Abstract
The zona pellucida (ZP) is a highly organized extracellular coat that surrounds all mammalian eggs. The mouse egg ZP is composed of three glycoproteins, called mZP1–3, that are synthesized, secreted, and assembled into a ZP exclusively by growing oocytes. Here, we microinjected epitope-tagged (Myc and Flag) cDNAs for mZP2 and mZP3 into the germinal vesicle (nucleus) of growing oocytes isolated from juvenile mice. Specific antibodies and laser scanning confocal microscopy were used to follow nascent, recombinant ZP glycoproteins in both permeabilized and nonpermeabilized oocytes. When such cDNAs were injected, epitope-tagged mZP2 (Myc-mZP2) and mZP3 (Flag-mZP3) were synthesized, packaged into large intracellular vesicles, and secreted by the vast majority of oocytes. Secreted glycoproteins were incorporated into only the innermost layer of the thickening ZP, and the amount of nascent glycoprotein in this region increased with increasing time of oocyte culture. Consistent with prior observations, the putative transmembrane domain at the C terminus of mZP2 and mZP3 was missing from nascent glycoprotein incorporated into the ZP. When the consensus furin cleavage site near the C terminus of mZP3 was mutated, such that it should not be cleaved by furin, secretion and assembly of mZP3 was reduced. On the other hand, mZP3 incorporated into the ZP lacked the transmembrane domain downstream of the mutated furin cleavage site, suggesting that some other protease(s) excised the domain. These results strongly suggest that nascent mZP2 and mZP3 are incorporated into only the innermost layer of the ZP and that excision of the C-terminal region of the glycoproteins is required for assembly into the oocyte ZP.
INTRODUCTION
All eggs are surrounded by one or more extracellular coats (Dumont and Brummett, 1985). Mammalian eggs are surrounded by a zona pellucida (ZP), a coat that plays important roles during oogenesis, fertilization, and preimplantation development (Gwatkin, 1977; Dietl, 1989; Yanagimachi, 1994). In mice, the ZP consists of three glycoproteins, called mZP1–3 (Wassarman et al., 1985; Wassarman, 1988). mZP3 is both a primary sperm receptor and acrosome reaction inducer; mZP2 is a secondary sperm receptor (Wassarman, 1999; Wassarman et al., 2001). In addition, each of these glycoproteins is an essential structural component of the extracellular coat. The ZP of eggs from all mammals, including human beings, consists of glycoproteins very similar to mZP1–3. Even the vitelline envelope surrounding eggs from fish, birds, and amphibians consists of glycoproteins related to mZP1–3 (Wassarman et al., 2001).
Expression of ZP glycoproteins in mice is initiated during oocyte growth and ends 2–3 wk later during ovulation when oocytes undergo meiotic maturation, arrest at metaphase II, and become unfertilized eggs. All three glycoproteins are synthesized and secreted concomitantly by growing oocytes (Wassarman, 1988; Epifano et al., 1995). The ZP increases in thickness as the oocyte increases in diameter. Even oocytes located at an ectopic site (i.e., the adrenal gland) assemble a ZP during their growth phase (Zamboni and Upadhyay, 1983). Interestingly, in some animals, such as fish and birds, certain vitelline envelope glycoproteins are synthesized by the liver and transported in the bloodstream to the ovary, whereas others are synthesized by oocytes or follicle cells in the ovary (Wassarman et al., 2001).
The mouse ZP is composed of long, interconnected filaments that form a thick (∼6.5 μm), porous coat around the oocyte (Greve and Wassarman, 1985; Wassarman and Mortillo, 1991; Wassarman et al., 1996; Green, 1997). Results of biochemical and electron microscopic studies suggest that ZP filaments are polymers of mZP2 and mZP3 that are cross-linked by mZP1. Consistent with this model, gene deletion experiments in mice strongly suggest that both mZP2 and mZP3 must be present to assemble a ZP around a growing oocyte. Eggs from mice that are homozygous nulls for either mZP3 or mZP2 lack a ZP and the females are infertile (Liu et al., 1996; Rankin et al., 1996, 2001). Eggs from mice that are heterozygous nulls for mZP3 have a ZP, but it is only about one-half the thickness of the ZP of eggs from wild-type animals (Wassarman et al., 1997). The latter suggests that the amount of mZP3 is limiting under these conditions, permitting formation of fewer mZP2 and mZP3 oligomers than present under wild-type conditions. Despite their eggs having a thin ZP, heterozygous females are as fertile as wild-type animals.
Examination of growing oocytes from mZP3−/− mice revealed that, in the complete absence of mZP3 synthesis, mZP2 is synthesized and secreted by oocytes (Qi and Wassarman, 1999). This is consistent with results of experiments in which antisense oligonucleotides directed against either mZP2 or mZP3 messenger-RNA were microinjected into growing mouse oocytes (Tong et al., 1995) and with experiments in which ZP3 was stably transfected into embryonal carcinoma (EC), Chinese hamster ovary, L, and CV-1 cells in the absence of ZP2 (Kinloch et al., 1991; Beebe et al., 1992; Litscher and Wassarman, 1996). Results of such experiments suggest that ZP glycoproteins may be synthesized and secreted independently of one another. Recently, it was reported that mZP2 and mZP3 are processed at a consensus furin cleavage site (CFCS) by the serine protease furin before assembly into the ZP (Litscher et al., 1999; Williams and Wassarman, 2001). Furin is a member of the protein convertase family and is involved in modifying and activating a variety of substrates (Molloy et al., 1999). This posttranslational modification may be essential for secretion, as well as for assembly of ZP glycoproteins into filaments by growing oocytes.
Many questions about ZP assembly during mammalian oogenesis remain unanswered. This is due, in part, to the fact that it has been technically difficult to study de novo assembly of the ZP in growing mouse oocytes. Here, in an attempt to overcome the difficulties often encountered, we microinjected epitope-tagged cDNAs for mZP2 and mZP3 into the germinal vesicle (GV) of growing oocytes isolated from ovaries of juvenile mice. By using monoclonal antibodies directed against the specific epitopes, Myc and Flag, we were able to follow nascent, recombinant mZP2 and mZP3 inside and outside mouse oocytes by laser scanning confocal microscopy (LSCM). Results of these experiments strongly suggest that the ZP is assembled from nascent glycoproteins incorporated into the innermost region of the ZP and that excision of the C-terminal region of the glycoproteins is required for their incorporation into the growing oocyte's thickening ZP. The methods described should continue to be extremely useful in determining the regions of ZP glycoproteins essential for their assembly into the egg extracellular coat.
MATERIALS AND METHODS
Construction of Epitope-tagged mZP2 and mZP3 Expression Plasmids
Total RNA was extracted from 20 ovaries excised from 14- to 15-d-old juvenile female mice (C57BL/6), using a QuickPrep total RNA extraction kit (Amersham Pharmacia Biotech, Piscataway, NJ). Full-length cDNAs of mZP2 and mZP3 genes were cloned by reverse transcription-PCR. Two pairs of primers flanking the entire coding regions of mZP2 and mZP3 were used: cZP2–5′, 5′-ATTGCGGGATCCGCTTTGGTGGTACCTTCCAA-3′ and cZP2–3′, 5′-GGGCGGGAATTCCTGCAGTCTCTTTATTTG-3′; cZP3–5′, 5′-ATTTCGGGATCCGCTGTACTCCAGGCGGGA-3′ and cZP3–3′, 5′-GCGGGGGAATTCTGAGTTTCTTCTTTTATTGCGG-3′. BamHI and EcoRI sites were introduced into the primers at the 5′- and 3′-ends, respectively (underlined nucleotides), and first-strand cDNAs were amplified from the total RNA using oligo-d(T) and reverse transcribed by Superscript reverse transcriptase (SuperScript Preamplification System, Invitrogen, Carlsbad, CA). Double-stranded cDNAs were completed with 30 cycles of PCR (94°C, 30 s; 58°C, 30 s; 72°C, 90 s for each cycle). Resulting cDNAs were digested with BamHI/EcoRI and cloned into the BamHI/EcoRI site of pBlueScript KS(+/−) phagemid (Stratagene, La Jolla, CA).
Myc-mZP2 and Flag-mZP3 were constructed using three-step overlapping PCR, essentially as previously described (Ho et al., 1989). Two primers, Myc, 5′-GAGCAGAAGCTCATCTCGGAAGAGGACTTG-TCCGAGAATCCTGCCTTCCCAGGCACTCTC-3′ and Flag, 5′-GACTACAAGGACGATGACGACAAGCTAGTTTCTCGAAACCGC-AGGCACGTGACC-3′, containing the Myc or Flag sequence (underlined nucleotides), were used to insert the epitope tags at the desired positions. The 10-amino acid Myc tag (-Glu-Gln-Lys-Leu-Ile-Ser-Glu-Glu-Asp-Leu-) was placed after the mZP2 signal sequence, between amino acids Gln-39 and Ser-40 (Figure 1). The eight-amino acid Flag tag (-Asp-Tyr-Lys-Asp-Asp-Asp-Asp-Lys-) was placed upstream of the CFCS, between amino acids Lys-346 and Leu-347 (Figure 1). Myc-mZP2 and Flag-mZP3 were digested with XbaI/SalI and subcloned into XbaI/SalI-digested pSI mammalian expression vector (Stratagene). The pSI vector contains a viral SV40 enhancer/promoter sequence, a chimeric intron sequence upstream of the multiple cloning site region, and an SV40 late polyadenylation signal.
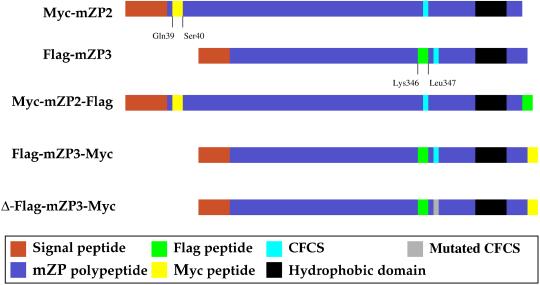
Figure 1.
Schematic representation of polypeptides encoded by cDNA constructs microinjected into growing mouse oocytes. Short peptide epitopes, Myc (yellow) and Flag (green), were inserted into mZP2 and mZP3 polypeptides. In singly tagged ZP glycoproteins, Myc (-Glu-Gln-Lys-Leu-Ile-Ser-Glu-Glu-Asp-Leu-) was inserted into mZP2 (Myc-mZP2), after the N-terminal signal sequence, between amino acids Gln-39 and Ser-40. Flag (-Asp-Tyr-Lys-Asp-Asp-Asp-Asp-Lys-) was inserted into mZP3 (Flag-mZP3) near its C terminus, before the CFCS, between amino acids Lys-346 and Leu-347. In doubly tagged ZP glycoproteins, epitope tags were inserted at the C terminus of Myc-mZP2 and Flag-mZP3, producing Myc-mZP2-Flag and Flag-mZP3-Myc, respectively. Also shown is Δ-Flag-mZP3-Myc, a construct in which the CFCS of mZP3 is mutated from -Arg-Asn-Arg-Arg- to -Arg-Asn-Gly-Glu- (gray). Several features of mZP2 and mZP3, such as the positions of the signal sequence (red), CFCS (royal blue), and transmembrane (hydrophobic) domain (black), are indicated.
Myc-mZP2-Flag was constructed using PCR with overhanging primers to add a coding sequence for the Flag epitope to the C terminus of Myc-mZP2 (underlined nucleotides). Primers used were 5′-GGGCGG-GAATTCTCACTTGTCGTCATCGTCCTTGTAGTCGTGATTGAAC-CTTATAGTTCTTTTCTTATA-3′ and 5′-ATTGCGGGATCCGCTTTGGTGGTACCTTCCAA-3′.
Flag-mZP3-Myc was constructed using PCR with overhanging primers to add a coding sequence for the Myc epitope to the C terminus of Flag-mZP3 (underlined nucleotides). Primers used were 5′-GGGCGGGAATTCTTACAAGTCCTCTTCCGAGATGAGCTTC-TGCTCTTGCGGAAGGGATACAAGGTAGGAA-3′ and 5′-ATTTCGGGATCCGCTGTACTCCAGGCGGGA-3′.
PCR mutagenesis was used on Flag-mZP3-Myc to convert the CFCS (-Arg-Asn-Arg-Arg-) to a noncleavable form (-Arg-Asn-Gly-Glu-; Volchkov et al., 1998), designated Δ-Flag-mZP3-Myc. Primers used were 5′-CTAGTTTCTCGAAACGGCGAGCACGTGACCGAT-GAAGCTG-3′ and 5′-CAGCTTCATCGGTCACGTGCTCGCCGTTTCGAGAAACTAG-3′.
The sequences of all five cDNA constructs were confirmed by DNA sequencing. Circular form plasmid DNA was purified using CsCl gradients. The concentration of purified DNA was measured by spectrophotometry (OD260/OD280 ∼ 1.9–2.0). After dialyzing against distilled water for 1 d at 4°C, followed by TE buffer (10 mM Tris, 0.1 mM EDTA, pH 8.0) for 1 d at 4°C, DNA was aliquoted and stored in 0.5 volume of sodium acetate (3 M, pH 5.4) and 2 volumes of ethanol at −20°C.
Collection, cDNA Microinjection, and Culture of Mouse Oocytes
Ovaries were excised from 13- to 16-d-old juvenile female mice (C57BL/6). Growing oocytes (50 ± 10 μm in diameter; Sorensen and Wassarman, 1976; Eppig and Telfer, 1993; Eppig et al., 1996) were released by puncturing ovaries with fine steel needles under a dissecting microscope in a culture dish containing prewarmed Earle's modified medium M199 (Invitrogen) supplemented with bovine serum albumin (BSA; 4 mg/ml) and sodium pyruvate (30 μg/ml; M199-M). Oocytes were collected with mouth-operated glass micropipettes and washed thoroughly through three drops of M199-M, equilibrated at 37°C in a humidified atmosphere of 5% CO2 in air, under mineral oil. Before microinjection, frozen cDNA samples were centrifuged for 10 min at 4°C. Pellets were washed with 70% ethanol and centrifuged for 5 min at 4°C. Pellets were air dried and dissolved in microinjection buffer (10 mM Tris, 0.25 mM EDTA, pH 7.4) at a final concentration of ∼1 mg/ml. All solutions in contact with DNA were filtered before use (0.22 μm; Millipore, Bedford, MA). cDNA constructs were back-loaded into microinjection pipettes and injected into the GV of growing mouse oocytes with a micromanipulator (model 5274; Eppendorf, Boulder, CO), essentially as previously described (DePamphilis et al., 1988). Oocytes were maintained in M2 medium (Speciality Media, Phillipsburg, NJ) during the microinjection process. Injected oocytes were incubated for 30 min in M199-M. Oocytes that survived this process were cultured for various lengths of time in 30 μl of M199-M, under mineral oil, at 37°C in a humidified atmosphere of 5% CO2 in air.
LSCM of Mouse Oocytes and Isolated ZP
Cultured mouse oocytes were fixed with 3.7% formaldehyde in phosphate-buffered saline (PBS)/ (poly)vinylpyrrolidone-40 (PVP-40), pH 7.2, for 15 min at room temperature (RT). During fixation, oocytes were either permeabilized in 0.1% Triton X-100 in 3.7% formaldehyde or left nonpermeabilized (3.7% formaldehyde without Triton X-100). To isolate ZP, cultured oocytes were incubated in 1% Nonidet P-40 (NP-40) in PBS/PVP-40, pH 7.2, and pipetted up and down (∼5 min at RT) through a glass pipette with a tip diameter smaller than that of oocytes. Isolated ZP were washed thoroughly through three drops of PBS/PVP-40 buffer (4 mg/ml PVP-40 in PBS, pH 7.2) and fixed with 3.7% formaldehyde. Fixed oocytes and ZP were then incubated in 50 μl of blocking buffer (2% BSA in PBS/PVP-40) at RT for 30 min. Oocytes were incubated at RT in blocking buffer containing anti-Flag (M2, Sigma, St. Louis, MO; 1:500 dilution; 1 h), anti-Myc (9E10, Sigma; 1:500 dilution, 1 h), or anti-Vamp (MAB 333, Chemicon International, Temecula, CA; 1:50 dilution, 2 h) monoclonal antibodies. After the oocytes were washed through three drops of blocking buffer, they were incubated in darkness with either fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse immunoglobulin (Ig) G (1:100 dilution; Molecular Probes, Eugene, OR), Texas Red-conjugated goat anti-mouse IgG (1:100 diluton; Molecular Probes), or FITC-conjugated goat anti-mouse IgM (1:50 dilution; CalTag Laboratories, Burlingame, CA). After staining, oocytes were placed in Slowfade Antifade equilibration buffer (Molecular Probes) at RT for 15 min. Oocytes were washed through three drops of PBS/PVP-40 and mounted onto a glass slide in Slowfade Antifade reagent in glycerol (Molecular Probes). Imaging was performed by LSCM using a TCS-SP (UV) microscope (Leica, Wetzlar, Germany). Fluorescence intensity was quantified at the same confocal microscope settings by calculating the mean pixel intensity for each stained ZP using IPLab for Macintosh (Scanalytics, Fairfax, VA).
Radioactive Labeling and Immunoprecipitation of ZP Glycoproteins
Myc-mZP2 was injected into the GV of 150–200 growing oocytes. Injected oocytes were cultured in M199-M for 4–6 h. This allowed time for conversion of supercoiled foreign DNA into transcribable forms and translation initiation. Oocytes were transferred into 25 μl of Met/Cys-depleted M199-M containing 4 mCi/ml 35S-labeled Met/Cys (ProMix 35S, cell-labeling mixture; Amersham Pharmacia Biotech). After 15 h, medium was collected and subjected to immunoprecipitation. Medium was diluted to 400 μl with IP buffer (150 mM NaCl, 50 mM Tris, pH 7.2, 0.1% Triton X-100, 1 mg/ml BSA, 10% glycerol). Samples were preabsorbed twice with 20 μl of protein G-agarose beads, rotating for 1 h at 4°C, and supernatants were incubated with a 1:200 dilution of anti-Myc, rotating for 2 h at 4°C. The immunocomplex was precipitated with 20 μl of protein G-agarose beads for 1 h, at 4°C. The medium was also immunoprecipitated with rabbit polyclonal anti-mZP2 (1:1000 dilution, 1 h, 4°C) and mixed with 20 μl of protein A-agarose beads for 1 h at 4°C. Immunoprecipitates were washed three times in IP buffer and once in PBS, dissolved in 20 μl of protein sample buffer (100 mM dithiothreitol, 50 mM Tris-HCl, pH 6.8, 10% glycerol, 2% SDS, 0.1% bromphenol blue), and subjected to electrophoresis, under reducing conditions, on 7.5% SDS-PAGE gels. After gels were incubated in Entensify solution (NEN, Boston, MA), as described by the manufacturer, and the dried gels were exposed to x-ray film with an intensifying screen, the fluorogram was developed.
RESULTS
Expression and Secretion of Epitope-tagged mZP2 and mZP3 by Microinjected Oocytes
To examine ZP glycoprotein expression and secretion, cDNAs encoding epitope-tagged mZP2 (Myc-mZP2) and mZP3 (Flag-mZP3) were microinjected into the GV of growing mouse oocytes (50 ± 10 μm in diameter) (Sorensen and Wassarman, 1976; Eppig and Telfer, 1993; Eppig et al., 1996). At this stage of growth, the oocyte GV remains intact during culture in vitro (Sorensen and Wassarman, 1976). cDNA constructs were placed under the control of an SV40 promoter, which has been shown to drive expression of reporter genes in isolated mouse oocytes cultured in vitro (Chalifour et al., 1986, 1987). A schematic representation of polypeptides encoded by cDNA constructs microinjected into growing mouse oocytes is presented in Figure 1.
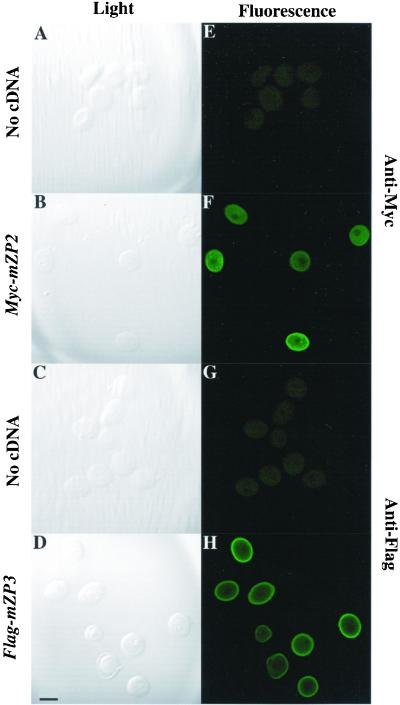
To examine expression of Myc-mZP2 and Flag-mZP3, LSCM was used with fixed and permeabilized injected and uninjected oocytes, as described in MATERIALS AND METHODS. Oocytes microinjected with either Myc-mZP2 or Flag-mZP3 were incubated in the presence of anti-Myc or anti-Flag monoclonal antibody, respectively, and subjected to LSCM. Compared with uninjected oocytes (Figure 2, A and E; C and G), injected oocytes showed an intense immunofluorescence signal (Figure 2, B and F; D and H) that was seen as early as ∼12 h postinjection. As shown in Table 1, a high percentage of injected oocytes survived the microinjection procedure and subsequent culturing in vitro (>90%), as determined by the presence of an intact GV and the absence of any cytoplasmic granulation or other visible abnormalities. Among surviving oocytes, ∼75% showed specific immunofluorescence signals during a 1- to 3-d culture period. These results demonstrate that microinjection of epitope-tagged mZP2 and mZP3 cDNAs into the GV of growing mouse oocytes is an effective method for detecting nascent, recombinant ZP glycoproteins.
Figure 2.
Immunofluorescence staining of Myc-mZP2 and Flag-mZP3 in growing mouse oocytes. Growing oocytes were isolated from juvenile mice, as described in MATERIALS AND METHODS. After microinjection of Myc-mZP2 and Flag-mZP3 cDNA (∼1 mg/ml) into the oocyte GV, oocytes were cultured for ∼20 h, as described in MATERIALS AND METHODS. Epitope-specific monoclonal antibodies, 9E10 (anti-Myc) and M2 (anti-Flag), were used to immunolabel oocytes at the end of the culture period. Goat anti-mouse IgG coupled to FITC was used as a secondary antibody. Light images of immunolabeled oocytes are presented in A–D and fluorescent images are presented in E–H. Whereas uninjected oocytes showed no staining (A and E [anti-Myc] and C and G [anti-Flag]), injected oocytes exhibited intense fluorescence (B and F [anti-Myc] and D and H [anti-Flag]). The most intense signal was seen at the surface of the oocytes over the plasma membrane/ZP region. In each case, 100–200 oocytes were examined by LSCM. Bar (in D), 50 μm.
Table 1.
Survival of and expression in growing mouse oocytes after cDNA microinjection
| cDNA injected | No. oocytes injected | Time of cuture (h) | % survival | % expressinga |
|---|---|---|---|---|
| 357 | 21–23 | 98 | 51 | |
| Myc-mZP2 | 295 | 43–48 | 96 | 85 |
| 246 | 72 | 92 | 73 | |
| 200 | 21–24 | 99 | 82 | |
| Flag-mZP3 | 292 | 44–50 | 95 | 96 |
| 210 | 72 | 95 | ndb |
Percentage of oocytes expressing recombinant ZP glycoprotein.
nd, not determined.
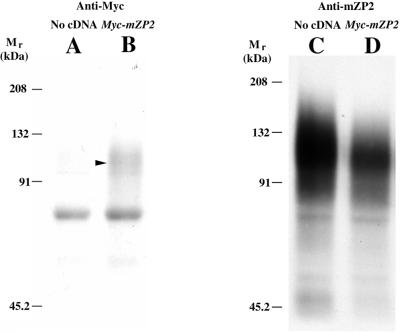
Fluorography of radiolabeled immunoprecipitates subjected to SDS-PAGE was used to detect secretion of epitope-tagged ZP glycoproteins into culture medium after microinjection of cDNAs. After microinjection, oocytes were incubated in Met/Cys-depleted medium supplemented with [35S]Met/Cys for 15 h. Medium from oocytes injected with Myc-mZP2 and from uninjected oocytes were immunoprecipitated with anti-Myc. As shown in Figure 3, a signal was seen in medium from oocytes microinjected with Myc-mZP2 (lane B, arrowhead; ∼120 kDa) but not in medium from uninjected oocytes (lane A). When supernatants from both samples were immunoprecipitated with anti-mZP2, a robust signal was seen with both samples (Figure 3, C and D; ∼120 kDa). Similar results were obtained with medium from oocytes microinjected with Flag-mZP3 and medium from uninjected oocytes immunoprecipitated with anti-Flag. Based on densitometry measurements of fluorograms, radiolabeled recombinant ZP glycoproteins represented ∼5% of total ZP glycoprotein synthesized during this period. This demonstrates that, although both injected and uninjected oocytes synthesize and secrete native mZP2 and mZP3, those oocytes injected with Myc-mZP2 or Flag-mZP3 also synthesize and secrete epitope-tagged ZP glycoproteins.
Figure 3.
Gel analysis of epitope-tagged ZP glycoproteins synthesized and secreted by growing mouse oocytes. Two hundred growing oocytes were isolated from 13-d-old mice, microinjected with Myc-mZP2, and cultured in M199-M for ∼5 h. Oocytes were then transferred to Met/Cys-depleted M199-M, supplemented with 4 mCi/ml [35S]Met/Cys. The culture medium was collected after ∼15 h and immunoprecipitated with monoclonal antibodies directed against Myc (9E10). Immunoprecipitates were subjected to 7.5% SDS-PAGE, as described in MATERIALS AND METHODS. Media from uninjected and injected oocytes probed with anti-Myc are shown in A and B, respectively. An arrowhead indicates the position of mZP2. Media were immunoprecipitated once again with an anti-mZP2 polyclonal antibody. As shown in C and D, both injected and noninjected oocytes secreted endogenous mZP2 glycoproteins. A band at ∼70 kDa was observed as a contaminant in all lanes. The positions of molecular mass standards are shown.
Cellular Localization of Epitope-tagged mZP2 and mZP3 in Microinjected Oocytes
In mice, the ZP is laid down by growing oocytes during a 2- to 3-wk period after birth, and the ZP increases in thickness as the oocyte increases in diameter (Wassarman et al., 1985). The epitope-tagged ZP glycoprotein expression system described above was used together with LSCM to localize recombinant ZP glycoproteins in growing oocytes.
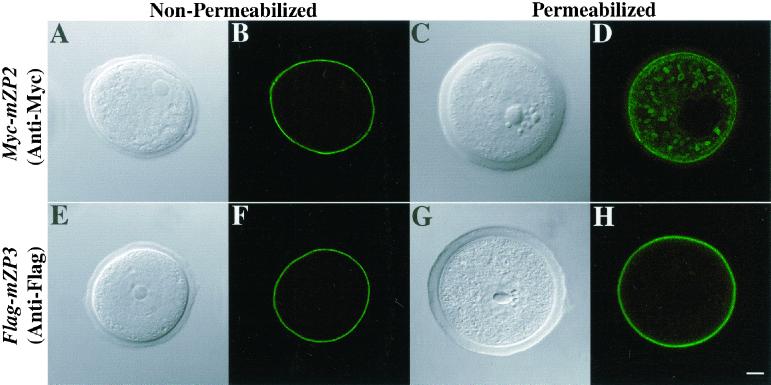
As shown above, epitope-tagged mZP2 and mZP3 glycoproteins were expressed and secreted by oocytes after microinjection with the corresponding cDNAs. To localize Myc-mZP2 and Flag-mZP3 in microinjected oocytes, oocytes were immunofluorescently labeled with anti-Myc and anti-Flag, respectively, and examined by LSCM at ∼21 h postinjection. With fixed, nonpermeabilized oocytes, Myc-mZP2 and Flag-mZP3 were seen concentrated primarily around the plasma membrane/ZP region of the oocytes (Figure 4, A and B; E and F). With fixed, permeabilized oocytes, Myc-mZP2 and Flag-mZP3 also were detected intracellularly in large (2.3 ± 0.32 μm diameter) vesicles (Figure 4, C and D; G and H). Oocytes microinjected with Myc-mZP2 and probed with anti-Myc (Figure 4, C and D) usually revealed a more pronounced vesicle staining pattern than Flag-mZP3 microinjected oocytes probed with anti-Flag (Figure 4, G and H).
Figure 4.
Localization of Myc-mZP2 and Flag-mZP3 in growing mouse oocytes. Oocytes from 14-d-old mice were microinjected with either Myc-mZP2 or Flag-mZP3. After ∼21 h of culture, the oocytes were stained with anti-Myc (A–D) or anti-Flag (E–H), followed by FITC-conjugated secondary antibody. Light (A, C, E, and G) and fluorescent (B, D, F, and H) images of oocytes are presented. In fixed, permeabilized oocytes, both epitope-tagged ZP proteins were detected, primarily over the plasma membrane/ZP region (D and H). In addition, intracellular vesicles were readily observed in Myc-mZP2-injected oocytes (D). Much less intracellular staining was detected in Flag-mZP3-injected oocytes (H), although in some cases, vesicles were detected. The plasma membrane/ZP staining appears to be on the outside of permeabilized growing oocytes, because fixed, nonpermeabilized samples (A, B, E, and F) exhibited very similar staining patterns. In each case, 100–200 oocytes were examined by LSCM. Bar (H), 10 μm.
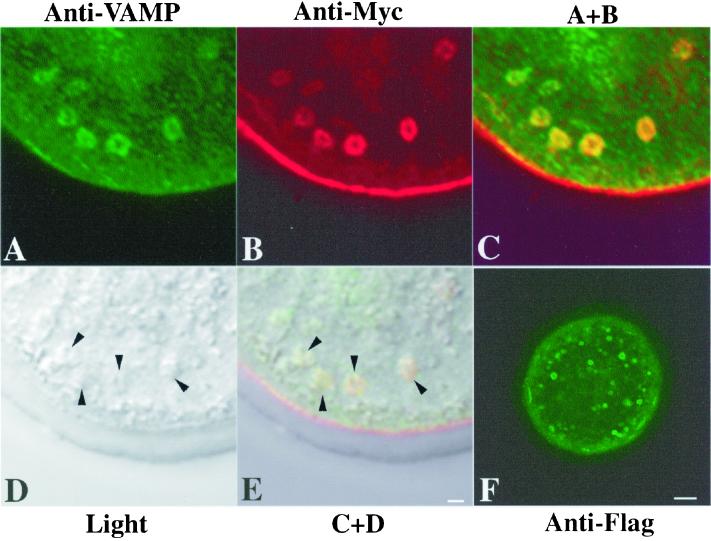
Use of a monoclonal antibody directed against VAMP (vescicle associated membrane protein; Südhof, 1995; Lin and Scheller, 2000) confirmed that intracellular mZP2 localized to membrane-bound secretory vesicles (Figure 5). It was noted that, in general, the vast majority of secretory vesicles were doughnut shaped, with a large nonfluorescent cavity in the middle (Figure 5, A–C). The colocalization of Myc-mZP2 and VAMP in these vesicles (Figure 5, C and E) strongly suggests that nascent, recombinant ZP glycoproteins are associated primarily with secretory vesicle membrane. When injected oocytes were immunolabeled without permeabilization, the most intense signal was found at the plasma membrane/ZP region; no significant intracellular signal was detected.
Figure 5.
Colocalization of Myc-mZP2 and VAMP in growing mouse oocytes. Oocytes from 14-d-old mice were microinjected with Myc-mZP2. After ∼21 h of culture, the oocytes were stained with anti-VAMP (IgM; A) and anti-Myc (IgG; B), followed by FITC-conjugated rabbit anti-mouse IgM and Texas Red-conjugated goat anti-mouse IgG secondary antibody. Light (D) and fluorescent (A–C) images of oocytes are presented. C, An overlap of A and B. Note the presence of VAMP (green) and Myc-mZP2 (red) in the large, doughnut-shaped vesicles present in the cortical region of the oocyte; overlap of VAMP and Myc-mZP2 is yellow (green plus red). E, An overlap of C and D. Arrowheads in D and E, the positions of large vesicles in the cortical region of the oocyte. In F, an oocyte microinjected with mZP2 tagged with Flag at its C terminus (Myc-mZP2-Flag) and probed with anti-Flag is shown. Note that the vesicles exhibit fluorescence along their membranes. In each case, 100–200 oocytes were examined by LSCM. Bars (E and F), 2 μm and 10 μm, respectively.
Epitope-tagged mZP2 and mZP3 Assemble into the ZP of Microinjected Oocytes
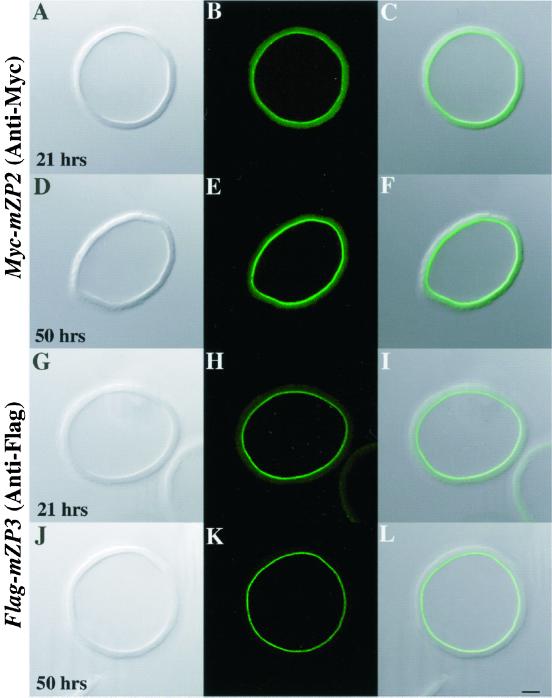
Having found that Myc-mZP2 and Flag-mZP3 localized to the plasma membrane/ZP region of nonpermeabilized oocytes, we examined whether epitope-tagged ZP glycoproteins were incorporated into the thickening ZP. Oocytes were microinjected with Myc-mZP2 and cultured for ∼21 h. ZP were isolated in the presence of 1% NP-40 (in PBS/PVP-40), followed by thorough washing in PBS/PVP-40 (Bleil and Wassarman, 1986; Litscher and Wassarman, 1999). This procedure removes adventitiously associated material from the ZP. Isolated ZP were fixed and immunolabeled with anti-Myc and examined by LSCM. Immunofluorescence was observed on the inner surface of isolated ZP (Figure 6, A–C), with no signal detected elsewhere in the ZP. Similar results were obtained when ZP were isolated from Flag-mZP3-microinjected oocytes and labeled with anti-Flag (Figure 6, G–I). These results suggest that newly synthesized Myc-mZP2 and Flag-mZP3 assemble into the thickening ZP only in the innermost region of the ZP.
Figure 6.
Assembly of Myc-mZP2 and Flag-mZP3 into ZP of growing mouse oocytes. Either Myc-mZP2 (A–F) or Flag-mZP3 (G–L) cDNA was microinjected into growing oocytes isolated from 13-d-old mice. After the oocytes were cultured for ∼21 (A–C and G–I) and ∼50 h (D–F and J–L), ZP were isolated in the presence of 1% NP-40 and immunolabeled with either anti-Myc or anti-Flag and FITC-conjugated secondary antibody. Light images are shown in A, D, G, and J and fluorescent images are shown in B, E, H, and K. Composites of light and fluorescent images are shown in C, F, I, and L. In all cases, epitope-tagged ZP glycoproteins were found only at the innermost layer of the ZP (A–F, Myc-mZP2; G–L, Flag-mZP3). The intensity of the immunofluorescence signal increased with increasing culture times (compare B and E, Myc-mZP2; compare H and K, Flag-mZP3; see text for details). More than 200 isolated ZP were stained in this manner. Bar (L), 10 μm.
To further examine incorporation of ZP glycoproteins, oocytes microinjected with either Myc-mZP2 or Flag-mZP3 were cultured for ∼50 h, and ZP were isolated from these oocytes and probed with the appropriate antibody. As seen with oocytes cultured for ∼21 h, the immunofluorescence signal was detected only along the inner surface of the ZP (Figure 6, D–F and J–L); however, longer culture times resulted in an increase in the intensity of the fluorescent signal; luminosity measurements confirmed the observed increase. Both Myc-mZP2 and Flag-mZP3 fluorescence intensities increased ∼1.5-fold (55% for Myc-mZP2 and 73% for Flag-mZP3) when the culture time was doubled. For example, the fluorescence intensity of Flag-mZP3 increased from 43 ± 4 U at ∼21 h to 74 ± 15 U at ∼50 h (10 isolated ZP examined for each sample; see MATERIALS AND METHODS). Collectively, these results strongly suggest that nascent, recombinant mZP2 and mZP3 are incorporated into the thickening ZP exclusively at its innermost surface.
Epitope-tagged mZP2 and mZP3 Are Proteolytically Processed before Incorporation into ZP of Microinjected Oocytes
Previously, it was suggested that the C termini of mZP2 and mZP3 are missing after incorporation of the glycoproteins into the ZP (Litscher et al., 1999). In addition, experiments with transfected cell lines suggested that cleavage of the mouse ZP glycoproteins at their CFCS is required for secretion (Williams and Wassarman, 2001). As shown in Figure 1, this site is located near the C termini of mZP2 and mZP3 and upstream of a predicted transmembrane region. We used the incorporation assay described above to determine whether cleavage of the C termini of mZP2 and mZP3 took place in microinjected oocytes.
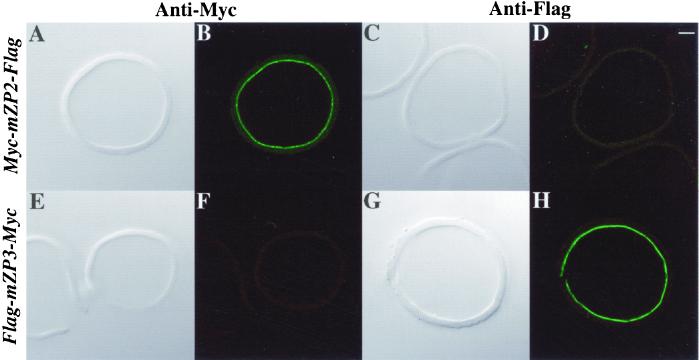
As shown in Figure 7, the regions upstream of the CFCS of both Myc-mZP2 and Flag-mZP3 are incorporated into isolated ZP after microinjection of the corresponding cDNAs. To probe the region downstream of the CFCS independently from the region upstream, a Flag-peptide sequence was placed at the extreme C-terminal end of Myc-mZP2 (Myc-mZP2-Flag) and a Myc-peptide sequence was placed at the extreme C-terminal end of Flag-mZP3 (Flag-mZP3-Myc; Figure 1). cDNA constructs encoding these doubly tagged ZP glycoproteins were individually microinjected into oocytes and, after culturing oocytes for ∼50 h, ZP were isolated in the presence of 1% NP-40, as described above. When ZP from oocytes microinjected with Myc-mZP2-Flag were probed with anti-Myc and examined by LSCM, a signal was present along the inner surface of the isolated ZP (Figure 7, A and B) but was absent when probed with anti-Flag (Figure 7, C and D). Similarly, when ZP from oocytes microinjected with Flag-mZP3-Myc were probed with anti-Flag, a signal was present along the inner surface of the isolated ZP (Figure 7, G and H) but was absent when probed with anti-Myc (Figure 7, E and F). As expected, antibody directed against the C-terminal epitope tag showed immunofluorescence only within the oocyte and not with isolated ZP. Consistent with previous findings (Litscher et al., 1999; Williams and Wassarman, 2001), these results suggest that in the ZP mZP2 and mZP3 lack their C-terminal transmembrane domains.
Figure 7.
Proteolytic processing of epitope-tagged ZP glycoproteins in growing mouse oocytes. Doubly tagged (Myc and Flag) cDNAs for mZP2 and mZP3 were microinjected into growing oocytes isolated from 14-d-old mice. After culture for ∼50 h, ZP were isolated in the presence of 1% NP-40 and stained with either anti-Myc or anti-Flag, followed by FITC-conjugated secondary antibody. Light images are shown in A, C, E, and G and fluorescent images are shown in B, D, F, and H. ZP from Myc-mZP2-Flag-injected oocytes (A–D) exhibited fluorescence only with anti-Myc (B), not with anti-Flag (D). ZP from Flag-mZP3-Myc-injected oocytes (E–H) exhibited fluorescence only with anti-Flag (H), not with anti-Myc (F). Bar (D), 10 μm.
Secretion and Assembly of Epitope-tagged mZP3, Mutated at its CFCS, by Microinjected Oocytes
A mutated form of Flag-mZP3-Myc, designated Δ-Flag-mZP3-Myc, was used to assess the potential role for cleavage at the mZP3 CFCS in secretion and assembly of nascent mZP3. In Δ-Flag-mZP3-Myc, the mZP3 CFCS was mutated from -Arg-Asn-Arg-Arg- to -Arg-Asn-Gly-Glu-. Previous results obtained with transfected cells suggest that this mutation should abolish the cleavage site for furin-like enzymes (Williams and Wassarman, 2001).
Oocytes were immunofluorescently labeled with either anti-Myc or anti-Flag after microinjection with either Flag-mZP3-Myc or Δ-Flag-mZP3-Myc and culture for ∼20 h. When probed with anti-Flag and subjected to LSCM, oocytes microinjected with either construct displayed strong signals along the plasma membrane/ZP region of the oocytes (Figure 8, A and B). On the other hand, very little signal was detected along this region when oocytes were probed with anti-Myc (Figure 8, C and D). This suggests that, as with wild-type glycoprotein, epitope-tagged mZP3 is proteolytically processed at its C terminus. It was noted that there was increased vesicular staining in oocytes microinjected with the mutant form of mZP3 and probed with either antibody (Figure 8, B and D), suggesting that mutation of the CFCS may slow down trafficking of mZP3 through the secretory pathway.
Figure 8.
Secretion of epitope-tagged mZP3, mutated at its CFCS, by growing mouse oocytes. Flag-mZP3-Myc and Δ-Flag-mZP3-Myc (mutated at its CFCS) were microinjected into growing oocytes isolated from 14- to 15-d-old mice. Oocytes were cultured for ∼20 h and then stained with either anti-Flag or anti-Myc, followed by FITC-conjugated secondary antibody. Intense fluorescence was evident over the plasma membrane/ZP region of oocytes injected with either cDNA construct after staining with anti-Flag (A and B) but not with anti-Myc (C and D). It was noted that, in almost all instances, there was a larger accumulation of fluorescently labeled vesicles in oocytes microinjected with Δ-Flag-mZP3-Myc (B and D) than in oocytes microinjected with Flag-mZP3-Myc (A and C). In each case, 100–200 oocytes were examined by LSCM. Bar (B), 10 μm.
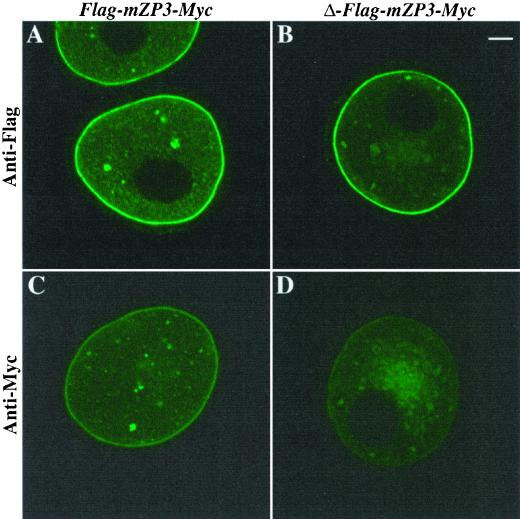
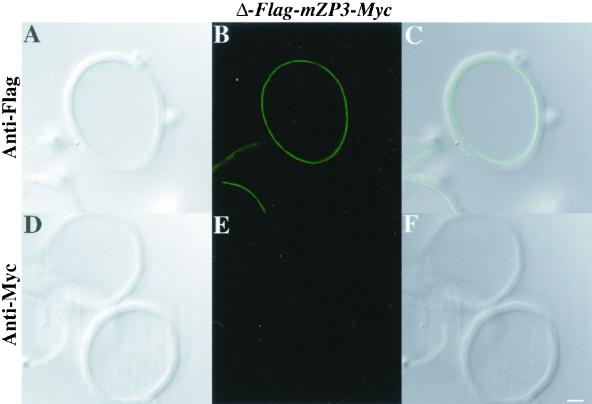
To determine whether the mutated form of mZP3 was incorporated into ZP, isolated ZP from oocytes microinjected with Δ-Flag-mZP3-Myc were probed with anti-Flag and subjected to LSCM. As with Flag-mZP3-Myc, a fluorescent signal was observed along the inner surface of the isolated ZP (Figure 9, A–C). When probed with anti-Myc, no labeling was observed (Figure 9, D–F). Taken together with results described above, this suggests that mutation of its CFCS causes an enhanced retention of mZP3 within the oocyte. However, the mutated mZP3 that escapes retention and is incorporated into the ZP undergoes proteolytic processing at its C terminus, possibly by protease(s) other than furin.
Figure 9.
Assembly of epitope-tagged mZP3, mutated at its consensus furin cleavage site, into the ZP of growing mouse oocytes. Δ-Flag-mZP3-Myc mutated at its CFCS was microinjected into growing oocytes isolated from 14-d-old mice. Oocytes were cultured for ∼50 h, and ZP were isolated in the presence of 1% NP-40 and stained with either anti-Flag or anti-Myc, followed by FITC-conjugated secondary antibody. Light (A and D) and fluorescent (B and E) images are shown for ZP treated with anti-Flag (A–C) or anti-Myc (D–F). C and F represent overlaps of A and B and D and E, respectively. Note that in B (anti-Flag), but not in E (anti-Myc), the innermost layer of the ZP is fluorescently labeled. Bar (F), 10 μm.
DISCUSSION
The mouse egg ZP is an extracellular matrix that plays vital roles during oogenesis, fertilization, and early embryogenesis (Gwatkin, 1977; Dietl, 1989; Yanagimachi, 1994). For example, during fertilization the egg ZP serves as a barrier preventing interactions between gametes from different species. The three constituent glycoproteins of the mouse ZP, mZP1-mZP3, are synthesized concomitantly and exclusively by growing oocytes during a 2- to 3-wk growth phase (Wassarman, 1988; Epifano et al., 1995). Ultrastructural studies of growing mouse oocytes revealed that the oocyte Golgi changes from flattened stacks of lamellae, with few, if any, vacuoles or granules, in the early stages of oocyte growth, to extensive arrays of swollen stacked lamellae with many large vacuoles in the late stages of growth (Wassarman and Josefowicz, 1978). These changes, as well as those of the endoplasmic reticulum, strongly suggest that growing mouse oocytes become actively engaged in trafficking of various glycoproteins, including those of the ZP.
The mouse ZP increases in thickness (to ∼6.5 μm; ∼3.5 ng of glycoprotein) as the oocyte increases in diameter (to ∼80 μm) during the 2- to 3-wk growth phase. The thickness of the ZP of eggs from different mammals varies considerably, from ∼2 μm for opossums to ∼25 μm for cows (Dunbar and O'Rand, 1991). The mouse egg ZP is a porous matrix, permeable to relatively large macromolecules, that consists of long filaments composed of mZP2 and mZP3, with a structural repeat present every 14–15 nm along the filaments (Greve and Wassarman, 1985; Wassarman et al., 1985; Wassarman and Mortillo, 1991). The filaments, in turn, are cross-linked by mZP1, a dimer of identical polypeptides held together by intermolecular disulfide bonds. Thus, the ZP is a highly organized three-dimensional matrix exhibiting a structural periodicity.
The cDNA expression system described here provided a sensitive means of assessing incorporation of nascent ZP glycoproteins into the thickening oocyte ZP. When epitope-tagged mZP2 and mZP3 cDNAs were placed under the control of an SV40 promoter and microinjected into the GV of growing mouse oocytes, no fluorescent signals were detectable ∼4 h after injection. This is consistent with the previous finding that expression of foreign genes usually is initiated ∼4–5 h after microinjection of cDNAs into mouse oocytes (Chalifour et al., 1986, 1987). On the other hand, in all experiments reported here, strong fluorescent signals were detected as early as ∼12 h after injection of oocytes. During this relatively short period, some of the expressed epitope-tagged ZP glycoprotein had already assembled into the innermost layer of the ZP. With increasing time of culture, microinjected oocytes assembled even more recombinant ZP glycoprotein into this region of the ZP (Figure 6), suggesting that the ZP thickens solely from the inside during oocyte growth. Results of previous experiments suggest that only about one-half of newly synthesized ZP glycoprotein is assembled into the ZP; the remainder exits through the ZP and follicle cell layer (Qi and Wassarman, 1999).
Finding that nascent mZP2 and mZP3 are deposited solely into the innermost layer of the ZP has several implications for its assembly. For example, as in the case of some other extracellular matrices (Henry and Campbell, 1998; Schwarzbauer and Sechler, 1999), thickening of the ZP from the inside could reflect a requirement for “cell-mediated” assembly. Some cellular components, possibly associated with oocyte plasma membrane, could be required for assembly of mZP2 and mZP3 into ZP filaments. If this is the situation, in the absence of cellular factors, it should not be possible to assemble purified ZP glycoproteins into filaments in vitro. In this same vein, it is possible that nascent ZP glycoproteins can only be incorporated at the growing ends of ZP filaments and that these ends are only present in the innermost layer of the ZP. Indeed, results of polarized light microscopy of hamster ZP suggest that filaments in its outer layer are oriented tangentially (parallel) to the oolemma, whereas those in its innermost layer (inner ∼6 μm of a ∼13-μm-thick ZP) are oriented radially (perpendicular) to the oolemma (Keefe et al., 1997). This could reflect a situation in which ZP filaments with growing ends are oriented toward the oocyte. It should be noted that, for many years, a multilayered ZP has been proposed for eggs from a variety of species, including rabbits, pigs, sheep, hamsters, and humans (Dickmann, 1965). This also would be consistent with the observation that the inner surface of the ZP is more closely packed than the outer surface (Dietl, 1989). Finally, as in the control of actin assembly and disassembly (Weber, 1999), it is possible that assembly of ZP glycoproteins is concentration dependent and that the concentration is highest near the site of secretion.
mZP1–3 are assembled into cross-linked filaments of the ZP using noncovalent bonds. The ZP is completely soluble in the presence of agents that do not disrupt covalent bonds, e.g., low pH, low ionic strength, or mild heat (Wassarman, 1988). It is of interest that all three glycoproteins contain a so-called “ZP domain” (Bork and Sander, 1992) that is present in a wide variety of proteins from both vertebrates and invertebrates (Wassarman et al., 2001). The domain is thought to participate directly in protein-protein interactions and is likely to be essential for ZP assembly. ZP domains share a conserved sequence of ∼260 amino acids, including 8 Cys residues, that is found in all egg coat glycoproteins from mammals, birds, amphibians, and fish. Interestingly, many other proteins such as transforming growth factor, type β receptor III, Tamm-Horsfall protein/uromodulin, ebnerin, tectorins α and β, and cuticlin also contain a ZP domain near their C termini (Wassarman et al., 2001) and, in most cases, are known to form oligomers. In this context, it has been reported that mouse ZP glycoproteins can be incorporated into the vitelline envelope of amphibian eggs injected with ZP glycoprotein messenger-RNAs (Doren et al., 1999). Presumably, the ZP domain of vitelline envelope glycoproteins can interact with that of ZP glycoproteins despite the evolutionary distance (∼350 million years) separating amphibians and mice.
Experiments presented here have extended to growing mouse oocytes studies of EC cells transfected with mouse ZP genes (Williams and Wassarman, 2001). Such studies suggested that cleavage at the CFCS is necessary for secretion of nascent ZP glycoproteins. In particular, the experiments presented here have permitted an analysis of posttranslational modification and trafficking of nascent ZP glycoproteins by mouse oocytes. In addition to a ZP domain, ZP glycoproteins possess a CFCS (-Arg-X-Lys/Arg-Lys/Arg-) and a potential transmembrane domain in their C-terminal region. For example, in mZP3 the CFCS (-Arg-Asn-Arg-Arg-) is located 34 amino acids upstream from a predicted transmembrane domain (23 amino acids) that is located 15 amino acids upstream of the C-terminal amino acid. Furin, a member of the protein convertase family, is associated with trans-Golgi and plasma membrane of virtually all cell types and participates in posttranslational modification and activation of many different substrates, including growth factors, receptors, and extracellular matrix proteins (Molloy et al., 1999).
It has been reported that mZP2 and mZP3 incorporated into the oocyte ZP have already undergone cleavage at their CFCS (Litscher et al., 1999), and studies with transfected EC cells suggest that proteolytic cleavage of mZP3 at its CFCS is, in fact, required for secretion of nascent mZP3 by cells (Williams and Wassarman, 2001). Failure of ZP glycoproteins to undergo proteolytic cleavage at the CFCS results in their accumulation in the endoplasmic reticulum of transfected cells. As seen here, peptide epitopes inserted upstream of the mZP2 and mZP3 CFCS are present in the oocyte ZP (e.g., Figure 7, B and H), whereas epitopes placed downstream of the transmembrane domain are absent (e.g., Figure 7, D and F). This is the case for mZP3 present in the ZP even when its CFCS is mutated to a sequence that should not be cleaved efficiently by furin (Figure 9). Mutation of the mZP3 CFCS may only partially abolish furin activity, resulting in the accumulation of nascent mZP3 in oocyte secretory vesicles. Alternatively, another protease(s) may cleave the C-terminal tail at the CFCS or some other site(s) (Plaimauer et al., 2001).
Epitopes placed downstream of the transmembrane domain of ZP glycoproteins are also present in oocyte secretory vesicles. ZP glycoproteins are associated with the membrane of secretory vesicles, not with the lumen, and give the immunostained vesicles a doughnut-shaped appearance (Figure 5F). These observations suggest that loss of the C-terminal tail of ZP glycoproteins is a late event in the secretory process. In fact, it was reported previously that a C-terminal epitope, downstream of the mZP3 CFCS and upstream of the transmembrane region, is present on mZP3 associated with the oocyte plasma membrane (Litscher et al., 1999). Therefore, it is likely that cleavage at the CFCS takes place once nascent ZP glycoprotein is located on the oocyte plasma membrane. However, it is possible that cleavage at the CFCS takes place earlier (e.g., in secretory vesicles) but that the region remains associated with the remainder of the polypeptide or with a membrane component. In any case, it remains to be determined whether this step is involved in the regulation of assembly of ZP glycoproteins into oligomers.
In conclusion, the evidence presented suggests that nascent ZP glycoproteins assemble solely into the innermost layer of the thickening ZP of growing oocytes. It also suggests that proteolytic processing of ZP glycoproteins in growing oocytes is required for secretion and assembly of the glycoproteins into the ZP. Because polypeptide downstream of the mZP3 CFCS is present on the membrane of oocyte secretory vesicles (e.g., Figure 5F) and on oocyte plasma membrane (Litscher et al., 1999), but not in the ZP (Figures 6 and 7), it is likely that this region of polypeptide is not removed until very late in the secretory process. It will be of great interest to determine whether the presence of the C-terminal tail of ZP glycoproteins prevents their oligomerization into ZP filaments.
ACKNOWLEDGMENTS
We thank our colleagues, Luca Jovine and Eveline Litscher, for lively discussions and constructive criticism throughout the course of this research. We thank Scott Henderson for discussion and expert instruction in confocal microscopy, Mitch Goldfarb for a generous gift of VAMP antibody, and Wei He for helpful instruction in microinjection technique. This research was supported in part by the National Institutes of Health (HD35105).
Abbreviations used:
- BSA
bovine serum albumin
- CFCS
consensus furin cleavage site
- EC
embryonal carcinoma
- FITC
fluorescein isothiocyanate
- GV
germinal vesicle
- Ig
immunoglobulin
- LSCM
laser scanning confocal microscopy
- NP-40
Nonidet P-40
- PBS
phosphate-buffered saline
- PVP-40
(poly)vinylpyrrolidone-40
- RT
room temperature
- VAMP
vesicle associated membrane protein
- ZP
zona(e) pellucida(e)
Footnotes
This paper is dedicated to the memory of Alan P. Wolffe, a friend and collaborator.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.01–09-0440. Article and publication date are at www.molbiolcell.org/cgi/10.1091/mbc.01–09-0440.
REFERENCES
- Beebe SL, Leyton L, Burks D, Ishikawa M, Fuerst T, Dean J, Saling P. Recombinant mouse ZP3 inhibits sperm binding and induces the acrosome reaction. Dev Biol. 1992;151:48–54. doi: 10.1016/0012-1606(92)90212-y. [DOI] [PubMed] [Google Scholar]
- Bleil JD, Wassarman PM. Autoradiographic visualization of the mouse egg's sperm receptor bound to sperm. J Cell Biol. 1986;102:1363–1371. doi: 10.1083/jcb.102.4.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bork P, Sander C. A large domain common to sperm receptors (ZP2 and ZP3) and TGF-β type III receptor. FEBS Lett. 1992;300:237–240. doi: 10.1016/0014-5793(92)80853-9. [DOI] [PubMed] [Google Scholar]
- Chalifour LE, Wirak DO, Hansen U, Wassarman PM, DePamphilis ML. Cis- and trans-acting sequences required for the expression of simian virus 40 genes in mouse oocytes. Genes Dev. 1987;1:1096–1106. doi: 10.1101/gad.1.10.1096. [DOI] [PubMed] [Google Scholar]
- Chalifour LE, Wirak DO, Wassarman PM, DePamphilis ML. Expression of simian virus 40 early and late genes in mouse oocytes and embryos. J Virol. 1986;59:619–627. doi: 10.1128/jvi.59.3.619-627.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePamphilis ML, Herman SA, Martinez-Salas E, Chalifour LE, Wirak DO, Cupo DY, Miranda M. Microinjecting DNA into mouse ova to study DNA replication and gene expression and to produce transgenic animals. BioTechniques. 1988;6:662–680. [PubMed] [Google Scholar]
- Dickmann Z. Sperm penetration into and through the zona pellucida of the mammalian egg. In: Wolstenholme GEW, O'Connor M, editors. Ciba Foundation Symposium, Preimplantation Stages of Pregnancy. Boston, MA: Little, Brown, and Co.; 1965. pp. 169–182. [Google Scholar]
- Dietl J, editor. The Mammalian Egg Coat: Structure and Function. Berlin: Springer-Verlag KG; 1989. [Google Scholar]
- Doren S, Landsberger N, Dwyer N, Gold L, Blanchette-Mackie J, Dean J. Incorporation of mouse zona pellucida proteins into the envelope of Xenopus laevis oocytes. Dev Genes Evol. 1999;209:330–339. doi: 10.1007/s004270050261. [DOI] [PubMed] [Google Scholar]
- Dumont JN, Brummett AR. Egg envelopes in vertebrates. In: Browder L, editor. Developmental Biology: A Comprehensive Synthesis. 1 (Oogenesis) New York: Plenum Press; 1985. pp. 235–288. [DOI] [PubMed] [Google Scholar]
- Dunbar BS, O'Rand MG, editors. A Comparative Overview of Mammalian Fertilization. New York: Plenum Press; 1991. [Google Scholar]
- Epifano O, Liang L, Familari M, Moos MC, Dean J. Coordinate expression of the three zona pellucida genes during mouse oogenesis. Development. 1995;121:1947–1956. doi: 10.1242/dev.121.7.1947. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, O'Brien M, Wigglesworth K. Mammalian oocyte growth and development in vitro. Mol Reprod Dev. 1996;44:260–273. doi: 10.1002/(SICI)1098-2795(199606)44:2<260::AID-MRD17>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, Telfer EE. Isolation and culture of oocytes. Methods Enzymol. 1993;225:77–84. doi: 10.1016/0076-6879(93)25008-p. [DOI] [PubMed] [Google Scholar]
- Green DPL. Three-dimensional structure of the zona pellucida. Rev Reprod. 1997;2:147–156. doi: 10.1530/ror.0.0020147. [DOI] [PubMed] [Google Scholar]
- Greve JM, Wassarman PM. Mouse egg extracellular coat is a matrix of interconnected filaments possessing a structural repeat. J Mol Biol. 1985;181:253–264. doi: 10.1016/0022-2836(85)90089-0. [DOI] [PubMed] [Google Scholar]
- Gwatkin RBL. Fertilization Mechanisms in Man and Mammals. New York: Plenum Press; 1977. [Google Scholar]
- Henry MD, Campbell KP. A role for distroglycan in basement membrane assembly. Cell. 1998;95:859–870. doi: 10.1016/s0092-8674(00)81708-0. [DOI] [PubMed] [Google Scholar]
- Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Keefe D, Tran P, Pellegrini C, Oldenbourg R. Polarized light microscopy and digital image processing identify a multilaminar structure of the hamster zona pellucida. Hum Reprod. 1997;12:1250–1252. doi: 10.1093/humrep/12.6.1250. [DOI] [PubMed] [Google Scholar]
- Kinloch RA, Mortillo S, Stewart CL, Wassarman PM. Embryonal carcinoma cells transfected with ZP3 genes differentially glyosylate similar polypeptides and secrete active mouse sperm receptor. J Cell Biol. 1991;115:655–664. doi: 10.1083/jcb.115.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin RC, Scheller RH. Mechanisms of synaptic vesicle exocytosis. Annu Rev Cell Dev Biol. 2000;16:19–49. doi: 10.1146/annurev.cellbio.16.1.19. [DOI] [PubMed] [Google Scholar]
- Litscher ES, Qi H, Wassarman PM. Mouse zona pellucida glycoproteins mZP2 and mZP3 undergo carboxy-terminal proteolytic processing in growing oocytes. Biochemistry. 1999;38:12280–12287. doi: 10.1021/bi991154y. [DOI] [PubMed] [Google Scholar]
- Litscher ES, Wassarman PM. Recombinant hamster sperm receptors that exhibit species-specific binding to sperm. Zygote. 1996;4:229–236. doi: 10.1017/s0967199400003142. [DOI] [PubMed] [Google Scholar]
- Litscher ES, Wassarman PM. Purification and functional analysis of mouse egg zona pellucida glycoproteins. In: Richter JD, editor. A Comparative Methods Approach to the Study of Oocytes and Embryos. Oxford, UK: Oxford University Press; 1999. pp. 10–22. [Google Scholar]
- Liu C, Litscher ES, Mortillo S, Sakai Y, Kinloch RA, Stewart CL, Wassarman PM. Targeted disruption of the mZP3 gene results in production of eggs lacking a zona pellucida and infertility in female mice. Proc Natl Acad Sci USA. 1996;93:5431–5436. doi: 10.1073/pnas.93.11.5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy SS, Anderson ED, Jean F, Thomas G. Bi-cycling the furin pathway from TGN localization to pathogen activation and embryogenesis. Trends Cell Biol. 1999;9:28–35. doi: 10.1016/s0962-8924(98)01382-8. [DOI] [PubMed] [Google Scholar]
- Plaimauer B, Mohr G, Wernhart W, Himmelspach M, Dorner F, Schlokat U. “Shed”furin: mapping of the cleavage determinants and identification of its C-terminus. Biochem J. 2001;354:689–695. doi: 10.1042/0264-6021:3540689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi H, Wassarman PM. Secretion of zona pellucida glycoprotein mZP2 by growing oocytes from mZP3+/+ and mZP3−/− mice. Dev Genet. 1999;25:95–102. doi: 10.1002/(SICI)1520-6408(1999)25:2<95::AID-DVG3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Rankin T, Familiari M, Lee E, Ginsberg A, Dwyer N, Blanchette-Mackie J, Drago J, Westphal H, Dean J. Mice homozygous for an insertional mutation in the ZP3 gene lack a zona pellucida and are infertile. Development. 1996;122:2903–2910. doi: 10.1242/dev.122.9.2903. [DOI] [PubMed] [Google Scholar]
- Rankin TL, O'Brien M, Lee E, Wigglesworth K, Eppig J, Dean J. Defective zonae pellucidae in ZP2-null mice disrupt folliculogenesis, fertility and development. Development. 2001;128:1119–1126. doi: 10.1242/dev.128.7.1119. [DOI] [PubMed] [Google Scholar]
- Schwarzbauer JE, Sechler JL. Fibronectin fibrillogenesis: a paradigm for extracellular matrix assembly. Curr Opin Cell Biol. 1999;11:622–627. doi: 10.1016/s0955-0674(99)00017-4. [DOI] [PubMed] [Google Scholar]
- Sorensen RA, Wassarman PM. Relationship between growth and meiotic maturation of the mouse oocyte. Dev Biol. 1976;50:531–536. doi: 10.1016/0012-1606(76)90172-x. [DOI] [PubMed] [Google Scholar]
- Südhof TC. The synaptic vesicle cycle: a cascade of protein-protein interactions. Nature. 1995;375:645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- Tong Z-B, Nelson LM, Dean J. Inhibition of zona pellucida gene expression by antisense oligonucleotides injected into mouse oocytes. J Biol Chem. 1995;270:849–853. doi: 10.1074/jbc.270.2.849. [DOI] [PubMed] [Google Scholar]
- Volchkov VE, Feldmann H, Vochova VA, Klenk H-D. Processing of the Ebola virus glycoprotein by the proprotein convertase furin. Proc Natl Acad Sci USA. 1998;95:5762–5767. doi: 10.1073/pnas.95.10.5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassarman PM. Zona pellucida glycoproteins. Annu Rev Biochem. 1988;57:415–442. doi: 10.1146/annurev.bi.57.070188.002215. [DOI] [PubMed] [Google Scholar]
- Wassarman PM. Mammalian fertilization: molecular aspects of gamete adhesion, exocytosis, and fusion. Cell. 1999;96:175–183. doi: 10.1016/s0092-8674(00)80558-9. [DOI] [PubMed] [Google Scholar]
- Wassarman PM, Bleil JD, Florman HM, Greve JM, Roller RJ, Salzmann GS, Samuels FG. The mouse egg's sperm receptor: what is it and how does it work? Cold Spring Harbor Symp Quant Biol. 1985;50:11–19. doi: 10.1101/sqb.1985.050.01.004. [DOI] [PubMed] [Google Scholar]
- Wassarman PM, Josefowicz WJ. Oocyte development in the mouse: an ultrastructural comparison of oocytes isolated at various stages of growth and meiotic competence. J Morphol. 1978;156:209–236. doi: 10.1002/jmor.1051560206. [DOI] [PubMed] [Google Scholar]
- Wassarman PM, Jovine L, Litscher ES. A profile of fertilization in mammals. Nat Cell Biol. 2001;3:E59–E64. doi: 10.1038/35055178. [DOI] [PubMed] [Google Scholar]
- Wassarman PM, Liu C, Litscher ES. Constructing the mouse egg zona pellucida: some new pieces of an old puzzle. J Cell Sci. 1996;109:2001–2004. doi: 10.1242/jcs.109.8.2001. [DOI] [PubMed] [Google Scholar]
- Wassarman PM, Mortillo S. Structure of the mouse egg extracellular coat, the zona pellucida. Int Rev Cytol. 1991;130:85–110. doi: 10.1016/s0074-7696(08)61502-8. [DOI] [PubMed] [Google Scholar]
- Wassarman PM, Qi H, Litscher ES. Mutant female mice carrying a single mZP3 allele produce eggs with a thin zona pellucida, but reproduce normally. Proc R Soc Lond B Biol Sci. 1997;264:323–328. doi: 10.1098/rspb.1997.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber A. Actin binding proteins that change extent and rate of actin monomer-polymer distribution by different mechanisms. Mol Cell Biochem. 1999;190:67–74. [PubMed] [Google Scholar]
- Williams Z, Wassarman PM. Secretion of mouse ZP3, the sperm receptor, requires cleavage of its polypeptide at a consensus furin cleavage site. Biochemistry. 2001;40:929–937. doi: 10.1021/bi002275x. [DOI] [PubMed] [Google Scholar]
- Yanagimachi R. Mammalian fertilization. In: Knobil E, Neill J, editors. The Physiology of Reproduction. Vol. 1. New York: Raven Press; 1994. pp. 189–317. [Google Scholar]
- Zamboni L, Upadhyay S. Germ cell differentiation in mouse adrenal glands. J Exp Zool. 1983;228:173–193. doi: 10.1002/jez.1402280204. [DOI] [PubMed] [Google Scholar]