Abstract
Acetate is a major source of energy and substrate for milk fat synthesis in the dairy cow. We recently reported a linear increase in milk fat yield and greater than a 30% net apparent transfer of acetate to milk fat with ruminal infusion of neutralized acetate. Additionally, ruminal acetate infusion linearly increases plasma ß-hydroxybutyrate. The objective of the current study was to investigate the ability of acetate and butyrate fed in a diet to increase milk fat synthesis. Twelve multiparous lactating Holstein cows were randomly assigned to treatments in a 3×3 Latin square design with 14 d periods that included a 7 d washout followed by 7 d of treatment. Cows were fed ad libitum a basal diet with a low risk for biohydrogenation-induced milk fat depression and treatments were mixed into the basal diet. Treatments were: 3.2% NaHCO3 (control), 2.9% sodium acetate, and 2.5% calcium butyrate (carbon equivalent to acetate treatment) as a percent of diet dry matter. Feeding sodium acetate increased DMI by 2.7 kg, had no effect on milk yield, and increased milk fat yield by 90 g/d and concentration by 0.2 percent units, compared to control. Calcium butyrate decreased DMI by 2.6 kg/d, milk yield by 1.65 kg/d, and milk fat yield by 60 g/d, compared to control. Sodium acetate increased concentration and yield of 16 carbon mixed source fatty acids (FA) and myristic acid, while decreasing the concentration of preformed FA, compared to control. Calcium butyrate had no effect on concentration of milk FA by source, but increased concentration of trans-10 C18:1 in milk by 18%, indicating a shift in rumen biohydrogenation pathways. Our data demonstrates that milk fat yield and concentration can be increased by feeding sodium acetate at 2.9% of diet DM, but not by feeding calcium butyrate at an equivalent carbon mass.
Keywords: acetate, butyrate, dairy cow, milk fat synthesis
INTRODUCTION
Milk fat production is important to the dairy industry because of its high market value and the properties milk fat imparts to dairy products. However, of all milk components, milk fat is the most variable, and is highly influenced by diet nutrient composition (Palmquist et al., 1993). Therefore, dietary strategies aiming to increase milk fat synthesis are of special interest.
Milk fatty acids (FA) originate either from de novo synthesis in the mammary gland or from uptake of plasma FA from dietary absorption or endogenous reserves (preformed FA). Acetate is the predominant substrate for de novo FA synthesis in the dairy cow because it serves as a 2-carbon donor for synthesis of malonyl Co-A and for NADPH synthesis through the isocitrate pathway (Bauman et al., 1970; Ingle et al., 1973; Smith, 1983). Additionally, in the well-fed cow, acetate is the major VFA produced in the rumen and provides 45% of the energy arising from VFA metabolism (Baldwin and Smith, 1971; Bergman, 1990). Therefore, acetate is fundamental for meeting energy requirements and for milk fat synthesis in the dairy cow.
Studies examining the role of acetate supply on milk fat synthesis were mostly conducted over 30 years ago [1955 to 1978; summarized in Urrutia and Harvatine (2017b)]. A meta-analysis primarily based on these early studies reported that acetate infusions linearly increased milk fat yield and concentration (Maxin et al., 2011), but these studies were small and used lower producing cows (14.3 ± 3.2 kg/d) fed diets very different from contemporary rations, therefore limiting extrapolation.
We recently reported that ruminal infusion of acetate neutralized to pH 6 increases milk fat yield (Urrutia and Harvatine, 2017a; Urrutia and Harvatine, 2017b). Specifically, in a 4 d dose response study, acetate linearly increased milk fat concentration and quadratically increased milk fat yield, with milk fat yield increasing 100, 217, and 185 g/d when providing 300, 600, and 900 g/d of sodium acetate respectively, compared to a sodium chloride control (Urrutia and Harvatine, 2017b). Acetate infusion also increased plasma BHB linearly at a rate of 5% per mole of acetate, possibly through conversion of acetate to butyrate in the rumen and rumen wall, and subsequent conversion to BHB (Sutton et al., 2003). BHB provides about half of the initial carbons to make the 4 carbon primer for de novo FA synthesis (Palmquist et al., 1969), therefore, it is possible that a portion of the increase in milk fat synthesis observed during rumen acetate infusions is due to increased plasma BHB. In the meta-analysis of Maxin et al., (2011), a greater milk fat response was observed for butyrate than acetate infusion; milk fat yield increased 127 g/d (R2adj = 0.68) and concentration increased 7.96 g/kg for each kg of additional butyrate (P < 0.001, n = 6). These data support a role for butyrate in stimulation of milk fat synthesis, but also provides a mechanism for increased milk fat synthesis observed during rumen infusion of acetate. The objective of the current experiment was to investigate the ability of the milk fat precursors acetate and butyrate to increase milk fat synthesis when fed in a TMR.
MATERIAL AND METHODS
Experimental Design and Treatments
All experimental procedures were approved by the Pennsylvania State University Institutional Animal Care and Use Committee. Twelve multiparous lactating Holstein cows (183 ± 45 DIM; 45 ± 4.7 kg milk/d) were randomly assigned to treatments in a 3×3 Latin square design with 14 d periods, which included a 7 d washout and 7 d treatment period. Cows were housed in a tie-stall barn located at the Pennsylvania State University Dairy Production Research and Teaching Center.
Cows were fed once daily at 0800 h at approximately 110% of expected intake and refusals were recorded daily. Treatments were sodium bicarbonate control (3.2% of diet DM), sodium acetate at 2.9% of diet DM (targeting 10 mol of acetate/d; NaAc; Food grade anhydrous sodium acetate, Kemin Industries Inc., Des Moines, IA), and calcium butyrate at 2.5% of diet DM (CaBu; Kemin Industries Inc.). The control was designed to provide equal moles of sodium as NaAc, and CaBu was designed to provide the same mass of carbon as NaAc. The basal TMR was balanced to have a low risk for biohydrogenation-induced milk fat depression as it contained adequate NDF and few rumen available unsaturated FA sources (Table 1). The basal TMR was mixed in a Kuhn RC 250 mixer and the treatments were mixed into the basal TMR in portable mixer cart (Rissler 1050) before delivery.
Table 1.
Ingredient and nutrient composition of experimental diet comparing sodium acetate to sodium bicarbonate DCAD control and equivalent carbon mass of butyrate as calcium butyrate1.
| Treatments2 |
|||
|---|---|---|---|
| Control | NaAc3 | CaBu4 | |
| Ingredients, % DM | |||
| Corn silage | 35.9 | 36.0 | 36.2 |
| Alfalfa haylage | 12.9 | 13.0 | 13.0 |
| Cracked corn | 10.9 | 11.0 | 11.0 |
| Canola meal | 10.5 | 10.6 | 10.6 |
| Bakery byproduct | 4.68 | 4.70 | 4.72 |
| Roasted soybeans | 4.68 | 4.70 | 4.72 |
| Molasses | 4.68 | 4.70 | 4.72 |
| Whole cottonseed | 4.29 | 4.30 | 4.32 |
| Grass hay | 3.90 | 3.91 | 3.93 |
| Ground corn | 2.34 | 2.35 | 2.36 |
| Mineral-vitamin mix5 | 1.61 | 1.61 | 1.61 |
| NPN6 | 0.39 | 0.39 | 0.39 |
| Sodium bicarbonate | 3.21 | ||
| Sodium acetate | 2.87 | ||
| Calcium butyrate | 2.47 | ||
| Nutrient composition, % DM | |||
| Crude protein | 14.6 ± 0.7 | 14.6 ± 0.7 | 14.7 ± 0.7 |
| Neutral detergent fiber | 30.7 ± 0.7 | 30.8 ± 0.7 | 30.9 ± 0.7 |
| Acid detergent fiber | 12.5 ± 0.1 | 12.5 ± 0.1 | 12.6 ± 0.1 |
| Starch | 18.7 ± 0.9 | 18.7 ± 0.9 | 18.8 ± 0.9 |
| Fatty acids | 4.31 ± 0.6 | 4.33 ± 0.6 | 4.35 ± 0.6 |
| Fatty acids, % of FA | |||
| C16:0 | 7.40 ± 1 | 7.43 ± 1 | 7.46 ± 1 |
| C18:0 | 2.87 ± 0.2 | 2.88 ± 0.2 | 2.89 ± 0.2 |
| C18:1, n-9 | 10.2 ± 1.5 | 10.2 ± 1.5 | 10.2 ± 1.5 |
| C18:2, n-6 | 18.8 ± 2.6 | 18.8 ± 2.6 | 18.9 ± 2.6 |
| C18:3, n-3 | 2.46 ± 0.09 | 2.47 ± 0.09 | 2.48 ± 0.09 |
| DCAD, mEq/100 g | 5.22± 0.17 | 4.78 ± 0.11 | 1.24 ± 0.002 |
Cows were fed the same basal TMR diet (1.67 Mcal / kg NEL) and treatments were mixed into the basal diet.
Treatments were sodium bicarbonate control (3.2% of diet DM), sodium acetate at 2.9% of diet DM (targeting 10 mol of acetate/d; NaAc; Food grade anhydrous sodium acetate, Kemin Industries, Inc., Des Moines, IA), calcium butyrate at 2.5% of diet DM (CaBu; Kemin Industries Inc.) to provide an equivalent carbon mass as NaAC treatment.
Acetate contained in NaAc provided 2.09 Mcal NEL/d
Butyrate contained in CaBu provided 2.65 Mcal NEL/d
Contained (%, as fed basis): 45.8 dried corn distillers grains with solubles; 35.8 limestone (38% Ca); 8.3 magnesium oxide (54% Mg); 6.4 salt; 1.73 vitamin ADE premix; 1.09 selenium premix (0.06% selenium); and 0.88 trace mineral mix.
Non protein nitrogen fed as a slow release urea (Optigen, Alltech Inc., Lexington, KY; 259% CP, DM basis).
Feed Sampling and Analysis
Forage and base diet DM concentration was determined weekly for diet adjustment and DMI determination (72 h in a forced-air oven at 55°C). All individual feed ingredients were sampled by period and forages and a representative composite of the grains was analyzed for CP, NDF, and ADF by wet chemistry procedures as described by Rico et al. (2014). The composition of each TMR differed slightly due to the small differences in treatment inclusion rates. This approach was selected as the treatments are water soluble and were not expected to change the fermentability or physical fill of the diet.
Milk Sampling and Analysis
Cows were milked twice daily at 0600 and 1800 h and milk yield was determined by an integrated milk meter (AfiMilk; SAE Afikim, Israel) as described by Urrutia and Harvatine (2017b). Milk was sampled at both milkings the day before treatment feeding and on d 3, 6, and 7 of treatment feeding. Samples were composited within day and analyzed for milk fat and protein by FTIR and milk FA profile by GLC as described by Urrutia and Harvatine (2017a). Milk composition on d 6 and 7 of feeding was averaged within period.
Blood Sampling and Analysis
Blood samples were collected from a coccygeal vessel using potassium EDTA tubes (Greiner Bio-One North America Inc., Monroe, NC) at 0500 and 1700 h on the last day of treatments to represent a low intake and high intake period of the day, respectively. Blood was immediately placed on ice, centrifuged within 30 min at 1300 x g for 15 min at 4°C, and plasma was harvested and stored at −20°C until laboratory analysis. Plasma samples were analyzed for glucose [PGO Enzyme procedure no. P 7119; Sigma-Aldrich, St. Louis, MO (17)], nonesterified fatty acids [NEFA; Wako HR Series NEFA-HR kit, Wako Chemicals USA, Richmond, VA as modified by Ballou et al. (2009)], BHB (procedure #2440, Stanbio Laboratory, Boerne, TX), and BUN (procedure #2050, Stanbio Laboratory, Boerne, TX).
Statistical Analysis
Data was analyzed as repeated measures in SAS (version 9.3) and the model included the random effect of cow, period, and treatment sequence and the fixed effects of a covariate (observed value for variable of interest on the day before treatment feeding), treatment, day of treatment, and their interaction. Subject was cow by period and the CS covariance structure was used. Denominator degrees of freedom were adjusted with the Kenwood Rogers method. Data points with Studentized residuals outside of ± 3.5 were considered outliers and excluded from analysis. A protected LSD separation was used within each timepoint to compare treatment means when the treatment or treatment by time interaction was significant. Differences were declared significant at P ≤ 0.05 and tendencies at 0.05 < P ≤ 0.10.
RESULTS AND DISCUSSION
There was a treatment by time interaction for DMI, as NaAc increased DMI over 2.5 kg/d and CaBu decreased DMI over 2.6 kg/d during treatment periods, compared to control (P < 0.05; Figure 1A). Reduced DMI in the CaBu treatment may have been due to palatability issues (Huhtanen et al., 1993), however, previous research where butyrate was incorporated into pelleted concentrates as calcium salts (Balch et al., 1967) or infused ruminally as sodium salts (~700 g of butyrate/d; Herrick et al., 2018), DMI was not affected. Providing butyric and acetic acid as diluted acids has reduced feed intake, presumably due to stimulation of satiety signals through chemoreceptors in the rumen wall, liver or portal system (Rook et al., 1965; Simkins et al., 1965).
Figure 1.
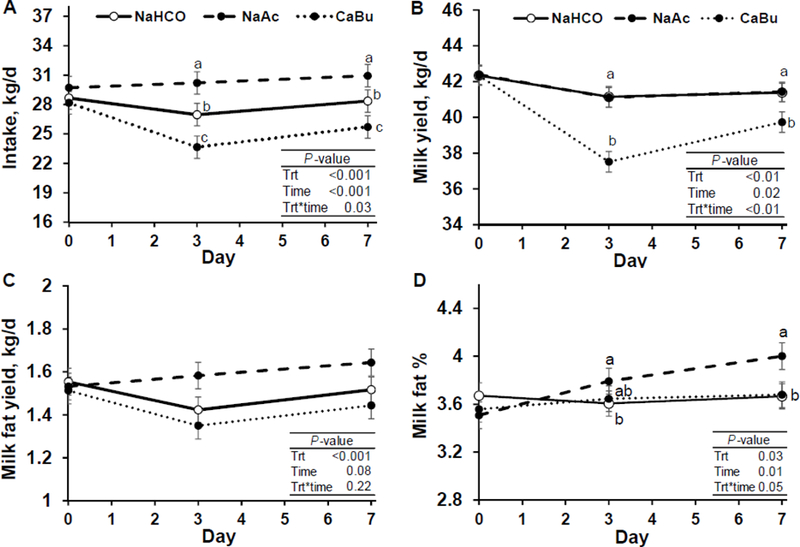
Time course of DMI (Panel A), milk yield (Panel B), milk fat yield (Panel C), and milk fat concentration (Panel D) of cows fed sodium bicarbonate at 3.2% of diet DM (control; NaHCO), sodium acetate at 2.9% of diet DM (NaAc), and calcium butyrate at 2.5% of diet DM (CaBu; equal carbon mass of NaAc; n = 12 per treatment). Least-square means and standard errors are shown and means that do not share a superscript differ within timepoint (P < 0.05).
There was a treatment by time interaction for milk yield, as NaAc had no effect on milk yield, but CaBu decreased milk yield by over 4% (−1.65 kg/d) during the treatment period compared to control (Treatment by time interaction P < 0.01; Figure 1B). The decrease in milk yield with CaBu is in agreement with the reduced DMI reported above.
Milk fat yield was increased 90 g/d by NaAc and decreased 60 g/d by CaBu compared to control (P < 0.05; Figure 1C). A similar amount of sodium acetate (10 mol/d) increased milk fat yield 217 g/d compared to a sodium chloride control when ruminally infused over 22 h/d (Urrutia and Harvatine, 2017b). The larger response with continuous infusion may be due to increased acetate availability over the entire day, while inclusion in TMR provides boluses with each meal. There was a treatment by time interaction for milk fat concentration as NaAc increased milk fat concentration on d 3 and 7 compared to control (0.20 and 0.34 percentage points, respectively; interaction P = 0.05, Figure 1D). Previous studies have shown increased milk fat concentration with rumen infusion of butyrate (Rook et al., 1965; Huhtanen et al., 1993), with only small nonsignificant reductions in DMI when providing high doses of butyric acid (Rook et al., 1965). As DMI was reduced with CaBu in the present experiment, other precursors for fat synthesis such as acetate, are expected to have also decreased and may have limited a positive effect of butyrate on milk fat synthesis.
Milk protein concentration did not differ between NaAc and control, but was decreased by CaBu compared to control (P < 0.05). Early and more recent research has not shown a consistent effect of increasing butyrate supply on milk protein content. For example, Huhtanen et al. (1993) when providing rumen infusion of increasing concentrations of butyrate (from 0 to 600 g/d) in isoenergetic mixture of volatile fatty acids, observed a positive linear response of milk protein content (from 3.27% to 3.46% milk protein); while Rook and Balch (1961) observed no effect of 880 g/d of butyrate infused ruminally on milk protein content. It is likely that milk protein content was reduced in CaBu due to reduced feed intake, as feed restriction results in decrease milk and milk component yield and concentration (Wilson et al., 1967). There was a treatment by time interaction for milk protein yield, as NaAc increased milk protein yield by 79 g/d on d 3 of treatments, while CaBu reduced milk protein yield over 100 g/d on d 3 and 7 of treatments, compared to control (Figure 2). Responses in milk protein yield were driven by increased milk yield for NaAc, but were a combination of reduced milk protein concentration and milk yield in CaBu. In previous research, milk protein concentration and yield was not affected by short (Urrutia and Harvatine, 2017a; Urrutia and Harvatine, 2017b) or long term (Sheperd and Combs, 1998) ruminal infusion of acetate.
Figure 2.
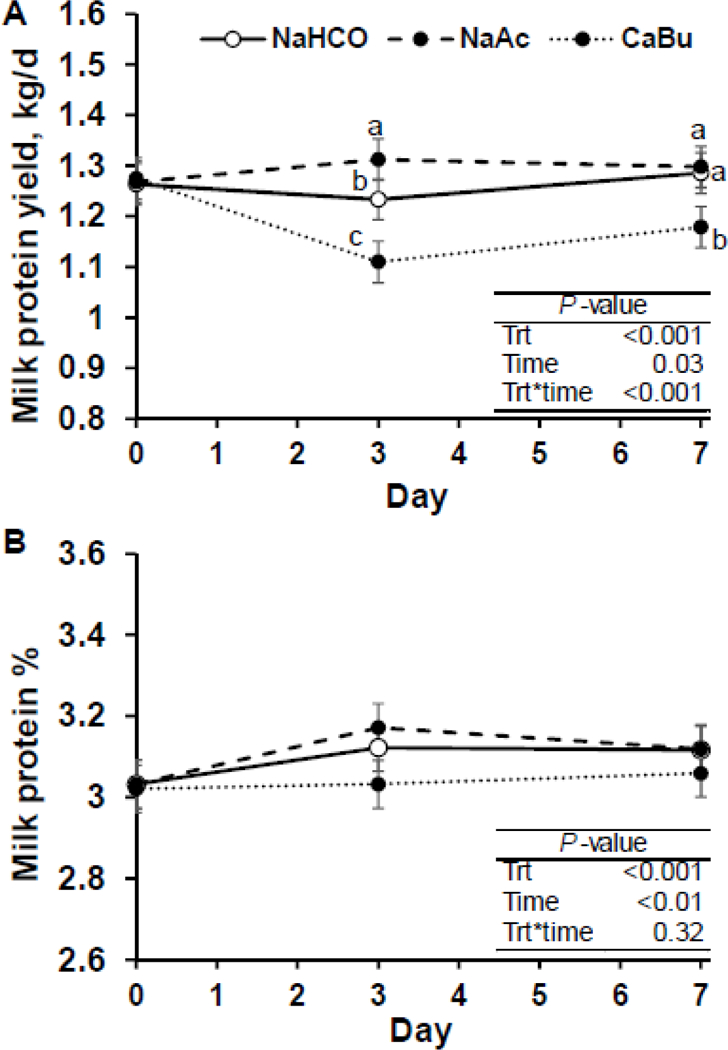
Time course of milk protein yield (Panel A) and concentration (Panel B) of cows fed sodium bicarbonate at 3.2% of diet DM (control; NaHCO), sodium acetate at 2.9% of diet DM (NaAc), and calcium butyrate at 2.5% of diet DM (CaBu; equal carbon mass of NaAc; n = 12 per treatment). Least-square means and standard errors are shown and means that do not share a superscript differ within timepoint (P < 0.05).
There were treatment by time interactions for yield of de novo and mixed origin FA (P = 0.09 and P = 0.02, respectively; Figure 3). De novo FA were increased 50 g/d by NaAc on d 3 (P < 0.05) compared to the control. Calcium butyrate decreased de novo FA by 12 g/d on d 7, compared to control. Sodium acetate also increased mixed source FA by over 50 g/d during the entire treatment period (d 3 to 7), compared to control. During ruminal infusion of neutralized acetate, Urrutia and Harvatine (2017b) reported increased yield of de novo (57 g/d) and mixed origin FA (87 g/d) in response to continuous rumen infusions of sodium acetate (10 mol/d), in a similar magnitude to the present experiment.
Figure 3.
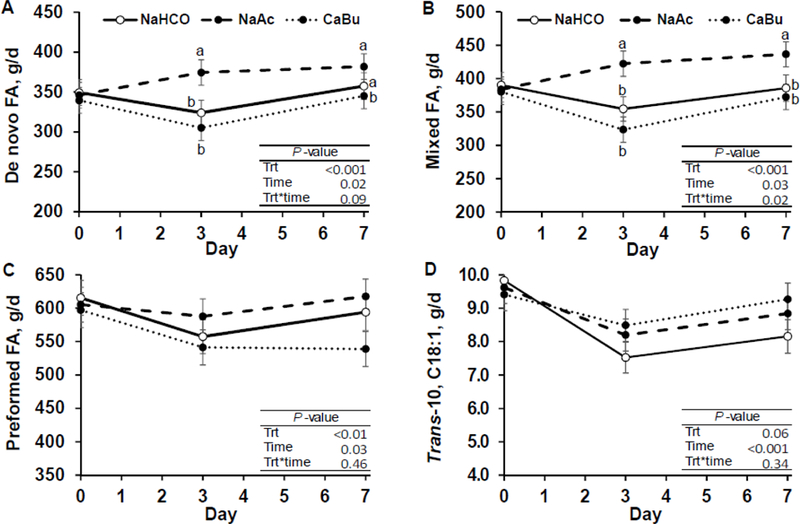
Time course of milk fatty acid yield of de novo (<16 carbons; Panel A), mixed source (16 carbons; Panel B), preformed (>16 carbons; Panel C) origin and trans-10, C18:1 (Panel D) of cows fed sodium bicarbonate at 3.2% of diet DM (control; NaHCO), sodium acetate at 2.9% of diet DM (NaAc), and calcium butyrate at 2.5% of diet DM (CaBu; equal carbon mass of NaAc), (n = 12 per treatment). Least-square means and standard errors are shown and means that do not share a superscript differ within timepoint (P < 0.05).
Treatment by time interactions were detected for the concentration of all FA grouped by source (de novo, mixed, and preformed) and for trans-10 C18:1 (all P < 0.01; Supplemental Figure S1). The concentration of de novo synthetized FA (<16 C FA; g/100g) were increased by NaAc on d 3 compared to control and by CaBu on d 7 compared to control. Concentration of mixed source FA (16 C FA) were increased by NaAc on day 3 and 7 and reduced by CaBu on d 3, compared to control. Concentration of preformed FA (>16 C FA) were reduced by NaAc on d 3 and d 7, and increased by CaBu on d 3 compared to control. CaBu increased concentration of milk trans-10 C18:1 on d 3 and 7 of treatment (treatment by time interaction P < 0.01; Supplemental Figure S1) indicating a shift in rumen biohydrogenation towards alternate pathways that result in biohydrogenation induced milk fat depression.
Analysis of individual FA identified some FA specific responses (Table 2). No treatment or treatment by time interactions effects were observed for C4:0, C6:0, and C8:0. NaAc decreased milk concentration of C17:0, trans-11 C18:1, cis-9, trans-11 conjugated linoleic acid (CLA), and total odd and branched chain FA (OBCFA) compared to control (all P < 0.05, Table 2). CaBu increased concentration of C17:0 and C17:1, compared to control (both P < 0.05). Several treatment by time interactions were detected (Supplemental Table S1). Importantly, NaAc increased concentration of palmitic acid (C16:0) and reduced concentration of iso C14:0, trans-9 C18:1, C18:2 n-6, and C18:3 n-3 in milk fat on d 3 and 7 of treatments, compared to control (P < 0.05). Also, NaAc increased concentration of C10:0, C12:0, and C14:0, and reduced concentration of ante-iso C15:0, ante-iso C17:0, and cis-9 C18:1 in milk fat on d 3, compared to control (all P < 0.05). Feeding CaBu increased milk concentration of iso C15:0, iso C17:0, C18:0 on d 3 and C10:0, C12:0, ante-iso C13:0, and C14:0 on d 7, compared to control (all P < 0.05). Also, CaBu reduced concentration of milk C14:0, iso C16:0, and C16:0 on d 3 and ante-iso C15:0, ante-iso C17:0, and C18:0 on d 7, compared to control (all P < 0.05). CaBu increased concentration of trans-9 18:1 on d 3 and 7 and reduced concentration of iso C14:0 on d 3 and 7, compared to control (P < 0.05). Overall, NaAc increased milk fat synthesis primarily through an increase in synthesis of de novo and mixed FA (C10:0, C12:0; C:14; C16:0), while CaBu, increased synthesis of C10:0 and C12:0 and modified FA of microbial origin, indicating a shift in rumen fermentation pattern (Vlaeminck et al., 2006).
Table 2.
Effect of feeding sodium acetate (NaAc) and an equal carbon mass of calcium butyrate (CaBu) on milk fatty acid composition.
| FA, g/100g | Treatment1 |
SE |
P-value |
||||
|---|---|---|---|---|---|---|---|
| NaHCO | NaAc | CaBu | trt | time | trt*time2 | ||
| C4:0 | 4.24 | 4.21 | 4.26 | 0.065 | 0.75 | <0.001 | 0.29 |
| C6:0 | 2.17 | 2.17 | 2.21 | 0.027 | 0.31 | <0.001 | 0.82 |
| C8:0 | 1.18 | 1.19 | 1.20 | 0.022 | 0.37 | <0.001 | 0.34 |
| C10:0 | 2.58 | 2.64 | 2.62 | 0.057 | 0.34 | <0.001 | 0.05 |
| C11:0 | 0.042 | 0.042 | 0.042 | 0.002 | 0.66 | <0.001 | 0.85 |
| C12:0 | 2.92 | 3.00 | 2.98 | 0.068 | 0.18 | <0.001 | 0.03 |
| iso C13:0 | 0.021 | 0.020 | 0.021 | 0.001 | 0.77 | 0.68 | 0.41 |
| ante-iso C13:0 | 0.056 | 0.056 | 0.059 | 0.001 | 0.16 | <0.001 | 0.02 |
| iso C14:0 | 0.102 | 0.091 | 0.082 | 0.004 | <0.001 | <0.001 | 0.06 |
| C14:0 | 10.1 | 10.4 | 10.1 | 0.12 | <0.01 | 0.12 | <0.001 |
| iso C15:0 | 0.199 | 0.201 | 0.202 | 0.003 | 0.39 | <0.001 | 0.03 |
| ante-iso C15:0 | 0.36 | 0.35 | 0.35 | 0.004 | 0.04 | 0.35 | 0.04 |
| C15:0 | 0.85 | 0.83 | 0.85 | 0.012 | 0.22 | <0.001 | 0.75 |
| iso C16:0 | 0.22 | 0.22 | 0.19 | 0.010 | 0.04 | <0.001 | 0.01 |
| C16:0 | 25.7 | 26.6 | 25.3 | 0.13 | <0.001 | 0.01 | <0.001 |
| iso C17:0 | 0.26 | 0.26 | 0.27 | 0.005 | 0.04 | <0.001 | <0.001 |
| ante-iso C17:0 | 0.34 | 0.33 | 0.34 | 0.004 | 0.12 | 0.002 | 0.002 |
| C17:0 | 0.46b | 0.45c | 0.47a | 0.006 | 0.001 | <0.001 | 0.22 |
| C17:1 | 0.128b | 0.124b | 0.133a | 0.002 | 0.01 | 0.001 | 0.11 |
| C18:0 | 12.7 | 13.0 | 12.9 | 0.17 | 0.42 | <0.01 | <0.001 |
| trans−9 C18:1 | 0.33 | 0.32 | 0.34 | 0.011 | <0.001 | 0.18 | <0.001 |
| trans−10 C18:1 | 0.59 | 0.59 | 0.66 | 0.020 | <0.001 | 0.04 | <0.01 |
| trans−11 C18:1 | 1.27a | 1.2b | 1.27a | 0.035 | 0.01 | <0.001 | 0.25 |
| cis−9 C18:1 | 19.5 | 18.8 | 19.4 | 0.56 | <0.01 | <0.001 | <0.001 |
| C18:2 n-6 | 3.12 | 2.95 | 3.17 | 0.097 | <0.001 | 0.06 | <0.01 |
| C18:3 n-3 | 0.47 | 0.44 | 0.47 | 0.017 | <0.01 | 0.25 | 0.01 |
| cis-9,trans C18:2 | 0.54a | 0.51b | 0.53a | 0.014 | 0.01 | <0.001 | 0.12 |
| Σ OBCFA3 | 3.05a | 2.96b | 3.03a | 0.026 | <0.001 | <0.001 | 0.32 |
Treatments were on a dry matter basis: 3.2% NaHCO3 (control), 2.9% NaAc, and 2.5 % CaBu (carbon equivalent to acetate treatment), n = 12 per treatment. Least-square means that do not share a superscript are significantly different (P < 0.05).
Main effects of treatment when a treatment by time interaction was detected are described in the text and shown in Supplemental Table S1.
Odd and branched chain FA.
Treatment by time interactions were detected for plasma NEFA and BUN (Figure 4A and 4B). CaBu reduced plasma NEFA by 18% and increased BUN by 12% at 1700 h compared to control (both P < 0.05). Also, both NaAc and CaBu increased BUN by over 24% at 0500 h compared to control (P < 0.05). Reduced plasma NEFA in CaBu may result from a BHB-mediated lipolysis inhibition through activation of adipose nutrient sensing receptors such as the Hydroxycarboxylic acid receptor 2 (Metz and Bergh, 1972; Mielenz, 2017). However, recent studies have not shown reductions in plasma NEFA in response to rumen infusion of butyrate (Herrick et al., 2018) or to high plasma BHB resulting from rumen acetate infusion (Urrutia and Harvatine, 2017b). Blood urea nitrogen is an indicator of efficiency of protein utilization and is related to energy balance and plasma NEFA (Broderick and Clayton, 1997; Rastani et al., 2006). Elevated BUN observed in CaBu may indicate inefficient dietary protein utilization or increased body protein mobilization and catabolism due to reduced feed intake.
Figure 4.
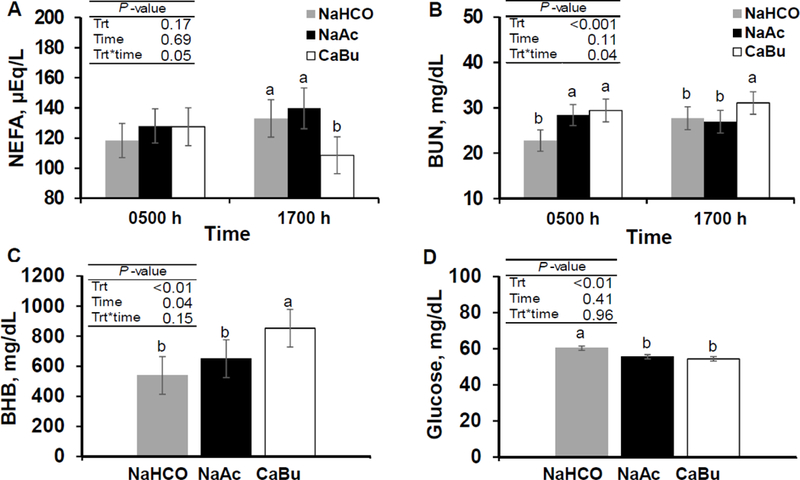
Effect of feeding sodium bicarbonate at 3.2% of diet DM (control; NaHCO), sodium acetate at 2.9% of diet DM (NaAc), and calcium butyrate at 2.5% of diet DM (CaBu; equal carbon mass of NaAc) on plasma NEFA (Panel A), BUN (Panel B), plasma glucose (Panel C), and plasma BHB (Panel D), (n = 12 per treatment). Least-square means and standard errors are shown and means that do not share a superscript differ within timepoint (P < 0.05).
Treatment impacted plasma glucose and BHB (Figure 4). Plasma BHB concentration was 539, 650, and 853 mg/dL in control, NaAc, and CaBu treatments, respectively (SE = 125; Figure 4C). NaAc did not affect BHB, while CaBu increased BHB by 58%, compared to control (P < 0.05). In previous studies (Urrutia and Harvatine, 2017a; Urrutia and Harvatine, 2017b), continuous acetate infusions resulted in increased plasma BHB compared to sodium chloride control, however, in the present study, feeding sodium acetate did not affect plasma BHB compared to a sodium bicarbonate control. Possible reasons may include a synchronized availability of rumen acetate and butyrate (made from acetate) with rumen wall energy demand after feeding in the present study, reducing transfer to blood circulation. Feeding butyrate (CaBu) in the current experiment resulted in a similar increase in plasma BHB compared to previous continuous ruminal acetate infusions (Urrutia and Harvatine, 2017b). This agrees with previous findings, as rumen butyrate, once absorbed is rapidly metabolized to BHB by rumen epithelial cells (Storry and Rook, 1965b). In the current study, both NaAc and CaBu reduced plasma glucose concentration by ~10% compared to control (P < 0.05; Figure 4D). Early reports have shown a role for butyrate in the regulation of glucose metabolism (Storry and Rook, 1965a), supported by in vitro studies where butyrate inhibits liver uptake of propionate (Demigné et al., 1986). Recently, Herrick et al. (2018) reported a 10% reduction in plasma glucose when providing ~700 g/d of butyrate as a continuous ruminal infusion. Although previous studies have not shown reductions in plasma glucose in response to acetate infusion (Storry and Rook, 1965a; Sheperd and Combs, 1998; Urrutia and Harvatine, 2017a; Urrutia and Harvatine, 2017b), it is possible that increased milk fat synthesis in NaAc resulted in an increase in glucose requirement for NADPH synthesis in the mammary gland.
Continuous sodium acetate infusions resulted in a linear increase in rumen pH compared to a sodium chloride control (Urrutia and Harvatine, 2017b), as sodium acetate infused at a pH of 6.0 provided buffering capacity to the rumen. The use of a sodium chloride control did not allow control for buffering capacity and for dietary cation-anion difference (DCAD). Interestingly, DCAD has been reported to increase milk fat yield and concentration [0.1% and 36 g/d for every 100mEq/kg increase in DCAD, respectively; (Iwaniuk and Erdman, 2015)] presumably through improved rumen buffering capacity, although the mechanism has not been demonstrated. Improved buffering capacity may result in a shift of rumen fermentation pathways towards reduced formation of alternate biohydrogenation intermediates that induce milk fat depression such as trans-10 C18:1 and trans-10, cis-12 C18:2 (Harvatine et al., 2009), therefore, an apparent increase in milk fat synthesis.
In the present experiment, a sodium bicarbonate treatment was used to provide a DCAD and buffering capacity control to sodium acetate. We observed no treatment or treatment by time interactions for NaAc on milk trans-10 C18:1, compared to control (Figure 3 and Supplemental Figure S1), and trans-10, cis-12 C18:2 was not detected in any sample, therefore we attribute the milk fat yield and concentration response observed in the current experiment to acetate supply and not a rumen buffering capacity or DCAD effect. Yet, the provision of sodium acetate as a feed supplement, would provide an additional benefit to the increase in milk fat synthesis, as it would improve rumen buffering capacity and therefore rumen health, especially when feeding more highly fermentable diets and during the high intake period of the day after delivery of fresh feed and during the afternoon when rumen pH is lowest (Salfer et al., 2018).
Several mechanisms may be involved in the increased milk fat synthesis observed with acetate supplementation, including simply an increase in substrate supply for milk fat synthesis or modification of metabolism as a bioactive nutrient. Molecular mechanisms exist for acetate to potentially modify metabolic signaling and regulation of gene expression (discussed by Urrutia and Harvatine, 2017b), but have not been well investigated in the cow. Feeding sodium acetate is expected to increase acetate supply more during the high intake period of the day (feeding to midnight). To determine if treatment effects differed at the morning and afternoon milking, milking time (AM/PM) and its interaction with treatment and experimental day were tested but were not significant (data not shown). This may be due to the timing of milking relative to the high intake period of the day and may require increased milking frequency to observe a temporal effect on milk fat yield.
CONCLUSION
In conclusion, milk fat yield and concentration can be increased by dietary supplementation of sodium acetate, and not calcium butyrate at the level investigated. Increased milk fat synthesis observed during acetate feeding may be due to either a greater substrate supply allowing greater synthesis of de novo and mixed FA, modification of metabolism by acetate, or a combination of these. Inclusion of sodium acetate into dairy cow diets will depend on the cost of a commercial supplement and market price for milk fat.
Supplementary Material
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the technical assistance of undergraduate and graduate students of the Harvatine Lab, Penn State University, University Park, PA. Gratitude is also expressed to the staff at the Pennsylvania State University Dairy Cattle Research and Education Center. Sodium acetate and calcium butyrate were graciously donated by Kemin Industries Inc. (Des Moines, IA). This project was partially supported by Penn State University including USDA National Institute of Food and Agriculture Federal Appropriations under Project number PEN04539 and accession number 1000803.
REFERENCES
- Balch C, Broster W, Johnson V, Line C, Rook J, Sutton J, and Tuck VJ. 1967. The effect on milk yield and composition of adding the calcium salts of acetic, propionic, butyric and lactic acids to the diets of dairy cows. J. Dairy Res. 34:199–206. [Google Scholar]
- Baldwin R and Smith N. 1971. Intermediary aspects and tissue interactions of ruminant fat metabolism. J. Dairy Sci. 54:583–595. [DOI] [PubMed] [Google Scholar]
- Ballou MA, Gomes RC, Juchem SO, and DePeters EJ. 2009. Effects of dietary supplemental fish oil during the peripartum period on blood metabolites and hepatic fatty acid compositions and total triacylglycerol concentrations of multiparous Holstein cows. J. Dairy Sci. 92:657–669. [DOI] [PubMed] [Google Scholar]
- Bauman DE, Brown RE, and Davis CL. 1970. Pathways of fatty acid synthesis and reducing equivalent generation in mammary gland of rat, sow, and cow. Arch. Biochem. Biophys. 140:237–244. [DOI] [PubMed] [Google Scholar]
- Bergman EN 1990. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev. 70:567–590. [DOI] [PubMed] [Google Scholar]
- Broderick GA and Clayton MK. 1997. A Statistical Evaluation of Animal and Nutritional Factors Influencing Concentrations of Milk Urea Nitrogen. J. Dairy Sci. 80:2964–2971. [DOI] [PubMed] [Google Scholar]
- Demigné C, Yacoub C, Rémésy C, and Fafournoux P. 1986. Propionate and butyrate metabolism in rat or sheep hepatocytes. Biochimica et Biophysica Acta (BBA) - Lipids and Lipid Metabolism 875:535–542. [DOI] [PubMed] [Google Scholar]
- Harvatine KJ, Boisclair YR, and Bauman DE. 2009. Recent advances in the regulation of milk fat synthesis. Animal 3:40–54. [DOI] [PubMed] [Google Scholar]
- Herrick KJ, Hippen AR, Kalscheur KF, Schingoethe DJ, Ranathunga SD, Anderson JL, Moreland SC, and van Eys JE. 2018. Infusion of butyrate affects plasma glucose, butyrate, and beta-hydroxybutyrate but not plasma insulin in lactating dairy cows. J. Dairy Sci. [DOI] [PubMed] [Google Scholar]
- Huhtanen P, Miettinen H, and Ylinen M. 1993. Effect of Increasing Ruminal Butyrate on Milk Yield and Blood Constituents in Dairy Cows Fed a Grass Silage-Based Diet. J. Dairy Sci. 76:1114–1124. [DOI] [PubMed] [Google Scholar]
- Ingle DL, Bauman DE, Mellenberger RW, and Johnson DE. 1973. Lipogenesis in the ruminant: effect of fasting and refeeding on fatty acid synthesis and enzymatic activity of sheep adipose tissue. J. Nutr. 103:1479–1488. [DOI] [PubMed] [Google Scholar]
- Iwaniuk M and Erdman R. 2015. Intake, milk production, ruminal, and feed efficiency responses to dietary cation-anion difference by lactating dairy cows. J. Dairy Sci. 98:8973–8985. [DOI] [PubMed] [Google Scholar]
- Maxin G, Rulquin H, and Glasser F. 2011. Response of milk fat concentration and yield to nutrient supply in dairy cows. Animal 5:1299–1310. [DOI] [PubMed] [Google Scholar]
- Metz SHM and Bergh S. G. v. d.. 1972. Effects of volatile fatty acids, ketone bodies, glucose, and insulin on lipolysis in bovine adipose tissue. FEBS Lett. 21:203–206. [DOI] [PubMed] [Google Scholar]
- Mielenz M 2017. Invited review: nutrient-sensing receptors for free fatty acids and hydroxycarboxylic acids in farm animals. animal 11:1008–1016. [DOI] [PubMed] [Google Scholar]
- Palmquist D, Beaulieu AD, and Barbano D. 1993. Feed and animal factors influencing milk fat composition. J. Dairy Sci. 76:1753–1771. [DOI] [PubMed] [Google Scholar]
- Rastani RR, Lobos NE, Aguerre MJ, Grummer RR, and Wattiaux MA. 2006. Relationships Between Blood Urea Nitrogen and Energy Balance or Measures of Tissue Mobilization in Holstein Cows During the Periparturient Period. The Professional Animal Scientist 22:382–385. [Google Scholar]
- Rico D, Ying Y, and Harvatine K. 2014. Effect of a high-palmitic acid fat supplement on milk production and apparent total-tract digestibility in high-and low-milk yield dairy cows. J. Dairy Sci. 97:3739–3751. [DOI] [PubMed] [Google Scholar]
- Rook JAF and Balch CC. 1961. The effects of intraruminal infusions of acetic, propionic and butyric acids on the yield and composition of the milk of the cow. Br. J. Nutr. 15:361–369. [DOI] [PubMed] [Google Scholar]
- Rook JAF, Balch CC, and Johnson VW. 1965. Further observations on the effects of intraruminal infusions of volatile fatty acids and of lactic acid on the yield and composition of the milk of the cow. Br. J. Nutr. 19:93–99. [DOI] [PubMed] [Google Scholar]
- Salfer IJ, Morelli MC, Ying Y, Allen MS, and Harvatine KJ. 2018. The effects of source and concentration of dietary fiber, starch, and fatty acids on the daily patterns of feed intake, rumination, and rumen pH in dairy cows. J. Dairy Sci. 101:10911–10921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheperd A and Combs D. 1998. Long-term effects of acetate and propionate on voluntary feed intake by midlactation cows. J. Dairy Sci. 81:2240–2250. [DOI] [PubMed] [Google Scholar]
- Simkins KL, Suttie JW, and Baumgardt BR. 1965. Regulation of Food Intake in Ruminants. 4. Effect of Acetate, Propionate, Butyrate, and Glucose on Voluntary Food Intake in Dairy Cattle1, 2, 3. J. Dairy Sci. 48:1635–1642. [Google Scholar]
- Smith SB 1983. Contribution of the pentose cycle to lipogenesis in bovine adipose tissue. Arch. Biochem. Biophys. 221:46–56. [DOI] [PubMed] [Google Scholar]
- Storry J and Rook J. 1965a. Effect in the cow of intraruminal infusions of volatile fatty acids and of lactic acid on the secretion of the component fatty acids of the milk fat and on the composition of blood. Biochem. J. 96:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storry J and Rook J. 1965b. Effects of intravenous infusions of acetate, β-hydroxybutyrate, triglyceride and other metabolites on the composition of the milk fat and blood in cows. Biochem. J. 97:879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton J, Dhanoa M, Morant S, France J, Napper D, and Schuller E. 2003. Rates of production of acetate, propionate, and butyrate in the rumen of lactating dairy cows given normal and low-roughage diets. J. Dairy Sci. 86:3620–3633. [DOI] [PubMed] [Google Scholar]
- Urrutia N and Harvatine KJ. 2017a. Effect of conjugated linoleic acid and acetate on milk fat synthesis and adipose lipogenesis in lactating dairy cows. J. Dairy Sci. 100:5792–5804. [DOI] [PubMed] [Google Scholar]
- Urrutia NL and Harvatine KJ. 2017b. Acetate Dose-Dependently Stimulates Milk Fat Synthesis in Lactating Dairy Cows. The Journal of Nutrition 147:763–769. [DOI] [PubMed] [Google Scholar]
- Vlaeminck B, Fievez V, Tamminga S, Dewhurst RJ, van Vuuren A, De Brabander D, and Demeyer D. 2006. Milk Odd- and Branched-Chain Fatty Acids in Relation to the Rumen Fermentation Pattern. J. Dairy Sci. 89:3954–3964. [DOI] [PubMed] [Google Scholar]
- Wilson GF, Davey AWF, and Dolby RM. 1967. Milk composition as affected by intra-ruminal infusion of volatile fatty acids to cows on a restricted ration. New Zealand Journal of Agricultural Research 10:215–225. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


