCTs) | ⊕⊕⊝⊝ LOW 2 4 | RR 1.41 (1.23 to 1.62) | Study population | |
| 77 per 1000 | 31 more per 1000 (18 more to 48 more) | ||||
| Heart failure follow‐up: median 9.7 years | 1214 (1 RCT) | ⊕⊕⊝⊝ LOW 2 4 | RR 1.90 (1.09 to 3.33) | Study population | |
| 30 per 1000 | 27 more per 1000 (3 more to 69 more) | ||||
| Global quality of life assessed with: EORTC QLQ‐C30 (version 3.0) Scale from: 0 to 100 follow‐up: median 5 years | 285 (1 RCT) | ⊕⊕⊕⊝ MODERATE 2 | ‐ | The mean global quality of life was 70.83 | MD 1.56 lower (4.5 lower to 1.38 higher) |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Downgraded by one level (−1) for performance bias
2 Downgraded by one level for performance and detection bias (−1)
3 Concern over selective reporting bias contributed to decision to downgrade by one level (−1)
4 Downgraded by one level (−1) for imprecision
a The control event rate for the low risk group was taken from TROG 03.06/VCOG PR 0103 which enrolled mostly patients with biochemically recurrent prostate without evidence of nodal or distant metastases (N0 and M0). At 5 years the rate of all cause mortality was 13.6% and the rate of prostate cancer mortality was approximated at 8.0%.
b The control event rate for the moderate risk group was from EORTC 30891 as a relatively contemporary study which enrolled mostly patients with locally advanced (T0‐4) and/or node positive (N0‐2) prostate without evidence of distant metastases (M0). At 5 years the rate of all cause mortality was 39.0% and the rate of prostate cancer mortality 21.8%.
Background
Description of the condition
Prostate cancer was diagnosed in 1.1 million men in 2012 and is the second most common cancer in men worldwide (GLOBOCAN 2012). An estimated 307,000 men died of prostate cancer in 2012, making it the fifth leading cause of death from cancer in men (GLOBOCAN 2012). Prostate cancer that is limited to the prostate gland (stage T1‐2, N0, M0) or that has spread locally outside the prostate gland but not to more distant organs (stage T3‐4, N0, M0), is considered to be amenable to potentially curative treatment. However, if the cancer is disseminated to regional lymph nodes (stage T1‐4, N1, M0), or has metastasised to the bones or to other areas (T1‐4, N0‐1, M1), prostate cancer is currently only amenable to palliative therapy such as androgen suppression therapy (EAU 2017).
Description of the intervention
Androgen suppression therapy is considered a mainstay of treatment for metastatic prostate cancer (EAU 2017). This treatment aims to inhibit or eliminate the production of the androgen testosterone which is important for the growth of prostate cells. Androgen suppression therapy leads to a decrease of testosterone circulating in the blood to very low — so‐called castrate — levels. The suppression of testosterone slows prostate cancer disease progression and leads to a decrease in PSA.
There are several different approaches to achieve androgen suppression in men with metastatic prostate cancer. Androgen suppression could be achieved by bilateral orchiectomy (surgical castration) or by medical castration using oestrogens, gonadotropin‐releasing hormone (GnRH) agonists, GnRH antagonists, antiandrogens (non‐steroidal antiandrogens and steroidal antiandrogens) or combination therapy of surgical or medical castration with antiandrogens.
Androgen suppression therapy can be either initiated early when disease is asymptomatic, with biochemical progression and tumours spreading only locally outside the prostate gland but not to more distant organs; or deferred until the patient suffers symptoms of disseminated prostate cancer or has radiological evidence of clinical tumour progression.
A Cochrane Review titled ‘Early versus deferred androgen suppression in the treatment of advanced prostatic cancer’ published in 2002 concluded that early androgen suppression for treatment of advanced prostate cancer might reduce disease progression and complications due to progression. Additionally, early androgen suppression may provide a small but statistically significant improvement in overall survival at 10 years (Nair 2002). Since then several relevant trials have been published making this update important.
Adverse effects of the intervention
The initiation of androgen suppression therapy at earlier stages of the disease presumably leads to an increase in the duration of hormone therapy and potentially, to an increased risk for treatment‐related adverse effects (Adolfsson 1999). Potential adverse events include psychological distress, injection side effects, fatigue, gynaecomastia, breast pain, hot flushes and cardiovascular side effects.
How the intervention might work
Androgens are necessary for the growth of prostate cancer cells. The secretion of the androgen testosterone is regulated by the hypothalamic‐pituitary‐gonadal axis. The hypothalamus secretes gonadotropin‐releasing hormone (GnRH; also known as luteinizing hormone‐releasing hormone (LHRH)) which stimulates the release of luteinizing hormone (LH) and follicle‐stimulating hormone (FSH) from the anterior pituitary gland. The distribution of LH stimulates the Leydig cells of the testes to secrete testosterone which is then converted within the prostate cell by 5‐α‐reductase enzyme to dihydrotestosterone (Gibbs 1996). Dihydrotestosterone is important for the normal development, growth and differentiation of cells of the prostate gland; it is also linked to the development of prostate cancer. Androgen suppression therapy aims to reduce or prevent testosterone secretion, which slows down disease progression (Huggins 2002). The suppression of testosterone also leads to a decrease of PSA.
Why it is important to do this review
This review is an update of the Cochrane Review titled ‘Early versus deferred androgen suppression in the treatment of advanced prostatic cancer’ published by Nair and colleagues in 2002 (Nair 2002; Wilt 2001). The debate concerning the value of different treatment options, especially the comparison between early and deferred androgen suppression therapy, has since continued. Since 2002, several randomised controlled trials have been published assessing the effects of primary therapy with early versus deferred androgen suppression therapy in men with advanced hormone‐sensitive prostate cancer (EORTC 30846; EORTC 30891; Granfors 2006). In 2013, a systematic review evaluated early versus deferred androgen suppression therapy for patients with lymph node‐positive prostate cancer after local therapy with curative intent which identified an improvement in survival and delayed disease progression but also found increased adverse events (Kunath 2013). However, there is still controversy concerning the ideal timing as to when to introduce hormonal therapy in asymptomatic metastatic patients (EAU 2017). As current guidelines are based on older literature and in part, outdated systematic reviews, there is a need to revisit the topic to update our understanding in light of the most recent data.
Objectives
To assess the effects of early versus deferred standard AST for advanced hormone‐sensitive prostate cancer.
Methods
Criteria for considering studies for this review
Types of studies
We included parallel‐grouped randomised controlled trials (RCTs) comparing early and deferred androgen suppression therapy for hormone‐sensitive advanced prostate cancer. We included all RCTs irrespective of their publication status or language of publication. We found no RCTs with a cross‐over design, which are also not feasible for this question. We did not consider non‐randomized trials as these were unlikely to provide high quality evidence and we were aware of an ample number of RCTs addressing this question.
Types of participants
We included trials if they enrolled men with advanced stages of prostate cancer who were not previously treated with hormonal therapy. We excluded no studies based on age or ethnicity of participants.
We defined advanced prostate cancer as any of the following stages.
Men with disseminated (metastatic) disease spread outside the prostate either to the lymph nodes (N1, M0) or other organs (M1).
Men with locally advanced disease spread outside the prostate gland but not to more distant organs (stage T3‐4, N0, M0) without local therapy (such as local radiation therapy, radical surgery or cryotherapy).
Men who had undergone local treatment with curative intent (such as local radiation therapy, radical surgery or cryotherapy) for prostate cancer with biochemical evidence of failure as documented by an elevated and/or rising PSA.
If studies included also men with localized disease (defined as prostate cancer within the prostate gland; T1‐2, N0, M0), we considered only data of the subgroup of men with advanced stages of prostate cancer (see Granfors 2006, EPCP). If this was not possible, we included only data regarding adverse events and quality of life in our meta‐analyses (see VACURG).
We included only patients with advanced hormone‐sensitive prostate cancer. Patients with castration‐resistant prostate cancer were not part of this review, and we did not include trials investigating systemic therapies for these patients in our analysis.
Types of interventions
We included studies evaluating standard androgen suppression therapies which are relevant to current clinical practice, such as surgical castration, medical castration using GnRH agonists (e.g. leuproreline, busereline, gosereline, triptoreline), GnRH antagonists (abarelix, degarelix), non‐steroidal or steroidal antiandrogens (e.g. bicalutamide, flutamide, cyproterone acetate), as well as combination therapy of surgical or medical castration with antiandrogens.
For this review, 'early AST' was defined as initiation of androgen suppression therapy at the time of:
initial diagnosis of asymptomatic locally advanced or advanced prostate cancer;
biochemical evidence of persistently elevated or rising PSA levels following local treatment with curative intent (such as local radiation therapy, radical surgery or cryotherapy) in asymptomatic patients with prostate cancer without evidence of metastatic disease.
We defined 'deferred AST' as treatment that was withheld until:
presentation of clinical prostate cancer related symptoms (such as bone pain, gross haematuria); or
radiological evidence of metastatic disease (such as bone scan, CT scan).
We excluded studies where androgen suppression was utilized as adjuvant treatment to local treatment with curative intent (such as local radiation therapy, radical surgery or cryotherapy).
We excluded studies evaluating oestrogens because this intervention is associated with severe side effects even at lower doses and therapy with oestrogens is now no longer considered standard of care therapy (EAU 2017) and rarely used.
5‐α‐reductase inhibitors (e.g. finasteride, dutasteride), as well as newer androgen suppression therapies such as abiraterone, darolutamide, enzalutamide or apalutamide, were not part of this review, and we did not include trials investigating these treatment options in our analysis.
We investigated the following comparisons of experimental intervention versus comparator intervention.
Experimental intervention
Early androgen suppression therapy.
Comparator interventions
Deferred androgen suppression therapy.
Comparisons
Early versus deferred androgen suppression therapy.
Types of outcome measures
We did not use measurement of outcomes assessed in this review as an eligibility criterion.
Primary outcomes
Time to death of any cause
Serious adverse events
Secondary outcomes
Time to death from prostate cancer
-
Adverse events
Skeletal events
Fatigue
Heart failure
Global quality of life
Time to disease progression
Method and timing of outcome measurement
Time to death of any cause: defined as the time from randomisation to the date of death.
Serious adverse events: defined as adverse events requiring hospitalisation or that were life‐threatening or fatal, or that were reported as serious adverse events by the authors of the original publication; measured at 6 months, 1 year, 2 years, or at the longest reported follow‐up.
Time to death from prostate cancer: defined as the time from randomisation to the date of cancer‐related death.
Adverse events: e.g. skeletal events, heart failure, fatigue etc.; measured at 6 months, 1 year, 2 years, or at the longest reported follow‐up. We defined these events based on the definitions used in the trials.
Global quality of life: assessed using validated generic and disease‐specific questionnaires; measured at baseline, 6 months, 1 year, 2 years, or at the longest reported follow‐up.
Time to disease progression: defined as the date from randomisation to disease progression; determined by appearance of new — or increase in existing — bone or extraskeletal metastases confirmed by imaging or physical examination. If data for time to disease progression were not available we assessed data for clinical progression (see Effects of interventions).
If we were unable to retrieve the necessary information to analyse time‐to‐event outcomes, we assessed the number of events per treatment group for these outcomes at 6 months, 1 year, 2 years, or at the longest reported follow‐up.
We compared and analysed each of these measures separately. To determine the validity of data synthesis across separate studies, the reviewer abstracted definitions used by each study to describe cancer‐specific survival and clinical progression‐free survival.
Main outcomes for 'Summary of findings' table
We presented a 'Summary of findings' table reporting the following outcomes.
Time to death of any cause.
Serious adverse events.
Time to death from prostate cancer.
Skeletal events.
Fatigue.
Heart failure.
Global quality of life.
Search methods for identification of studies
We performed a comprehensive systematic search with no restrictions on the language of publication or publication status.
Electronic searches
We searched the following sources from inception of each database.
-
Cochrane Library (2018, Issue 11; last searched 20 November 2018)
Cochrane Database of Systematic Reviews (CDSR)
Cochrane Central Register of Controlled Trials (CENTRAL)
Database of Abstracts of Reviews of Effects (DARE)
Health Technology Assessment Database (HTA)
MEDLINE (via Ovid; 1946 onward to 20 November 2018)
Embase (1947 onwards to 20 November 2018)
Web of Science (Thomson Reuters Web of Knowledge; 1970 onward to 20 November 2018)
Additionally, we also searched the following trial registries.
ClinicalTrials.gov (www.clinicaltrials.gov); last searched 2 January 2019.
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) search portal (apps.who.int/trialsearch); last searched 2 January 2019.
A librarian developed the search strategy after input and feedback from the research team. We applied the search to the Cochrane Library via Wiley, MEDLINE via Ovid, Embase via Embase.com, and the Web of Science via Clarivate Analytics on 20 November 2018. When appropriate we used controlled vocabulary, such as Medical Subject Headings and Emtree terms, in combination with keywords for the concepts of prostatic neoplasms, time factors, and androgen suppression therapies, including specific drug names. We made an effort to account for plurals, acronyms, and synonyms. We did not limit the search by language or date. We first ran the search on 2 November 2015, followed by updates on 23 January 2018 and 20 November 2018. We retrieved all articles meeting the inclusion criteria and reviewed the full text. For details on the search strategy, see Appendix 1; Appendix 2; Appendix 3; Appendix 4; Appendix 5. We checked every included study for a trial registry entry and presented the results in the 'Characteristics of included studies' tables.
Searching other resources
We also searched the reference lists of retrieved included trials, reviews, meta‐analyses and health technology assessment reports and contacted experts in the field to identify any further studies that we might have missed.
We also searched the electronically available abstract books from the following conferences.
American Society of Clinical Oncology (ASCO; jco.ascopubs.org; last searched 2 January 2019).
American Urological Association (AUA; www.jurology.com; 2008 onward to 2 January 2019).
We used the following keywords for this search: 'early androgen'; 'immediate androgen'; 'prostate cancer'.
Data collection and analysis
Selection of studies
We used the reference management software Endnote to collate references and remove potential duplicate records. Two reviewers (AK, FK) independently scanned the abstracts, titles, or both, of remaining records retrieved, to determine which studies should be assessed further as full texts. The review authors (AK, FK or MP) investigated independently all potentially relevant records and classified studies as included studies, excluded studies, studies awaiting classification, or ongoing studies in accordance with the criteria for each provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). We resolved any disagreements through discussion or through consensus reached by recourse to a third review author (PD). We documented reasons for exclusion of studies in a 'Characteristics of excluded studies' table. We have presented a PRISMA flow diagram showing the process of study selection (Liberati 2009).
Data extraction and management
We used a data abstraction form that was already pilot tested during data assessment of previous evaluations (Kunath 2012; Kunath 2014).
For studies that fulfilled inclusion criteria, two review authors (AK, FK) independently abstracted the following information, which we provide in the 'Characteristics of included studies' table.
Study design.
Study dates.
Study settings and country.
Participant inclusion and exclusion criteria.
Participant details, such as baseline demographics and disease characteristics.
The number of participants by study and by study arm.
Details of relevant experimental and comparator interventions such as dose, route, frequency, and duration.
Definitions of relevant outcomes, method and timing of outcome measurement, as well as any relevant subgroups.
Study funding sources.
Declarations of interest by primary investigators.
Two review authors extracted outcome data relevant to this review as needed for calculation of summary statistics and measures of variance (FK/AK, KJ). For time‐to‐event outcomes, we obtained hazard ratios (HRs) with corresponding measures of variance or data necessary to calculate this information using an indirect estimation method (Tierney 2006). For dichotomous outcomes, we obtained numbers of events and totals for population of a 2 × 2 table, as well as summary statistics with corresponding measures of variance. For the continuous outcome (quality‐of‐life outcome), we extracted the mean difference with corresponding 95% confidence interval. We resolved any disagreements by discussion; or, if required, by consultation with a third review author (PD).
We provide information, including trial identifier, about potentially relevant ongoing studies in the table 'Characteristics of ongoing studies'.
Dealing with duplicate and companion publications
In the event of duplicate publications, companion documents or multiple reports of a primary study, we maximized yield of information by mapping all publications to unique studies and collating all available data. We used the most complete dataset aggregated across all known publications. In case of doubt, we gave priority to the publication reporting the longest follow‐up associated with our primary or secondary outcomes.
Assessment of risk of bias in included studies
Two review authors (MP, FK) assessed the risk of bias of each included study independently. We resolved disagreements by discussion, or reached a consensus by consultation with a third review author (PD).
We assessed risk of bias using Cochrane's 'Risk of bias' tool for RCTs (Higgins 2011c). We assessed the following domains.
Random sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Other sources of bias.
We judged risk of bias domains as 'low risk', 'high risk' or 'unclear risk' and evaluated individual bias items as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c). We present a 'Risk of bias summary' figure to illustrate these findings.
For performance bias (blinding of participants and personnel) and detection bias (blinding of outcome assessment), we evaluated the risk of bias separately for each outcome, and we grouped outcomes according to whether measured subjectively or objectively when reporting our findings in the 'Risk of bias' tables.
We also assessed attrition bias (incomplete outcome data) on an outcome‐specific basis, and grouped outcomes with judgements when reporting our findings in the 'Risk of bias' tables. We defined that risk of attrition bias is likely to be rated as 'low' if the proportion of patients is less than 10%, 'unclear' if between 11% and 20% and 'high' if greater than 20%; we know, however, that this is a simplification and that the event rate carries impact in this calculation.
We further summarized the risk of bias across domains for each outcome in each included study, as well as across studies and domains for each outcome.
We defined the following endpoints as subjective outcomes as determined by their susceptibility to detection bias and the importance of blinding outcome assessors.
Serious adverse events.
Tme‐to‐disease progression.
Time to death from prostate cancer.
Adverse events.
Global quality of life.
We defined the following endpoint as an objective outcome.
Time to death of any cause.
Concomitant interventions had to be the same in the experimental and comparator groups to establish valid comparisons. If not, or if not explicitly reported, we considered this in our 'Risk of bias' analysis and performed sensitivity analyses (see Sensitivity analysis).
Measures of treatment effect
We expressed time‐to‐event data as hazard ratios (HRs) with 95% confidence intervals (CIs). We expressed dichotomous data as risk ratios (RRs) with 95% CIs, and continuous data as mean difference with 95% CIs.
Unit of analysis issues
The unit of analysis was the individual participant. We did not identify cross‐over trials. We treated included trials with more than two intervention groups in accordance with guidance provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
Dealing with missing data
We performed intention‐to‐treat (ITT) analyses if data were available. We investigated attrition rates (e.g. dropouts, losses to follow‐up and withdrawals) and critically appraised issues of missing data. We did not impute missing data.
Assessment of heterogeneity
We identified heterogeneity through visual inspection of forest plots to assess the amount of overlap of CIs; and with the I² statistic, which quantifies heterogeneity across studies (Higgins 2002; Higgins 2003). We interpreted I² as follows.
0% to 40%: may not be important.
30% to 60%: may indicate moderate heterogeneity.
50% to 90%: may indicate substantial heterogeneity.
75% to 100%: considerable heterogeneity.
When we found heterogeneity, we determined possible reasons for it by examining individual study and subgroup characteristics.
Assessment of reporting biases
If available, we obtained study protocols to assess for selective outcome reporting. We used funnel plots to assess small study effects only if we included at least 10 studies (see Analysis 1.1).
1.1. Analysis.
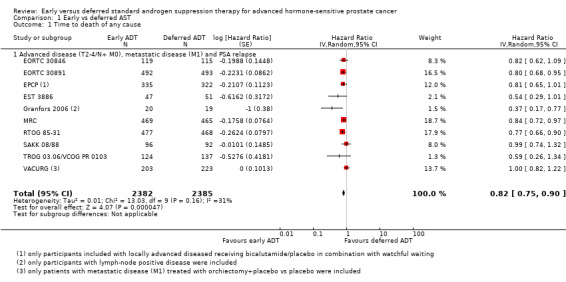
Comparison 1 Early vs deferred AST, Outcome 1 Time to death of any cause.
Data synthesis
We summarized data using a random‐effects model. We interpreted random‐effects meta‐analyses with consideration of the whole distribution of effects. In addition, we performed statistical analyses according to the statistical guidelines contained in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). For dichotomous outcomes, we used the Mantel‐Haenszel method. We displayed continuous outcomes graphically in a forest plot without need of pooling. For time‐to‐event outcomes, we used the generic inverse variance method. We used the most up‐to‐date Review Manager 5 (RevMan 5) software to perform analyses (Review Manager 2014).
Subgroup analysis and investigation of heterogeneity
We expected the following characteristics to introduce clinical heterogeneity, and carried out subgroup analyses for our primary outcomes with investigation of interactions.
Metastatic disease (M1) versus advanced but non‐metastatic disease (T2‐4/ N+ M0) versus PSA relapse.
We used the test for subgroup differences in RevMan 5 to compare subgroup analyses if there were sufficient studies (Review Manager 2014).
Sensitivity analysis
We performed sensitivity analyses for our primary outcomes in order to explore the influence of the following factors on effect sizes.
Restricting the analysis by taking into account risk of bias, by excluding studies at 'high risk' or 'unclear risk' (one 'high risk' study or two 'unclear risk' studies) to establish the extent to which they dominate the results.
'Summary of findings' tables
We presented the overall quality of the evidence for each outcome according to the GRADE approach, which takes into account five criteria not only related to internal validity (risk of bias, inconsistency, imprecision, publication bias) but also to external validity, such as directness of results (Guyatt 2008). For each comparison, two review authors (FK, MP) independently rated the quality of evidence for each outcome as 'high', 'moderate', 'low', or 'very low' using GRADEproGDT; discrepancies were resolved by discussion or, if needed, by arbitration by a third review author (PD). We present a summary of the evidence for the main outcomes in Table 1, which provides key information about the best estimate of the magnitude of the effect, in relative terms and absolute differences for each relevant comparison of alternative management strategies; numbers of participants and studies addressing each important outcome; and the rating of the overall confidence in effect estimates for each outcome (Guyatt 2011; Schünemann 2011). If meta‐analysis was not possible, we presented results in a narrative 'Summary of findings' table.
Results
Description of studies
Results of the search
We identified 22,374 records following our database search; and after screening by title and abstract, we evaluated 127 full‐text articles for eligibility. The flow of literature through the assessment process is shown in the study flow diagram (Figure 1). We identified seven new randomised controlled trials since publication of the original review in 2002 (Nair 2002/Wilt 2001 included EST 3886; MRC; VACURG; note: the EST 3886 was labelled as 'ECOG' by Wilt and colleagues) and finally included a total of 10 trials (53 references) in this review (EORTC 30846; EORTC 30891; EPCP; EST 3886; Granfors 2006; MRC; RTOG 85‐31; SAKK 08/88; TROG 03.06/VCOG PR 0103; VACURG). All records were published in English. We did not identify any relevant ongoing trials.
1.
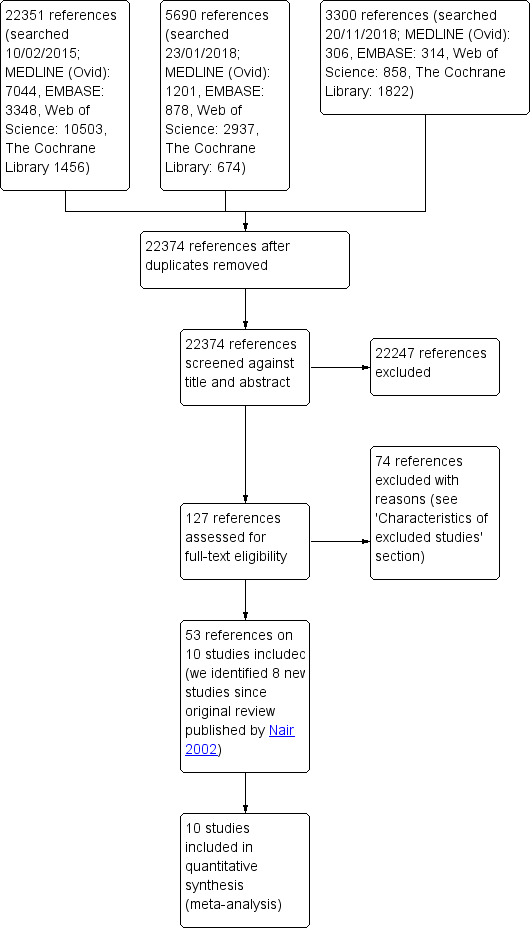
Study flow diagram.
Included studies
For a detailed description of the baseline characteristics and participants of the included studies see Characteristics of included studies; Table 2; Table 3.
1. Description of interventions.
| Intervention(s) (route, frequency, total dose of injection or total dose/day) | Intervention(s) appropriate as applied in a clinical practice setting a (description) | Comparator(s) (route, frequency, total dose/day) | Comparator(s) appropriate as applied in a clinical practice setting a (description) | |
| EORTC 30846 | Gosereline (Zoladex) (s.c., every 4 weeks, 3.6 mg) and cryptoterone acetate (p.o., 3 times per day for the first 4 weeks of treatment, 50 mg) or orchiectomy (surgery, once, n.a.) | s.c. injections and p.o. or surgical intervention | Same treatment starting at clinical or subjective progression | s.c. injections and p.o. or surgical intervention |
| EORTC 30891 | Subcapsular orchiectomy or buserelin (s.c. every 2 months, 6.3 mg) and cyproterone acetate (p.o. for the first 2 weeks, 50 mg) | Surgical intervention or s.c. injections | Same treatment starting at symptomatic disease progression | Surgical intervention or s.c. injections |
| ECPC | Bicalutamide (p.o., once daily, 150 mg) and watchful waiting (for oncological outcomes); bicalutamide (p.o., once daily, 150 mg) and standard care including radical prostatectomy, radiotherapy, watchful waiting, or cryotherapy/cryosurgery (for adverse events) | p.o. | Placebo (p.o., once daily, n.a.) in addition to standard care | p.o. |
| EST 3886 | Goserelin (Zoladex) (s.c., every 4 weeks, 3.6 mg) or orchiectomy (surgery, once, n.a.) | s.c. injections or surgical intervention | Same treatment starting at disease progression | s.c. injections or surgical intervention |
| Granfors 2006 | Orchiectomy (surgery, once 3 weeks after the staging operation, n.a.) | Surgical intervention | Same treatment starting at disease progression (in 4 cases: LHRH analogues) | Surgical intervention (in 4 cases: s.c. injections) |
| MRC | Total or subcapsular orchiectomy (surgery, once, n.a.) or LHRH analogues (s.c., ‐, ‐); if for any reason either of these options became inappropriate an alternative form of effective hormone therapy was allowed: cryptoterone acetate, oestrogens, flutamide (‐, ‐, ‐) | Surgical intervention or s.c. injections | Same treatment starting at disease progression | Surgical intervention or s.c. injections |
| RTOG 85‐31 | Goserelin (s.c., every 4 weeks, 3.6 mg) | s.c. injections | Same treatment starting at disease progression | s.c. injections |
| SAKK 08/88 | Subcapsular orchiectomy (surgery, once, n.a.) | Surgical intervention | Same treatment starting at disease progression | Surgical intervention |
| TROG 03.06/ VCOG PR 0103 | LHRH analogues (s.c., ‐, ‐), LHRH antagonists (s.c., ‐, ‐) | s.c. injections (intermittent ADT: 171/261; continuous ADT: 90/261) | Same treatment starting at disease progression (symptoms, occurrence of metastases, PSA doubling times decreased to 6 months or less) or at least 2 years after randomisation | s.c. injections (intermittent ADT: 171/261; continuous ADT: 90/261) |
| VACURG | Orchiectomy (surgery, once, n.a.) and placebo (p.o., ‐, ‐) | Surgical intervention and p.o. | Placebo (p.o., ‐, ‐) | p.o. |
| ‐ denotes not reported; a The term 'clinical practice setting' refers to the specification of the intervention/comparator as used in the course of a standard medical treatment (such as dose, dose escalation, dosing scheme, provision for the contraindications and other important features); C: comparator; I: intervention; N/CPS: no specification of clinical practice setting possible; s.c.: subcutaneous; p.o.: per os; n.a.: not applicable; LHRH: luteinizing hormone‐releasing hormone; PSA: prostate‐specific antigen | ||||
2. Baseline characteristics.
| Duration of follow‐up | Description of participants | Trial period | Country | Setting | Ethnic groups | |
| EORTC 30846 | Median 13 years | Prostate cancer T2‐3 N1‐3 M0, no local treatment of the primary tumour | 02/1986 to 11/1998 | The Netherlands, Norway, Sweden, Austria, Switzerland, Belgium, France, Denmark, Spain, Russia, Poland, Italy | Multicentric | ‐ |
| ‐ | ||||||
| EORTC 30891 | Median 7.8 years | Prostate cancer T0‐4, N0‐2, M0 without previous treatment | 02/1990 to 01/1999 | Switzerland, United Kingdom, Austria, the Netherlands, Spain, Belgium | Multicentric | ‐ |
| ‐ | ||||||
| EPCP | Median 9.7 years | Prostate cancer T1‐4, any N, M0 | ‐ | North America, Europe, South Africa, Australia, Israel, Mexico, Scandinavia | Multicentric | Caucasian 95.3%, Black 0.9%, Other 3.7% |
| Caucasian 94.7%, Black 0.7%, Other 4.6% | ||||||
| EST 3886 | Median 11.9 years | Prostate cancer T1‐T2, N+, M0 ( after radical prostatectomy and bilateral pelvic lymphadenectomy) | 1988 to 1993 | USA | Multicentric | ‐ |
| ‐ | ||||||
| Granfors 2006 | Median 9.7 years | Prostate cancer T1‐4, pN0‐3, M0 (only patients with lymph node involvement were included) | 1986 to 1991 | Sweden | Multicentric | ‐ |
| ‐ | ||||||
| MRC | ‐ | Prostate cancer T2‐T4, M0‐M1, Mx | 1985 to 1993 | United Kingdom | Multicentric | ‐ |
| ‐ | ||||||
| RTOG 85‐31 | Median 7.6 years | Prostate cancer T1/T2 N+ or T3 ± N+ | 1987 to 1992 | USA | Multicentric | ‐ |
| ‐ | ||||||
| SAKK 08/88 | ‐ | Prostate cancer T0‐4, N0‐2, M0‐1 (asymptomatic, without previous treatment not suitable or unwilling for local curative therapy) | 1988 to 1992 | Switzerland | Multicentric | ‐ |
| ‐ | ||||||
| TROG 03.06/ VCOG PR 0103 | Median 5 years | Prostate cancer with PSA relapse after previous attempted curative therapy or asymptomatic in patients not considered suitable for curative treatment | 2004 to 2012 | Australia, New Zealand, and Canada | Multicentric | ‐ |
| ‐ | ||||||
| VACURG | ‐ | Prostate cancer stage I ‐ IV (only data from patients with metastatic disease (M1 = stage IV) were included) | 1960 to 1975 | USA | Multicentric | ‐ |
| ‐ | ||||||
| ‐ denotes not reported | ||||||
We included a total of 10 trials (EORTC 30846; EORTC 30891; EPCP; EST 3886; Granfors 2006; MRC; RTOG 85‐31; SAKK 08/88; TROG 03.06/VCOG PR 0103; VACURG).
Participant characteristics by study
The EORTC 30846 trial recruited participants with lymph node‐positive (pN1‐3) prostate cancer without local treatment of the primary tumour.
The EORTC 30891 trial recruited participants with newly diagnosed prostate cancer T0‐4, N0‐2, M0 without previous treatment.
The EPCP trial recruited participants with localized (T1‐2, NO/Nx) or locally advanced (T3‐4, any N; or any T, N+) prostate cancer (all M0). Participants received either radiotherapy (1317 participants), radical prostatectomy (4454 participants), watchful waiting (2285 participants), or other treatments (e.g. cryotherapy, cryosurgery, systemic therapy with flutamide plus LHRH‐analogue; 4 participants). However, we included only data of adverse events, time to disease progression and time to death of any cause for the subgroup of patients with locally advanced diseased (T3‐4, any N; or any T, N+; all M0) treated with bicalutamide plus watchful waiting versus placebo plus watchful waiting (657 of 8113 patients).
The EST 3886 trial recruited participants with clinically localized node‐positive prostate cancer (no more than stage T2).
The Granfors 2006 trial recruited participants with newly diagnosed clinical localized prostate cancer with or without pelvic lymph node involvement. We included only data of the subgroup of patients with lymph node‐positive prostate cancer (39 patients (43%) had lymph node‐positive disease).
The MRC trial recruited participants with locally advanced or asymptomatic metastatic prostate cancer.
The RTOG 85‐31 trial recruited participants with clinical T3 tumour or involvement of the regional lymph nodes. Lymph node assessment was mandatory and could be performed by either lymphangiogram, computed tomography, or lymphadenectomy. Authors also presented data regarding time to disease progression with PSA level less than 1.5 ng/ml. However, we did not include these results because approximately 40% of patients had no initial PSA values. PSA testing was not mandatory at the inception of the study because it was not widely available.
The SAKK 08/88 trial recruited participants with T0‐4, N0‐2, M0‐1 newly diagnosed asymptomatic prostate cancer without previous treatment not suitable or unwilling to undergo local curative therapy.
The TROG 03.06/VCOG PR 0103 trial recruited participants with a histologically confirmed diagnosis of adenocarcinoma of the prostate who either had a PSA relapse after previous attempted curative therapy or asymptomatic men who were not considered suitable for curative treatment.
The VACURG trial recruited participants with histologically confirmed prostate cancer stage I to IV whose condition had been newly diagnosed. The trial consisted of three prospective randomised clinical trials that were analysed separately (for details see 'Characteristics of included studies' table). For time to death of any cause, we included only data from study 1 for prostate cancer patients with metastatic disease (M1 = stage IV) treated with placebo or with orchiectomy plus placebo. For time to death of any cause, we did not include patients receiving oestrogens (study 1, 2, 3) or patients with locally advanced disease (T3‐4, M0 = stage III) because it was unclear if these patients received also local therapy (e.g. prostatectomy). For death from heart or vascular disease, we included data from study 1 for prostate cancer patients with locally advanced (T3‐4, M0 = stage III) or metastatic disease (M1 = stage IV) treated with placebo or with orchiectomy plus placebo. We did not include data for time to progression, or time to death from prostate cancer because the analyses of these outcomes included locally advanced and metastatic patients (stage III and IV) and it is unclear if stage III patients also had local therapy.
Intervention characteristics by study
Three trials used surgical castration (subcapsular orchiectomy) or subcutaneous (s.c.) injections using GnRH‐agonists (EORTC 30891; EST 3886; MRC); one trial used surgical castration and a per os (p.o.) therapy (placebo; VACURG); one trial used s.c. injections, p.o. therapy or surgical castration (EORTC 30846); one trial used p.o. therapy using bicalutamide (EPCP); two trials used s.c. injections using GnRH‐agonists (RTOG 85‐31; TROG 03.06/VCOG PR 0103); and two trials used surgical castration (Granfors 2006; SAKK 08/88). For details see Characteristics of included studies tables.
Definition of deferred AST by study
In the EORTC 30846 trial, participants received identical treatment starting at the time of clinical progression or subjective progression, based on a rise of serum prostate‐specific antigen (PSA) or an increase in the T category or prostatic volume.
In the EORTC 30891 trial participants received identical treatment starting at the time of symptomatic disease progression (defined as one of the following: new symptomatic metastases or metastases whose location threatened to produce serious complications, such as pathologic fractures or paralysis; increase in pain score due to the prostate cancer by more than or equal to two categories; deterioration in World Health Organization (WHO) performance status by two levels due to prostate cancer; and evidence of ureteric obstruction caused either by the primary tumour or metastases). In the absence of symptoms, deferred treatment was not to be initiated on a rise in serum PSA or alkaline phosphatase, or asymptomatic new hot spots in the bone scan or soft tissue metastases.
In the EPCP trial participants received a placebo in addition to standard care. The duration of randomised therapy was 2 years in Trial 23 (or until disease progression if earlier) and until disease progression in Trials 24 and 25 (less or equal to 5 years recommended for adjuvant therapy in Trial 24). At disease progression further therapy was initiated at the investigators' discretion.
In the EST 3886 trial participants received identical treatment starting at the time of disease recurrence (detection of local or disseminated disease (or both) on a computed tomographic scan, a chest x‐ray film, a bone scan, physical examination, or biopsy).
In the Granfors 2006 trial participants underwent orchiectomy or, in four cases, were treated with luteinizing hormone‐releasing hormone analogues when progression was diagnosed. Progression was defined as the occurrence of clinically evident local tumour growth or bone or other distant metastases.
In the MRC trial participants received identical treatment starting at the time of: pain from, or complications of, bone metastases; local progression; increasing tumour marker level; general systemic effects; or patient preferences.
In the RTOG 85‐31 trial participants received identical treatment starting at relapse, defined as: local failure (reappearance of palpable tumour after initial clearance, progression of palpable tumour at any time, persistence of palpable tumour beyond 24 months after study entry, biopsy‐proven presence of carcinoma ≥ 2 years after study entry); or regional failure (clinical radiographic evidence of tumour in the pelvis with or without palpable tumour in the prostate by digital examination).
In the SAKK 08/88 trial participants received identical treatment at the onset of symptoms caused by metastases or when ureteric obstruction or new asymptomatic metastases were likely to cause severe complications (pathologic fractures, spinal palsy etc.). Biochemical progression — such as increasing prostate‐specific antigen or phosphatase, new hot spots, or soft tissue metastases during follow‐up — did not justify deferred orchiectomy as long as the patient remained asymptomatic and did not have a decrease in performance status.
In the TROG 03.06/VCOG PR 0103 trial participants received identical treatment starting at least 2 years after randomisation, unless symptoms or metastases developed or PSA doubling times decreased to 6 months or less.
The VACURG study consisted of three prospective RCTs that were analysed separately. We included only data of trial 1. If patients showed progression of the disease, then the clinicians treating them were free to change their therapy. Time to progression was defined as follows: time until first metastases; or first increase in acid phosphatase; or death from prostate cancer. Patients in the placebo group were able to change their therapy so that they could receive oestrogens later. The comparison can be thought of as an orchiectomy versus delayed endocrine therapy.
Excluded studies
We present a detailed description of the excluded studies in Characteristics of excluded studies below; (also see Figure 1). We excluded 74 references after assessing for eligibility.
Risk of bias in included studies
We assessed the risk of bias of the included studies according to the seven domains outlined in the Cochrane 'Risk of bias' tool (Higgins 2011a). We extracted the methodological details of the studies from the published data. For details on risk of bias, see Figure 2 and Characteristics of included studies section.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
Information regarding random sequence generation was not reported in seven studies, leading to unclear risk of bias (EORTC 30846; EORTC 30891; EST 3886; Granfors 2006; MRC; SAKK 08/88; VACURG). Three studies reported an adequate method of sequence generation and we rated them at low risk of bias (EPCP; RTOG 85‐31; TROG 03.06/VCOG PR 0103).
Allocation concealment
We did not identify information on allocation concealment for four studies and rated them at unclear risk of bias (EORTC 30891; Granfors 2006; MRC; VACURG). Six studies reported an adequate method of allocation concealment leading to low risk of bias (EORTC 30846; EPCP; EST 3886; RTOG 85‐31; SAKK 08/88; TROG 03.06/VCOG PR 0103).
Blinding
There was no blinding in nine studies (EORTC 30846; EORTC 30891; EST 3886; Granfors 2006; MRC; RTOG 85‐31; SAKK 08/88; TROG 03.06/VCOG PR 0103; VACURG). Only the EPCP trial was double‐blinded.
Blinding of participants and personnel (Objective Outcome)
We defined only 'Time to death of any cause' as an objective outcome. Participants and personnel were blinded in the EPCP trial but blinding was broken by the committee due to statistically significant differences in time to disease progression. We rated that there is an unclear risk of performance bias in all included studies.
Blinding of participants and personnel (Subjective Outcomes)
For our subjective outcomes (serious adverse events, time to disease progression, time to death from prostate cancer, adverse events and quality of life), we rated nine studies as having high risk of performance bias (EORTC 30846; EORTC 30891; EST 3886; Granfors 2006; MRC; RTOG 85‐31; SAKK 08/88; TROG 03.06/VCOG PR 0103; VACURG). Participants and personnel were only blinded in the EPCP trial but blinding was broken by the committee due to statistically significant differences in time to disease progression. We therefore concluded that there is an unclear risk of bias (EPCP).
Blinding of outcome assessment (objective outcome)
We defined as an objective outcome only 'Time to death of any cause'. We judged the risk of bias as low for all included trials.
Blinding of outcome assessment (subjective outcomes)
There was a high risk of detection bias for our subjective outcomes (serious adverse events; time to disease progression; time to death from prostate cancer; adverse events; and quality of life) in nine studies (EORTC 30846; EORTC 30891; EST 3886; Granfors 2006; MRC; RTOG 85‐31; SAKK 08/88; TROG 03.06/VCOG PR 0103; VACURG). Blinding of participants and personnel in the EPCP trial was broken by the committee due to statistically significant differences in time to disease progression, and we rated it as having an unclear risk of bias (EPCP).
Incomplete outcome data
Incomplete outcome data for oncological outcomes (time to death of any cause, time to disease progression, time to death from prostate cancer)
We rated seven studies as having low risk of attrition bias (EORTC 30846; EORTC 30891; EST 3886; MRC; RTOG 85‐31; SAKK 08/88; TROG 03.06/VCOG PR 0103). In the EPCP trial, missing outcome data were balanced in numbers across intervention groups with similar reasons for missing data across groups. However, we only included participants with locally advanced disease receiving bicalutamide/placebo in combination with watchful waiting for evaluation of time to death of any cause and time to disease progression (N = 657 of 8113 participants). In Granfors 2006 trial, we found also no evidence for missing outcome data for all patients. However, we included only patients with lymph‐node positive disease (N = 39 of 91 participants). In the VACURG trial, we found also no evidence for missing outcome data for all participants but included only data for prostate cancer patients with metastatic disease treated with placebo or with orchiectomy plus placebo (N = 953 of 3433 participants). We did not include patients receiving oestrogens or patients with locally advanced disease (T3‐4 M0 = stage III) because it was unclear if these patients received also local therapy (e.g. prostatectomy). We rated three studies as having an unclear risk of attrition bias (EPCP; Granfors 2006; VACURG).
Incomplete outcome data for adverse events (serious and other adverse events)
We rated five studies as having an unclear risk bias because the assessment of attrition bias for adverse events was not applicable (EORTC 30846; Granfors 2006; MRC; RTOG 85‐31; VACURG).
Incomplete outcome data for quality of life
Only one study reported quality of life (TROG 03.06/VCOG PR 0103). More than 90% of participants completed quality‐of‐life questionnaires at each visit, with no differences in completion rates between the two arms leading to low risk of attrition bias.
Selective reporting
We rated that there is high risk for reporting bias in four studies (EORTC 30846; Granfors 2006; RTOG 85‐31; VACURG).
In the EORTC 30846 trial there was no assessment of adverse events (except for the serious adverse event of death due to cardiovascular events or infection) but it could have been expected or adverse events were measured but not reported. Data for the predefined outcome 'Time to clinical progression' were evaluated but not reported.
In the Granfors 2006 trial, adverse events were not reported. We contacted the authors but did not receive a response. Data regarding time to disease progression and time to death from prostate cancer were not reported for lymph node‐positive patients.
In the RTOG 85‐31 trial there was no assessment of adverse events but it could have been expected or adverse events were measured but not reported. Adverse events were only reported incompletely for a minor subgroup of patients. However, data could not be included in this review.
In the VACURG trial there was no assessment of adverse events (only for death due to heart or vascular disease) but it could have been expected or adverse events were measured but not reported.
The methodology of the MRC study was not planned for evaluating adverse events. However, it could have been expected for a randomised controlled trial, leading to unclear risk of bias. Adverse events were measured in the SAKK 08/88 study but we assume that they have been only partially reported, leading to unclear risk of bias. The study protocol was not available for EST 3886 study, leading to unclear risk of bias.
Other potential sources of bias
We identified no other potential sources of bias (unclear risk of bias for all studies).
Effects of interventions
See: Table 1
For details see: Data and analyses; Table 1; Figure 3; Figure 4; Figure 5; Figure 6
3.
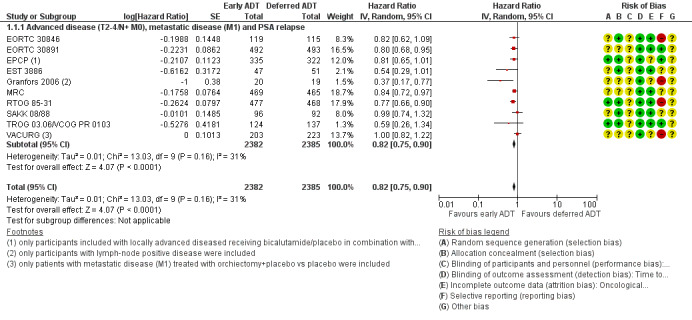
Forest plot of comparison: 1 Early vs deferred AST, outcome: 1.1 Time to death of any cause.
4.
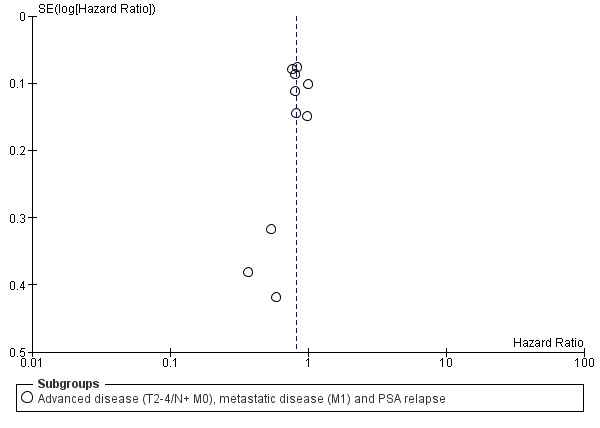
Funnel plot of comparison: 1 Early vs deferred AST, outcome: 1.1 Time to death of any cause.
5.
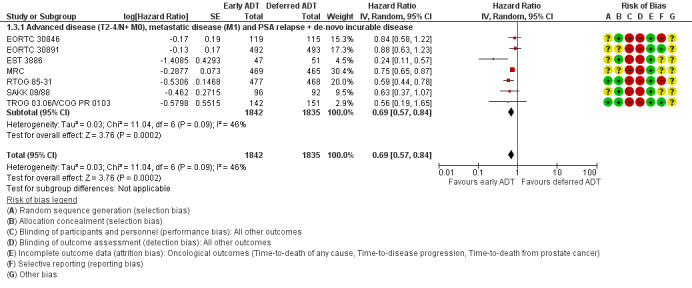
Forest plot of comparison: 1 Early vs deferred AST, outcome: 1.3 Time to death from prostate cancer.
6.
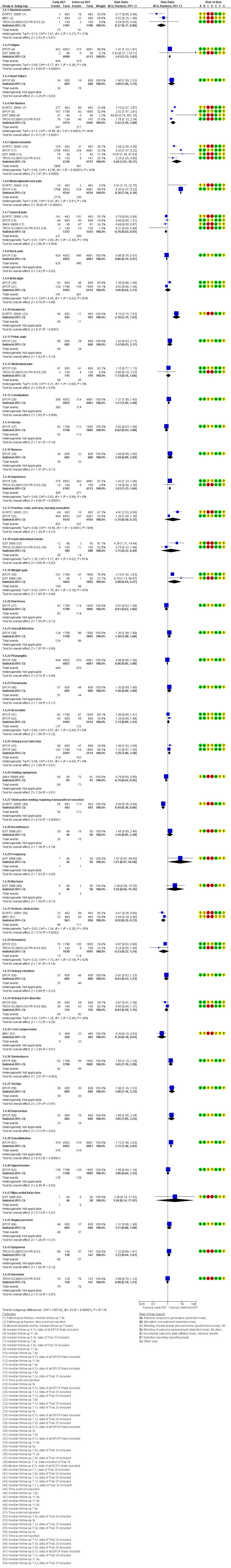
Forest plot of comparison: 1 Early vs deferred AST, outcome: 1.4 Adverse events.
Primary outcomes
Time to death of any cause
Early AST probably reduces the risk of death from any cause over time (HR 0.82, 95% CI 0.75 to 0.90; moderate‐certainty evidence; 4767 participants).
We derived the control event rate at 5 years for a group that we considered moderate risk from EORTC 30891 as a relatively contemporary study, which enrolled mostly patients with locally advanced (T0‐4) and/or node positive (N0‐2) prostate cancer without evidence of distant metastases (M0). At 5 years the rate of all‐cause mortality was 39.0%. Therefore, this corresponds to 57 fewer deaths (95% CI 80 fewer to 31 fewer) per 1000 men at 5 years for the moderate‐risk group (Table 1).
The control event rate for the low risk group was taken from TROG 03.06/ VCOG PR 0103, which enrolled mostly men with biochemically recurrent prostate cancer without evidence of nodal or distant metastases (N0 and M0). At 5 years the rate of all‐cause mortality was 13.6%. Using this number, the effect size corresponded to 23 fewer deaths (95% CI 32 fewer to 13 fewer) per 1000 men at 5 years. We downgraded for study limitations (Table 1).
Serious adverse events
Early versus deferred AST may makes little or no difference in serious adverse events (RR 1.05, 95% CI 0.95 to 1.16; 5 RCTs; 10,575 participants; 5 to 13 years' follow‐up; Analysis 1.2; low‐certainty evidence). We downgraded for study limitations and reporting bias. This corresponded to 110 serious adverse events per 1000 participants with deferred AST and 6 more (6 fewer to 18 more) serious adverse events per 1000 participants with early AST (Table 1).
1.2. Analysis.
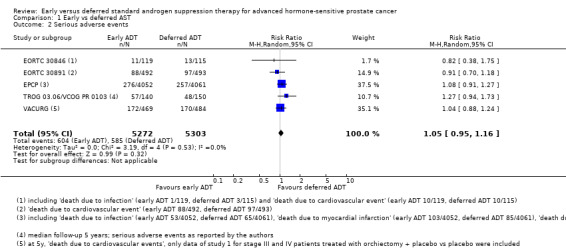
Comparison 1 Early vs deferred AST, Outcome 2 Serious adverse events.
We included adverse events that were labelled serious by the authors (TROG 03.06/VCOG PR 0103); or that lead to death (EORTC 30846: death due to infection or cardiovascular events; EPCP: death due to infection, myocardial infarction, cerebrovascular events, heart failure or cerebral infarction; EORTC 30891: death due to cardiovascular events; VACURG: death due to cardiovascular disease).
Secondary outcomes
Time to death from prostate cancer
Early AST probably reduces the risk of death from prostate cancer over time (HR 0.69, 95% CI 0.57 to 0.84; moderate‐certainty evidence).
Using a control event rate for moderate risk of 21.8% derived from EORTC 30891, this corresponds to 62 fewer prostate cancer deaths per 1000 (95% CI 87 fewer to 31 fewer) after 5 years (Table 1). We downgraded for study limitations.
Based on a control event rate of 8.0% for low risk based on TROG 03.06/ VCOG PR 0103, this corresponds to 24 fewer death from prostate cancer (95% CI 34 fewer to 12 fewer) per 1000 men.
Skeletal events
Early AST may slightly decreases the rate of skeletal events (RR 0.37, 95% CI 0.17 to 0.80; 3 RCTs; 2209 participants; low‐certainty evidence; Analysis 1.4; Figure 6). This corresponds to 23 fewer skeletal events (95% CI 31 fewer to 7 fewer) per 1000 participants with early AST. We downgraded for study limitations and imprecision (Table 1).
1.4. Analysis.
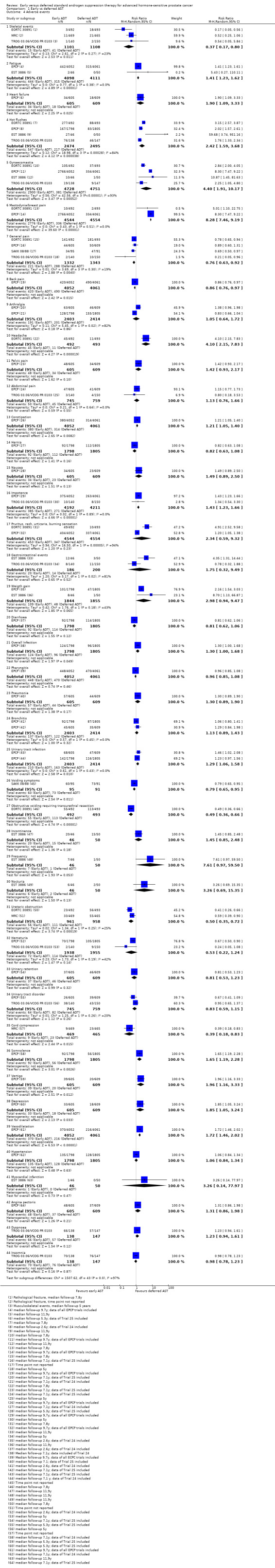
Comparison 1 Early vs deferred AST, Outcome 4 Adverse events.
Fatigue
Early AST may slightly increases the rate of fatigue (RR 1.41, 95% CI 1.23 to 1.62; 2 RCTs; 8209 participants; low‐certainty evidence; Analysis 1.4; Figure 6). This corresponds to 31 more men with fatigue (95% CI 18 more to 48 more) per 1000 participants with early AST. We downgraded for study limitations and imprecision (Table 1).
Heart failure
Early AST may slightly increases the rate of heart failure (RR 1.90, 95% CI 1.09 to 3.33; 1 RCT; 1214 participants; median 9.7 years follow‐up; low‐certainty evidence; Analysis 1.4; Figure 6). This corresponded to 27 more heart failures (95% CI 3 more to 69 more) per 1000 participants with early AST. We downgraded for study limitations and imprecision (Table 1).
Other adverse events
We further reported the following additional adverse events that we included post hoc, since we perceived them to be patient‐important.
Early androgen suppression therapy may slightly increase the rate of hot flushes, gynaecomastia, mastodynia/breast pain, headache, constipation, impotence, overall infection, urinary tract infection, somnolence, vertigo, depression and vasodilatation (for details see Analysis 1.4; Figure 6).
Early androgen suppression therapy may slightly decrease the rate of general pain, back pain, voiding symptoms, obstructive voiding requiring transurethral resection, ureteric obstruction and cord compression (for details see Analysis 1.4; Figure 6).
There was no difference between early and deferred androgen suppression therapy for arthralgia, abdominal pain, hernia, nausea, pruritus/rash/urticaria/burning sensation, gastrointestinal events, weight gain, diarrhoea, pharyngitis, pneumonia, bronchitis, incontinence, frequency, nocturia, haematuria, urinary retention, urinary tract disorder, hypertension, myocardial infarction, angina pectoris, dyspnoea and insomnia (for details see Analysis 1.4; Figure 6).
Global quality of life
Early versus deferred AST probably makes little or no difference in global quality of life after 2 years assessed with the EORTC QLQ‐C30 (version 3.0) questionnaire (mean difference −1.56, 95% CI −4.50 to 1.38; 1 RCT; 285 participants; moderate‐certainty evidence; Analysis 1.5). This corresponded to a mean global quality of life score of 70.83, measured on a scale from 0 to 100 with deferred AST and a mean difference of 1.56 lower (4.5 lower to 1.38 higher) mean global quality of life scores per 1000 participants with early AST (Table 1). We downgraded for study limitations (Table 1). The change in mean difference for global quality of life is trivial and does not appear clinically important (mean difference from −5 to 5 is interpreted as trivial according to Cocks 2012).
1.5. Analysis.
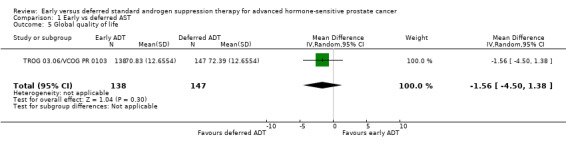
Comparison 1 Early vs deferred AST, Outcome 5 Global quality of life.
Authors reported additional results for quality of life subcategories. There were no differences in physical functioning (MD −0.19, 95% CI −2.48 to 2.11; not shown), role functioning (MD −0.97, 95% CI −4.37 to 2.42; not shown), emotional functioning (MD −1.30, 95% CI −4.07 to 1.47; not shown) or sexual function (MD −0.34, 95% CI −10.48 to 9.80; not shown) but early androgen suppression therapy decreased sexual activity (MD −10.72, 95% CI −14.28 to −7.15) and increased hormone‐treatment‐related symptoms (MD 4.41, 95% CI 2.51 to 6.30).
Time to disease progression
Early AST may increases slightly time to disease progression (HR 0.51, 95% CI 0.44 to 0.60; 6 RCTs; 2718 participants; Analysis 1.6). One study (Granfors 2006) reported only dichotomous data for clinical progression for advanced but non‐metastatic prostate cancer (T2‐4/ N+M0) and could therefore not be included in the meta‐analysis. After 9.3 years, early AST decreased the rate of clinical progression (RR 0.36, 95% CI 0.18 to 0.72; early ADT 6/20, deferred ADT 16/19; not shown).
1.6. Analysis.
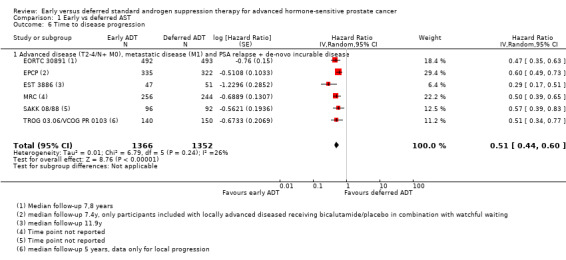
Comparison 1 Early vs deferred AST, Outcome 6 Time to disease progression.
Subgroup analyses
Time to death of any cause based on disease stage
For details see Analysis 2.1. Two thousand, nine hundred and fifty‐eight participants had an advanced but non‐metastatic disease (T2‐4/ N+ M0), 426 participants metastatic disease (M1), and 261 participants had a PSA relapse. Overall, we did not identify a subgroup difference between advanced but non‐metastatic disease (T2‐4/ N+ M0) versus metastatic disease (M1) versus PSA relapse although the test for interaction approaches statistical significance (P = 0.06). This subgroup analysis was exclusively based on comparisons across different trials.
2.1. Analysis.
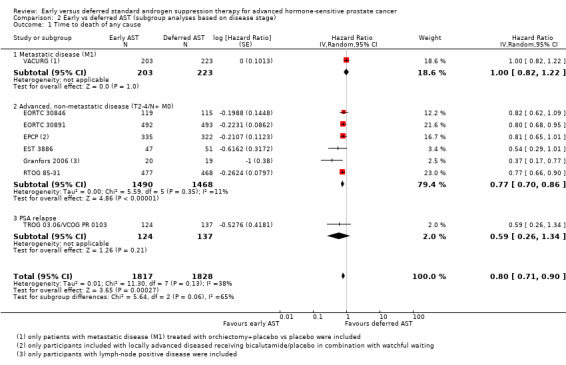
Comparison 2 Early vs deferred AST (subgroup analyses based on disease stage), Outcome 1 Time to death of any cause.
Serious adverse events based on disease stage
For details see Analysis 2.2. Nine thousand, three hundred and thirty‐two participants had an advanced but non‐metastatic disease (T2‐4/N+ M0) and 953 participants had a metastatic disease (M1). We did not identify a subgroup difference between disease stage (P = 0.79; I² = 0%)
2.2. Analysis.
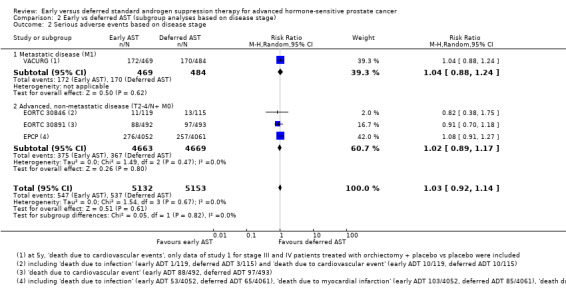
Comparison 2 Early vs deferred AST (subgroup analyses based on disease stage), Outcome 2 Serious adverse events based on disease stage.
Sensitivity analyses
Time to death of any cause
After exclusion of studies with unclear risk for attrition bias (EPCP; Granfors 2006; VACURG), early androgen suppression therapy continued to extend time to death of any cause (HR 0.81, 95% CI 0.75 to 0.88; not shown). Heterogeneity was decreased to 0%.
Discussion
Summary of main results
We identified 10 randomised controlled trials comparing early versus deferred standard androgen suppression therapy (AST) for treatment of advanced hormone‐sensitive prostate cancer.
Early AST probably extends time to death of any cause and time to death from prostate cancer (both moderate‐certainty evidence); and may decrease slightly the rate of skeletal events (low‐certainty evidence). It may result in little or no difference in serious adverse events (low‐certainty evidence) overall and probably results in little or no difference in global quality of life (moderate‐certainty evidence).
On the 'harm' side, early AST may slightly increase fatigue (low evidence certainty) and may increase the risk of heart failure (low evidence certainty).
Predefined subgroup analysis was suggestive (P value for test of interaction: 0.06) of a possible subgroup effect based on disease staging with a larger effect on all‐cause mortality seen in patients with biochemically recurrent disease versus locally advanced, non‐metastatic disease versus metastatic disease. Given that this finding was based on across‐trial comparisons it should be interpreted with caution and viewed as hypothesis‐generating.
Overall completeness and applicability of evidence
Several limitations deserve consideration by the reader.
First, this review pools trial evidence that dates as far back as the 1960s. Participants enrolled in these trials differed substantially from today's prostate cancer patients who are often detected by PSA screening and may have a lower disease burden throughout their disease course. While the GnRH agonists used in most of the trials remain the mainstay of androgen suppression therapy today, antiandrogens such as cyproterone acetate that were part of the treatment regimen are no longer used. In aggregate, these issue raise concerns about the applicability of this body of evidence to today's patients.
Second, the spectrum of disease represented in these trials is wide, ranging from clinically localized to distant metastatic disease. As stipulated in our plans to conduct subgroup analyses, it is plausible that the effects of treatment may differ based on disease stage. While our subgroup analyses provides some suggestion of a subgroup effect, the test of interaction (P = 0.06) did not strictly speaking meet the threshold for statistical significance. While recognizing the potential for spurious findings and type I statistical errors of such analyses, especially when applied to trials that did not stratify for a given subgroup, the analysis may also have been underpowered. As a result, our conclusions with regards to subgroups are limited.
Third, definitions of outcomes such as skeletal events, fatigue and heart failure were inconsistently defined thereby presenting another potential source of heterogeneity.
Lastly, we recognize that the management of advanced prostate cancer is rapidly advancing. Newer agents such as abiraterone or combined early chemo‐hormonal therapy (chemotherapy with docetaxel and LHRH agonists) are now used early on. Enzalutamide is used in metastatic, castration‐resistant prostate cancer patients in combination with standard androgen suppression therapy; and apalutamide has been approved by the FDA for patients with non‐metastatic castration‐resistant prostate cancer. Other drugs such as darolutamide are being evaluated in phase III clinical trials. These novel developments will impact the future role of AST.
Quality of the evidence
We consistently downgraded the certainty of evidence, resulting in ratings that ranged from moderate to low. The main concerns were as follows.
Study limitations, mostly related to performance bias. None of the studies included in this review blinded patients or personnel, which may have impacted the intensity of follow‐up and the type of care they received.
In addition, we had concerns about detection bias for outcomes other than time to death from any cause.
Furthermore, allocation concealment was unclear in several trials and we had concerns about the possibility of selective reporting.
Potential biases in the review process
We performed an extensive literature search using a comprehensive search strategy without language or publication status restrictions, and additionally searched trial registries for unpublished, planned, or ongoing studies. While it is theoretically possible that additional studies may have been conducted but not yet published, it is unlikely that we may have missed studies published in languages other than English or in non‐indexed journals. Should any such studies be identified, we will include them in further updates of this review.
Agreements and disagreements with other studies or reviews
Several systematic reviews exist addressing the issue of early versus deferred androgen deprivation therapy. However, none of them applied the same methodological rigour; rated the quality of evidence on a 'per outcome' basis using GRADE or provided a summary of findings, reporting both relative and absolute effect size estimates.
Boustead 2007 provided a systematic review assessing the effects of treatments for locally advanced prostate such as radical prostatectomy, radiotherapy, and/or watchful waiting with androgen deprivation therapy (corresponding to early ADT) versus these treatments with androgen deprivation therapy initiated at the time of disease progression. Their results indicated that early androgen suppression therapy leads to decreased mortality and disease progression. No undesirable outcomes such as treatment‐related adverse events were assessed nor did the review assess risk of bias of the included studies. Also since that time several additional relevant trials have been published.
Prezioso 2014 conducted a similar systematic review of early versus deferred androgen suppression therapy in men with locally advanced prostate cancer and/or asymptomatic metastasis. They found a reduction of all‐cause mortality, prostate‐cancer‐specific mortality, overall progression and distant progression using early androgen suppression therapy. Similarly, this study failed to both address potential undesirable effects of treatment nor did it quantify the certainty of evidence according to GRADE.
A related Cochrane Review by our working group focused on the effects of early versus deferred androgen suppression therapy in men with lymph‐node‐positive prostate cancer after local therapy with curative intent (Kunath 2013). We found an improvement in survival and delayed disease progression but also found early treatment associated with increased adverse events. The certainty of evidence supporting these findings was low.
Authors' conclusions
Implications for practice.
In men with clinically localized prostate cancer who are either unable or unwilling to undergo local treatment with curative intent, or who have locally advanced prostate cancer, node positive disease and/or (asymptomatic) metastatic disease, findings of this review favours early over delayed androgen suppression therapy in terms of all‐cause survival and other oncological outcomes. This benefit may come at the expense of increased individual non‐serious adverse events. It appears important to share this information on both desirable and undesirable effects with patients considering AST and to facilitate shared decision‐making to resolve the resulting trade‐offs.
Implications for research.
This Cochrane Review update focused on standard androgen suppression therapies. Newer androgen suppression therapies, such as abiraterone, darolutamide, enzalutamide or apalutamide, were not part of this review, and trials investigating these treatment options were not included in our analysis. We identified seven new RCTs since publication of the original review in 2002. Finally, 10 RCTs were identified to support the findings of this Cochrane Review. Conclusions are limited primarily by imprecision, and performance and detection bias, and further research is likely to have an important impact on credibility of results. High‐quality randomised controlled trials with long‐term follow‐up should be conducted evaluating quality of life. However, due to newer medical drugs and expanded treatment indications it is questionable if further research will be conducted evaluating early versus deferred standard AST for advanced hormone‐sensitive prostate cancer.
What's new
| Date | Event | Description |
|---|---|---|
| 4 June 2019 | New citation required and conclusions have changed | This is an update of a Cochrane Review initially published in 2002. In contrast to this review, we adapted methodology to the new standards of Cochrane Urology, developed a new search strategy and performed a new systematic review with meta‐analysis of available literature. |
| 2 January 2019 | New search has been performed | This is an update of a Cochrane Review initially published in 2002. In contrast to this review, we adapted methodology to the new standards of Cochrane Urology, developed a new search strategy and performed a new systematic review with meta‐analysis of available literature. |
Notes
Parts of the Methods section of this review were based on a standard template developed by the Cochrane Metabolic and Endocrine Disorders Group that has been modified and adapted for use by the Cochrane Urology Group.
Acknowledgements
We would like to thank the peer reviewers (Gunhild von Amsberg, Mark Tuthill, Mark Klein) and members of Cochrane Urology for their comments and suggestions in writing this review. We would like to thank Alina Kessel (AK) for her assistance in literature screening and obtaining full‐text manuscripts for this Cochrane Review.
Appendices
Appendix 1. MEDLINE search strategy
1 randomized controlled trial.pt. (411918)
2 controlled clinical trial.pt. (91700)
3 randomized.ab. (333738)
4 placebo.ab. (168173)
5 drug therapy.fs. (1838416)
6 randomly.ab. (240773)
7 trial.ab. (347576)
8 groups.ab. (1501496)
9 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 (3661001)
10 exp animals/ not humans.sh. (4121693)
11 9 not 10 (3149532)
12 exp prostatic neoplasms/ (101605)
13 (prostat* adj3 (cancer* or tumo* or neoplas* or carcinom* or malign*)).mp. (126130)
14 12 or 13 (126130)
15 (time or time factors).sh. (1065104)
16 (earl* or late or later or initia* or defer* or delay* or immedia* or post* or adjuvant* or progress* or symptom* or asymptom* or after* or time or chrono* or date or long term or short term or longterm or shortterm or date or dates or watch* or wait*).mp. (9705587)
17 15 or 16 (9705587)
18 exp androgen antagonists/ or exp gonadotropin‐releasing hormone/ or exp castration/ or exp orchiectomy/ (89838)
19 (androgen receptor antagonists or nonsteroidal anti‐androgens).sh. (961)
20 ((androg* or antiandrog*) adj3 (antagonist* or suppress* or depriv*)).mp. (14027)
21 (hormone therapy or hormone therapies or hormonal therapy or hormonal therapies or hormone treatment or hormone treatments or orchiectom* or orchidectom*or castrat* or orchectom* or orcheotom* or testectom* or androgen receptor antagonist or androgen receptor antagonists or androgen receptor blocker or androgen receptor blockers or androgen receptor blocking agent or androgen receptor blocking agents or antigonadorelin or anti gonadorelin or lrf antagonist or lrf antagonists or AST or ADT or androgen antagonist or androgen antagonists or anti androgen or anti androgens or antiandrogen or anti‐androgen or antiandrogenic or antiandrogenics or anti‐androgenic or antiandrogenics or anti‐androgenics or antiandrogens or anti‐androgens or bicalutamide or cyoctol or cyproterone or flutamide or hydroxyflutamide or nilutamide or nonsteroidal anti androgen or nonsteroidal anti androgens or nonsteroidal antiandrogen or nonsteroidal antiandrogens or buserelin or cryptocur or cystorelin or decapeptyl or dirigestran or d‐trp‐6‐lh‐rh or eligard or enantone or factrel or fertagyl or fertiral or fsh releasing hormone or fsh‐releasing hormone or fsh releasing hormones or fsh‐releasing hormones or gn rh or gnrh or gonadoliberin or gonadorelin or gonadotrophin releasing factor or gonadotrophin releasing hormone or gonadotropin release factor or gonadotropin releasing factor or gonadotropin releasing hormone or gonadotropin releasing hormones or gonadotrophin releasing factors or gonadotrophin releasing hormones or gonadotropin release factors or gonadotropin releasing factors or gonadotropin releasing hormones or gonadotropin releasing hormones or goserelin or leuprolide or leuprorelin or lfrh or lh fsh releasing hormone or lh releasing hormone or lhfsh releasing hormone or lh‐fsh releasing hormone or lh fsh releasing hormones or lh releasing hormones or lhfsh releasing hormones or lh‐fsh releasing hormones or lhfshrh or lh‐releasing hormone or lh‐releasing hormones or lh releasing hormone or lh releasing hormones or lhrf or lhrh or lh‐rh or lh‐rf or lh rh or lh rf or lrh or luforan or luliberin or luliberine or lupron or lutal or lutamin or luteinising hormone release factor or luteinizing hormone release factors or luteinising hormone releasing factor or luteinising hormone releasing factors or luteinizing hormone releasing hormone or luteinising hormone releasing hormones or luteinizing hormone release factor or luteinizing hormone release factors or luteinizing hormone releasing factor or luteinizing hormone releasing factors or luteinizing hormone releasing hormone or luteinizing hormone releasing hormones or luteinizing hormone‐releasing hormone or luteinizing hormone‐releasing hormones or profact or pulstim or zoladex or abarelix* or anandron* or apimid*or bicalutamid* or casodex* or casudex* or chimax* or cytamid* or degarelix* or drogenil* or eligard* or euflex* or eulexin* or firmagon* or fluken* or flulem* or flumid* or fluta* or flutexin* or fugerel* or grisetin* or iftolid* or nilandron* or nilutamid* or oncosal* or plenaxis* or prostacur* or prostica* or prostogenat* or restotard* or trimestral*).mp. (105192)
22 18 or 19 or 20 or 21 (145105)
23 11 and 14 and 17 and 22 (7044)
Appendix 2. Embase search strategy
#1 ('crossover procedure'/exp or 'double blind procedure'/exp or 'randomized controlled trial'/exp or 'single blind procedure'/exp) or (random* or factorial* OR crossover* OR 'cross over' or 'cross‐over' OR placebo* or assign* or allocate* or volunteer*):ti,ab,de or (doubl* near/3 blind*):ti,ab,de or (singl* near/3 blind*):ti,ab,de
#2 'prostate tumor'/de OR (prostat* near/3 (cancer* or tumo* or neoplas* or carcinom* or malign*)):ti,ab,de,tn
#3 'time'/de OR (earl* or 'late' or 'later' or initia* or defer* or delay* or immedia* or post* or adjuvant* or progress* or symptom* or asymptom* or after* or chrono* or 'date' or 'long term' or 'short term' or 'longterm' or 'shortterm' or 'time' or 'date' or 'dates' or watch* or wait*):ti,ab,de,tn
#4 ((androg* or antiandrog*) near/3 (antagonist* or suppress* or depriv*)):ti,ab,de,tn
#5 'antiandrogen'/de or 'gonadorelin'/exp or 'castration'/de or 'orchiectomy'/de or 'androgen receptor antagonist'/exp or ('hormone therapy' or 'hormone therapies' or 'hormonal therapy' or 'hormonal therapies' or 'hormone treatment' or 'hormone treatments' or orchiectom* or orchidectom* or castrat* or orchectom* or orcheotom* or testectom* or 'androgen receptor antagonist' or 'androgen receptor antagonists' or 'androgen receptor blocker' or 'androgen receptor blockers' or 'androgen receptor blocking agent' or 'androgen receptor blocking agents' or 'antigonadorelin' or 'anti gonadorelin' or 'lrf antagonist' or 'lrf antagonists' or 'AST' or 'ADT' or 'androgen antagonist' or 'androgen antagonists' or 'anti androgen' or 'anti androgens' or 'antiandrogen' or 'anti‐androgen' or 'antiandrogenic' or 'antiandrogenics' or 'anti‐androgenic' or 'antiandrogenics' or 'anti‐androgenics' or 'antiandrogens' or 'anti‐androgens' or 'bicalutamide' or 'cyoctol' or 'cyproterone' or 'flutamide' or 'hydroxyflutamide' or 'nilutamide' or 'nonsteroidal anti androgen' or 'nonsteroidal anti androgens' or 'nonsteroidal antiandrogen' or 'nonsteroidal antiandrogens' or 'buserelin' or 'cryptocur' or 'cystorelin' or 'decapeptyl' or 'dirigestran' or 'd‐trp‐6‐lh‐rh' or 'eligard' or 'enantone' or 'factrel' or 'fertagyl' or 'fertiral' or 'fsh releasing hormone' or 'fsh‐releasing hormone' or 'fsh releasing hormones' or 'fsh‐releasing hormones' or 'gn rh' or 'gnrh' or 'gonadoliberin' or 'gonadorelin' or 'gonadotrophin releasing factor' or 'gonadotrophin releasing hormone' or 'gonadotropin release factor' or 'gonadotropin releasing factor' or 'gonadotropin releasing hormone' or 'gonadotropin releasing hormones' or 'gonadotrophin releasing factors' or 'gonadotrophin releasing hormones' or 'gonadotropin release factors' or 'gonadotropin releasing factors' or 'gonadotropin releasing hormones' or 'gonadotropin releasing hormones' or 'goserelin' or 'leuprolide' or 'leuprorelin' or 'lfrh' or 'lh fsh releasing hormone' or 'lh releasing hormone' or 'lhfsh releasing hormone' or 'lh‐fsh releasing hormone' or 'lh fsh releasing hormones' or 'lh releasing hormones' or 'lhfsh releasing hormones' or 'lh‐fsh releasing hormones' or 'lhfshrh' or 'lh‐releasing hormone' or 'lh‐releasing hormones' or 'lh releasing hormone' or 'lh releasing hormones' or 'lhrf' or 'lhrh' or 'lh‐rh' or 'lh‐rf' or 'lh rh' or 'lh rf' or 'lrh' or 'luforan' or 'luliberin' or 'luliberine' or 'lupron' or 'lutal' or 'lutamin' or 'luteinising hormone release factor' or 'luteinising hormone release factors' or 'luteinising hormone releasing factor' or 'luteinising hormone releasing factors' or 'luteinising hormone releasing hormone' or 'luteinising hormone releasing hormones' or 'luteinizing hormone release factor' or 'luteinizing hormone release factors' or 'luteinizing hormone releasing factor' or 'luteinizing hormone releasing factors' or 'luteinizing hormone releasing hormone' or 'luteinizing hormone releasing hormones' or 'luteinizing hormone‐releasing hormone' or 'luteinizing hormone‐releasing hormones' or 'profact' or 'pulstim' or 'zoladex' or abarelix* or anandron* or apimid*or bicalutamid* or casodex* or casudex* or chimax* or cytamid* or degarelix* or drogenil* or eligard* or euflex* or eulexin* or firmagon* or fluken* or flulem* or flumid* or fluta* or flutexin* or fugerel* or grisetin* or niftolid* or nilandron* or nilutamid* or oncosal* or plenaxis* or prostacur* or prostica* or prostogenat* or restotard* or trimestral*):ti,ab,de,tn
#6 #4 OR #5
#7 #1 AND #2 AND #3 AND #6
Appendix 3. Web of Science search strategy
#1 TS=(prostat* NEAR/3 (cancer* OR tumo* OR neoplas* OR carcinom* OR malign*))
#2 TS=(earl* OR "late" OR "later" OR initia* OR defer* OR delay* OR immedia* OR post* OR adjuvant* OR progress* OR symptom* OR asymptom* OR after* OR "time" OR chrono* OR "date" OR "long term" OR "short term" OR "longterm" OR "shortterm" OR "date" OR "dates" OR watch* OR wait*)
#3 TS=((androg* OR antiandrog*) NEAR/3 (antagonist* OR suppress* OR depriv*))
#4 TS=("hormone therapy" OR "hormone therapies" OR "hormonal therapy" OR "hormonal therapies" OR "hormone treatment" OR "hormone treatments" OR orchiectom* OR orchidectom*or castrat* OR orchectom* OR orcheotom* OR testectom* OR "androgen receptor antagonist" OR "androgen receptor antagonists" OR "androgen receptor blocker" OR "androgen receptor blockers" OR "androgen receptor blocking agent" OR "androgen receptor blocking agents" OR "antigonadorelin" OR "anti gonadorelin" OR "lrf antagonist" OR "lrf antagonists" OR "AST" OR "ADT" OR "androgen antagonist" OR "androgen antagonists" OR "anti androgen" OR "anti androgens" OR "antiandrogen" OR "anti‐androgen" OR "antiandrogenic" OR "antiandrogenics" OR "anti‐androgenic" OR "antiandrogenics" OR "anti‐androgenics" OR "antiandrogens" OR "anti‐androgens" OR "bicalutamide" OR "cyoctol" OR "cyproterone" OR "flutamide" OR "hydroxyflutamide" OR "nilutamide" OR "nonsteroidal anti androgen" OR "nonsteroidal anti androgens" OR "nonsteroidal antiandrogen" OR "nonsteroidal antiandrogens" OR "buserelin" OR "cryptocur" OR "cystorelin" OR "decapeptyl" OR "dirigestran" OR "d‐trp‐6‐lh‐rh" OR "eligard" OR "enantone" OR "factrel" OR "fertagyl" OR "fertiral" OR "fsh releasing hormone" OR "fsh‐releasing hormone" OR "fsh releasing hormones" OR "fsh‐releasing hormones" OR "gn rh" OR "gnrh" OR "gonadoliberin" OR "gonadorelin" OR "gonadotrophin releasing factor" OR "gonadotrophin releasing hormone" OR "gonadotropin release factor" OR "gonadotropin releasing factor" OR "gonadotropin releasing hormone" OR "gonadotropin releasing hormones" OR "gonadotrophin releasing factors" OR "gonadotrophin releasing hormones" OR "gonadotropin release factors" OR "gonadotropin releasing factors" OR "gonadotropin releasing hormones" OR "gonadotropin releasing hormones" OR "goserelin" OR "leuprolide" OR "leuprorelin" OR "lfrh" OR "lh fsh releasing hormone" OR "lh releasing hormone" OR "lhfsh releasing hormone" OR "lh‐fsh releasing hormone" OR "lh fsh releasing hormones" OR "lh releasing hormones" OR "lhfsh releasing hormones" OR "lh‐fsh releasing hormones" OR "lhfshrh" OR "lh‐releasing hormone" OR "lh‐releasing hormones" OR "lh releasing hormone" OR "lh releasing hormones" OR "lhrf" OR "lhrh" OR "lh‐rh" OR "lh‐rf" OR "lh rh" OR "lh rf" OR "lrh" OR "luforan" OR "luliberin" OR "luliberine" OR "lupron" OR "lutal" OR "lutamin" OR "luteinising hormone release factor" OR "luteinising hormone release factors" OR "luteinising hormone releasing factor" OR "luteinising hormone releasing factors" OR "luteinising hormone releasing hormone" OR "luteinising hormone releasing hormones" OR "luteinizing hormone release factor" OR "luteinizing hormone release factors" OR "luteinizing hormone releasing factor" OR "luteinizing hormone releasing factors" OR "luteinizing hormone releasing hormone" OR "luteinizing hormone releasing hormones" OR "luteinizing hormone‐releasing hormone" OR "luteinizing hormone‐releasing hormones" OR "profact" OR "pulstim" OR "zoladex" OR abarelix* OR anandron* OR apimid*or bicalutamid* OR casodex* OR casudex* OR chimax* OR cytamid* OR degarelix* OR drogenil* OR eligard* OR euflex* OR eulexin* OR firmagon* OR fluken* OR flulem* OR flumid* OR fluta* OR flutexin* OR fugerel* OR grisetin* OR niftolid* OR nilandron* OR nilutamid* OR oncosal* OR plenaxis* OR prostacur* OR prostica* OR prostogenat* OR restotard* OR trimestral*)
#1 AND #2 AND (#3 OR #4)
Appendix 4. The Cochrane Library search strategy
#1 MeSH descriptor: [Prostatic Neoplasms] explode all trees 3580
#2 (prostat* near/3 (cancer* or tumo* or neoplas* or carcinom* or malign*)):ti,ab,kw 6388
#3 #1 or #2 6388
#4 MeSH descriptor: [Time] this term only 443
#5 MeSH descriptor: [Time Factors] this term only 51540
#6 (earl* or late or later or initia* or defer* or delay* or immedia* or post* or adjuvant* or progress* or symptom* or asymptom* or after* or time or chrono* or date or long term or short term or longterm or shortterm or date or dates or watch* or wait*):ti,ab,kw 577793
#7 #4 or #5 or #6 577793
#8 MeSH descriptor: [Androgen Antagonists] explode all trees 758
#9 MeSH descriptor: [Gonadotropin‐Releasing Hormone] explode all trees 2037
#10 MeSH descriptor: [Castration] explode all trees 765
#11 MeSH descriptor: [Orchiectomy] explode all trees 333
#12 MeSH descriptor: [Androgen Receptor Antagonists] this term only 9
#13 MeSH descriptor: [Nonsteroidal Anti‐Androgens] this term only 0
#14 (androg* or antiandrog*) near/3 (antagonist* or suppress* or depriv*):ti,ab,kw 1265
#15 ("hormone therapy" or "hormone therapies" or "hormonal therapy" or "hormonal therapies" or "hormone treatment" or "hormone treatments" or orchiectom* or orchidectom*or castrat* or orchectom* or orcheotom* or testectom* or "androgen receptor antagonist" or "androgen receptor antagonists" or "androgen receptor blocker" or "androgen receptor blockers" or "androgen receptor blocking agent" or "androgen receptor blocking agents" or "antigonadorelin" or "anti gonadorelin" or "lrf antagonist" or "lrf antagonists" or "AST" or "ADT" or "androgen antagonist" or "androgen antagonists" or "anti androgen" or "anti androgens" or "antiandrogen" or "anti‐androgen" or "antiandrogenic" or "antiandrogenics" or "anti‐androgenic" or "antiandrogenics" or "anti‐androgenics" or "antiandrogens" or "anti‐androgens" or "bicalutamide" or "cyoctol" or "cyproterone" or "flutamide" or "hydroxyflutamide" or "nilutamide" or "nonsteroidal anti androgen" or "nonsteroidal anti androgens" or "nonsteroidal antiandrogen" or "nonsteroidal antiandrogens" or "buserelin" or "cryptocur" or "cystorelin" or "decapeptyl" or "dirigestran" or "d‐trp‐6‐lh‐rh" or "eligard" or "enantone" or "factrel" or "fertagyl" or "fertiral" or "fsh releasing hormone" or "fsh‐releasing hormone" or "fsh releasing hormones" or "fsh‐releasing hormones" or "gn rh" or "gnrh" or "gonadoliberin" or "gonadorelin" or "gonadotrophin releasing factor" or "gonadotrophin releasing hormone" or "gonadotropin release factor" or "gonadotropin releasing factor" or "gonadotropin releasing hormone" or "gonadotropin releasing hormones" or "gonadotrophin releasing factors" or "gonadotrophin releasing hormones" or "gonadotropin release factors" or "gonadotropin releasing factors" or "gonadotropin releasing hormones" or "gonadotropin releasing hormones" or "goserelin" or "leuprolide" or "leuprorelin" or "lfrh" or "lh fsh releasing hormone" or "lh releasing hormone" or "lhfsh releasing hormone" or "lh‐fsh releasing hormone" or "lh fsh releasing hormones" or "lh releasing hormones" or "lhfsh releasing hormones" or "lh‐fsh releasing hormones" or "lhfshrh" or "lh‐releasing hormone" or "lh‐releasing hormones" or "lh releasing hormone" or "lh releasing hormones" or "lhrf" or "lhrh" or "lh‐rh" or "lh‐rf" or "lh rh" or "lh rf" or "lrh" or "luforan" or "luliberin" or "luliberine" or "lupron" or "lutal" or "lutamin" or "luteinising hormone release factor" or "luteinising hormone release factors" or "luteinising hormone releasing factor" or "luteinising hormone releasing factors" or "luteinising hormone releasing hormone" or "luteinising hormone releasing hormones" or "luteinizing hormone release factor" or "luteinizing hormone release factors" or "luteinizing hormone releasing factor" or "luteinizing hormone releasing factors" or "luteinizing hormone releasing hormone" or "luteinizing hormone releasing hormones" or "luteinizing hormone‐releasing hormone" or "luteinizing hormone‐releasing hormones" or "profact" or "pulstim" or "zoladex" or abarelix* or anandron* or apimid*or bicalutamid* or casodex* or casudex* or chimax* or cytamid* or degarelix* or drogenil* or eligard* or euflex* or eulexin* or firmagon* or fluken* or flulem* or flumid* or fluta* or flutexin* or fugerel* or grisetin* or niftolid* or nilandron* or nilutamid* or oncosal* or plenaxis* or prostacur* or prostica* or prostogenat* or restotard* or trimestral*):ti,ab,kw 9929
#16 #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 10490
#17 #3 and #7 and #16 1456
Appendix 5. Clinicaltrials.gov and ICTRP search portal
We used the following keywords for this search: 'early androgen', 'immediate androgen', 'prostate cancer'.
Data and analyses
Comparison 1. Early vs deferred AST.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to death of any cause | 10 | 4767 | Hazard Ratio (Random, 95% CI) | 0.82 [0.75, 0.90] |
| 1.1 Advanced disease (T2‐4/N+ M0), metastatic disease (M1) and PSA relapse | 10 | 4767 | Hazard Ratio (Random, 95% CI) | 0.82 [0.75, 0.90] |
| 2 Serious adverse events | 5 | 10575 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.95, 1.16] |
| 3 Time to death from prostate cancer | 7 | 3677 | Hazard Ratio (Random, 95% CI) | 0.69 [0.57, 0.84] |
| 3.1 Advanced disease (T2‐4/N+ M0), metastatic disease (M1) and PSA relapse + de‐novo incurable disease | 7 | 3677 | Hazard Ratio (Random, 95% CI) | 0.69 [0.57, 0.84] |
| 4 Adverse events | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Skeletal events | 3 | 2209 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.17, 0.80] |
| 4.2 Fatigue | 2 | 8209 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [1.23, 1.62] |
| 4.3 Heart failure | 1 | 1214 | Risk Ratio (M‐H, Random, 95% CI) | 1.90 [1.09, 3.33] |
| 4.4 Hot flushes | 4 | 4969 | Risk Ratio (M‐H, Random, 95% CI) | 2.42 [1.59, 3.68] |
| 4.5 Gynaecomastia | 4 | 9479 | Risk Ratio (M‐H, Random, 95% CI) | 4.40 [1.91, 10.17] |
| 4.6 Mastodynia/breast pain | 2 | 9098 | Risk Ratio (M‐H, Random, 95% CI) | 8.28 [7.46, 9.19] |
| 4.7 General pain | 4 | 2675 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.63, 0.92] |
| 4.8 Back pain | 1 | 8113 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.76, 0.97] |
| 4.9 Arthralgia | 1 | 4817 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.64, 1.72] |
| 4.10 Headache | 1 | 985 | Risk Ratio (M‐H, Random, 95% CI) | 4.10 [2.15, 7.83] |
| 4.11 Pelvic pain | 1 | 1214 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.93, 2.17] |
| 4.12 Abdominal pain | 2 | 1504 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.76, 1.66] |
| 4.13 Constipation | 1 | 8113 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [1.05, 1.40] |
| 4.14 Hernia | 1 | 3603 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.63, 1.08] |
| 4.15 Nausea | 1 | 1214 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.89, 2.50] |
| 4.16 Impotence | 2 | 8403 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [1.23, 1.66] |
| 4.17 Pruritus, rash, urticaria, burning sensation | 2 | 9098 | Risk Ratio (M‐H, Random, 95% CI) | 2.34 [0.59, 9.32] |
| 4.18 Gastrointestinal events | 2 | 386 | Risk Ratio (M‐H, Random, 95% CI) | 1.75 [0.32, 9.49] |
| 4.19 Weigth gain | 2 | 3699 | Risk Ratio (M‐H, Random, 95% CI) | 2.98 [0.94, 9.47] |
| 4.20 Diarrhoea | 1 | 3603 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.62, 1.06] |
| 4.21 Overall Infection | 1 | 3603 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [1.00, 1.68] |
| 4.22 Pharyngitis | 1 | 8113 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.85, 1.08] |
| 4.23 Pneumonia | 1 | 1214 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.89, 1.90] |
| 4.24 Bronchitis | 1 | 4817 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.89, 1.43] |
| 4.25 Urinary tract infection | 1 | 4817 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [1.06, 1.58] |
| 4.26 Voiding symptoms | 1 | 186 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.65, 0.95] |
| 4.27 Obstructive voiding requiring transurethral resection | 1 | 985 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.36, 0.66] |
| 4.28 Incontinence | 1 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.85, 2.48] |
| 4.29 Frequency | 1 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 7.61 [0.97, 59.50] |
| 4.30 Nocturia | 1 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 3.26 [0.69, 15.35] |
| 4.31 Ureteric obstruction | 2 | 1919 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.35, 0.72] |
| 4.32 Hematuria | 2 | 3893 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.22, 1.24] |
| 4.33 Urinary retention | 1 | 1214 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.53, 1.23] |
| 4.34 Urinary tract disorder | 2 | 1504 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.59, 1.15] |
| 4.35 Cord compression | 1 | 934 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.18, 0.83] |
| 4.36 Somnolence | 1 | 3603 | Risk Ratio (M‐H, Random, 95% CI) | 1.65 [1.19, 2.28] |
| 4.37 Vertigo | 1 | 1214 | Risk Ratio (M‐H, Random, 95% CI) | 1.96 [1.16, 3.33] |
| 4.38 Depression | 1 | 1214 | Risk Ratio (M‐H, Random, 95% CI) | 1.85 [1.05, 3.24] |
| 4.39 Vasodilatation | 1 | 8113 | Risk Ratio (M‐H, Random, 95% CI) | 1.72 [1.46, 2.02] |
| 4.40 Hypertension | 1 | 3603 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.84, 1.34] |
| 4.41 Myocardial infarction | 1 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 3.26 [0.14, 77.97] |
| 4.42 Angina pectoris | 1 | 1214 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.86, 1.98] |
| 4.43 Dyspnoea | 1 | 285 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.94, 1.61] |
| 4.44 Insomnia | 1 | 285 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.78, 1.23] |
| 5 Global quality of life | 1 | 285 | Mean Difference (IV, Random, 95% CI) | ‐1.56 [‐4.50, 1.38] |
| 6 Time to disease progression | 6 | 2718 | Hazard Ratio (Random, 95% CI) | 0.51 [0.44, 0.60] |
| 6.1 Advanced disease (T2‐4/N+ M0), metastatic disease (M1) and PSA relapse + de‐novo incurable disease | 6 | 2718 | Hazard Ratio (Random, 95% CI) | 0.51 [0.44, 0.60] |
1.3. Analysis.
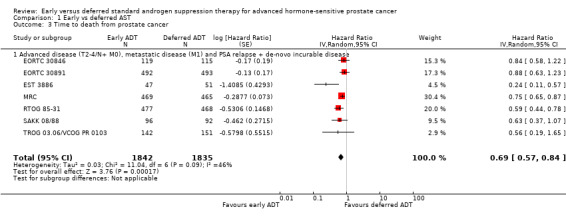
Comparison 1 Early vs deferred AST, Outcome 3 Time to death from prostate cancer.
Comparison 2. Early vs deferred AST (subgroup analyses based on disease stage).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to death of any cause | 8 | 3645 | Hazard Ratio (Random, 95% CI) | 0.80 [0.71, 0.90] |
| 1.1 Metastatic disease (M1) | 1 | 426 | Hazard Ratio (Random, 95% CI) | 1.0 [0.82, 1.22] |
| 1.2 Advanced, non‐metastatic disease (T2‐4/N+ M0) | 6 | 2958 | Hazard Ratio (Random, 95% CI) | 0.77 [0.70, 0.86] |
| 1.3 PSA relapse | 1 | 261 | Hazard Ratio (Random, 95% CI) | 0.59 [0.26, 1.34] |
| 2 Serious adverse events based on disease stage | 4 | 10285 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.92, 1.14] |
| 2.1 Metastatic disease (M1) | 1 | 953 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.88, 1.24] |
| 2.2 Advanced, non‐metastatic disease (T2‐4/N+ M0) | 3 | 9332 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.89, 1.17] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
EORTC 30846.
| Methods |
Design: randomised controlled trial Setting: multicentric (27 institutions) Recruiting period: February 1986 to November 1998 Sample size: 302 recruited, 234 randomised patients Follow‐up (months): median 13 years |
|
| Participants |
Population description: patients with lymph‐node‐positive (pN1‐3) cancer without local treatment of the primary tumour Inclusion criteria:
Exclusion criteria: not reported Tumour stage: T2‐3, N1‐3, M0 Previous treatment: no previous treatment other than lymph node dissection or lymph node biopsy Number randomised: 234 patients (Early ADT: 119; included in analysis 119. Deferred ADT: 115; included in analysis 115) Withdrawals and exclusions: no exclusions Subgroup measured: not reported Subgroup reported: not reported Age:
Baseline imbalances: The 2 groups were well balanced except for small differences for some factors: the median age was 66.6 years for the EET arm and 64.3 years for the DET arm. In the EET arm, 29.4% of the tumours were poorly differentiated (WHO grade 3) versus 33.9% in the DET arm, but T3‐4 (TNM 1972) were seen in 68.1% of the patients in the EET arm versus 62.6% in the DET arm. |
|
| Interventions |
Early ADT (intervention group):
Deferred ADT (control group):
|
|
| Outcomes |
Primary outcome(s):
Secondary outcome(s):
|
|
| Funding sources |
|
|
| Declaration of interest | The author certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (e.g. employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: none. | |
| Notes | The trial is underpowered to reach its goal of showing non‐inferiority ("The trial was designed to prove non‐inferiority of deferred ADT to early ADT [...] Three hundred twenty patients were considered required [...] Since the trial was launched, the conception of what might be called equivalence or non‐inferiority has evolved and now allows only much smaller survival losses and smaller false error rates [...], so that the original sample size calculation would now be considered unethical. Furthermore, the recruitment was difficult, so that not even the originally planned 320 evaluable men were recruited. Reliable information concerning the treatment modalities, which were applied at the investigators' discretion at the time of progression under endocrine treatment, is not available. However, yearly follow‐up indicates that 50% of the patients in the delayed and early group, respectively, continued the same treatment as per protocol after they reached the end of the protocol treatment.") | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Information from publication: Information was not reported. Comment: We assume that randomisation was performed adequately at the EORTC Data Centre. However, information was not reported and there is therefore unclear risk of bias. |
| Allocation concealment (selection bias) | Low risk |
Information from publication: "centrally". Comment: Randomisation was performed centrally at the EORTC Data Centre. |
| Blinding of participants and personnel (performance bias) Time to death of any cause | Unclear risk |
Information from publication: There was no blinding. Comment: Time to death of any cause was measured and reported. It might be conceivable that even objective outcomes are influenced by lack of blinding. We finally judge that there is an unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All other outcomes | High risk |
Information from publication: There was no blinding. Comment: Time to death from prostate cancer and few adverse events were measured and reported. We judge that subjective outcomes are likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Time to death from any cause | Low risk |
Information from publication: There was no blinding of outcome assessment (or it was not reported). Comment: Time to death of any cause was measured and reported. Blinding of outcome assessment could have been expected. However, we judge that it is not likely that outcome assessment for objective outcomes is influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All other outcomes | High risk |
Information from publication: There was no blinding of outcome assessment (or it was not reported). Comment: Time to death from prostate cancer and few adverse events were measured and reported. Blinding of outcome assessment could have been expected. We judge that outcome assessment of subjective outcomes is likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) Oncological outcomes (Time‐to‐death of any cause, Time‐to‐disease progression, Time‐to‐death from prostate cancer) | Low risk | All patients randomised were included in the analysis for time to death of any cause and time to death from prostate cancer. |
| Incomplete outcome data (attrition bias) Adverse events (Serious and other adverse events) | Unclear risk | Not applicable (outcome not measured/reported). Only deaths due to cardiovascular events or infection were reported. |
| Incomplete outcome data (attrition bias) Quality of life | Unclear risk | Not applicable (outcome not measured/reported). |
| Selective reporting (reporting bias) | High risk | There was no assessment of adverse events (except death due to cardiovascular events or infection) but it could have been expected or adverse events were measured but not reported. Data for the predefined outcome 'Time to clinical progression' were evaluated but not reported. |
| Other bias | Unclear risk | We identified no other sources of bias. |
EORTC 30891.
| Methods |
Design: randomised controlled trial Setting: multicentric Recruiting period: February 1990 to January 1999 Sample size: 985 patients Follow‐up: median follow‐up 7.8 years |
|
| Participants |
Population description: newly diagnosed prostate cancer T0‐4, N0‐2, M0 without previous treatment Inclusion criteria:
Exclusion criteria:
Tumour stage: T0‐4, N0‐2, M0 Previous treatment: no previous local or systemic treatment Number randomised: 1002 patients Withdrawals and exclusions: 17 patients from 2 centres were excluded because of non‐availability of source documentation (+ 25 patients were ineligible (see below), but remained in the analysis)
Subgroup measured: not reported Subgroup reported: not reported Age:
Baseline imbalances: no significant differences in the baseline characteristics of patients in the 2 arms |
|
| Interventions |
Early ADT (intervention group):
Deferred ADT (control group):
|
|
| Outcomes |
Primary outcome(s):
Secondary outcome(s):
|
|
| Funding sources | Buserelin was in part supplied free by the Hoechst‐Company (now Sanofi‐Aventis) | |
| Declaration of interest | The authors indicated no potential conflicts of interest | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Information from publication: Information was not reported. Comment: We assume that randomisation was performed adequately at the EORTC Data Centre. However, information was not reported and there is therefore unclear risk of bias. |
| Allocation concealment (selection bias) | Unclear risk |
Information from publication: Information was not reported. Comment: We assume that allocation concealment was performed adequately at the EORTC Data Centre. However, information was not reported and there is therefore unclear risk of bias. |
| Blinding of participants and personnel (performance bias) Time to death of any cause | Unclear risk |
Information from publication: There was no blinding. Comment: It might be conceivable that time to death of any cause is influenced by lack of blinding. We finally judge that there is an unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All other outcomes | High risk |
Information from publication: There was no blinding. Comment: We judge that time to disease progression, time to death from prostate cancer and adverse events are likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Time to death from any cause | Low risk |
Information from publication: There was no blinding of outcome assessment (or it was not reported). Comment: We judge that it is not likely that outcome assessment for time to death of any cause is influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All other outcomes | High risk |
Information from publication: There was no blinding of outcome assessment (or it was not reported). Comment: We judge that outcome assessment of time to disease progression, time to death from prostate cancer and adverse events are likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) Oncological outcomes (Time‐to‐death of any cause, Time‐to‐disease progression, Time‐to‐death from prostate cancer) | Low risk | The reasons for missing outcome data are unlikely to be related to true outcome (17 of 985 patients were excluded because of non‐availability of source documentation). |
| Incomplete outcome data (attrition bias) Adverse events (Serious and other adverse events) | Low risk | The reasons for missing outcome data are unlikely to be related to true outcome (17 of 985 patients were excluded because of non‐availability of source documentation). |
| Incomplete outcome data (attrition bias) Quality of life | Unclear risk | Not applicable (outcome not measured/reported). |
| Selective reporting (reporting bias) | Low risk | We identified a pre‐defined protocol and the relevant outcomes were reported and analysed as planned. |
| Other bias | Unclear risk | We identified no other sources of bias. |
EPCP.
| Methods |
Design: 3 randomised placebo‐controlled double‐blind trials Setting: multicentric (North America (Trial 23, 3292 men); Europe, South Africa, Australia, Israel, Mexico (Trial 24, 3603 men); and Scandinavia (Trial 25, 1218 men)) Recruiting period: not reported Sample size: 8113 patients Follow‐up (months): median follow up: 9,7 years (range: 0 to 12.87 years) |
|
| Participants |
Population description: patients with localized (T1‐2, NO/Nx) or locally advanced (T3‐4, any N; or any T, N+) prostate cancer (all M0) Inclusion criteria:
Exclusion criteria:
Tumour stage: T1‐4, any N, M0 Previous treatment:
Number randomised: 8113 patients Withdrawals and exclusions:
Subgroup measured: ‐ Subgroup reported: ‐ Age:
Baseline imbalances: The treatment groups were well balanced, with differences between trials relating to differences in entry criteria |
|
| Interventions |
Early ADT (intervention group):
Deferred ADT (control group):
|
|
| Outcomes |
Primary outcome(s):
Secondary outcome(s)
|
|
| Funding sources | The EPC programme was funded by AstraZeneca. Casodex® and Zoladex® are registered trademarks of the AstraZeneca group of companies. | |
| Declaration of interest | Peter Iversen, David McLeod, William See and Manfred Wirth are investigators for AstraZeneca‐sponsored studies, and are engaged as paid consultants and lecturers for AstraZeneca. William See has also provided expert testimony for, and received research funding from, AstraZeneca. Thomas Morris and Jon Armstrong are employees and stock holders of AstraZeneca. | |
| Notes | We included only data on adverse events, objective progression‐free survival and overall survival for the subgroup of patients with locally advanced diseased treated with bicalutamide and watchful waiting or placebo and watchful waiting (657 of 8113 patients). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Information from publication: "Randomisation schemes were produced by computer software incorporating a standard procedure for generating random numbers". Comment: Adequate random sequence generation. |
| Allocation concealment (selection bias) | Low risk |
Information from publication: "balanced to treatment in balanced blocks (using a block size of four)". Comment: Adequate allocation concealment. |
| Blinding of participants and personnel (performance bias) Time to death of any cause | Unclear risk |
Information from publication: "The trial was double‐blinded"..."Patients were randomised in a 1:1 basis to receive either 150 mg bicalutamide daily or placebo". Comment: Blinding was broken by the committee due to statistically significant differences in time to disease progression in trials 24/25. |
| Blinding of participants and personnel (performance bias) All other outcomes | Unclear risk |
Information from publication: "The trial was double‐blinded"..."Patients were randomised in a 1:1 basis to receive either 150 mg bicalutamide daily or placebo". Comment: Blinding was broken by the committee due to statistically significant differences in time to disease progression in trials 24/25. |
| Blinding of outcome assessment (detection bias) Time to death from any cause | Low risk |
Information from publication: "An independent Data and Safety Monitoring Committee reviewed blinded data on an ongoing basis during follow‐up". Comment: Time to death of any cause was assessed. Blinding was broken by the committee due to statistically significant differences in time to disease progression in trials 24/25. However, we judge that it is not likely that outcome assessment for objective outcomes is influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All other outcomes | Unclear risk |
Information from publication: "An independent Data and Safety Monitoring Committee reviewed blinded data on an ongoing basis during follow‐up". Comment: Time to disease progression, time to death from prostate cancer and adverse events were assessed. Blinding was broken by the committee due to statistically significant differences in time to disease progression in trials 24/25. |
| Incomplete outcome data (attrition bias) Oncological outcomes (Time‐to‐death of any cause, Time‐to‐disease progression, Time‐to‐death from prostate cancer) | Unclear risk | Missing outcome data balanced in numbers across intervention groups with similar reasons for missing data across groups. However, we only included participants with locally advanced diseased receiving bicalutamide/placebo in combination with watchful waiting for evaluation of time to death of any cause and time to disease progression (N = 657 of 8113 participants). |
| Incomplete outcome data (attrition bias) Adverse events (Serious and other adverse events) | Low risk | All participants were included in analyses. |
| Incomplete outcome data (attrition bias) Quality of life | Unclear risk | Not applicable (outcome not measured/reported). |
| Selective reporting (reporting bias) | Low risk | The relevant outcomes were reported and analysed as planned. |
| Other bias | Unclear risk | We identified no other sources of bias. |
EST 3886.
| Methods |
Design: prospective randomised controlled trial Setting: multicentric Recruiting period: 1988 to 1993 Sample size: 98 patients Follow‐up: 11.9 years |
|
| Participants |
Population description: clinically localized node‐positive prostate cancer (no more than stage T2) Inclusion criteria:
Exclusion criteria: not reported Tumour stage: T1‐T2, N+, M0 Previous treatment: radical prostatectomy and bilateral pelvic lymphadenectomy, no previous hormonal therapy Number randomised: 100 patients Withdrawals and exclusions: • 1 did not undergo prostatectomy • 1 did not undergo lymphadenectomy Subgroup measured: ‐ Subgroup reported: ‐ Age: • median all patients (n = 98): 65.6 years (range: 45 to 78 years) • median immediate group (n = 47): 65.1 years (range: 52 to 75 years) • median observation group (n = 51): 66.6 years (range: 45 to 78 years) Baseline imbalances: ‐ |
|
| Interventions |
Early ADT (intervention group):
Deferred ADT (control group):
|
|
| Outcomes |
Primary outcome(s):
Secondary outcome(s):
|
|
| Funding sources | This study was supported in part by Public Health Service grants from the National Cancer Institute, National Institutes of Health, and the Department of Health and Human Services | |
| Declaration of interest | The authors declare no conflicts of interest. | |
| Notes | The trial was underpowered: the trial was initially planned for 220 lymph node‐positive patients but was stopped early after inclusion of 100 of which only 98 were randomised. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Information from publication: "randomly assigned by use of a permuted blocks algorithm that was balanced by institution and stratified by choice of type of ADT". Comment: We assume that randomisation was performed adequately at the central randomisation desk of the Eastern Cooperative Oncology Group (ECOG). However the process of selecting the blocks was not specified and there is therefore unclear risk of bias. |
| Allocation concealment (selection bias) | Low risk |
Information from publication: "centrally by telephone by personnel at the central randomisation desk of the Eastern Cooperative Oncology Group (ECOG), who had no further role in the trial. Participants and investigators could not foresee assignment". Comment: Adequate allocation concealment. |
| Blinding of participants and personnel (performance bias) Time to death of any cause | Unclear risk |
Information from publication: There was no blinding. Comment: It might be conceivable that even time to death of any cause is influenced by lack of blinding. We finally judge that there is an unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All other outcomes | High risk |
Information from publication: There was no blinding. Comment: We judge that time to disease progression, time to death from prostate cancer and adverse events are likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Time to death from any cause | Low risk |
Information from publication: There was no blinding of outcome assessment (or it was not reported). Comment: Blinding of outcome assessment could have been expected (only pathologists were blinded). However, we judge that it is not likely that outcome assessment for time to death of any cause is influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All other outcomes | High risk |
Information from publication: There was no blinding of outcome assessment (or it was not reported). Comment: Blinding of outcome assessment could have been expected (only pathologists were blinded). We judge that outcome assessment of time to disease progression, time to death from prostate cancer and adverse events is likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) Oncological outcomes (Time‐to‐death of any cause, Time‐to‐disease progression, Time‐to‐death from prostate cancer) | Low risk | All participants were included in analyses. |
| Incomplete outcome data (attrition bias) Adverse events (Serious and other adverse events) | Low risk | All participants were included in analyses. |
| Incomplete outcome data (attrition bias) Quality of life | Unclear risk | Not applicable (outcome not measured/reported). |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available. |
| Other bias | Unclear risk | We identified no other sources of bias. |
Granfors 2006.
| Methods |
Design: randomised controlled trial Setting: multicentric Recruiting period: 1986 to 1991 Sample size: 91 patients Follow‐up (months): median follow‐up: 9.7 years for all patients, 16.5 years for survivors |
|
| Participants |
Population description: newly diagnosed clinical localized prostate cancer with or without pelvic lymph node involvement (only patients with lymph node involvement were included in this review) Inclusion criteria: patients < 76 years old with newly diagnosed, clinically localized prostatic adenocarcinoma Exclusion criteria:
Tumour stage: T1‐4, pN0‐3, M0 Previous treatment: no previous curative treatment but all patients underwent bilateral staging pelvic lymphadenectomy as an open procedure Number randomised: 91 patients (only patients with lymph‐node positive disease were included: early ADT n = 20; deferred ADT n = 19). Withdrawals and exclusions: not reported Subgroup measured: not reported Subgroup reported: not reported Age: Mean: 68.8 years (range: 49.2 to 75.3 years) Baseline imbalances: not reported |
|
| Interventions |
Early ADT (intervention group):
Deferred ADT (control group):
|
|
| Outcomes |
Primary outcome(s):
Secondary outcome(s)
|
|
| Funding sources | not reported | |
| Declaration of interest | not reported | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Information from publication: Information not reported. Comment: Unclear random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk |
Information from publication: Information not reported. Comment: Unclear allocation concealment. |
| Blinding of participants and personnel (performance bias) Time to death of any cause | Unclear risk |
Information from publication: There was no blinding. Comment: It might be conceivable that even time to death of any cause is influenced by lack of blinding. We finally judge that there is an unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All other outcomes | High risk |
Information from publication: There was no blinding. Comment: Time to disease progression was not reported for the subgroup of patients with lymph‐node‐positive disease. Data for clinical progression are reported descriptively and are not included in meta‐analysis. We judge that clinical progression is likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Time to death from any cause | Low risk |
Information from publication: There was no blinding of outcome assessment (or it was not reported). Comment: Blinding of outcome assessment could have been expected. However, we judge that it is not likely that outcome assessment for time‐to‐death of any cause is influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All other outcomes | High risk |
Information from publication: There was no blinding of outcome assessment (or it was not reported). Comment: Time to disease progression was not reported for the subgroup of patients with lymph‐node positive disease. Data for clinical progression are reported descriptively and are not included in meta‐analysis. Blinding of outcome assessment could have been expected. We judge that outcome assessment of clinical progression is likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) Oncological outcomes (Time‐to‐death of any cause, Time‐to‐disease progression, Time‐to‐death from prostate cancer) | Unclear risk | We found no evidence for missing outcome data for all patients. However, we included only patients with lymph‐node positive disease leading to unclear risk of bias. |
| Incomplete outcome data (attrition bias) Adverse events (Serious and other adverse events) | Unclear risk | Not applicable (outcome not measured/reported). |
| Incomplete outcome data (attrition bias) Quality of life | Unclear risk | Not applicable (outcome not measured/reported). |
| Selective reporting (reporting bias) | High risk | There was no assessment of adverse events but it could have been expected or adverse events were measured but not reported. Data regarding time to disease progression and time to death from prostate cancer were not reported for lymph‐node‐positive patients. |
| Other bias | Unclear risk | We identified no other sources of bias. |
MRC.
| Methods |
Design: randomised controlled trial Setting: multicentric Recruiting period: 1985 to 1993 Sample size: 934 patients Follow‐up (months): each year, shortly after the anniversary of entry (duration of follow‐up is not reported) |
|
| Participants |
Population description: locally advanced or asymptomatic metastatic prostate cancer Inclusion criteria:
Exclusion criteria:
Tumour stage: T2‐T4, M0‐M1, Mx (patients with no evidence of metastatic disease, but with no confirmation by a bone scan ) Previous treatment: patients could undergo a therapeutic or diagnostic TURP or radiotherapy Number randomised: 934 patients Withdrawals and exclusions: analysis by intention‐to‐treat Subgroup measured: metastatic disease (M1), advanced but non‐metastatic disease (M0) and patients with no evidence of metastatic disease, but with no confirmation by a bone scan (Mx; n = 174). Because of the number of patients with uncertain disease classification, we included data of all patients irrespective of subgroups. Subgroup reported: metastatic disease (M1), advanced but non‐metastatic disease (M0) and patients with no evidence of metastatic disease, but with no confirmation by a bone scan (Mx) Age: not reported Baseline imbalances: a ‘minimization’ algorithm used to limit chance differences between groups in age, T category and metastatic status |
|
| Interventions |
Early ADT (intervention group):
Deferred ADT (control group):
|
|
| Outcomes |
Primary outcome(s):
|
|
| Funding sources | not reported | |
| Declaration of interest | not reported | |
| Notes | Participants otherwise managed their patients according to their clinical practice. In the hope that a substantial number of busy working urologists could be recruited, entry and follow‐up were simplified as much as possible, and only data considered relevant to the main issue were collected. As an aid to recruitment, it was intended to simplify registration and to allow investigators to adopt as much of their routine practice as possible. It transpired that many British urologists did not have ready access to bone‐scan facilities. Thus, the simple stratification into M0 and M1 disease envisaged in the protocol had to be modified. An additional category, Mx, was introduced and the categories defined as: M0, patients with no evidence of metastatic disease, confirmed by a negative bone scan; Mx, patients with no evidence of metastatic disease, but with no confirmation by a bone scan; M1, patients with definite scintigraphic, radiological or other evidence of metastatic disease. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Information from publication: Information not reported. Comment: It was only reported that during the registration/randomisation telephone call essential baseline details were recorded on computer and a ‘minimization’ algorithm used to limit chance differences between groups in age, T category and metastatic status. |
| Allocation concealment (selection bias) | Unclear risk |
Information from publication: Information not reported. Comment: It was only reported that patients were registered and randomised by a single telephone call to the trial office. |
| Blinding of participants and personnel (performance bias) Time to death of any cause | Unclear risk |
Information from publication: There was no blinding. Comment: It might be conceivable that even time to death of any cause is influenced by lack of blinding. We finally judge that there is an unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All other outcomes | High risk |
Information from publication: There was no blinding. Comment: We judge that time to disease progression, time to death from prostate cancer and adverse events are likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Time to death from any cause | Low risk |
Information from publication: There was no blinding of outcome assessment (or it was not reported). Comment: Blinding of outcome assessment could have been expected. However, we judge that it is not likely that time to death of any cause is influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All other outcomes | High risk |
Information from publication: There was no blinding of outcome assessment (or it was not reported). Comment: Blinding of outcome assessment could have been expected. We judge that outcome assessment of time to disease progression, time to death from prostate cancer and adverse events is likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) Oncological outcomes (Time‐to‐death of any cause, Time‐to‐disease progression, Time‐to‐death from prostate cancer) | Low risk | There is no evidence for missing outcome data; all patients randomised were included in the analyses. However, no data for disease progression were reported for all included participants (M1+M0); only participants with M0 disease were reported. |
| Incomplete outcome data (attrition bias) Adverse events (Serious and other adverse events) | Unclear risk | Not applicable (outcome was not measured/reported). |
| Incomplete outcome data (attrition bias) Quality of life | Unclear risk | Not applicable (outcome was not measured/reported). |
| Selective reporting (reporting bias) | Unclear risk | The protocol is not available but we think that all outcomes were reported. The methodology of the study was not planned for evaluating adverse events. However, it could have been expected for a randomised controlled trial leading to unclear risk of bias. |
| Other bias | Unclear risk | We identified no other sources of bias. |
RTOG 85‐31.
| Methods |
Design: prospective randomised controlled trial Setting: multicentric Recruiting period: 1987 to 1992 Sample size: 977 patients Follow‐up (months):
|
|
| Participants |
Population description: patients with clinical T3 tumour or involvement of the regional lymph nodes. Lymph node assessment was mandatory and could be performed by either lymphangiogram, computed tomography, or lymphadenectomy. Inclusion criteria: Patients with histologically confirmed adenocarcinoma of the prostate who:
Exclusion criteria:
Tumour stage:
Previous treatment:
Number randomised: 977 patients Withdrawals and exclusions: 32 patients (retrospectively classified as ineligible) Subgroup measured: patients with node positive adenocarcinoma Subgroup reported: patients with node positive adenocarcinoma Age: not reported Baseline imbalances: not reported |
|
| Interventions |
Early ADT (intervention group):
Deferred ADT (control group):
|
|
| Outcomes |
Primary outcome(s):
Secondary outcome(s)
|
|
| Funding sources | Not reported | |
| Declaration of interest | The authors indicated no potential conflicts of interest | |
| Notes | Authors also present data regarding progression‐free survival with PSA level less than 1.5 ng/ml. However, we did not include these results because approximately 40% of patients had no initial PSA values. PSA testing was not mandatory at the inception of the study because it was not widely available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Information from publication: "random number generator". Comment: Adequate random sequence generation. |
| Allocation concealment (selection bias) | Low risk |
Information from publication: "central allocation". Comment: Adequate allocation concealment. |
| Blinding of participants and personnel (performance bias) Time to death of any cause | Unclear risk |
Information from publication: There was no blinding. Comment: It might be conceivable that even time to death of any cause is influenced by lack of blinding. We finally judge that there is an unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All other outcomes | High risk |
Information from publication: There was no blinding. Comment: We judge that time to death from prostate cancer is likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Time to death from any cause | Low risk |
Information from publication: There was no blinding of outcome assessment (or it was not reported). Comment: Blinding of outcome assessment could have been expected. However, we judge that it is not likely that outcome assessment for time to death of any cause is influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All other outcomes | High risk |
Information from publication: There was no blinding of outcome assessment (or it was not reported). Comment: Blinding of outcome assessment could have been expected. We judge that outcome assessment of time to death from prostate cancer is likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) Oncological outcomes (Time‐to‐death of any cause, Time‐to‐disease progression, Time‐to‐death from prostate cancer) | Low risk | For early ADT, 488 patients were randomised and 477 (97.7%) were in analysis. For deferred ADT, 489 patients were randomised and 468 (95.7%) were in analysis. The proportion of patients that were not in analysis is less than 10% and risk of attrition bias is therefore likely to be low. |
| Incomplete outcome data (attrition bias) Adverse events (Serious and other adverse events) | Unclear risk | Not applicable (outcome not measured/reported). |
| Incomplete outcome data (attrition bias) Quality of life | Unclear risk | Not applicable (outcome not measured/reported). |
| Selective reporting (reporting bias) | High risk | There was no assessment of adverse events but it could have been expected or adverse events were measured but not reported. Adverse events were only reported incompletely for a minor subgroup of patients. However, data could not be included in this review. |
| Other bias | Unclear risk | We identified no other sources of bias. |
SAKK 08/88.
| Methods |
Design: randomized controlled trial Setting: multicentric Recruiting period: 1988 to 1992 Sample size: 197 patients Follow‐up (months): not reported |
|
| Participants |
Population description: patients with T0‐4, N0‐2, M0‐1 newly diagnosed asymptomatic prostate cancer without previous treatment not suitable or unwilling for local curative therapy Inclusion criteria:
Exclusion criteria:
Tumour stage: T0‐4, N0‐2, M0‐1 Previous treatment: no previous treatment Number randomised: 197 patients Withdrawals and exclusions: 9 patients (4 in immediate arm; 5 in deferred arm) Subgroup measured: M0 vs. M1, WHO performance 0‐1 vs. 2, tumour stage T0‐2 vs. T3‐4, lymph node status N0 vs N1‐2 Subgroup reported: not reported Age:
Baseline imbalances: no significant differences between the groups |
|
| Interventions |
Early ADT (intervention group):
Deferred ADT (control group):
|
|
| Outcomes |
Primary outcome(s):
Secondary outcome(s):
|
|
| Funding sources | Not reported | |
| Declaration of interest | The author indicated no potential conflicts of interest | |
| Notes | Patient accrual was stopped prematurely because of similar competing trial: the trial was closed in February 1992 because the European Organization for Research and Treatment of Cancer trial 30891 with a similar objective, but including only M0 patients, was opened at that time. To avoid selection bias with predominantly M1 patients in this SAKK 08/88 trial, it was closed prematurely, but the observation time was prolonged until more than 90% of patients had died. This allowed the acquisition of the necessary number of events for an adequate statistical power of 88%. The power analysis was based on a sample of 188 patients, the achieved total of 172 events, an accrual duration of 4 years, and a hypothesized difference of 15% in 5‐year overall survival. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Information from publication: Information was not reported. Comment: We assume that randomisation was performed adequately at the Swiss Group for Clinical Cancer Research (SAKK) coordinating centre. However, information was not reported and there is therefore unclear risk of bias. |
| Allocation concealment (selection bias) | Low risk |
Information from publication: "Central allocation"..."Registration was performed at the Swiss Group for Clinical Cancer Research (SAKK) coordinating centre (Bern, Switzerland) by telephone". Comment: Adequate allocation concealment. |
| Blinding of participants and personnel (performance bias) Time to death of any cause | Unclear risk |
Information from publication: There was no blinding. Comment: It might be conceivable that even time to death of any cause is influenced by lack of blinding. We finally judge that there is an unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All other outcomes | High risk |
Information from publication: There was no blinding. Comment: We judge that time to disease progression, time to death from prostate cancer and adverse events are likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Time to death from any cause | Low risk |
Information from publication: There was no blinding of outcome assessment (or it was not reported). Comment: Blinding of outcome assessment could have been expected. However, we judge that it is not likely that outcome assessment for time to death of any cause is influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All other outcomes | High risk |
Information from publication: There was no blinding of outcome assessment (or it was not reported). Comment: Blinding of outcome assessment could have been expected. We judge that outcome assessment of time to disease progression, time to death from prostate cancer and adverse events is likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) Oncological outcomes (Time‐to‐death of any cause, Time‐to‐disease progression, Time‐to‐death from prostate cancer) | Low risk | For early ADT, 100 patients were randomised and 96 (96%) were in analysis. For deferred ADT, 97 patients were randomised and 92 (94.8%) were in analysis. The proportion of patients that were not in analysis is less than 10% and risk of attrition bias is therefore likely to be low. |
| Incomplete outcome data (attrition bias) Adverse events (Serious and other adverse events) | Low risk | For early ADT, 100 patients were randomised and 96 (96 %) were in analysis. For deferred ADT, 97 patients were randomised and 92 (94.8%) were in analysis. The proportion of patients that were not in analysis is less than 10% and risk of attrition bias is therefore likely to be low. |
| Incomplete outcome data (attrition bias) Quality of life | Unclear risk | Not applicable (outcome not measured/reported). |
| Selective reporting (reporting bias) | Unclear risk | Adverse events were measured but we assume that they are only partially reported leading to unclear risk of bias. |
| Other bias | Unclear risk | We identified no other sources of bias. |
TROG 03.06/VCOG PR 0103.
| Methods |
Design: randomised phase 3 trial, randomly assigned in 1:1 ratio Setting: multicentric (29 public and private cancer centres across Australia, New Zealand, and Canada) Recruiting period: 2004 to 2012 Sample size: 293 patients Follow‐up (months): median follow‐up: 5 years |
|
| Participants |
Population description: patients with a histologically confirmed diagnosis of adenocarcinoma of the prostate who either had a PSA relapse after previous attempted curative therapy or asymptomatic men who were not considered suitable for curative treatment Inclusion criteria:
Exclusion criteria:
Tumour stage:
Previous treatment:
Number randomised: 293 patients (group 1: 261; group 2: 32) Withdrawals and exclusions: group 1: 2 withdrawals; group 2: 1 withdrawal
Subgroup measured: Subgroup analysis of overall survival of patients in group 1 and group 2 were planned. But because of the small numbers accrued to group 2, an analysis of overall survival for this subgroup was not performed. Subgroup reported: ‐ Age:
Baseline imbalances:
|
|
| Interventions |
Early ADT (intervention group):
Deferred ADT (control group):
|
|
| Outcomes |
Primary outcome(s):
Secondary outcome(s):
|
|
| Funding sources | Australian National Health and Medical Research Council and Cancer Councils, The Royal Australian and New Zealand College of Radiologists, educational grant for data management from Mayne Pharma Australia Role of funding sources The sponsor employed staff involved in the conduct and analysis of data in this report. The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all of the data and the final responsibility to submit for publication. |
|
| Declaration of interest | GD reports grants from the NHMRC, grants from various Cancer Councils, grants from the RANZCR, grants from Mayne Pharma, during conduct of study. HW reports personal fees from Janssen for panel participation, personal fees from Astellas for speaking, and travel expenses as an invited conference speaker from GlaxoSmithKline. AL reports personal fees for CME talks from AstraZeneca, personal fees for CME talks, travel, and advisory board membership from AbbVie; advisory board membership from Ferring; and grants and advisory board membership from Sanofi. NS reports grants from Abbot Pharma and Tolmar during the conduct of the study and personal fees from AstraZeneca. MS reports grants and personal fees for travel from Astellas. All other authors declare no competing interests. |
|
| Notes | "At study commencement we used the American Society for Radiation Oncology (ASTRO) definition of PSA failure for men who relapsed after radiotherapy (three successive PSA rises after the nadir, with the date of relapse back‐dated to midway between nadir and the first rise). In 2009 we amended this to the Phoenix definition (≥2 μg/L above nadir) to reflect contemporary practice." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Information from publication: "randomisation was coordinated by the Cancer Council Victoria (Melbourne, VIC, Australia) using a database‐embedded, dynamically balanced, randomisation method"..."The computer system algorithm balanced the stratification factors without need for permuted blocks". Comment: Adequate random sequence generation. |
| Allocation concealment (selection bias) | Low risk |
Information from publication: "A computer algorithm randomly assigned the participants to groups centrally". Comment: Adequate allocation concealment. |
| Blinding of participants and personnel (performance bias) Time to death of any cause | Unclear risk |
Information from publication: There was no blinding. Comment: It might be conceivable that even time to death of any cause is influenced by lack of blinding. We finally judge that there is an unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All other outcomes | High risk |
Information from publication: There was no blinding. Comment: We judge that time to disease progression, time to death from prostate cancer, adverse events and quality of life are likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Time to death from any cause | Low risk |
Information from publication: There was no blinding of outcome assessment (or it was not reported). Comment: Blinding of outcome assessment could have been expected. However, we judge that it is not likely that outcome assessment for time to death of any cause is influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All other outcomes | High risk |
Information from publication: There was no blinding of outcome assessment (or it was not reported). Comment: Blinding of outcome assessment could have been expected. We judge that outcome assessment of time to disease progression, time to death from prostate cancer, adverse events and quality of life is likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) Oncological outcomes (Time‐to‐death of any cause, Time‐to‐disease progression, Time‐to‐death from prostate cancer) | Low risk | No evidence for missing outcome data for time to death and time to death from prostate cancer. For time to disease progression, missing outcome data are balanced in numbers across intervention groups with similar reasons for missing data across groups (Randomised: early ADT: 142, deferred: 151. In evaluation: early ADT: 140, deferred ADT: 150). We judge that this number of withdrawals is not enough to have a clinically relevant effect. |
| Incomplete outcome data (attrition bias) Adverse events (Serious and other adverse events) | Low risk | Missing outcome data are balanced in numbers across intervention groups with similar reasons for missing data across groups (Randomised: early ADT: 142, deferred: 151. In evaluation: early ADT: 140, deferred ADT: 150). We judge that this number of withdrawals is not enough to have a clinically relevant effect. |
| Incomplete outcome data (attrition bias) Quality of life | Low risk | More than 90% of participants completed quality‐of‐life questionnaires at each visit, with no differences in completion rates between the 2 arms. |
| Selective reporting (reporting bias) | Low risk | All predefined outcomes were reported. |
| Other bias | Unclear risk | We identified no other sources of bias. |
VACURG.
| Methods |
Design: 3 prospective randomised clinical trials Setting: multicentric Recruiting period: 1960 to 1975 (study 1: 1960 to 1967; study 2: 1967 to 1969; study 3: 1969 to 1975) Sample size: 3433 patients (study 1: 1902; study 2: 508; study 3: 1023) Follow‐up (months): not reported |
|
| Participants |
Population description: patients with histologically confirmed prostate cancer stage I to IV whose condition had been newly diagnosed Inclusion criteria:
No patients had staging laparotomies and bone scans were not used in staging. Exclusion criteria: not reported Tumour stage: stage I to IV Previous treatment: no previous treatment reported Number randomised: 3433 patients Withdrawals and exclusions: study 2 had to stop after a few years because 5.0 mg oestrogens were too hazardous Subgroup measured: not reported Subgroup reported: not reported Age: not reported Baseline imbalances: no baseline imbalances reported |
|
| Interventions | VACURG study consisted of 3 prospective randomised clinical trials that were analysed separately (we included only study 1): STUDY 1 Early ADT (intervention group):
Deferred ADT (control group):
STUDY 2 (not included in this review) Early ADT (intervention group)
Deferred ADT (control group):
STUDY 3 (not included in this review) Early ADT (intervention group)
Deferred ADT (control group):
|
|
| Outcomes |
Primary outcome(s):
|
|
| Funding sources | Grant R10 CA12443 from the National Cancer Institute, National Institutes of Health, Public Health Service, Bethesda, MD. | |
| Declaration of interest | not reported | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Information from publication: Information was not reported. Comment: Unclear random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk |
Information from publication: Information was not reported. Comment: Unclear allocation concealment. |
| Blinding of participants and personnel (performance bias) Time to death of any cause | Unclear risk |
Information from publication: "Patients received a placebo treatment" (placebo with orchiectomy vs. placebo without orchiectomy). Comment: However, there was no blinding regarding orchiectomy (such as a placebo operation). It might be conceivable that even time to death of any cause is influenced by lack of blinding. We finally judge that there is an unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All other outcomes | High risk |
Information from publication: "Patients received a placebo treatment" (placebo with orchiectomy vs. placebo without orchiectomy). Comment: However, there was no blinding regarding orchiectomy (such as a placebo operation). Patients received a placebo treatment (orchiectomy + placebo vs. placebo). However, blinding was not reported and there was no blinding for orchiectomy. We judge that adverse events are likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Time to death from any cause | Low risk |
Information from publication: There was no blinding of outcome assessment (or it was not reported). Comment: Blinding of outcome assessment could have been expected. However, we judge that it is not likely that outcome assessment for time to death of any cause is influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All other outcomes | High risk |
Information from publication: There was no blinding of outcome assessment (or it was not reported). Comment: Blinding of outcome assessment could have been expected. We judge that outcome assessment of adverse events is likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) Oncological outcomes (Time‐to‐death of any cause, Time‐to‐disease progression, Time‐to‐death from prostate cancer) | Unclear risk | There is no evidence for missing outcome data for time to death of any cause. However, we included only prostate cancer patients from study 1 with metastatic disease treated with placebo or with orchiectomy + placebo. We did not include patients from study 2 or 3 or patients receiving oestrogens for treating prostate cancer. There is therefore unclear risk of bias. |
| Incomplete outcome data (attrition bias) Adverse events (Serious and other adverse events) | Unclear risk | Not applicable (outcome not measured/reported). Only death due to heart or vascular disease was reported. |
| Incomplete outcome data (attrition bias) Quality of life | Unclear risk | Not applicable (outcome not measured/reported). |
| Selective reporting (reporting bias) | High risk | There was no assessment of adverse events (only for death due to heart or vascular disease) but it could have been expected or adverse events were measured but not reported. |
| Other bias | Unclear risk | We identified no other sources of bias. |
p.o. = per os
s.c. = subcutaneous
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ahmed 2002 | Wrong study design |
| Akaza 2003 | Wrong study design |
| Allepuz Losa 1999 | Wrong study design |
| Alyea 1945 | Wrong study design |
| Anderson 1999 | Wrong study design |
| Anderson 2004 | Wrong study design |
| Barnes 1981 | Wrong study design |
| Bennett 1999 | Wrong study design |
| Bennett 2008 | Wrong study design |
| Bertaccini 2012 | Wrong study design |
| Bertelli 1990 | Wrong study design |
| Bex 1998 | Wrong study design |
| Bhayani 1999 | Wrong study design |
| Bishop 2003 | Wrong study design |
| Black 2007 | Wrong indication |
| Blasko 1997 | Wrong study design |
| Blom 1992 | Wrong study design |
| Blood 2010 | Wrong patient population |
| Boccon‐Gibod 2003 | Wrong study design |
| Boccon‐Gibod 2005 | Wrong study design |
| Boccon‐Gibod 2010 | Wrong study design |
| Boehmer 2008 | Wrong study design |
| Bolla 1997 | Wrong intervention |
| Bolla 1999a | Wrong intervention |
| Bolla 1999b | Wrong intervention |
| Bolla 2002 | Wrong intervention |
| Bolla 2010 | Wrong intervention |
| Bolla 2012 | Wrong intervention |
| Bonard 1966 | Wrong study design |
| Bott 2004 | Wrong study design |
| Bourke 2013 | Wrong study design |
| Boustead 2007 | Wrong study design |
| Boyer 1996 | Wrong study design |
| Brower 2008 | Wrong study design |
| Bruce 2012 | Wrong study design |
| Christensen 1990 | Wrong study design |
| Cookson 1994 | Wrong study design |
| D'Amico 2004 | Wrong intervention |
| D'Amico 2008 | Wrong intervention |
| deKernion 1990 | Wrong study design |
| Duchesne 2006 | Wrong study design |
| Garcia‐Albeniz 2015 | Observational study |
| Grossman 1986 | Wrong study design |
| Herr 1993 | Wrong study design |
| Hinkelbein 1998 | Wrong study design |
| Horwitz 2008 | Wrong intervention |
| Kim 2010 | Wrong study design |
| Konski 2005 | Wrong intervention |
| Kozlowski 1991 | Wrong study design |
| Lawton 2008 | Wrong patient population |
| Makarov 2006 | Wrong study design |
| Mickisch 2001 | Wrong study design |
| Newling 2001 | Wrong study design |
| Newling 2003 | Wrong study design |
| Pilepich 1995 | Wrong intervention |
| Pilepich 2001 | Wrong intervention |
| Prezioso 2014 | Wrong study design |
| Richie 1997 | Wrong study design |
| Schellhammer 2006 | Wrong study design |
| Scher 1997 | Wrong study design |
| Schröder 1989 | Wrong study design |
| Schröder 2004 | Wrong intervention |
| Shipley 2001 | Wrong intervention |
| Sieber 2004 | Wrong intervention |
| Tyrrell 1998 | Wrong study design |
| van Aubel 1985 | Wrong study design |
| Van Cangh 2000 | Wrong study design |
| Wirth 2003a | Wrong study design |
| Wirth 2003b | Wrong study design |
| Wirth 2003c | Wrong patient population (Patients with locally advanced disease treated with adjuvant androgen suppression therapy after local therapy not fitting to predefined inclusion criteria) |
| Zagars 1988 | Wrong study design |
| Zierhut 1998 | Wrong study design |
| Zlotta 2006 | Wrong study design |
| Zubek 2009 | Wrong study design |
Differences between protocol and review
This is an update of a Cochrane Review initially published in 2002 (Nair 2002). For this update we adapted methodology to current Cochrane standards, which required extensive changes including a new search strategy, the use of GRADE and the inclusion of a 'Summary of findings' table for the most patient‐important outcomes. During data extraction, we renamed the outcome 'quality of life' to 'global quality of life'. We identified seven new randomised controlled trials since the original review was published in 2002 (Nair 2002). We changed the title to 'Early versus deferred standard androgen suppression therapy for advanced hormone‐sensitive prostate cancer'.
Contributions of authors
Frank Kunath: development methodology, trial selection, literature screening, data extraction, data analysis, risk of bias assessment, data interpretation, clinical expertise
Katrin Jensen: data analysis, data interpretation
Mariona Pinart: literature screening, trial selection, data extraction, 'Risk of bias' assessment
Andreas Kahlmeyer: update of literature screening, interpretation of data, clinical expertise
Stefanie Schmidt: trial selection, data extraction, 'Risk of bias' assessment
Carrie L Price: search strategy development, literature search
Verena Lieb: coordination of work, interpretation of data, clinical expertise
Philipp Dahm: data interpretation, consultation to resolve discrepancies or disagreements, clinical expertise
All review authors contributed to review drafting.
Sources of support
Internal sources
-
University Hospital Erlangen, Germany.
Salary support for Frank Kunath, Andreas Kahlmeyer, Verena Lieb
-
University of Minnesota, Minenapolis, USA.
Salary support for Philipp Dahm
-
Deutsche Gesellschaft für Urologie (German Association of Urology), Germany.
Salary support for Stefanie Schmidt
-
Welch Medical Library, John Hopkins Medical Institution, Baltimore, Maryland, USA.
Slary support for Carrie L. Price
External sources
-
German Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung, BMBF, 01KG1706), Germany.
Grant support for Mariona Pinart, Katrin Jensen
Declarations of interest
The present work was supported by a grant from the German Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung, BMBF, Förderkennzeichen 01KG1706).
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
EORTC 30846 {published data only}
- Kurth K, Schröder F, Fossa S, Hoekstra W, Karthaus P, Prijk L, et al. Early versus delayed endocrine treatment of T2‐T3 pN1‐3 M0 prostate cancer without local treatment of the primary tumour: final results of European organisation for the Research and Treatment of Cancer protocol 30846 after 13 years of follow‐up. Urology 2009;74:S34‐S35. [DOI] [PubMed] [Google Scholar]
- Schröder FH, Kurth K‐H, Fossa SD, Hoekstra W, Karthaus PP, Prijck L, et al. Early versus delayed endocrine treatment of T2‐T3 pN1‐3 M0 prostate cancer without local treatment of the primary tumour: Final results of European organisation for the research and treatment of cancer protocol 30846 after 13 years of follow‐up (a randomised controlled trial). European Urology 2009;55(1):14‐22. [DOI] [PubMed] [Google Scholar]
- Schröder FH, Kurth KH, Fossa SD, Hoekstra W, Karthaus PPM, Debois M, at al. Early versus delayed endocrine treatment of pN1‐3 M0 prostate cancer without local treatment of the primary tumor: Results of European Organisation for the Research and Treatment of Cancer 30846 ‐ A phase III study. Journal of Urology 2004;172(3):923‐7. [DOI] [PubMed] [Google Scholar]
EORTC 30891 {published data only}
- Studer U, Whelan P, Albrecht W, Wimpissinger F, Casselman J, Reijke TM, et al. Long term results of immediate versus deferred androgen deprivation in patients with no local treatment for T0‐4 N0‐2 M0 prostate cancer (EORTC 30891). Journal of Urology 2011;185:e144. [Google Scholar]
- Studer UE, Collette L, Whelan P, Albrecht W, Casselman J, Reijke T, et al. Which subgroups of patients with newly diagnosed T0‐4 N0‐2 mo prostate cancer not suitable for local treatment with curative intent (EORTC 30891) are at risk to die from prostate cancer and benefit from immediate androgen deprivation?. European Urology Supplements 2007;6(2):27. [Google Scholar]
- Studer UE, Collette L, Whelan P, Albrecht W, Casselman J, Reijke T, et al. Which subgroups of patients are at risk to die from prostate cancer and benefit from immediate androgen deprivation if they are not suitable for local treatment with curative intent of newly diagnosed prostate cancer T0‐4 N0‐2 M0 (EORTC 30891)?. Journal of Urology 2007;177:127. [Google Scholar]
- Studer UE, Hauri D, Dietrich D. Immediate vs deferred hormonal therapy for prostate cancer patients not suitable for curative local treatment. Journal of Urology 2002;167:303. [Google Scholar]
- Studer UE, Whelan P, Albrecht W, Casselman J, Reijke T, Hanri D, et al. Patients with asymptomatic prostate cancer T.0‐4 N.0‐2 M.0 not suitable for local definitive treatment: Do they need immediate androgen deprivation?. European Urology Supplements. 2005; Vol. 4:78.
- Studer UE, Whelan P, Albrecht W, Casselman J, Kurth K, Hauri D, at al. Immediate versus deferred androgen deprivation in patients with asymptomatic prostate cancer T0‐4 NO‐2 MO not suitable for local definitive treatment. Journal of Urology 2005;173:450. [Google Scholar]
- Studer UE, Whelan P, Albrecht W, Casselman J, Reijke T, Hauri D, et al. Immediate or deferred androgen deprivation for patients with prostate cancer not suitable for local treatment with curative intent: European Organisation for Research and Treatment of Cancer (EORTC) Trial 30891. Journal of Clinical Oncology 2006;24(12):1868‐76. [DOI] [PubMed] [Google Scholar]
- Studer UE, Whelan P, Albrecht W, Wimpissinger F, Casselman J, Reijke Th, et al. Immediate or deferred androgen deprivation for patients with prostate cancer and no local treatment of the prostate: Long term results of EORTC 30891. European Urology Supplements 2011;10:254. [Google Scholar]
EPCP {published data only}
- Brawer MK. Bicalutamide as immediate therapy either alone or as adjuvant to standard care of patients with localized or locally advanced prostate cancer: first analysis of the Early Prostate Cancer Program. BJU International 2003;91(6):465‐6. [DOI] [PubMed] [Google Scholar]
- Iversen P. Bicalutamide 150 mg in addition to standard care in patients with early, non‐metastatic prostate cancer: Results from the SPCG‐6 study at a median follow‐up of 5.3 years. Journal of Urology 2004;171:311‐2. [DOI] [PubMed] [Google Scholar]
- Iversen P, Johansson J‐E, Lodding P, Kylmala T, Lundmo P, Klarskov P, et al. Bicalutamide 150 mg in addition to standard care for patients with early non‐metastatic prostate cancer: updated results from the Scandinavian Prostate Cancer Period Group‐6 Study after a median follow‐up period of 7.1 years. Scandinavian Journal of Urology and Nephrology 2006;40(6):441‐52. [DOI] [PubMed] [Google Scholar]
- Iversen P, Johansson J‐E, Lodding P, Lukkarinen O, Lundmo P, Klarskov P, et al. Bicalutamide (150 mg) versus placebo as immediate therapy alone or as adjuvant to therapy with curative intent for early nonmetastatic prostate cancer: 5.3‐year median follow up from the Scandinavian Prostate Cancer Group Study Number 6. Journal of Urology 2004;172(5 Pt 1):1871‐6. [DOI] [PubMed] [Google Scholar]
- Iversen P, McLeod DG, See WA, Morris T, Armstrong J, Wirth MP, et al. Antiandrogen monotherapy in patients with localized or locally advanced prostate cancer: final results from the bicalutamide Early Prostate Cancer programme at a median follow‐up of 9.7 years. BJU International 2010;105(8):1074‐81. [DOI] [PubMed] [Google Scholar]
- Iversen P, Tammela TLJ, Vaage S, Lukkarinen O, Lodding P, Bull‐Njaa T, et al. A randomised comparison of bicalutamide ('Casodex') 150 mg versus placebo as immediate therapy either alone or as adjuvant to standard care for early non‐metastatic prostate cancer ‐ First report from the Scandinavian Prostatic Cancer Group Study No. 6. European Urology 2002;42:204‐11. [DOI] [PubMed] [Google Scholar]
- McLeod DG, Iversen P, See WA, Morris T, Armstrong J, Wirth MP. Bicalutamide 150 mg plus standard care vs standard care alone for early prostate cancer. British Journal of Urology International 2006;97(2):247‐54. [DOI] [PubMed] [Google Scholar]
- See W, McLeod DG, Wirth MP, Iversen P, Morris T, Armstrong J. Bicalutamide 150 mg in addition to standard care delays progression to bone metastases in patients with locally advanced prostate cancer: Analyses from the second analysis of the Early Prostate Cancer program. International Journal of Radiation Oncology, Biology, Physics 2005;63:S286‐S287. [Google Scholar]
- See WA, Iversen P, McLeod DG, Wirth MP, Carroll K, Morris T, et al. Bicalutamide 150 mg in addition to standard care significantly improves prostate specific antigen progression‐free survival in patients with early, non‐metastatic prostate cancer: Median 5.4 years' follow‐up. Journal of Urology 2004;171:280‐1. [Google Scholar]
- See WA, Iversen P, McLeod DG, Wirth MP, Morris T, Carroll K. Bicalutamide 150 mg alone or as adjuvant to standard care significantly improves progression‐free survival in patients with early, non‐metastatic prostate cancer (median 5.4 years' follow‐up). Journal of Urology 2004;71:214. [Google Scholar]
- See WA, Wirth MP, McLeod DG, Iversen P, Klimberg I, Gleason D, et al. Bicalutamide as immediate therapy either alone or as adjuvant to standard care of patients with localized or locally advanced prostate cancer: First analysis of the early prostate cancer program. Journal of Urology 2002;168(2):429‐35. [PubMed] [Google Scholar]
- See WA, Wirth MP, McLeod DG, Iversen P, Klimberg I, Gleason D, et al. Bicalutamide as immediate therapy either alone or as adjuvant to standard care of patients with localized or locally advanced prostate cancer: First analysis of the early prostate cancer program. Journal of Urology 2002;168:2558. [PubMed] [Google Scholar]
- Tyrrell C, Payne H, See W, McLeod D, Wirth MW, Iversen P, et al. Bicalutamide ("Casodex") 150mg as adjuvant to radiotherapy in localized or locally advanced prostate cancer. International Journal of Radiation Oncology, Biology, Physics 2001;51(3 Suppl 1):15‐6. [Google Scholar]
- Tyrrell CJ, Payne H, See WA, McLeod DG, Wirth MP, Iversen P, et al. Bicalutamide ('Casodex') 150 mg as adjuvant to radiotherapy in patients with localised or locally advanced prostate cancer: Results from the randomised Early Prostate Cancer Programme. Radiotherapy and Oncology 2005;76(1):4‐10. [DOI] [PubMed] [Google Scholar]
- Wirth M, See W, McLeod D, Iversen P, Persson B, Carroll K. Bicalutamide ('Casodex') 150 mg as immediate or adjuvant therapy in 8113 men with localized or locally advanced prostate cancer. Proceedings of the American Society of Clinical Oncology 2001;20(Pt 1):177a, Abstract 705. [Google Scholar]
- Wirth M, See WA, McLeod DG, Iversen P, Morris T, Carroll K, et al. Bicalutamide 150 mg in addition to standard care in patients with localized or locally advanced prostate cancer: results from the second analysis of the early prostate cancer program at median followup of 5.4 years. Journal of Urology 2004;172(5 Pt 1):1865‐70. [DOI] [PubMed] [Google Scholar]
- Wirth M, Tyrrell C, Delaere K, Sanchez‐Chapado M, Ramon J, Wallace D, et al. Adjuvant therapy with bicalutamide 150 mg versus standard care alone: Third analysis results from trial 24 of the early prostate cancer programme. European Urology Supplements 2006;5:251. [Google Scholar]
- Wirth M, Tyrrell C, Delaere K, Sanchez‐Chapado M, Ramon J, Wallace DMA, et al. Bicalutamide ('Casodex') 150mg in addition to standard care in patients with nonmetastatic prostate cancer: Updated results from a randomised double‐blind phase III study (median follow‐up 5.1y) in the early prostate cancer programme. Prostate Cancer and Prostatic Diseases 2005;8(2):194‐200. [DOI] [PubMed] [Google Scholar]
- Wirth M, Tyrrell C, Delaere K, Sánchez‐Chapado M, Ramon J, Wallace DM, et al. Bicalutamide (Casodex) 150 mg plus standard care in early non‐metastatic prostate cancer: Results from Early Prostate Cancer Trial 24 at a median 7 years' follow‐up. Prostate Cancer and Prostatic Diseases 2007;10(1):87‐93. [DOI: 10.1038/sj.pcan.4500916] [DOI] [PubMed] [Google Scholar]
- Wirth M, Tyrrell C, Wallace M, Delaere KP, Sánchez‐Chapado M, Ramon J, et al. Bicalutamide (Casodex) 150 mg as immediate therapy in patients with localized or locally advanced prostate cancer significantly reduces the risk of disease progression. Urology 2001;58(2):146‐50. [DOI] [PubMed] [Google Scholar]
EST 3886 {published data only}
- Messing EM, Manola J, Sarosdy M, Wilding G, Crawford D, Kiernan M, et al. Immediate hormonal therapy versus observation after radical prostatectomy and pelvic lymphadenectomy for node positive prostate cancer: At 10 years results of EST3886. Journal of Clinical Oncology 2004;22:399S‐S. [Google Scholar]
- Messing EM, Manola J, Sarosdy M, Wilding G, Crawford ED, Trump D. Immediate hormonal therapy compared with observation after radical prostatectomy and pelvic lymphadenectomy in men with node‐positive prostate cancer. New England Journal of Medicine 1999;341(24):1781‐8. [DOI] [PubMed] [Google Scholar]
- Messing EM, Manola J, Yao J, Kiernan M, Crawford D, Wilding G, et al. Immediate versus deferred androgen deprivation treatment in patients with node‐positive cancer after radical prostatectomy and pelvic lympadenectomy. Lancet Oncology 2006;7(6):472‐9. [DOI] [PubMed] [Google Scholar]
- Messing EM, Monola J, Yao J, Kiernan M, Crawford ED, Wilding G, et al. Immediate vs delayed hormonal therapy (HT) in patients with nodal positive (N+) prostate cancer who had undergone radical prostatectomy (RP) plus pelvic lymphadenectomy (LND): Results of central pathology review (CPR). Journal of Urology 2004;171:383. [Google Scholar]
Granfors 2006 {published data only}
- Granfors T, Modig H, Damber JE, Tomic R. Combined orchiectomy and external radiotherapy versus radiotherapy alone for nonmetastatic prostate cancer with or without pelvic lymph node involvement: A prospective randomized study. Journal of Urology 1998;159(6):2030‐4. [DOI] [PubMed] [Google Scholar]
- Granfors T, Modig H, Damber JE, Tomic R. Long‐Term Followup of a Randomized Study of Locally Advanced Prostate Cancer Treated With Combined Orchiectomy and External Radiotherapy Versus Radiotherapy Alone. Journal of Urology 2006;176(2):544‐7. [DOI] [PubMed] [Google Scholar]
MRC {published data only}
- The Medical Reasearch Council Prostate Cancer Working Party Investigators Group. Immediate versus deferred treatment for advanced prostatic cancer: Initial results of the Medical Research Council trial. British Journal of Urology 1997;79(2):235‐46. [DOI] [PubMed] [Google Scholar]
RTOG 85‐31 {published data only}
- Lawton CA, Winter K, Byhardt R, Sause WT, Hanks GE, Russell AH, et al. Androgen suppression plus radiation versus radiation alone for patients with D1 (pN+) adenocarcinoma of the prostate (results based on a national prospective randomized trial, RTOG 85‐31). International Journal of Radiation Oncology, Biology, Physics 1997;38(5):931‐9. [DOI] [PubMed] [Google Scholar]
- Lawton CA, Winter K, Grignon D, Pilepich MV. Androgen suppression plus radiation versus radiation alone for patients with stage D1/pathologic node‐positive adenocarcinoma of the prostate: Updated results based on national prospective randomized trial Radiation Therapy Oncology Group 85‐31. Journal of Clinical Oncology 2005;23(4):800‐7. [DOI] [PubMed] [Google Scholar]
- Lawton CA, Winter K, Murray K, Machtay M, Mesic JB, Hanks GE, et al. Updated results of the phase III radiation therapy oncology group (RTOG) trial 85‐31 evaluating the potential benefit of androgen suppression following standard radiation therapy for unfavourable prognosis carcinoma of the prostate. International Journal of Radiation Oncology, Biology, Physics 2001;49(4):937‐46. [DOI] [PubMed] [Google Scholar]
- Pilepich MV, Winter K, Lawton C, Krisch RE, Wolkov H, Movsas B, et al. Androgen suppression adjuvant to radiotherapy in carcinoma of the prostate. Long‐term results of Phase III RTOG Study 85‐31. International Journal of Radiation Oncology, Biology, Physics 2003;57(2 Suppl):172‐3. [DOI] [PubMed] [Google Scholar]
SAKK 08/88 {published data only}
- Studer UE, Hauri D, Hanselmann S, Chollet D, Leisinger H‐J, Gasser T, et al. Immediate versus deferred hormonal treatment for patients with prostate cancer who are not suitable for curative local treatment: results of the randomized trial SAKK 08/88. Journal of Clinical Oncology 2004;22(20):4109‐10. [DOI] [PubMed] [Google Scholar]
TROG 03.06/VCOG PR 0103 {published data only}
- Duchesne GM, Bassett J, D'Este C, Frydenberg M, Ledwich L, Millar JL, et al. TROG 03.06 and VCOG PR 0103: The “timing of androgen deprivation therapy in prostate cancer patients with a rising PSA (TOAD)” collaborative randomised phase III trial. Journal of Clinical Oncology 2015;33(15_suppl):5007. [Google Scholar]
- Duchesne GM, Woo HH. The 'Timing of Androgen‐Deprivation therapy in incurable prostate cancer' protocol (TOAD)‐‐where are we now? Synopsis of the Victorian Cooperative Oncology Group PR 01‐03 and Trans‐Tasman Radiation Oncology Group 03.06 clinical trial. BJU International 2014;114(Suppl 1):9‐12. [DOI] [PubMed] [Google Scholar]
- Duchesne GM, Woo HH, Bassett JK, Bowe SJ, D'Este C, Frydenberg M, et al. Timing of androgen‐deprivation therapy in patients with prostate cancer with a rising PSA (TROG 03.06 and VCOG PR 01‐03 [TOAD]): a randomised, multicentre, non‐blinded, phase 3 trial. Lancet Oncology 2016;17(6):727‐37. [DOI] [PubMed] [Google Scholar]
- Duchesne GM, Woo HH, King M, Bowe SJ, Stockler MR, Ames A, et al. Health‐related quality of life for immediate versus delayed androgen‐deprivation therapy in patients with asymptomatic, non‐curable prostate cancer (TROG 03.06 and VCOG PR 01‐03 [TOAD]): a randomised, multicentre, non‐blinded, phase 3 trial. Lancet Oncology 2017;18(9):1192‐201. [DOI] [PubMed] [Google Scholar]
VACURG {published data only}
- Blackard CE, Byar DP, Jordan WP, and the Veterans Administration Cooperative Urological Research Group. Orchiectomy for advanced prostatic carcinoma: a reevaluation. Urology 1973;1(6):553‐60. [DOI] [PubMed] [Google Scholar]
- Byar DP. VACURG studies of conservative treatment. Scandinavian Journal of Urology and Nephrology 1980;55:99‐102. [PubMed] [Google Scholar]
- Byar DP, Corle DK. Hormone therapy for prostate cancer: results of the Veterans Administration Cooperative Urological Research Group studies. NCI monographs: a publication of the National Cancer Institute 1988;7:165‐70. [PubMed] [Google Scholar]
- Hurst KS, Byar DP, and the Veterans Administration Cooperative Urological Research Group. An analysis of the effects of changes from the assigned treatment in a clinical trial of treatment for prostatic cancer. Journal of Chronic Diseases 1973;26(5):311‐24. [DOI] [PubMed] [Google Scholar]
- Veterans Administration Cooperative Urological Research Group. Carcinoma of the prostate: a continuing cooperative study. Journal of Urology 1964;91:590‐4. [Google Scholar]
- Veterans Administration Cooperative Urological Research Group. Treatment and survival of patients with cancer of the prostate. Surgery, Gynecology & Obstetrics 1967;124:1011‐7. [PubMed] [Google Scholar]
References to studies excluded from this review
Ahmed 2002 {published data only}
- Ahmed S, Trump DL. The case for early androgen deprivation: the data should not be ignored. Urologic Oncology 2002;7(2):77‐80. [DOI] [PubMed] [Google Scholar]
Akaza 2003 {published data only}
- Akaza H. Early androgen suppression may reduce disease progression and improve long term survival compared with deferred androgen suppression in locally advanced prostate cancer. Cancer Treatment Reviews 2003;29(3):224‐5. [DOI: 10.1016/S0305-7372(03)00119-1] [DOI] [PubMed] [Google Scholar]
Allepuz Losa 1999 {published data only}
- Allepuz Losa C, Gil Martínez P, Gil Sanz MJ, Rioja Sanz LA. Early versus late hormonal treatment in advanced prostate cancer [Tratamiento hormonal precoz vs tardio en el cancer avanzado de prostata.]. Actas Urologicas Espanolas 1999;23(7):557‐64. [PubMed] [Google Scholar]
Alyea 1945 {published data only}
- Alyea EP. Early or late orchiectomy for carcinoma of the prostate. Journal of Urology 1945;53:143‐53. [Google Scholar]
Anderson 1999 {published data only}
- Anderson JB. Early versus deferred hormone therapy. European Urology 1999;36 Suppl 2:9‐13. [DOI] [PubMed] [Google Scholar]
Anderson 2004 {published data only}
- Anderson J. Bicalutamide 150 mg: practical prescribing in patients with early prostate cancer. BJU international 2004;94:758‐9. [DOI] [PubMed] [Google Scholar]
Barnes 1981 {published data only}
- Barnes R, Hadley H, Bergman RT. Immediate versus delayed endocrine therapy for prostatic carcinoma. Western Journal of Medicine 1981;134:345‐6. [PMC free article] [PubMed] [Google Scholar]
Bennett 1999 {published data only}
- Bennett CL, Tosteson TD, Schmitt B, Weinberg PD, Ernstoff MS, Ross SD. Maximum androgen‐blockade with medical or surgical castration in advanced prostate cancer: A meta‐analysis of nine published randomized controlled trials and 4128 patients using flutamide. Prostate Cancer and Prostatic Diseases 1999;2(1):4‐8. [DOI] [PubMed] [Google Scholar]
Bennett 2008 {published data only}
- Bennett CL, Sartor O, McLeod DG, Halabi S. Effects of age, health‐related quality of life, and education level on management after biochemical failure with watchful waiting versus hormonal therapy in men with prostate cancer: Results from the compare registry. Journal of Urology 2008;179(4, Supplement):109. [DOI: 10.1016/S0022-5347(08)60317-1] [DOI] [Google Scholar]
Bertaccini 2012 {published data only}
- Bertaccini A, Marchiori D. The efficacy of degarelix on LUTS (Lower urinary tract symptoms) relief in patients with prostate cancer. Urologia 2012;79(3):197‐9. [DOI: 10.5301/RU.2012.9687] [DOI] [PubMed] [Google Scholar]
Bertelli 1990 {published data only}
- Bertelli A. Introduction: recent progress in therapy of prostatic carcinoma and other hormone dependent pathology with the use of agonistic analogs of LHRH [Introduzione: recenti progressi nella terapia del carcinoma prostatico e di altre patologie ormonodipendenti con l'impiego di agonisti analoghi all'LHRH]. Drugs under Experimental and Clinical Research 1990;16 Suppl:1‐2. [PubMed] [Google Scholar]
Bex 1998 {published data only}
- Bex A, Rübben H. When to begin with androgen deprivation? [Wann ist mit einer Androgendeprivation zu beginnen?]. Der Urologe. Ausg. A 1998;37(2):133‐4. [DOI] [PubMed] [Google Scholar]
Bhayani 1999 {published data only}
- Bhayani SB, Andriole GL. Hormonal manipulation for rising PSA after radical prostatectomy. Seminars in Urologic Oncology 1999;17(3):148‐53. [PubMed] [Google Scholar]
Bishop 2003 {published data only}
- Bishop M. The role of anti‐androgen monotherapy in the treatment of prostate cancer. BJU International 2003;92(6):653‐4. [DOI] [PubMed] [Google Scholar]
Black 2007 {published data only}
- Black PC, Basen‐Engquist K, Wang X, Swartz RJ, Eddings T, Matin SF, et al. A randomized prospective trial evaluating testosterone, haemoglobin kinetics and quality of life, during and after 12 months of androgen deprivation after prostatectomy: Results from the Postoperative Adjuvant Androgen Deprivation trial. BJU International 2007;100(1):63‐9. [DOI: ] [DOI] [PubMed] [Google Scholar]
Blasko 1997 {published data only}
- Blasko JC, Lange PH. Prostate cancer‐‐the therapeutic challenge of locally advanced disease. New England Journal of Medicine 1997;337(5):340‐1. [DOI] [PubMed] [Google Scholar]
Blom 1992 {published data only}
- Blom JH, Schröder FH. On the management of metastatic prostate cancer with LH‐RH analogs. Recent Results in Cancer Research 1992;124:33‐41. [DOI] [PubMed] [Google Scholar]
Blood 2010 {published data only}
- Blood PA. Neo‐adjuvant androgen deprivation therapy does not increase the risk of cardiovascular death in men treated for prostate cancer with curative intent. International Journal of Radiation Oncology, Biology, Physics 2010;78(3):S150‐1. [Google Scholar]
Boccon‐Gibod 2003 {published data only}
- Boccon‐Gibod L, Bertaccini A, Bono AV, Dev Sarmah B, Höltl W, Mottet N, et al. Management of locally advanced prostate cancer: a European consensus. International Journal of Clinical Practice 2003;57(3):187‐94. [PubMed] [Google Scholar]
Boccon‐Gibod 2005 {published data only}
- Boccon‐Gibod L. Optimising hormone therapy in advanced disease. European Urology Supplements 2005;4:21‐9. [DOI: 10.1016/j.eursup.2005.08.001] [DOI] [Google Scholar]
Boccon‐Gibod 2010 {published data only}
- Boccon‐Gibod L, Richaud P, Coloby P, Coulange C, Culine S, Davin JL, et al. First line indications for hormonal therapy in prostate cancer. Progres en Urologie 2010;20(2):109‐15. [DOI: ] [DOI] [PubMed] [Google Scholar]
Boehmer 2008 {published data only}
- Boehmer D. Combined radiotherapy and hormonal therapy in the treatment of prostate cancer. Frontiers of Radiation Therapy and Oncology 2008;41:26‐31. [DOI: ] [DOI] [PubMed] [Google Scholar]
Bolla 1997 {published data only}
- Bolla M, Gonzalez D, Warde P, Dubois JB, Mirimanoff R, Storme G, et al. Immediate hormonotherapy with an LHRH analogue improves local control and survival in patients with locally advanced prostate cancer treated by radiotherapy. A randomized phase III clinical trial of the EORTC. European Journal of Cancer 1997;33:115. [Google Scholar]
Bolla 1999a {published data only}
- Bolla M, Collette L, Gonzalez D, Warde P, Dubois JB, Mirimanoff R, et al. Long term results of immediate adjuvant hormonal therapy with goserelin in patients with locally advanced prostate cancer treated with radiotherapy ‐ A phase III EORTC study. European Journal of Cancer 1999;35:S82. [DOI: 10.1016/S0959-8049(99)80699-6] [DOI] [Google Scholar]
Bolla 1999b {published data only}
- Bolla M, Collette L, Gonzalez D, Warde P, Dubois JB, Mirimanoff R, et al. Long term results of immediate adjuvant hormonal therapy with goserelin in patients with locally advanced prostate cancer treated with radiotherapy. A phase III EORTC study [abstract]. International Journal of Radiation Oncology Biology Physics 1999;45(3 Suppl):147. [Google Scholar]
Bolla 2002 {published data only}
- Bolla M, Collette L, Blank L, Warde P, Dubois JB, Mirimanoff RO, et al. Long‐term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): A phase III randomised trial. Lancet 2002;360(9327):103‐8. [DOI] [PubMed] [Google Scholar]
Bolla 2010 {published data only}
- Bolla M, Tienhoven G, Warde P, Dubois JB, Mirimanoff RO, Storme G, et al. External irradiation with or without long‐term androgen suppression for prostate cancer with high metastatic risk: 10‐year results of an EORTC randomised study. Lancet Oncology 2010;11(11):1066‐73. [DOI] [PubMed] [Google Scholar]
Bolla 2012 {published data only}
- Bolla M, Laramas M, Association of Radiotherapy and Oncology of the Mediterranean arEa (AROME). Combined hormone therapy and radiation therapy for locally advanced prostate cancer. Critical Review in Oncology and Hematology 2012;84(Suppl 1):30‐34. [DOI] [PubMed] [Google Scholar]
Bonard 1966 {published data only}
- Bonard EC. Hormone and adjuvant therapy in advanced cancers [Le traitement hormonal et adjuvant des cancers avances]. Praxis 1966;55:676‐9. [PubMed] [Google Scholar]
Bott 2004 {published data only}
- Bott SRJ. Management of recurrent disease after radical prostatectomy. Prostate cancer and prostatic diseases 2004;7:211‐6. [DOI: 10.1038/sj.pcan.4500732] [DOI] [PubMed] [Google Scholar]
Bourke 2013 {published data only}
- Bourke L, Kirkbride P, Hooper R, Rosario AJ, Chico TJ, Rosario DJ. Endocrine therapy in prostate cancer: time for reappraisal of risks, benefits and cost‐effectiveness?. British Journal of Cancer 2013;108(1):9‐13. [DOI: 10.1038/bjc.2012.523] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boustead 2007 {published data only}
- Boustead G, Edwards SJ. Systematic review of early vs deferred hormonal treatment of locally advanced prostate cancer: a meta‐analysis of randomized controlled trials. BJU International 2007;99:1383‐9. [DOI] [PubMed] [Google Scholar]
Boyer 1996 {published data only}
- Boyer M. The management of prostate cancer. Australian Prescriber 1996;19(1):22‐4. [Google Scholar]
Brower 2008 {published data only}
- Brower V. Watchful waiting beats androgen deprivation therapy in early prostate cancer. Journal of the National Cancer Institute 2008;100:1494‐6. [DOI] [PubMed] [Google Scholar]
Bruce 2012 {published data only}
- Bruce JY, Lang JM, McNeel DG, Liu G. Current controversies in the management of biochemical failure in prostate cancer. Clinical Advances in Hematology & Oncology 2012;10(11):716‐22. [PubMed] [Google Scholar]
Christensen 1990 {published data only}
- Christensen MM, Aagaard J, Madsen PO. Reasons for delay of endocrine treatment in cancer of the prostate (until symptomatic metastases occur). Progress in Clinical and Biological Research 1990;359:7‐14; discussion 15‐1424. [PubMed] [Google Scholar]
Cookson 1994 {published data only}
- Cookson MS, Sarosdy MF. Hormonal‐therapy for metastatic prostate‐cancer ‐ issues of timing and total androgen ablation. Southern Medical Journal 1994;87(1):1‐6. [DOI] [PubMed] [Google Scholar]
D'Amico 2004 {published data only}
- D'Amico AV, Manola J, Loffredo M, Renshaw AA, DellaCroce A, Kantoff PW. 6‐month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer ‐ A randomized controlled trial. Journal of the American Medical Association 2004;292(7):821‐7. [DOI: 10.1001/jama.292.7.821] [DOI] [PubMed] [Google Scholar]
D'Amico 2008 {published data only}
- D'Amico AV, Chen MH, Renshaw AA, Loffredo M, Kantoff PW. Androgen suppression and radiation vs radiation alone for prostate cancer ‐ A randomized trial. Journal of the American Medical Association 2008;299(3):289‐95. [DOI: 10.1001/jama.299.3.289] [DOI] [PubMed] [Google Scholar]
deKernion 1990 {published data only}
- deKernion JB, Neuwirth H, Stein A, Dorey F, Stenzl A, Hannah J, et al. Prognosis of patients with stage D1 prostate carcinoma following radical prostatectomy with and without early endocrine therapy. Journal of Urology 1990;144:700‐3. [DOI] [DOI] [PubMed] [Google Scholar]
Duchesne 2006 {published data only}
- Duchesne GM, Syme R, Howell D. How early is early: androgen deprivation for prostate‐specific antigen relapse in prostate cancer. Journal of Clinical Oncology 2006;24(18):2964‐5. [DOI] [PubMed] [Google Scholar]
Garcia‐Albeniz 2015 {published data only}
- Garcia‐Albeniz X, Chan JM, Paciorek A, Logan RW, Kenfield SA, Cooperberg MR, et al. Immediate versus deferred initiation of androgen deprivation therapy in prostate cancer patients with PSA‐only relapse. An observational follow‐up study. European Journal of Cancer 2015;51(7):817‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia‐Albeniz X, Chan JM, Paciorek AT, Logan RW, Kenfield SA, Cooperberg MR, et al. Immediate versus deferred initiation of androgen deprivation therapy in prostate cancer patients with PSA‐only relapse. Journal of Clinical Oncology 2014;32(5S):Suppl, abstract 5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grossman 1986 {published data only}
- Grossman HB. Hormonal‐therapy of prostatic‐carcinoma ‐ is there a rationale for delayed treatment. Urology 1986;27:199‐204. [DOI] [PubMed] [Google Scholar]
Herr 1993 {published data only}
- Herr H, Kornblith AB, Ofman U. A comparison of the quality of life of patients with metastatic prostate cancer who received or did not receive hormonal therapy. Cancer 1993;71(3 Suppl):1143‐50. [DOI: 10.1002/1097-0142(19930201)71:3+%3C1143::AID-CNCR2820711437%3E3.0.CO; 2‐I] [DOI] [PubMed] [Google Scholar]
Hinkelbein 1998 {published data only}
- Hinkelbein W. Adjuvant or therapeutic androgen suppression in locoregional advanced prostatic carcinoma (RTOG 85‐31) [Adjuvante oder therapeutische Androgensuppression beim lokoregionar fortgeschrittenen Prostatakarzinom (RTOG 85‐31)]. Strahlentherapie und Onkologie 1998;174:385‐6. [DOI] [PubMed] [Google Scholar]
Horwitz 2008 {published data only}
- Horwitz EM, Bae K, Hanks GE, Porter A, Grignon DJ, Brereton HD, et al. Ten‐year follow‐up of radiation therapy oncology group protocol 92‐02: A phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. Journal of Clinical Oncology 2008;26(15):2497‐504. [DOI: ] [DOI] [PubMed] [Google Scholar]
Kim 2010 {published data only}
- Kim JO, Vaid M, Tyldesley S, Woods R, Pickles T. A population‐based study of cardiovascular (CV) mortality among patients with prostate cancer (PCa) treated with radical external beam radiation therapy (EBRT) with and without adjuvant androgen deprivation therapy (ADT) at a provincial cancer agency. Journal of Clinical Oncology 2010;28(15_suppl):4656. [DOI] [PubMed] [Google Scholar]
Konski 2005 {published data only}
- Konski A, Sherman E, Krahn M, Bremner K, Beck JR, Watkins‐Bruner D, et al. Economic analysis of a phase III clinical trial evaluating the addition of total androgen suppression to radiation versus radiation alone for locally advanced prostate cancer (Radiation Therapy Oncology Group protocol 86‐10). International Journal of Radiation Oncology, Biology, Physics 2005;63(3):788‐94. [DOI: ] [DOI] [PubMed] [Google Scholar]
Kozlowski 1991 {published data only}
- Kozlowski JM, Ellis WJ, Grayhack JT. Advanced prostatic carcinoma. Early versus late endocrine therapy. Urologic Clinics of North America 1991;18(1):15‐24. [PubMed] [Google Scholar]
Lawton 2008 {published data only}
- Lawton CA, Bae K, Pilepich M, Hanks G, Shipley W. Long‐term treatment sequelae after external beam irradiation with or without hormonal manipulation for adenocarcinoma of the prostate: analysis of radiation therapy oncology group studies 85‐31, 86‐10, and 92‐02. International Journal of Radiation Oncology, Biology, Physics 2008;70(2):437‐41. [DOI: 10.1016/j.ijrobp.2007.06.050] [DOI] [PMC free article] [PubMed] [Google Scholar]
Makarov 2006 {published data only}
- Makarov DV, Trock BJ, Humphreys EB, Mangold LA, Carducci MA, Partin AW, et al. Factors influencing prostate cancer specific mortality in patients receiving delayed androgen deprivation therapy for metastasis after biochemical recurrence following radical prostatectomy. Journal of Clinical Oncology 2006;24:abstract 4571. [Google Scholar]
Mickisch 2001 {published data only}
- Mickisch GH. Early versus deferred hormonal treatment for asymptomatic prostate cancer. Onkologie 2001;24:214‐220. [DOI] [PubMed] [Google Scholar]
Newling 2001 {published data only}
- Newling D. Advanced prostate cancer: Immediate or deferred hormone therapy?. European Urology 2001;39:15‐21. [DOI: 10.1159/000052545] [DOI] [PubMed] [Google Scholar]
Newling 2003 {published data only}
- Newling DW. Immediate or deferred hormonal therapy?. Scandinavian Journal of Urology and Nephrology 2003;37:16‐9. [DOI: 10.1080/03008880310006896] [DOI] [PubMed] [Google Scholar]
Pilepich 1995 {published data only}
- Pilepich MV, Krall JM, al‐Sarraf M, John MJ, Doggett RL, Sause WT, et al. Androgen deprivation with radiation‐therapy compared with radiation‐therapy alone for locally advanced prostatic‐aarcinoma ‐ a randomized comparative trial of the radiation‐therapy oncology group. Urology 1995;45:616‐23. [DOI: 10.1016/S0090-4295(99)80053-3] [DOI] [PubMed] [Google Scholar]
Pilepich 2001 {published data only}
- Pilepich MV, Winter K, John MJ, Mesic JB, Sause W, Rubin P, et al. Phase III radiation therapy oncology group (RTOG) trial 86‐10 of androgen deprivation adjuvant to definitive radiotherapy in locally advanced carcinoma of the prostate. International Journal of Radiation Oncology Biology Physics 2001;50(5):1243‐52. [DOI: ] [DOI] [PubMed] [Google Scholar]
Prezioso 2014 {published data only}
- Prezioso D, Iacono F, Romeo G, Ruffo A, Russo N, Illiano E. Early versus delayed hormonal treatment in locally advanced or asymptomatic metastatic prostatic cancer patient dilemma. World Journal of Urology 2014;32(3):661‐7. [DOI: 10.1007/s00345-013-1144-x] [DOI] [PubMed] [Google Scholar]
Richie 1997 {published data only}
- Richie JP. Stage D1 prostate cancer ‐ delayed androgen deprivation. Urology 1997;50:838‐9. [DOI: 10.1016/S0090-4295(97)00544-X] [DOI] [PubMed] [Google Scholar]
Schellhammer 2006 {published data only}
- Schellhammer PF. Timing of androgen deprivation therapy: Some questions answered, others not. Journal of the National Cancer Institute 2006;98(12):802‐3. [DOI] [PubMed] [Google Scholar]
Scher 1997 {published data only}
- Scher HI, Liebertz C, Kelly WK, Mazumdar M, Brett C, Schwartz L, et al. Bicalutamide for advanced prostate cancer: the natural versus treated history of disease. Journal of Clinical Oncology 1997;15(8):2928‐38. [DOI] [PubMed] [Google Scholar]
Schröder 1989 {published data only}
- Schröder FH. Early versus delayed endocrine treatment in metastatic prostatic cancer. Therapeutic Progress in Urological Cancers 1989;303:253‐60. [PubMed] [Google Scholar]
Schröder 2004 {published data only}
- Schröder FH, Whelan P, Reijke TM, Kurth KH, Pavone‐Macaluso M, Mattelaer J, et al. Metastatic prostate cancer treated by flutamide versus cyproterone acetate ‐ Final analysis of the "European organization for research and treatment of cancer" (EORTC) protocol 30892. European Urology 2004;45:457‐64. [DOI: 10.1016/j.eururo.203.11.016] [DOI] [PubMed] [Google Scholar]
Shipley 2001 {published data only}
- Shipley W, Heydon K, Pilepich M. A secondary analysis of RTOG 86‐10: does the extent of progression at the time of initiating salvage hormone therapy influence survival in patients with prostate cancer who failed initial treatment with irradiation? [abstract]. Journal of Clinical Oncology 2001;20 (Pt 1):182a, Abstract 726. [Google Scholar]
Sieber 2004 {published data only}
- Sieber PR, Keiller DL, Kahnoski RJ, Gallo J, McFadden S. Bicalutamide 150 mg maintains bone mineral density during monotherapy for localized or locally advanced prostate cancer. Journal of Urology 2004;171(6 I):2272‐6. [DOI: ] [DOI] [PubMed] [Google Scholar]
Tyrrell 1998 {published data only}
- Tyrrell CJ, Kaisary AV, Iversen P, Anderson JB, Baert L, Tammela T, et al. A randomised comparison of 'Casodex' (R) (bicalutamide) 150 mg monotherapy versus castration in the treatment of metastatic and locally advanced prostate cancer. European Urology 1998;33(5):447‐56. [DOI] [PubMed] [Google Scholar]
van Aubel 1985 {published data only}
- Aubel OG, Hoekstra WJ, Schröder FH. Early orchiectomy for patients with stage D1 prostatic carcinoma. Journal of Urology 1985;134(2):292‐4. [DOI] [PubMed] [Google Scholar]
Van Cangh 2000 {published data only}
- Cangh PJ, Gala JL, Tombal B. Immediate vs. delayed androgen deprivation for prostate cancer. Prostate Supplement 2000;10:19‐25. [DOI] [PubMed] [Google Scholar]
Wirth 2003a {published data only}
- Wirth MP, Weissbach L, Marx FJ, Heckl W, Jellinghaus W, Riedmiller H, et al. Prospective randomized trial comparing flutamide as adjuvant treatment versus observation after radical prostatectomy for locally advanced, lymph node‐negative prostate cancer. European Urology 2004;45(3):267‐70. [DOI] [PubMed] [Google Scholar]
Wirth 2003b {published data only}
- With M, Froehner M.A. A review of studies of hormonal adjuvant therapy in prostate cancer. European Urology 1999;36(Suppl. 2):14‐9. [DOI] [PubMed] [Google Scholar]
Wirth 2003c {published data only}
- Wirth MP, Weissbach L, Marx FJ, Heckl W, Jellinghaus W, Riedmiller H, et al. Prospective randomised trial comparing flutamide as adjuvant treatment versus observation after radical prostatectomy for stage pT3pN0 prostate cancer. Journal of Urology 2003;169:343. [DOI] [PubMed] [Google Scholar]
- With M, Froehner MA. A review of studies of hormonal adjuvant therapy in prostate cancer. European Urology 1999;36(Suppl. 2):14‐19. [DOI] [PubMed] [Google Scholar]
Zagars 1988 {published data only}
- Zagars GK, Johnson DE, Eschenbach AC, Hussey DH. Adjuvant estrogen following radiation therapy for stage C adenocarcinoma of the prostate: Long‐term results of a prospective randomized study. International Journal of Radiation Oncology, Biology, Physics 1988;14(6):1085‐91. [DOI] [PubMed] [Google Scholar]
Zierhut 1998 {published data only}
- Zierhut D. Therapy of nodal positive prostatic carcinoma: when should hormone therapy be started? [Therapie des nodal positiven Prostatakarzinomas: Wann sollte mit der Hormontherapie begonnen werden?]. Strahlentherapie und Onkologie 1998;174(7):382‐3. [DOI] [PubMed] [Google Scholar]
Zlotta 2006 {published data only}
- Zlotta AR, Schulman C. Immediate versus deferred androgen‐deprivation therapy for prostate cancer: the jury is still out. Nature Clinical Practice. Urology 2006;3(9):474‐5. [DOI: 10.1038/ncpuro0579] [DOI] [PubMed] [Google Scholar]
Zubek 2009 {published data only}
- Zubek VB, Konski A. Cost Effectiveness of Risk‐Prediction Tools in Selecting Patients for Immediate Post‐Prostatectomy Treatment. Molecular Diagnosis & Therapy 2009;13(1):31‐47. [DOI] [PubMed] [Google Scholar]
Additional references
Adolfsson 1999
- Adolfsson J. The natural history of early prostate cancer and the impact of endocrine treatment. European Urology 1999;36(Suppl 2):3‐8. [DOI] [PubMed] [Google Scholar]
Cocks 2012
- Cocks K, King MT, Velikova G, Castro Jr. G, Martyn St‐James M, Fayers PM, et al. Evidence‐based guidelines for interpreting change scores for the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30. European Journal of Cancer 2012;48(11):1713–21. [DOI] [PubMed] [Google Scholar]
EAU 2017
- Mottet N, Bellmunt J, Briers E, Bolla M, Bourke L, Cornford P, et al. ESTRO – ESUR – SIOG Prostate Cancer Guidelines Panel. EAU – ESTRO – ESUR – SIOG Guidelines on Prostate Cancer. uroweb.org/guideline/prostate‐cancer (accessed 26 June 2017).
Endnote [Computer program]
- Thomson Reuters; www.endnote.com. Endnote. Version X5 or X6 or X7. Thomson Reuters; www.endnote.com, 1988‐2016.
Gibbs 1996
- Gibbs SJ, Plowman PN. Androgen deprivation and antagonism in the treatment of advanced prostatic carcinoma. Clinical Oncology 1996;8(6):346‐52. [DOI] [PubMed] [Google Scholar]
GLOBOCAN 2012
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. (editors). GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. globocan.iarc.fr (accessed 26 June 2017).
GRADEproGDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEproGDT. Version accessed prior to 19 March 2019. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Schünemann HJ. GRADE: what is "quality of evidence" and why is it important to clinicians?. BMJ (Clinical Research Ed.) 2008;336(7651):995‐8. [DOI: 10.1136/bmj.39490.551019.BE] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [DOI: 10.1016/j.jclinepi.2010.04.026] [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. British Medical Journal 2003;327(7414):557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from handbook.cochrane.org.
Higgins 2011c
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Huggins 2002
- Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. 1941. Journal of Urology 2002;167(2 Pt 2):948‐51. [PubMed] [Google Scholar]
Kunath 2012
- Kunath F. LHRH antagonists versus standard androgen suppression therapy for advanced prostate cancer: a systematic review with comparative safety data analysis. www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42012002751 (accessed 5 April 2015). [DOI] [PMC free article] [PubMed]
Kunath 2013
- Kunath F, Keck B, Rücker G, Motschall E, Wullich B, Antes G, Meerpohl JJ. Early versus deferred androgen suppression therapy for patients with lymph node‐positive prostate cancer after local therapy with curative intent: a systematic review. BMC Cancer 2013;13:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kunath 2014
- Kunath F, Grobe HR, Rücker G, Motschall E, Antes G, Dahm P, et al. Non‐steroidal antiandrogen monotherapy compared with luteinising hormone–releasing hormone agonists or surgical castration monotherapy for advanced prostate cancer. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD009266.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 2009;6(7):e1000100. [DOI: 10.1371/journal.pmed.1000100] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Tierney 2006
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;8:16. [DOI: 10.1186/1745-6215-8-16] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Nair 2002
- Nair B, Wilt T, MacDonald R, Rutks I. Early versus deferred androgen suppression in the treatment of advanced prostatic cancer. Cochrane Database of Systematic Reviews 2002, Issue 1. [DOI: 10.1002/14651858.CD003506] [DOI] [PubMed] [Google Scholar]
Wilt 2001
- Wilt TJ, Nair B, MacDonald R, Rutks I. Early versus deferred androgen suppression in the treatment of advanced prostatic cancer. Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD003506] [DOI] [PubMed] [Google Scholar]


