Despite the epidemic of childhood obesity, effective and sustainable intervention approaches to help children lose weight or maintain their weight in low-income families remain elusive.
Abstract
BACKGROUND:
Our primary aim was to evaluate the effects of 2 family-based obesity management interventions compared with a control group on BMI in low-income adolescents with overweight or obesity.
METHODS:
In this randomized clinical trial, 360 urban-residing youth and a parent were randomly assigned to 1 of 2 behaviorally distinct family interventions or an education-only control group. Eligible children were entering the sixth grade with a BMI ≥85th percentile. Interventions were 3 years in length; data were collected annually for 3 years. Effects of the interventions on BMI slope (primary outcome) over 3 years and a set of secondary outcomes were assessed.
RESULTS:
Participants were primarily African American (77%), had a family income of <25 000 per year, and obese at enrollment (68%). BMI increased over time in all study groups, with group increases ranging from 0.95 to 1.08. In an intent-to-treat analysis, no significant differences were found in adjusted BMI slopes between either of the family-based interventions and the control group (P = .35). No differences were found between the experimental and control groups on secondary outcomes of diet, physical activity, sleep, perceived stress, or cardiometabolic factors. No evidence of effect modification of the study arms by sex, race and/or ethnicity, household income, baseline levels of child and parent obesity, or exposure to a school fitness program were found.
CONCLUSIONS:
In this low-income, adolescent population, neither of the family-based interventions improved BMI or health-related secondary outcomes. Future interventions should more fully address poverty and other social issues contributing to childhood obesity.
What’s Known on This Subject:
Adolescent obesity continues to be a concerning issue in the United States. The need for effective interventions to reduce overweight and obesity is critical in low-income, minority families who suffer greater levels of obesity but are generally less responsive to intervention.
What This Study Adds:
In a 3-year randomized trial, family-based interventions did not improve BMI or secondary health-related outcomes in a low-income, adolescent population. Future interventions should more fully address poverty and other social issues contributing to childhood obesity.
Adolescent obesity continues to be a concerning issue in the United States.1 With 1 in 3 children today being overweight or obese2 and significant disparities by race, ethnicity, and socioeconomic status,3,4 it is critical to identify effective methods for weight management in these children to reduce their risk for future health issues, such as hypertension, diabetes, lipid abnormalities, and early mortality.5 Currently, direct medical costs of obesity in the United States are $149 billion.6
Despite the epidemic of childhood obesity, effective and sustainable interventions to help children lose or maintain their weight as they grow remain elusive.7–9 Evidence demonstrates that behavioral interventions to reduce overweight and obesity are less effective among prepubertal, young adolescents; boys; low-income families; single-parent households; and African American or Hispanic adolescents; many of whom have the highest rates of obesity.10–15 In particular, low-income, urban-dwelling families have been shown to engage in less physical activity; often live in areas with less access to affordable, healthy food options; and frequently live in highly stressful environments.16,17 In addition, adolescents with parents with overweight or obesity are more difficult to treat,18 likely because of social norms and the family environment. Peer relationships, family eating and activity patterns, and community culture also have been shown to influence adolescents’ weight status and response to weight-management interventions.19–21
This article reports the primary and secondary outcome results of the Ideas Moving Parents and Adolescents to Change Together (IMPACT) trial.22 The IMPACT trial assessed the effects of 2 distinct family-based behavioral obesity management interventions compared with an education-only control group on BMI in middle school, low-income, urban adolescents with overweight and obesity. The effects of the interventions on a set of secondary outcomes also were evaluated: (1) healthy weight behaviors (diet, physical activity, sedentary activity, sleep, and perceived stress) and (2) cardiometabolic risk factors. IMPACT was 1 of 4 intervention trials of the Childhood Obesity Prevention and Treatment Research Consortium (COPTR), funded by the National Institutes of Health.23 Each of 4 sites tested distinct 3-year interventions in low-income populations and was supported by a coordinating center (University of North Carolina at Chapel Hill). The study was monitored by a data and safety monitoring board appointed by the National Heart, Lung, and Blood Institute and received University Hospitals of Cleveland Human Subjects Institutional Review Board approval.
Methods
Study Design, Population, and Recruitment
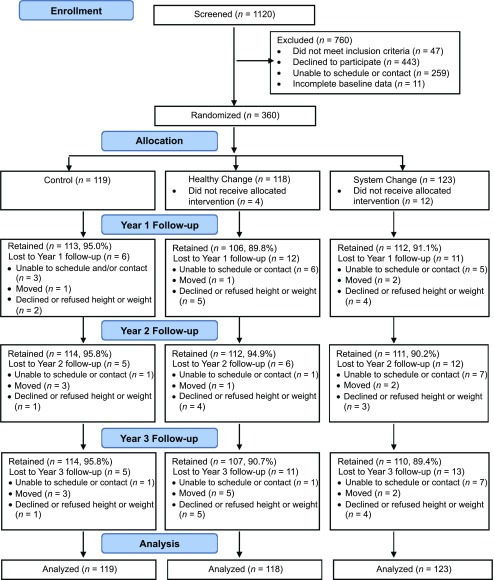
In this 3-group randomized controlled trial, 360 middle school, low-income, minority adolescents were recruited as part of an existing BMI and blood pressure (BP) screening program in the Cleveland Metropolitan School District and 5 charter schools. With parent permission, the schools provided the results of the BMI screening and parent contact information to the research team, who then consecutively contacted parents of all eligible adolescents (BMI ≥85th percentile and entering the sixth grade) for participation in the study. One parent or guardian per child also was enrolled. Children were excluded if they were taking medications that alter appetite or weight, had stage 2 hypertension or stage 1 hypertension with end organ damage,24 had type 1 or 2 diabetes, had sickle cell disease (conditions that are primarily treated with medication rather than lifestyle interventions), or had a known medical condition that itself causes obesity (eg, Prader-Willi syndrome). Participants were recruited between May 2012 and January 2014 and followed for 3 years. Figure 1 shows the Consolidated Standards of Reporting Trials diagram of participant screening, random assignment, and retention.
FIGURE 1.
Consolidated Standards of Reporting Trials diagram.
Randomization Procedures
Participants were randomly assigned to 1 of 3 intervention study arms within 37 days of the baseline visit. A computerized minimization program25,26 was used to help ensure that groups assigned to each study arm were similar in terms of BMI (overweight or obese), sex (male or female), and school location in Cleveland (east or west side).
Intervention Descriptions
Interventions
The effects of 2 theoretically different family-based interventions, Healthy Change and System Change, were assessed against a control group. The Healthy Change intervention consisted of behavior change strategies commonly used in cognitive behavioral and motivational interviewing interventions, such as problem-solving, goal setting, self-monitoring, and relapse-prevention skills. The System Change intervention was based on process improvement techniques and emphasized restructuring family daily routines (systems) to establish new healthy living habits. Participants were taught to use a series of small, family self-designed experiments to design new routines. Families also charted their daily routines associated with home, school, and work and used a storyboard to track their family change processes. Descriptions of and distinctions between the 2 experimental interventions are described in detail elsewhere.22,27
In the 3-year interventions, both experimental interventions focused on the same healthy living behaviors (diet, physical activity, sedentary activity, sleep, and stress management). The intervention modes of delivery were the same across the 2 interventions, consisting of small group sessions of 12 to 15 families who met in 25 face-to-face sessions in Year 1, alternating monthly face-to-face group and individualized telephone sessions in Year 2, and 4 face-to-face and 8 telephone sessions in Year 3. Each intervention session was delivered by 2 trained interventionists (1 man and 1 woman), at least 1 of whom was of minority race and/or ethnicity. The interventionists were generally school teachers or recreation center personnel who were independently contracted for this role and were trained by using a structured protocol. All intervention materials and curricula for both parent and child were developed at the fifth-grade reading level. All didactic sessions (group and telephone coaching) were audiotaped, and 10% were randomly selected for review of fidelity of content delivery.
Interventions were tailored for adolescent participants in both intervention arms by using a responsive intervention design28,29 in which a set of tailoring variables and decision rules for their application were specified a priori. In this responsive intervention protocol, adolescents received up to 60 minutes of personal coaching each month of the study in addition to the usual standard intervention if they met any of the following 4 criteria: identified as a binge eater, morbidly obese at baseline (>99.5 BMI percentile), low parent and/or family involvement (adolescent attending >50% of sessions alone without a parent), or excessive weight gain during the study (>2 lb per month in a 3-month period resulting in an increase in BMI).
Control Group
A control group of brief education and social interaction only comprised the third study arm. Parent and child participants in this arm received 1 hour of private coaching from a registered dietitian on healthy eating and physical activity in Year 1 as well as a social telephone call and social event in all study years to enhance study retention.
Data Collection and Measurements
Participant outcome assessment data were collected at baseline and annually for 3 years by trained and certified personnel at the Clinical Research Units at 2 Cleveland hospital systems and, in some cases, in the children’s schools. Written informed consent and assent were obtained from the parent and child, respectively, before any data collection. Interview-based data were obtained in private interviews by using audio-assisted survey software in English and Spanish. All measurements were collected by masked, certified staff who were not involved in the intervention. Descriptions of all data collection methods and measures are described in detail elsewhere22 and in the Supplemental Information.
Primary Outcomes
BMI was the primary outcome and was calculated as weight in kilograms divided by the square of the height in meters. Height was measured to the nearest 0.1 cm by using a wall-mounted Harpenden Stadiometer. Weight was measured to the nearest 0.1 kg. Measures were collected in duplicate and averaged.
Secondary Outcomes
Secondary outcomes measured in the adolescent participants included waist circumference, tricep skinfold thickness, dietary intake (three 24-hour dietary recalls using the Nutrition Data System for Research30,31 software to calculate daily intake of calories, percentage of calories from fat, number of fruits and vegetables, and sodium), physical activity (using ActiGraph GT3X+ monitors), sleep, fitness, BP, and a set of cardiometabolic variables: fasting blood glucose, insulin resistance (homeostatic model assessment for insulin resistance), hemoglobin A1c, C-reactive protein, total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides.
Exposure to an Existing School-Based Fitness Program
The We Run This City (WRTC) Youth Marathon program32 is an existing school-based fitness program offered by the local YMCA in >30 Cleveland schools each year (one-third of study participants’ schools). Thus, an opportunity presented itself to assess the effect of this school fitness program on BMI in addition to the effects of the IMPACT interventions. In WRTC, students join teams led by school personnel and train for ∼14 weeks. Study-supported navigators (masked to study-arm assignment) encouraged IMPACT participants attending WRTC schools to enroll and stay active in the program. Participants were documented as being exposed to the WRTC program if they enrolled in the program and attended at least 1 training session (yes or no).
Demographic Characteristics
Self-reports by parents provided information on parent education, marital status, household income, number of people living in the household, employment status, food-program participation, and access to a vehicle. Standardized surveys were used to measure depressive symptoms and food security. Census-tract poverty level, unemployment, and crime rates were assessed along with the number of supermarkets and small grocery stores within one-half mile of the participant’s home. Throughout the study, residential and school changes were documented (see the Supplemental Information for details).
Statistical Analyses
Our primary hypothesis was that over a 3-year period, both System Change and Healthy Change would have greater impact on BMI slope than the education-alone control after adjusting for BMI at baseline and a random effect to account for clustering by school (at study enrollment). A BMI slope (trajectory over 3 years) was created for each participant. An F test with 2 degrees of freedom was used to test for between-group differences by using an α of .05. An a priori power analysis indicated that a minimum of 288 subjects (96 per arm) would provide 94% power to detect an effect size as small as 5% (considered a clinically significant effect).33 The primary outcome analysis was replicated by the coordinating center.
Secondary outcomes were analyzed by using the same approach used for the primary outcomes. All secondary outcomes were analyzed in the continuous form as slopes. Additional planned analyses using BMI slope as the outcome examined effect modification of study arms by sex, race and/or ethnicity, household income, baseline level of child and parent obesity, and exposure to the WRTC school fitness program.
Missing Data and Imputation Process
Our intent-to-treat analysis included all index children. Interpolation and imputation were used in the calculation of slopes for BMI and the secondary outcome variables. Imputation was conducted for participants missing all BMI follow-up data by using 13 prespecified imputation variables and 1000 imputations. For the BMI measures available at multiple assessment points, we interpolated slopes. For all secondary outcomes, 100 runs were used for the computation of slopes.
Results
Sample Characteristics
Table 1 provides a description of the study sample’s baseline characteristics. There were no differences across study groups. Participants were primarily African Americans living in single-parent households with low household incomes. A high percentage of the parents and children were obese at baseline. One-quarter of the families reported no access to a car, and nearly 36% lacked a grocery store within one-half mile of their home. The neighborhood crime rates were 3 times that of the larger county rate. The children experienced a high rate of residential and school changes during the 3-year study (Table 2). Approximately 35% of the study participants were enrolled in a school that offered the WRTC school-based fitness program, of whom, on average, 50% were enrolled in the program.
TABLE 1.
Baseline Characteristics by Study Group
| Total (n = 360) | Control (n = 119) | Healthy Change (n = 118) | System Change (n = 123) | |
|---|---|---|---|---|
| Child female sex, No. (%) | 208 (57.8) | 69 (58.0) | 66 (55.9) | 73 (59.3) |
| Child’s age, y, mean (SD) | 11.6 (0.6) | 11.6 (0.6) | 11.6 (0.6) | 11.5 (0.6) |
| Child’s race and/or ethnicity, No. (%) | ||||
| Non-Hispanic white | 14 (3.9) | 5 (4.2) | 4 (3.4) | 5 (4.1) |
| Non-Hispanic African American | 276 (76.7) | 93 (78.2) | 91 (77.1) | 92 (74.8) |
| Hispanic | 59 (16.4) | 18 (15.1) | 17 (14.4) | 24 (19.5) |
| Multiracial | 8 (2.2) | 2 (1.7) | 5 (4.2) | 1 (0.8) |
| Other | 3 (0.8) | 1 (0.8) | 1 (0.8) | 1 (0.8) |
| Child’s pubertal status (yes), No. (%) | 210 (61.4) | 66 (59.5) | 66 (57.9) | 78 (66.7) |
| Child’s wt, kg, mean (SD) | 64.4 (14.3) | 63.8 (15.0) | 64.6 (12.9) | 65.0 (14.8) |
| Child’s height, cm, mean (SD) | 153.7 (7.6) | 153.6 (7.4) | 153.8 (8.1) | 153.7 (7.4) |
| Child’s BMI, mean (SD) | 27.1 (4.9) | 26.8 (4.7) | 27.3 (4.8) | 27.4 (5.1) |
| Child’s BMI percentile, mean (SD) | 95.7 (3.7) | 95.5 (4.0) | 95.8 (3.6) | 95.8 (3.6) |
| Child’s BMI category, No. (%) | ||||
| Overweight | 117 (32.5) | 38 (31.9) | 37 (31.4) | 42 (34.1) |
| Obese | 243 (67.5) | 81 (68.1) | 81 (68.6) | 81 (65.9) |
| Waist-to-height ratio, mean (SD) | 0.6 (0.1) | 0.6 (0.1) | 0.6 (0.1) | 0.6 (0.1) |
| Body fat, %, mean (SD) | 38.5 (5.8) | 38.0 (5.5) | 38.8 (5.9) | 38.6 (5.9) |
| Child’s systolic BP, mm Hg, mean (SD) | 108.1 (8.3) | 108.3 (8.6) | 108.1 (7.7) | 108.1 (8.6) |
| Child’s diastolic BP, mm Hg, mean (SD) | 63.9 (6.9) | 63.6 (7.2) | 64.2 (6.3) | 63.8 (7.1) |
| Child’s BP category, No. (%) | ||||
| Normal | 326 (90.6) | 108 (90.8) | 109 (92.4) | 109 (88.6) |
| Prehypertensive | 20 (5.6) | 5 (4.2) | 6 (5.1) | 9 (7.3) |
| Hypertensive | 14 (3.8) | 6 (5.0) | 3 (2.5) | 5 (4.1) |
| Child depressive symptoms, mean (SD) | 16.0 (10.6) | 15.1 (10.3) | 16.5 (10.8) | 16.5 (10.7) |
| Parent’s BMI, mean (SD) | 35.7 (8.8) | 36.3 (9.0) | 35.6 (9.1) | 35.2 (8.3) |
| Parent’s BMI category, No. (%) | ||||
| Underweight (<18.5) | 3 (0.9) | 2 (1.8) | 1 (0.9) | 0 (0.0) |
| Normal wt (18.5–24.9) | 31 (9.0) | 8 (7.1) | 12 (10.5) | 11 (9.4) |
| Overweight (25.0–29.9) | 60 (17.5) | 17 (15.2) | 20 (17.5) | 23 (19.7) |
| Obese (>30) | 249 (72.6) | 85 (75.9) | 81 (71.1) | 83 (70.9) |
| People living in household, mean (SD) | ||||
| Total | 4.5 (1.5) | 4.5 (1.6) | 4.3 (1.3) | 4.5 (1.6) |
| Adults | 1.6 (0.7) | 1.6 (0.7) | 1.5 (0.6) | 1.6 (0.7) |
| Children | 2.8 (1.4) | 2.9 (1.5) | 2.8 (1.2) | 2.9 (1.5) |
| Parent’s education level, No. (%) | ||||
| No high school diploma | 65 (18.0) | 23 (19.3) | 17 (14.4) | 25 (20.3) |
| High school diploma | 101 (28.1) | 31 (26.1) | 38 (32.2) | 32 (26.0) |
| Some college, technical training, or associate’s degree | 162 (45.0) | 53 (44.5) | 54 (45.8) | 55 (44.7) |
| Bachelor’s degree or more | 32 (8.9) | 12 (10.1) | 9 (7.6) | 11 (8.9) |
| Parent marital status (single), No. (%) | 239 (66.8) | 84 (70.6) | 81 (69.2) | 74 (60.7) |
| Parent employment status, No. (%) | ||||
| Working full-time | 136 (37.8) | 48 (40.3) | 44 (37.3) | 44 (35.8) |
| Working part-time | 64 (17.8) | 22 (18.5) | 18 (15.3) | 24 (19.5) |
| Not working for pay | 160 (44.4) | 49 (41.2) | 56 (47.5) | 55 (44.7) |
| Annual household income, $, No. (%) | ||||
| ≤14 999 | 105 (29.2) | 34 (28.6) | 33 (28.0) | 38 (30.9) |
| 15 000–24 999 | 71 (19.7) | 22 (18.5) | 26 (22.0) | 23 (18.7) |
| 25 000–34 999 | 47 (13.1) | 16 (13.4) | 13 (11.0) | 18 (14.6) |
| 35 000–49 999 | 36 (10.0) | 12 (10.1) | 15 (12.7) | 9 (7.3) |
| 50 000–74 999 | 25 (6.9) | 10 (8.4) | 8 (6.8) | 7 (5.7) |
| ≥75 000 | 10 (2.8) | 4 (3.3) | 3 (2.5) | 3 (2.4) |
| Prefer not to answer | 20 (5.6) | 5 (4.2) | 6 (5.1) | 9 (7.3) |
| Do not know | 46 (12.8) | 16 (13.4) | 14 (11.9) | 16 (13.0) |
| Food assistance via WIC, No. (%) | 58 (16.1) | 20 (16.8) | 18 (15.3) | 20 (16.3) |
| Food assistance via SNAP, No. (%) | 254 (70.6) | 81 (68.1) | 88 (74.6) | 85 (69.1) |
| Child’s food security, No. (%) | ||||
| Secure | 238 (68.0) | 80 (69.6) | 82 (71.3) | 76 (63.3) |
| Insecure, no hunger | 102 (29.1) | 33 (28.7) | 28 (24.3) | 41 (34.2) |
| Insecure, with hunger | 10 (2.9) | 2 (1.7) | 5 (4.3) | 3 (2.5) |
| Access to a car (no), No. (%) | 89 (24.7) | 24 (20.2) | 33 (28.0) | 32 (26.0) |
| Neighborhood factorsa | ||||
| In poverty, %, mean (SD) | 37.4 (15.9) | 35.8 (15.6) | 37.8 (16.8) | 38.5 (15.3) |
| Unemployed, %, mean (SD) | 22.7 (10.2) | 22.5 (10.2) | 23.0 (10.4) | 22.5 (10.0) |
| Violent crime rate (per 100 000),b mean (SD) | 1873.9 (964.4) | 1835.1 (938.4) | 1916.6 (905.8) | 1869.2 (1044.6) |
| Participants without a full service or small grocery within one-half mile of home, No. (%) | 114 (35.5) | 40 (38.8) | 36 (34.3) | 38 (33.6) |
SNAP, Supplemental Nutrition Assistance Program; WIC, Women, Infants, and Children program.
Neighborhood indicators are reported at the census-tract level.
For comparison, the violent crime rate (per 100 000) for the county is 560.5 per 100 000.
TABLE 2.
Residential and School Changes Reported by Participants
| Subjects, % | ||||
|---|---|---|---|---|
| Total (n = 360) | Control (n = 119) | Healthy Change (n = 118) | System Change (n = 123) | |
| Year 1 | ||||
| Changed schools | 32.1 | 30.5 | 31.6 | 34.2 |
| Changed residence | 24.6 | 28.0 | 18.8 | 26.9 |
| Year 2 | ||||
| Changed schools | 29.2 | 28.8 | 27.2 | 31.6 |
| Changed residence | 25.7 | 25.0 | 25.0 | 27.1 |
| Year 3 | ||||
| Changed schoolsa | 52.3 | 50.0 | 55.8 | 51.3 |
| Changed residence | 25.2 | 27.0 | 23.4 | 25.2 |
| Total (years 1–3)b | ||||
| Did not change schools or residence outside of HS transition | 34.7 | 34.2 | 37.5 | 32.5 |
| Changed residence but not schools | 19.7 | 23.9 | 17.0 | 17.9 |
| Changed schools but not residence | 16.5 | 14.5 | 17.9 | 17.1 |
| Changed schools and residences at least once | 29.2 | 27.4 | 27.7 | 32.5 |
HS, high school.
A significant portion of students transitioned from kindergarten through eighth grade to high school by the time of the third-year assessment. All other changes in schools were within kindergarten–through–eighth-grade schools.
Any participant who experienced 1 move or school change in each. Overall school change was limited to those who experienced school change in the first 2 years.
Study Retention and Intervention Participation
Participant retention for measurement of BMI was 91.9%, 93.3%, and 91.9% in years 1, 2 and 3, respectively. Of the 360 enrolled participants, 14 had BMI available only at baseline and had a BMI slope imputed. Intervention participation was measured as the number of intervention contacts and the number of hours of intervention exposure over 3 years. Intervention participation rates were not statistically different between the 2 intervention arms. Rates of intervention participation averaged 58% with a mean intervention exposure time of 22 hours (range = 0–56). Forty-three percent (n = 103) of the adolescents in the intervention arms of the study qualified to receive the extra coaching sessions, in which tailored information was provided for the following components: binge eating (51%), excess weight gain (49%), morbid obesity (16%), and lack of parent and/or guardian involvement (2%). These participants received an average of 1.4 hours (SD = 1.1; range = 0.25–5.25) of additional intervention exposure. Fidelity assessments of the intervention content delivery indicated a 99% compliance with the intervention protocols.
Primary and Secondary Outcomes
Results showed that over the 3-year study, there were no significant differences in BMI among the 3 study groups. As shown in Table 3, no differences were found in the adjusted BMI slopes between either the Healthy Change and the control groups or the System Change and the control groups. BMI increased over time with unadjusted annual estimates of 0.95 in the education-only control group and 0.82 and 1.08 in the Healthy Change and the System Change groups, respectively. The primary outcome was null, and the adjusted differences in annual BMI change (slope) were small (<0.2). Also shown in Table 3, no differences were found between the experimental groups and control group in any of the other anthropometric variables studied or secondary outcomes: diet, physical activity, sleep, perceived stress, or cardiometabolic factors. Using BMI slope as the outcome, we found no evidence of effect modification of the study arms by sex, race and/or ethnicity, household income, and baseline levels of child and parent obesity. Analysis of the effects of exposure to the school fitness program also yielded null direct and indirect effects on BMI slope.
TABLE 3.
Annualized Changes in Key Outcome Variables and Adjusted Differences in Annualized Change Between the Healthy Change or System Change Group and the Education-Only Controls
| Variable | Unadjusted Annualized Change,a Mean (SD) | HC or SC Versus Education-Only, P | Adjusted Difference in Annualized Change (95% CI) | |||
|---|---|---|---|---|---|---|
| Education Only | HC | SC | F test | HC Versus Education-Only | SC Versus Education-Only | |
| Primary outcome: BMI | 0.952 (1.318) | 0.821 (1.363) | 1.083 (1.129) | 0.35 | −0.132 (−0.390 to 0.126) | 0.137 (−0.071 to 0.346) |
| Secondary outcomes | ||||||
| Anthropometric | ||||||
| BMI percentile | −1.101 (3.206) | −1.363 (3.836) | −0.260 (1.555) | 0.11 | −0.309 (−1.034 to 0.416) | 0.785 (0.497 to 1.072) |
| Waist-to-height ratio | −0.001 (0.020) | −0.003 (0.023) | 0.002 (0.017) | 0.34 | −0.003 (−0.007 to 0.002) | 0.002 (−0.001 to 0.005) |
| Waist circumference | 2.200 (3.288) | 1.814 (3.584) | 2.339 (2.839) | 0.58 | −0.368 (−1.045 to 0.309) | 0.134 (−0.393 to 0.661) |
| Body fat (Stevens equation), % | −0.044 (2.170) | −0.246 (2.376) | 0.299 (1.889) | 0.39 | −0.217 (−0.672 to 0.238) | 0.335 (−0.019 to 0.689) |
| BP, mm Hg | ||||||
| Systolic | 0.886 (2.787) | 1.245 (3.319) | 1.130 (3.075) | 0.51 | 0.301 (−0.326 to 0.928) | 0.184 (−0.387 to 0.755) |
| Diastolic | −0.162 (2.378) | −0.136 (2.796) | −0.074 (2.726) | 0.65 | 0.069 (−0.459 to 0.598) | −0.038 (−0.544 to 0.469) |
| Systolic percentile | −2.028 (8.095) | −1.450 (9.206) | −1.053 (8.801) | 0.60 | 0.325 (−1.415 to 2.065) | 0.607 (−1.027 to 2.241) |
| Diastolic percentile | −2.132 (6.645) | −2.335 (8.228) | −2.044 (8.063) | 0.72 | −0.087 (−1.642 to 1.468) | −0.347 (−1.844 to 1.150) |
| Diet | ||||||
| Calories per d | 24.03 (250.44) | 12.49 (226.65) | 6.99 (211.04) | 0.34 | −26.584 (−70.613 to 17.445) | −11.781 (−51.486 to 27.925) |
| Calories from fat, % | −0.018 (2.601) | 0.139 (2.905) | 0.050 (2.589) | 0.55 | 0.039 (−0.526 to 0.603) | 0.138 (−0.349 to 0.625) |
| No. fruit servings per d | −0.055 (0.360) | −0.061 (0.239) | −0.050 (0.234) | 0.38 | −0.010 (−0.056 to 0.037) | −0.028 (−0.072 to 0.016) |
| No. vegetable servings per d | −0.008 (0.253) | 0.013 (0.276) | 0.008 (0.263) | 0.14 | 0.020 (−0.034 to 0.073) | 0.016 (−0.033 to 0.066) |
| Sodium intake per d, mg | 39.15 (446.28) | −0.42 (425.7 9) | 26.46 (409.34) | 0.32 | −30.046 (−112.759 to 52.667) | 32.241 (−44.774 to 109.257) |
| Physical activity | ||||||
| Moderate or vigorous, min per d | −3.523 (8.493) | −3.370 (10.123) | −4.619 (7.486) | 0.346 | 0.050 (−1.964 to 2.065) | −0.788 (−2.249 to 0.673) |
| Bed rest or sedentary, min per d | 11.022 (16.961) | 10.501 (16.365) | 13.052 (14.561) | 0.62 | −0.066 (−3.322 to 3.191) | −1.099 (−3.941 to 1.743) |
| Wear time in moderate-to-vigorous activity, % | −0.481 (1.149) | −0.426 (1.319) | −0.609 (0.986) | 0.167 | 0.054 (−0.209 to 0.316) | −0.087 (−0.280 to 0.105) |
| Wear time in bed rest or sedentary activities, % | 1.638 (2.231) | 1.610 (2.228) | 1.900 (2.059) | 0.53 | −0.033 (−0.476 to 0.411) | 0.232 (−0.169 to 0.634) |
| Sleep | ||||||
| Adolescent sleep, wake | −0.273 (2.089) | −0.239 (2.350) | −0.361 (2.618) | 0.10 | −0.019 (−0.465 to 0.427) | 0.284 (−0.204 to 0.772) |
| Daytime sleepiness | −1.184 (7.749) | −1.061 (8.937) | −1.552 (9.755) | 0.33 | 0.158 (−1.539 to 1.855) | 1.029 (−0.791 to 2.848) |
| Sleep on weekends, min | −13.101 (65.529) | −9.032 (51.553) | −4.474 (48.285) | 0.72 | 0.695 (−9.047 to 10.438) | −0.638 (−9.643 to 8.367) |
| Sleep on weekdays, min | −21.034 (35.040) | −19.867 (39.697) | −14.146 (31.819) | 0.62 | 0.231 (−7.271 to 7.733) | 2.949 (−2.985 to 8.883) |
| Stress | ||||||
| Child’s perceived stress | −0.044 (2.489) | 0.311 (2.584) | −0.040 (2.744) | 0.53 | 0.075 (−0.420 to 0.570) | 0.588 (0.076 to 1.100) |
| Fitness | ||||||
| Resting pulse rate | −1.392 (3.713) | −1.139 (3.312) | −1.279 (3.280) | 0.33 | 0.047 (−0.579 to 0.672) | 0.099 (−0.510 to 0.708) |
| Shuttle-run laps | 1.744 (3.498) | 1.126 (2.639) | 0.906 (2.155) | 0.10 | −0.573 (−1.085 to 0.060) | −0.742 (−1.155 to −0.329) |
| Cardiometabolic factors | ||||||
| Fasting glucose, mg/dL | −0.490 (3.135) | −0.146 (2.864) | −0.520 (3.805) | 0.90 | 0.116 (−0.383 to 0.615) | 0.155 (0.497 to 0.807) |
| Hemoglobin A1c, % | −0.017 (0.093) | −0.040 (0.210) | −0.021 (0.304) | 0.45 | −0.027 (−0.075 to 0.021) | 0.018 (−0.052 to 0.088) |
| HDL cholesterol, mg/dL | −0.385 (2.906) | −0.3 (3.433) | −0.6 (2.526) | 0.58 | 0.183 (−0.364 to 0.730) | −0.237 (−0.698 to 0.224) |
| High-sensitivity C-reactive protein, mg/L | −0.0185 (0.123) | −0.013 (0.163) | −0.002 (0.141) | 0.73 | 0.003 (−0.032 to 0.038) | 0.017 (−0.009 to 0.043) |
| Insulin, μU/mL | 0.228 (4.568) | 0.772 (4.761) | 0.406 (6.875) | 0.44 | 0.065 (−0.898 to 1.028) | 0.510 (0.868 to 1.888) |
| HOMA-IR | 0.004 (1.009) | 0.032 (1.003) | 0.037 (1.281) | 0.55 | −0.000 (−0.229 to 0.228) | 0.063 (−0.210 to 0.336) |
| LDL cholesterol (derived), mg/dL | −2.357 (6.645) | −1.449 (7.915) | −1.970 (7.295) | 0.439 | 1.384 (−0.053 to 2.821) | 0.486 (−0.666 to 1.638) |
| Total cholesterol, mg/dL | −2.94 (7.201) | −2.409 (9.500) | −3.651 (8.280) | 0.73 | 0.985 (−0.612 to 2.582) | −0.342 (−1.678 to 0.994) |
| Triglycerides, mg/dL | −1.516 (13.910) | −3.280 (17.847) | −5.054 (17.573) | 0.56 | −2.585 (−5.832 to −0.662) | −2.538 (−5.672 to 0.596) |
| Alanine aminotransferase, U/L | −0.775 (3.603) | −1.875 (5.115) | −0.811 (3.055) | 0.46 | −0.601 (−1.741 to 0.539) | 0.049 (−0.553 to 0.651) |
HC, Healthy Change group; HOMA-IR, homeostatic model assessment for insulin resistance; SC, System Change group.
Analyses controlled for baseline BMI and school clustering at randomization. With the exception of the cardiometabolic factors involving blood samples, the sample described here, before imputation for most variables, includes 346 subjects, including 117 education-only, 112 Healthy Change, and 117 System Change subjects. Total enrollment was 360. At least 344 subjects are included in each row for the anthropometric and BP variables. At least 316 subjects are included for the cardiometabolic variables.
Discussion
Neither of the long-term family interventions tested in this randomized trial improved BMI or weight- and health-related secondary outcomes, nor was there any effect of exposure to the school fitness program. Although the study findings are disappointing, we believe them to be robust because of the considerable strengths of this randomized controlled trial. These strengths include the use of theory-based interventions, a lengthy follow-up period, and excellent study retention. The study was also strengthened by investigator participation in the larger COPTR and an independent coordinating center that facilitated strong quality control of data collection, management, and analyses. Limitations of the study include a moderate level of intervention participation and generalizability restricted to a low-resource, minority, urban population.
Several factors may have contributed to these null findings. Families in the study led challenging lives, which may have interfered with their ability to act on the healthy living information and behavior change techniques taught in the interventions. As shown in Table 1, many lived in high-poverty, sometimes violent, and economically depressed neighborhoods. As examples, during the study, 1 child participant was shot during a drive-by shooting, and several participants were friends of a youth killed in a high-profile police shooting. The disrupted lives of the participants were further indicated by the frequent residence changes of the families; nearly half of the study families changed residences at least once during the 3-year study, and 10% moved 3 or more times. We also found that >30% of children reported being food insecure, and most lived in neighborhoods with limited healthy food options.
It is possible that the lack of effect of the interventions tested was due to the high adolescent baseline BMIs (mean: 95.5 percentile), suggesting that the weight-management behaviors taught in the interventions required a considerable lifestyle behavior change. Also, 76% of the parents were obese, possibly contributing to the lack of effect of our interventions, and nearly one-quarter of parents of children with obesity (>95th percentile) did not believe their child’s weight was a concern at baseline. Although the interventions used in this study were codesigned with families before the trial34 and an intervention-tailoring protocol was included in the interventions, it is possible that more precise tailoring to specific family factors and environmental contexts is needed. Lastly, although the interventions were over a considerable period of time (3 years) and at a dose consistent with recommendations,35 the timing and dose of childhood obesity interventions have yet to be determined.
Future research should include analyses of stratified subgroups, such as socioeconomic status, sex, age, or race and/or ethnicity. This would provide greater insight into how different groups may respond differently to the same intervention and help tailor future interventions to maximize their benefits. More attention to perceptions of obesity and motivation to make lifestyle changes could be useful before the introduction of other intervention components.
Lessons learned in this study that are relevant to practitioners include the following: (1) education alone is not sufficient to change lifestyle behaviors related to weight management in children; and (2) interventions for low-income, urban-dwelling, minority families likely need to be tailored specifically to the family’s personal and environmental characteristics, and referral of families to weight-management programs that provide this personalized, tailored approach is important for sustained lifestyle change.
The null findings from this study are consistent with those of other major trials addressing childhood obesity in low-resourced, minority populations,14,36,37 including those of the other recently completed COPTR studies.38,39 This suggests that new approaches are needed to discover effective childhood obesity prevention and treatment interventions for this population. In addition to greater understanding of assisting behavior change that is tailored to specific populations, more research is needed that addresses underlying issues such as poverty and other social determinants of health-promoting behaviors. This may entail more policy-related and whole-community research. Additionally, the effect of biological influences was not taken into account in this study, such as microbiome, metabolic, and genetic influences on body weight.40,41
Conclusions
In this randomized controlled trial, we found no effect of 2 interventions to improve BMI in low-income, urban youth. Family, school, peers, community, and policy provide environmental contexts that shape children’s energy intake and expenditure and therefore together influence the development of obesity and its comorbidities. Viewing childhood obesity from this socioecological perspective will assist in developing interventions that are likely to be more powerful in reducing obesity in low-income, urban youth.
Acknowledgments
We acknowledge the conceptual and leadership contributions to the IMPACT project of Leona Cuttler. We also acknowledge the important contributions of Carolyn Ievers-Landis, Eileen Seeholzer, Julie Hewitt, Ryan Kofron, Barbara Clint, Tara Taylor, Desiree Powell, and Deborah Aloshen. Two major community partners, the Cleveland Metropolitan School District and the YMCA of Greater Cleveland, also provided invaluable support to the IMPACT study.
Glossary
- BP
blood pressure
- COPTR
Childhood Obesity Prevention and Treatment Research Consortium
- HDL
high-density lipoprotein
- IMPACT
Ideas Moving Parents and Adolescents to Change Together
- LDL
low-density lipoprotein
- WRTC
We Run This City
Footnotes
Drs Moore and Borawski conceptualized and designed the study, supervised the conduction of the study and the analysis of results, and wrote the initial draft of the manuscript; Drs Love and Stevens and Mr Thomas designed the analysis plan and conducted, interpreted, and wrote the analysis of the study results; Ms Casey, Ms McAleer, and Drs Uli and Plow designed the data collection instruments for the study, coordinated and supervised data collection, and reviewed and revised the manuscript; Dr Truesdale, Ms Jones, and Mr Long designed and coordinated the data management systems and reviewed and revised the manuscript; Ms Clara Adegbite-Adeniyi and Dr Trapl designed and supervised the interventions and their delivery and reviewed and revised the manuscript; Drs Hardin and Pratt and Ms Nevar assisted in the conceptualization, drafting, and reviews and revisions of the manuscript; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute; the Eunice Kennedy Shriver National Institute of Child Health and Human Development, or the National Institutes of Health. Deidentified individual participant data will not be made available at this time because this project is part of a National Institutes of Health multiple-site project initiative. Data and documents for data sharing are currently being developed.
This trial has been registered at www.clinicaltrials.gov (identifier NCT01514279).
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by the National Heart, Lung, and Blood Institute; the Eunice Kennedy Shriver National Institute of Child Health and Human Development; and the National Institutes of Health Office of Behavioral and Social Sciences Research (grants U01 HL103622 and U01 HL 103561). The collection of environmental data used in this article was supported by the Centers for Disease Control and Prevention (grant U48DP005030; Prevention Research Centers Program). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
COMPANION PAPER: A companion to this article can be found online at www.pediatrics.org/cgi/doi/10.1542/peds.2019-0839.
References
- 1.Ogden CL, Carroll MD, Lawman HG, et al. Trends in obesity prevalence among children and adolescents in the United States, 1988-1994 through 2013-2014. JAMA. 2016;315(21):2292–2299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Flegal KM. Prevalence of obesity in the United States. JAMA. 2014;312(2):189–190 [DOI] [PubMed] [Google Scholar]
- 3.Rogers R, Eagle TF, Sheetz A, et al. The relationship between childhood obesity, low socioeconomic status, and race/ethnicity: lessons from Massachusetts. Child Obes. 2015;11(6):691–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watts AW, Mason SM, Loth K, Larson N, Neumark-Sztainer D. Socioeconomic differences in overweight and weight-related behaviors across adolescence and young adulthood: 10-year longitudinal findings from Project EAT. Prev Med. 2016;87:194–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelsey MM, Zaepfel A, Bjornstad P, Nadeau KJ. Age-related consequences of childhood obesity. Gerontology. 2014;60(3):222–228 [DOI] [PubMed] [Google Scholar]
- 6.Kim DD, Basu A. Estimating the medical care costs of obesity in the United States: systematic review, meta-analysis, and empirical analysis. Value Health. 2016;19(5):602–613 [DOI] [PubMed] [Google Scholar]
- 7.Pratt CA, Loria CM, Arteaga SS, et al. A systematic review of obesity disparities research. Am J Prev Med. 2017;53(1):113–122 [DOI] [PubMed] [Google Scholar]
- 8.Bleich SN, Segal J, Wu Y, Wilson R, Wang Y. Systematic review of community-based childhood obesity prevention studies. Pediatrics. 2013;132(1). Available at: www.pediatrics.org/cgi/content/full/132/1/e201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Cai L, Wu Y, et al. What childhood obesity prevention programmes work? A systematic review and meta-analysis. Obes Rev. 2015;16(7):547–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Norman G, Huang J, Davila EP, et al. Outcomes of a 1-year randomized controlled trial to evaluate a behavioral ‘stepped-down’ weight loss intervention for adolescent patients with obesity. Pediatr Obes. 2016;11(1):18–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beauchamp A, Backholer K, Magliano D, Peeters A. The effect of obesity prevention interventions according to socioeconomic position: a systematic review. Obes Rev. 2014;15(7):541–554 [DOI] [PubMed] [Google Scholar]
- 12.Patel MR, McGuire DK. Pounds of prevention: obesity therapy. Am Heart J. 2001;142(3):388–390 [DOI] [PubMed] [Google Scholar]
- 13.Robinson LE, Webster EK, Whitt-Glover MC, Ceaser TG, Alhassan S. Effectiveness of pre-school- and school-based interventions to impact weight-related behaviours in African American children and youth: a literature review. Obes Rev. 2014;15(suppl 4):5–25 [DOI] [PubMed] [Google Scholar]
- 14.Robinson TN, Matheson DM, Kraemer HC, et al. A randomized controlled trial of culturally tailored dance and reducing screen time to prevent weight gain in low-income African American girls: Stanford GEMS. Arch Pediatr Adolesc Med. 2010;164(11):995–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klesges RC, Obarzanek E, Kumanyika S, et al. The Memphis Girls’ Health Enrichment Multi-site Studies (GEMS): an evaluation of the efficacy of a 2-year obesity prevention program in African American girls. Arch Pediatr Adolesc Med. 2010;164(11):1007–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwarteng JL, Schulz AJ, Mentz GB, Israel BA, Perkins DW. Independent effects of neighborhood poverty and psychosocial stress on obesity over time. J Urban Health. 2017;94(6):791–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dubowitz T, Zenk SN, Ghosh-Dastidar B, et al. Healthy food access for urban food desert residents: examination of the food environment, food purchasing practices, diet and BMI. Public Health Nutr. 2015;18(12):2220–2230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bushnik T, Garriguet D, Colley R. Parent-Child association in body weight status. Health Rep. 2017;28(6):12–19 [PubMed] [Google Scholar]
- 19.Berge JM, Wall M, Larson N, Forsyth A, Bauer KW, Neumark-Sztainer D. Youth dietary intake and weight status: Healthful neighborhood food environments enhance the protective role of supportive family home environments. Health Place. 2014;26:69–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopes VP, Gabbard C, Rodrigues LP. Physical activity in adolescents: examining influence of the best friend dyad. J Adolesc Health. 2013;52(6):752–756 [DOI] [PubMed] [Google Scholar]
- 21.Stok FM, de Vet E, de Wit JB, Luszczynska A, Safron M, de Ridder DT. The proof is in the eating: subjective peer norms are associated with adolescents’ eating behaviour. Public Health Nutr. 2015;18(6):1044–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore SM, Borawski EA, Cuttler L, Ievers-Landis CE, Love TE. IMPACT: a multi-level family and school intervention targeting obesity in urban youth. Contemp Clin Trials. 2013;36(2):574–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pratt CA, Boyington J, Esposito L, et al. Childhood Obesity Prevention and Treatment Research (COPTR): interventions addressing multiple influences in childhood and adolescent obesity. Contemp Clin Trials. 2013;36(2):406–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2, suppl 4th report):555–576 [PubMed] [Google Scholar]
- 25.Conlon M, Anderson GC. Three methods of random assignment: comparison of balance achieved on potentially confounding variables. Nurs Res. 1990;39(6):376–379 [PubMed] [Google Scholar]
- 26.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31(1):103–115 [PubMed] [Google Scholar]
- 27.Moore SM, Schiffman R, Waldrop-Valverde D, et al. Recommendations of common data elements to advance the science of self-management of chronic conditions. J Nurs Scholarsh. 2016;48(5):437–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Collins LM, Murphy SA, Bierman KL. A conceptual framework for adaptive preventive interventions. Prev Sci. 2004;5(3):185–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy SA, Collins LM, Rush AJ. Customizing treatment to the patient: adaptive treatment strategies. Drug Alcohol Depend. 2007;88(suppl 2):S1–S3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conway JM, Ingwersen LA, Vinyard BT, Moshfegh AJ. Effectiveness of the US Department of Agriculture 5-step multiple-pass method in assessing food intake in obese and nonobese women. Am J Clin Nutr. 2003;77(5):1171–1178 [DOI] [PubMed] [Google Scholar]
- 31.McPherson RS, Hoelscher DM, Alexander M, Scanlon KS, Serdula MK. Dietary assessment methods among school-aged children: validity and reliability. Prev Med. 2000;31(2):S11–S33 [Google Scholar]
- 32.Borawski EA, Jones SD, Yoder LD, et al. We Run This City: impact of a community–school fitness program on obesity, health, and fitness. Prev Chronic Dis. 2018;15:160471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.US Preventive Services Task Force Final recommendation statement: obesity in children and adolescents: screening. 2017. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/obesity-in-children-and-adolescents-screening1. Accessed June 10, 2018
- 34.Moore SM, Killion CM, Andrisin S, et al. Use of appreciative inquiry to engage parents as codesigners of a weight management intervention for adolescents. Child Obes. 2017;13(3):182–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Connor EA, Evans CV, Burda BU, Walsh ES, Eder M, Lozano P. Screening for obesity and intervention for weight management in children and adolescents: evidence report and systematic review for the us preventive services task force. JAMA. 2017;317(23):2427–2444 [DOI] [PubMed] [Google Scholar]
- 36.Butte NF, Hoelscher DM, Barlow SE, et al. Efficacy of a community- versus primary care-centered program for childhood obesity: TX CORD RCT. Obesity (Silver Spring). 2017;25(9):1584–1593 [DOI] [PubMed] [Google Scholar]
- 37.Woo Baidal JA, Nelson CC, Perkins M, et al. Childhood obesity prevention in the women, infants, and children program: outcomes of the MA-CORD study. Obesity (Silver Spring). 2017;25(7):1167–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barkin SL, Heerman WJ, Sommer EC, et al. Effect of a behavioral intervention for underserved preschool-age children on change in body mass index: a randomized clinical trial. JAMA. 2018;320(5):450–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.French SA, Sherwood NE, Veblen-Mortenson S, et al. Multicomponent obesity prevention intervention in low-income preschoolers: primary and subgroup analyses of the NET-works randomized clinical trial, 2012-2017. Am J Public Health. 2018;108(12):1695–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. JAMA. 2009;301(21):2252–2259 [DOI] [PubMed] [Google Scholar]
- 41.Greiner T, Bäckhed F. Effects of the gut microbiota on obesity and glucose homeostasis. Trends Endocrinol Metab. 2011;22(4):117–123 [DOI] [PubMed] [Google Scholar]