Abstract
Gastric cancer is a leading cause of cancer worldwide. Our previous studies showed that aberrant activation of JAK/STAT3 signaling confer epigenetically silences STAT3 target genes in gastric cancer. To further investigate the clinical significance of this phenomenon, we performed Illumina 850K methylation microarray analysis in AGS gastric cancer cells, and cells depleted of STAT3. Integrative computational analysis identified SPG20 as a putative STAT3 epigenetic target, showing promoter hypomethylation in STAT3-depleted AGS cells. Bisulphite pyrosequencing and qRT-PCR confirmed that SPG20 is epigenetically silenced by promoter hypermethylation in a panel of gastric cancer cell lines including AGS cells, but not in immortalized gastric epithelial GES cells. Expression of SPG20 could be restored by the treatment with a DNMT inhibitor, further suggesting that SPG20 is epigenetically silenced by promoter methylation. Clinically, a progressive increase in SPG20 methylation was observed in tissues samples from gastritis (n = 34), to intestinal metaplasia (IM, n = 33), to gastric cancer (n = 53). Importantly, SPG20 methylation could be detected in cell-free DNA isolated from serum samples of gastritis, IM and gastric cancer patients, having a progressive similar to tissues. Taken together, SPG20, a potential STAT3 target, is frequently methylated in gastric cancer, representing a novel noninvasive biomarker for early detection of this deadly disease.
Introduction
Gastric cancer is a leading cause of cancer deaths worldwide [1]. Despite advances in cancer therapy, gastric cancer patients still have a poor 5-year survival of less than 15% [2], likely due to a lack of biomarkers for early detection. Although endoscopic screening for early gastric cancer may improve patient’s survival, noninvasive, sensitive, and specific population screening assays are currently not available and are therefore urgently needed.
Epigenetic modifications, including DNA methylation, play an important role in transcriptional regulation, and embryonic and disease development [3, 4]. Due to its stability, methylation at the cytosine of CG dinucleotides has been found both in tissues and bodily fluids such as plasma and serum. These attributes make DNA methylation an attractive target for non-invasive cancer detection in cell-free DNA (cfDNA, also known as liquid biopsy) [5, 6].
Our laboratory has long been dedicated to the development of DNA methylation as a biomarker for disease monitoring [7–9]. Additionally, we previously demonstrated that DNA methylation could be detected in bodily fluids, in several human diseases, including cancers [10, 11]. However, promising DNA methylation biomarkers, for noninvasive early detection of gastric cancer, remain lacking.
Our previous studies demonstrated that aberrant activation of JAK/STAT signaling, triggered by H. pylori infection, can lead to epigenetic silencing of tumor suppressors in gastric cancer [12, 13]. In this study, we examine additional STAT3 targets that are epigenetically silenced by DNA methylation in gastric cancer, representing sensitive biomarkers for noninvasive detection of gastric cancer.
Materials and methods
Patient samples
Patient samples (biopsy and serum) were obtained from Chang Gung Memorial Hospital, Chiayi, Taiwan or the Medical School of Zhejiang University, Hangzhou, China from March 2013 to February 2016 (Table 1). All samples were stored at -80°C before subsequent processing for analysis. All human subject assessments were approved by the Institutional Review Board (IRB) of the Chang Gung Memorial Hospital, Chiayi, Taiwan and the ethics committee of Zhejiang University, Hangzhou, China. The study was carried out in strict accordance with approved guidelines. Written informed consent was obtained from all participants.
Table 1. Summary of clinico-pathological data of patients samples.
| Gastric cancer (n = 53) | Non-cancer (n = 107) | |
|---|---|---|
| Age Median Range |
69 43~87 |
57 25~87 |
| Sex Male Female |
37 16 |
60 47 |
| Disease type normal gastritis intestinal metaplasia(IM) cancer |
53 |
40 34 33 |
|
H. pylori infection positive negative |
12 28 |
40 55 |
| Median survival | 8.2 (n = 17) | |
| Stage (for cancer) I II III IV |
12 10 20 12 |
|
| Metastasis (for cancer) Yes No |
21 32 |
Cell culture
Gastric cancer cell line (AGS, KATO III, MKN28, MKN45, SNU1, SNU16, purchased from ATCC, Manassas, VA) and an immortalized gastric epithelial cell line, GES (a kind gift from Dr. Jun Yu, The Chinese University of Hong Kong, Hong Kong) were maintained in RPMI 1640 (Gibco, Waltham, MA) supplemented with 10% FBS (Gibco) and 1% Penicillin-Streptomycin (Gibco). All cells were maintained at 37°C, with 5% CO2, under a humidified incubator. Cells were treated with 0.5μM 5-aza-2’-deoxycytidine (5-azaDC, Sigma, St. Louis, MO) for 3 days and harvested for RNA extraction.
DNA extraction, RNA extraction, and quantitative reverse transcription-PCR
DNA was extracted using a Genomic DNA Mini Kit (Geneaid, Taiwan), according to the manufacturer’s instructions. DNA was then eluted in 50μl distilled water and stored at -20°C until use. Cell-free DNA was extracted from 500ml serum samples using QIAamp DNA Blood Mini Kit (Qiagen GmbH, Germany). RNA was extracted using TriZol reagent (Invitrogen, Carlsbad, CA), according to the manufacturer’s protocol. To remove potential contaminating DNA from the complementary DNA, 1μg of total RNA was treated with DNase I (Amplification Grade, Invitrogen), prior to reverse transcription (Superscript ll RT, Invitrogen). Real-time PCR reactions were performed on an ABI Step-One real-time PCR system (Applied Biosystems, Foster City, CA) with specific primers (Table 2). Relative gene expression was determined by comparing the threshold cycle (Ct) of the test gene against the Ct value of GAPDH, in a given sample. (i.e., the comparative Ct method).
Table 2. Primer sequence used in this study.
| Sequence 5’-3’ | |
|---|---|
| RT-PCR | |
| GAPDH forward GAPDH reverse LPHN3 forward LPHN3 reverse SOX5 forward SOX5 reverse ABR forward ABR reverse SPG20 forward SPG20 reverse |
CCCCTTCATTGACCTCAACTACAT CGCTCCTGGAAGATGGTGA GAAATATTCTTTGGATTTTGGACC TAGCTCAGAGTCAAGGTGAATTG GGGAACAACAGGTGCTTGAT CTCCACTCAGATTGAAATCCATC AGCGCCTGAAGAAGAAGATGTT TGAATTGCTTCTCTCCACTCTGA AACTAGACCCTCCTCTGACCAA TTTCTTCTGGAACTGGCTCA |
| Pyrosequencing | |
| SPG20 forward SPG20 reverse SPG20 sequencing |
AAGTAATAGAAGATTGTATTTATATGGAATTATTTTTTGA ACAAATTAATCTAATACCTATTTAACCCTTTACCA GGAAGTTTTTTTGTAATGTG |
| Methylation-Specific PCR | |
| Methylated SPG20 MF SPG20 MR Unmethylated SPG20 UF SPG20 UR |
ATTTTTTGATTCGTAGTTTTTTATAATC AAAAATAAAACCGACCCGA GAATTATTTTTTGATTTGTAGTTTTT AAAATAAAACCAACCCAAAA |
Bisulphite conversion and pyrosequencing
0.5μg genomic DNA was bisulphite modified using an EZ DNA Methylation Kit (Zymo Research, Orange, CA) according to the manufacturer’s protocol. The bisulphite modified DNA was then subjected to PCR amplification, using a tailed reverse primer, including a biotin-labeled universal primer, PCR, and sequencing primers were designed using PyroMark assay Design 2.0 software (Qiagen). The SPG20 transcription start site (+1094 to +1387) was PCR amplified using specific primer (Table 3), and pyrosequencing was performed using PyroMark Q24 (Qiagen) Pyro Gold Reagents (Qiagen), according to the manufacturer’s protocol. Methylation percentage of six CpG sites located from +1209 to +1229 bp was determined by the fluorescence intensity of cytosines and thymines at each CpG site. In vitro methylated DNA (IVD, Merck Millipore, MA) was included as positive control for bisulphite pyrosequencing.
Table 3. Association between clinical parameters and SPG20 methylation in 53 gastric cancer patient samples.
| SPG20 methylation (n) | P | |
|---|---|---|
| Age ≥60 <60 |
24.40 ± 11.81, (43) 24.27 ± 11.93, (10) |
0.48 |
| Sex Male Female |
24.27 ± 11.93, (37) 24.40 ± 11.81, (16) |
0.48 |
|
H. pylori infection Yes No |
25.97 ± 12.52, (12) 24.33 ± 11.82, (27) |
0.27 |
| Stage High stage (I ~ II) Low stage (III ~ IV) |
24.27 ± 11.93, (28) 24.40 ± 11.81, (23) |
0.48 |
| Metastasis Yes No |
24.27 ± 11.93, (20) 24.70 ± 11.72, (30) |
0.43 |
Methylation-specific PCR (MSP)
Bisulphite-modified DNA was subjected to MSP for SPG20 methylation analysis using specific primers (Table 2). 4μl of bisulphite-converted DNA were amplified in a total volume of 20μl containing 10x PCR Buffer, 0.25mM dNTPs, 2mM MgCl2, 0.2μM of each primer and 1.25U of Platinum Taq DNA polymerase (Invitrogen) at 95°C for 2 min, followed by 40 cycles of denaturing at 95°C for 30 sec, annealing at 60°C for 30 sec, and extension at 72°C for 30 sec, followed by followed by a final extension step of 72°C for 10 min. In vitro methylated DNA (IVD, Merck Millipore) was included as positive control and normal blood (NB) was included as a negative control of MSP. 10μl of PCR products were loaded onto 10% polyacrylamide gels, which were then stained with ethidium bromide, and visualized under UV illumination.
Infinium microarray DNA methylation analysis
Bisulphite-modified DNA from AGS gastric cancer cells and cells depleted of STAT3 was subject to methylation analysis, using an Illumina 850K methylation microarray. The methylation level of each probe (β-value) was defined by the intensity of the methylated allele (M) / (intensity of the unmethylated allele (U) + the intensity of the methylated allele (M) + 100). The microarray data has been deposited in the Gene Expression Omnibus database (accession number: GSE109541).
Statistical analysis
Unpaired t-tests were used to compare parameters of the various groups. All statistical calculations were performed using the SPSS statistical package (version 18.0) for Windows (IBM, Chicago, IL, USA). In this study, P values <0.05 were considered statistically significant.
Results
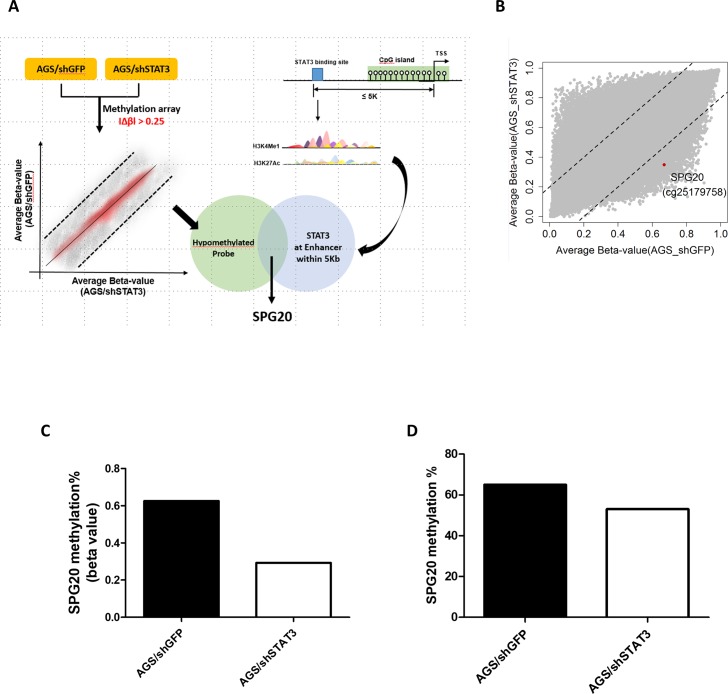
Our previous studies found that aberrant activation of JAK/STAT signaling could lead to epigenetic silencing of STAT3 targets in gastric cancer [12, 13]. We therefore hypothesized that binding of STAT3 to promoter-proximal CpG islands may affect their methylation status. In this regard, we performed Illumina 850K methylation microarray analysis in bisulphite-treated genomic DNA from AGS gastric cancer cells, and cells depleted of STAT3. Computational predictions were also performed to identify all STAT3-binding sites located in open chromatin regions (as demarcated by H3K4me1 and H3K27Ac) in close proximity to promoter CpG islands (Fig 1A). One probe (cg25179758, Fig 1B and 1C) within the promoter region of SPG20, showing differential hypomethylation in STAT3-depleted AGS cells, was identified. Bisulphite pyrosequencing further confirmed that knock-down of STAT3 decreased SPG20 methylation in AGS cells (Fig 1D).
Fig 1. Integrated microarray and bioinformatics analyses identifies SPG20 as an epigenetically silenced target of STAT3, in gastric cancer.
(A) Schematic diagram showing the experimental scheme of this study. Stable transfectants of AGS gastric cancer cells depleted of STAT3 (shSTAT3) or control (shGFP) were subjected to Illumina 850K methylation microarray analysis. Probes showing a changes in β values (|Δβ|) of at least 0.25 (25% methylation difference) were selected. In addition, bioinformatics analysis was performed to identify potential STAT3 targets by filtering genes with at least one STAT3-binding site in potential enhancer region (as enriched with H3K4me1 and H3K27Ac) of the promoter CpG island. One hypomethylated probe and a potential STAT3 target, SPG20, was selected for further analysis. (B) Scatter plot showing the average beta-value of each probe in AGS cells depleted of STAT3 or GFP (as control). One of the probes (cg25179758, red dot) showing hypomethylation, located within the SPG20 promoter region, was selected for further analysis. Methylation% of SPG20 at the promoter region as determined by (C) methylation microarray (beta value) or (D) bisulphite pyrosequencing.
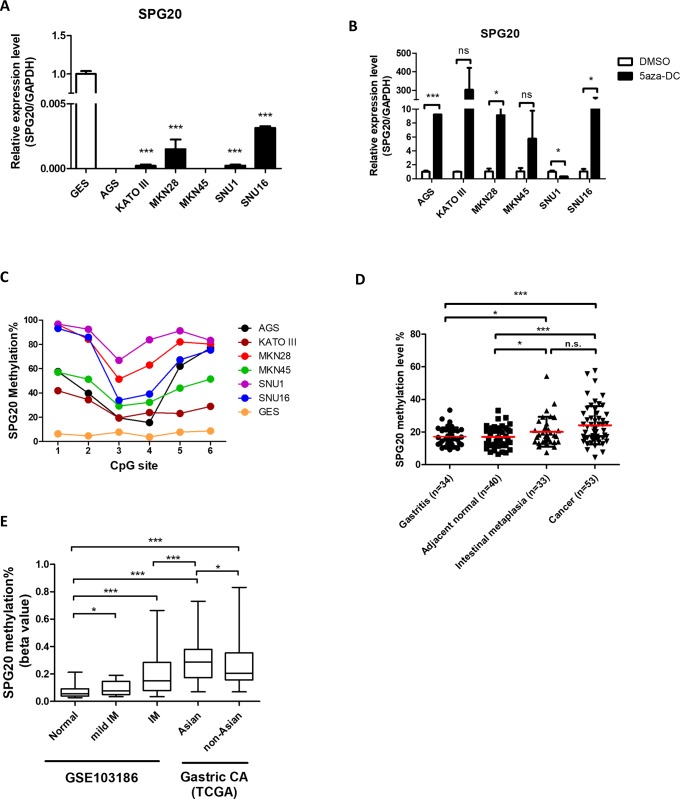
To further examine the role of DNA methylation in regulating expression of SPG20, we assessed its expression in an immortalized gastric epithelial cell (GES), and a panel of gastric cancer cell lines. Except for GES cells, downregulation of SPG20 was observed in all cancer cell lines (Fig 2A). Treatment with the DNMT inhibitor (5aza-DC) resulted in robust re-expression of SPG20 in those cells (Fig 2B), due to DNA demethylation of its promoter as confirmed by bisulphite pyrosequencing (Fig 2C).
Fig 2. SPG20 is epigenetically silenced by promoter methylation in gastric cancer.
Relative expression of SPG20 in (A) immortalized GES cells, and a separate panel of gastric cancer cell lines, and (B) 5aza-DC-treated gastric cancer cells, as determined by qRT-PCR. Each bar represents the means ± SD of duplicate experiments. (C) Methylation levels of six CpG sites within the SPG20 promoter region in GES cells and various gastric cell lines, as determined by bisulphite pyrosequencing. (D) Promoter methylation of SPG20 in gastritis, tumor adjacent normal, intestinal metaplasia (IM), and gastric cancer tissue samples, as determined by bisulphite pyrosequencing. Red lines denote median values. (E) Promoter methylation of SPG20 in IM and gastric cancer, as retrieved from GSE103186 (normal and IM) and TCGA (cancer). Significant differences between groups are indicated by *P<0.05, **P<0.01, ***P<0.005, as determined by Mann-Whitney U-test.
We then examined the clinical significance of SPG20 methylation in a cohort of gastritis, intestinal metaplasia (IM), and paired gastric cancer patient samples (Table 1). Although no clinical parameters significantly associated with DNA hypermethylation (Table 3), results from bisulphite pyrosequencing showed higher SPG20 methylation in gastric tumors, compared to tumor-adjacent normal and gastritis tissues (Fig 2D). Interestingly, IM tissues also showed a higher SPG20 methylation, compared to adjacent normal and gastritis tissues (Fig 2D).
We also compared SPG20 methylation in datasets (GSE103186 and TCGA) from normal gastric epithelial, IM, and gastric cancer tissues obtained from two publicly available databases. Consistently, cancer tissues showed higher SPG20 methylation than normal and IM samples (Fig 2E). As demonstrated in our cohort, samples from IM also showed higher SPG20 methylation than those from normal tissues. It is interesting to note that SPG20 methylation, in cancer tissues from Asian populations, were higher than those from non-Asian populations, suggesting population-specific underlying epigenotypes.
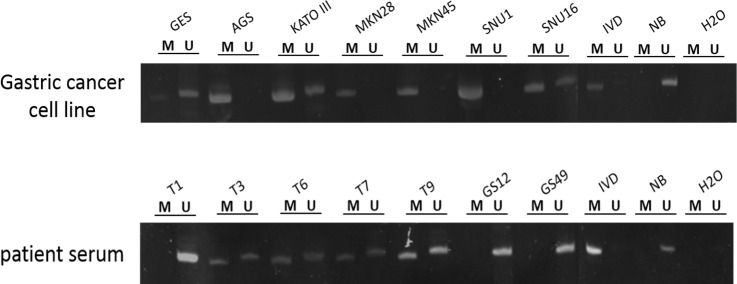
To further examine the feasibility of SPG20 methylation, as a noninvasive methylation biomarker for the early detection of gastric cancer, we performed conventional methylation-specific PCR to amplify short fragment of methylated DNA from cell-free DNA (cfDNA) obtained from serum samples of non-cancer individuals, and patients with IM or gastric cancer. In agreement with our bisulphite pyrosequencing results, SPG20 methylation could be detected in all gastric cancer cell lines by MSP (Fig 3). Overall, the sensitivity and specificity of gastric cancer detection using SPG20 methylation, were 88.6% and 75%, respectively (Table 4). Importantly, the sensitivity of cancer or IM detection could remain as high as 87.5% (Table 4).
Fig 3. Methylation analysis of SPG20 in cell lines and cell-free DNA from serum patient samples.
Representative gel picture from methylation-specific PCR (MSP) analysis to determine SPG20 methylation in gastric cancer cell lines (top panel) and patient serum samples (bottom panel). Bisulphite-modified DNA was PCR-amplified using specific primers. “M” and “U” indicate the presence of methylated and unmethylated alleles, respectively. IVD (in vitro methylated DNA) was a positive control for methylation and NB (normal blood) was a negative control for methylation. Water (H2O) was used as a negative control for PCR.
Table 4. Sensitivity and specificity of cancer detection using serum SPG20 methylation.
| Disease type1 | Cancer only (n = 53) | Cancer or IM (n = 56) |
|---|---|---|
| Sensitivity Specificity PPV2 NPV3 |
88.6% (47/53) 75.0% (15/20) 90.3% 71.4% |
87.5% (49/56) 75.0% (15/20) 90.7% 68.1% |
1As compared with serum samples from non-cancer (n = 20)
2Positive predictive value
3Negative predictive value
Discussion
DNA methylation, an “epigenetic” mode of transcriptional regulation, is altered in numerous pathologies. Consequently, due to its chemical uniqueness and stability (e.g., vs. RNA), disease-associated methylated DNA sequences represent promising tissue and liquid biomarkers. In the current study, by methylation microarray, we identified that a potential STAT3 target, SPG20, is differentially methylated in gastric cancer. Cell line studies further confirmed that SPG20 is epigenetically silenced, by DNA methylation, in gastric cancer cell lines. Clinical studies also demonstrated SPG20 hypermethylation in tissues and serum samples from IM and gastric cancer patients. Importantly, a progressive increase in SPG20 methylation, from gastritis to gastric cancer, was observed.
SPG20, encoding the multifunctional protein Spartin, has been shown to be involved in several cellular processes. Several studies have found that SPG20, containing a MIT (microtubule interacting and trafficking) domain, is involved in cytokinesis [14]. Cells depleted of SPG20 showed cytokinesis arrest and convoluted midbodies. More recently, SPG20 has been found to be involved in EGFR trafficking and MAPK signaling pathway [15, 16]. Specifically, gastric cancer cells depleted of SPG20 showed increased EGFR expression and phosphorylation of kinase involved in the MAPK signaling. Taken together, these studies suggest that SPG20 may act in early carcinogenesis and proliferation of human cancer. In agreement with those observations, SPG20 methylation was observed in intestinal metaplasia (IM), an early lesion of gastric cancer, from our own cohort and published data [17]. Although gene silencing of SPG20 methylation has been demonstrated previously in several cancers [18, 19], including gastric cancer [20], we believe this is the first study to demonstrate SPG20 methylation in IM, suggesting a role in early gastric carcinogenesis.
Infection by H. pylori is considered as a major risk factor for gastric cancer [21], capable of activating multiple signaling pathways including the JAK/STAT signaling [22, 23]. In this regard, activation of JAK/STAT signaling plays an important role in gastric carcinogenesis [24, 25]. However, the role of STAT3 in the epigenetic silencing of its targets is not fully elucidated. In this study, we found that the putative STAT3 target, SPG20, is epigenetically silenced in gastric cancer, similar to our previous findings of two other STAT3-epigenetically silenced targets, NR4A3 [13] and GATA3 [12]. Although a putative STAT3-binding site, as determined by ENCODE data, is few kb away from the promoter CpG island, the presence of the enhancer histone mark, H3K4me1, suggest that STAT3 might affect promoter methylation by higher-order chromatin structure. Such postulate is supported by the observation that Asian gastric cancer patients with more virulent and inflammatory-inducing strain of H. pylori strains [26–28] have higher SPG20 methylation than non-Asian populations with less virulent strains (Fig 2E). However, further experiments are required to demonstrate the role of STAT3 in the epigenetic silencing of SPG20.
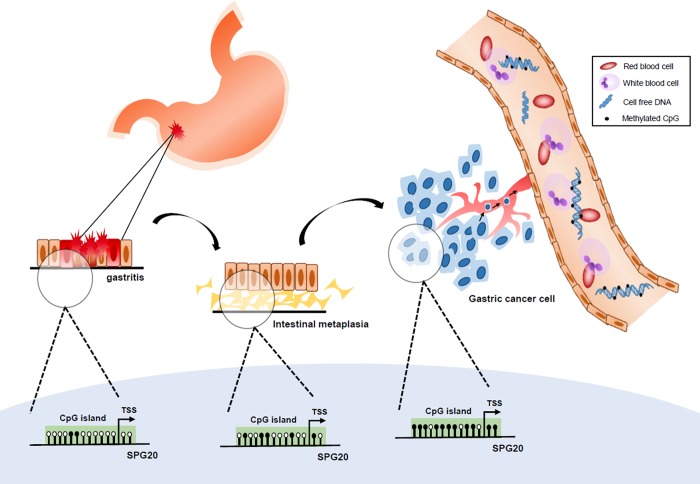
In this study, we also demonstrated that SPG20 methylation could be detected in cell-free DNA isolated from serum samples of intestinal metaplasia (IM) and gastric cancer patients (Illustrated in Fig 4). Importantly, a progressive increase of SPG20 methylation detection was observed from gastritis, to IM, and to cancer. However, more samples are required to determine the sensitivity and specificity of SPG20 methylation in cancer diagnosis especially in the detection of early gastric cancer lesions.
Fig 4. Schematic diagram showing a progressive increase of SPG20 methylation in the development of gastric cancer.
During the development of gastric cancer from gastritis to intestinal metaplasia to gastric cancer, a progressive increase in SPG20 promoter methylation, probably due to increased activation of JAK/STAT signaling, is observed. Methylated DNA released into the peripheral blood as cell-free DNA, by necrosis or tumor metastasis, may then serve as a biomarker for the early detection of gastric cancer.
In conclusion, we herein demonstrate that the putative STAT3 target, SPG20, is epigenetically silenced by promoter methylation in gastric cancer. and that such methylation is detectable in tissues and cfDNA from patients with gastric cancer as well as intestinal metaplasia. Consequently, SPG20 methylation may be able to serve as a non-invasive biomarker for the early detection of gastric cancer.
Acknowledgments
The authors would like to thank the Tissue Bank at the Department of Medical Research, Chang Gung Memorial Hospital at Chiayi for providing patient samples. This work was partially supported by the Center for Nano Bio-Detection from The Featured Research Areas College Development Plan of National Chung Cheng University and the Center for Innovative Research on Aging Society (CIRAS) from The Featured Areas Research Center Program under the framework of the Higher Education Sprout Project by Ministry of Education, Taiwan.
Data Availability
The microarray data has been deposited in the Gene Expression Omnibus database (accession number: GSE109541).
Funding Statement
This study was supported by research grants from Chang Gung Memorial Hospital, Taiwan under grant numbers CORPG6D0031~33 and CORPG6G0031~32 to KLW and research grants from the Ministry of Science and Technology, Taiwan (MOST) under grant numbers: 107-2314-B-194 -001 and 106-2314-B-194 -001 to MWYC. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. 10.3322/caac.21208 . [DOI] [PubMed] [Google Scholar]
- 2.Hochwald SN, Kim S, Klimstra DS, Brennan MF, Karpeh MS. Analysis of 154 actual five-year survivors of gastric cancer. J Gastrointest Surg. 2000;4(5):520–5. . [DOI] [PubMed] [Google Scholar]
- 3.Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet. 2012;13(7):484–92. 10.1038/nrg3230 . [DOI] [PubMed] [Google Scholar]
- 4.Jones PA, Issa JP, Baylin S. Targeting the cancer epigenome for therapy. Nat Rev Genet. 2016;17(10):630–41. 10.1038/nrg.2016.93 . [DOI] [PubMed] [Google Scholar]
- 5.Dietrich D. DNA Methylation Analysis from Body Fluids. Methods Mol Biol. 2018;1655:239–49. 10.1007/978-1-4939-7234-0_18 . [DOI] [PubMed] [Google Scholar]
- 6.De Rubis G, Krishnan SR, Bebawy M. Circulating tumor DNA—Current state of play and future perspectives. Pharmacol Res. 2018;136:35–44. 10.1016/j.phrs.2018.08.017 . [DOI] [PubMed] [Google Scholar]
- 7.Chou JL, Su HY, Chen LY, Liao YP, Hartman-Frey C, Lai YH, et al. Promoter hypermethylation of FBXO32, a novel TGF-beta/SMAD4 target gene and tumor suppressor, is associated with poor prognosis in human ovarian cancer. Lab Invest. 2010;90(3):414–25. 10.1038/labinvest.2009.138 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsieh HY, Jou YC, Tung CL, Tsai YS, Wang YH, Chi CL, et al. Epigenetic silencing of the dual-role signal mediator, ANGPTL4 in tumor tissues and its overexpression in the urothelial carcinoma microenvironment. Oncogene. 2018;37(5):673–86. 10.1038/onc.2017.375 . [DOI] [PubMed] [Google Scholar]
- 9.Chou JL, Huang RL, Shay J, Chen LY, Lin SJ, Yan PS, et al. Hypermethylation of the TGF-beta target, ABCA1 is associated with poor prognosis in ovarian cancer patients. Clin Epigenetics. 2015;7:1 10.1186/s13148-014-0036-2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yeh CM, Chen PC, Hsieh HY, Jou YC, Lin CT, Tsai MH, et al. Methylomics analysis identifies ZNF671 as an epigenetically repressed novel tumor suppressor and a potential non-invasive biomarker for the detection of urothelial carcinoma. Oncotarget. 2015;6(30):29555–72. 10.18632/oncotarget.4986 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tseng KC, Chou JL, Huang HB, Tseng CW, Wu SF, Chan MW. SOCS-1 promoter methylation and treatment response in chronic hepatitis C patients receiving pegylated-interferon/ribavirin. J Clin Immunol. 2013;33(6):1110–6. 10.1007/s10875-013-9903-4 . [DOI] [PubMed] [Google Scholar]
- 12.Wu CS, Wei KL, Chou JL, Lu CK, Hsieh CC, Lin JM, et al. Aberrant JAK/STAT Signaling Suppresses TFF1 and TFF2 through Epigenetic Silencing of GATA6 in Gastric Cancer. Int J Mol Sci. 2016;17(9). 10.3390/ijms17091467 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeh CM, Chang LY, Lin SH, Chou JL, Hsieh HY, Zeng LH, et al. Epigenetic silencing of the NR4A3 tumor suppressor, by aberrant JAK/STAT signaling, predicts prognosis in gastric cancer. Sci Rep. 2016;6:31690 10.1038/srep31690 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lind GE, Raiborg C, Danielsen SA, Rognum TO, Thiis-Evensen E, Hoff G, et al. SPG20, a novel biomarker for early detection of colorectal cancer, encodes a regulator of cytokinesis. Oncogene. 2011;30(37):3967–78. 10.1038/onc.2011.109 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou Z, Wang W, Xie X, Song Y, Dang C, Zhang H. Methylation-induced silencing of SPG20 facilitates gastric cancer cell proliferation by activating the EGFR/MAPK pathway. Biochem Biophys Res Commun. 2018;500(2):411–7. 10.1016/j.bbrc.2018.04.089 . [DOI] [PubMed] [Google Scholar]
- 16.Bakowska JC, Jupille H, Fatheddin P, Puertollano R, Blackstone C. Troyer syndrome protein spartin is mono-ubiquitinated and functions in EGF receptor trafficking. Mol Biol Cell. 2007;18(5):1683–92. 10.1091/mbc.E06-09-0833 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang KK, Ramnarayanan K, Zhu F, Srivastava S, Xu C, Tan ALK, et al. Genomic and Epigenomic Profiling of High-Risk Intestinal Metaplasia Reveals Molecular Determinants of Progression to Gastric Cancer. Cancer Cell. 2018;33(1):137–50 e5. 10.1016/j.ccell.2017.11.018 . [DOI] [PubMed] [Google Scholar]
- 18.Lind GE, Danielsen SA, Ahlquist T, Merok MA, Andresen K, Skotheim RI, et al. Identification of an epigenetic biomarker panel with high sensitivity and specificity for colorectal cancer and adenomas. Mol Cancer. 2011;10:85 10.1186/1476-4598-10-85 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frazzi R, Zanetti E, Pistoni M, Tamagnini I, Valli R, Braglia L, et al. Methylation changes of SIRT1, KLF4, DAPK1 and SPG20 in B-lymphocytes derived from follicular and diffuse large B-cell lymphoma. Leuk Res. 2017;57:89–96. 10.1016/j.leukres.2017.02.012 . [DOI] [PubMed] [Google Scholar]
- 20.Zhang H, Song Y, Xia P, Cheng Y, Guo Q, Diao D, et al. Detection of aberrant hypermethylated spastic paraplegia-20 as a potential biomarker and prognostic factor in gastric cancer. Med Oncol. 2014;31(2):830 10.1007/s12032-013-0830-2 . [DOI] [PubMed] [Google Scholar]
- 21.Polk DB, Peek RM Jr. Helicobacter pylori: gastric cancer and beyond. Nat Rev Cancer. 2010;10(6):403–14. 10.1038/nrc2857 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bronte-Tinkew DM, Terebiznik M, Franco A, Ang M, Ahn D, Mimuro H, et al. Helicobacter pylori cytotoxin-associated gene A activates the signal transducer and activator of transcription 3 pathway in vitro and in vivo. Cancer Res. 2009;69(2):632–9. 10.1158/0008-5472.CAN-08-1191 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee IO, Kim JH, Choi YJ, Pillinger MH, Kim SY, Blaser MJ, et al. Helicobacter pylori CagA phosphorylation status determines the gp130-activated SHP2/ERK and JAK/STAT signal transduction pathways in gastric epithelial cells. J Biol Chem. 2010;285(21):16042–50. 10.1074/jbc.M110.111054 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giraud AS, Menheniott TR, Judd LM. Targeting STAT3 in gastric cancer. Expert Opin Ther Targets. 2012;16(9):889–901. 10.1517/14728222.2012.709238 . [DOI] [PubMed] [Google Scholar]
- 25.Ernst M, Thiem S, Nguyen PM, Eissmann M, Putoczki TL. Epithelial gp130/Stat3 functions: an intestinal signaling node in health and disease. Semin Immunol. 2014;26(1):29–37. 10.1016/j.smim.2013.12.006 . [DOI] [PubMed] [Google Scholar]
- 26.Fu HY, Asahi K, Hayashi Y, Eguchi H, Murata H, Tsujii M, et al. East Asian-type Helicobacter pylori cytotoxin-associated gene A protein has a more significant effect on growth of rat gastric mucosal cells than the Western type. J Gastroenterol Hepatol. 2007;22(3):355–62. 10.1111/j.1440-1746.2006.04531.x . [DOI] [PubMed] [Google Scholar]
- 27.Ohno T, Sugimoto M, Nagashima A, Ogiwara H, Vilaichone RK, Mahachai V, et al. Relationship between Helicobacter pylori hopQ genotype and clinical outcome in Asian and Western populations. J Gastroenterol Hepatol. 2009;24(3):462–8. 10.1111/j.1440-1746.2008.05762.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ando T, Wassenaar TM, Peek RM Jr., Aras RA, Tschumi AI, van Doorn LJ, et al. A Helicobacter pylori restriction endonuclease-replacing gene, hrgA, is associated with gastric cancer in Asian strains. Cancer Res. 2002;62(8):2385–9. . [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The microarray data has been deposited in the Gene Expression Omnibus database (accession number: GSE109541).