Abstract
Background/Aims
Blue laser imaging (BLI) is a new technique for detailed examination of upper gastrointestinal lesions. This study aimed to evaluate the diagnostic value of BLI combined with magnifying endoscopy for precancerous and early gastric cancer lesions.
Materials and Methods
A total of 249 gastric lesions detected via conventional white light endoscopy (WLE) based on assessments of mucosal shape and color were included in this study. The accuracy of diagnosis of precancerous or early cancer lesions white light magnification alone, BLI-contrast magnification, and BLI-bright magnification was determined according to the VS criteria.
Results
For white light magnification alone, BLI-contrast magnification, and BLI-bright magnification, the concordance rates for lesions were 76.7%, 85.1%, and 86.7%, respectively, and the Kappa values were 0.571, 0.730, and 0.760, respectively. For the screening of high-grade intraepithelial neoplasia or early gastric cancer, the diagnostic sensitivities of white light magnification alone, BLI-contrast magnification, and BLI-bright magnification were 72.0%, 92.0%, and 92.0%, respectively; the specificities were 95.5%, 98.2%, and 99.1%, respectively; the consistencies were 93.2%, 97.6%, and 98.4%, respectively; and the Kappa values were 0.642, 0.871, and 0.911, respectively. For diagnoses of high-grade intraepithelial neoplasia or early gastric cancer, the concordance between endoscopic and pathological diagnosis was significantly higher for BLI-contrast and BLI-bright magnification than for white light magnification alone (p<0.05).
Conclusion
BLI combined with magnifying endoscopy may improve diagnostic accuracy for early gastric cancer and precancerous lesions.
Keywords: Stomach neoplasms, diagnosis, blue laser imaging
INTRODUCTION
Among gastrointestinal cancers, gastric cancer is one of the most malignant and has the highest morbidity and mortality in China. The early identification and diagnosis of gastric cancer can permit endoscopic radical resection, which significantly improves patients’ survival and prognosis. However, because early gastric cancer lacks specific clinical manifestations and signs, it is difficult to accurately diagnose this disease via ordinary endoscopy. Blue laser imaging (BLI) laser endoscopy is a new endoscopic technique that has been used in recent years. This technique uses a laser as the light source. It not only has white light observation functionality but also allows for narrow-band observation using a high-contrast mode (BLI-contrast mode). Moreover, in combination with magnifying endoscopy, this technique’s highlight mode (BLI-bright mode) can clearly show the mucosal microvascular and microtubule structure of a lesion, which can be useful to determine the nature of the lesion and its pathological features. This study aimed to explore the value of BLI combined with magnifying endoscopy in the diagnosis of early gastric cancer and precancerous lesions.
MATERIALS AND METHODS
Materials
After obtaining approval from the ethics committee, 235 patients were screened using normal white light endoscopy (WLE) based on abnormal changes in mucosal morphology or color from September 2015 to May 2017. These patients included 144 males and 91 females aged between 40 and 80 years, with a median age of 56 years. They had 249 focal lesions in total. The major clinical manifestations were abdominal pain, abdominal discomfort, heartburn, and belching. All selected patients signed the relevant informed consent form, and the study was approved by the Human Research Ethics Committee of Renmin Hospital of Wuhan University.
Methods
Endoscopy procedures
An EG-L590ZW electronic magnification endoscope was used in combination with a LASEREO blue laser endoscope system, an LL-4450 laser source, and a VP-4450HD image-processing device. Before each assessment, scopolamine butoxide 20 mg was intravenously administered, and 50 ml of mucus removal solution (chymotrypsin, simethicone, 5% sodium bicarbonate) was orally administered. The apex of the endoscope was fitted with a black rubber cap (MAJ-1990)[FujifilmMedical Co. Ltd., Tokyo, Japan] to fix the focal length from the magnifying endoscope to the gastric mucosal surface at 3 mm. First, color differences in the lesion’s morphology and border as well as between the lesion and the surrounding area were observed for the suspected lesions found using WLE. Then, white light magnification alone, BLI-contrast magnification, and BLI-bright magnification were used. Morphological changes in mucocutaneous microvessels and microvessels were observed. These morphological changes were compared with those in adjacent normal areas, and microglandular and microvascular structures were evaluated. After the completion of the above examination, disposable biopsy forceps were used to biopsy the lesion at four points. The biopsy samples were sent for histopathological examination; lesion sections were stained with HE, and two doctors at our hospital with experience in pathological analysis examined these sections and determined pathological diagnoses. Endoscopic performance of typical cases is shown in Figures 1 to 4.
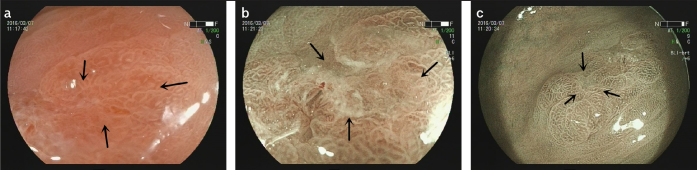
Figure 1. a–c.
The microscope characteristics of high-grade intraepithelial neoplasia lesions in pathological diagnosis.
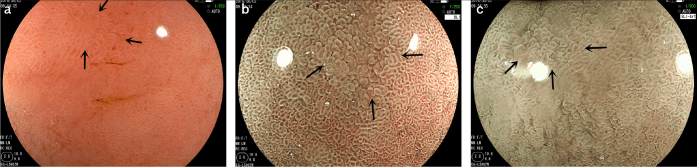
Figure 4. a–c.
The microscope characteristics of chronic gastritis in pathological diagnosis.
Endoscopic image analysis
Endoscopic diagnosis can involve subjectivity; therefore, to avoid potential selectivity bias and improve the quality of this study, relevant endoscopic images for each lesion were randomly selected, separately displayed, and interpreted by four individuals with rich endoscopy-related operating experience and complete knowledge of biopsy pathology. Endoscopic diagnostic results were determined based on the consensus reached after discussion and consultation. With respect to BLI endoscopic diagnostic criteria, lesions can be classified into three types based on microvascular and microtubule morphology: regular, irregular, and absent. In addition, according to Yao et al. (1) and other proposed VS diagnostic criteria, lesions can be categorized based on whether a boundary between the lesion and the surrounding normal mucosa can be clearly recognized. We defined endoscopic cancerous lesions as those with irregular/absent microvascular morphology and a dividing line or those with irregular/absent microtubule morphology and a dividing line.
Pathological diagnosis
All lesion biopsies obtained were immediately placed in 10% formalin solution for fixation, and the two aforementioned experienced gastrointestinal specialist pathologists determined the pathological diagnoses. In this study, according to the revised Vienna criteria for gastric cancer diagnosis (2), high-grade intraepithelial neoplasia (C4) was defined as gastric cancer, and low-grade intraepithelial neoplasia (C3) was defined as a non-cancerous lesion. This study used pathological diagnosis as the gold standard for gastric cancer diagnosis.
Statistical analysis
The SPSS 16.0 statistical software was used to process study data. A Kappa consistency test was used to evaluate consistency between the gold standard of pathological diagnosis and diagnoses obtained using white light alone, BLI-contrast magnification, and BLI-bright magnification. The McNemar paired chi-square test was performed. BLI-contrast magnification, BLI-bright magnification and white light magnification alone were compared with respect to consistency between endoscopic and pathological diagnoses, with P<0.05 used as the threshold for statistical significance.
RESULTS
Clinical features and pathological diagnosis
The 249 focal lesions included 26, 44, and 179 lesions in the gastric cardia, the gastric angle, and the gastric antrum, respectively. With respect to pathological diagnoses, 149, 67, 8, 25, and 0 lesions were diagnosed as chronic gastritis, intestinal metaplasia, low-grade intraepithelial neoplasia, high-grade intraepithelial neoplasia/early gastric cancer, and multiple types of cancer, respectively.
Correlations between white light magnification and pathological findings
In analyses of images obtained using ordinary white light magnification alone, approximately 84.0% (21/25) of the cancerous lesions had irregular or absent microvascular morphology, whereas approximately 79.5% of the non-cancerous lesions (178/224) had regular microvascular morphology. Irregular or absent microtubular morphology was observed for approximately 80.0% (20/25) of the cancerous lesions but only approximately 21.0% (47/224) of the non-cancerous lesions. A clear demarcation line was observed for approximately 76.0% (19/25) of the cancerous lesions, whereas no such line was observed for approximately 91.1% (204/224) of the non-cancerous lesions. These results are shown in Table 1.
Table 1.
Relationship between lesion morphology and pathological diagnosis under different endoscopic magnification methods [%].
| Endoscopic performance | Patients | Pathological diagnosis | |||
|---|---|---|---|---|---|
|
| |||||
| Chronic gastritis (n=149) | Intestinal metaplasia (n=67) | Low-grade intraepithelial neoplasia (n=8) | High-grade Intraepithelial neoplasia or early gastric cancer (n=25) | ||
| White light magnification | |||||
| Microvascular morphology | |||||
| Regular | 182 | 121 (81.2) | 53 (79.1) | 4 (50.0) | 4 (16.0) |
| Irregular/absent | 67 | 28 (18.8) | 14 (20.9) | 4 (50.0) | 21 (84.0) |
| Microtubule morphology | |||||
| Regular | 182 | 118 (81.2) | 54 (80.6) | 5 (62.5) | 5 (20.0) |
| Irregular/absent | 67 | 31 (18.8) | 13 (19.4) | 3 (37.5) | 20 (80.0) |
| Dividing line | |||||
| Exist | 39 | 12 (8.1) | 4 (6.0) | 4 (50.0) | 19 (76.0) |
| Not exist | 210 | 137 (91.9) | 63 (94.0) | 4 (50.0) | 6 (24.0) |
| BLI-contrast magnification | |||||
| Microvascular morphology | |||||
| Regular | 215 | 143 (96.0) | 66 (98.5) | 5 (62.5) | 1 (4.0) |
| Irregular/absent | 34 | 6 (4.0) | 1 (1.5) | 3 (37.5) | 24 (96.0) |
| Microtubule morphology | |||||
| Regular | 215 | 145 (97.3) | 65 (97.0) | 4 (50.0) | 1 (4.0) |
| Irregular/absent | 34 | 4 (2.7) | 2 (3.0) | 4 (50.0) | 24 (96.0) |
| Dividing line | |||||
| Exist | 25 | 0 (0.0) | 0 (0.0) | 2 (25.0) | 23 (92.0) |
| Not exist | 224 | 149 (100.0) | 67 (100.0) | 6 (75.0) | 2 (8.0) |
| BLI-bright magnification | |||||
| Microvascular morphology | |||||
| Regular | 214 | 144 (96.6) | 64 (95.5) | 6 (75.0) | 0 (0.0) |
| Irregular/absent | 35 | 5 (3.4) | 3 (4.5) | 2 (25.0) | 25 (100.0) |
| Microtubule morphology | |||||
| Regular | 215 | 143 (96.0) | 66 (98.5) | 5 (62.5) | 1 (4.0) |
| Irregular/absent | 34 | 6 (4.0) | 1 (1.5) | 3 (37.5) | 24 (96.0) |
| Dividing line | |||||
| Exist | 28 | 0 (0.0) | 0 (0.0) | 3 (37.5) | 25 (100.0) |
| Not exist | 221 | 149 (100.0) | 67 (100.0) | 5 (62.5) | 0 (0.0) |
Correlations between BLI-contrast magnification and pathological findings
Analyses of images obtained using BLI-contrast magnification showed that microvascular morphology was irregular or absent for 96.0% (24/25) of the cancerous lesions but was regular for approximately 95.5% (214/224) of the non-cancerous lesions. Irregular or absent microtubule morphology was found for 96.0% (24/25) of the cancerous lesions but only approximately 4.4% (10/224) of the non-cancerous lesions. A clear demarcation line was present for 92.0% (23/25) of the cancerous lesions but absent for approximately 99.1% (222/224) of the non-cancerous lesions.
Correlations between BLI-bright magnification and pathological findings
Analyses of images obtained using BLI-bright magnification showed that microvascular morphology was irregular or absent for 100.0% (25/25) of the cancerous lesions but was regular for approximately 95.5% (214/224) of the non-cancerous lesions. Irregular or absent microtubule morphology was found for 96.0% (24/25) of the cancerous lesions but only approximately 4.4% (10/224) of the non-cancerous lesions. A clear demarcation line was present for 100.0% (25/25) of the cancerous lesions but absent for approximately 98.7% (221/224) of the non-cancerous lesions.
Evaluating the consistency between pathological diagnoses and diagnoses obtained using BLI combined with magnifying endoscopy
The consistencies of diagnoses determined using white light magnification alone, BLI-contrast magnification, and BLI-bright magnification with the gold standard of pathological diagnosis were 76.7%, 85.1%, and 86.7%, respectively, and the Kappa values were 0.571, 0.730, and 0.760, respectively (all P<0.001). Pathological diagnoses were most consistent with diagnoses based on visible, BLI-contrast magnification and BLI-bright magnification findings but were not strongly consistent with white light magnification-based diagnoses. These results are shown in Table 2.
Table 2.
Comparison of diagnosis between pathology and different endoscopic magnification methods.
| Endoscopic magnification method | Patients | Pathological diagnosis | |||
|---|---|---|---|---|---|
|
| |||||
| Chronic gastritis (n=149) | Intestinal metaplasia (n=67) | Low-grade intraepithelial neoplasia (n=8) | High-grade Intraepithelial neoplasia or early gastric cancer (n=25) | ||
| White light magnification | |||||
| Chronic gastritis | 163 | 132 | 26 | 2 | 3 |
| Intestinal metaplasia | 49 | 9 | 37 | 1 | 2 |
| Low-grade intraepithelial neoplasia | 9 | 3 | 0 | 4 | 2 |
| High-grade intraepithelial neoplasia or early gastric cancer | 28 | 5 | 4 | 1 | 18 |
| BLI-contrast magnification | |||||
| Chronic gastritis | 156 | 138 | 18 | 0 | 0 |
| Intestinal metaplasia | 58 | 7 | 47 | 4 | 0 |
| Low-grade intraepithelial neoplasia | 8 | 2 | 0 | 4 | 2 |
| High-grade intraepithelial neoplasia or early gastric cancer | 27 | 2 | 2 | 0 | 23 |
| BLI-bright magnification | |||||
| Chronic gastritis | 155 | 139 | 16 | 0 | 0 |
| Intestinal metaplasia | 60 | 7 | 50 | 3 | 0 |
| Low-grade intraepithelial neoplasia | 9 | 3 | 0 | 4 | 2 |
| High-grade intraepithelial neoplasia or early gastric cancer | 25 | 0 | 1 | 1 | 23 |
Chronic gastritis, intestinal metaplasia, and low-grade intraepithelial neoplasia were grouped into non-cancerous lesions; and early gastric cancer and high-grade intraepithelial neoplasia were grouped into cancerous lesions. For the screening of gastric cancer, the diagnostic sensitivities of white light magnification alone, BLI-contrast magnification, and BLI-bright magnification were 72.0%, 92.0%, and 92.0%, respectively; the specificities were 95.5%, 98.2%, and 99.1%, respectively; the consistencies were 93.2%, 97.6%, and 98.4%, respectively; and the Kappa values were 0.642, 0.871, and 0.911, respectively. Further evaluations of BLI combined with magnifying endoscopy revealed that BLI-contrast and BLI-bright magnification increased the sensitivity of microscopic diagnoses of cancerous lesions to over 90%, with significantly elevated Kappa values of over 0.80 for microscopic and pathological diagnoses. For the early diagnosis of microscopic cancerous lesions, white light magnification alone had a sensitivity of less than 80% and a Kappa value of approximately 0.642. These results are shown in Table 3. It is evident that compared with white light magnification alone, BLI combined with magnifying endoscopy may significantly improve the consensus between microscopic and pathological diagnoses for early gastric cancer and precancerous lesions.
Table 3.
The consistencies of different endoscopic magnification methods and pathology for diagnosis of gastric cancer.
| Endoscopic methods | Patients | Pathological diagnosis | Sensitivity (%) | Specificity (%) | Consistency (%) | Kappa | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Non-cancerous lesions (n=224) | Cancerous lesions (n=25) | ||||||
| White light magnification | 72.0 | 95.5 | 93.2 | 0.642 | |||
| Non-cancerous lesions | 221 | 214 | 7 | ||||
| Cancerous lesions | 28 | 10 | 18 | ||||
| BLI-contrast magnification | 92.0 | 98.2 | 97.6a | 0.871 | |||
| Non-cancerous lesions | 222 | 220 | 2 | ||||
| Cancerous lesions | 27 | 4 | 23 | ||||
| BLI-bright magnification | 92.0 | 99.1 | 98.4a | 0.911 | |||
| Non-cancerous lesions | 224 | 222 | 2 | ||||
| Cancerous lesions | 25 | 2 | 23 | ||||
Compared with the single magnification of white light,
p<0.05
DISCUSSION
Clinically, the early onset of gastric cancer is insidious and is not accompanied by obvious symptoms. It is easy to misdiagnose tiny, depressed, or flat raised lesions. Most gastric cancer lesions are already at an advanced stage at diagnosis, and the five-year survival rate for advanced gastric cancer is less than 30% (3). Therefore, in the face of high mortality from gastric cancer, our endoscopists are focused on not only secondary prevention of this disease but also the key aspects of improving survival and reducing mortality for patients who undergo gastric cancer surgery. However, at present, the detection rate for early gastric cancer in our country is only 5%–10%, and diagnosis of early gastric cancer primarily depends on biopsies obtained during endoscopy. However, endoscopy using a common gastroscope produces a low positive rate because of this instrument’s inadequate capabilities. Certain studies (4) have indicated that during the process of malignant transformation of the gastric mucosa, microglial and microvascular morphology will change; therefore, in the assessment of early gastric cancer, observation of microvascular and microglial morphology is extremely important. BLI endoscopy is a new endoscopic technique that has been used in recent years. When used in combination with magnifying endoscopy, BLI can allow for improved observations of fine mucosal surface images. In this context, accurately assessing lesion morphology and structure (5–11) and correctly diagnosing early gastric cancer are of great significance.
In BLI endoscopy, two different types of lasers are used as light sources. A short-wavelength narrow-band laser (410±10 nm) can be used to obtain information about deep mucosal microvessels and superficial microtubules and observe narrow-band light. A long-wavelength laser (450±10 nm) can produce white light via fluorescence stimulation, allowing for white light observation. BLI has two observation modes: the BLI-contrast and BLI-bright modes. Both modes can be used to observe a lesion’s microvascular and microtubule structures; however, the BLI-contrast mode is mainly used to observe detailed features of the lesion’s microvessels and microtubules in near-field and magnified fields of view, whereas the BLI-bright mode has slightly increased white light composition and is thus used primarily for long-range observation of contrast enhancement. Research (7) has shown that the average observation distances for the BLI-contrast and BLI-bright modes were 31.3 mm and 24.7 mm, respectively (P<0.01), indicating that the BLI-bright approach can be used to obtain high-resolution contrast images from a long-distance perspective. Thus, this mode can be used for the long-distance observation of a lesion and to help find lesions during screening endoscopy (7). Moreover, BLI endoscopy can clearly show the boundaries of early gastric cancer. In BLI, there is a high degree of color contrast between malignant brown lesions and the surrounding mucosa without enlargement (11), and the magnification provided by the endoscope clearly reveals microvascular and microtubule structures on the lesion surface, which contributes to determining the boundaries of malignant lesions and performing endoscopic radical resection of early gastric cancer. And many researches confirmed that the diagnostic effectiveness of early gastric cancer in magnifying BLI(M-BLI) is similar to that of magnifying endoscopy with narrow-band imaging (M-NBI), and it also solves the shortcomings of NBI of light dark in observing whole gastric mucosa and poor at visualizing mucosal microstructure (12–15).
Using the VS diagnostic criteria, we compared abnormal microstructures of gastric mucosa (i.e., microvascular abnormalities, microtubule abnormalities, and microvessel density) observed via BLI-bright magnification and BLI-contrast magnification. We found that BLI-contrast magnification and BLI-bright magnification had significantly better diagnostic accuracy than white light magnification alone with respect to a cancerous lesion’s microvascular and microtubule abnormalities and the existence of a line of demarcation. Diagnoses determined using BLI-contrast magnification and BLI-bright magnification also exhibited better concordance with pathological diagnoses (Kappa>0.7, p<0.001) than diagnoses determined using white light magnification. The improvements in accuracy of microscopic diagnosis achieved using BLI-contrast magnification and BLI-bright magnification compared with the accuracy of white light magnification alone were significant (p<0.05). In particular, for early gastric cancer (or high-grade intraepithelial neoplasia), the sensitivities of microscopic diagnoses determined using the BLI approaches were over 90%, and the consistency between microscopic and pathological diagnoses was significantly superior for these approaches than for white light alone (p<0.05). In addition, no significant difference between the accuracies of BLI-contrast magnification and BLI-bright magnification for diagnosing early gastric cancer was detected (p>0.05); however, both of these approaches are included in the observation modes of BLI endoscopy, and the combination of the two techniques will help to improve the diagnosis of early gastric cancer and precancerous lesions.
The data used for this study are from a single center, and the study involved a relatively small sample and lacked follow-up observations. Therefore, additional large multicenter investigations are needed to confirm the study’s results.
This research shows that when applying the VS diagnostic criteria, the use of BLI laser endoscopy, with its unique combination of a narrow-band laser and a white light source, can significantly improve diagnostic accuracy for early gastric cancer and precancerous lesions in a simple, effective, and inexpensive manner.
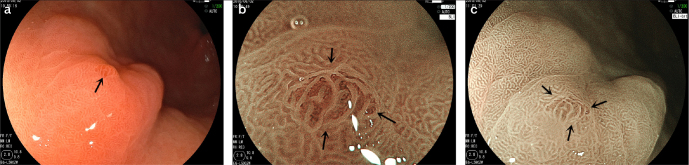
Figure 2. a–c.
The microscope characteristics of low-grade intraepithelial neoplasia lesions in pathological diagnosis.
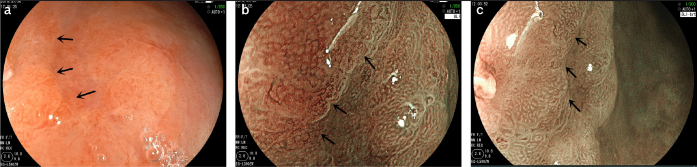
Figure 3. a–c.
The microscope characteristics of intestinal metaplasia in pathological diagnosis.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the Ethics Committee of Renmin Hospital of Wuhan University.
Informed Consent: Written informed consent was obtained from the patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - S.L.; Design - S.L.; Supervision - S.L.; Materials - Y.Z.; Data Collection and/or Processing - Y.Z.; Analysis and/or Interpretation - Y.Z.; Literature Search - Y.Z.; Writing Manuscript - Y.Z.; Critical Review - S.L.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Yao K, Anagnostopoulos GK, Ragunath K. Magnifying endoscopy for diagnosing and delineating early gastric cancer. Endoscopy. 2009;41:462–7. doi: 10.1055/s-0029-1214594. [DOI] [PubMed] [Google Scholar]
- 2.Dixon MF. Gastrointestinal epithelial neoplasia: Vienna revisited. Gut. 2002;51:130–1. doi: 10.1136/gut.51.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan YK, Fielding JW. Early diagnosis of early gastric cancer. Eur J Gastroenterol Hepatol. 2006;18:821–9. doi: 10.1097/00042737-200608000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Tajiri H, Matsuda K, Fujisaki J. What can we see with the endoscope? Present status and future perspectives. Dig Endosc. 2002;14:131–7. doi: 10.1046/j.0915-5635.2002.00191.x. [DOI] [Google Scholar]
- 5.Osawa H, Yamamoto H. Present and future status of flexible spectral imaging color enhancement and blue laser imaging technology. Dig Endosc. 2014;1:105–15. doi: 10.1111/den.12205. [DOI] [PubMed] [Google Scholar]
- 6.Yoshida N, Yagi N, Inada Y, et al. Ability of a novel blue laser imaging system for the diagnosis of colorectal polyps. Dig Endosc. 2014;26:250–8. doi: 10.1111/den.12127. [DOI] [PubMed] [Google Scholar]
- 7.Kaneko K, Oono Y, Yano T, et al. Effect of novel bright image enhanced endoscopy using blue laser imaging (BLI) Endoscopy international open. 2014;2:E212–E219. doi: 10.1055/s-0034-1390707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshida N, Hisabe T, Hirose R, et al. Improvement in the visibility of colorectal polyps by using blue laser imaging. Gastrointest Endosc. 2015;82:542–9. doi: 10.1016/j.gie.2015.01.030. [DOI] [PubMed] [Google Scholar]
- 9.Yoshida N, Hisabe T, Inada Y, et al. The ability of a novel blue laser imaging system for the diagnosis of invasion depth of colorectal neoplasms. J Gastroenterol. 2014;49:73–80. doi: 10.1007/s00535-013-0772-7. [DOI] [PubMed] [Google Scholar]
- 10.Togashi K, Nemoto D, Utano K, et al. Blue laser imaging endoscopy system for the early detection and characterization of colorectal lesions: a guide for the endoscopist. Therap Adv Gastroenterol. 2016;40:94–101. doi: 10.1177/1756283X15603614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osawa H, Yamamoto H, Miura Y, et al. Blue Laser Imaging Provides Excellent Endoscopic Images of Upper Gastrointestinal Lesions. Video J Encyclopedia of GI Endosc. 2014;1:607–10. doi: 10.1016/j.vjgien.2014.01.001. [DOI] [Google Scholar]
- 12.Yagi N, Naito Y, Dohi O, et al. Mo1649 The Efficacy of a Novel Blue LASER Imaging System for the Diagnosis of Early Gastric Cancers; a Prospective Single Center Open Trial. Gastrointest Endosc. 2013;77:AB458. doi: 10.1016/j.gie.2013.03.397. [DOI] [Google Scholar]
- 13.Osawa H, Yamamoto H. Present and future status of flexible spectral imaging color enhancement and blue laser imaging technology. Dig Endosc. 2014;26:105–15. doi: 10.1111/den.12205. [DOI] [PubMed] [Google Scholar]
- 14.Kimura-Tsuchiya R, Dohi O, Fujita Y, et al. Magnifying Endoscopy with Blue Laser Imaging Improves the Microstructure Visualization in Early Gastric Cancer: Comparison of Magnifying Endoscopy with Narrow-Band Imaging. Gastroenterol Res Pract. 2017;2017 doi: 10.1155/2017/8303046. 8303046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dohi O, Yagi N, Yoshida S, et al. Magnifying Blue Laser Imaging versus Magnifying Narrow-Band Imaging for the Diagnosis of Early Gastric Cancer: A Prospective, Multicenter, Comparative Study. Digestion. 2017;96:127–34. doi: 10.1159/000479553. [DOI] [PubMed] [Google Scholar]