Abstract
Xenopus oocytes and embryos are model systems optimally suited for quantitative proteomics. This is due to the availability of large amount of protein material and the ease of physical manipulation. Furthermore, facile in vitro fertilization provides superbly synchronized embryos for cell cycle and developmental stages. Here, we detail protocols developed over the last few years for sample preparation of multiplexed proteomics with TMT-tags followed by quantitative mass spectrometry analysis using the MultiNotch MS3 approach. In this approach, each condition is barcoded with an isobaric tag at the peptide level. After barcoding, samples are combined and the relative abundance of ~100,000 peptides is quantified on a mass spectrometer. High reproducibility of the sample preparation process prior to peptides being tagged and combined is of upmost importance for obtaining unbiased data. Otherwise, differences in sample handling can inadvertently appear as biological changes. We detail and exemplify the application of our sample workflow on an embryonic time-series of ten developmental stages of Xenopus laevis embryos ranging from the egg to stage 35 (just before hatching). Our accompanying paper (Chapter 14) details a bioinformatics pipeline to analyze the quality of the given sample preparation and strategies to convert spectra of X. laevis peptides into biologically interpretable data.
Keywords: Proteomics, Xenopus laevis, Development, Sample preparation, Multiplexing, Mass spectrometry, Yolk, TMT, Protein dynamics
1. Introduction
Amphibian embryos and oocytes are classic models to study cellular and developmental processes [1–4]. Due to inexpensive maintenance, resistance to diseases, and year-round availability of eggs and embryos, Xenopus laevis has become the predominant amphibian model in the laboratories. Recently, the genome of this quasitetraploid frog has been sequenced [5], which will greatly benefit systems-level analysis on the genome, RNA, and protein levels. Xenopus is particularly attractive for proteomics studies. This is due to the very large amount of protein that is easily obtainable. Depending on the method of choice, proteomic experiments are typically performed with ~1 μg to ~1 mg of proteins. One egg/embryo contains ~25 μg of non-yolk protein [6], therefore, a collection of at most ~40 embryos would suffice for experiments that require the most material. For comparison, this is equivalent to ~2000 fly embryos [7], or ~60,000 mouse oocytes [8]. Single cell proteomics with the Xenopus eggs and oocytes is compatible with standard proteomics protocols [9]. More recently, the Nemes group has pushed the limits of sample limited analysis and is able to quantify ~400 proteins of individual cells in the 16-cell stage [10]. Besides providing large amounts of material, Xenopus oocytes and embryos can be easily physically manipulated. For example, we recently published a paper on nucleocytoplasmic partitioning where we manually isolated the nuclei of oocytes [11]. Similarly, we have manually isolated and defined the composition of the Balbiani body [12]. Another major advantage of the Xenopus model is the superb synchrony of developmental stages achievable with in vitro fertilization. This benefit has been used in multiple papers to study the changes in protein abundances during development [13–15]. With egg activation via electric shock, synchrony can be improved to within a few seconds, which allowed us to follow the change of protein abundance and phospho dynamics in the metaphase-anaphase transition [16]. Further, the ability to make essentially undiluted cytoplasm from Xenopus eggs and embryos has made it a classical biochemical system. These lysates are still “alive” in many aspects as is evident in their ability to form mitotic spindles and nuclei in the test tube [17–19]. We suspect that the combination of classical biochemical approaches like isolation of organelles or fractionation with proteomics is an emerging and powerful tool to study the organization of cytoplasm [20, 21].
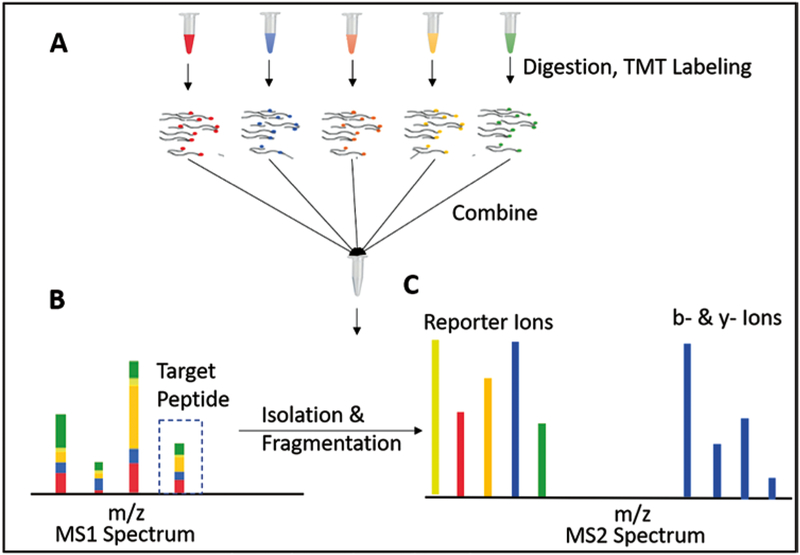
Since the mid-1990s, mass spectrometry-based proteomics has undergone an impressive evolution starting with the ability to identify a few proteins to experiments that regularly identify ~10,000 proteins [22–24]. Diverse options for experimental design on quantification of protein abundances are available. Among them are SILAC, label-free, multiplexed proteomics, and DIA [25–27, 39]. Due to a beneficial combination of sensitivity, measurement quality, and the ability to compare many samples at once, our method of choice is multiplexed proteomics. For multiplexed proteomics, peptides of the different conditions are labeled with a barcode, i.e., an isobaric tag (Fig. 1). The conditions (currently up to 11) are combined and ionized. In the MS1 spectrum the peptides from the different conditions are indistinguishable. Upon fragmentation, the reporter ions are released and encode the different conditions. The relative signal can be used to quantify relative protein abundance. However, multiplexed proteomics’ data quality was initially limited by the artifacts that arise from co-isolation and co-fragmentation of unintended peptides. Nonetheless, MultiNotch MS3, QuantMode, and TMTc can overcome this problem [28–31, 40]. MultiNotch MS3 (TMT-MS3) is currently the most widely used method and has allowed detection of protein abundance changes of less than 10% as significant [11].
Fig. 1.
Principle of multiplexed proteomics. (A) Proteins from multiple conditions (replicates, time points, etc.) are digested and labeled with isobaric tags (e.g., TMT). The distinct colors represent different tags, which serve as barcodes for the sample origin. After tagging, different conditions are mixed and together ionized onto a mass spectrometer. (B) In the MS1 spectrum the isobaric tags have the identical mass, thus peptide peaks from different conditions are indistinguishable. (C) The isolated peptides are fragmented and produce condition-specific reporter ions. The intensities of these reporter ions can be used for relative quantification of the associated protein. The b- and y-ions, which result from breakage of the peptide’s backbone, are used for identification
Here, we detail the sample preparation and data acquisition for a TMT-MS3 experiment exemplified with the quantification of protein dynamics of an embryonic time-series. We collected ten developmental stages spanning from egg to stage 35 which is the stage just before hatching. Importantly, for multiplexed proteomics labeling with the isobaric tag occurs at the peptide level. Upstream preparation is performed in parallel. Hence, it is extremely important for any of these sample manipulations to be as reproducible as possible. Here, we detail how we achieve reproducible sample preparation to the best of our knowledge. The accompanying paper (Chapter 14) discusses how to analyze the acquired spectra and use the information in these to perform sample preparation quality analysis and how to convert collections of spectra into biologically interpretable data.
2. Materials
2.1. Embryo Lysis and Yolk Removal
10 mM Combretastatin: stock solution in acetonitrile (store at −80 °C).
10 mM Cytochalasin D: stock solution in acetonitrile (store at −80 °C).
Lysis Buffer: 250 mM Sucrose,1% Nonidet P-40 (NP 40), 10 mM Ethylenediaminetetraacetic acid (EDTA), 25 mM HEPES pH = 7.2, 10 μM Cytochalasin D, Complete Roche Mini (1 tablet per 10 ml). Lysis buffer should be freshly prepared.
Sodium Dodecyl Sulfate (SDS): Prepare 10% stock solution.
Liquid nitrogen.
2.2. Cysteine Protection
Dithiothreitol (DTT): 500 mM stock solution in HPLC grade water, adjust pH to ~7 (store at −80 °C).
N-Ethylmaleimide (NEM): 1 M stock solution in acetonitrile (HPLC grade) (store at −80 °C).
2.3. Methanol–Chloroform Precipitation [32]
Methanol (HPLC Grade).
Chloroform (HPLC grade).
Water (HPLC grade).
10 mM 3-[4-(2-Hydroxyethyl)-1-piperazinyl]propanesulfonic acid (EPPS), pH 8.5: Prepare in HPLC grade water.
6 M Guanidine Hydrochloride (GuaCl) (e.g., Chem-Implex International, Pure GuaCl >99.5%), in 10 mM EPPS pH 8.5 (use HPLC grade water).
Ultrasonic cleaner (e.g. Branson M2800).
2.4. Estimate Protein Amount (BCA Assay)
Pierce BCA Protein Assay Kit (Thermo Scientific).
Multi-Mode Microplate Reader (e.g., Synergy HT, Biotek).
2.5. Digestion
Lysyl Endopeptidase (LysC) (Wako chemicals, 10 AU, store at −80 °C): Prepare aliquots of 2 μg/μl in HPLC grade water and flash freeze them in liquid nitrogen. Check lot specifications, typically 10 AU is ~2 mg of LysC.
Trypsin (Promega, sequencing grade, 0.5 μg/μl, store at −80 °C).
10 mM EPPS, pH 8.5: Prepare in HPLC grade water.
Vacuum concentrator, consisting of centrifugal evaporator SC100 (Savant), refrigerated vapor trap RVT4104 (Savant), vacuum pump RV8 (Edwards).
2.6. TMT Labeling
200 mM EPPS, pH 8.0.
TMT10-plex reagent set (Thermo Fisher Scientific): Prepare aliquots of 20 μg/μl in dry acetonitrile (Sigma), flash freeze, and store at −80 °C until use. Recently, an 11-plex TMT kit has become available.
Hydroxylamine solution, 50% wt (Sigma, store at 4 °C). We replace hydroxylamine yearly.
Vacuum concentrator, consisting of centrifugal evaporator SC100 (Savant), refrigerated vapor trap RVT4104 (Savant), vacuum pump RV8 (Edwards).
2.7. Reverse Phase Solid Phase Extraction/Sep-Pak
Phosphoric acid.
Methanol.
70% acetonitrile + 1% formic acid solution.
35% acetonitrile + 1% formic acid solution.
-
1% formic acid.
All the reagents should be prepared in HPLC grade water and stored in glass scintillation vials (e.g., Sci Spec, 20 ml). Solutions are stable at room temperature but the vials should be tightly closed to prevent evaporation of acetonitrile. For corrosive solutions like formic acid, we place a parafilm sheet between cap and vial before closing.
C-18 Sep-Pak cartridges (Waters Corporation, 50 mg, 1.3 ml column volume).
Solid Phase Extraction Vacuum Manifold (e.g. Sigma 57250-U).
Vacuum concentrator, consisting of centrifugal evaporator SC100 (Savant), refrigerated vapor trap RVT4104 (Savant), vacuum pump RV8 (Edwards).
2.8. Medium pH Reverse Phase Prefractionation
- HPLC buffers
- Buffer A: 10 mM ammonium bicarbonate, pH 8.0, 0.5% acetonitrile.
- Buffer B: 10 mM ammonium bicarbonate, pH 8.0, 95% acetonitrile:
- Sonicate the buffers for ~ 30 min to degas them. Solutions are stable at room temperature but the vials should be tightly closed to prevent evaporation of acetonitrile. All buffers should be prepared with HPLC grade water.
2c (TFE) (Acros Organics, 99.8%).
C18 column (Agilent, ZORBAX Extend-C18, 4.6 × 150 MM, 3.5-μm, 80 A, Part # 763953–902).
Injection Loop (Agilent Technologies, 500 μl, Part # 0101–1246).
Syringe (HAMILTON, 500 μl, Part # 81216).
96-well Deep well plates (e.g., Axygen Scientific, 1.1 ml, Part # 391-01-101).
HPLC (Agilent Technologies,1260 Infinity II LC System).
Vacuum concentrator, consisting of centrifugal evaporator SC100 (Savant), refrigerated vapor trap RVT4104 (Savant), vacuum pump RV8 (Edwards).
2.9. Stage Tip
Methanol.
1% formic acid.
35% acetonitrile + 1% formic acid solution.
-
70% acetonitrile + 1% formic acid solution.
All the reagents should be prepared with HPLC grade solvents. Solutions are stable at room temperature but the vials should be tightly closed to prevent evaporation.
Empore C-18 Solid Phase Extraction Disc (Fischer Scientific, 47 mm, Cat # 2216-C18).
Blunt needle (Monoject, 16 × 1–1/2 BLUNT, CE0123).
Paper Clips (e.g., Acco., Stock # 72380).
Vacuum concentrator, consisting of centrifugal evaporator SC100 (Savant), refrigerated vapor trap RVT4104 (Savant), vacuum pump RV8 (Edwards).
2.10. Liquid Chromatography-Mass Spectrometry Analysis
1% formic acid: Prepare in HPLC grade water.
Vial inserts (Agilent Technologies, 250 μl, Part # 5181–8872).
Glass vials (Agilent Technologies, 2 ml, Part# 5181–3375).
Seal (SUN-Sri, 11 mm, Part # 200100).
Crimper (Agilent Technologies, 11 mm, Part # 5040–4667).
Decapper (Agilent Technologies, 11 mm, Part # 5040–4668).
Easy-nLC 1200 (Thermo Fisher Scientific).
Orbitrap Fusion Lumos (Thermo Fisher Scientific).
3. Methods
3.1. Embryo Lysis and Yolk Removal
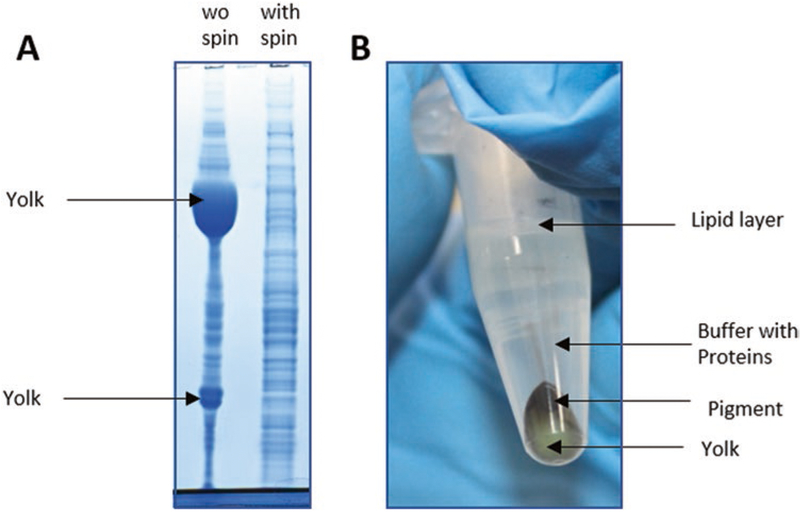
Efficient lysis, extraction, and denaturing of proteins from the cells is necessary for accessibility of proteins in the sample. However, yolk proteins constitute approximately 90% of the Xenopus proteins in eggs and early embryos. Having yolk in the sample can hinder the identification of other proteins which are less abundant. For this reason, we remove most yolk via a soft spin (Fig. 2A, B). We optimized the lysis buffer to be as destructive as possible and solubilize proteins to the maximum extent possible, while keeping yolk platelets mostly intact, so that we can remove them via centrifugation.
Fig. 2.
Yolk spin out. (A) ~90% of the protein mass in Xenopus embryos are yolk. Shown is a Coomassie-stained gel of a lysed Xenopus egg (left) and egg proteins after removing yolk via centrifugation (right). (B) Eppendorf tube after centrifugation step as detailed in the paper. Yolk and pigments sediment. Solubilized proteins can be removed from the supernatant
The buffer’s pH at 7.2 is compatible with subsequent cysteine protection with NEM, higher pH would lead to solubilization of yolk and side-reactions of NEM. The nonionic NP-40 is the harshest detergent we could find that does not significantly solubilize yolk. We add the chelator EDTA, and protease inhibitors to prevent protein degradation. Combretastatin and Cytochalasin depolymerize the microtubules and the actin cytoskeleton respectively and are added to prevent the spin out of the cytoskeletal macro assemblies. After the yolk is spun out, addition of SDS denatures proteins and keeps them inactivated during cysteine protection.
Collect 20 embryos (approximately 20 × 25 μg) per stage, and remove the buffer. Be careful not to lyse the embryos while collecting them. Lysing them at this point might destroy consistency across different conditions.
Flash freeze the embryos in liquid nitrogen. This allows downstream handling of all collected samples in parallel, which improves reproducibility.
After the collections are complete, lyse every condition in 1 ml of lysis buffer by pipetting up and down with a 1 ml pipette at least 15 times.
Incubate on ice for approximately 10 min to help disassemble large structures such as the cytoskeleton.
Vortex for 10 s. Do not vortex longer as it might dissolve the yolk proteins.
Spin down the yolk at 2500 rcf for 4 min at 4 °C. This step will spin out yolk and a thin layer of pigment granules (Fig. 2B). Flick the tubes gently to resuspend the proteins that are associated with lipids collected on the surface. Extract most of the supernatant without disturbing the yolk layer (~950 μl). It is acceptable to leave some buffer behind.
Add HEPES to 100 mM (pH 7.2). High salt concentration tends to solubilize yolk. Therefore, we keep the salt concentration in the lysis buffer low and add additional buffering capacity before cysteine protection.
Denature the proteins in lysed samples by adding SDS to 2%.
3.2. Cysteine Protection
The thiol side chain of cysteines is very reactive. The unspecific modifications resulting from reactions with, e.g., oxygen make it hard or impossible to identify cysteine containing peptides. Therefore, the cysteines are protected with NEM, which makes them inert (see Note 1). DTT is first added to break disulfide bonds. Please note that one DTT molecule will react with two NEM molecules.
Reduce the sample by adding DTT to 5 mM. Vortex followed by a quick spin. Incubate at 60 °C for 20 min to help solubilize membrane proteins. Make sure that the pH is below 7.5 (see Note 2).
Cool down to room temperature and add NEM to 20 mM. Vortex followed by a quick spin.
Incubate it at room temperature for 20 min.
Add additional 10 mM of DTT to consume the unreacted NEM in the sample. Vortex followed by a quick spin.
Incubate at room temperature for 10 min.
3.3. Methanol-Chloroform Precipitation
We include the Methanol-Chloroform precipitation to remove detergents and salts that could interfere with the digestion, prefractionation, or the mass spectrometer [32]. MeOH precipitation is our method of choice due to the high reproducibility between multiple samples. However, it requires at least 100 μg of proteins. For samples with less material we use trichloroacetic acid precipitation [33].
Take 200 μl of the sample per condition in a 2 ml Eppendorf tube.
Add 800 μl of methanol. Vortex well.
Add 400 μl of chloroform. Vortex well.
Add 600 μl of water. Vortex well.
Centrifuge at 20,000 rcf for 2 min at room temperature.
Carefully remove the supernatant and discard the upper layer without disturbing the interphase.
Add 600 μl of methanol. Vortex well.
Centrifuge again at 20,000 rcf for 2 min at room temperature to pellet the protein.
Remove the supernatant.
Repeat the methanol wash with 500 μl of methanol. Vortex well.
Centrifuge again at 20,000 rcf for 2 min at room temperature.
Remove the supernatant and air-dry the pellet to evaporate the left over organic solvents.
Take up the pellet in 6 M GuaCl, 10 mM EPPS, pH 8.5 to approximately 5 mg/ml protein concentration. The pH of 8.5 seems to be optimal for efficient and reproducible digestion. To help redissolve the protein pellet, heat to 60 °C and sonicate indirectly in water bath.
3.4. Estimate Protein Amount (BCA Assay)
To determine how much protease and isobaric tags to add, we need to know the approximate protein amount. We often skip this step and approximate that one embryo/egg will produce ~25 μg of non-yolk protein.
Prepare a working reagent stock solution by mixing 50 parts of reagent A with 1 part of reagent B.
Prepare BSA standard for a range of concentrations 2, 1, 0.5, 0.25, 0 mg/ml (approximately 20 μl each).
Add 5 μl of sample (well within the working range of 2–0.1 mg/ml) and the standard BSA solution to 150 μl of the working reagent in the wells of the plate. Measure BSA results in triplicates.
Incubate for 30 min at 37 °C.
Cool plate to RT. Measure the absorbance at or near 562 nm on a plate reader.
Subtract the average 562 nm absorbance measurement of the blank standard replicates from the 562 nm measurements of all other individual standard and unknown sample replicates.
Prepare a standard curve by plotting the average blank-corrected 562 nm measurement for each BSA standard vs. its concentration. Use the standard curve to determine the protein concentration of each unknown sample.
3.5. Digestion
Digestion occurs in parallel for different conditions. Our main goal for this step is reproducibility. Therefore, we perform a two-step digestion. The first step of digestion is carried out with 2 M GuaCl and LysC only. High concentration of chaotrope will (partly) unfold most proteins and allow LysC to digest them into smaller pieces. While LysC is active at 2 M GuaCl, it will miss many potential cleavage sites. The GuaCl is diluted to 0.5 M and fresh LysC and Trypsin are added. In comparison with Trypsin, LysC is superior in digesting peptides ending with lysine. Trypsin is added to digest arginine. For the highest quality MultiNotch MS3 data, one can perform a LysC digest only. This will result in all b- and y-ions to be TMT-tagged and will suppress interference. However, a LysC only digest will come at the cost of quantifying fewer proteins. For the embryonic time-series example, we omitted the trypsin digestion.
Dilute sample with 10 mM EPPS (pH 8.5) to 2 M GuaCl (see Note 3), vortex well and spin.
Add LysC (Based on whatever is larger: 1/100 per protein weight or 20 ng/μl LysC concentration).
Gently flick the tubes to mix the LysC well in the sample. Do not vortex to avoid denaturation of protease.
Incubate at room temperature overnight.
Dilute with 10 mM EPPS (pH 8.5) to 0.5 M GuaCl (see Note 3). Vortex well and spin.
Add LysC to 20 ng/μl. Optionally, if besides cleavage after lysine, also cleavage after arginine is desired, add Trypsin to 10 ng/μl.
Gently flick the tubes to mix the proteases well in the sample. Do not vortex.
Incubate at 37 °C overnight. To prevent condensation on lid, heat the entire tube.
Vacuum concentrate the samples. This reduces the amount of hydroxyl ions, which interfere with TMT-labeling.
3.6. TMT Labeling
The reaction of isobaric TMT tags with the peptide N-terminus or alpha amino group of lysine to form an amide linkage is pH sensitive. At too low of a pH, the free amines get protonated and will no longer react. At too high a pH, the TMT tags react with hydroxyl ions in solution and are degraded. We found that a pH of 8.0 is optimal for this reaction to reach completion (see Note 4). In the previous step we removed water to minimize competing hydroxyl ions. Acetonitrile concentrations significantly higher than 30% will decrease reaction efficiency. We use at least 4 μg of TMT to label 1 μg of peptide and at least 2 μg of TMT per 1 μl of added 200 mM EPPS.
Resuspend 100 μg with 50 μl of 200 mM EPPS, pH 8.0.
Add 20 μl of TMT-stock solution (20 μg/μl in acetonitrile). Flick to mix well.
Incubate at room temperature for 2 h.
Quench with hydroxylamine. Hydroxylamine degrades the excess TMT and removes TMT that reacted with tyrosine. Hydroxylamine is pre-diluted to 5%. Add 5 μl of 5% to the reaction. Incubate for 30 min at room temperature.
Combine all conditions into one tube. Mix well.
Acidify with 5% phosphoric acid. DO NOT use formic acid, as we have previously observed formaldehyde adducts on peptides. We suspect that formaldehyde formed by formic acid reacting with hydroxylamine.
Vacuum concentrate the sample to remove acetonitrile from TMT labeling step. Acetonitrile would interfere with the following reverse phase purification step.
3.7. Reverse Phase Solid Phase Extraction/Sep-Pak
At this stage, sample contains lots of salt (GuaCl) and might contain some undigested proteins that can interfere with the down-stream prefractionation step, e.g., by clogging the column. The disposable Sep-Pak will desalt the sample and retain (most) undigested proteins (see Note 5).
Prepare sample: The sample is resuspended in 500 μl of HPLC water. Check the pH (it should be below 2) (see Note 6). If the pH is not approximately 1, add additional phosphoric acid.
- Condition/Equilibrate the column:
- Wet the column with 100% methanol, run through at a flow rate of 5–10 ml/min for ~15 s.
- Equilibrate the column with 1% formic acid, run through quickly at a flow rate of 5–10 ml/min for ~15 s.
Load sample: Load the sample under gravity or low vacuum at a flow rate of 0.2–1 ml/min for ~5 min.
- Wash:
- Wash the tube with 500 μl of 1% formic acid for ~1 min.
- Wash with 500 μl 1% formic acid for ~1 min.
- Wash with 500 μl 1% formic acid for ~1 min once again.
- Elute: Remember to elute into a fresh tube.
- Elute with 1 ml of 35% acetonitrile, 1% formic acid under gravity or low vacuum for ~5 min.
- Elute with 1 ml of 70% acetonitrile, 1% formic acid under gravity or low vacuum for ~5 min.
Dry: Vacuum concentrate the sample to remove acetonitrile.
3.8. Quality Control (Detailed in Accompanying Paper)
The multiple sample preparation steps allow many possibilities for errors. Mass spectrometry data is rich in information and can, to some extent, inform us about problems. At this point, we evaluate the quality of the prepared sample before committing significant additional time for pre-fractionation and mass spectrometry analysis of pre-fractionated samples.
The analysis of the data to identify problems with labeling or digestion efficiency is detailed out in the accompanying paper (Chapter 14).
Take 1 μg of the sample after Sep-Pak or perform Stage tip (see Subheading 3.10) and analyze on the mass spectrometer (see Subheading 3.11 on how to do the liquid chromatography-mass spectrometry analysis).
3.9. Medium pH Reverse Phase Prefractionation
Fractionating the sample prior to mass spectrometry analysis has the capability of increasing the depth of proteome coverage by reducing the sample complexity of the eluate. The performance of this two-dimensional chromatographic technique depends on separation efficiency in both dimensions and orthogonality of separation. A reverse phase system with significantly different pH in both dimensions performs well and is highly robust. The change in the separation selectivity on modifying the pH is attributed to the alteration in charge distribution of the peptides. Pooling multiple early, middle, and late fractions further provides an advantage in terms of time efficiency, better proteome coverage, and orthogonality. Therefore, an offline medium pH reverse phase (RP) HPLC separation with a concatenation strategy is employed prior to a low pH RP in front of the mass spectrometer. This procedure is designed for total protein content of ~1000 μg in the sample.
Run blank sample: Load 450 μl TFE and run through the column [34]. Elute with the two-gradient buffer system. TFE helps elute potential contaminations from the previous experiments. Refer to Table 2 for the gradients of the buffers, pressure limits and the flow rates used.
Prepare sample: Dissolve sample in ~450 μl of buffer A. The volume of the sample is limited by the injection loop volume.
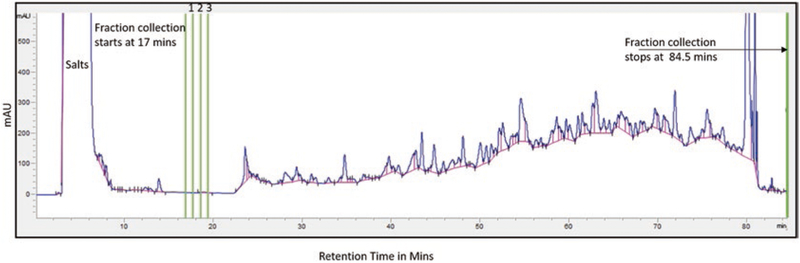
Fractionate sample: Load the sample and fractionated (for gradient see Table 1). Collect 96 fractions starting at 17 min every 42 s, until 84.5 min (Fig. 3).
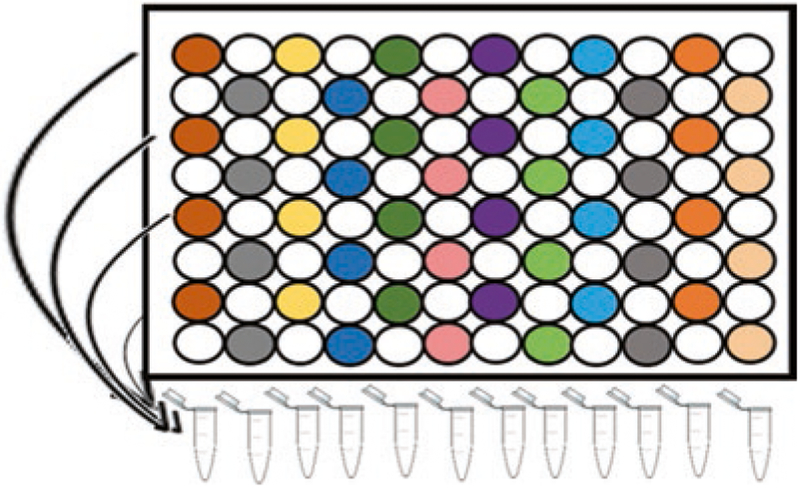
Combine fractions: Combine the 96 fractions into 24 fractions (Fig. 4) [35].
Dry: Vacuum concentrate the 24 combined fractions to remove acetonitrile.
Table 1.
Gradients for elution of peptide fractions
| Time [min] | A [%] | B [%] | Flow [ml/min] |
|---|---|---|---|
| 0.00 | 100.0 | 0.0 | 0.500 |
| 17.00 | 100.0 | 0.0 | 0.500 |
| 18.00 | 93.0 | 7.0 | 0.500 |
| 75.00 | 65.0 | 35.0 | 0.500 |
| 76.00 | 0.0 | 100.0 | 0.500 |
| 80.00 | 0.0 | 100.0 | 0.500 |
| 86.00 | 100.0 | 0.0 | 0.500 |
| 90.00 | 100.0 | 0.0 | 0.500 |
Fig. 3.
Elution profile of the peptides during prefractionation. The green lines mark the fractions collected at various times starting at 17 min with 42 s increments until the 96th fraction at 84.5 mins (The first three fractions are marked as 1, 2, and 3). The large peak at the beginning corresponds to small molecules
Fig. 4.
Schematic representation of concatenation strategy. Each color in the vertical column represents fractions that are combined into a sample, which is analyzed via LC-MS. After the first 12 collections, collect another 12 by following the same scheme using the uncolored wells in the vertical rows
3.10. Stage Tip [36]
The sample contains ammonium bicarbonate from the pre-fractionation step. While ammonium bicarbonate is volatile and should mostly decompose on the mass spectrometer inlet tube. However, some will accumulate inside the mass spectrometer, which can severely hamper instrument performance.
- Column production.
- Column holder: Take a 0.5 ml Eppendorf tube, cut off bottom half. Also cut bottom half of a 200 μl. Insert cut pipette tip into cut Eppendorf tube.
- Column: Press the blunt needle into the C18 disc to cut out the desired column material. Bend open the paper clip to obtain a straight wire. Insert the needle, with C18 material inside, into a new 200 μl tip. With the wire inserted into the blunt needle, push out the C18 material into the 200 μl tip. Repeat this procedure with a second disc. We use one disc per ~25 μg of peptides. Press the wire down on the C18 disc, to generate a tight seal between the C18 material and the 200 μl tip. Make sure that there are no gaps, which would allow the sample to pass by the C18 material.
- Column assembly: Insert the column into the column holder. We discard columns after use, but preserve the column holders for future use.
Prepare sample: Acidify the samples by taking them up in 30 μl of 5% phosphoric acid. Make sure the pH is close to 1.
- Condition/equilibrate the column:
- Wet the column with 30 μl of 100% methanol. Centrifuge at ~420 rcf for 15 s. Visually inspect the column to observe if methanol is still present above the C18. If all the methanol has already flowed through, the C18 disc will not effectively bind the peptides and a new stage-tip needs to be prepared. Centrifuge at ~420 rcf for 2 min. Check the column. If some liquid is still present spin down for 30 s more and check the column. Repeat the process until all the liquid flows through. The disc should not dry out, it should look pale like wax (see Note 7).
- Equilibrate the column with 30 μl of 1% formic acid. Spin at 1700 rcf for 1 min. Spin more if needed as described above. Do not dry the disc.
Load sample: Load the sample. Spin at 1700 rcf for 1 min. Spin more if needed as described above. Do not dry the disc.
- Wash:
- Wash with 30 μl of 1% formic acid. Spin at 1700 rcf for 1 min. Spin more if needed as described above. Do not dry the disc.
- Wash again with 30 μl of 1% formic acid. Spin at 1700 rcf for 1 min. Spin more if needed as described above. Do not dry the disc.
- Elute:
- Insert the vial insert into a 2 ml Eppendorf. Take the entire column assembly and transfer it to a torpedo tube for elution.
- Elute with 30 μl of 35% acetonitrile + 1% formic acid. Spin at 3800 rcf for 1 min. Spin more if needed.
- Elute one more time with 30 μl of 70% acetonitrile + 1% formic acid. Spin at 3800 rcf for 1 min. Spin more if needed. Drying the disc at this point is acceptable.
Dry: vacuum concentrate the samples to remove acetonitrile.
3.11. Liquid Chromatography–Mass Spectrometry Analysis
Resuspend the dry sample in 1% formic acid to approximately 1 μg/μl (see Note 8).
Transfer the vial inserts into the mass spectrometer vials.
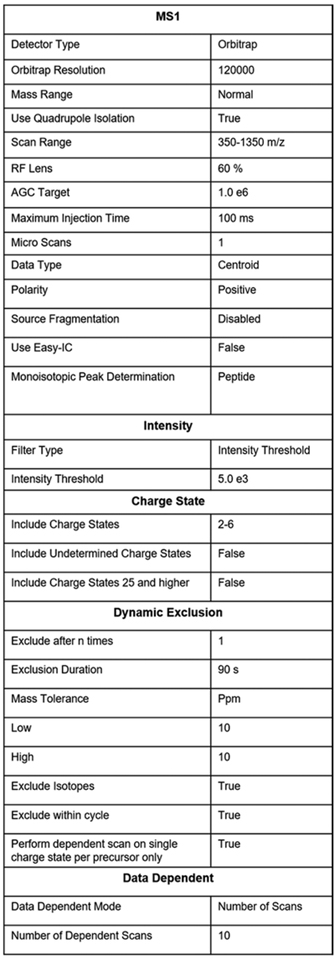
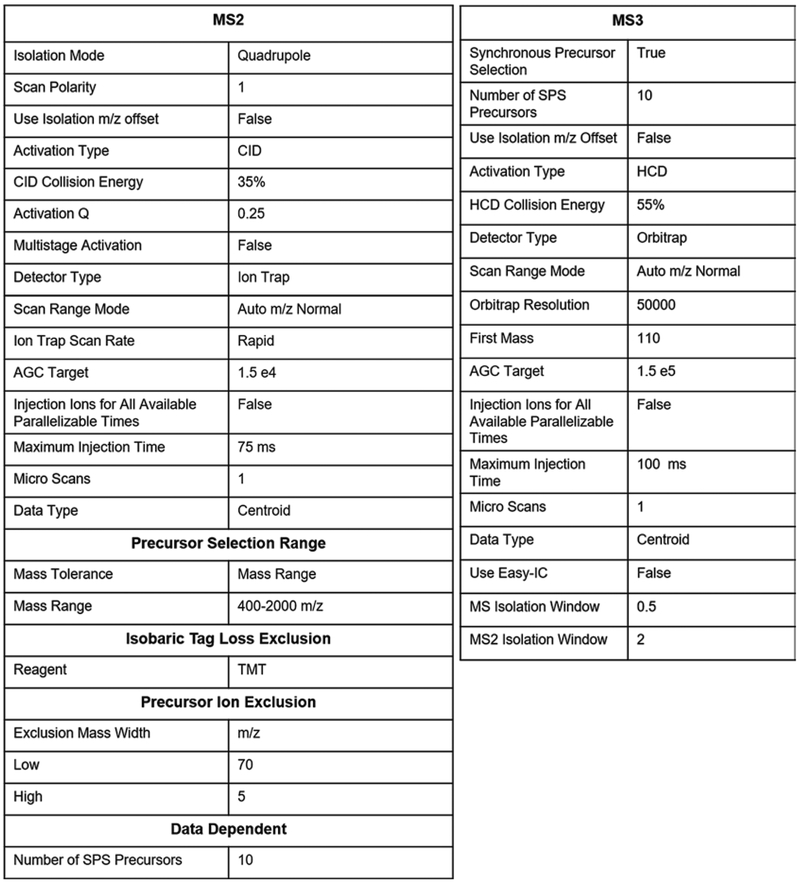
Create an instrument method with the settings described in Fig. 5.
Aim to shoot about 3 μg of the total protein per prefractionated sample.
Fig. 5.
Parameter setting for TMT-MS3 method
4. Notes
We recently learnt that maleimide ring hydrolyzes over time [37]. It therefore might be advantageous to replace the NEM with other alkylating reagents like iodoacetamide.
A higher pH can deprotonate the lysines. NEM may react with deprotonated amines or undergo hydrolysis at a more alkaline pH.
Higher chaotrope concentration decreases protease activity.
Though we found pH 8.0 to be optimal for this reaction, the alpha amino groups of N-terminus cysteines frequently do not react.
We have recently started to replace this step with ultracentrifugation of the acidified sample at 200,000 rcf at 4 °C for an hour. The undigested proteins aggregate with the low pH. These aggregates, together with other large assemblies like glycogen, spin out [38]. The supernatant is collected and subjected to medium pH reverse phase prefractionation. The prefractionation step simultaneously acts as a desalting step. We therefore load the sample with continuous flow of buffer A and only after 17 min start to increase the acetonitrile concentration and fractionation collection (Fig. 3). However, we still don’t know if desalting with the medium pH reverse phase column is a sustainable option. Even occasional clogging of these very expensive columns would not be acceptable.
pH of the sample should be lower than pKa of the carboxyl groups. This makes the peptides more hydrophobic and helps them to stick better to the columns.
Repeat the process in ~30 s time increments for additional spins to avoid the disc drying out. Different stage-tips will vary by how much centrifugation force/time is required for all liquid to flow through. If the flow rate is too slow, we often have to increase centrifugation speeds at various steps from ~420 rcf to e.g., ~1000 rcf or higher. However, we try to load the sample in at least 2 min so that peptides have enough time to bind to the C18 material.
A fraction of the sample is lost in the multistep purification process. Therefore, the protein content at this stage is just an estimate.
Acknowledgments
We thank Thao Nguyen for help collecting the Xenopus embryonic time series and Felix Keber for comments and suggestions on the manuscript. M.P. was supported by NIH grant R01GM103785. M.S. was supported by NIH F31 predoctoral fellowship 5F31GM116451. This work was supported by NIH grant 1R35GM128813 and Princeton University startup funding.
References
- 1.Swammerdam J (1737) Bibilia Naturae; Sive historia insectorum, in classes certas redact 2 [Google Scholar]
- 2.Prevost JL, Dumas J-B (1824) Nouvelle théorie de la génération. Ann Sci Nat 2 [Google Scholar]
- 3.Gurdon JB, Elsdale TR, Fischberg M (1958) Sexually mature individuals of Xenopus laevis from the transplantation of single somatic nuclei. Nature 182(4627):64–65 [DOI] [PubMed] [Google Scholar]
- 4.Paine PL, Moore LC, Horowitz SB (1975) Nuclear envelope permeability. Nature 254(5496):109–114 [DOI] [PubMed] [Google Scholar]
- 5.Session AM, Uno Y, Kwon T, Chapman JA, Toyoda A, Takahashi S, Fukui A, Hikosaka A, Suzuki A, Kondo M, van Heeringen SJ, Quigley I, Heinz S, Ogino H, Ochi H, Hellsten U, Lyons JB, Simakov O, Putnam N, Stites J, Kuroki Y, Tanaka T, Michiue T, Watanabe M, Bogdanovic O, Lister R, Georgiou G, Paranjpe SS, van Kruijsbergen I, Shu S, Carlson J, Kinoshita T, Ohta Y, Mawaribuchi S, Jenkins J, Grimwood J, Schmutz J, Mitros T, Mozaffari SV, Suzuki Y, Haramoto Y, Yamamoto TS, Takagi C, Heald R, Miller K, Haudenschild C, Kitzman J, Nakayama T, Izutsu Y, Robert J, Fortriede J, Burns K, Lotay V, Karimi K, Yasuoka Y, Dichmann DS, Flajnik MF, Houston DW, Shendure J, DuPasquier L, Vize PD, Zorn AM, Ito M, Marcotte EM, Wallingford JB, Ito Y, Asashima M, Ueno N, Matsuda Y, Veenstra GJ, Fujiyama A, Harland RM, Taira M, Rokhsar DS (2016) Genome evolution in the allotetraploid frog Xenopus laevis. Nature 538(7625):336–343. 10.1038/nature19840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gurdon JB, Wakefield L (1986) Microinjection of amphibian oocytes and eggs for the analysis of transcription. Microinjection and Organelle Transplantation Techniques 269–299. [Google Scholar]
- 7.Markow TA, Beall S, Matzkin LM (2009) Egg size, embryonic development time and ovoviviparity in Drosophila species. J Evol Biol 22(2):430–434. 10.1111/j.1420-9101.2008.01649.x [DOI] [PubMed] [Google Scholar]
- 8.Tartia AP, Rudraraju N, Richards T, Hammer MA, Talbot P, Baltz JM (2009) Cell volume regulation is initiated in mouse oocytes after ovulation. Development 136(13):2247–2254. 10.1242/dev.036756 [DOI] [PubMed] [Google Scholar]
- 9.Smits AH, Lindeboom RG, Perino M, van Heeringen SJ, Veenstra GJ, Vermeulen M (2014) Global absolute quantification reveals tight regulation of protein expression in single Xenopus eggs. Nucleic Acids Res 42(15):9880–9891. 10.1093/nar/gku661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lombard-Banek C, Moody SA, Nemes P (2016) High-sensitivity mass spectrometry for probing gene translation in single embryonic cells in the early frog (Xenopus) embryo. Front Cell Dev Biol 4:100 10.3389/fcell.2016.00100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wühr M, Güttler T, Peshkin L, McAlister GC, Sonnett M, Ishihara K, Groen AC, Presler M, Erickson BK, Mitchison TJ, Kirschner MW, Gygi SP (2015) The nuclear proteome of a vertebrate. Curr Biol 25(20):2663–2671. 10.1016/j.cub.2015.08.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boke E, Ruer M, Wühr M, Coughlin M, Lemaitre R, Gygi SP, Alberti S, Drechsel D, Hyman AA, Mitchison TJ (2016) Amyloid-like self-assembly of a cellular compartment. Cell 166(3):637–650. 10.1016/j.cell.2016.06.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peshkin L, Wühr M, Pearl E, Haas W, Freeman RM Jr, Gerhart JC, Klein AM, Horb M, Gygi SP, Kirschner MW (2015) On the relationship of protein and mRNA dynamics in vertebrate embryonic development. Dev Cell 35(3):383–394. 10.1016/j.devcel.2015.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lombard-Banek C, Moody SA, Nemes P (2016) Single-cell mass spectrometry for discovery proteomics: quantifying translational cell heterogeneity in the 16-cell frog (Xenopus) embryo. Angew Chem Int Ed Engl 55(7):2454–2458. 10.1002/anie.201510411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun L, Bertke MM, Champion MM, Zhu G, Huber PW, Dovichi NJ (2014) Quantitative proteomics of Xenopus laevis embryos: expression kinetics of nearly 4000 proteins during early development. Sci Rep 4:4365 10.1038/srep04365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Presler MS, Van Itallie E, Klein AM, Kunz R, Coughlin P, Peshkin L, Gygi S, Wühr M, Kirschner M (2017) Proteomics of phosphorylation and protein dynamics during fertilization and meiotic exit in the Xenopus egg. Proc Natl Acad Sci U S A. 2017 Dec 12;114(50):E10838–E10847. doi: 10.1073/pnas.1709207114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sawin KE, Mitchison TJ (1991) Mitotic spindle assembly by two different pathways in vitro. J Cell Biol 112(5):925–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reinsch S, Karsenti E (1997) Movement of nuclei along microtubules in Xenopus egg extracts. Curr Biol 7(3):211–214 [DOI] [PubMed] [Google Scholar]
- 19.Wühr M, Chen Y, Dumont S, Groen AC, Needleman DJ, Salic A, Mitchison TJ (2008) Evidence for an upper limit to mitotic spindle length. Curr Biol 18(16):1256–1261. 10.1016/j.cub.2008.07.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gache V, Waridel P, Winter C, Juhem A, Schroeder M, Shevchenko A, Popov AV (2010) Xenopus meiotic microtubule-associated interactome. PLoS One 5(2):e9248 10.1371/journal.pone.0009248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liska AJ, Popov AV, Sunyaev S, Coughlin P, Habermann B, Shevchenko A, Bork P, Karsenti E, Shevchenko A (2004) Homology-based functional proteomics by mass spectrometry: application to the Xenopus microtubule-associated proteome. Proteomics 4(9):2707–2721. 10.1002/pmic.200300813 [DOI] [PubMed] [Google Scholar]
- 22.Wühr M, Freeman RM Jr, Presler M, Horb ME, Peshkin L, Gygi S, Kirschner MW (2014) Deep proteomics of the Xenopus laevis egg using an mRNA-derived reference database. Curr Biol 24(13):1467–1475. 10.1016/j.cub.2014.05.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deshmukh AS, Murgia M, Nagaraj N, Treebak JT, Cox J, Mann M (2015) Deep proteomics of mouse skeletal muscle enables quantitation of protein isoforms, metabolic pathways, and transcription factors. Mol Cell Proteomics 14(4):841–853. 10.1074/mcp.M114.044222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eng JK, McCormack AL, Yates JR (1994) An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J Am Soc Mass Spectrom 5(11):976–989. 10.1016/1044-0305(94)80016-2 [DOI] [PubMed] [Google Scholar]
- 25.Ong SE, Blagoev B, Kratchmarova I, Kristensen DB, Steen H, Pandey A, Mann M (2002) Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol Cell Proteomics 1(5):376–386 [DOI] [PubMed] [Google Scholar]
- 26.Cox J, Hein MY, Luber CA, Paron I, Nagaraj N, Mann M (2014) Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. Mol Cell Proteomics 13(9):2513–2526. 10.1074/mcp.M113.031591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenberger G, Bludau I, Schmitt U, Heusel M, Hunter CL, Liu Y, MacCoss MJ, MacLean BX, Nesvizhskii AI, Pedrioli PGA, Reiter L, Rost HL, Tate S, Ting YS, Collins BC, Aebersold R (2017) Statistical control of peptide and protein error rates in large-scale targeted data-independent acquisition analyses. Nat Methods 14(9):921–927. 10.1038/nmeth.4398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McAlister GC, Nusinow DP, Jedrychowski MP, Wühr M, Huttlin EL, Erickson BK, Rad R, Haas W, Gygi SP (2014) Multinotch MS3 enables accurate, sensitive, and multiplexed detection of differential expression across cancer cell line proteomes. Anal Chem 86(14):7150–7158. 10.1021/ac502040v [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wenger CD, Lee MV, Hebert AS, McAlister GC, Phanstiel DH, Westphall MS, Coon JJ (2011) Gas-phase purification enables accurate, multiplexed proteome quantification with isobaric tagging. Nat Methods 8(11):933–935. 10.1038/nmeth.1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ting L, Rad R, Gygi SP, Haas W (2011) MS3 eliminates ratio distortion in isobaric multiplexed quantitative proteomics. Nat Methods 8(11):937–940. 10.1038/nmeth.1714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wühr M, Haas W, McAlister GC, Peshkin L, Rad R, Kirschner MW, Gygi SP (2012) Accurate multiplexed proteomics at the MS2 level using the complement reporter ion cluster. Anal Chem 6;84(21):9214–9221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wessel D, Flugge UI (1984) A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem 138 (1):141–143. doi:0003-2697(84)90782-6 [pii] [DOI] [PubMed] [Google Scholar]
- 33.Chevallet M, Diemer H, Van Dorssealer A, Villiers C, Rabilloud T (2007) Toward a better analysis of secreted proteins: the example of the myeloid cells secretome. Proteomics 7(11):1757–1770. 10.1002/pmic.200601024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mitulovic G, Stingl C, Steinmacher I, Hudecz O, Hutchins JR, Peters JM, Mechtler K (2009) Preventing carryover of peptides and proteins in nano LC-MS separations. Anal Chem 81(14):5955–5960. 10.1021/ac900696m [DOI] [PubMed] [Google Scholar]
- 35.Edwards A, Haas W (2016) Multiplexed quantitative proteomics for high-throughput comprehensive proteome comparisons of human cell lines. Methods Mol Biol 1394:1–13. 10.1007/978-1-4939-3341-9_1 [DOI] [PubMed] [Google Scholar]
- 36.Rappsilber J, Mann M, Ishihama Y (2007) Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat Protoc 2(8):1896–1906. 10.1038/nprot.2007.261 [DOI] [PubMed] [Google Scholar]
- 37.Lyon RP, Setter JR, Bovee TD, Doronina SO, Hunter JH, Anderson ME, Balasubramanian CL, Duniho SM, Leiske CI, Li F, Senter PD (2014) Self-hydrolyzing maleimides improve the stability and pharmacological properties of antibody-drug conjugates. Nat Biotechnol 32(10):1059–1062. 10.1038/nbt.2968 [DOI] [PubMed] [Google Scholar]
- 38.Dworkin MB, Dworkin-Rastl E (1989) Metabolic regulation during early frog development: glycogenic flux in Xenopus oocytes, eggs, and embryos. Dev Biol 132(2):512–523 [DOI] [PubMed] [Google Scholar]
- 39.Thompson A, Schafer J, Kuhn K, Kienle S, Schwarz J, Schmidt G, Neumann T, Johnstone R, Mohammed AK, Hamon C (2003) Tandem mass tags: a novel quantification strategy for comparative analysis of complex protein mixtures by MS/MS. Anal Chem 75(8):1895–1904 [DOI] [PubMed] [Google Scholar]
- 40.Sonnett M, Yeung E, Wühr M (2018) Accurate, Sensitive, and Precise Multiplexed Proteomics Using the Complement Reporter Ion Cluster. Analytical Chemistry 90(8): 5032–5039 [DOI] [PMC free article] [PubMed] [Google Scholar]