Abstract
The process through which macromolecules penetrate the plasma membrane of mammalian cells remains poorly defined. We have examined whether natural cellular events modulate the capacity of cells to take up agents applied extraneously. Herein, we report that during mitosis and in a cell type-independent manner, cells exhibit a natural ability to absorb agents present in the extracellular environment up to 150 kDa as assessed using fluorescein isothiocyanate-dextrans. This event is exclusive to the mitotic period and not observed during G0, G1, S, or G2 phase. During mitosis, starting in advanced prophase, oligonucleotides, active enzymes, and polypeptides are efficiently taken into mitotic cells. This uptake of macromolecules during mitosis still takes place in the presence of cytochalasin D or nocodazole, showing no requirement for intact microtubules or actin filaments in this process. However, cell rounding up, which still takes place in the presence of either of these drugs in mitotic cells, appears to be a key event in this process. Indeed, limited trypsinization of adherent cells mimics both the cell retraction and macromolecule uptake observed as cells enter mitosis. A plasmid DNA encoding green fluorescent protein (3.3Mda) coated with an 18 amino acid peptide is efficiently expressed when applied onto synchronized G2/M fibroblasts, whereas little or no expression is observed when the coated plasmid is applied onto asynchronous cell cultures. This shows that such coating peptides are only efficient for their encapsulating and protective effect on the plasmid DNA to be “vectorized” rather than acting as true vectors.
INTRODUCTION
An important challenge in the development of biomedical applications based on gene therapy involves the identification of reliable, low-cost, and harmless gene delivery systems and protocols that lead to the efficient internalization of active molecules (Wilke et al., 1996). Currently, a number of techniques are in routine laboratory use without a clear understanding of the entry mechanism being known. Examples include calcium phosphate and diethylaminoethyl dextran-dependent methods of cell transfection and the use of lipid micelle-dependent transfection agents. The development of reliable macromolecular transfer techniques for use in gene therapy requires an improved understanding of the ways in which cells internalize macromolecules.
Eucaryotic cells possess the intrinsic capacity to internalize macromolecules as part of natural processes that can follow several routes. Cells can internalize small molecules and particles through potocytosis, which occurs via intracellular caveolae (Anderson et al., 1992). Pinocytosis (Berlin et al., 1978; Berlin and Oliver, 1980) and endocytosis (Racoosin and Swanson, 1992) are well-documented example of routes for trafficking material between cells and their environment. Macropinocytosis is a specific form of pinocytosis that is more generally linked to the presence of ruffles on plasma membrane (Swanson, 1989; Dowrick et al., 1993; Francis et al., 1993; Hacker et al., 1997) and strong actin mobilization. For example, platelet-derived growth factor induces ruffles and actin ring structure formation very rapidly into fibroblasts (Mellström et al., 1988; Hedberg and Bell, 1995). Macropinosomes are large cytoplasmic vesicles with a diameter up to 2.5 μm, contrasting with the smaller pinosomes or endocytic vacuoles that have diameters of 0.1–0.9 μm (Hewlett et al., 1994).
Fibroblasts also can internalize macromolecules by using phagocytic pathways associated with collagen fibrils (McCullogh and Knowles, 1993; Everts et al., 1996), although phagocytosis is not the principal function of collagen fibers as in macrophages. In addition, under particular experimental conditions the internalization of dextran after mechanical stress has been reported (Lin et al., 1997). Under these circumstances fibroblasts, fixed on a collagen bed retract up as a response to a mechanical stress (when the collagen film is detached), leading to entry of fluorescein isothiocyanate (FITC)-dextrans of various sizes.
One of the key objectives of gene therapy is to identify harmless bioactive molecules that can induce the internalization of ectopically applied agents into cells. The final goals will involve identifying specific targeting agents that lead to the cell type-dependent internalization. Despite many attempts to construct peptide- or oligonucleotide-dependent vectors, little is known of the natural capacity of cells to take up exogenously applied materials. In this report we have examined the capacity of cells to internalize molecules during the cell cycle. We show that exclusively during mitosis, fibroblasts can internalize proteins and oligonucleotides but not plasmids. This internalization does not require an intact microtubule or actin cytoskeletal network. Material translocated into cells during mitosis enters the cytoplasm in a size-dependent manner. Plasmid DNA can be translocated into cytoplasm if the DNA is previously incubated with a coating peptide therefore considered as a “vector” or “carrier” (Midoux et al., 1993; Hart et al., 1995; reviewed in Lindgren et al., 2000). However, we show herein that this facilitation is only effective during mitosis and not at other times in the cell cycle. These results suggest a simple test, i.e., using cells in G1 to prove that a potential transporter for intracellular delivery is a true carrier and not just a “wrapping” agent. Together, our data provide the first evidence that cells going through mitosis have a spontaneous capacity to take up exogenous macromolecules.
MATERIALS AND METHODS
Chemicals and Reagents
FITC-dextrans, demecolcine, nocodazole, cytochalasin D, and biotinylated horseradish peroxidase (HRP) were purchased from Sigma Chemicals (L'isle d'Abeau, France). The molecular mass of dextrans is 4, 10, 20, 40, 70, and 150 kDa, which corresponds in average to particle diameters of 1.4, 2.2, 3.2, 4.1, 5.75, and 8.9 nm, respectively, as given by the manufacturer. Trypsin was stock solution at 2.5% in physiological serum (Eurobio, Les Vlis, France). FITC 5′-labeled phosphodiesteric and phosphorothioate oligonucleotides (5′-CGGCGCTACGTCTTTAT-3′) were from Eurogentec (Brussels, Belgium). Biotinylated-PKI peptide (PkI-6-24) is described in Fernandez et al. (1991). The Texas Red-labeled streptavidin was purchased from Roche Molecular Biochemicals (Mannheim, Germany). Green fluorescent protein (GFP) plasmid was described previously in Zernicka-Goetz et al. (1996). The peptide used for transfection assays was acetyl-1GALFLGFLG10-AWGSPKKR18K-cysteamid. Sequence 1–10 comes from fusion peptide of HIV-1 gp41 described for its self-assembly propensity (Rafalski et al., 1990) and sequence 12–18 derived from DNA binding site of H1 nucleoprotein (Poccia et al. 1990). Transfection control was done using FuGENE6 (Fugene in the text) transfection reagent (Roche Molecular Biochemicals).
Fibroblast Cultures and Treatments
Rat embryo fibroblasts (REF52) were cultured at 37°C in Dulbecco's modified Eagle's medium supplemented with 2 mM l-Gln, 100 IU/ml penicillin-streptavidin, and 12% fetal calf serum as described previously (Girard et al., 1995). Cells at passages between 18 and 25 were used. Cells were plated on glass coverslips and synchronized by serum starvation for 36 h. After refeeding into the proliferative cycle, cells reach a mitotic index of 10–18% after 24 h. Macromolecules solutions of FITC-dextrans were incubated onto the cells for 15 min at a 10 mg/ml final concentration at 37°C, rinsed twice in phosphate-buffered saline (PBS) and then the coverslips are fixed with 3.7% formalin in 150 mM NaCl, 8 mM KH2PO4, pH 7.2, for 5 min, before being rinsed in water and mounted in Airvol 205 as described elsewhere (Girard et al., 1995). To compare FITC-dextrans between them, the concentrations analyzed for the representation of fluorescence level versus FITC-dextran molecular weight were normalized to a constant number of fluorescein per glucose (given by the provider). Biotinylated HRP at concentration between 5 and 10 mg/ml in PBS was incubated with the cells for 15 min as described above then revealed by fluorescein-labeled streptavidin. 17-mer oligonucleotides were used at a concentration of 5 μM. To assay HRP activity, revelation was performed on PBS-rinsed cells by using 4-chloronaphtol (0.03% in PBS + H2O2 at 1/500) as colorimetric substrate. Trypsinization was performed at room temperature for 1 min by using 0.25% trypsin in Hanks' saline.
To localize the FITC-dextrans or HRP-biotinylated Texas Red-streptavidin fluorescence, cells were observed by fluorescence microscopy with a Zeiss Axiophot microscope with an objective of 40×/1.30 oil and an FT 580 filter (excitation 320–380, emission 430 nm). The total fluorescence was quantified as follows: each picture cell was analyzed using histogram according a threshold level calculated from fluorescence level of interphase cells and the fluorescence intensity quantified using ImageQuant software (Molecular Dynamics, Sunnyvale, CA). The fluorescence intensity (F.I.) is the relative fluorescence [F.I. (mitotic cells) vs. F.I. (interphase cells)] observed in the same microscopic field.
RESULTS
FITC-Dextrans Uptake
To date, there is considerable ambiguity concerning the efficiency with which molecules applied ectopically to cells undergo internalization. To address this question, we have begun a systematic analysis of the process of internalization by determining whether internalization is modulated in a cell cycle-, size-, and substance-dependent manner.
To examine the mode through which molecules become internalized, we initially analyzed synchronized fibroblasts to determine whether internalization was modulated in a cell cycle-dependent manner. Asynchronous rat embryo fibroblasts, REF52, were treated with several sizes of fluorescent dextran for 15 min before fixation and analysis for fluorescence uptake. As shown in Figure 1, the only cells that showed uptake of fluorescence in this period were those in mitosis. More detailed analysis with synchronized fibroblasts revealed that cells in G0, G1, S, and G2 did not take up fluorescent dextran to a significant extent by using comparable experimental conditions to those described herein. However, cells in very early G1 incubated with FITC-dextran (immediately after serum reactivation of G0 cells) show limited dextran uptake as small pinocytotic vesicles near the edges of the cells (our unpublished results). This FITC-dextran uptake at the G0/G1 transition remained at a very low level in comparison with that observed in mitotic cells and is consistent with the described increase in membrane ruffling and endocytosis accompanying cell mitogenic activation (Mellström et al., 1988; Hedberg and Bell, 1995).
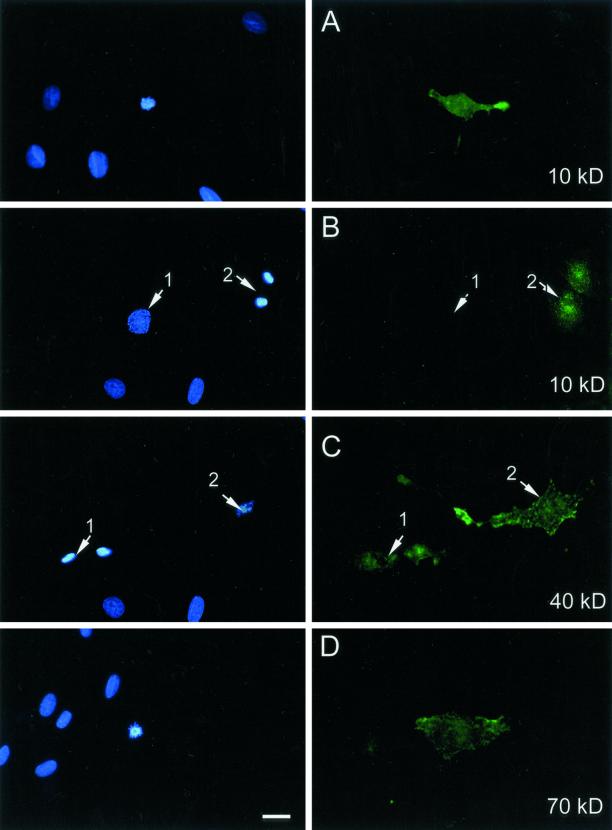
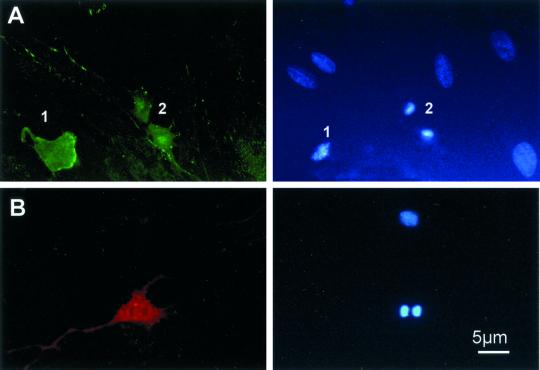
Figure 1.
Uptake of different sizes of FITC-dextrans in REF52 cells. FITC-dextrans were applied onto cultured REF52 cells at a concentration of 10 mg/ml in Dulbecco's modified Eagle's medium at 37°C for 15 min before washing, fixation, and DNA staining as described under MATERIALS AND METHODS. Left column shows the staining for DNA with Hoechst and right column shows FITC-dextran fluorescence. From top to bottom: uptake of 10-kDa FITC-dextran into an advanced prophase cell; fluorescence from 10-kDa FITC-dextran taken up into telophase cells. There is no FITC-dextran fluorescence in an adjacent early prophase cell (1), whereas the telophase cells (2) show clear fluorescence uptake; fluorescence from 40-kDa dextran taken up into telophase (1) and metaphase (2) cells; and uptake of 70-kDa FITC-dextran into a prometaphase cell. Bar, 5 μm.
Detailed examination of uptake in mitotic cells reveals it takes place from advanced prophase to early telophase periods of mitosis (Figure 1, top 4 panels). This mitosis-specific internalization was observed over a wide range of sizes for dextran from 4 to >150 kDa (Figure 2). Interestingly, considering a constant FITC/glucose ratio, the level of fluorescence uptake was markedly reduced when using the larger dextrans, suggesting that they penetrated more slowly and/or there was an upper size restriction in the process. We have observed a similar mitosis-specific uptake of fluorescent dextrans in a variety of cell types, including human primary fibroblasts HS68, HeLa-transformed epithelial cells, and murine C2 myoblasts.
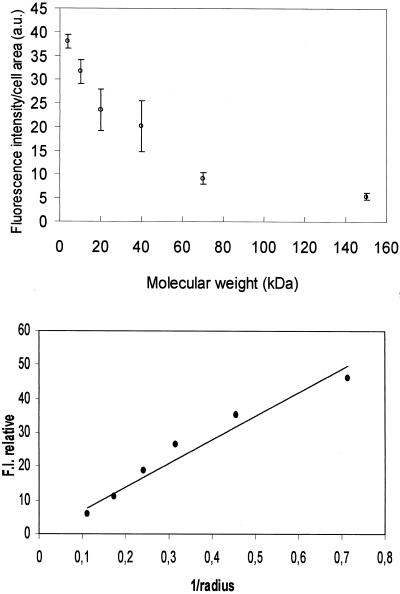
Figure 2.
Quantitative representation of FITC-dextran penetration versus particles size. First, fluorescence intensity was corrected and normalized for the number of FITC per glucose. Top, average F.I. level per mitotic cell versus FITC-dextran molecular weight. F.I. values were calculated from at least 50 cells for each dextran size. a.u., arbitrary unit. Bottom, log of F.I. versus log of FITC-dextran radius as supplied by the provider (1–9 nm). The curve shows a linear relation between F.I. and the inverse ratio of the radius (1/r). Bar, 5 μm.
Treatment with Trypsin or Cytoskeletal-Targeted Drugs
Mitosis differs significantly from the other periods of the cell cycle by the rounding of cells. To examine whether this change in cell shape was important, we artificially rounded cells after a brief treatment with trypsin as described under MATERIALS AND METHODS. As shown in Figure 3A, artificially rounded cells take up dextrans, although to a lesser extent than seen during mitosis. In addition, we observed that dextran uptake still occurred in cells treated with cytochalasin D for 30 min before dextran application, a treatment that disrupts actin filaments (Figure 3B). This result shows that mitotic uptake of macromolecules involves a mechanism independent of F-actin. Similarly, treatment of cells with nocodazole (5 μM) for 30 min before dextran application and during the time of incubation of the FITC-dextrans did not prevent dextran uptake in treated mitotic cells, which were rounded in a metaphase block (Figure 3C). In addition, the effect of nocodazole on microtubules was fully reversed upon removal of the drug: treated cells resumed mitosis and cell growth within 3–4 h after nocodazole removal (our unpublished results). As an additional control, we examined whether uptake occurred at 4°C, conditions under which active processes are considered to be stopped. For this, REF52 cells were placed at 4°C for 30 min before incubation with FITC-dextran at 4°C for 15, 30, and 60 min and checked for dextran uptake at the end of each 4°C incubation. Although cells incubated for 15 and 30 min at 4°C show no detectable uptake of fluorescent dextrans, a very faint level of uptake could be observed again specifically in mitotic cells after 60 min (Figure 3D). Fluorescence intensity measurement on the photomicrographs shows that this uptake of FITC-dextran in 60 min at 4°C represents <8–10% of the level of fluorescence uptake in 10 min at 37°C.
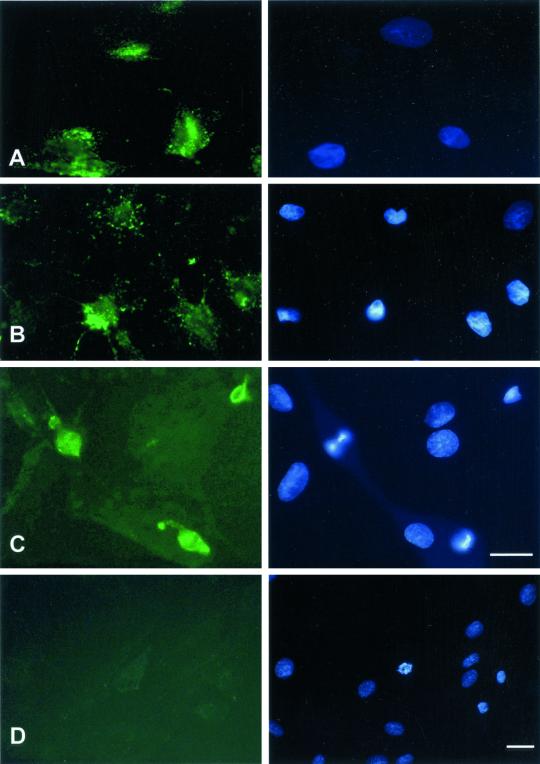
Figure 3.
Macromolecular internalization after drug treatment or low temperature. Right column shows Hoechst staining; left column shows FITC staining. REF52 cells were treated either for 1 min with 0.25% trypsin, for 30 min with 10 μM cytochalasin D, or for 30 min with 5 μM nocodazole, before application of FITC-dextrans for 15 min at 37°C, rinsed, and fixed as described in Figure 1. For cytochalasin D or nocodazole treatments, the drug was also included at the same concentration together with the dextrans during the 15-min incubation time. Alternatively, cells were placed at 4°C for 30 min and further incubated at 4°C in the presence of FITC-dextran for 60 min. (A) Fluorescence of 20-kDa dextran after incubation onto trypsinized cells. (B) Fluorescence of 20-kDa FITC-dextran after incubation onto cytochalasin D-treated cells. (C) Fluorescence of 40-kDa FITC-dextran after incubation onto nocodazole-treated cells. (D) Fluorescence uptake of 20-kDa FITC-dextran after incubation at 4°C for 60 min. Bar, 10 μm.
Taken together, these results show that a mitosis-specific mechanism allows effective uptake of a wide size range of macromolecules into cells, which occurs upon rounding up of mitotic cells. This cell rounding up can be mimicked by a short trypsinization of the adherent cells, a treatment that also induces dextran uptake into rounding up cells. Both cell shape remodeling and macromolecular uptake still occur at mitosis when either the actin filament or the microtubule network is disassembled by specific drugs, and the process still occurs, although considerably slowed down, in cells placed at 4°C.
We next examined whether this mitosis-specific event could be extended to a variety of other macromolecules, including globular proteins and oligonucleotides.
Horseradish Peroxidase
To analyze whether cells could effectively take up enzymatic activities during mitosis we followed the uptake of HRP, a 44-kDa glycoprotein. Asynchronous REF52 cells were incubated with biotinylated HRP for 15 min before fixation and staining for HRP with Texas Red-conjugated streptavidin or colorimetric assays for HRP activity by using 4-chloronaphtol. As shown in Figure 4, A and B, HRP is efficiently internalized in mitotic REF52 cells. In contrast, no staining is seen in surrounding interphase cells. In mitotic cells, the staining for HRP appears clearly concentrated at the cell borders. As shown in Figure 4C, right, internalized HRP retains peroxidase activity. Granular deposits can be observed on the membrane neighborhood in contrast with Figure 4C, left, which presents assay performed without HRP. Moreover, it also confirms that the lack of staining for HRP in interphase cells results from a lack of uptake because during the HRP color development reaction no staining associates with the surrounding interphase cells. Taken together, these results confirm that active proteins up to at least 44 kDa can be internalized into cells in a mitosis-specific manner.
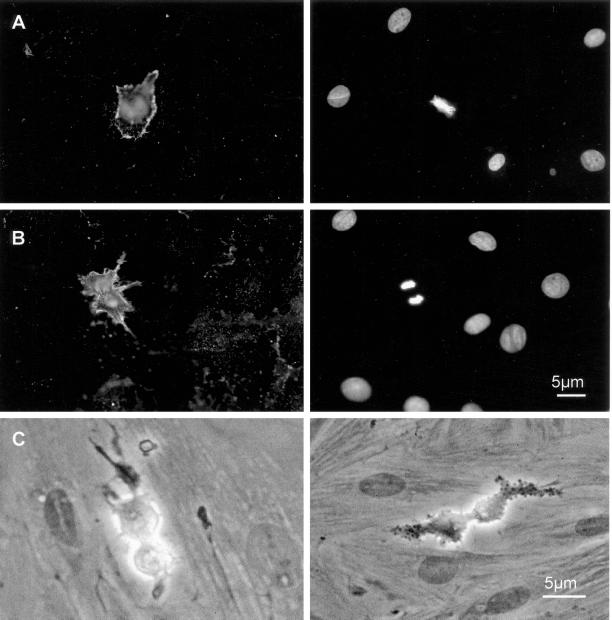
Figure 4.
HRP penetration in mitotic cells. (A) Biotinylated HRP was incubated for 15 min onto REF52 cells at 5 mg/ml before washing and fixation/permeabilization for incubation with streptavidin-Texas Red. Shown is the streptavidin-associated fluorescence (left) and DNA staining with Hoechst (right). (B) Same experiment as in A, showing HRP uptake in anaphase cells. (C) Assay for HRP enzymatic activity (15-min incubation time before colorimetric assay) without (left) or with (right) 4-chloronaphtol colorimetric substrate treatment. The vesicular aspect of internalized HRP is visible.
Oligonucleotide and PKI Peptide
We next examined whether oligonucleotides and peptides, as biomolecules of potential therapeutic interest, could enter cells during mitosis. For this we used a 17-base pair phosphorothioate oligonucleotide encoding 17 bases of cdk5 kinase or an 18-mer PKI peptide (a potent inhibitor of cAMP-dependent protein kinase), which was biotinylated (Fernandez et al., 1991). Both the oligonucleotide and the peptide are totally cell impermeant. These two reagents were used at 5 μM and 0.2 mg/ml, respectively, onto synchronized cells for 15 min at 37°C. Cells were rinsed with PBS and fixed in formalin at the end of this incubation time. Subsequent incubation with Texas Red-streptavidin was used to visualize the PKI peptide. The oligonucleotide was synthesized with two terminal fluorescein residues. As seen with FITC-dextran molecules, the cdk5-FITC oligonucleotide was found efficiently internalized into cells from late prometaphase to anaphase B (Figure 5, top) but not at other times of the cell cycle. The internalized fluorescent oligo was found evenly distributed throughout the cytoplasm with a small proportion of granular staining near the cell membrane. Similar results were also observed with an oligonucleotide encoding the same sequence but with phosphodiester as the stabilizing group (our unpublished results). For the PKI peptide, Figure 5, bottom, shows the efficient uptake of the peptide into an anaphase cell, whereas no uptake was detected into the adjacent interphase cells.
Figure 5.
Internalization of oligonucleotide and impermeant peptide. Synchronized cells were incubated for 15 min at 37°C in the presence of either 0.2 mg/ml FITC-labeled 15-mer oligonucleotide, or 1 mg/ml biotinylated PKI peptide (18 amino acids), before rinsing three times with PBS and fixation in formalin. The oligonucleotide taken up was directly visible by fluorescence microscopy, whereas visualization of the biotinylated peptide required a further 30-min incubation with Texas Red-conjugated streptavidin. Right column shows DNA staining by Hoechst. (A) Uptake of FITC-phosphorothioate oligonucleotide. Shown is the fluorescence taken up in a metaphase (1) and a pair of telophase (2) cells. (B) Biotinylated-PKI peptide uptake in mitotic cells: shown is the fluorescence from uptake into an early anaphase cell.
Transfection by Using Carrier Peptide Technology
The results we have presented herein show that cells can take up a variety of macromolecules or different sizes up to 150 kDa. A number of reports have described the direct vectorization of macromolecules, particularly plasmid DNA into cells by synthetic oligopeptides (Midoux et al., 1993; Hart et al., 1995; Chaloin et al., 1997; reviewed in Lindgren et al., 2000). Because we have identified a spontaneous uptake of macromolecules at mitosis, we examined whether such a vector peptide, designed to bind, coat, and condense DNA plasmids, could influence the effective penetration of plasmids into mitotic cells. For these studies, plasmid DNA encoding GFP was preincubated for 30 min in the presence of a DNA-binding peptide potentially acting as a vector peptide and the complex was then incubated with cells synchronized at the G2/mitosis boundary. After incubation for 90 min, cells were washed and further grown for 16 h before examining the expression of GFP. The peptide we have chosen is a chimerical amphiphilic peptide designed to bind DNA according to an “SPKK” nucleoprotein site (peptideA). We have previously studied and described some of the properties of such amphiphilic peptide, including its binding and vectorization of retinoids and its ability to self-aggregate (Pellegrin et al., 1998). The experiment was designed to assess the vectorization activity of the peptide versus a simple mitosis-dependent entry because plasmid uptake and expression were compared after incubation onto asynchronous or G2-M–synchronized cells. As shown in Figure 6, incubation of the DNA with peptideA leads to the expression of GFP protein in a mitosis-dependent manner. The photomicrograph shows that the DNA entered the cytoplasm and expressed (Figure 6, A and B). It was noticeable that the majority of GFP-expressing cells was in pairs, most likely as a result of the DNA penetrating the cells during mitosis. The expression analysis shows that an efficient transfection and expression of the plasmid occurs when peptide-A–DNA complex is incubated with cells synchronized in G2-M with a mitotic index approaching 18–20%. In typical experiments, 15% of cells express GFP after 16 h, which reflects an average transfection level of 80% of the mitotic cells in the synchronized culture. In contrast, when the peptide is applied onto asynchronous cell cultures, no >1% GFP-expressing cells is obtained. This level of transfection corresponds to the mitotic index in asynchronous cells. As a control, a GFP-plasmid incubated in the absence of peptide shows no detectable expression, a result that suggests that plasmid DNA is by itself too long and wrongly charged to be spontaneously taken up as intact DNA for expression, even in cells at mitosis (our unpublished results). In contrast, when using a standard lipid-based transfection agent, herein Fugene (Roche Molecular Biochemicals), we observed no difference in GFP expression between cells synchronized in mitosis and asynchronously growing cells. In both cases, GFP expression was detected in ∼7–8% of the cells (Figure 6C). These data show that the DNA-binding peptide allows coating and entry of plasmid DNA and its subsequent expression in mitotic cells but not in cells in interphase. This observation indicates that such DNA-binding peptide acts more as a coating and stabilizing agent on the plasmid DNA rather than as a true vector. These data are important to keep in mind when assessing the potential cellular delivery activity of any carrier or “translocating” peptide.
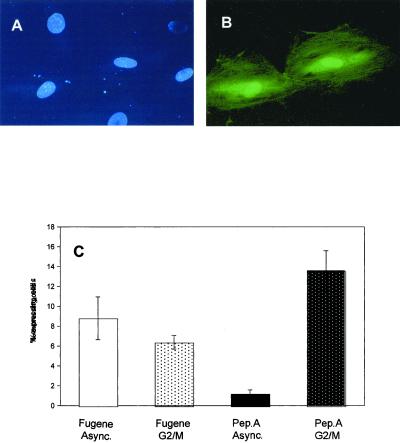
Figure 6.
Expression of a GFP plasmid transfected in the presence of a carrier DNA-binding peptide. REF52 cells were grown and either synchronized in G2-M phase or left asynchronous before addition of GFP plasmid (1–2 μg/ml) either premixed with peptide A (0.3 mg/ml) or with the transfection agent Fugene6. PeptideA-plasmid DNA mixture was applied onto cells for 90 min and cells were further grown for 16 h before examining GFP expression. Fugene was used as specified by the manufacturer. Microphotographs show Hoechst nuclear DNA staining (A) and GFP expression (B) after peptideA-GFP plasmid application. In A, the Hoechst fluorescence shows the nuclei of two daughter cells after division of the same mother cell. In B, only these cells present green fluorescence from expressed GFP protein. In C, the expression levels are given for transfection by using either Fugene or peptideA with plasmid DNA. There is no significant difference between synchronous (G2-M) or asynchronous (interphase) cells in the case of Fugene-mediated transfection. In contrast, when using peptide-DNA mixture, up to 15% of the G2-M cells expressed GFP (when the mitotic index in these cells approached 20%), whereas in asynchronous cells (with <2% cells in mitosis at any one time), GFP-plasmid expressed in ∼1% cells.
DISCUSSION
We have shown that a wide range of macromolecules (proteins, dextrans, oligonucleotides, and cell-impermeant peptides) can penetrate cells specifically during mitosis. The window of internalization seems to be from late prophase to late anaphase B and is related to cell shape rounding. Detailed analysis of synchronized cells reveals that such internalization occurs uniquely during mitosis and is never seen during other phases of the cell cycle (G0, G1, S, and G2). Mitotic internalization is limited in our experiments to an upper size limit of 150 kDa. Although the precise mechanism through which mitotic cells internalize macromolecules remains to be determined these observations provide a new basis for analyzing the capacity of molecules to vectorize substances into cells.
Several mechanisms are described for classical uptake and internalization of molecules, but none precisely fit our observations during mitosis. There could be sufficient changes in actin organization and membrane remodeling to support a macropinocytotic mechanism, which could also account for the punctuate staining pattern observed with fluorescent dextran. However, our observations that mitotic uptake still occurs in the presence of cytochalasin D effectively discounts macropinocytosis. Alternatively, fibroblasts are capable of phagocytosis (McCullogh and Knowles, 1993) although it is unknown whether this activity persists in mitotic cells.
Using dextrans of different molecular mass, we observed a rapid decrease of internalization rate with size molecules. The linear relation between F.I. to the radius of the dextrans indicates a mechanism that can be interpreted with a simple diffusion model (Ussing, 1978). The slowest of successive steps (always considered as the only observable) is therefore the diffusion of a particle approaching the plasma membrane and its intake into the membrane invagination. But it is important to underline that we observed an effective process that needs <15 min at 37°C to allow the internalization of macromolecules. Several reports (Dowrick et al., 1993; Sit et al., 1993; Hewlett et al., 1994) have described invagination of the plasma membrane internal ruffling and internalization of the extracellular medium corresponding to some kind of phagocytosis. Although cells generally show significant changes in membrane dynamics during mitosis through blebs, ruffles, and filipodiae (Harri and Low, 1975; Gordon et al., 1983), it is well known that some processes such as endocytosis are strongly reduced during mitosis (Berlin et al., 1978; Berlin and Oliver, 1980). Hence, the experimental results found herein seem rather surprising. However, a number of previous reports (Sit et al. 1992, 1993; Sit, 1996) have shown the rapid (<2 min) mitotic uptake of large particles by human Chang liver cells during mitosis. Plasma membrane infolding, and internal ruffles with internalization of outer medium are events that accompany mitotic cell shape remodeling. As shown in Figure 3A, for trypsinized cells, it can be assumed that a similar cell rounding is involved together with membrane remodeling, a process allowing macromolecules to be effectively taken up into cells. Our hypothesis is that such dynamic membrane remodeling can act as a “trap” for macromolecules close enough to the plasma membrane. Hence, membrane internalization in mitosis corresponds to the creation of membrane infolding and a subsequent internalization of nearby molecules at the beginning of the process. As a consequence, a concentration depletion/gradient is formed, entailing the observed diffusion. Such mechanism would not require the endocytic pathway and takes into account all our observations, i.e., internalization through large structures (∼1–2 μm) and diffusion rate-controlled process. It is worth noting that in cells placed at 4°C, the process is considerably slowed down (at least 60-fold slower). However, mitosis itself and all membrane dynamics are also slowed down to a similar extent by such low temperatures.
Our observations that cells spontaneously internalize many biomolecules during mitosis have a number of important implications 1) for the use of a number of drugs and macromolecules as anticancer agents, and 2) for future studies on molecules implicated on induction or facilitation of internalization.
Whereas cells in interphase are normally impermeable to most potentially therapeutic and/or cytotoxic agents, they become cell permeant when cells reach mitosis. Indeed, we have observed this process for a small toxic molecule that is actively kept out of nondividing interphase cells, i.e., ethidium bromide (EtBr). Cells incubated in the presence of low concentrations (10 μM) of EtBr in the culture medium show a massive entry of this molecule into mitotic cells (clearly visible through the red endogenous fluorescence of EtBr), whereas all surrounding nonmitotic cells remain totally impermeable to this molecule (our unpublished observation). Although cells in interphase are also capable of taking up macromolecules through endocytosis (as confirmed herein with dextran at the GO/G1 transition), this remains on a much lower scale and far less efficient than the internalization observed in mitotic cells.
It is clear that any demonstration for a true cellular delivery peptide should show uptake in G1- and S-phase cells because any test passing mitosis will show uptake independently of a true “vectorization” of the macromolecule. In addition, as we have shown herein, it is important to address the biological activity of vectorized molecules. Under the conditions we described, we show that DNA binding peptides can help to internalize a sufficient number of plasmid copies for subsequent efficient expression of the fluorescent GFP protein to be visualized. Furthermore, when using oligonucleotides (for instance, in antisense experiments) the efficiency of uptake against the loss of oligonucleotides due to their degradation in the medium may be greatly enhanced by using cells synchronized at the G2/mitosis boundary.
Clearly, a naked plasmid DNA cannot enter the cells under normal circumstances and we have observed no expression of GFP plasmid alone even when applied onto cultured cells with a high mitotic index. The global charge of the plasmid is negative, as is the membrane, effectively preventing plasmid/membrane interaction. Naked DNA must also be more susceptible to being nicked in the culture medium such that it may not be taken up as intact plasmid for subsequent expression into cells. However, coating the plasmid with a cationic peptide may be sufficient, first to protect the plasmid from being nicked, and second to neutralize or reduce the charge of the DNA and bring compaction properties, due to the ionic interactions between residues from the peptide and negative charges from the DNA. As shown in Figure 6, under these circumstance, the plasmid enters, but only efficiently at mitosis. Under the conditions we have used (1.0 μg of DNA/ml of culture media) each cell is in contact with considerably more than a single copy of the plasmid. The data we describe in Figure 6 strongly suggest that some peptides previously described as internalizing or vector (Midoux et al., 1993; Hart et al., 1995; Chaloin et al., 1997) or “cell-penetrating” peptides (reviewed in Lindgren et al., 2000) for DNA or RNA transfection may be efficient as encapsulating, protective, and cell membrane-interacting agents for the plasmid DNA to be delivered rather than as true cell penetrating or so-called translocating peptides.
ACKNOWLEDGMENTS
We thank all the members of the Cell Biology group for critical comments during this work. This work was supported by grants from Association pour la Recherche contre le Cancer (no. 9484) and Hoechst-Marion-Roussel to N.J.C.L. and Association Française contre les Myopathies to A.F. P.P. was supported by the Fondation pour la Recherche Médicale.
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.01–06-0280. Article and publication date are at www.molbiolcell.org/cgi/10.1091/mbc.01–06-0280.
REFERENCES
- Anderson RG, Kamen BA, Rothberg KG, Lacey SW. Potocytosis: sequestration and transport of small molecules by caveolae. Science. 1992;255:410–411. doi: 10.1126/science.1310359. [DOI] [PubMed] [Google Scholar]
- Berlin RD, Oliver JM. Surface functions during mitosis. J Cell Biol. 1980;85:660–671. doi: 10.1083/jcb.85.3.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin DR, Oliver JM, Walter RJ. Surface functions during mitosis I: phagocytosis, pinocytosis and mobility of surface-bound ConA. Cell. 1978;15:327–341. doi: 10.1016/0092-8674(78)90002-8. [DOI] [PubMed] [Google Scholar]
- Chaloin L, Vidal P, Heitz A, Van Mau N, Mery J, Divita G, Heitz F. Conformations of primary amphipathic carrier peptides in membrane mimicking environment. Biochemistry. 1997;36:11179–11187. doi: 10.1021/bi9708491. [DOI] [PubMed] [Google Scholar]
- Dowrick P, Kenworthy P, McCann B, Warn R. Circular ruffle formation and closure lead to macropinocytosis in hepatocyte growth factor/scatter factor-treated cells. Eur J Cell Biol. 1993;61:44–53. [PubMed] [Google Scholar]
- Everts V, van der Zee E, Creemers L, Beertsen W. Phagocytosis and intra cellular digestion of collagen, its role in turnover and remodelling. Histochem J. 1996;28:229–245. doi: 10.1007/BF02409011. [DOI] [PubMed] [Google Scholar]
- Fernandez A, Mery J, Vandromme M, Basset M, Cavadore JC, Lamb NJ. Effective intracellular inhibition of the cAMP-dependent protein kinase by microinjection of a modified form of the specific inhibitor peptide PKi in living fibroblasts. Exp Cell Res. 1991;195:468–477. doi: 10.1016/0014-4827(91)90398-e. [DOI] [PubMed] [Google Scholar]
- Francis CL, Ryan TA, Jones BD, Smith SJ, Falkow S. Ruffles induced by Salmonella and other stimuli direct macropinocytosis of bacteria. Nature. 1993;364:639–642. doi: 10.1038/364639a0. [DOI] [PubMed] [Google Scholar]
- Girard F, Fernandez A, Lamb N. Delayed cyclin A and B1 degradation in non-transformed mammalian cells. J Cell Sci. 1995;168:2599–2608. doi: 10.1242/jcs.108.7.2599. [DOI] [PubMed] [Google Scholar]
- Gordon SR, Rothstein H, Harding CV. Studies on corneal endothelial growth and repair. IV. Changes in the surface during cell division as revealed by scanning electron microscopy. Eur J Cell Biol. 1983;31:26–33. [PubMed] [Google Scholar]
- Hacker U, Albrecht R, Maniak M. Fluid-phase uptake by macropinocytosis in Discotellium. J Cell Sci. 1997;110:105–112. doi: 10.1242/jcs.110.2.105. [DOI] [PubMed] [Google Scholar]
- Harri JE, Low FN. Mitotic cells and their microappendages in the primitive streak of the chick embryo. Am J Anat. 1975;144:249–255. doi: 10.1002/aja.1001440210. [DOI] [PubMed] [Google Scholar]
- Hart SL, Hartbottle RP, Cooper R, Miller A, Williamson R, Coutelle C. Gene delivery and expression mediated by an integrin-binding peptide. Gene Ther. 1995;2:552–554. [PubMed] [Google Scholar]
- Hedberg KM, Bell PB. The effect on neomycin on PDGF-induced mitogenic response and actin organization in cultured human fibroblasts. Exp Cell Res. 1995;219:266–275. doi: 10.1006/excr.1995.1227. [DOI] [PubMed] [Google Scholar]
- Hewlett LJ, Prescott AR, Watts C. The coated pit and macropinocytic pathways serve distinct endosome populations. J Cell Biol. 1994;124:689–703. doi: 10.1083/jcb.124.5.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y-C, Ho Y-C, Grinnell F. Fibroblasts contracting collagen matrices form transient plasma membrane passages through which the cells take up fluorescein isothiocyanate-dextran and Ca++ Mol Biol Cell. 1997;8:59–71. doi: 10.1091/mbc.8.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren M, Hallbrink M, Prochiantz A, Langel U. Cell penetrating peptides. Trends Pharmacol Sci. 2000;21:99–103. doi: 10.1016/s0165-6147(00)01447-4. [DOI] [PubMed] [Google Scholar]
- McCullogh CA, Knowles GC. Deficiencies in collagen phagocytosis by human fibroblasts in vitro: a mechanism for fibrosis? J Cell Physiol. 1993;155:461–471. doi: 10.1002/jcp.1041550305. [DOI] [PubMed] [Google Scholar]
- Mellström K, Heldin C-H, Westermark B. Induction of circular membrane ruffling on human fibroblasts by platelet-derived growth factor. Exp Cell Res. 1988;177:347–359. doi: 10.1016/0014-4827(88)90468-5. [DOI] [PubMed] [Google Scholar]
- Midoux P, Mendes C, Legrand A, Raimond J, Mayer R, Monsigny M, Roche AC. Specific gene transfer mediated by lactosylated poly-L-lysine into hepatoma cells. Nucleic Acids Res. 1993;21:871–878. doi: 10.1093/nar/21.4.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrin P, Chaloin L, Roustan C, Méry J, Bennes R. Aggregates of an amphiphilic synthetic peptide bind and deliver all-trans retinol and all-trans retinoic acid into fibroblast cells. Eur J Biochem. 1998;251:480–486. doi: 10.1046/j.1432-1327.1998.2510480.x. [DOI] [PubMed] [Google Scholar]
- Poccia D, Pavan W, Green GR. 6DMAP inhibits chromatin decondensation but not sperm histone kinase in sea urchin male pronuclei. Exp Cell Res. 1990;188:226–234. doi: 10.1016/0014-4827(90)90164-6. [DOI] [PubMed] [Google Scholar]
- Racoosin EL, Swanson JA. M-CSF-mediated macroinocytosis increases solute endocytosis but not receptor-mediated endocytosis in mouse macrophages. J Cell Sci. 1992;102:867–880. doi: 10.1242/jcs.102.4.867. [DOI] [PubMed] [Google Scholar]
- Rafalski M, Lear JD, DeGrado WF. Phospholipid interactions of synthetic peptides representing the N-terminus of HIV gp41. Biochemistry. 1990;29:7917–7922. doi: 10.1021/bi00486a020. [DOI] [PubMed] [Google Scholar]
- Sit KH. Cell rounding with “ripp-off” detachment. Histol Histopathol. 1996;11:215–227. [PubMed] [Google Scholar]
- Sit KH, Bay BH, Wong KP. Reduced surface area in mitotic cell rounding of human Chang liver cells. Anat Rec. 1993;235:183–190. doi: 10.1002/ar.1092350202. [DOI] [PubMed] [Google Scholar]
- Sit KH, Bay BH, Wong KP. Distinctive uptake of neutral red by mitotic cancer cells. Biotech Histochem. 1992;67:196–201. doi: 10.3109/10520299209110066. [DOI] [PubMed] [Google Scholar]
- Swanson JA. Phorbol esters stimulate macropinocytosis and solute flow through macrophages. J Cell Sci. 1989;94:135–142. doi: 10.1242/jcs.94.1.135. [DOI] [PubMed] [Google Scholar]
- Ussing HH. Membrane transport in biology. Chapter 1. In: Tosteson DCE, editor. Membrane Transport in Biology. I. Concepts and Models. Berlin: Springer Verlag; 1978. [Google Scholar]
- Wilke, M., Fortunati, E., van der Broeek, M., Hoogeven, A.T., and Scholte, B.J. (1996). Efficacy of a peptide-based gene delivery system depends on mitotic activity. 3, 1133–1142. [PubMed]
- Zernicka-Goetz M, Pines J, Ryan K, Siemering KR, Haseloff J, Evans MJ, Gurdon JB. An indelible lineage marker for Xenopus using a mutated green fluorescent protein. Development. 1996;122:3719–3724. doi: 10.1242/dev.122.12.3719. [DOI] [PubMed] [Google Scholar]