Abstract
Few studies have been conducted examining cytokines in cerebrospinal fluid of patients compared to healthy volunteers. The goals of this study were: 1) to report original data detailing cytokine levels in the cerebrospinal fluid (CSF) of 10 patients with a schizophrenia spectrum disorder (SSD) diagnosis and 10 healthy controls and 2) to conduct a meta-analysis of the available data on cytokine levels in the CSF of patients with SSD compared to healthy controls, including our new data. Cytokine concentrations were measured using the Q-plex Human Cytokine Screen array in CSF of 10 patients with SSD and 10 healthy volunteers. For the meta-analysis, an electronic PubMed and Google Scholar search without restrictions was conducted for articles that reported on cytokine levels in CSF in patients with an SSD compared to healthy controls. Our original data revealed statistically significant increases in levels of interleukin-8 (IL-8) and interleukin-1 beta (IL-1β) in the CSF of patients with an SSD compared to healthy volunteers. Our meta-analysis showed statistically significant increases in interleukin-6 (IL-6) and IL-8 in patients compared to healthy volunteers. Effect sizes between treated and untreated patients for IL-6 were of similar magnitude. However, IL-6 levels were higher in early stage schizophrenia patients compared to chronic schizophrenia patients. Studies with larger sample sizes, comprehensive assessments and ideally in the context of a randomized controlled intervention to minimize the impact of confounding factors are needed to fully understand the role of cytokines and inflammatory markers in the pathophysiology and treatment of schizophrenia.
Keywords: cytokines, schizophrenia, cerebrospinal fluid, inflammation
1. Introduction
Several lines of evidence suggest that dysfunction in inflammatory processes may be implicated in the pathophysiology of schizophrenia. First, large epidemiological studies have found an association between maternal infections during pregnancy, such as influenza and rubella, and schizophrenia in offspring (Buka et al., 2001; Limosin et al., 2003). Though the mechanism linking infection and schizophrenia is uncertain, the leading hypothesis involves the release of maternal cytokines to the fetus via the placenta, leading to abnormal fetal brain development (Brown and Patterson, 2011).
Second, recent GWAS studies have consistently shown genome-wide significant associations between schizophrenia and single nucleotide polymorphisms in a large locus on chromosome 6 that includes the major histocompatibility complex, a group of key molecules involved in the inflammatory response (Ripke et al., 2014). A follow-up analysis of this region showed that genetic variation of the complement component 4 (C4) gene, which codes for a protein involved in the classical activation pathway of the complement system, causes greater expression of C4a alleles, and in turn, increased risk for schizophrenia (Sekar et al., 2015).
Third, the availability of Food and Drug Administration-approved non-steroidal anti-inflammatory medications like celecoxib and aspirin for the use in pain and other inflammatory conditions has facilitated the investigation of these medications as add-on treatments to antipsychotic medications in schizophrenia, with the effect of modest improvement of psychotic symptoms in individuals with early stage psychosis (Sommer et al., 2014).
Further work in support of the hypothesis has been conducted by the examination of peripheral biomarkers of inflammation, such as cytokines, in the blood and cerebrospinal fluid (CSF) of people with schizophrenia (Barak et al., 1995; Coughlin et al., 2016; Garver et al., 2003; Hayes et al., 2014). For example, Miller and colleagues completed a meta-analysis of cytokine alterations in blood and CSF of patients with schizophrenia (Miller et al., 2011). Blood analysis showed increases in IL-1β, IL-6 and transforming growth factor- β (TGF-β) in acute relapsed patients and first episode patients compared to healthy controls. CSF analysis showed decreased levels of IL-1β in patients compared to controls and no differences in other cytokines. Similar results were found by Upthegrove and colleagues, who subsequently completed a meta-analysis focusing on cytokine levels in the blood of medication-naive first-episode individuals and found significant elevation in pro-inflammatory cytokines IL-1β, sIL-2r, IL-6, and TNF-α (Upthegrove et al., 2014). Despite early results by Miller and colleagues indicating a decrease in IL-1β in CSF, a subsequent meta-analysis by Wang and Miller found increased levels of IL-1β, in addition to increased levels of IL-6 and IL-8, in the CSF of patients with schizophrenia (Miller et al., 2011; Wang and Miller, 2018).
While several studies have identified cytokine profiles in the blood of patients with schizophrenia spectrum disorders (SSD) compared to healthy controls, fewer studies have been conducted to compare cytokine profiles in CSF. Given that CSF is in direct contact with the brain, it may be that inflammatory abnormalities in brain tissue are more closely reflected in CSF as compared to peripheral blood. Further, although blood and CSF are both sources of peripheral cytokine levels, the transportation of cytokines across the blood brain barrier is conducted by transporters that are specific for unique families of cytokines and can be affected by a variety of factors like circadian rhythm, brain disease or injury (Banks, 1995; Banks et al., 2009). Although evidence suggests blood brain barrier dysfunction in schizophrenia (Vasic et al., 2012) it is yet unclear how blood brain barrier dysfunction may affect cytokine levels in cerebrospinal fluid compared to peripheral blood. For these reasons, we hypothesized that there is a unique cytokine profile in CSF that may provide a better understanding of how inflammation affects the central nervous system in SSD. Therefore, and given that a small number of studies on this topic have been published, we conducted an updated meta-analysis using additional studies, including our own original dataset, and performed subgroup analysis to further determine the effects of variables such as antipsychotic exposure and length of illness on cytokine levels.
2. Materials and Methods
2.1. The Zucker Hillside Hospital study
2.1.1. Inclusion and Exclusion Criteria
Patients were included in the study if they fulfilled DSM-IV criteria for schizophrenia, schizophreniform, schizoaffective disorder or psychosis Not Otherwise Specified (NOS) and were willing and capable of providing informed consent. Subjects were excluded from participation if they were prescribed an anti-coagulant, had a history of an organic brain disorder, a clinically significant thrombocytopenia or coagulopathy based on screening blood tests, acute medical problems or if they met criteria for substance dependence or abuse within six months of consent based on DSM-IV criteria. Healthy controls were also excluded if they had an Axis I diagnosis, a first-degree relative with a known or suspected Axis I disorder, or a significant medical problem based on a self-report questionnaire.
2.1.2. Recruitment and Consent
Patients were recruited from the inpatient and outpatient departments at The Zucker Hillside Hospital. Healthy controls were recruited from the general population via word of mouth, newspaper and internet advertisements, and poster flyers. All subjects provided written informed consent to a protocol approved by the Institutional Review Board of the North Shore-Long Island Jewish Health System. Informed consent of the participants was obtained after the nature of the procedures had been fully explained. After providing informed consent, subjects underwent baseline ratings, including demographic information and the Brief Psychiatric Rating Scale (BPRS).
2.1.3. CSF collection
Subjects underwent a lumbar puncture, performed using a standard technique with a 25 gauge, Whitacre point spinal needle after subcutaneous lidocaine was applied. The procedure was conducted with patients sitting up and all procedures took place at 2 pm. 15–25 cc of CSF were obtained from each subject. The first 2–3 mls obtained after collecting the fluid were sent for routine testing (cell count, proteins, glucose, and VDRL). CSF samples were then flash frozen with liquid nitrogen and stored at −80 Celsius.
2.1.4. Cytokine Analysis
The following cytokines were quantified using the multiplex ELISA-based Q-Plex™ Human Cytokine array (Quansys Biosciences): IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12p70, IL-13, IL-15, IL-17, IL-23, IFNγ, TNFα and TNFβ. We utilized the Q-Plex™ Human Cytokine array given our prior experience with this array, its convenience and cost-effectiveness. All samples were analyzed in triplicates and the average of each sample was taken. The Q-PlexTM technology involves the micro-spotting of individual groups of capture antibody in either a cartesian or polar coordinate system on the bottom of a 96 well plate, each spot being its own micro ELISA. The lower limit of detection for the multiplex assay was the lowest concentration of an analyte where its signal was distinguishable from the background (2 times the standard deviation of the mean of the background). Quantification was performed by S. H-K and M. F at The Feinstein Institute for Medical Research.
2.2. The Meta-analysis
2.2.1. Search Strategy
An electronic Medline (PubMed) search without restrictions was conducted until May 2016 for articles published at any time. A systematic review was initially conducted in PubMed using the following search strategy: the Boolean operator “AND” was used to connect the words “Schizophrenia and Disorders with Psychotic Features”[Mesh] AND (cerebrospinal fluid OR CSF) AND (cytokines OR interleukins OR interferon). Additionally, a non-systematic search to find additional studies in Google Scholar was conducted, along with a manual search of reference lists of included articles and other relevant articles, including prior meta-analyses. Additional information was obtained through direct correspondence with Drs. Engberg,Van Kammen, Pomper, and Coughlin (Van Kammen et al., 1999; Soderlund et al., 2009; Coughlin et al., 2016).
2.2.2. Study Selection
All individual studies that measured quantitative levels of cytokines in cerebrospinal fluid in both patients with an SSD diagnosis (schizophrenia, schizoaffective disorder or schizophreniform disorder) and healthy volunteers were included in the analyses.
2.2.3. Data Extraction
The following data were extracted from each study and for both patients and healthy controls: age; sex; race; cytokines studied; sample size; mean pg/ml and standard deviation for each cytokine; or the median and interquartile if the mean and SD were not available. We used the same approach used by Wang and Miller to estimate mean and SD from median and IQR and as follows: (1) mean = (2m + a + b)/4, where m is the median and a and b are the 25th and 75th percentiles, respectively and (2) IQR = 1.35 × SD (Wang and Miller, 2018). Information was extracted by one investigator (PM) and verified by a second investigator (JdO). We contacted first and last authors for additional information in the case that published articles were missing necessary information for the meta-analysis.
2.3. Statistical Analysis
Analysis of the original data obtained at The Zucker Hillside Hospital was conducted as follows: baseline characteristics were compared between groups using t-tests for continuous variables or chi-square tests for categorical variables. Cytokine levels were compared between groups using t-test for normally distributed cytokine levels or the Wilcoxon rank-sum test in the case of non-normally distributed cytokine levels. Adjustment for multiple comparisons was conducted using the Simes method (Simes, 1986).
For the meta-analysis, pooled standardized mean differences and standard deviation for each cytokine were compared between patients and healthy controls using a random effects model. Publication bias was assessed using Funnel plot and the Egger linear regression test. Subgroup analyses were conducted to determine the role of other variables such as antipsychotic exposure and length of illness in the outcome. Sensitivity analysis were conducted to determine the influence of individual studies on the outcome. Data were analyzed using STATA 11.2 (STATA Statistical Software, College Station, TX, USA, www.stata.com) software. Cohen’s d was used as an effect size measure.
3. Results
3.1. The Zucker Hillside Hospital study
3.1.1. Subjects
Ten patients with an SSD diagnosis and 10 healthy controls matched to patients in age, sex and race were included in this analysis. There were no statistically significant differences in age, sex and race between groups. The mean age of patients was 43 years (SD=8.1; range: 32–56) and the mean age of healthy controls was 40 years (SD=8.8; range: 23–56). Patients had a median BPRS total score of 33 (IQR=27–35). Forty percent of patients (n=4) had a diagnosis of schizoaffective disorder, 30% (n=3) had a diagnosis of schizophrenia, and 30% (n=3) had a diagnosis of psychosis NOS. Patients were comprised of individuals with a chronic diagnosis who were on antipsychotic medication at the time of the lumbar puncture (Table 1). All urine toxicology screenings for patients and controls were negative, excluding one patient who tested positive for benzodiazepines.
Table 1.
Baseline Characteristics
| Patients (n=10) | Healthy Controls (n=10) | p-value | |
|---|---|---|---|
| Mean age, n(SD) | 43.04 (8.12) | 40.02 (8.78) | 0.44 |
| Sex, male, n(%) | 9(90) | 9(90) | >0.9 |
| Race, black, n(%) | 4(40) | 6(60) | 0.49 |
| Median BPRS, n(IQR) | 33(27–35) [5] | 0(0) | |
| Medications | |||
| Antipsychotic n(%) | 6(100) [6]* | NA | |
| Mood stabilizer n(%) | 2(33) [6] | NA | |
| Anti-depressant n(%) | 3(50) [6] | NA | |
| Benzodiazepine n(%) | 4(67) [6] | NA | |
| Outpatient recruitment setting | 9(100) [9] | NA | |
| MATRICS Consensus Cognitive Battery | |||
| Composite Score, median (IQR) | 27(10–34) [7] | 43(41–44) [7] | 0.004 |
| Speed of Processing, median (IQR) | 30(22–32) [7] | 51(42–56) [7] | 0.002 |
| Attention/Vigilance, median (IQR) | 27(16–41) [7] | 53(49–66) [7] | 0.004 |
| Working Memory, median (IQR) | 37(25–40) [7] | 37(34.47) [7] | 0.28 |
| Verbal Learning, median (IQR) | 36(33–39) [7] | 44(37–54) [7] | 0.06 |
| Visual Learning, median (IQR) | 41(25–44) [7] | 36(26–48) [7] | 0.95 |
| Reasoning/Problem Solving, median (IQR) | 36(32–37) [7] | 45(33–48) [7] | 0.15 |
| Social Cognition, median (IQR) | 40(22–45) [7] | 51(45–57) [7] | 0.02 |
IQR- Interquartile Range BPRS- Brief Psychiatric Rating Scale NA- Not Applicable
[ ] -Number of subjects with available data
3.1.2. Cytokine Analysis
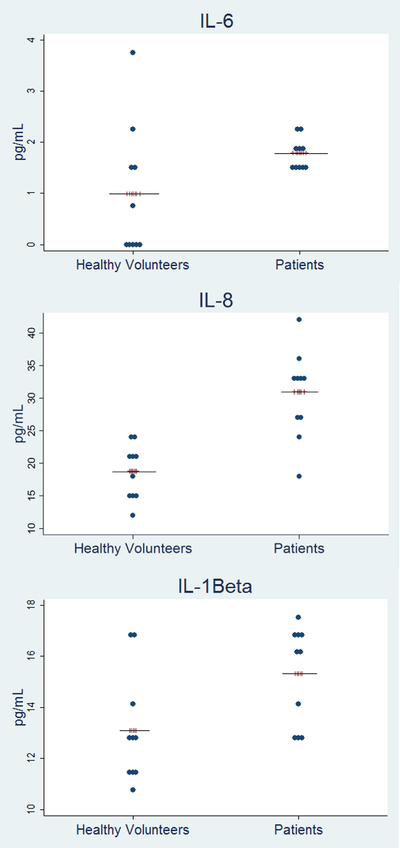
Out of the 16 cytokines tested, only 3 of them were reliably detected in CSF (IL-1β, IL-6 and IL-8). The lower limit of quantification (LLOQ) was a concentration of our analyte at a dilution 1:10. Levels below the lower limit of quantification were set to LLOQ/2 rather than values of zero. Given that the LLOQ was 0.1 for IL-6, we entered 0.05 for the analysis to account for the mean value between 0 and 0.1 (LLOQ). Levels were statistically significantly higher in patients vs. healthy controls for IL-1β (median= 16.25 pg/ml, IQR= 13.13–16.64 vs. median= 12.73 pg/ml, IQR= 11.53–13.85, adjusted-p=0.04) and IL-8 (median= 33.39 pg/ml, IQR= 27.33–34.41 vs. median=19.33 pg/ml, IQR=15.75–22.14, adj-p= 0.01) whereas there were no statistically significant differences in IL-6 levels (median= 1.7 pg/ml, IQR= 1.48–2.06 vs. median= 0.45 pg/ml, IQR= 0.05–1.66, adj-p= 0.06) (Figure 1). Raw data are provided in the supplementary file (Supplementary File; Table S1).
Figure 1.
Cytokine Levels in CSF – Zucker Hillside Study
3.2. The Meta-analysis
3.2.1. Studies
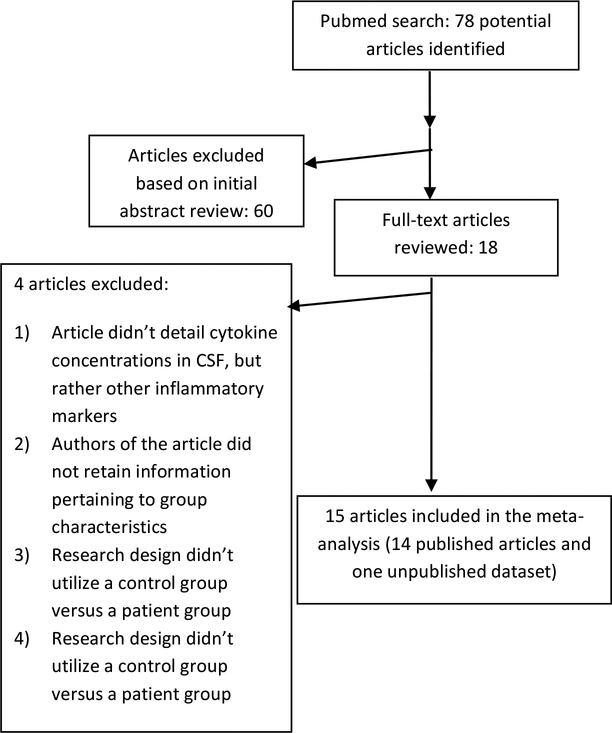
After conducting the systematic search in Pubmed, 78 studies were initially identified. Of those, 60 were excluded at the abstract level. Of the remaining 18 studies, 4 were excluded (Figure 2). A nonsystematic search in Google scholar did not provide any additional studies.
Figure 2.
QUOROM Flow Chart
In the end, fourteen published articles plus the new data collected for this study were included in the meta-analysis (Table 2 and Table S2). For subgroup analyses, studies were classified into a chronic schizophrenia group or an early schizophrenia group. The former group comprised of studies that included patients who had been ill for five or more years while those in the early schizophrenia group included patients who had been ill for less than five years, an approach used by other investigators (Coughlin et al., 2017). Similarly, studies were classified into an antipsychotic treated (“AP treated”) group or a non-antipsychotic treated (“NAP-treated”) group. Study sample sizes ranged from nine participants to 86 participants. In all, 421 patients with a schizophrenia spectrum disorder and 290 healthy volunteers were included in the meta-analysis (Table 2; Table S2).
Table 2.
Characteristics of studies included in the meta-analysis
| First Author | Cytokines analyzed | Mean duration of illness in years ± SD | Antipsychotic Exposure | Sample Size, patients (n) | Mean Age, patients (mean, SD) | Male Sex, patient (%) | Sample Size, controls (n) | Mean Age, controls (mean, SD) | Male Sex, controls (%) | Schizophrenia diagnosis n(%) | Schizoaffective disorder diagnosis n(%) | Recruitment Setting | Symptom Score ± SD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| El-Mallakh et al., 1992 | IL-2 | “Chronic”* | Half unmedicated for 4–6 weeks | 35 | 31.6 (xx) | 74.07 | 11 | 30.3 | 81.81 | 34(97) | 1(3) | Inpatient | N/A |
| Licinio et al., 1993 | IL-1α, IL-2 | 17.4± NA* | AP free for 2 weeks | 10 | 38.5 (12.8) | 100 | 10 | 35.1(13.5) | 100 | 10(100) | 0(0) | Inpatient | N/A |
| Katila et al., 1994 | IL-1β | 18.5±12.2* | On AP | 4 | 42.8 (11) | 64.28 | 5 | 38.3 (7.8) | 55.55 | 4(100) | 0(0) | Inpatient | 27.1 ±8.6 (BPRS) |
| Barak et al., 1995 | IL-1β, IL-2, IL-6 | 12.1±6.3* | On AP | 16 | 42.7 (9.2) | 100 | 10 | 44.8 (9.1) | 100 | 16(100) | 0(0) | Inpatient | N/A |
| Vawter et al., 1996 | TGFβ1, TGFβ2 | “Postmortem” | N/A | 12 | 58.2 (5.5) | 58.33 | 16 | 72.7 (3.1) | 68.75 | 12(100) | 0(0) | Postmortem | N/A |
| Rapaport et al., 1997 | IL-1α, IL-2 | N/A* | On AP | 60 | 29 (5) | 56.6 | 21 | 27 (4) | 52.38 | 60(100) | 0(0) | Inpatient | 54.95±10.89 (BPRS) |
| Vawter et al., 1997 | TGFβ1, TGFβ2 | N/A* | N/A | 44 | 34.5 (1.1) | 77.27 | 19 | 30.2 (2.2) | 68.42 | 44(100) | 0(0) | Inpatient | N/A |
| Van Kammen et al., 1999 | IL-6 | “Chronic”* | On AP at baseline. Follow up was drug free but not entered in the meta-analysis | 61 | 38.1 (7.2) | 100 | 25 | 35.0 (10.2) | 100 | 54(89) | 7(11) | Inpatient | N/A |
| Garver et al., 2003 | IL-6 | N/A* | AP free for 2–7 days | 31 | 34.1 (10.1) | 90.32 | 14 | 32.9 (12.1) | 71.43 | 31(100) | 0(0) | Inpatient | N/A |
| Soderlund et al., 2009 | IL-1β, IL-6, IL-8 | 1 st episode | On AP | 26 | 27.5 (6.6) | 100 | 30 | 25.4 (7.2) | 100 | 26(100) | 0(0) | Inpatient | N/A |
| Sasayama et al.,2013 | IL-6 | 16.2±7.9* | On AP | 32 | 40.8 (8.8) | 62.5 | 35 | 41.3 (16.4) | 60 | 32(100) | 0(0) | Outpatient | |
| Hayes et al., 2014 | IL-6, IL-8 | N/A* | AP free | 46 | 25.8 (0.8) | 78.2 | 35 | 26.4 (0.6) | 78.2 | 46(100) | 0(0) | N/A | N/A |
| Schwieler et al.,2015 | IL-6, IL-8 | median(IQR): 11(5–15.25)* | On AP | 23 | median (IQR): 35.0 (32–41) | 65.2 | 37 | median (IQR): 23.0 (22–25.5) | 62.2 | 21(91) | 2(9) | Outpatient | median (IQR): 33.0 (27–35) (PANSS) |
| Coughlin JM et al., 2016 | IL-6 | Recent Onset (within the first 5 years of illness) | On AP | 11 | 24.1(3.1) | 79 | 12 | 24.9 (4.7) | 56 | 11(100) | 0(0) | N/A | N/A |
| Gallego et al., 2017 | IL-1β, IL-6, IL-8 | “Chronic”* | On AP | 10 | 43(8.1) | 90 | 10 | 40(8.8) | 90 | 3(30) | 4(40) | Inpatient/Outpatient | 30.4(5.8) (BPRS) |
-Uploaded separately for formatting purposes
AP free Anti-psychotic medication free
On AP On anti-psychotic medication
N/A Not Available
BPRS Brief Psychiatric Rating Scale
IQR Interquartile Range
IL Interleukin
TGFβ1 Transforming growth factor beta 1
PANSS Positive and Negative Syndrome Scale
TGFβ2 Transforming growth factor beta 2
SD Standard Deviation
Denotes a chronic patient sample
3.2.2. IL-6 analysis
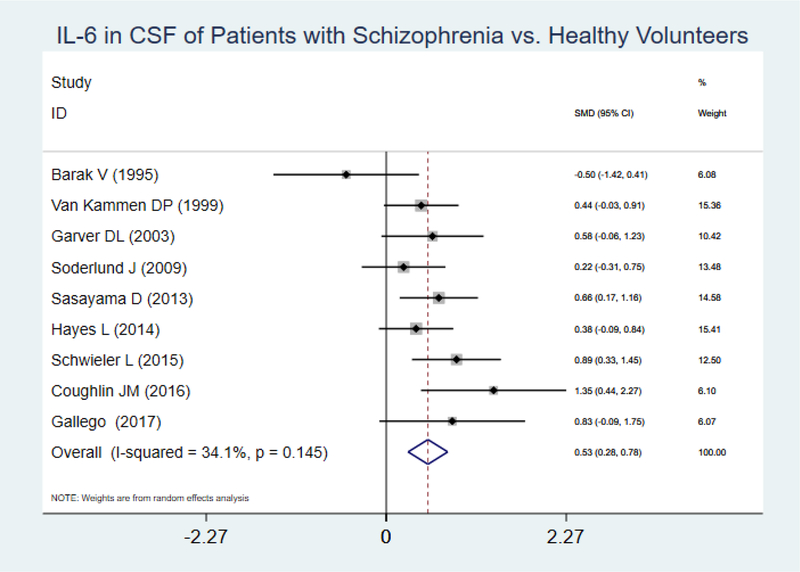
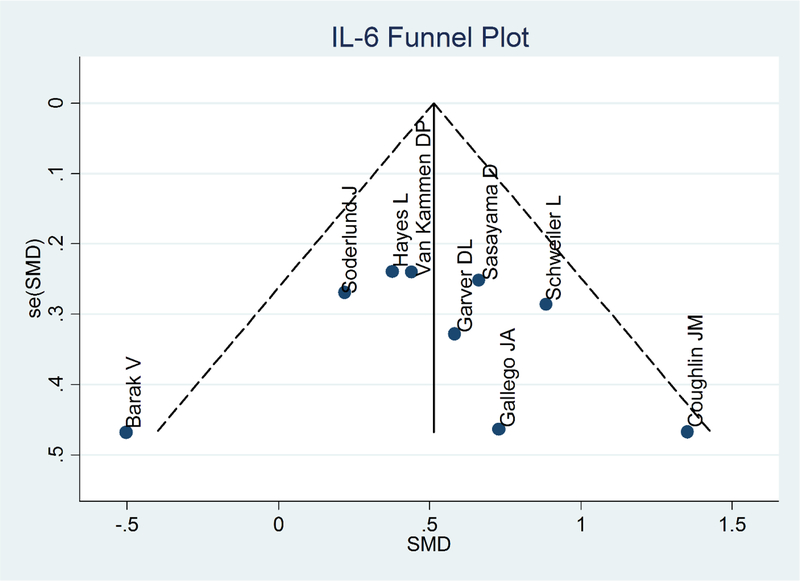
Eight articles and one unpublished dataset of our own were included. Analysis using a random effects model showed that IL-6 levels were statistically significantly higher in patients compared to healthy volunteers (SMD= 0.53, 95% CI =0.28–0.78, p<0.001) (I2 : 34.1%, p=0.145)(Figure 3). Inspection of the forest plot revealed a potential outlier (Barak et al., 1995). Sensitivity analysis conducted after removing this study showed no changes in significance level and a minor increase in SMD (SMD= 0.57, 95% CI =0.37–0.77, p<0.001). Publication bias was not evident after examining the Funnel plot and the Egger test (Figure 4).
Figure 3.
IL-6 in CSF of Patients with Schizophrenia vs. Healthy Volunteers
Figure 4.
IL-6 Funnel Plot
Subgroup analysis by antipsychotic exposure (AP-treated and NAP-treated) was completed using a random effects model. Pooled SMD in both strata were statistically significantly higher and of similar magnitude in patients vs. controls (AP-treated: SMD= 0.55, 95% CI=0.22–0.89, p=0.001, n=7; NAP-treated: SMD= 0.45, 95% CI =0.07–0.83, p=0.02, n=2).
Subgroup analysis by stage of illness was completed using a random effects model. The pooled SMD between groups (patients vs. controls) was higher in the early psychosis sample (SMD=0.72, 95% CI= [−0.38]–1.83, p=0.2, n=2) compared to the chronic sample (SMD= 0.52, 95% CI=0.27–0.77, p<0.001, n=7)
3.2.3. IL-8 analysis
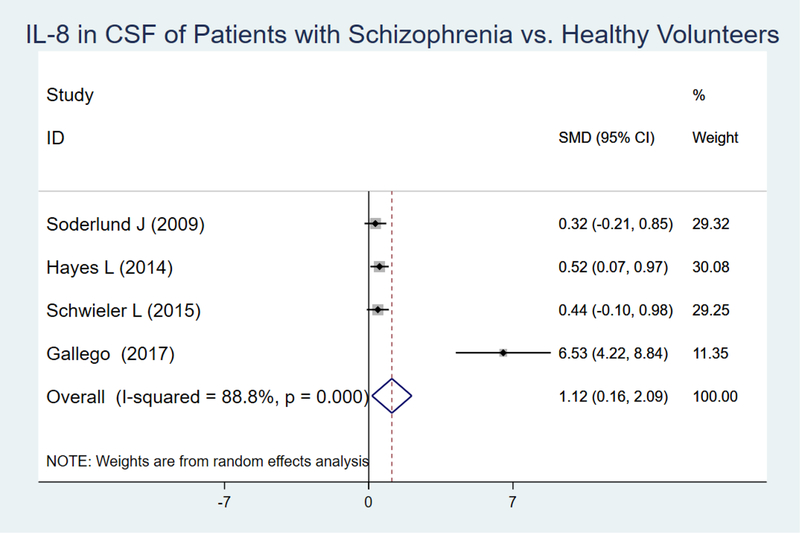
Three articles and our original data were included in the analysis. After conducting a random effects model the pooled SMD between patients with an SSD diagnosis and healthy controls for IL-8 was 1.12 (95% CI =0.16–2.09, p=0.02) (I2 : 88.8%, p<0.001) (Figure 5). Inspection of the Funnel plot suggested the possibility of publication bias (Supplementary File; Figure S1). Further assessment using the Egger test revealed small-study effects (p=0.039).
Figure 5.
IL-8 in CSF of Patients with Schizophrenia vs. Healthy Volunteers
Inspection of the forest plot showed a potential outlier (our own original data). Sensitivity analyses were then conducted, and after removing our study, the main effect remained statistically significant but the SMD decreased to 0.44 (95% CI=0.15–0.73, p=0.003). Moreover, the small study effects present in the overall IL-8 meta-analysis and detected by the Egger test were no longer present (p=0.41).
3.2.4. IL-1β analysis
Three articles and our original data were included in the analysis. A random effects model was used, and no statistically significant difference was observed between groups (SMD= −0.11, 95% CI = [−0.11] – [−1.84], p=0.91, n=4) (I2 : 92.9%; p<0.001) (Supplementary File; Figure S2). A funnel plot suggested the presence of publication bias but the small number of studies included limits the interpretation (Supplementary File; Figure S3). Subgroup analysis could not be conducted given the lack of NAP-treated or early-stage SSD studies for this specific meta-analysis.
3.2.5. IL-2 analysis
Four articles were included in the analysis. A random effects model was used and no statistically significant differences were noted between groups (SMD=0.085, CI 95%= [−0.43]–0.60, p=0.75, n=4) (I2 : 49.9%; p=0.112) (Supplementary File; Figures S4 and S5).
3.2.6. Other Cytokines
Too few articles were available to conduct meaningful meta-analysis for IL-1α (n=2), TGFβ1 (n=2) and TGFβ2 (n=2).
4. Discussion
In the original data section of this manuscript, we report statistically significant increases in levels of IL-1β and IL-8 in the cerebrospinal fluid of patients with SSD compared to healthy volunteers. Although differences in IL-6 levels were not statistically significant, increased levels in patients were observed compared to healthy volunteers. In the meta-analysis, we report statistically significant elevations in IL-6 and IL-8 in CSF of patients with an SSD compared to healthy volunteers but no statistically significant differences in IL-1β.
The results of our meta-analysis confirm some of the findings of our original data as well as the results of the meta-analysis conducted by Wang and Miller (Wang and Miller, 2018). We were able to update the cytokine portion of Wang and Miller’s meta-analysis by including datasets from Coughlin and colleagues as well as our own original data (Coughlin et al., 2016; Wang and Miller, 2018). Our meta-analytic finding that IL-6 and IL-8 are increased in those with an SSD are consistent with their results but our meta-analysis did not replicate their IL-1β results. This discrepancy may be due to differences in the model used for analysis, as we used a random effects model whereas Wang and Miller utilized a fixed effects model (Wang and Miller, 2018). We chose a random effects model based on high heterogeneity between studies, likely due to individual studies having small sample sizes and different study designs. This high heterogeneity was particularly shown by IL-1β results demonstrating an I2 value of 92.95% (p<0.0001).
Data thus far strongly supports the finding of elevated IL-6 levels in CSF of SSD patients. All but one study observing IL-6 (Barak et al., 1995) demonstrate increased IL-6 levels of a similar magnitude in patients with an SSD compared to healthy volunteers (Figure 3). Two critical questions are whether this increase is a marker of acute psychosis vs. a trait marker and whether antipsychotic exposure affects the levels in CSF. Our IL-6 subgroup analysis suggest that antipsychotic exposure did not have a meaningful effect on IL-6 levels, whereas being in the early stage of the illness was associated with higher IL-6 values. A similar finding was reported by Miller and colleagues, who reported that the association between abnormalities in IL-6 measured in peripheral blood and acute relapse is independent of antipsychotic treatment, although a more recent meta-analysis by Goldsmith and colleagues reported decreases in IL-6 levels in blood after treatment (Goldsmith et al., 2016; Miller et al., 2011). Interestingly, Van Kammen and colleagues, in one of the few longitudinal studies that performed follow up lumbar punctures in the same subjects, found that plasma IL-6 levels change with haloperidol withdrawal and subsequent relapse whereas CSF levels remain elevated despite relapse status or AP exposure (Van Kammen et al., 1999).
Unfortunately, we could not conduct subgroup analysis given the limited number of studies for IL-8. Very limited data is available to understand the relationship between treatment and length of illness variables and IL-8 levels in CSF. Two recent meta-analyses of cytokine alterations in peripheral blood in schizophrenia did not find any effects of AP exposure on blood IL-8 levels or did not report any data (Goldsmith et al., 2016; Tourjman et al., 2013). Moreover, IL-8 levels have not been measured in blood of first episode psychosis patients (Upthegrove et al., 2014).
The direct relationship between abnormalities in IL-6, IL-1β and IL-8 and a diagnosis of schizophrenia is unclear. However, IL-6 is thought to have an epigenetic effect by enhancing the nuclear translocation of DNA Cytosine-5-Methyltransferase (DNMT1), which in turn increases the methylation of DNA sequences and represses the GAD67 promoter (Hodge et al., 2007; Kundakovic et al., 2009). Given GAD67’s role in GABA synthesis and signaling, the repression of GAD67 by IL-6 implicates IL-6 as having a role in GABAergic neuronal dysfunction. Increased levels of IL-1B are thought to have an effect on the expression of genes that regulate glutamate neurotransmission and to be partially responsible for increased intracellular glutamate resulting in decreased neuronal viability (Soderlund et al., 2009; Ye et al., 2013). Lastly, fetal exposure to IL-8 in the second and third trimesters of pregnancy has been associated with obstetric complications, a future diagnosis of schizophrenia in offspring and disruptions in fetal neuroanatomical development commonly associated with schizophrenia (Ellman et al., 2010). On the other hand, there is evidence that cytokine abnormalities are not specific to schizophrenia and that these are encountered in other psychiatric disorders (Wang and Miller, 2018). Interestingly, Wang and Miller (2018) found an increase of 1L-1β levels in the CSF of those with bipolar disorder and schizophrenia compared to healthy controls and an increase of IL-6 and IL-8 in the CSF of those with schizophrenia and depression compared to healthy controls. These results suggest then that despite overlapping findings, there are also specific cytokine abnormalities that are more prominent in certain psychiatric disorders.
Our original study has limitations. It utilized a small sample size, which included only adults and mostly male subjects. In addition, all patients were on antipsychotic treatment and 30% of patients had a diagnosis of psychosis NOS. However, we did not find any statistically significant differences in cytokine levels between patients with psychosis NOS versus patients with either schizophrenia or schizoaffective disorder (data not shown). Our findings are also limited by the fact that we did not systematically collect data about smoking, which has been shown to affect cytokine levels peripherally (Zhang et al., 2008). Therefore, the generalizability of our original study is limited by the small sample size and the restricted characteristics of our subjects.
Given widespread regulatory and research challenges in conducting CSF research, studies included in the meta-analysis had similar limitations compared to our own study. Therefore, the number of included studies is small and the sample sizes of those studies are typically small. In addition, data related to other potential confounding/interacting factors like antipsychotic type and dose, duration of treatment, body mass index, smoking status, severity of illness, and chronic medical complications were not consistently collected or reported, which prevented us from conducting more comprehensive statistical analysis to better understand the impact of these other factors on cytokine levels. Therefore, the results of our meta-analysis, and especially the subgroup analyses, should be regarded as preliminary. Lastly, an important limitation, not only of our original study but also of studies included in the meta-analysis, is that analyses were not performed using advanced CSF analytic methods as they are more commonly done in neurology (Jarius et al., 2010; Reiber and Peters, 2011; Gastaldi et al., 2017). These advanced methods include measuring all immune globulin classes in paired blood-CSF samples, measuring albumin in CSF, providing individual CSF reports and using parameters with well stablished cut-offs (Reiber and Peters, 2011; Gastaldi et al., 2017). Unfortunately, we did not measure immunoglobulins or albumin in CSF or collect blood samples from subjects, and therefore we could not perform these advanced CSF analyses. This was also true for all studies included in the meta-analysis except one (Vawter et al., 1996) which used some but not all of the advanced CSF analytic methods.
Moving forward, a critical question to explore is whether cytokine levels in peripheral blood correlate with cytokine levels in cerebrospinal fluid. Given the fact that that certain cytokines are transported across the blood brain barrier and that transporters are unique for certain groups of cytokines, it is possible that the degree of correlation is far from perfect (Banks et al., 1995; Banks, 2009). An additional factor to consider is the fact that the blood brain barrier permeability increases in the presence of inflammation, a process that it is believed to occur in schizophrenia (Muller and Ackenheil, 1995; Varatharaj and Galea, 2017). Notably, Sasayama and colleagues obtained CSF and peripheral blood samples from schizophrenia patients and found no correlation between CSF and serum IL-6 levels (Sasayama et al., 2013). Additionally, and as reported by Van Kammen and colleagues, blood levels of IL-6 may be considered more a state marker while CSF levels of IL-6 seem independent of state variables as it remains elevated regardless antipsychotic exposure or clinical status (Van Kammen et al., 1999).
In summary, a subset of pro-inflammatory cytokines is elevated in the cerebrospinal fluid of patients with a SSD diagnosis, although some findings are inconsistent across individual studies, likely due in part to a small number of studies and subjects. Moreover, cytokine levels are likely affected by other genetic, illness, and environmental factors, which were not accounted for in the majority of studies. Therefore, future studies would benefit from systematically collecting information about these factors and conducting advanced CSF analytic methods to better account for the effect of these factors in cytokine levels. Future studies would also benefit from recruiting a larger number of subjects, including antipsychotic-free and first-episode SSD patients, conducting follow-up lumbar punctures to help us understand the impact of length of illness and AP exposure on cytokine levels, and concomitantly collecting peripheral blood samples to help us understand the relationship between peripheral blood and CSF cytokine levels in patients with SSD diagnoses.
Supplementary Material
Acknowledgements
The authors would like to thank Drs. Engberg, Van Kammen, Pomper, and Coughlin for sharing information about their studies.
Funding
This study was supported in parts by two NARSAD Young Investigator Grants (PI: J. Gallego; PI: S. Husain-Krautter) from the Brain & Behavior Research Foundation and a K23MH100264 from the National Institute of Mental Health (PI: J. Gallego). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Brain & Behavior Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest
Drs. Gallego, Husain-Krautter, Moreno, Del Ojo, Lencz, Rothstein and Ahmed have nothing to disclose. Emily Blanco and E. Madeline Fagen have nothing to disclose. Dr. Malhotra is a consultant to Genomind Inc, InformedDNA, and Concert Pharmaceuticals.
References
- Banks WA, Kastin AJ, Broadwell RD, 1995. Passage of Cytokines across the Blood-Brain Barrier. Neuroimmunomodulation. 2, 241–248. [DOI] [PubMed] [Google Scholar]
- Banks WA, 2009. Characteristics of compounds that cross the blood-brain barrier. BMC Neurology. 9(Suppl 1), S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak V, Levine J, Nisman B, Nolsman I, 1995. Changes in Interleukin-1B and Soluble Interleukin-2 Receptor Levels in CSF and Serum of Schizophrenic Patients. J Basic and Clin Physiol and Pharmacol. 6, 61–69. [DOI] [PubMed] [Google Scholar]
- Brown AS, Patterson PH, 2011. Maternal Infection and Schizophrenia: Implications for Prevention. Schizophr Bull. 37, 284–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buka SL, Tsaung MT, Torrey EF, Klebanoff MA, Bernstein D, Yolken RH, 2001. Maternal Infections and Subsequent Psychosis Among Offspring. Arch Gen Psych. 58, 1032–1037. [DOI] [PubMed] [Google Scholar]
- Coughlin JM, Wang Y, Ambinder EB, Ward RE, Minn I, Vranesic M, Kim PK, Ford CN, Higgs C, Hayes LN, Schretlen DJ, Dannals RF, Sawa A, Pomper MG, 2016. In vivo markers of inflammatory response in recent-onset schizophrenia: a combined study using [11C]DPA-713 PET and analysis of CSF and plasma. Transl Psychiatry. 6, e777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin JM, Hayes LN, Tanaka T, Xiao M, Yoklen RH, Worley P, Leweke FM, Sawa A, 2017. Reduced superoxide dismutase-1 (SOD1) in cerebrospinal fluid of patients with early psychosis in association with clinical features. Schizophr Res. 183, 64–69. [DOI] [PubMed] [Google Scholar]
- Ellman LM, Deicken RF, Vinogradov S, Kremen WS, Poole JH, Kern DM, Tsai WY, Schaefer CA, Brown AS, 2010. Structural brain alterations in schizophrenia following fetal exposure to the inflammatory cytokine interleukin-8. Schizophr Res. 121, 46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Mallakh RS, Suddath RL, Wyatt RJ, 1992. Interleukin 1-a and Interleukin-2 in Cerebrospinal Fluid of Schizophrenic Subjects. NeuroPsychopharmacol Biol Psychiatry. 13, 383–391. [DOI] [PubMed] [Google Scholar]
- Garver D, Tamas RL, Holcomb J, 2003. Elevated Interleukin-6 in the Cerebrospinal Fluid of a Previously Delineated Schizophrenia Subtype. Neuropsychopharm. 28, 1515–1520. [DOI] [PubMed] [Google Scholar]
- Gastaldi M, Zardini E, Leante R, Ruggieri M, Costa G, Cocco E, De Luca G, Cataldo I, 2017. Cerebrospinal fluid analysis and the determination of oligoclonal bands. Neurol Sci. (Suppl 2): S217–S224. [DOI] [PubMed] [Google Scholar]
- Goldsmith DR, Rapaport MH, Miller BJ, 2016. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatr. 21, 1696–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes L, Severance EG, Leek JT, Gressit KL, Rohleder C, Coughlin JM, Leweke FM, Yolken RH, Sawa A, 2014. Inflammatory Molecular Signature with Infectious Agents in Psychosis. Schizophr Bull. 40, 963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge DR, Cho E, Copeland TD, Guszczynski T, Yang E, Seth AK, Farrar WL, 2007. IL-6 enhances the nuclear translocation of DNA cytosine-5-methyltransferase 1 (DNMT1) via phosphorylation of the nuclear localization sequence by the AKT kinase. Cancer Genomics Proteomics. 6, 387–98. [PubMed] [Google Scholar]
- Jarius S, Franciotta D, Paul F, Ruprecht K, Bergamaschi R, Rommer PS, Reuss R, Probst C, Kristoferitsch W, Wandingerm KP, Wildemann B, 2010. Cerebrospinal fluid antibodies to aquaporin-4 in neuromyelitis optica and related disorders: frequency, origin, and diagnostic relevance. J Neuroinflammation. 7, 52–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katila H, Hurme M, Wahlbeck K, Appelberg B, Rimon R, 1994. Plasma and Interleukin-1B and Interleukin-6 in Hospitalized Schizophrenia Patients. Neuropsychobiology. 30, 20–23. [DOI] [PubMed] [Google Scholar]
- Kundakovic M, Chen Y, Guidotti A, Grayson DR, 2009. The Reelin and GAD67 Promoters Are Activated by Epigenetic Drugs That Facilitate the Disruption of Local Repressor Complexes. Mol. Pharmacol 75, 342–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licinio J, Seibyl JP, Altemus M, Charney DS, Krystal JH, 1993. Elevated CSF Levels of Interleukin-2 in Neuroleptic-Free Schizophrenia Patients. Am J Psychiatry. 150, 1408–10. [DOI] [PubMed] [Google Scholar]
- Limosin F, Rouillon F, Payan C, Cohen JM, Strub N, 2003. Prenatal exposure to influenza as a risk factor for adult schizophrenia. Acta Psychiatr Scand. 107, 331–335. [DOI] [PubMed] [Google Scholar]
- McAllister CG, Van Kammen DP, Rehn TJ, Miller AL, Gurklis J, Kelley ME, Yao J, Peters JL, 1995. Increases in CSF levels of interleukin-2 in schizophrenia: effects of recurrence of psychosis and medication status. Am J Psychiatry. 152, 1291–7. [DOI] [PubMed] [Google Scholar]
- Muller N, Ackenheil M, 1995. Immunoglobulin and albumin content of cerebrospinal fluid in schizophrenic patients: relationship to negative symptomatology. Schizophr Res. 14, 223–8. [DOI] [PubMed] [Google Scholar]
- Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B, 2011. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol. Psychiatry 70, 663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport MH, McAllister CG, Pickar D, Tamarkin L, Kirch DG, Paul SM, 1997. CSF IL-l and IL-2 in medicated schizophrenic patients and normal volunteers. Schizophr Res. 25, 123–129. [DOI] [PubMed] [Google Scholar]
- Reiber H, Peter JB, 2001. Cerebrospinal fluid analysis: disease-related data patterns and evaluation programs. J. Neurol. Sci 184, 101–122. [DOI] [PubMed] [Google Scholar]
- Ripke S, Neale BM, Corvin A, Walters JT, Farh KH, Holmans PA, Lee P, Bulik-Sullivan B, et al. , 2014. Biological Insights From 108 Schizophrenia-Associated Genetic Loci. Nat Genet. 511, 421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasayama D, Hattroi K, Wakabayashi C, Teraishi T, Hori H, Ota M, Yoshida S Arima K, Hiquchi T, Amano N, Kunugi H, 2013. Increased cerebrospinal fluid interleukin-6 levels in patients with schizophrenia and those with major depressive disorder. J of Psychiatr Res. 47, 401–6. [DOI] [PubMed] [Google Scholar]
- Schwieler L, Larsson MK, Skogh E, Kegel ME, Orhan F, Abdelmoaty S, Finna A, Bhat M, Samuelsson M, Lundberg K, Dahl ML, Sellgren C, Schuppr-Koistinen I, Svensson C, Erhardt S, Engberg G, 2015. Increased levels of IL-6 in the cerebrospinal fluid of patients with chronic schizophrenia — significance for activation of the kynurenine pathway. J Psychiatry Neurosci. 40, 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, Kamitaki N, Tooley K, Presumey J, Baum M, Van Doren V, Genovese G, Rose SA, Handsaker RE, Schizophrenia Working Group of the Psychiatric Genomics Consortium, Daly MJ, Carroll MC, Stevens B, McCarroll SA, 2016. Schizophrenia risk from complex variation of complement component 4. Nature. 530, 177–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simes RJ 1986. An improved Bonferroni procedure for multiple tests of significance. Biometrika. 73, 751–754. [Google Scholar]
- Soderlund J, Schroder J, Nordin C, Samuelsson M, Walther-Jallow L, Karlsson H, Erhardt S, Engberg G, 2009. Activation of brain interleukin-1b in schizophrenia. Mol. Psychiatry 14, 1069–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer IE, van Westrhenen R, Begemann MJH, de Witte LD, Leucht S, Kahn RS, 2014. Efficacy of Anti-inflammatory Agents to Improve Symptoms in Patients With Schizophrenia: An Update. Schizophr Bull. 40, 181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourjman V, Kouassi E, Koué ME, Rocchetti M, Fortin-Fournier S, Fusar-Poli P, Potvin S, 2013. Antipsychotics’ effects on blood levels of cytokines in schizophrenia: a metaanalysis. Schizophr Res. 151, 43–47. [DOI] [PubMed] [Google Scholar]
- Upthegrove R, Manzanares-Teson N, Barnes NM, 2014. Cytokine function in medication-naive first episode psychosis: a systematic review and meta-analysis. Schizophr Res. 155, 101–108. [DOI] [PubMed] [Google Scholar]
- Van Kammen DP, McAllister-Sistilli CG, Kelley ME, Gurklis JA, Yao JK, 1999. Elevated interleukin-6 in schizophrenia. Psychiatry Res. 87, 129–136. [DOI] [PubMed] [Google Scholar]
- Vawter MP, Dillon-Carter O, Tourtellotte WW, Carvey P, Freed WJ, 1996. TGFb1 and TGFb2 Concentrations Are Elevated in Parkinson’s Disease in Ventricular Cerebrospinal Fluid. Exp. Neurol 142, 313–322. [DOI] [PubMed] [Google Scholar]
- Vawter MP, Dillon-Carter O, Issa F, Wyatt RJ, Freed WJ, 1997. Transforming growth factors beta 1 and beta 2 in the cerebrospinal fluid of chronic schizophrenic patients. Neuropsychopharmacology. 16, 83–87. [DOI] [PubMed] [Google Scholar]
- Varatharaj A, Galea I, 2017. The blood-brain barrier in systemic inflammation. Brain Behav Immun. 60, 1–12. [DOI] [PubMed] [Google Scholar]
- Vasic N, Connemann BJ, Wolf RC, Tumani H, Brettschneider J, 2012. Cerebrospinal fluid biomarker candidates of schizophrenia: where do we stand? Eur Arch Psychiatry and Clin Neurosci. 262, 375–391. [DOI] [PubMed] [Google Scholar]
- Wang AK, Miller BJ, 2018. Meta-analysis of Cerebrospinal Fluid Cytokine and Tryptophan Catabolite Alterations in Psychiatric Patients: Comparisons Between Schizophrenia, Bipolar Disorder, and Depression. Schizophr Bull. 44, 75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, Huang Y, Zhao L, Li Y, Sun L, Zhou Y, Qian G, Zheng JC, 2013. IL-1β and TNF-α induce neurotoxicity through glutamate production: a potential role for neuronal glutaminase. J Neurochem. 125, 897–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XY, Cao LY, Song C, Wu GY, Chen DC, Qi LY Wang F, Xiu MH, Chen S, Zhang Y, Lu L, Kosten TA, Kosten TR, 2008. Lower serum cytokine levels in smokers than nonsmokers with chronic schizophrenia on long-term treatment with antipsychotics. Psychopharmacology. 201, 383–389. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.