Abstract
Protein kinases in the Cot-1/Orb6/Ndr/Warts family are important regulators of cell morphogenesis and proliferation. Cbk1p, a member of this family in Saccharomyces cerevisiae, has previously been shown to be required for normal morphogenesis in vegetatively growing cells and in haploid cells responding to mating pheromone. A mutant of PAG1, a novel gene in S. cerevisiae, displayed defects similar to those of cbk1 mutants. pag1 and cbk1 mutants share a common set of suppressors, including the disruption of SSD1, a gene encoding an RNA binding protein, and the overexpression of Sim1p, an extracellular protein. These genetic results suggest that PAG1 and CBK1 act in the same pathway. Furthermore, we found that Pag1p and Cbk1p localize to the same polarized peripheral sites and that they coimmunoprecipitate with each other. Pag1p is a conserved protein. The homologs of Pag1p in other organisms are likely to form complexes with the Cbk1p-related kinases and function with those kinases in the same biological processes.
INTRODUCTION
Cell morphogenesis and proliferation are complex processes controlled by intricate signaling pathways. In recent years, serine/threonine protein kinases in the Cot-1/Orb6/Ndr/Warts family have emerged as important regulators of cell morphogenesis and cell proliferation. In the filamentous fungus Neurospora crassa, a mutation in cot-1 results in excessive numbers of branched hyphal tips at a restrictive temperature, but these tips fail to elongate (Yarden et al., 1992). orb6 is an essential gene in fission yeast (Verde et al., 1998). Depletion of the Orb6 protein leads to loss of polarized cell shape and to mitotic advance. The member of this kinase family in the dimorphic fungus Ustilago maydis is encoded by ukc1 (Durrenberger and Kronstad, 1999). Disruption of ukc1 causes a change of cell shape from elongated to rounded and prevents cells from forming filamentous colonies. During evolution, this kinase family diverged into two subfamilies in metazoans: the Ndr kinases (Millward et al., 1995) and the Warts/Lats kinases (Justice et al., 1995; Xu et al., 1995). The Ndr kinases are more closely related to the fungal kinases. The Drosophila melanogaster Ndr kinase is encoded by tricornered (Geng et al., 2000). Mutations in tricornered result in splitting of surface structures, including epidermal hairs, the shafts of sensory bristles, larval denticles, and the lateral branches of the arista. The Caenorhabditis elegans Ndr kinase is encoded by sax-1 (Zallen et al., 2000). sax-1 mutants have expanded cell bodies and ectopic neurites in many classes of neurons.
In Saccharomyces cerevisiae, Cbk1p is the protein kinase belonging to this family. cbk1 mutants were first isolated in a screen for mutations that reduce transcriptional repression by Sin3p (Dorland et al., 2000). Further studies showed that cbk1Δ cells fail to degrade the septa connecting mother and daughter cells, resulting in the formation of cell aggregates in liquid cultures (Racki et al., 2000; Bidlingmaier et al., 2001). These cells also display other defects in cell morphogenesis, such as round cell shapes, random budding patterns, and abnormal mating projections.
The cell separation defect of cbk1Δ cells is likely due to reduced activity of the transcription factor Ace2p, which is required for expression of chitinase. Because dominant mutations in ACE2 suppress the cell separation defect but not the cell shape and budding pattern defects of cbk1Δ, CBK1 probably regulates both an Ace2p-dependent pathway and an Ace2p-independent pathway (Racki et al., 2000). Whole genome transcriptional analysis of cbk1Δ revealed that, in addition to the chitinase gene, the expression levels of many other genes participating in cell wall physiology were altered, suggesting that other transcriptional regulators besides Ace2p may act downstream of Cbk1p (Bidlingmaier et al., 2001).
In a genetic screen, we isolated a mutant of a novel gene, PAG1 (YIL129C). The pag1 mutant exhibited defects in morphogenesis similar to those of the cbk1 mutant. Herein, we report our genetic and biochemical analysis demonstrating that Pag1p and Cbk1p function together in a protein complex.
MATERIALS AND METHODS
Strains and Plasmids
Table 1 lists the genotypes of the yeast strains used in this study. Standard yeast genetics methods were used in the construction of the strains (Adams et al., 1997). pag1-1 strains with the Genome Deletion Project background were made by backcrossing three times into that background. The PAG1 sequence in all of the plasmids containing PAG1 was derived from a genomic library plasmid that can complement the pag1-1 mutant. To delete the PAG1 gene, we subcloned a HindIII/XhoI DNA fragment upstream of the PAG1 coding region and a XbaI/HindIII fragment from the 3′ end of the coding region into an integrating vector pRS305 (LEU2). The resulting plasmid was cut by HindIII and transformed into a diploid yeast strain to replace most of the PAG1 coding sequence with the vector sequence. After the replacement a 10-base pair coding region remained at the 5′ end and 90 base pairs remained at the 3′ end. To add tags to the N terminus of Pag1p, a BamHI site was inserted at the 5′ end of the coding region by polymerase chain reaction (PCR). DNA sequences encoding an 8xHA tag made by PCR or the yeast codon-optimized green fluorescent protein (GFP) (Cormack et al., 1997) were cloned in frame into this BamHI site. An 860-base pair sequence upstream of the PAG1 coding region, the tag sequence, and a 1.2-kb PAG1 5′ coding region were cloned into pRS306 (URA3) to make the tagging plasmids. A BglII site inside the coding region was used to target the integration of the tagging plasmid to the PAG1 locus so that the tagged Pag1p was the only full-length Pag1p expressed by the cell. The plasmid sequence in the HA-PAG1 strains was removed by selecting pop-out events on 5-fluoroorotic acid (5-FOA) plates. CBK1-GFP strains were made by PCR amplification of the DNA containing the kanMX marked CBK1-GFP locus from WR154 (Racki et al., 2000) and then introducing the PCR product into our strains by transformation.
Table 1.
List of yeast strains used in this study
| Strains | Genotype |
|---|---|
| NY2331 | MATα gyp1Δ∷LEU2 leu2-3,112 ura3-52 ade2 ade3 his3 |
| NY2340 | MATa pag1Δ∷LEU2 ura3-52 leu2-3,112 his3-Δ200 lys2 [CEN LYS2 PAG1]. |
| NY2341 | MATα pag1Δ∷LEU2 ura3Δ0 leu2Δ0 his3Δ1 lys2Δ0 met15Δ0 [CEN LYS2 PAG1] |
| NY13 | MATa ura3-52 |
| NY2333 | MATa pag1-1 ura3-52 leu2-3,112 trp1 |
| NY2334 | MATa/α pag1Δ∷LEU2/PAG1 ura3-52/ura3-52 his3-Δ200/his3-Δ200 leu2-3,112/leu2-3,112 |
| NY1523 | MATa/α ura3-52/ura3-52 leu2-3,112/leu2-3,112 his3-Δ200/his3-Δ200 |
| NY2342 | MATa/α pag1-1/pag1-1 ura3-52/URA3 leu2-3,112/LEU2 trp1/TRP1 his4-619/HIS4 |
| NY2337ab | MATa met15Δ0 |
| NY2344ab | MATa ssd1Δ∷kanMX met15Δ0 |
| NY2343a | MATa pag1-1 met15Δ0 |
| NY2345a | MATa pag1-1 ssd1Δ∷kanMX met15Δ0 |
| NY2349ab | MATa/α LYS2/lys2Δ0 MET15/met15Δ0 |
| NY2351ab | MATa/α ssd1Δ∷kanMX/ssd1Δ∷kanMX LYS2/lys2Δ0 MET15/met15Δ0 |
| NY2362a | MATa/α pag1-1/pag1-1 MET15/met15Δ0 |
| NY2363a | MATa/α pag1-1/pag1-1 ssd1Δ∷kanMX/ssd1Δ∷kanMX MET15/met15Δ0 |
| NY2350ab | MATa/α CBK1/cbk1Δ∷kanMX LYS2/lys2Δ0 MET15/met15Δ0 |
| NY2361a | MATa/α cbk1Δ∷kanMX/CBK1 ssd1Δ∷kanMX/MET15/met15Δ0 LYS2/lys2Δ0 |
| NY2346a | MATa pag1Δ∷LEU2 ssd1Δ∷kanMX met15Δ0 |
| NY2347a | MATa cbk1Δ∷kanMX ssd1Δ∷kanMX met15Δ0 |
| NY2348a | MATa pag1Δ∷LEU2 cbk1Δ∷kanMX ssd1Δ∷kanMX met15Δ0 |
| NY2364ab | MATa cts1Δ∷kanMX met15Δ0 |
| NY2365ab | MATa ace2Δ∷kanMX met15Δ0 |
| NY2352a | MATa/α pag1Δ∷LEU2/pag1Δ∷LEU2 ssd1Δ∷kanMX/ssd1Δ∷kanMX MET15/met15Δ0 LYS2/lys2Δ0 |
| NY2353a | MATa/α cbk1Δ∷kanMX/cbk1Δ∷kanMX ssd1Δ∷kanMX/ssd1Δ∷MET15/met15Δ0 LYS2/lys2Δ0 |
| NY2354a | MATa/α pag1Δ∷LEU2/pag1Δ∷LEU2 cbk1Δ∷kanMX/cbk1Δ∷kanMX ssd1Δ∷kanMX/ssd1Δ∷kanMX MET15/met15Δ0 LYS2/lys2Δ0 |
| NY2336a | MATa pag1Δ∷LEU2 met15Δ0 [2μ URA3 PAG1] |
| NY2355a | MATa/α MET15/met15Δ0 LYS2/lys2Δ0 [2μ URA3 SIM1] |
| NY2356a | MATa/α pag1Δ∷LEU2/pag1Δ∷LEU2 MET15/met15Δ0 lys2Δ0/lys2Δ0 [2μ URA3 SIM1] |
| NY2357a | MATa/α cbk1Δ∷kanMX/cbk1Δ∷kanMX MET15/met15Δ0 LYS2/lys2Δ0 [2μ URA3 SIM1] |
| NY2338ab | MATa sim1Δ∷kanMX met15Δ0 |
| NY2339a | MATa met15Δ0 [2μ URA3 SIM1] |
| NY2335 | MATa ura3-52 [URA3, PAG1p-GFP-PAG1] |
| NY2358 | MATa HA-PAG1 ura3-52 |
| NY2359 | MATa CBK1-GFP ura3-52 |
| NY2360 | MATa HA-PAG1 CBK1-GFP ura3-52 |
These strains have the background of the Saccharomyces Genome Deletion Project strains and share the ura3Δ0, leu2Δ0, and his3Δ1 markers, which are not indicated in the table.
These strains are Saccharomyces Genome Deletion Project strains obtained from Research Genetics, Huntsville, AL.
Genetic Screens
Mutants synthetically lethal with gyp1Δ were selected with a colony-sectoring assay (Bender and Pringle, 1991) from ethylmethane sulfonate-mutagenized NY2331 cells containing a plasmid [CEN URA3 ADE3 GYP1]. Cells that could not lose the ADE3 plasmid formed nonsectoring colonies, i.e., colonies that remained uniformly red on YPD. The nonsectoring mutants were further screened for their dependence on GYP1 by their failure to grow on 5-FOA plate and by their ability to form sectored colonies again after the transformation of another GYP1 plasmid with a HIS3 marker. From 25,000 mutagenized colonies, 22 mutants were obtained and named pag mutants (perish in the absence of GYP1). All of these mutants were recessive. They were grouped into 12 complementation groups. Eleven of the PAG genes were cloned by complementation with a YCp50-based genomic library (Table 2).
Table 2.
Genes complementing the mutants synthetically lethal with gyp1Δ
| pag mutant | No. of isolates | Complementing gene | Protein encoded |
|---|---|---|---|
| pag1 | 1 | YIL129Ca | Unknown ORF, 2376 amino acids |
| pag2 | 2 | GOS1a | Golgi SNARE protein |
| pag3 | 4 | ERG2 | Enzyme of the ergosterol biosynthesis pathway |
| pag4 | 2 | ARL1a | ARF-like protein |
| pag5 | 2 | ERG28 | Enzyme of the ergosterol biosynthesis pathway |
| pag6 | 1 | KRE9 | Glycoprotein involved in β-1,6-glucan assembly |
| pag7 | 1 | ERG11 | Enzyme of the ergosterol biosynthesis pathway |
| pag8 | 2 | ERG24 | Enzyme of the ergosterol biosynthesis pathway |
| pag9 | 1 | WBP1 | Oligosaccharyl transferase beta subunit |
| pag10 | 4 | VPS1 | Dynamin like protein localized on Golgi |
| pag11 | 1 | PTC1 | Ser/Thr protein phosphatase, PP2C family |
ORF, open reading frame; SNARE, soluble N-ethylmaleimide-sensitive factor attachment protein receptor.
These three genes were confirmed to be linked to the mutant alleles by linkage analysis.
To screen for bypass suppressors of pag1Δ, a yeast genomic library mutagenized by an mTn-3xHA/lacZ transposon (Ross-Macdonald et al., 1997) was used to tranform NY2340. A total of ∼48,000 transformants was obtained on SC-URA plates. The colonies were replica-plated onto α-aminoadipate (α-AA) plates to select for mutants that could survive without the PAG1 plasmid. Colony PCR analysis showed that 5 of the 18 colonies growing on α-AA had lost the PAG1 gene. To confirm that their ability to grow without PAG1 depended on the transposon, these five mutants were crossed to NY2341. Tetrad analysis showed that two of the bypass mutants cosegregated the Ura+ phenotype conferred by the transposon with the α-AA resistance. The DNA sequences adjacent to the transposon were retrieved by inverse PCR (Adams et al., 1997). Sequencing of the PCR products showed that the orientations of the two transposons were opposite to each other and that the insertion sites were at coordinates 1047219 and 1048066 of chromosome IV, respectively.
To identify high-copy suppressors of pag1-1, we transformed pag1-1 cells with a yeast genomic library based on YEp24. From 3 × 105 transformants, 694 colonies that could grow at 34°C on YPD were obtained. Of these colonies, 71 were unable to grow on 5-FOA at 34°C and therefore required a plasmid for growth at restrictive temperature. The plasmids in these cells were rescued and then examined by restriction analysis or DNA sequencing. We obtained two plasmids that did not contain PAG1 and could suppress the growth defect at both 34 and 37°C. They had overlapping genomic inserts. After subcloning, the suppressor gene in these plasmids was determined to be SIM1.
Microscopy
For light microscopy, samples were viewed on a Zeiss Axiophot 2 microscope (Zeiss, Pberkochen, Germany) by using a 63 or 100× oil-immersion objective (numerical aperture 1.4). Images were acquired with a Photometrics Quantix charge-coupled device camera (Tuscon, AZ) by using IPLab for Macintosh software (Scanalytics, Fairfax, VA). The lengths of the major and minor axis of the cell were measured with Adobe Photoshop (Adobe Systems, Mountain View, CA). Phalloidin staining was done as described (Adams et al., 1997). Samples for electron microscopy were prepared with a permanganate fixation method (Kaiser and Schekman, 1990).
β-1,3-Glucanase Sensitivity Assay
Quantazyme ylg, a recombinant β-1,3-glucanase, was purchased from Qbiogene (Carlsbad, CA). Yeast cells growing exponentially in liquid media were washed with water and then resuspended in 50 mM Tris-HCl, pH 7.5, 0.15 M NaCl, 5 mM EDTA, 10 mM diothiothreitol. Quantazyme ylg was added to 10 U/ml to start the digestion. Cell lysis was monitored by the decrease of OD600.
Chitinase Assay
Yeast cells were inoculated from a stationary culture to YPD media to an initial OD600 of 0.01. They were grown overnight at 25°C until the OD600 reached ∼1. NaN3 was added to the culture to a final concentration of 10 mM. Media was separated from the cells by centrifugation. Cells were resuspended in water and split in two; one half was assayed directly, representing the cell surface fraction, and the other half was lysed by freeze-thawing in the presence of 1% Triton X-100 (Munn et al., 1999). Chitinase activity was assayed essentially as described (Kuranda and Robbins, 1991). Culture media, cell suspension, or cell lysate (30 μl) were mixed with 20 μl of 250 μM 4-methylumbelliferyl β-d-N,N′,N"-triaceylchitotrioside (Sigma, St. Louis, MO) in 0.25 M sodium citrate buffer, pH 3.0, and incubated at room temperature for 20, 60, and 100 min. The reaction was stopped by the addition of 50 μl of 0.5 M glycine-NaOH buffer, pH 10.5. The liberated 4-methylumbelliferone was measured with a Hitachi F-3010 fluorescence spectrophotometer (excitation at 360 nm, emission at 450 nm). Intracellular chitinase activity was calculated from the difference between the total cell-associated activity (from lysates) and the surface activity (from whole cells).
Antibodies
To make an antibody against Sim1p, a 585-base pair DNA sequence encoding a C-terminal fragment of Sim1p was amplified by PCR and cloned into the pMAL-c2 vector (New England Biolabs, Beverly, MA). The Sim1p peptide fused with maltose-binding protein was expressed and purified from Escherichia coli and used as antigen to immunize rabbits. The anti-GFP polyclonal antibody was a gift from Dr. S. Ferro-Novick (Yale University School of Medicine, New Haven, CT). 12CA5 monoclonal antibody against the hemagglutinin (HA) tag was from Roche Molecular Biochemicals (Indianapolis, IN).
Immunoprecipitation
NY2358 (HA-PAG1), NY2359 (CBK1-GFP), and NY2360 (HA-PAG1 CBK1-GFP) were grown in YPD to log phase. Cells (200 OD600 units) were washed once with 20 mM HEPES-NaOH, pH 7.5, 20 mM NaN3, 20 mM NaF. Cells were then resuspended in lysis buffer (20 mM HEPES-NaOH, pH 7.5, 100 mM NaCl, 1 mM EDTA, 10 μM antipain, 1 μg/ml aprotinin, 30 μM leupeptin, 30 μM chymostatin, 20 μM pepstatin A, 2 mM benzamidine, 1 mM phenylmethylsulfonyl fluoride). Cell lysis was accomplished by beating with 0.5-mm zirconia/silica beads (BioSpec Products, Bartlesville, OK) as described (Grote and Novick, 1999). Cell lysates were mixed with 1/10 volume of 20% nonionic detergent IGEPAL CA-630 (Sigma) and rocked for 1 h at 4°C. After centrifugation at 14,000 rpm for 6 min in a microcentrifuge, supernatants were transferred to new tubes and mixed with anti-HA or anti-GFP antibodies and incubated at 4°C for 1 h. Protein A-Sepharose beads (Sigma) were then added and the tubes were rocked for another 1 h at 4°C. Lysis buffer containing 1% IGEPAL CA-630 was used to wash the beads five times. The beads were then boiled in SDS-PAGE sample buffer. Samples were separated by SDS-PAGE with a 4.8% gel for HA-Pag1p and an 8% gel for Cbk1p-GFP, transferred to nitrocellulose membranes and probed with anti-HA and anti-GFP antibodies.
RESULTS
A pag1 Mutant Displays Morphogenesis Defects Similar to those of cbk1 Mutants
In a genetic screen for mutants synthetically lethal with gyp1Δ, we isolated a mutant of a novel gene, PAG1 (YIL129C). Pag1p has been conserved during evolution. We were able to find homologs in every sequenced eukaryotic genome by a BLAST search (Table 3 ). All of its homologs are large proteins with >2000 amino acids. Budding yeast, fission yeast, Drosophila, and Caenorhabditis elegans each have a single Pag1p-related protein. The Drosophila homolog is encoded by furry, which is an essential gene required for maintaining the integrity of cellular extensions (Cong et al., 2001). The C. elegans homolog is encoded by sax-2, a gene that regulates neuronal cell shape (M. Gallegos and C. Bargmann, personal communication; Zallen et al., 2000). Pag1p homologs also exist in mammals. A gene encoding a human homolog of Pag1p was found during the construction of a transcription map (Couch et al., 1996). The gene CG003 encodes a 10.7-kb transcript expressed in many tissues. Sequence analysis predicted that Pag1p does not have a signal peptide sequence or other sorting sequences, and is is not likely to have a transmembrane domain. No known structural motifs could be found in the Pag1p sequence.
Table 3.
Homologs of Pag1p in different organisms revealed by a BLAST search A BLAST search was performed using the Pag1p (YIL129C) protein sequence as query to search nonredundant protein sequence database at the NCBI WWW BLAST server (http://www.ncbi.nlm.nih.gov/blast/). The program is BLASTP 2.1.2 with default parameters. Scores and expectation values (E values) are as determined by the BLAST algorithm.
| GenBank ID | Organism | Predicted protein length | Score | E value |
|---|---|---|---|---|
| 12044481 | Schizosaccharomyces pombe | 2196 | 692 | 0 |
| 11357426 | Arabidopsis thaliana | 2163 | 127 | 2e-27 |
| 11907986 | D. melanogaster | 3479 | 102 | 4e-20 |
| 4902699 | Homo sapiens | 3012 | 100 | 2e-19 |
| 9802901 | C. elegans | 2858 | 90.9 | 2e-16 |
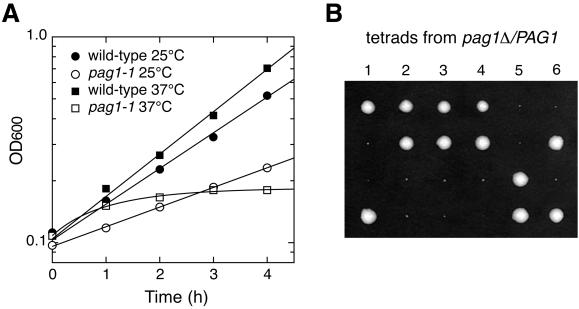
The single pag1 mutant isolated from the gyp1Δ synthetic lethal screen was named pag1-1. Its growth was temperature sensitive. In liquid YPD media, the doubling time of the wild-type strain at 25°C was 1.8 h, whereas the doubling time of pag1-1 at 25°C was 3.1 h (Figure 1A). After shifting to 37°C, wild-type cells doubled every 1.5 h, whereas pag1-1 cells stopped growing after 1 h. To examine the phenotype of a null allele, PAG1 was deleted in a wild-type diploid strain of our lab strain background, and the resulting strain was sporulated. The pag1Δ cells were defective in growth (Figure 1B); they formed tiny colonies after 5 d on a YPD plate at 25°C and did not form any visible colony at 30°C. Therefore, PAG1 is important for vegetative growth of cells, and pag1-1 is a partial loss-of-function allele. Because pag1Δ cells were too sick to work with, we decided to characterize only the phenotype of pag1-1. By backcrossing, we obtained a pag1-1 strain with the background of the Saccharomyces Genome Deletion Project (Winzeler et al., 1999). Our lab strain background and the Genome Deletion Project strain background were both derived from S288C. All the phenotypes we characterized were qualitatively similar in these two backgrounds.
Figure 1.
PAG1 is essential for growth. (A) Growth properties of the temperature-sensitive mutant pag1-1. Wild-type cells (NY13) or pag1-1 cells (NY2333) growing exponentially at 25°C were shifted to fresh YPD medium at 25 or 37°C. Optical density at 600 nm (OD600) was determined at 1-h intervals. (B) Tetrads derived from the sporulation of pag1Δ/PAG1 (NY2334) were dissected onto a YPD plate and incubated at 25°C for 5 d. Six tetrads are shown. The smaller colonies are pag1Δ cells.
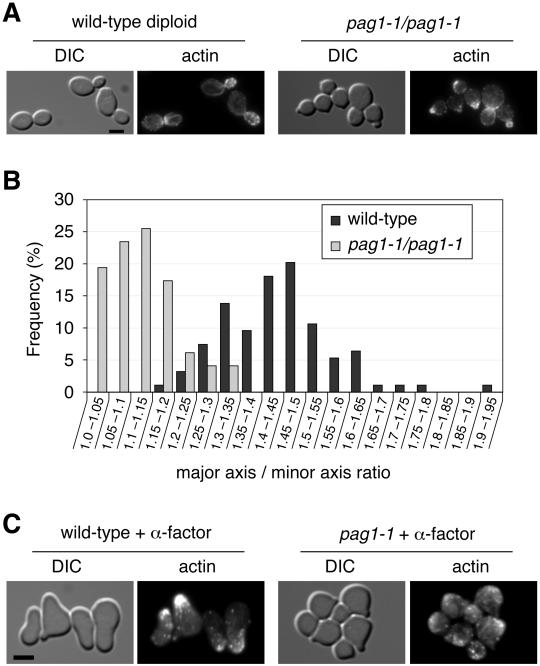
Light microscopy showed that pag1-1 cells appeared rounder than wild-type cells. Because diploid cells have a more elongated shape than haploid cells, we examined the shape of pag1-1 diploid cells. pag1-1 diploid cells grown at 30°C were significantly rounder than the wild-type diploid cells (Figure 2A). The average major axis/minor axis ratio of wild-type diploid cells was 1.44 ± 0.13 (n = 94), whereas the average ratio of pag1-1 diploid cells was 1.12 ± 0.08 (n = 98) (Figure 2B). Therefore, pag1-1 cells have a cell shape defect. Because the actin cytoskeleton is essential for establishment of normal cell morphology, we examined the localization of actin in pag1-1 cells with phalloidin staining (Figure 2A). We found that actin localization was relatively normal in pag1-1 diploid cells, indicating that the cell shape defect was not due to a gross disorganization of the actin cytoskeleton.
Figure 2.
pag1-1 is defective in cell shape and mating projection formation. (A) Wild-type diploid cells (NY1523) and pag1-1 diploid cells (NY2342) growing exponentially in YPD at 30°C were fixed and stained with fluorescently labeled phalloidin. Cells were imaged in differential interference contrast (DIC) mode and fluorescence mode, respectively. Bar, 4 μm. (B) pag1-1 diploid cells are significantly rounder than wild-type cells. From the DIC images, we measured the lengths of the major axis and minor axis of budded cells of the two strains shown in A. Distributions of the major axis/minor axis ratio are plotted as a histogram. (C) Wild-type MATa cells (NY13) and pag1-1 MATa cells (NY2333) grown in YPD at 30°C were treated with α-factor (5 μg/ml) at 30°C for 3 h then fixed and stained with fluorescently labeled phalloidin. Bar, 4 μm.
During mating, yeast cells undergo dramatic morphological changes. When MATa cells are exposed to α-mating factor, they generate elongated projections. Many genes involved in morphogenesis during vegetative growth also act in mating projection formation. Therefore, we examined whether pag1-1 cells had a defect in mating projection formation. When we treated wild-type MATa cells with α-factor for 3 h, 93% (n = 115) of them formed mating projections longer than half the cell diameter. In contrast, only 3% of pag1-1 cells (n = 120) under the same treatment formed mating projections longer than half the cell diameter (Figure 2C). Many pag1-1 cells did form small protrusions like tiny buds. Interestingly, most of these protrusions were not stained by phalloidin, whereas the mating projections of wild-type cells were invariably brightly stained. This observation suggested that the small protrusions could be due to an aborted attempt by pag1-1 cells to form normal mating projections or that the actin cytoskeleton could not be assembled normally during mating in pag1-1 cells.
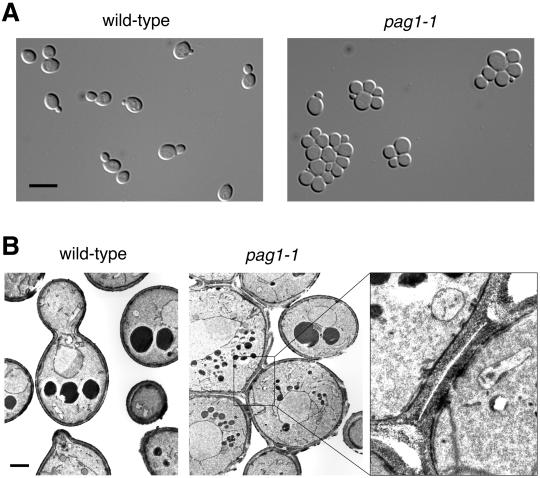
Light microscopy revealed another morphological defect of pag1-1 cells: they formed aggregates in liquid media (Figure 3A). To examine the nature of the connection between aggregated cells, we carried out electron microscopy analysis with a fixation method that preserves the cell wall. Electron micrographs showed that aggregated pag1-1 cells were connected by septa (Figure 3B). These observations suggested that pag1-1 cells have a cell separation defect.
Figure 3.
pag1-1 cells have a separation defect. (A) pag1-1 cells form aggregates in liquid culture. Wild-type cells (NY13) and pag1-1 cells (NY2333) growing exponentially in YPD at 25°C were examined by light microscopy. Bar, 8 μm. (B) Electron microscopy shows that aggregated pag1-1 cells are connected by septa. Bar, 1 μm. A septum between two pag1-1 cells is shown at a higher magnification at right.
Although electron microscopy did not reveal any gross changes in the cell wall morphology of pag1-1 cells, the separation defect of these cells led us to examine whether they had additional cell wall defects. On treatment with 10 U/ml β-1,3-glucanase, the OD600 of a wild-type cell suspension decreased 77% within 40 min, whereas during the same period the OD600 of the pag1-1 sample only dropped ∼10%. Microscopic examination confirmed that the reduction of OD600 was due to cell lysis. Almost all of the wild-type cells were lysed after 70 min, whereas most of the pag1-1 cells remained intact. The resistance of pag1-1 cells to β-1,3-glucanase indicated that the cell wall structure or composition of pag1-1 cells was different from that of wild-type cells. β-1,3-glucanase is the main ingredient of commonly used yeast-digesting enzymes, such as zymolyase. Accordingly, we found that pag1-1 cells were more resistant to zymolyase than wild-type cells (our unpublished observation). Resistance to zymolyase has been observed before in cell wall mutants such as the gas1Δ mutant (Popolo et al., 1993). GAS1 encodes a protein involved in the cross-linking of β-1,3-glucan in the cell wall (Mouyna et al., 2000).
The defects of pag1–1 cells in cell separation, cell shape, and mating projection formation were similar to the known defects of cbk1Δ mutants (Racki et al., 2000; Bidlingmaier et al., 2001). This similarity led us to hypothesize that PAG1 and CBK1 may have related functions. Although the cbk1Δ strains used in the previous studies had no growth defect, CBK1 was shown to be an essential gene by the Saccharomyces Genome Deletion Project (record number 22051) (Winzeler et al., 1999). PAG1 is also an essential gene in the background of the Genome Deletion Project (our unpublished observation and Genome Deletion Project record number 22288). Therefore, in a standard strain background, both PAG1 and CBK1 are required for cell proliferation. However, this requirement can be bypassed in some other genetic backgrounds.
PAG1 and CBK1 Act in the Same Pathway
We used a transposon insertion mutagenesis method to identify the mutations that can bypass the requirement of PAG1 for growth. From this screen, we obtained two mutants that could grow without PAG1. Sequencing the DNA adjacent to the transposons showed that the transposons in the two mutants independently inserted into the same gene, SSD1. The insertion sites were located at 1317 base pairs and 2164 base pairs downstream of the start codon of the 3753-base pair open reading frame of SSD1. Because the C-terminal conserved region of Ssd1p is required for its function (Uesono et al., 1997), both transposon insertions were likely to result in loss-of-function alleles of SSD1. SSD1 is known to genetically interact with many different genes but its physiological function is not clear.
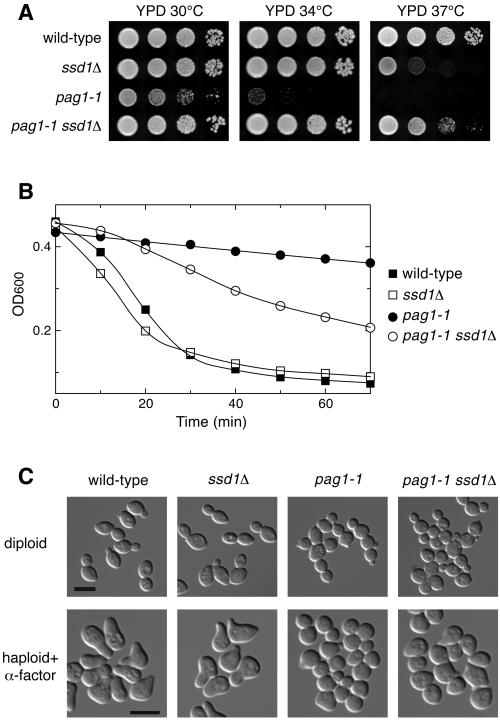
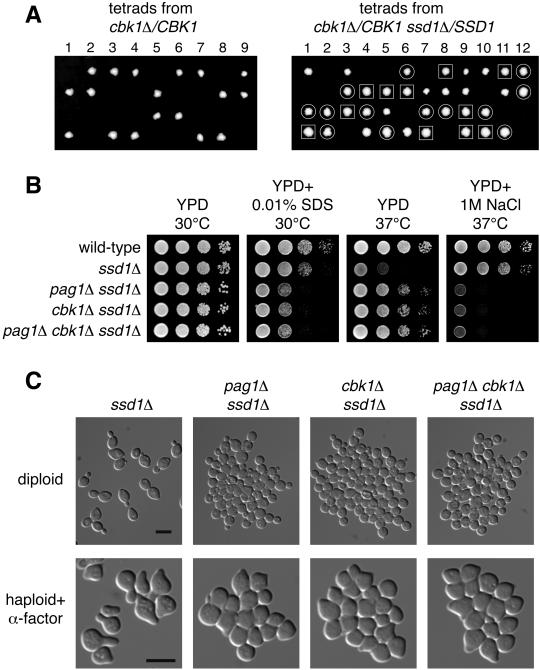
Because the pag1-1 mutant displayed many defects, we then examined which defects could be suppressed by the disruption of SSD1. All the strains used in this analysis had the background of the Genome Deletion Project. SSD1 is not essential for growth and ssd1Δ displayed only a mild growth defect when grown at 37°C on YPD (Figure 4A). We found that ssd1Δ strongly suppressed the growth defect of pag1-1. Interestingly, the pag1-1 ssd1Δ double mutant grew better than either single mutant at 37°C on YPD, indicating that PAG1 and SSD1 act antagonistically against each other. ssd1Δ also partially reversed the β-1,3-glucanase resistance phenotype of pag1-1 (Figure 4B). When we examined the morphology of these strains, we found that ssd1Δ was in every way the same as the wild type. However, ssd1Δ did not significantly suppress the cell separation defect or the diploid cell shape defect of pag1-1 (Figure 4C). It only weakly suppressed the mating projection formation defect. After treatment with α-factor for 3 h, 78, 78, 3, and 18% (n = 200) of the wild-type, ssd1Δ, pag1-1, and pag1-1 ssd1Δ cells, respectively, formed mating projections longer than half the cell diameter.
Figure 4.
Deletion of SSD1 strongly suppresses the growth defect of pag1-1 but does not significantly rescue the defects in cell morphogenesis. (A) ssd1Δ suppresses the growth defect of pag1-1 and its sensitivity to SDS and 1 M NaCl. Tenfold serial dilutions of wild-type (NY2337), ssd1Δ (NY2344), pag1-1 (NY2343), and pag1-1 ssd1Δ (NY2345) cells were spotted on plates and incubated at the indicated temperatures. Photos were taken 40 h later. (B) pag1-1 is resistant to β-1,3-glucanase treatment and ssd1Δ partially suppresses this phenotype. The same yeast strains as used in A were grown in YPD at 30°C and then treated with β-1,3-glucanase (10 U/ml Quantazyme ylg). Cell lysis was monitored by the decrease of OD600. (C) ssd1Δ does not significantly suppress the defects of pag1-1 in cell separation and polarized growth. Wild-type (NY2349), ssd1Δ (NY2351), pag1-1 (NY2362), and pag1-1 ssd1Δ (NY2363) homozygous diploid cells growing exponentially in YPD at 30°C were fixed and photographed. The same MATa strains as used in A were grown in YPD at 30°C, treated with α-factor (5 μg/ml) for 3 h at 30°C, and then fixed and photographed. Bar, 8 μm.
If PAG1 and CBK1 have related functions, they may share the same suppressors. Therefore, we examined whether ssd1Δ can suppress the growth defect of cbk1Δ. We dissected the tetrads derived from a diploid strain heterozygous for both cbk1Δ and ssd1Δ and found that cbk1Δ ssd1Δ double mutants grew at the wild-type rate on YPD at 25°C, whereas cbk1Δ cells did not grow (Figure 5A). Suppression of the lethality of both pag1Δ and cbk1Δ by ssd1Δ supports the hypothesis that PAG1 and CBK1 have related functions.
Figure 5.
cbk1Δ ssd1Δ cells display the same phenotype as pag1Δ ssd1Δ cells. (A) The lethality of cbk1Δ in the genetic background of the genome deletion project can be suppressed by ssd1Δ. Tetrads derived from cbk1Δ/CBK1 (NY2350) and cbk1Δ/CBK1 ssd1Δ/SSD1 (NY2361) diploid cells were dissected onto YPD plates and incubated at 25°C. Photos were taken 4 d later. The genotypes of the haploid progeny were determined by PCR analysis. ssd1Δ colonies are circled. cbk1Δ ssd1Δ colonies are enclosed in squares. Unmarked colonies are wild-type. (B) pag1Δ ssd1Δ, cbk1Δ ssd1Δ, and pag1Δ cbk1Δ ssd1Δ cells are equally hypersensitive to SDS and NaCl. Tenfold serial dilutions of haploid cells were spotted on plates and incubated at the indicated temperatures. Photos were taken 40 h later for the YPD plates and the YPD plate containing SDS and 90 h later for the YPD plate containing NaCl. (C) pag1Δ ssd1Δ, cbk1Δ ssd1Δ, and pag1Δ cbk1Δ ssd1Δ cells display similar defects in cell separation and polarized growth. The homozygous diploid cells growing exponentially in YPD at 30°C were fixed and photographed. The MATa strains grown in YPD at 30°C were treated with α-factor (5 μg/ml) for 3 h at 30°C and then fixed and photographed. Bar, 8 μm.
We then compared the phenotypes of pag1Δ ssd1Δ, cbk1Δ ssd1Δ, and pag1Δ cbk1Δ ssd1Δ. By all criteria examined, these three strains displayed the same phenotypes (Figure 5, B and C). They grew at the same rates on YPD plates at 30°C or 37°C. They were hypersensitive to SDS to the same extent. The growth of these three strains was inhibited to the same extent by 1 M NaCl at 37°C. These three strains were also defective in cell separation. In fact, they had a more severe aggregation phenotype than pag1-1, especially so with the diploid strains (Figures 4C and 5C). All three strains were defective in diploid cell shape and mating projection formation. After treatment with α-factor for 3 h, 80, 9, 11, and 11% (n = 200) of the ssd1Δ, pag1Δ ssd1Δ, cbk1Δ ssd1Δ, and pag1Δ cbk1Δ ssd1Δ cells, respectively, formed mating projections longer than half the cell diameter. These results suggest that PAG1 and CBK1 function in the same pathway.
cbk1Δ was shown to have reduced expression of yeast chitinase (Cts1p) and the low level of chitinase is probably a main cause of the cell separation defect (Racki et al., 2000; Bidlingmaier et al., 2001). Hence, we examined the expression levels of Cts1p in our strains with a quantitative chitinase assay (Table 4). Wild-type cells secrete a majority of the chitinase into the growth medium (Kuranda and Robbins, 1991). Therefore, we measured the chitinase activity in the medium, on the cell surface, and internal to the cells. cts1Δ had no activity at all. ssd1Δ had a total chitinase activity 36% more than the wild type, whereas all the mutants with a cell separation defect had much lower chitinase activity than the wild type. The chitinase activities of pag1-1 and pag1-1 ssd1Δ were 16 and 27% of the wild-type level. pag1Δ ssd1Δ, cbk1Δ ssd1Δ, and pag1Δ cbk1Δ ssd1Δ produced even less chitinase, at 6, 5, and 5% of the wild-type level, respectively. Therefore, the chitinase levels of these strains largely correlated with the severity of their cell separation defects but not their growth defects. These results are consistent with the model that both PAG1 and CBK1 function in the pathway that controls the expression of chitinase.
Table 4.
Chitinase activity of haploid yeast strains grown at 25°C in YPD Unit, nanomole of 4-methylumbelliferone released per hour per OD600 unit
| Medium | Surface | Intracellular | Total | |
|---|---|---|---|---|
| Wild-type | 2.69 | 0.33 | 0.25 | 3.26 |
| ssd1Δ | 3.51 | 0.53 | 0.39 | 4.43 |
| pag1-1 | 0.32 | 0.15 | 0.06 | 0.53 |
| pag1-1 ssd1Δ | 0.44 | 0.29 | 0.14 | 0.87 |
| pag1Δ ssd1Δ | 0.07 | 0.08 | 0.06 | 0.20 |
| cbk1Δ ssd1Δ | 0.03 | 0.07 | 0.06 | 0.15 |
| pag1Δ cbk1Δ ssd1Δ | 0.04 | 0.07 | 0.05 | 0.16 |
| cts1Δ | 0.00 | 0.00 | 0.00 | 0.00 |
| ace2Δ | 0.00 | 0.01 | 0.00 | 0.01 |
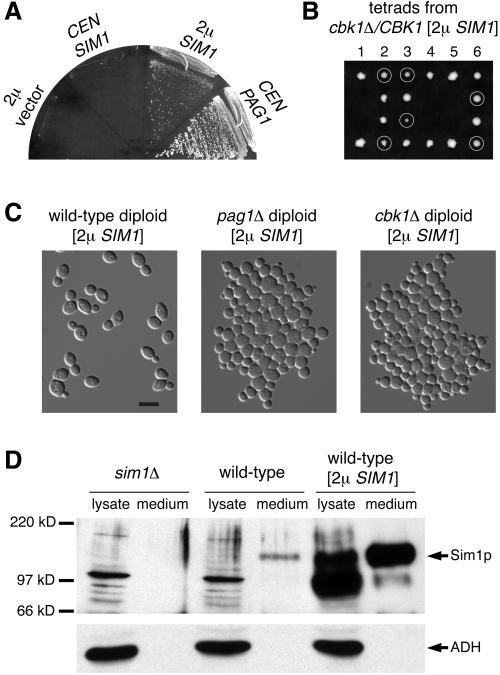
From a screen for high-copy suppressors of pag1-1, we identified a suppressor that could support the growth of pag1-1 at 37°C. The suppressor gene SIM1 could also completely bypass the requirement of PAG1 for growth when expressed from a high-copy plasmid, but not when expressed from a low-copy plasmid (Figure 6A). We introduced the high-copy SIM1 plasmid into a heterozygous cbk1Δ diploid strain with the Genome Deletion Project background. Many cbk1Δ spores derived from the sporulation of this diploid could form colonies, and the growth of these cells was dependent on the SIM1 plasmids they carried (Figure 6B). Not all spores were able to grow, probably due to plasmid loss during growth in the rich presporulation medium. Although the high-copy SIM1 plasmid suppressed the growth defects of pag1Δ and cbk1Δ, it did not rescue the cell separation defect or cell shape defect (Figure 6C). A Sim1p antibody detected a 150 kD protein that accumulated in the medium of wild-type cells but not in the medium of sim1Δ cells, indicating that Sim1p is an extracellular protein (Figure 6D).
Figure 6.
Overexpression of an extracellular protein, Sim1p, suppresses the growth defects of both pag1Δ and cbk1Δ. (A) SIM1 is a dosage-dependent suppressor of pag1Δ. pag1Δ [2 μ URA3 PAG1] (NY2336) with the HIS3-containing plasmids shown in the figure streaked onto a 5-FOA plate and incubated at 25°C. (B) A multicopy [2 μ URA3 SIM1] plasmid suppresses the lethality of cbk1Δ. Tetrads were dissected onto a YPD plate and incubated at 25°C. Photo was taken 4 d later. G-418 resistant colonies (cbk1Δ) are circled. They are Ura+ and cannot grow on a 5-FOA plate. (C) pag1Δ and cbk1Δ cells with a multicopy SIM1 plasmid display defects in cell separation and polarized growth. Wild-type (NY2355), pag1Δ (NY2356), and cbk1Δ (NY2357) homozygous diploid cells containing a [2 μ URA3 SIM1] plasmid growing exponentially in SC-URA at 30°C were fixed and photographed. (D) Sim1p is a secreted protein. Cells were grown to log phase in synthetic media. Lysates were made by vortexing with glass beads. Proteins secreted into the medium were precipitated with trichloroacetic acid. Samples corresponding to materials from cultures containing 0.4 OD600 unit cells were separated by SDS-PAGE. Western blot was probed with antibodies against Sim1p and a cytosolic protein, alcohol dehydrogenase (ADH).
Pag1p Is Physically Associated with Cbk1p
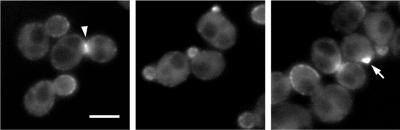
To study the localization of Pag1p, we tagged it with green fluorescent protein (GFP). GFP-Pag1p is fully functional since it could support growth as well as the wild-type Pag1p. GFP-Pag1p expressed from the PAG1 promoter exhibited a punctate fluorescent pattern at the cell periphery and also an evenly distributed cytoplasmic staining (Figure 7). The localization of peripheral staining was cell cycle dependent. Cells in early phases of the cell cycle displayed concentrated and bright staining at the presumptive budding sites and within the tiny buds. In cells with small-to-medium buds, GFP-Pag1p was found in punctate structures covering the entire inner surface of the buds. In cells with large buds that were probably undergoing cytokinesis, GFP-Pag1p concentrated at the mother-daughter necks. This cell cycle dependent distribution is essentially the same as the published localization pattern of GFP-tagged Cbk1p (Racki et al., 2000), suggesting that Pag1p and Cbk1p function at the same sites and may directly associate with each other.
Figure 7.
Pag1p exhibits a polarized localization to the cell periphery. Localization of GFP-tagged Pag1p was detected in living cells (NY2335) grown in YPD. GFP-Pag1p expressed from the PAG1 promoter was the sole copy of the full-length Pag1p in this strain. Bar, 4 μm. A mother-daughter neck and a presumptive budding site are indicated by an arrowhead and an arrow, respectively.
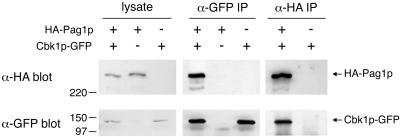
To examine whether Pag1p physically interacts with Cbk1p, we constructed strains expressing N-terminally HA-tagged Pag1p (HA-Pag1p), C-terminally GFP-tagged Cbk1p (Cbk1p-GFP), or both. HA-Pag1p and Cbk1p-GFP could support growth as well as the wild-type proteins and therefore were fully functional. Anti-GFP and anti-HA antibodies were used for immunoprecipitation (IP) from yeast lysates (Figure 8). The anti-GFP IP recovered 22% of the Cbk1p-GFP in the lysates. From the lysate containing both tagged proteins, 9% of the HA-Pag1p was coprecipitated by the anti-GFP antibody. As a control, no HA-Pag1p was precipitated by the anti-GFP antibody from the lysate containing HA-Pag1p but not Cbk1p-GFP. The anti-HA IP recovered 18% of the HA-Pag1p in the lysates. From the lysate containing both tagged proteins, 7% of the Cbk1p-GFP was coprecipitated by the anti-HA antibody. These results indicated that significant portions of Pag1p and Cbk1p in the cell lysate were associated with each other. In conjunction with the localization data, we conclude that Pag1p and Cbk1p are likely to act together in a protein complex in the cell.
Figure 8.
HA-tagged Pag1p coimmunoprecipitates with GFP-tagged Cbk1p. Lysates were prepared from yeast cells expressing HA-Pag1p, Cbk1p-GFP or both. Lysates and immunoprecipitates with anti-HA and anti-GFP antibodies were separated by SDS-PAGE and probed with anti-HA and anti-GFP antibodies.
DISCUSSION
Our characterization of the pag1-1 mutant revealed that it had the same morphogenesis defects as previously characterized cbk1Δ mutants, such as a cell separation defect, a rounder cell shape, and a defect in mating projection formation. In addition, we showed that the bypass suppressor and the high-copy suppressor of pag1Δ also suppressed cbk1Δ. Furthermore, the identification of these suppressors made it possible to compare the phenotypes of pag1Δ and cbk1Δ, which are both lethal in the strain background that we used. We found that in the presence of a suppressor, pag1Δ and cbk1Δ cells had the same defects in sensitivity to SDS and NaCl, cell shape, mating projection formation, cell separation, and chitinase expression level. In addition, the double mutant of pag1Δ and cbk1Δ exhibited the same phenotype as the two single mutants. These results strongly suggest that PAG1 and CBK1 function in the same pathway. This conclusion is strengthened and further extended by our localization and immunoprecipitation results, which provide evidence that Pag1p and Cbk1p are physically associated with each other in the cell.
The physiological functions of many Cbk1p homologs, such as SAX-1 in C. elegans and Tricornered in Drosophila, have been revealed by the characterization of mutants (Geng et al., 2000; Zallen et al., 2000). During the preparation of this manuscript, we learned that sax-2, a gene that acts in the same pathway as sax-1, encodes the homolog of Pag1p in C. elegans (Gallegos and Bargmann, personal communication). Furry, the Drosophila homolog of Pag1p, also functions in the same pathway as the Drosophila homolog of Cbk1p, Tricornered (Cong et al., 2001). Therefore, the functional link between the Pag1p-like proteins and the Cbk1p-like proteins is conserved from budding yeast to C. elegans and Drosophila. Our coimmunoprecipitation result is the first evidence that a Cbk1p-like protein is physically associated with a Pag1p-like protein. Based on the strong conservation of both the protein sequence and the functional relationship, it is likely that the Cbk1p homologs and Pag1p homologs in metazoans also act together in protein complexes.
Previous studies found that cbk1Δ had no growth defect (Racki et al., 2000; Bidlingmaier et al., 2001). However, we showed that CBK1 is essential for cell proliferation in a standard yeast strain and that the lethality of cbk1Δ can be suppressed by disruption of the SSD1 gene. SSD1 has two polymorphic forms in commonly used lab yeast strains. The SSD1-V (viable) allele encodes a protein with an apparent molecular weight of 160 kDa; the ssd1-d (dead) allele found in strains such as W303 and YPH499 expresses an 83-kDa protein that is likely to be a C-terminally truncated fragment with no function (Uesono et al., 1997). SSD1-V has been cloned as a single-copy suppressor of many different mutants in the strains with the ssd1-d background. Therefore, a loss-of-function mutation in SSD1 is synthetically lethal or sick with mutations affecting diverse cellular processes. However, we found that disruption of SSD1 rescues the growth defect of pag1Δ and cbk1Δ. This means that pag1Δ and cbk1Δ would not have severe growth defects in strains carrying the ssd1-d allele, such as in strains of the W303 or YPH499 backgrounds. Therefore, the allelic difference in SSD1 is the likely reason that previous studies did not find any growth defect of cbk1Δ.
Cbk1p has been shown to control two separate morphogenesis pathways (Racki et al., 2000; Bidlingmaier et al., 2001): an Ace2p-dependent pathway that promotes efficient cell separation by up-regulating chitinase expression, and an Ace2p-independent pathway required for polarized cell growth. Our observation that cbk1Δ is lethal to strains with the SSD1-V allele suggests that Cbk1p is involved in an additional pathway that controls cell proliferation. Because disruption of SSD1 suppressed the growth defect but did not significantly alter the cell separation or the polarized growth defects of pag1-1, SSD1 probably does not act in the two morphogenesis pathways previously defined. SSD1 encodes a cytoplasmic protein with in vitro RNA binding ability (Uesono et al., 1997). The RNA binding ability of Ssd1p suggests that it may regulate gene expression at the posttranscriptional level. Our genetic data indicate that SSD1 inhibits cell proliferation and this inhibitory effect is normally counteracted by CBK1 and PAG1. When SSD1 is disrupted, cells no longer require CBK1 or PAG1 for growth. Interestingly, Ssd1p and Ace2p were both identified as Cbk1p-interacting proteins in a two-hybrid screen (Racki et al., 2000). It is conceivable that Ssd1p and Ace2p are kinase substrates of Cbk1p. Consistent with this idea, it has been shown that Ssd1p is a phosphoprotein (Uesono et al., 1994).
We identified SIM1 as a high-copy suppressor of the pag1-1 mutant. Overexpression of Sim1p suppresses both pag1Δ and cbk1Δ, but does not suppress their morphogenesis defects. SIM1 was originally identified in a screen for cell cycle mutants (Dahmann et al., 1995), but its physiological function is not clear. We found that Sim1p is an extracellular protein. Therefore, Sim1p may have a cell surface related function, and the suppression of pag1Δ and cbk1Δ by its overexpression may be due to the compensation of some cell surface defects.
The localization of Pag1p and Cbk1p to the polarized peripheral sites may have important functional implications. This localization indicates that they may be regulated by cell surface sensors that monitor cell wall function. It also places them in proximity to proteins specifically involved in polarized cell growth such as Spa2p, Pea2p, Bu6p, and Bni1p (Sheu et al., 2000). We speculate that the Cbk1p-Pag1p complex may act on some of these proteins to control polarized growth.
The length of Pag1p (2376 amino acids) implies that it has the potential to interact with many binding partners simultaneously. It may serve as a scaffold to recruit other regulatory proteins or downstream targets. We observed that Pag1p and Cbk1p from a detergent lysate sedimented much faster than a 670-kDa marker protein in a sucrose gradient (our unpublished observation). Therefore, the size of the Cbk1p protein complex seemed to be much larger than the sum of one Pag1p molecule and one Cbk1p molecule. One protein that may be associated with the Cbk1p protein complex is Hym1p. hym1Δ has the same transcriptional repression defect (Dorland et al., 2000) and morphogenesis defects (Bidlingmaier et al., 2001) as cbk1Δ. The double mutant of hym1Δ and cbk1Δ does not have more severe defects than the single mutants, indicating that the two genes are involved in the same pathway. In the Saccharomyces Genome Deletion Project background, HYM1 is essential (record number 25039). Disruption of SSD1 or overexpression of Sim1p suppresses the growth defect of hym1Δ in that background (our unpublished observation). Hym1p has homologs in all sequenced eukaryotic genomes and its homolog in the filamentous fungus Aspergillus nidulans is important for vegetative growth and morphological development (Karos and Fischer, 1999). It is likely that Hym1p-related proteins in different organisms function together with the Cbk1p- and Pag1p-related proteins. In addition to Hym1p, there may be other proteins functionally related or even physically associated with the Cbk1p complex. With its facile biochemical and genetic tools, S. cerevisiae is probably an ideal system to uncover unknown subunits in this protein complex.
Note added in proof. It was recently brought to our attention that PAG1 was previously identified in a screen for mutants that activate OCH1 transcription by Drs. Joseph Horecka and Yoshifumi Jigami (The Institute of Molecular and Cell Biology, National Institute of Advanced Science and Technology, Japan) and entered into the Saccharomyces Genome Database (1999) under the name TAO3.
ACKNOWLEDGMENTS
We thank Dr. Christopher J. Herbert for providing the WR154 strain, Elaine Downie and Dr. Susan Ferro-Novick for making anti-Sim1p antibody, and Kimberly Zichichi and Dr. Marc Pypaert for electron microscopy sample preparation and image acquisition. We are grateful for the critical reading of the manuscript by Drs. Eric Grote, Andreas Wiederkehr, Charles Boyd, and Meng-Qiu Dong. This work was supported by grants from the National Institutes of Health to P.N.
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.01–07-0365. Article and publication date are at www.molbiolcell.org/cgi/10.1091/mbc.01–07-0365.
REFERENCES
- Adams A, Gottschling DE, Kaiser CA, Stearns T. Methods in Yeast Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1997. [Google Scholar]
- Bender A, Pringle JR. Use of a screen for synthetic lethal and multicopy suppressee mutants to identify two new genes involved in morphogenesis in Saccharomyces cerevisiae. Mol Cell Biol. 1991;11:1295–1305. doi: 10.1128/mcb.11.3.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidlingmaier S, Weiss EL, Seidel C, Drubin DG, Snyder M. The Cbk1p pathway is important for polarized cell growth and cell separation in Saccharomyces cerevisiae. Mol Cell Biol. 2001;21:2449–2462. doi: 10.1128/MCB.21.7.2449-2462.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong J, Geng W, He B, Liu J, Charlton J, Adler PN. The furry gene of Drosophila is important for maintaining the integrity of cellular extensions during morphogenesis. Development. 2001;128:2793–2802. doi: 10.1242/dev.128.14.2793. [DOI] [PubMed] [Google Scholar]
- Cormack BP, Bertram G, Egerton M, Gow NA, Falkow S, Brown AJ. Yeast-enhanced green fluorescent protein (yEGFP) Microbiology. 1997;143:303–311. doi: 10.1099/00221287-143-2-303. [DOI] [PubMed] [Google Scholar]
- Couch FJ, et al. Generation of an integrated transcription map of the BRCA2 region on chromosome 13q12–q13. Genomics. 1996;36:86–99. doi: 10.1006/geno.1996.0428. [DOI] [PubMed] [Google Scholar]
- Dahmann C, Diffley JF, Nasmyth KA. S-phase-promoting cyclin-dependent kinases prevent re-replication by inhibiting the transition of replication origins to a pre-replicative state. Curr Biol. 1995;5:1257–1269. doi: 10.1016/s0960-9822(95)00252-1. [DOI] [PubMed] [Google Scholar]
- Dorland S, Deegenaars ML, Stillman DJ. Roles for the Saccharomyces cerevisiae SDS3, CBK1 and HYM1 genes in transcriptional repression by. SIN3. Genetics. 2000;154:573–586. doi: 10.1093/genetics/154.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrenberger F, Kronstad J. The ukc1 gene encodes a protein kinase involved in morphogenesis, pathogenicity and pigment formation in Ustilago maydis. Mol Gen Genet. 1999;261:281–289. doi: 10.1007/s004380050968. [DOI] [PubMed] [Google Scholar]
- Geng W, He B, Wang M, Adler PN. The tricornered gene, which is required for the integrity of epidermal cell extensions, encodes the Drosophila nuclear DBF2-related kinase. Genetics. 2000;156:1817–1828. doi: 10.1093/genetics/156.4.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote E, Novick PJ. Promiscuity in Rab-SNARE interactions. Mol Biol Cell. 1999;10:4149–4161. doi: 10.1091/mbc.10.12.4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice RW, Zilian O, Woods DF, Noll M, Bryant PJ. The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 1995;9:534–546. doi: 10.1101/gad.9.5.534. [DOI] [PubMed] [Google Scholar]
- Kaiser CA, Schekman R. Distinct sets of SEC genes govern transport vesicle formation and fusion early in the secretory pathway. Cell. 1990;61:723–733. doi: 10.1016/0092-8674(90)90483-u. [DOI] [PubMed] [Google Scholar]
- Karos M, Fischer R. Molecular characterization of HymA, an evolutionarily highly conserved and highly expressed protein of Aspergillus nidulans. Mol Gen Genet. 1999;260:510–521. doi: 10.1007/s004380050924. [DOI] [PubMed] [Google Scholar]
- Kuranda MJ, Robbins PW. Chitinase is required for cell separation during growth of Saccharomyces cerevisiae. J Biol Chem. 1991;266:19758–19767. [PubMed] [Google Scholar]
- Millward T, Cron P, Hemmings BA. Molecular cloning and characterization of a conserved nuclear serine(threonine) protein kinase. Proc Natl Acad Sci USA. 1995;92:5022–5026. doi: 10.1073/pnas.92.11.5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouyna I, Fontaine T, Vai M, Monod M, Fonzi WA, Diaquin M, Popolo L, Hartland RP, Latge JP. Glycosylphosphatidylinositol-anchored glucanosyltransferases play an active role in the biosynthesis of the fungal cell wall. J Biol Chem. 2000;275:14882–14889. doi: 10.1074/jbc.275.20.14882. [DOI] [PubMed] [Google Scholar]
- Munn AL, Heese-Peck A, Stevenson BJ, Pichler H, Riezman H. Specific sterols required for the internalization step of endocytosis in yeast. Mol Biol Cell. 1999;10:3943–3957. doi: 10.1091/mbc.10.11.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popolo L, Vai M, Gatti E, Porello S, Bonfante P, Balestrini R, Alberghina L. Physiological analysis of mutants indicates involvement of the Saccharomyces cerevisiae GPI-anchored protein gp115 in morphogenesis and cell separation. J Bacteriol. 1993;175:1879–1885. doi: 10.1128/jb.175.7.1879-1885.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racki WJ, Becam AM, Nasr F, Herbert CJ. Cbk1p, a protein similar to the human myotonic dystrophy kinase, is essential for normal morphogenesis in Saccharomyces cerevisiae. EMBO J. 2000;19:4524–4532. doi: 10.1093/emboj/19.17.4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross-Macdonald P, Sheehan A, Roeder GS, Snyder M. A multipurpose transposon system for analyzing protein production, localization, and function in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1997;94:190–195. doi: 10.1073/pnas.94.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu YJ, Barral Y, Snyder M. Polarized growth controls cell shape and bipolar bud site selection in Saccharomyces cerevisiae. Mol Cell Biol. 2000;20:5235–5247. doi: 10.1128/mcb.20.14.5235-5247.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uesono Y, Fujita A, Toh-e A, Kikuchi Y. The MCS1/SSD1/SRK1/SSL1 gene is involved in stable maintenance of the chromosome in yeast. Gene. 1994;143:135–138. doi: 10.1016/0378-1119(94)90618-1. [DOI] [PubMed] [Google Scholar]
- Uesono Y, Toh-e A, Kikuchi Y. Ssd1p of Saccharomyces cerevisiae associates with RNA. J Biol Chem. 1997;272:16103–16109. doi: 10.1074/jbc.272.26.16103. [DOI] [PubMed] [Google Scholar]
- Verde F, Wiley DJ, Nurse P. Fission yeast orb6, a ser/thr protein kinase related to mammalian rho kinase and myotonic dystrophy kinase, is required for maintenance of cell polarity and coordinates cell morphogenesis with the cell cycle. Proc Natl Acad Sci USA. 1998;95:7526–7531. doi: 10.1073/pnas.95.13.7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winzeler EA, et al. Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science. 1999;285:901–906. doi: 10.1126/science.285.5429.901. [DOI] [PubMed] [Google Scholar]
- Xu T, Wang W, Zhang S, Stewart RA, Yu W. Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development. 1995;121:1053–1063. doi: 10.1242/dev.121.4.1053. [DOI] [PubMed] [Google Scholar]
- Yarden O, Plamann M, Ebbole DJ, Yanofsky C. cot-1, a gene required for hyphal elongation in Neurospora crassa, encodes a protein kinase. EMBO J. 1992;11:2159–2166. doi: 10.1002/j.1460-2075.1992.tb05275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zallen JA, Peckol EL, Tobin DM, Bargmann CI. Neuronal cell shape and neurite initiation are regulated by the Ndr kinase SAX-1, a member of the Orb6/COT-1/warts serine/threonine kinase family. Mol Biol Cell. 2000;11:3177–3190. doi: 10.1091/mbc.11.9.3177. [DOI] [PMC free article] [PubMed] [Google Scholar]