Sarcoplasmic/endoplasmic reticulum Ca2+ ATPase 2a (SERCA2a, the major isoform of SERCA expressed in cardiomyocytes) plays an essential role in the regulation of cardiac contractility1. It transports cytoplasmic Ca2+ into the sarcoplasmic reticulum (SR). Ca2+ stored within the SR is released with each action potential to induce contraction and muscle contraction is terminated when this Ca2+ is transported back into the SR. The rate of SR Ca2+ uptake dictates the rate of myocardial relaxation and the amount of SR Ca2+ loading determines the amount of Ca2+ stored for the next contraction. In this way regulation of SERCA2a activity controls both cardiac contractility and lusitropy. This is exemplified by stress responses that drive activation of the sympathetic/β-adrenergic system1, which indirectly activates SERCA2a by relieving phospholamban (PLB)-mediated inhibition. The resultant increases in SERCA2a activity leads to both increased myocardial contractility and cardiac relaxation. While control of SERCA2 function in acute stress responses are well-defined, regulatory mechanisms that control SERCA2a function in chronic stress states such as Heart Failure (HF) is less well understood.
HF is a syndrome in which the heart is unable to pump a sufficient amount of blood to meet the needs of the tissues. It is often characterized by reduced cardiac contractility. A number of studies have shown that reduced expression and/or activity of SERCA2a is a significant contributing factor loss of contractility in HF. Another cause of decreased SERCA2a activity in HF is reduced PLB phosphorylation. Multiple approaches have been used to determine if correction of the SERCA2a deficiency in HF can improve cardiac function. Several groups, including the Hajjar group2, have tested if restoring SERCA2a protein abundance in cardiomyocytes prevents or ameliorates cardiac function in hearts stressed by pressure overloaded or infarcted hearts3, 4. Transgenic and gene therapy approaches have been used with rodent and large mammalian models to test this modality4. Some of these animal studies demonstrated that restoring SERCA2a expression improved cardiac metabolism, reduced arrhythmias, and enhanced coronary blood flow5. However, other studies showed that the beneficial effects could be diminished by limited energy supply in hypertrophied myocardium6. The Hajjar group and other groups advanced this gene therapy approach to human clinical trials2, 5. While Phase I and phase IIa studies both showed reduced cardiac events in small cohorts of heart failure patients receiving high dose of AAV1.SERCA2a, a larger double-blind, placebo-controlled and randomized phase IIb clinical trial (CUPID2) did not show significant improvement of primary and secondary endpoints5. The results of the CUPID2 trial led to the suspension of two other clinical trials (AGENT-HF and SERCA-LVAD) utilizing SERCA2a gene therapy.
More recent studies have shown that post translational modifications (PTMs) of the SERCA2a protein, rather than its regulatory protein PLB, contributes to decreased SERCA2a protein activity1. Previously identified PTMs include glutathionylation7, nitration and SUMOylation2. In the current issue of Circulation Research8 the role of acetylation of SERCA2a in HF is reported for the first time. In contrast to the stimulatory effects of glutathionylation on C6747 and SUMOylation on K480 and K5852 on SERCA2a activity, Gorski et al.8 demonstrate that acetylation of K492 decreases both SERCA2a activity and myocyte contractility. Furthermore, they discovered that the acetylation of K492 was increased in failing human tissue and pressure-overloaded mouse hearts, which was due to decreased sirtuin 1 (SIRT1). Activating SIRT1 by β-lapachone improved cardiac function and ameliorated cardiac remodeling in wild-type mice with pressure overload (transaortic constriction, TAC) but not in SIRT1 knockdown or knockout mice, suggesting that SIRT1 is the specific deacetylase for SERCA2a K492. Additional evidence was presented showing that the transcription cofactor p300 is required for SERCA2a acetylation.
While there are many potential explanations for these findings, one possibility is that post-translational modifications, acetylation in this case, of SERCA2a8 can dominate SERCA2 function even if the protein is overexpressed. If true, future investigations focused on controlling rather than overexpressing SERCA2a may yield more promising outcomes.
Early investigations on control of SERCA2a activity focused on controlling PLB. The knockout/knockdown of PLB or increasing PLB phosphorylation by increasing constitutive protein phosphatase inhibitor I1 improved cardiac function in HF models1, 5. However, double knockout of the negative SERCA regulators PLB and sarcolipin worsened cardiac function in ageing and pressure overloaded hearts9. S100A1, a protein abundantly expressed in cardiomyocytes, is able to promote SERCA2a activity. Gene therapy with S100A1 improved cardiac function in a preclinical model of ischemic heart disease10. The advent of micro-RNA research identified miR-25 as a down-regulator of SERCA2a. Its expression is upregulated in failing human and animal tissues. Anti-miR-25 antagomir was able to restore the expression of SERCA2a and halted heart failure development in mice subjected to TAC11. Most recently, a micropeptide encoded by a presumed long noncoding RNA, DWORF, has been shown to displace PLB, increasing SERCA2a activity and improving cardiac function in dilated cardiomyopathy12. As the delivery of small molecule inhibitors such as antagomirs or micropeptides provide a much easier therapeutic modality than gene delivery, it offers a practical hope for heart failure treatment.
The discoveries that post-translational modifications directly alter the activity of SERCA2a offer a new avenue for HF treatment via enhancing SERCA2a activity. Glutathionylation on C6747, 13 and SUMOylation on K480 and K5852 of SERCA2a increase SERCA2a activity but nitration of Tyr294, Tyr295 and Tyr753, oxidization of the thiol group on C674 and other amino acids14, and the newly identified acetylation of K492 decrease activity. Increasing SERCA2a SUMOylation by either SUMO1 overexpression2 or small molecule-mediated activation of SUMO115 is able to treat heart failure. In the recently published paper by the same group in Circulation Research, the authors found another PTM of SERCA2a, acetylation of K492, was increased in failing and stressed hearts, resulting in SERCA2a activity inhibition8. More importantly, the authors found that increased acetylation of SERCA2a was specifically caused by the decrease of SIRT1 expression. This provides the opportunity to specifically activate SIRT1 activity to decrease SERCA2a acetylation and increase SERCA2a activity. As small molecules activating SIRT1 already exist and the acetylation site is different from the beneficial SUMOylation sites, targeting acetylation represents an ideal opportunity to manipulate SERCA2a function towards the treatment of failing hearts. Further, as we continue to develop more insight into mechanisms in control of SERCA2a function, combination therapies become increasingly realistic. Hence, it may be possible to utilize small molecules to both increase SUMOylation and decrease acetylation of SERCA2a simultaneously. Alternatively, PTM modifiers like these could dramatically improve SERCA2a gene therapy; if so, this would also provide insight into why previous efforts to use gene therapy to rescue HF were unsuccessful. That said, one limiting factor of the SIRT1 activation approach is that SIRT1 is widely expressed and is involved in many signaling processes. Hence, the side effects of SIRT1 activators will need to be considered, despite prior investigations demonstrating that SIRT1 activation is generally beneficial16.
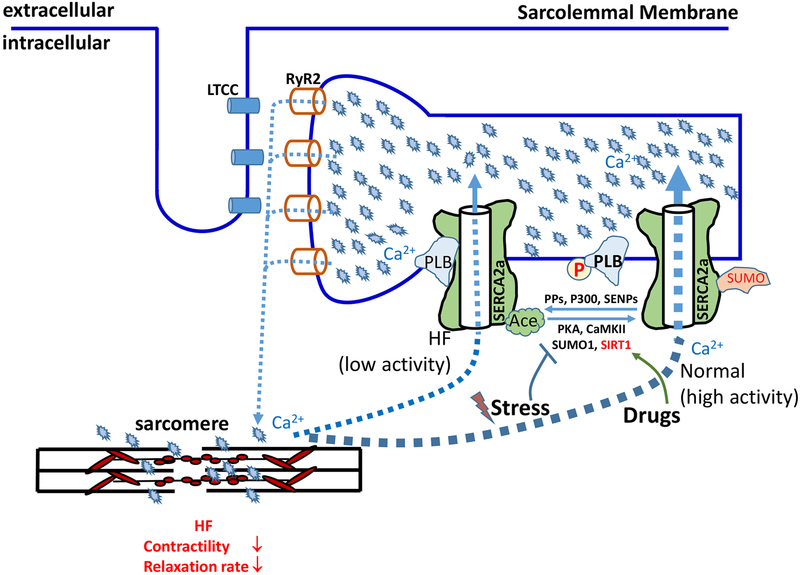
Prior studies have established that fluctuation of SERCA2a activity through regulatory mechanisms are beneficial to the heart and loss of this fluctuation causes adverse cardiac remodeling when ageing or subjected to TAC9. Further, treating heart failure by enhancing myocyte contractility must proceed with caution as overloading with Ca2+ of the SR can lead to ER stress and spontaneous Ca2+ release, resulting in mitochondrial Ca2+ overloading. Ultimately this can lead to myocyte death, cardiac hypertrophy, and arrhythmia17, 18, especially at very high SERCA2a levels. In addition, enhancing cardiac contractility by augmenting SERCA2a activity will increase the demand of energy supply, which is often limited in diseased hearts. In such cases, cardiac contractility enhancement may exacerbate the development of heart disease. Considered collectively, it seems clear that more information is needed to determine how best to utilize SERCA2a targeting in heart treatment. Our current understanding of how regulation of SERCA2a function controls cardiac contractility and HF is summarized in figure 1. Future investigations either defining as yet unidentified mechanisms and/or designing therapeutic strategies based on SERCA2a expression and function represent amongst the more promising approaches towards the treatment of this deadly disease.
Figure 1: Manipulating SERCA2a post-translational modifications for HF treatment.
Cardiac contraction is initiated upon the release of SR Ca2+ through RyR2, which is determined by the amount of Ca2+ in the SR. SERCA2a is a cardiac-specific isoform and serves as the primary facilitator of SR Ca2+ loading, making it a critical regulator of both contractility and lusitropy. HF is characterized by loss of contractility; loss of SERCA2a expression and/or activity is a well-established contributing factor to HF. The activity of SERCA2a is regulated by PLB along with a combination of post-translational modifications. Whereas either binding to dephosphorylated PLB or p300-mediated acetylation decrease SERCA activity, phosphorylation of PLB by PKA or CAMKII and SUMOylation by SUMO1 increase it. SERCA2a in failing hearts has increased binding to dephosphorylated PLB and acetylation, but decreased SUMOylation, all leading to decreased activity. Reversing these SERCA2a PTM abnormalities in HF by drugs holds the promise for HF treatment. Ace – Acetylation; CaMKII – Calmodulin Kinase II; HF – Heart Failure; LTCC – L-Type Ca2+ channels; PKA – Protein Kinase A; PLB – Phospholamban; PPs – protein phosphatases; RYR – Ryanodine Receptors; SERCA2a – Sarco/Endoplasmic Reticulum Ca2+-ATPase 2a; SIRT1 – NAD+-dependent deacetylase sirtuin-1; SUMO - small ubiquitin-like modifier, SENPs - SUMO specific proteases.
Acknowledgements:
This work was supported by NIH grants R01HL088243 & R01HL140071 (XC), R01HL033921, R01HL119229, R01HL132391, P01HL134608, R01HL139960 & P01HL091799 (SRH) and R01GM117907 & R56AI143256 (JS).
Footnotes
Disclosures:
None.
References:
- 1.Kranias EG, Hajjar RJ. Modulation of cardiac contractility by the phospholamban/serca2a regulatome. Circ Res 2012;110:1646–1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayward C, Banner NR, Morley-Smith A, Lyon AR, Harding SE. The current and future landscape of serca gene therapy for heart failure: A clinical perspective. Hum Gene Ther 2015;26:293–304 [DOI] [PubMed] [Google Scholar]
- 3.Mariani JA, Smolic A, Preovolos A, Byrne MJ, Power JM, Kaye DM. Augmentation of left ventricular mechanics by recirculation-mediated aav2/1-serca2a gene delivery in experimental heart failure. Eur J Heart Fail 2011;13:247–253 [DOI] [PubMed] [Google Scholar]
- 4.Hulot JS, Ishikawa K, Hajjar RJ. Gene therapy for the treatment of heart failure: Promise postponed. Eur Heart J 2016;37:1651–1658 [PubMed] [Google Scholar] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pinz I, Tian R, Belke D, Swanson E, Dillmann W, Ingwall JS. Compromised myocardial energetics in hypertrophied mouse hearts diminish the beneficial effect of overexpressing serca2a. J Biol Chem 2011;286:10163–10168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adachi T, Weisbrod RM, Pimentel DR, Ying J, Sharov VS, Schoneich C, Cohen RA. S-glutathiolation by peroxynitrite activates serca during arterial relaxation by nitric oxide. Nat Med 2004;10:1200–1207 [DOI] [PubMed] [Google Scholar]
- 7.Kho C, Lee A, Jeong D, Oh JG, Chaanine AH, Kizana E, Park WJ, Hajjar RJ. Sumo1-dependent modulation of serca2a in heart failure. Nature. 2011;477:601–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gorski PA, Jang SP, Jeong D, Lee A, Lee P, Oh JG, Chepurko V, Yang DK, Kwak TH, Eom SH, Park ZY, Yoo YJ, Kim DH, Kook H, Sunagawa Y, et al. Role of sirt1 in modulating acetylation of the sarco-endoplasmic reticulum ca(2+)-atpase in heart failure. Circ Res 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shanmugam M, Gao S, Hong C, Fefelova N, Nowycky MC, Xie LH, Periasamy M, Babu GJ. Ablation of phospholamban and sarcolipin results in cardiac hypertrophy and decreased cardiac contractility. Cardiovasc Res 2011;89:353–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pleger ST, Shan C, Ksienzyk J, Bekeredjian R, Boekstegers P, Hinkel R, Schinkel S, Leuchs B, Ludwig J, Qiu G, Weber C, Raake P, Koch WJ, Katus HA, Muller OJ, et al. Cardiac aav9-s100a1 gene therapy rescues post-ischemic heart failure in a preclinical large animal model. Sci Transl Med 2011;3:92ra64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wahlquist C, Jeong D, Rojas-Munoz A, Kho C, Lee A, Mitsuyama S, van Mil A, Park WJ, Sluijter JP, Doevendans PA, Hajjar RJ, Mercola M. Inhibition of mir-25 improves cardiac contractility in the failing heart. Nature. 2014;508:531–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Makarewich CA, Munir AZ, Schiattarella GG, Bezprozvannaya S, Raguimova ON, Cho EE, Vidal AH, Robia SL, Bassel-Duby R, Olson EN. The dworf micropeptide enhances contractility and prevents heart failure in a mouse model of dilated cardiomyopathy. Elife. 2018;7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lancel S, Zhang J, Evangelista A, Trucillo MP, Tong X, Siwik DA, Cohen RA, Colucci WS. Nitroxyl activates serca in cardiac myocytes via glutathiolation of cysteine 674. Circ Res 2009;104:720–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vangheluwe P, Raeymaekers L, Dode L, Wuytack F. Modulating sarco(endo)plasmic reticulum ca2+ atpase 2 (serca2) activity: Cell biological implications. Cell Calcium. 2005;38:291–302 [DOI] [PubMed] [Google Scholar]
- 15.Kho C, Lee A, Jeong D, Oh JG, Gorski PA, Fish K, Sanchez R, DeVita RJ, Christensen G, Dahl R, Hajjar RJ. Small-molecule activation of serca2a sumoylation for the treatment of heart failure. Nat Commun 2015;6:7229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nogueiras R, Habegger KM, Chaudhary N, Finan B, Banks AS, Dietrich MO, Horvath TL, Sinclair DA, Pfluger PT, Tschop MH. Sirtuin 1 and sirtuin 3: Physiological modulators of metabolism. Physiol Rev 2012;92:1479–1514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakayama H, Chen X, Baines CP, Klevitsky R, Zhang X, Zhang H, Jaleel N, Chua BH, Hewett TE, Robbins J, Houser SR, Molkentin JD. Ca2+- and mitochondrial-dependent cardiomyocyte necrosis as a primary mediator of heart failure. J Clin Invest 2007;117:2431–2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eisner D Calcium in the heart: From physiology to disease. Exp Physiol 2014;99:1273–1282 [DOI] [PubMed] [Google Scholar]