Abstract
Background
Toxoplasma gondii (T. gondii) is an obligate intracellular opportunistic parasite that is the causative agent of toxoplasmosis. This parasite accounts for mental disorders; however, the relationship between T. gondii infection and depressive disorder is unclear. Regarding this, the present systematic review and meta-analysis was conducted to investigate the scientific evidence regarding the potential association between major depression disorder (MDD) and Toxoplasma infection.
Methods
For the purpose of the study, the articles related to the subject of interest were systematically searched in seven electronic databases. Special attention was given to the studies examining T. gondii seropositivity level in depressed patients and controls.
Results
The search process resulted in the identification of a total of 30 publications meeting the inclusion criteria and published up to April 2018 for the systematic review. Furthermore, 29 studies met the inclusion criteria to be entered into meta-analysis. Our meta-analysis involved the review of cross-sectional studies including 1657 depressed patients and 19565 individuals as controls and case-control studies entailing 1311 depressed cases and 6015 controls without depression. 1582 depressed people participated in cross-sectional studies whose results were reported as odds ratio (OR). In addition, the total number of participants was 15068 in this type of studies. Statistical analysis indicated that the pooled OR of the risk of anti-T. gondii IgG antibody in depressed individuals in case-control and cross-sectional studies was 1.15 (95% confidence interval (CI): 0.95–1.39).
Conclusions
As the findings of the reviewed articles indicated, toxoplasmosis is not a risk factor for MDD. However, it is necessary to perform further research to clarify the detailed association between T. gondii and dysthymia or mild and moderate depression. Furthermore, it is recommended to better investigate the effect of antibody titers on the relationship between depression and T. gondii infection.
Introduction
Toxoplasma gondii (T. gondii) is an obligate neurotropic protozoan parasite that forms cysts in some tissues, including the brain of warm-blooded mammals like humans [1]. Cats are the final hosts of this parasite, and human infection occurs often via the ingestion of oocyst in water or tissue cyst in undercooked or raw meat [2]. Toxoplasma gondii infects about 25–30% of the people in developed and developing countries [1]. Most of T. gondii infections in immunocompetent individuals are asymptomatic. Nevertheless, in congenital disorders and immunocompromised patients, the infection may lead to the eye, lymph node, and central nervous system diseases [1, 3, 4].
The neurotropic nature and other specifications of T. gondii have made it a potential causative agent for psychiatric and behavioral disorders. T. gondii uses a complicated mechanism to gain access to the brain. When T. gondii reaches the brain, it invades different brain cells, including astrocytes and neurons, where it forms cysts [5]. According to the evidence, latent toxoplasmosis causes behavioral disorders not only in mice but also in humans [6, 7].
Recently published systematic review articles have proven the relationship between T. gondii infection and some psychiatric disorders such as bipolar disorder [8, 9], schizophrenia [9, 10], and epilepsy [11]. Among behavioral disorders, depression as the most common mental disorder, is coupled with remarkable morbidity and mortality. According to the Diagnostic and Statistical Manual of Mental Disorder (DSM-V) criteria, major depressive disorder (MDD) is a mental disorder characterized by at least two weeks of feeling low mood and disappointment. It is often accompanied by decreased self-esteem, loss of interest, cognitive performance, delight, dream, appetite, energy level, feelings of worthlessness, changes in weight, and having suicidal ideation and attempt for suicide [12–14].
So far, two systematic reviews have evaluated the relationship between toxoplasmosis and depression. One study has indicated that depression might be associated with microbial infections, such as those caused by T. gondii, human herpesvirus, hepatitis B virus, Chlamydiaceae, and Borna disease virus [15]. In addition, in a recent systematic review, several psychiatric disorders, namely schizophrenia, bipolar disorder, obsessive-compulsive disorder, and addiction, have been reported to be in association with toxoplasmosis. However, in the mentioned study, no significant relationship was found between depression and toxoplasmosis [9]. Since the results of the available original articles are contradictory, the aim of this new systematic review was to comprehensively assess the association between MDD and toxoplasmosis adding the latest studies on this matter. This study was also targeted toward the investigation of the susceptibility of toxoplasmosis patients to depression.
Methods
Design and protocol registration
The preferred reporting items for systematic reviews and meta-analysis (PRISMA) was used for performing this study (S1 Table) [16]. The study protocol (CRD42017069169) was registered on the site of the international prospective register of systematic reviews (PROSPERO) [17].
Search strategy
For the purpose of the study, the original studies investigating the association between toxoplasmosis and depression and published up to April 2018 were systematically searched in several databases, including “PubMed”, “Science Direct”, “Scopus”, “ProQuest”, “EMBASE”, “Web of Science”, and “Google Scholar” with no language restriction. The search process was accomplished using the following keywords in combination or alone: “Toxoplasma gondii”, “Toxoplasmosis”, “Prevalence”, “Seroprevalence”, “Depression”, “Depressed”, “Depressive”, “Psychic disorder”, “Mental disorder”, “Systematic review”, and “Meta-analysis”. In order to achieve additional eligible articles, the reference lists of the retrieved articles were checked. In addition, unpublished studies were not investigated in the review process.
Inclusion and exclusion criteria
We included articles based on the following criteria: 1) cross-sectional and case-control studies investigating the relationship between toxoplasmosis and MDD, 2) the studies performed only on humans, 3) original articles with available full texts in all languages, and 4) studies providing detailed information on the prevalence of toxoplasmosis using serology (i.e., IgG and/or IgM antibodies) and molecular tests in people with the diagnosis of depression according to the DSM-5 criteria [12]. On the other hand, the exclusion criteria entailed: 1) studies not representative for our target population, 2) studies performed on animal models; 3) low-quality data, 4) case reports or case series, and 5) studies with incomplete data.
Study selection and data extraction
All identified titles and abstracts were independently assessed for eligibility by two authors using a pilot form. Any disagreements in the selected studies were resolved by discussion, and the arbitration of the third author. The included papers were attentively studied, and the information about the study location, number and percentage of the positive and negative cases of toxoplasmosis in patients and controls, number and percentage of depressed patients and controls, age, gender, method of studies, all laboratory results, and Odds ratio (OR) were extracted using a data extraction form.
Quality assessment
Assessment of methodological quality was performed by two investigators using the Newcastle-Ottawa Scale [18] with separate criteria for case-control and cross-sectional studies to evaluate the selection, comparability, and outcome of the included studies. In the case-control studies, the scores of 7–9, 4–6, and ≤ 3 were representative of high, moderate, and low quality, respectively. Furthermore, regarding the cross-sectional studies, the scores of 6–7, 3–5, and 1–2 indicated high, moderate, and low quality, respectively. In this regard, the higher scores were awarded to the studies of higher quality. The quality of the included studies is reported in S2 Table.
Statistical analysis
The meta-analysis was performed to evaluate the potential for the association between T. gondii infection and MDD using Stata software, version 14 (StataCorp, College Station, TX, USA) [19]. The heterogeneity index among different studies was determined using Cochran’s Q test [19]. The ORs of the risk of anti-T. gondii IgG and IgM antibodies in depressed patients were estimated using a random effects model. The relationship between toxoplasmosis and MDD was expressed as OR for seven cross-sectional studies. For the other five cross-sectional studies presenting results as number and percentage, the OR was calculated. In addition, for the 17 case-control studies not reporting the association between toxoplasmosis and depression as OR, we calculated OR and 95% confidence interval (CI) using the raw number of individuals in case and control groups. Finally, 29 articles were analyzed. An OR > 1 indicates the positive effect of Toxoplasma on MDD, and an OR < 1 shows that toxoplasmosis has a protective effect against MDD.
A funnel plot and Egger’s regression test were used to check for the presence of publication bias, and the significance level was less than 0.1 [20]. Furthermore, a sensitivity analysis was performed to identify the effect of each study on the overall results through removing each study. The subgroup analysis was conducted according to the type of the study. Also, analysis of the effect of study quality on the overall effect was performed.
Results
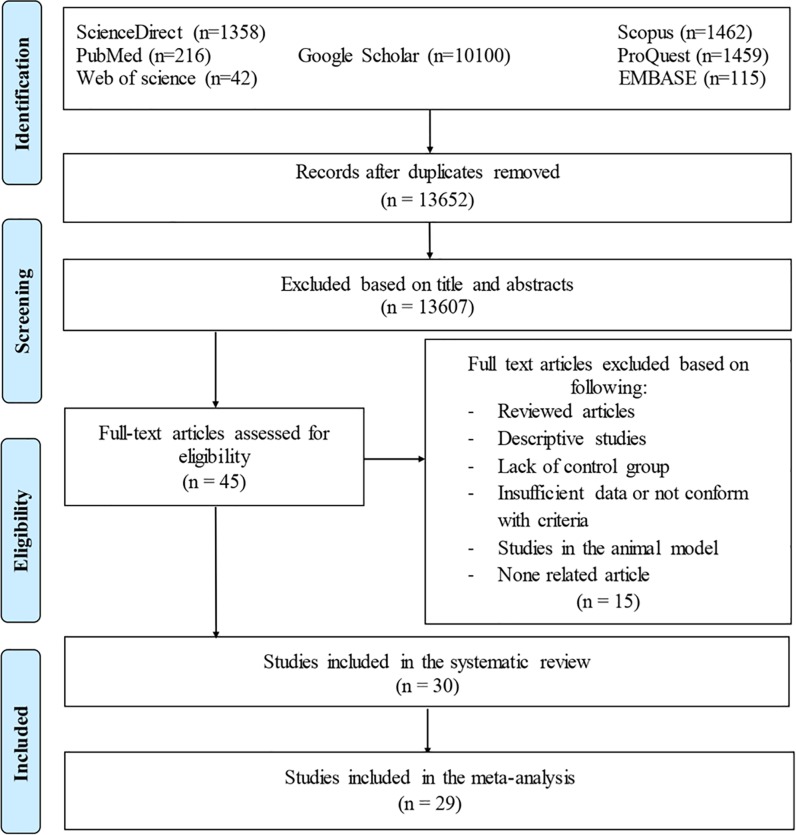
As shown in Fig 1, the literature search resulted in the identification of 13,606 related articles. After eliminating the duplicate publications, a total of 12,587 articles remained. Following a thorough examination of the titles and abstracts of the papers, a total of 45 studies were extracted. Finally, out of 45 unique records, 30 articles were eligible to be included in this systematic review, 29 cases of which met our inclusion criteria for meta-analysis. A study conducted by Groër was not analyzed due to the lack of a control group [21].
Fig 1. The PRISMA flow diagram of the search strategy, study selection, and data management procedure of T. gondii infection and depression.
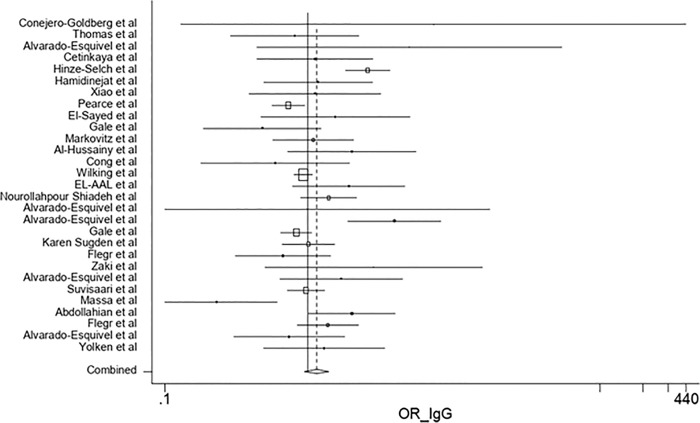
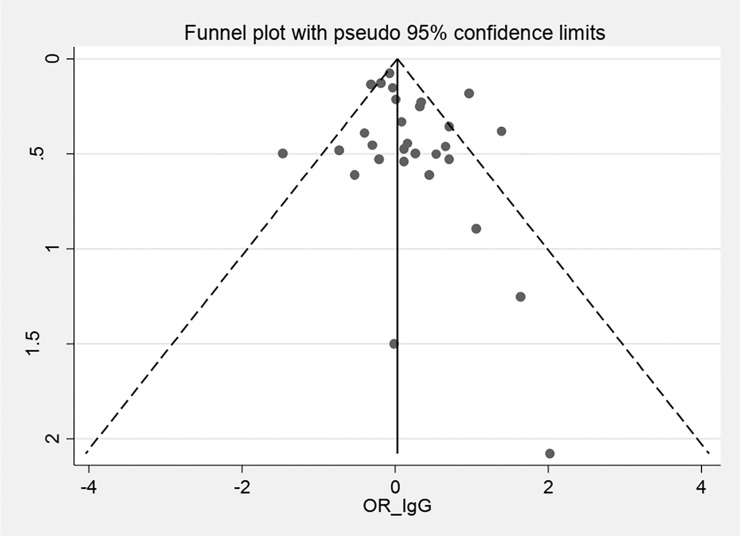
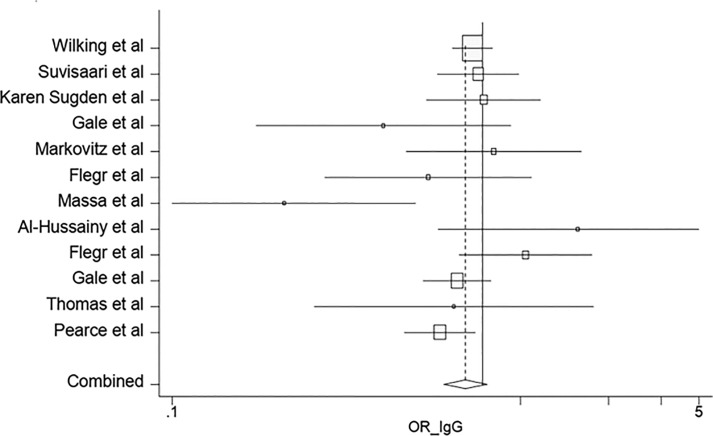
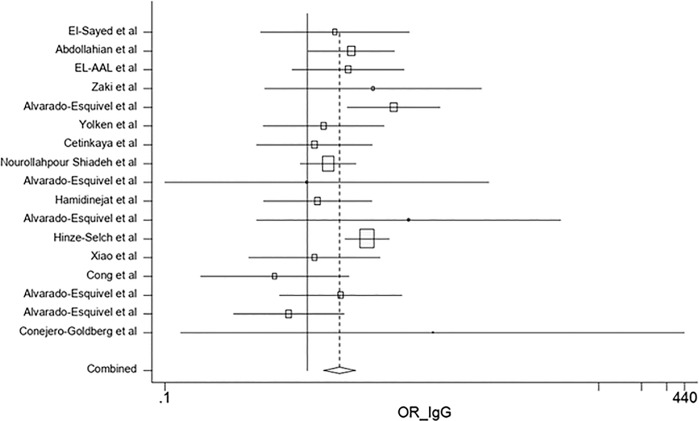
According to the results of the meta-analysis, the pooled OR of the risk of anti-T. gondii IgG antibody in the depressed subjects investigated in the included studies was 1.15 (95% CI: 0.95–1.39) (Fig 2). The test of heterogeneity revealed a significant heterogeneity among the studies (Q = 80.58%, p = 0.000). Publication bias was evaluated using the Egger's test (p = 0.148). On the other hands, as funnel plot shows the graph is symmetric and there is no publication bias (Fig 3). Furthermore, the OR of the risk of anti-T. gondii IgM antibody in depressed individuals was estimated at 1.69 (95% CI: 0.72–3.96).
Fig 2. Forest plot diagram of studies showing IgG seropositivity rates of T. gondii.
Perpendicular discontinuous line indicates the odds ratio index. The perpendicular continuous line represents the null hypothesis. The horizontal lines illustrate 95% CI for ORs. A study that its confidence interval (horizontal line) interrupts the vertical continuous line (line null hypothesis), the odd ratio of this study is not statistically significant.
Fig 3. A bias assessment plot from Egger.
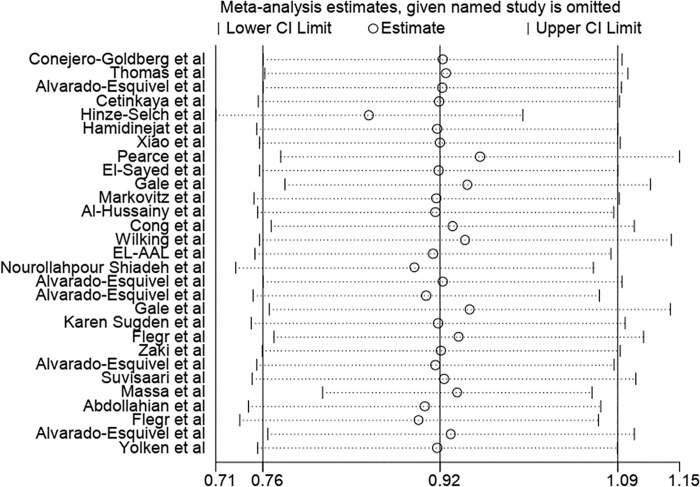
The results of the sensitivity analysis demonstrated that the impact of each study on overall estimates was not significant (Fig 4). According to the random effects model, the combined ORs of the risk of anti-T. gondii IgG antibody in depressed subjects based on the type of research in cross-sectional and case-control studies were 0.88 (95% CI: 0.75–1.03) and 1.67 (95% CI: 1.29–2.16), respectively (Figs 5 and 6). The quality assessment scores were evaluated as effective factors in overall estimation using meta regression; it has been shown that the impact of quality of each study on the overall estimates was not statistically significant (p = 0.275).
Fig 4. Sensitivity analysis for assessing the effect of each primary study on the total estimates.
Fig 5. Forest plot diagram of cross-sectional studies showing IgG seropositivity rates of T. gondii.
Fig 6. Forest plot diagram of case-control studies showing IgG seropositivity rates of T. gondii.
A total of 1657 patients with MDD and 19565 controls in cross-sectional studies and 1311 depressed cases and 6015 controls in case-control studies were included in the meta-analysis. Furthermore, 1582 depressed people participated in cross-sectional studies and their results were reported as OR. The total number of participants in this type of study was 15068.
Information and characteristics of the included publications are shown in Tables 1 and 2. The studies reviewed in this research were from 13 countries. Most of the studies were conducted in the USA (n = 7), followed by Mexico (n = 5), Iran (n = 3), Germany, Egypt, Saudi Arabia, Czech Republic, China (n = 2), United Kingdom, Turkey, Austria, New Zealand, and Finland (n = 1). Furthermore, the most widely used diagnostic test to evaluate Toxoplasma antibodies was the enzyme-linked immunosorbent assay (ELISA) method.
Table 1. Characteristics of the included studies for T. gondii infection in depressed patients and controls.
| No | First author | Publication year | Place of study | Type of study | Method | Test | Results | Age (years ± SD) | Sex (N) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Conejero-Goldberg et al. [22] | 2003 | USA | Case control | Nested-PCR | - | Not significant | P: 39.9 C: 48.7 |
— |
| 2 | Thomas et al. [23] | 2004 | United Kingdom | Cross sectional | Eiken latex agglutination test, Dye test, IgM ELISA, IFA | IgG and IgM | Not significant | P: <70 C: <70 |
— |
| 3 | Alvarado-Esquivel et al. [24] | 2006 | Mexico | Case control | ELISA | IgG and IgM | Not significant | P: 43.7±13.8 C: 42± 20.2 |
C: (F:55, M:125) |
| 4 | Cetinkaya et al. [25] | 2007 | Turkey | Case control | ELISA | IgG and IgM | Not significant | P: 37.4 ± 01 C: 37.17 ± 11.54 |
P: (F:24, M:26) C: (F:27, M:23) |
| 5 | Hinze-Selch et al. [26] | 2007 | Germany | Case control | Toxo-Spot IF | IgG | Significant | P: 46.0 ±15.5 C: 38.9 ± 13.3 |
— |
| 6 | Hamidinejat et al.[27] | 2010 | Iran | Case control | ELISA | IgG and IgM | Not significant | P: 18–58 C: 18–58 |
— |
| 7 | Xiao et al.[28] | 2010 | China | Case control | ELISA | IgG | Not significant | P: 15–65 C: 15–65 |
— |
| 8 | Groër et al.[21] | 2011 | Austria | Cross sectional | ELISA | IgG | Significant | P: 18–45 C: 18–45 |
F:414 |
| 9 | Pearce et al. [29] | 2012 | USA | Cross sectional | EIA | IgG | Significant | P: 15–39 C: — |
|
| 10 | El-Sayed et al. [30] | 2012 | Egypt | Case control | ELISA | IgG | Not significant | P: 37 ± 11 C: 37.76 ± 10.50 |
P: (F:11, M:19) C: (F:12, M:18) |
| 11 | Gale et al.[31] | 2014 | USA | Cross sectional | ELISA | IgG | Not significant | 29.7 ± 0.4 | — |
| 12 | Markovitz et al.[32] | 2015 | USA | Cross sectional | ELISA | IgG | Not significant | P: ≥18 C: — |
C: (F:217, M:139) |
| 13 | Al-Hussainy et al. [33] | 2015 | Saudi Arabia | Cross-sectional | ELISA | IgG and IgM | Not significant | P: ≥15 C: ≥15 |
P: (F:21, M:18) |
| 14 | Cong et al. [34] | 2015 | China | Case control | ELISA | IgG and IgM | Not significant | P: 16–91 C: 16–91 |
C: (F:238, M:207) |
| 15 | Wilking et al. [35] | 2016 | Germany | Cross sectional | ELFA | IgG | Not significant | P: 18–79 C: — |
— |
| 16 | El-Aal et al. [36] | 2016 | Egypt | Case control | ELISA | IgG | Not significant | P: 2–46 C: 2–46 |
P: (F:73, M:45) C: (F:33, M:27) |
| 17 | Shiadeh et al. [37] | 2016 | Iran | Case control | ELISA | IgG | Not significant | P: 28.9 ± 5.6 C: 28.2 ± 5.4 |
F:116 F:244 |
| 18 | Alvarado-Esquivel et al. [38] | 2016a | Mexico | Case control | ELISA | IgG and IgM | Not significant | P: 38.65 ± 12.93 C: 38.66 ± 12.89 |
P: (F:64, M:25) C: (F:260, M:96) |
| 19 | Alvarado-Esquivel et al. [39] | 2016b | Mexico | Case control | EIA | IgG and IgM | Significant | P: 39.43 ± 14.05 C: 39.45 ±13.98 |
P: (F:42, M:23) C: (F:168, M:92) |
| 20 | Gale et al.[40] | 2016 | USA | Cross sectional | EIA | IgG | Not significant | P: 20–80 C: — |
— |
| 21 | Sugden et al. [41] | 2016 | New Zealand | Cross sectional | EIA | IgG | Not significant | P: 38 C: — |
— |
| 22 | Zaki et al. [42] | 2016 | Saudi Arabia | Case control | ELISA | IgG and IgM | Not significant | P: 35.3±9.1 C: 34.7±8.9 |
C: (F:94, M:68) |
| 23 | Flegr and Escudero [43] | 2016 | Czech Republic | Cross sectional | CFT and ELISA | IgG and IgM | Not significant | P: M: 34.0±10.5, F: 36.5±12.3 C: M: 34·8±12·7, F: 32,4±11·0 |
P: (F:41, M:6) C: (F:356, M:853) |
| 24 | Alvarado-Esquivel et al. [44] | 2017a | Mexico | Case control | ELISA and PCR | IgG and IgM | Not significant | P: 69.08±11.39 C: 68.56±10.08 |
C: (F:105, M:90) |
| 25 | Suvisaari et al. [45] | 2017 | Finland | Cross sectional | ELISA | IgG | Not significant | P: ≥30 C: — |
— |
| 26 | Massa et al. [46] | 2017 | USA | Cross sectional | ELISA | IgG | Significant | P: 44.02±11.89 C: — |
— |
| 27 | Abdollahian et al. [47] | 2017 | Iran | Case control | ELISA | IgG and IgM | Significant | P: 35±11.61 C: 38±13.2 |
C: (F:180, M:170) |
| 28 | Flegr and Horáček [48] | 2017 | Czech Republic | Cross sectional | CFT and ELISA | IgG and IgM | Not significant | M: 35.6±12.4 F: 32.9±12.3 |
— |
| 29 | Alvarado-Esquivel et al.[49] | 2017b | Mexico | Case control | EIA and ELFA | IgG and IgM | Not significant | P: 23.40 ± 8.36 C: 23.01 ± 7.55 |
F:200 F:200 |
| 30 | Yolken et al. [50] | 2017 | USA | Case control | ELISA | IgG | Not significant | P: 37.8 C:32.7 |
P: (F:40, M:24) C: (F:358, M:213) |
ELISA: Enzyme-linked immunosorbent assay, IFA: Indirect immunofluorescence assay, CFT: Complement fixation test, EIA: Enzyme immunoassay, Nested-PCR: Nested-polymerase chain reaction, IgG: Immunoglobulin G, IgM: Immunoglobulin M, P: Pateint, C: Control, F: Female, M: Male, N: Number
Table 2. Characteristics of the included studies for T. gondii serological analysis in depressed patients and controls.
| No | First author | N | Case: MDD+ (n) | Control: MDD- (n) | MDD+ & T+ (n, %) | MDD- & T+ (n, %) | OR (95% CI) | p-value |
|---|---|---|---|---|---|---|---|---|
| 1 | Conejero-Goldberg et al. [22] | 29 | 3 | 26 | 0 (0%) | 0 (0%) | 7.57 (0.13–445.73) | — |
| 2 | Thomas et al. [23] | 18 | 18 | — | 7 (38.9%) | — | 0.81 (0.29–2.3) | — |
| 3 | Alvarado-Esquivel et al. [24] | 183 | 3 | 180 | 1 (33.33%) | 16 (8.9%) | 5.13 (0.44–59.67) | p = 0.25 |
| 4 | Cetinkaya et al. [25] | 100 | 50 | 50 | 12 (24%) | 11 (22%) | 1.12 (0.44–2.84) | — |
| 5 | Hinze-Selch et al. [26] | 679 | 465 | 214 | 221 (47.5%) | 55 (25.70%) | 2.62 (1.83–3.74) | — |
| 6 | Hamidinejat et al.[27] | 94 | 46 | 48 | 15 (32.6%) | 14 (29.2%) | 1.18 (0.49–2.82) | — |
| 7 | Xiao et al. [28] | 2663 | 29 | 2634 | 4 (13.8%) | 329 (12.5%) | 1.12 (0.39–3.24) | — |
| 8 | Groër et al. [21] | 414 | 414 | — | 44 (10.63%) | — | — | — |
| 9 | Pearce et al. [29] | 515 | 515 | — | 65 (12.62%) | — | 0.73 (0.56–0.95) | p>0.05 |
| 10 | El-Sayed et al. [30] | 50 | 30 | 20 | 12 (40%) | 6 (30%) | 1.56 (0.47–5.18) | — |
| 11 | Gale et al. [31] | 129 | 129 | — | — | — | 0.48 (0.19–1.26) | — |
| 12 | Markovitz et al. [32] | 76 | 76 | — | — | — | 1.09 (0.57–2.10) | — |
| 13 | Al-Hussainy et al. [33] | 94 | 39 | 55 | 10 (25.64%) | 8 (14.55%) | 2.03 (0.72–5.72) | p = 0.001 |
| 14 | Cong et al. [34] | 484 | 39 | 445 | 3 (7.7%) | 55 (12.36%) | 0.59 (0·18–1.98) | — |
| 15 | Wilking et al. [35] | 6515 | 768 | 5,747 | 409 (53.25%) | 3161 (55%) | 0.93 (0·8–1.08) | …. |
| 16 | El-Aal et al. [36] | 178 | 118 | 60 | 24 (20.3%) | 7 (11.7%) | 1.93 (0.78–4.79) | — |
| 17 | Shiadeh et al. [37] | 360 | 116 | 244 | 69 (59.5%) | 125 (51.23%) | 1.4 (0.89–2.19) | p = 0.142 |
| 18 | Alvarado-Esquivel et al. [38] | 363 | 7 | 356 | 0 (0%) | 22 (6.2%) | 0.99 (0.05–17.91) | — |
| 19 | Alvarado-Esquivel et al. [39] | 325 | 65 | 260 | 15 (23.1%) | 18 (6.9%) | 4.03 (1.91–8.54) | p<0.001 |
| 20 | Gale et al.[40] | 1001 | 1001 | — | — | — | 0.83 (0.64, 1.06) | — |
| 21 | Sugden et al. [41] | 127 | 127 | — | 35 (27.56) | — | 1.01 (0.66–1.54) | — |
| 22 | Zaki et al. [42] | 168 | 6 | 162 | 2 (33.3%) | 24 (14.8%) | 2.88 (0.66–16.58) | p = 0.387 |
| 23 | Flegr and Escudero [43] | 1256 | 47 | 1209 | 8 (17.02%) | 285 (23.57%) | 0.67 (0.31–1.44) | p = 0·109 |
| 24 | Alvarado-Esquivel et al. [44] | 230 | 35 | 195 | 6 (17.1%) | 21 (10.8%) | 1.71 (0.64–4.61) | p = 0.02 |
| 25 | Suvisaari et al. [45] | 5917 | 288 | 5629 | 70 (20.35%) | 1331 (19.67%) | 1.04 (0.8–1.37) | p = 0.75 |
| 26 | Massa et al. [46] | 153 | 153 | — | — | — | 0.23 (0.087–0.613) | — |
| 27 | Abdollahian et al. [47] | 385 | 35 | 350 | 18 (51.43%) | 120 (34.28%) | 2.03 (1.01–4.08) | — |
| 28 | Flegr and Horáček [48] | 78 | 78 | — | — | — | 1.38 (0.84–2.25) | p = 0.203 |
| 29 | Alvarado-Esquivel et al. [49] | 400 | 200 | 200 | 9 (4.5%) | 12 (6.0%) | 0.74 (0.3–1.79) | p = 0.50 |
| 30 | Yolken et al.[50] | 635 | 64 | 571 | 5 (7.8%) | 35 (6.1%) | 1.3 (0.49–3.44) | — |
N and n: Number, CI: Confidence interval; MDD+: Individuals with depression; MDD-: Individuals without depression; MDD+ & T+: Individuals with depression and Toxoplasma positive; MDD- & T+: Individuals without depression and Toxoplasma positive; OR: Odds ratio
Discussion
This systematic review and meta-analysis aimed to quantify the pooled ORs of anti-T. gondii IgG and IgM antibodies in depressed patients and compare them with those of control groups. The results obtained from the current study showed that the overall ORs of anti-T. gondii IgG and IgM antibodies in patients with MDD were 1.15 (95% CI: 0.95–1.39) and 1.69 (95% CI: 0.72–3.96), respectively. Based on these results, toxoplasmosis is not considered a risk factor for depressed patients. There are several reasons accounting for these negative findings: 1) infectious agent does not play an etiologic role in major depression, 2) the infectious agent has attended earlier in individuals’ life but are no longer detectable [22].
Based on the results of the meta-analysis, the depressed patients had a lower seroprevalence of T. gondii as compared to the controls. Major depression showed no association with anti-Toxoplasma IgG and anti-Toxoplasma IgM. Our results are inconsistent with those of the systematic reviews evaluating the association between toxoplasmosis and psychiatric disorders, including epilepsy (OR = 2.25) [11], bipolar disorder (OR = 1.26) [8], obsessive-compulsive disorder (OR = 1.96) [51], schizophrenia (OR = 1.81) [9], and addiction (OR = 1.91) [9].
On the other hand, our results are in line with those of a couple of previous meta-analyses that showed no association between T. gondii infection and depression [9, 15]. Wang et al. in 2014 [15] evaluated the relationship between different infectious agents and depression. In this meta-analysis, only three studies had addressed the relationship between T. gondii infection and depression. After analyzing various studies, no significant association was found, and the OR was calculated as 1.36. All three studies were included in the current meta-analysis.
In a unique meta-analysis published in 2015 [9], Sutterland et al. assessed the prevalence of T. gondii infection in several psychiatric disorders using both published and unpublished studies. They obtained a non-significant OR of 1.2 indicating no general difference between healthy subjects and depressed patients in terms of T. gondii infection prevalence. Since the present study only included the published studies, 7 out of the 9 studies reviewed in the mentioned study were investigated in the current meta-analysis. One of the two non-investigated studies was unpublished, and the other study was a summary of the paper presented at a congress [52]. In addition, 22 studies were not investigated in the mentioned meta-analysis; however, they were included in the current study due to meeting our inclusion and exclusion criteria.
The advantages of this meta-analysis are as follows: 1) the investigation of a larger number of cases and controls, compared to those of the previous studies that improves the statistical power to assess the association between toxoplasmosis and depression, 2) inclusion of studies from eight other countries in the meta-analysis that intensifies the consistency of the association, 3) analysis of case-control and cross-sectional studies based on OR, and 4) exclusive attention to MDD.
According to Fig 2, the lowest and highest ORs are related to the studies performed by Massa et al. [46] and Conejero-Goldberg et al. [22], respectively. This difference could be due to the sole use of postmortem brain samples by Conejero-Goldberg et al. [22]. In addition, the quality of the mentioned study was low, and the number of the subjects was small. However, Massa et al. used a larger sample size with a different set of psychiatric diagnoses. Moreover, race, educational level, and economic status can result in different ORs [46].
Difference in the sensitivity and specificity of detection methods, different geographic regions, age, gender, and ethnic groups are the main reasons for the difference in the prevalence of T. gondii [6, 9, 53]. Given the incomplete data of the studies assessing the relationship between different variables (e.g., age, gender, ethnic, diet, family history, parasite strain, socioeconomic level) and the prevalence of toxoplasmosis, meta-analysis was not applicable for these variables. Furthermore, due to the lack of the evaluation of these risk factors in some studies, these variables cannot be analyzed; accordingly, this is considered as a basic gap.
For example, the gender agent has an apparent impact on the psychomotor performance of humans [54]. Female sex hormones are known to manipulate dopaminergic actions in some parts of the brain. The occurrence of degenerative disorders in women, such as Parkinsonism, decreases the protective effect of dopamine [55]. Conversely, higher levels of testosterone in men can cause toxoplasmosis associated with changes in human and animal behaviors [56]. Therefore, this variable is important in evaluating the relationship between depression and T. gondii and needs to be addressed.
The possible mechanism underlying the behavioral changes correlated with latent toxoplasmosis is the presence and spread of T. gondii cysts in the central nervous system. Berenreiterová et al. [57] reported that Toxoplasma latent cysts in mice were distributed all over the brain; it would seem possible that parasitic cysts in these areas in humans could alter both frontal and limbic regions which could then result in behavioral and emotional changes. Tryptophan catabolic shunt and serotonin during reactivation stage of T. gondii infection may contribute to the development of depressive-like behavior [58]. In addition, neuronal function and immune-mediated dopamine and serotonin synthesis may be the mechanism of contribution of T. gondii infection to behavioral disorders [59]. The production of a large amount of dopamine by parasite increases the destruction of the cyst walls and production of tachyzoites [60]. In a study, the treatment of rats with latent toxoplasmosis by means of a dopamine-2 antagonist resulted in the reduction of their risky behaviors [61].
T helper 1 (Th1), natural killer cells, and nitric oxide interfere in host response to T. gondii infection. The Th1 cells secrete interferon-γ (IFN-γ) and pro-inflammatory cytokines that are important in impeding tachyzoite replication and preventing the reactivation of the tissue cysts of the central nervous system [62]. Inflammatory cytokines, such as IFN-c, tumor necrosis factor-a, and interleukin 6, are parts of the inflammatory response to T. gondii, leading to the production of glucocorticoids, which affects neuroplasticity [63]. Reduced serotonin production and tryptophan depletion in the brain may contribute to the incidence of depression caused by such cytokines as IFN-γ that could lead to the activation of indoleamine 2, 3-dioxygenase [64, 65]. Activation of guanosine-triphosphate-cyclohydrolase-1 by IFN-γ and other pro-inflammatory cytokines (e.g., IFN-α and IFN-β) increases the production of neopterin, enhances nitrate concentration through the production of tetrahydrobiopterin, and decreases phenylalanine and tyrosine levels. Amine acid phenylalanine has a key role in the biosynthesis of norepinephrine and dopamine. Sleep disturbance, fatigue, and disorders of the gastrointestinal and musculoskeletal systems are associated with decreased dopamine synthesis [64]. In a study, it was reported that the signs and symptoms of depression were ameliorated after the treatment of T. gondii infection although patient was unresponsive to the conventional anti-depressants [66].
Limitations
There are several restrictions in our research. One of these limitations was the adoption of different sampling techniques, such as Facebook-based snowball method, in various studies. Investigation of different age groups in different studies was another limitation since individuals with a longer duration of Toxoplasma seropositivity could have a higher level of depression. The type of studies reviewed is also a limitation of this study as the nature of cross-sectional study did not allow us to investigate possible causal relationships between T. gondii and depression. Also, in most case-control studies, the case and control groups were matched for age and sex, but cross-sectional studies cannot be matched for age and sex and therefore may be one of the reasons for the difference in the significance level of studies. In some cross sectional studies, despite having a large population-based study sample, the number of participants with depression was still limited, and the lack of significant association of T. gondii with depression despite higher prevalence in seropositivity and higher serointensity may reflect limited statistical power. Another limitation was that most of the included studies did not have sufficient information on disease status or severity. In addition, the studies had variable quality. Furthermore, a few studies were conducted on limited population, such as pregnant women; therefore, the results cannot be extrapolated to the general population. As the final limitation, the reviewed studies did not evaluate the effect of Toxoplasma strains on depression.
Conclusions
Based on the results of this meta-analysis, no statistically significant association was observed between toxoplasmosis and MDD. However, according to the results of the reviewed studies, especially those of the case-control studies, the potential role of toxoplasmosis in depression cannot be completely ruled out. Therefore, it is necessary to perform further research to clarify the role of T. gondii exposure in the clinical characteristics of MDD and determine the detailed association between T. gondii and dysthymia or mild and moderate depression. In addition, it is recommended to evaluate the effect of the antibody titers on the association between depression and Toxoplasma.
Supporting information
(DOC)
(DOCX)
Acknowledgments
This article is an approved plan (No.3167) from the Deputy of Research, Mazandaran University of Medical Sciences, Sari, Iran.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Montoya JG LO. Toxoplasmosis. Lancet. 2004;12(363):1965–76. 10.1016/S0140-6736(04)16412-X PMID: 15194258. [DOI] [PubMed] [Google Scholar]
- 2.Guo M, Dubey JP, Hill D, Buchanan RL, Gamble HR, Jones JL, et al. Prevalence and risk factors for Toxoplasma gondii infection in meat animals and meat products destined for human consumption. J Food Prot. 2015;78(2):457–76. 10.4315/0362-028X.JFP-14-328 [DOI] [PubMed] [Google Scholar]
- 3.Maenz M, Schlüter D, Liesenfeld O, Schares G, Gross U, Pleyer U. Ocular toxoplasmosis past, present and new aspects of an old disease. Prog Retin Eye Res. 2014;39:77–106. 10.1016/j.preteyeres.2013.12.005 [DOI] [PubMed] [Google Scholar]
- 4.Tack DM, Holman RC, Folkema AM, Mehal JM, Blanton JD, Sejvar JJ. Trends in encephalitis-associated deaths in the United States, 1999–2008. Neuroepidemiology. 2014;43(1):1–8. 10.1159/000362688 [DOI] [PubMed] [Google Scholar]
- 5.Carruthers VB, Suzuki Y. Effects of Toxoplasma gondii infection on the brain. Schizophr Bull. 2007;33(3):745–51. 10.1093/schbul/sbm008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kocazeybek B, Oner YA, Turksoy R, Babur C, Cakan H, Sahip N, et al. Higher prevalence of toxoplasmosis in victims of traffic accidents suggest increased risk of traffic accident in Toxoplasma-infected inhabitants of Istanbul and its suburbs. Forensic Sci Int. 2009;187(1):103–8. 10.1016/j.forsciint.2009.03.007 [DOI] [PubMed] [Google Scholar]
- 7.Yagmur F, Yazar S, Temel HO, Cavusoglu M. May Toxoplasma gondii increase suicide attempt-preliminary results in Turkish subjects? Forensic Sci Int. 2010;199(1):15–7. [DOI] [PubMed] [Google Scholar]
- 8.De Barros JLVM Barbosa IG, Salem H Rocha NP, Kummer A Okusaga OO, et al. Is there any association between Toxoplasma gondii infection and bipolar disorder? A systematic review and meta-analysis. J Affect Disord 2017;209:59–65. 10.1016/j.jad.2016.11.016 [DOI] [PubMed] [Google Scholar]
- 9.Sutterland A, Fond G, Kuin A, Koeter M, Lutter R, Gool T, et al. Beyond the association. Toxoplasma gondii in schizophrenia, bipolar disorder, and addiction: systematic review and meta‐analysis. Acta Psychiatrica Scandinavica. 2015; 132(3):161–79. 10.1111/acps.12423 [DOI] [PubMed] [Google Scholar]
- 10.Torrey EF, Bartko JJ, Lun ZR, Yolken RH. Antibodies to Toxoplasma gondii in patients with schizophrenia: a meta-analysis. Schizophr Bull. 2007;33(3):729–36. 10.1093/schbul/sbl050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ngoungou EB, Bhalla D, Nzoghe A, Darde ML, Preux PM. Toxoplasmosis and epilepsy—systematic review and meta analysis. PLoS neglected tropical diseases. 2015;9(2):e0003525 10.1371/journal.pntd.0003525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Association AP. Highlights of changes from DSM-IV-TR to DSM-5. http://wwwpsychiatryorg/File%20Library/Practice/DSM/DSM-5/Changes-from-DSM-IV-TR–toDSM-5pdf (retrieved 040115). 2013.
- 13.Pratt LA, Brody DJ. Depression in the United States household population, 2005–2006 NCHS Data Brief (7):1–8. 2008. [PubMed] [Google Scholar]
- 14.Arling TA, Yolken RH, Lapidus M, Langenberg P, Dickerson FB, Zimmerman SA, et al. Toxoplasma gondii antibody titers and history of suicide attempts in patients with recurrent mood disorders. J Nerv Ment Disv. 2009;3(12):905–8. 10.1097/NMD.0b013e3181c29a23 PMID: 20010026 [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Zhang L, Lei Y, Liu X, Zhou X, Liu Y, et al. Meta-analysis of infectious agents and depression. Sci Rep. 2014;4:4530 10.1038/srep04530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG. Reprint—preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Phys Ther. 2009;89(9):873–80. 10.1093/ptj/89.9.873 [DOI] [PubMed] [Google Scholar]
- 17.Daryani A, Montazeri M, Nayeri Chegeni T. 2017. Is there any association between Toxoplasma gondii infection and depression? A systematic review and meta-analysis. PROSPERO: [cited 2017]. [DOI] [PMC free article] [PubMed]
- 18.Wells G, Shea B, O’connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses Ottawa Hospital Research Institute; 2011. oxford. asp; 2015. [Google Scholar]
- 19.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21(11):1539–58. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 20.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997; 315(7109):629–34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Groër MW, Yolken RH, Xiao J-C, Beckstead JW, Fuchs D, Mohapatra SS, et al. Prenatal depression and anxiety in Toxoplasma gondii–positive women. Am J Obstet Gynecol. 2011; 204(5):433. e1.–e7. 10.1016/j.ajog.2011.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conejero-Goldberg C, Torrey EF, Yolken RH. Herpesviruses and Toxoplasma gondii in orbital frontal cortex of psychiatric patients. Schizophr Res. 2003; 60(1):65–9. 10.1016/S0920-9964(02)00160-3 [DOI] [PubMed] [Google Scholar]
- 23.Thomas HV, Thomas DR, Salmon RL, Lewis G, Smith AP. Toxoplasma and coxiella infection and psychiatric morbidity: a retrospective cohort analysis. BMC psychiatry. 2004; 4(1):32 10.1186/1471-244X-4-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alvarado-Esquivel C, Alanis-Quiñones O-P, Arreola-Valenzuela M-Á, Rodríguez-Briones A, Piedra-Nevarez L-J, Duran-Morales E, et al. Seroepidemiology of Toxoplasma gondii infection in psychiatric inpatients in a northern Mexican city. BMC Infect Dis. 2006;6(1):178 10.1186/1471-2334-6-178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cetinkaya Z, Yazar S, Gecici O, Namli MN. Anti-Toxoplasma gondii antibodies in patients with schizophrenia—preliminary findings in a Turkish sample. Schizophr Bull. 2007; 33(3):789–91. 10.1093/schbul/sbm021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hinze-Selch D, Däubener W, Eggert L, Erdag S, Stoltenberg R, Wilms S. A controlled prospective study of Toxoplasma gondii infection in individuals with schizophrenia: beyond seroprevalence. Schizophr Bull. 2007; 33(3):782–8. 10.1093/schbul/sbm010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamidinejat H, Ghorbanpoor M, Hosseini H, Alavi SM, Nabavi L, Jalali MHR, et al. Toxoplasma gondii infection in first-episode and inpatient individuals with schizophrenia. Int J Infect Dis. 2010; 14(11):e978–e81. 10.1016/j.ijid.2010.05.018 [DOI] [PubMed] [Google Scholar]
- 28.Xiao Y, Yin J, Jiang N, Xiang M, Hao L, Lu H, et al. Seroepidemiology of human Toxoplasma gondii infection in China. BMC Infect Dis. 2010;10(1):4 10.1186/1471-2334-10-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pearce BD, Kruszon-Moran D, Jones JL. The relationship between Toxoplasma gondii infection and mood disorders in the third National Health and Nutrition Survey. Biol Psychiatry. 2012; 72(4):290–5. 10.1016/j.biopsych.2012.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El-Sayed NM, Ismail KA, Ahmed SA-E-G, Ezz-El-Din HM, Azzam HME-E-D. Possible association between Toxoplasma gondii infection and schizophrenia: Egyptian study. Infect Dis Clin Pract. 2012;20(6):394–9. 10.1097/IPC.0b013e31826991aa [DOI] [Google Scholar]
- 31.Gale SD, Brown BL, Berrett A, Erickson LD, Hedges DW. Association between latent toxoplasmosis and major depression, generalised anxiety disorder and panic disorder in human adults. Folia Parasitol. 2014;61(4):285 [PubMed] [Google Scholar]
- 32.Markovitz AA, Simanek AM, Yolken RH, Galea S, Koenen KC, Chen S, et al. Toxoplasma gondii and anxiety disorders in a community-based sample. Brain Behav Immun. 2015; 43:192–7. 10.1016/j.bbi.2014.08.001 [DOI] [PubMed] [Google Scholar]
- 33.Al-Hussainy NH, Al-saedi AM, Al-lehaibi JH, Al-lehaibi YA, Al-Sehli YM, Afifi MA. Serological evidences link toxoplasmosis with schizophrenia and major depression disorder. J Microsc Ultrastruct. 2015; 3(3):148–53. 10.1016/j.jmau.2015.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cong W, Dong W, Bai L, Wang X-Y, Ni X-T, Qian A-D, et al. Seroprevalence and associated risk factors of Toxoplasma gondii infection in psychiatric patients: a case-control study in eastern China. Epidemiol Infect. 2015;143(14):3103–9. 10.1017/S0950268814003835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilking H, Thamm M, Stark K, Aebischer T, Seeber F. Prevalence, incidence estimations, and risk factors of Toxoplasma gondii infection in Germany: a representative, cross-sectional, serological study. Sci Rep. 2016; 6:22551 10.1038/srep22551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.El-Aal A, Fathy N, Saber M, Fawzy N, Ashour WR. Sero-prevalence of anti-Toxoplasma gondii antibodies among patients with neuropsychiatric disorders: epilepsy and depression. J Egypt Soc Parasitol. 2016; 240(4028):1–8. PMID: 30230768 [PubMed] [Google Scholar]
- 37.Shiadeh MN, Rostami A, Pearce B, Gholipourmalekabadi M, Newport D, Danesh M, et al. The correlation between Toxoplasma gondii infection and prenatal depression in pregnant women. Eur J Clin Microbiol Infect Dis. 2016; 35(11):1829–35. 10.1007/s10096-016-2734-5 [DOI] [PubMed] [Google Scholar]
- 38.Alvarado-Esquivel C, Sánchez-Anguiano LF, Hernández-Tinoco J, Berumen-Segovia LO, Torres-Prieto YE, Estrada-Martínez S, et al. Toxoplasma gondii infection and depression: a case—control seroprevalence study. Eur J Microbiol Immunol. 2016a; 6(2):85–9. 10.1556/1886.2016.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alvarado-Esquivel C, Sanchez-Anguiano LF, Hernandez-Tinoco J, Berumen-Segovia LO, Torres-Prieto YE, Estrada-Martinez S, et al. Toxoplasma gondii infection and mixed anxiety and depressive disorder: a case-control seroprevalence study in Durango, Mexico. J Clin Med Res. 2016b;8(7):519 10.14740/jocmr2576w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gale SD, Berrett AN, Brown B, Erickson LD, Hedges DW. No association between current depression and latent toxoplasmosis in adults. Folia Parasitol. 2016; 63:032 10.14411/fp.2016.032 [DOI] [PubMed] [Google Scholar]
- 41.Sugden K, Moffitt TE, Pinto L, Poulton R, Williams BS, Caspi A. Is Toxoplasma gondii infection related to brain and behavior impairments in humans? Evidence from a population-representative birth cohort. PLoS One. 2016; 11(2):e0148435 10.1371/journal.pone.0148435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zaki WM, Hofdi RY, Shebiley AA, Saadi ZA, Ageel AH. Seroprevalence of Toxoplasma gondii infection and its associated risk factors in neuropsychiatric patients in Jazan province, Saudi Arabia. J Egypt Soc Parasitol. 2016;240(4028):1–8. 10.12816/0033966 PMID: 30230742 [DOI] [PubMed] [Google Scholar]
- 43.Flegr J, Escudero D. Impaired health status and increased incidence of diseases in Toxoplasma-seropositive subjects–an explorative cross-sectional study. Parasitology. 2016;143(14):1974–89. 10.1017/S0031182016001785 [DOI] [PubMed] [Google Scholar]
- 44.Alvarado-Esquivel C, Martínez-Martínez AL, Sánchez-Anguiano LF, Hernández-Tinoco J, Castillo-Orona JM, Salas-Martínez C, et al. Lack of association between Toxoplasma gondii exposure and depression in pregnant women: a case-control study. BMC Infect Dis. 2017; 17(1):190 10.1186/s12879-017-2292-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suvisaari J, Torniainen-Holm M, Lindgren M, Härkänen T, Yolken RH. Toxoplasma gondii infection and common mental disorders in the Finnish general population. J Affect Disord. 2017; 223:20–5. 10.1016/j.jad.2017.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Massa NM, Duncan E, Jovanovic T, Kerley K, Weng L, Gensler L, et al. Relationship between Toxoplasma gondii seropositivity and acoustic startle response in an innercity population. Brain Behav Immun. 2017; 61:176–83. 10.1016/j.bbi.2016.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abdollahian E, Shafiei R, Mokhber N, Kalantar K, Fata A. Seroepidemiological study of Toxoplasma gondii infection among psychiatric patients in Mashhad, northeast of Iran. Iran J Parasitol. 2017; 12(1):117 [PMC free article] [PubMed] [Google Scholar]
- 48.Flegr J, Horáček J. Toxoplasma-infected subjects report an obsessive-compulsive disorder diagnosis more often and score higher in obsessive-compulsive inventory. Eur Psychiatry. 2017;40:82–7. 10.1016/j.eurpsy.2016.09.001 [DOI] [PubMed] [Google Scholar]
- 49.Alvarado-Esquivel C, Méndez-Hernández EM, Salas-Pacheco JM, Ruano-Calderón LÁ, Hernández-Tinoco J, Arias-Carrión O, et al. Toxoplasma gondii exposure and Parkinson's disease: a case–control study. BMJ open. 2017;7(2):e013019 10.1136/bmjopen-2016-013019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yolken R, Torrey EF, Dickerson F. Evidence of increased exposure to Toxoplasma gondii in individuals with recent onset psychosis but not with established schizophrenia. PLoS Negl Trop Dis. 2017; 11(11):e0006040 10.1371/journal.pntd.0006040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nayeri Chegeni T, Sarvi S, Amouei A, Moosazadeh M, Hosseininejad Z, A Aghayan S, et al. Relationship between toxoplasmosis and obsessive compulsive disorder: A systematic review and meta-analysis. PLoS neglected tropical diseases. 2019;13(4):e0007306 10.1371/journal.pntd.0007306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yonghua Z, Yue C, Lei Z, Qi G. Analysis of probable correlation between Toxoplasma gondii and schizophrenia: a seroepidemiological longitudinal investigation from 2002 to 2007 in Suzhou and Wuxi regions, Jiangsu, China. Trop Med Int Health. 2011;16:367–8. [Google Scholar]
- 53.Duffy AR, Beckie TM, Brenner LA, Beckstead JW, Seyfang A, Postolache TT, et al. Relationship between Toxoplasma gondii and mood disturbance in women veterans. Mil Med. 2015; 180(6):621–5. 10.7205/MILMED-D-14-00488 [DOI] [PubMed] [Google Scholar]
- 54.Havlicek J, Gašová Z, Smith AP, Zvára K, Flegr J. Decrease of psychomotor performance in subjects with latent asymptomatic toxoplasmosis. Parasitology. 2001; 122(05):515–20. [DOI] [PubMed] [Google Scholar]
- 55.Becker JB. Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacol Biochem Behav. 1999; 64(4):803–12. [DOI] [PubMed] [Google Scholar]
- 56.Hodková H, Kolbeková P, Skallová A, Lindová J, Flegr J. Higher perceived dominance in Toxoplasma infected men—a new evidence for role of increased level of testosterone in toxoplasmosis-associated changes in human behavior. Neuro Endocrinol Lett. 2007; 28(2):110–4. [PubMed] [Google Scholar]
- 57.Berenreiterová M, Flegr J, Kuběna AA, Němec P. The distribution of Toxoplasma gondii cysts in the brain of a mouse with latent toxoplasmosis: implications for the behavioral manipulation hypothesis. PloS one. 2011; 6(12):e28925 10.1371/journal.pone.0028925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mahmoud ME, Ihara F, Fereig RM, Nishimura M, Nishikawa Y. Induction of depression-related behaviors by reactivation of chronic Toxoplasma gondii infection in mice. Behav Brain Res. 2016; 298:125–33. 10.1016/j.bbr.2015.11.005 [DOI] [PubMed] [Google Scholar]
- 59.Zhu S. Psychosis may be associated with toxoplasmosis. Med Hypotheses. 2009; 73(5):799–801. 10.1016/j.mehy.2009.04.013 [DOI] [PubMed] [Google Scholar]
- 60.Strobl JS, Goodwin DG, Rzigalinski BA, Lindsay DS. Dopamine stimulates propagation of Toxoplasma gondii tachyzoites in human fibroblast and primary neonatal rat astrocyte cell cultures. J Parasitol. 2012; 98(6):1296–9. 10.1645/GE-2760.1 [DOI] [PubMed] [Google Scholar]
- 61.Webster J, Lamberton P, Donnelly C, Torrey E. Parasites as causative agents of human affective disorders? The impact of anti-psychotic, mood-stabilizer and anti-parasite medication on Toxoplasma gondii's ability to alter host behaviour. Proc Biol Sci. 2006; 273(1589):1023–30. 10.1098/rspb.2005.3413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McConkey GA, Martin HL, Bristow GC, Webster JP. Toxoplasma gondii infection and behaviour–location, location, location? J Exp Biol. 2013; 216(1):113–9. 10.1242/jeb.074153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fabiani S, Pinto B, Bonuccelli U, Bruschi F. Neurobiological studies on the relationship between toxoplasmosis and neuropsychiatric diseases. J Neurol Sci. 2015; 351(1):3–8. 10.1016/j.jns.2015.02.028 [DOI] [PubMed] [Google Scholar]
- 64.Hsu PC, Groer M, Beckie T. New findings: Depression, suicide, and Toxoplasma gondii infection. J Am Assoc Nurse Pract. 2014; 26(11):629–37. 10.1002/2327-6924.12129 [DOI] [PubMed] [Google Scholar]
- 65.Webster JP, McConkey GA. Toxoplasma gondii-altered host behaviour: clues as to mechanism of action. Folia Parasitol. 2010; 57(2):95 [DOI] [PubMed] [Google Scholar]
- 66.Kar N, Misra B. Toxoplasma seropositivity and depression: a case report. BMC psychiatry. 2004; 4(1):1 10.1186/1471-244X-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.