Abstract
Objective:
Lung cancer is the leading cause of cancer death in people living with HIV (PWH). Surgical resection is a key component of potentially curative treatment regimens for early stage lung cancers, but its safety is unclear in the setting of HIV. From a national cohort, we assessed potential differences in the risk of major lung cancer surgery complications by HIV status.
Design:
We linked clinical and cancer data from the Veterans Aging Cohort Study (VACS) and Veterans Affairs Corporate Data Warehouse to outcomes from the Veterans Affairs Surgical Quality Improvement Program (VASQIP) and identified 8,371 patients (137 PWH, 8,234 uninfected) who underwent lung cancer surgeries between 2000 and 2016.
Methods:
We compared rates of 15 major short-term surgical complications by HIV status.
Results:
Use of surgical resection for early stage lung cancer did not differ by HIV status. Lung cancer surgery postoperative (30-day) mortality was 2.0% for PWH and did not differ by HIV status (p=0.9). Pneumonia was the most common complication for both PWH and uninfected Veterans, but did not differ significantly in prevalence between groups (11.0% for PWH versus 9.4%; p=0.5). The frequency of complications did not differ by HIV status for any complication (all p>0.3). There were no significant predictors of post-operative complications for PWH.
Conclusions:
In a national antiretroviral-era cohort of lung cancer patients undergoing surgical lung resection, short-term outcomes following surgery did not differ significantly by HIV status. Concerns regarding short-term surgical complications should have limited influence on treatment decisions for PWH with lung cancer.
Keywords: Lung cancer, Non-AIDS malignancy, Lung cancer surgery, Non-small cell lung cancer, Veterans Aging Cohort Study, Surgical outcomes
Background
Lung cancer is the leading cause of cancer death and a major source of morbidity among persons living with HIV infection (PWH) in the United States[1, 2]. The significant lung cancer burden observed among PWH is partly attributable to high smoking rates in this group, but also due to independent, HIV-related factors increasing lung cancer risk[3].
Despite major improvements in health outcomes during the antiretroviral therapy (ART) era, PWH experience worse lung cancer survival compared to uninfected persons[4]. The reasons for these poorer outcomes are unclear, but may be associated with disparities in lung cancer care[5]. Population-based studies have shown lower rates of lung cancer treatment in PWH than uninfected individuals[5–7]. These disparities may be related to clinician apprehension regarding increased risks of treatment-related complications (particularly major surgery-related adverse events) among PWH[8, 9].
Appropriate lung cancer treatment is dictated by cancer stage, tumor characteristics and patient factors. Overall, approximately 35% of lung cancer cases in PWH are diagnosed at a potentially resectable (I-IIIA) cancer stage[5, 10]. However, adoption of lung cancer screening among PWH smokers[11–13] will substantially increase the proportion of resectable cancers[14]. Optimal management of early stage lung cancer is therefore critical, as these are the only patients for whom long-term survival can be achieved[15]. Surgical resection is the major component of lung cancer treatment for stage I-IIIA and can be curative in some circumstances.
The short-term risks of lung cancer surgery in PWH are unknown. Improved understanding of these risks is critical for clinical decision-making regarding the use of optimal lung cancer treatment in this group, and may help addressing treatment disparities. In this study, we used data from a national cohort of PWH and uninfected comparators in the Veterans Health Administration to evaluate the use of and short-term outcomes for lung cancer surgery, hypothesizing that in the ART-era PWH with lung cancer would experience similar harms associated with surgery compared to demographically similar uninfected patients.
Methods
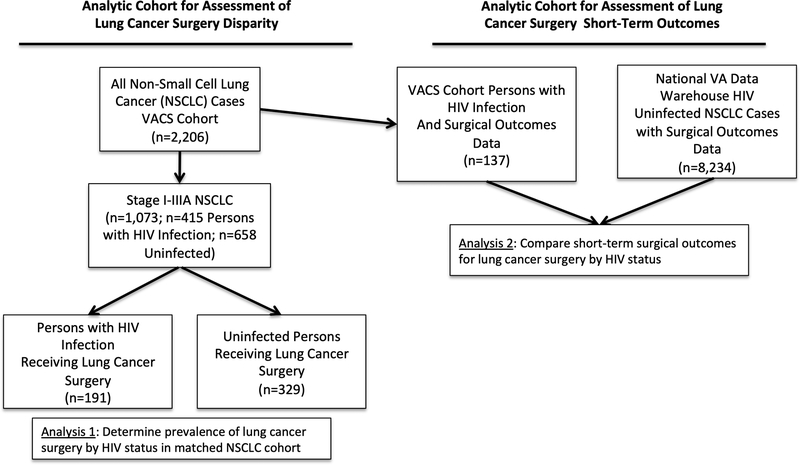
We used data from the Veterans Aging Cohort Study (VACS), a large cohort of PWH who, upon enrollment, are matched (by age, race/ethnicity, sex and VA clinical site) to two uninfected comparators assembled from Veterans Affairs clinical and administrative data. During the years 2000–2016 we identified all cases of non-small cell lung cancer (NSCLC) within VACS (Figure 1; n=2,206) using linked data from the Corporate Data Warehouse Oncology (CDW Oncology) repository, a national database of cancer registry information collected at VA hospitals by trained cancer registrars. We then determined which NSCLC patients from VACS underwent surgical resection (“Analysis 1”; n=520). The purpose of this analysis was to use the VACS cohort to determine the proportion of Veterans who underwent surgical resection for lung cancer by HIV status, to assess for treatment disparities. In the second analysis (Figure 1, “Analysis 2”) we evaluated the risk of major surgical complications by HIV status, our primary study objective. To improve the study power for this objective, we included additional lung cancer cases by collecting all available uninfected NSCLC patients who underwent surgery during the same timeframe from the CDW Oncology registry (n=8,234). We then merged our expanded cancer cohort with data from the Veterans Affairs Surgical Quality Improvement Program (VASQIP), which provided prospectively collected information on preoperative characteristics and post-operative outcomes for patients who underwent lung cancer surgery.
Figure 1.
Flowchart describing the design of analytic cohorts for each study objective.
Study Outcomes and Variables
For our initial analysis determining the overall rate of lung cancer resection by HIV status in the VACS cohort, we used both registry data and procedure codes (which allowed for identification of surgeries that occurred at non-VA hospitals) to identify lung cancer surgeries for all NSCLC patients in VACS. For our analysis of surgical complications by HIV status, our primary analytic cohort consisted of patients undergoing lung cancer resection surgery as identified by procedure codes in VASQIP. However, VASQIP data was not available for all patients, as it is only captured for surgeries performed in VA facilities.
All primary and secondary outcomes were reported in VASQIP. Our primary outcome of interest was 30-day mortality after lung cancer surgery. Secondary outcomes included 30-day surgical complications, such as pneumonia, sepsis, reintubation, reoperation, and myocardial infarction.
For our analysis of surgical complications we then collected data on age, gender, race and ethnicity from VA administrative data. We collected comorbidity (such as chronic obstructive pulmonary disease [COPD]) information from all available data sources which included both relevant diagnostic codes and data recorded in VASQIP. Other preoperative variables that we included from VASQIP were American Society of Anesthesiologists (ASA) class, functional status impairment, significant alcohol use and smoking status. From linked cancer registry data we determined cancer diagnosis date, tumor stage and histologic subtype. For HIV+ patients we ascertained CD4 cell count, HIV viral suppression (<500 copies/ml) and VACS index values[16], a validated prognosis score for PWH (incorporating age, CD4 cell count, HIV viral load, aspartate transaminase concentration (AST), alanine transaminase concentration (ALT), platelet count, estimated glomerular filtration rate, hemoglobin level, and hepatitis C serostatus), at the time of cancer diagnosis.
Statistical Analysis
We first compared the overall prevalence of lung cancer surgery by HIV status among VACS lung cancer patients to evaluate for the presence of treatment disparities. We then compared baseline characteristics for patients undergoing lung cancer surgery in the larger cohort (VACS lung cancer cases merged to overall national VA lung cancer cases) by HIV status, using Wilcoxon’s test for continuous variables and the chi-square test or Fisher’s exact test (where appropriate) for categorical variables. The probability of death within 30 days of surgery and other major adverse events were then compared using Fisher’s exact test or the chi-square test, where appropriate. We then fitted logistic regression models for each adverse outcome with HIV status as our primary predictor, adjusting for age, sex, race/ethnicity, year of surgery, alcohol use, and surgical approach. As an alternative, confirmatory approach, we employed inverse probability weighting (IPW) in separate models to account for potential selection bias in the use of surgery for PWH versus uninfected Veterans.[17] Weights represented the inverse of the probability that a subject would be sampled, and therefore included in the study, to estimate an ideal “pseudo-population” where no selection bias occurred. Weights were calculated by fitting a logistic regression model including all available baseline covariates (similar to the adjusted models) predicting HIV status. We excluded subjects with weights greater than 99th percentile and fitted weighted models evaluating HIV as a predictor of all study outcomes. We then used adjusted logistic models limited to PWH to determine predictors of 30-day mortality or the combined 30-day outcome of death or any other major adverse event. Multiple imputation methods were employed to account for missing data, which was limited (4% of subjects were missing race/ethnicity information, <1% had no data regarding smoking status and/or ASA class). All analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
Using the observed 30-day mortality and major complication rates from HIV+ patients and available sample size, we calculated that we had 80% power to detect a >5% absolute difference in mortality and a >10% difference in major complications associated with HIV infection. The Institutional Review Board of the Yale University School of Medicine approved this research.
Results
In our initial analysis of stage I-IIIA NSCLC cases in the VACS cohort, we found that PWH (n=191) were equally likely to be treated with surgical resection as uninfected Veterans (191/415 = 46% for PWH vs 329/658 = 50% for uninfected; p=0.3; results not otherwise shown) during 2000–2016.
In the larger combined cohort (including additional comparator cases from the national VA sample), PWH lung cancer surgery patients were younger, more likely to be African-American or Hispanic, and were less likely to have COPD, but otherwise did not differ significantly in comorbid conditions, anesthesia-risk class, or functional status impairment (Table 1). The majority of PWH had viral suppression (71%) and CD4 counts greater than 200 cells/mm3 (75%) at lung cancer diagnosis. Cancer histologic type differed by HIV status, with PWH being less likely to have squamous cell subtypes (35% versus 40%) and more likely to be categorized as undefined NSCLC (6% versus 1%; p<0.001 for histologic subtype comparisons). Surgical procedure types also differed by HIV status; PWH were more likely to receive pneumonectomy (9% versus 4%) and less likely to undergo limited lung resection (16% versus 19%) than uninfected patients (p=0.01).
Table 1.
Characteristics of Non-Small Cell Lung Cancer Patients by HIV Status
| Characteristic | PWH N=137 |
Uninfected N=8,234 |
P-value |
|---|---|---|---|
| Age, years (mean, sd) | 60 (8.3) | 66 (8.0) | <0.0001 |
| Female, N (%) | 2 (1.5) | 242 (2.9) | 0.3 |
| Race/Ethnicity, N (%) | <0.0001 | ||
| White | 64 (47) | 5,983 (73) | |
| African-American | 59 (43) | 1,208 (15) | |
| Hispanic | 10 (7) | 171 (2) | |
| Other | 4 (3) | 510 (6) | |
| Unknown | 0 (0) | 362 (4) | |
| Smoking Status, N, (%) | 0.2 | ||
| Yes | 86 (63) | 4,733 (57) | |
| No | 51 (37) | 3,499 (42) | |
| Missing | 0 (0) | 2 (1) | |
| Congestive Heart Failure, N, (%) | 0 (0) | 59 (1) | 0.32 |
| Chronic Obstructive Pulmonary Disease N, (%) | 44 (32) | 3,938 (48) | 0.0003 |
| Diabetes, N, (%) | 23 (17) | 1,739 (21) | 0.21 |
| ASA Class, N, (%) | 0.6 | ||
| 1 | 0 (0) | 2 (1) | |
| 2 | 4 (3) | 309 (4) | |
| 3 | 105 (77) | 6,643 (81) | |
| 4 | 28 (20) | 1,275 (15) | |
| 5 | 0 (0) | 2 (1) | |
| Missing | 0 (0) | 3 (1) | |
| Functional Status Impairment, N (%) | 6 (4) | 254 (3) | 0.39 |
| Moderate/Significant Alcohol Use*, N, (%) | 11 (8) | 1,132 (14) | 0.053 |
| Year of Lung Cancer Diagnosis, N (%) | <0.0001 | ||
| 2000–2005 | 44 (32) | 497 (6) | |
| 2006–2010 | 56 (41) | 4143 (50) | |
| 2011–2016 | 37 (27) | 3,594 (44) | |
| Histologic Subtype, N (%) | <0.0001 | ||
| Adenocarcinoma | 74 (54) | 4,618 (58) | |
| Squamous cell carcinoma | 48 (35) | 3,157 (40) | |
| Large cell carcinoma | 6 (4) | 143 (2) | |
| NSCLC, NOS | 8 (6) | 34 (1) | |
| Tumor Stage, N (%) | 0.96 | ||
| I | 86 (63) | 5,028 (63) | |
| II | 26 (19) | 1,518 (19) | |
| IIIA | 13 (10) | 754 (9) | |
| IIIB | 4 (3) | 262 (3) | |
| IV | 5 (4) | 420 (5) | |
| Surgical Technique, N, (%) | 0.013 | ||
| Lobectomy | 102 (75) | 6084 (74) | |
| Limited Resection | 22 (16) | 1561 (19) | |
| Pneumonectomy | 13 (9) | 352 (4) | |
| Missing | 0 (0) | 237 (3) | |
| HIV RNA, N (%) | |||
| <500 copies/ml | 97 (71) | ||
| >500 copies/ml | 20 (15) | ||
| Missing | 20 (15) | ||
| CD4 Count, N (%) | |||
| <200 cells/cc3 | 15 (11) | ||
| 200–500 cells/cc3 | 56 (41) | ||
| >500 cells/cc3 | 47 (34) | ||
| Missing | 19 (14) | ||
| VACS Index, median (IQR)* | 34 (24–47) |
Defined as more than 2 alcoholic drinks per day in 2 weeks prior to surgery
PHW: Person living with HIV; COPD: Chronic obstructive pulmonary disease; ASA: American Society of Anesthesiologists; NSCLC: Non-small cell lung cancer; NSCLC, NOS: Non-small cell lung cancer, not otherwise specified
IQR: Interquartile range;
Increasing VACS Index values are associated with higher 5-year mortality.
Perioperative mortality for NSCLC surgery was 2.2% (95% confidence interval [CI]: 0.5–6.3%) for PWH and 2.5% (95% CI: 2.1–2.8%) for uninfected patients (p=0.8). Major complications occurred in 17.5% of procedures for HIV+ vs. 19.4% of uninfected patients (p=0.6); none of the rates of complications significantly differed by HIV status. Common post-operative complications included pneumonia (11.0% for PWH versus 9.4% for uninfected; p=0.5) followed by reoperation (9.5% for PWH versus 7.5%; p=0.4) and reintubation (7.3% for PWH versus 8.3%; p=0.7).
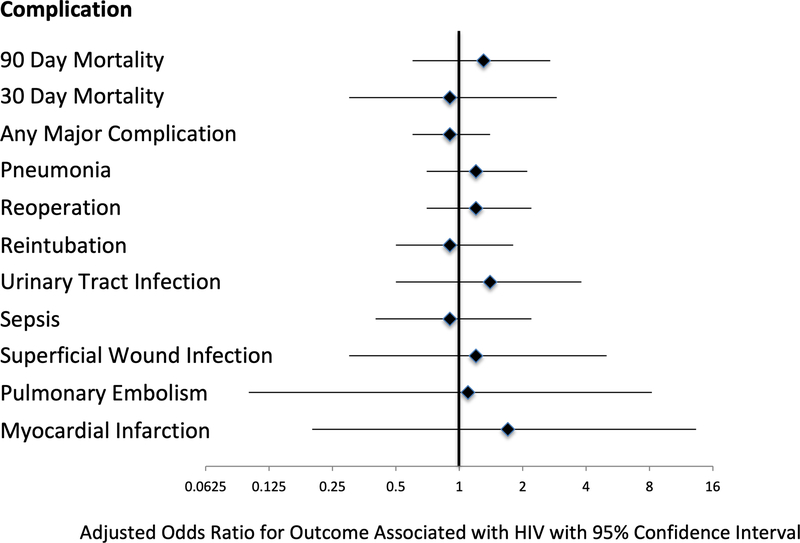
Adjusted analyses showed that HIV was not significantly associated with either 90-day (Figure 2, Table 3: adjusted odds ratio [AOR] for HIV 1.3, 95% CI: 0.6–2.7), 30-day mortality (Table 3; AOR 0.9, 95% CI: 0.3–2.9) or any major complication (AOR 0.9, 95% CI: 0.6–1.4). Confirmatory analyses using IPW methods also did not demonstrate any significant associations between HIV infection and adverse short-term surgical outcomes. We also performed analyses limited to PWH evaluating the association of preoperative characteristics and short-term outcomes; no baseline characteristics (including HIV-related variables) were predictive of any adverse outcome in either unadjusted or adjusted analyses (data not otherwise shown).
Figure 2.
Forest plot depicting adjusted odds ratios the association of HIV infection with major outcomes.
Table 3.
Adjusted analyses of HIV status and major surgical outcomes
| Complication | Adjusted* Odds Ratio for HIV | 95% CI | IPW Adjusted Odds Ratio for HIV | 95% CI |
|---|---|---|---|---|
| 90-Day Mortality | 1.35 | 0.66–2.77 | 0.50 | 0.16–1.58 |
| 30-Day Mortality | 0.92 | 0.28–3.08 | 0.49 | 0.08–3.19 |
| Any Major Complication | 0.93 | 0.59–1.46 | 0.5 | 0.3–1.1 |
| Pneumonia | 1.22 | 0.70–2.13 | 0.75 | 0.30–1.87 |
| Reoperation | 1.23 | 0.68–2.23 | 0.83 | 0.37–1.84 |
| Reintubation | 0.96 | 0.49–1.87 | 0.61 | 0.23–1.60 |
| Urinary Tract Infection | 1.37 | 0.49–3.84 | 0.44 | 0.12–1.57 |
| Sepsis | 0.90 | 0.36–2.24 | 0.38 | 0.13–1.13 |
| Superficial Wound Infection | 1.21 | 0.29–5.06 | 0.51 | 0.09–2.81 |
| Pulmonary Embolism | 1.18 | 0.16–8.78 | ** | |
| Deep Wound Infection | ** | ** | ||
| Myocardial Infarction | 1.85 | 0.24–14.50 | 1.90 | 0.25–14.21 |
| Bleeding Requiring >4 Units Transfused | ** | ** | ||
| Cerebrovascular Accident | ** | ** |
IPW: Inverse probability weighted; CI: Confidence interval;
Model adjusted for age, sex, race/ethnicity, year of surgery, COPD, alcohol use and surgical approach ;
Too few events to calculate
Discussion
Surgical resection for early stage lung cancer is potentially curative. The burden of lung cancer among PWH is expected to grow[18], and with increases in lung cancer screening, the prevalence of localized cancers is also likely to increase in this group[19]. Establishing the safety of lung cancer surgery in PWH is important to decrease existing disparities in cancer treatment. In our study of PWH and uninfected Veterans with lung cancer, we did not find significant differences in the utilization of major surgical resection by HIV status in patients with early stage lung cancer in a well-matched cohort, suggesting that in the VA system there are limited disparities in the use of lung cancer surgery in patients with HIV. Additionally, using prospectively collected surgical outcomes data, we found no major differences in post-surgical adverse events according to HIV status. These findings suggest that concerns regarding short-term surgical outcomes associated with the presence of HIV infection should not play a role in treatment decision-making.
Several recent population-based studies have found lower rates of stage-appropriate lung cancer treatment (including surgery) in individuals with HIV infection, with differences ranging from 12–17%[5, 7, 20]. A survey of oncologic providers found that a majority of clinicians felt there was insufficient guidance regarding appropriate cancer treatment for PWH, and that concerns regarding adverse effects and efficacy of cancer treatments deterred appropriate therapy for PWH[21]. In contrast, we found no differences in the use of surgical resection (the gold standard treatment) for early stage lung cancer among Veterans. As the VACS cohort enrolls all PWH Veterans engaged in care, our findings are likely an accurate picture of treatment patterns for this group within the VA system, but may not necessarily represent the experiences of PWH less engaged in medical care. As the VA is the largest single provider of HIV care in the US, it is possible that providers have a higher comfort level in treating this population, possibly explaining the differences that have been seen with other population-based studies. Alternatively, some sociodemographic groups that are overrepresented among the US PWH (minorities, lower income persons) may experience structural barriers to appropriate lung cancer treatment, and this is likely diminished within the VA system.
HIV infection has been associated with higher rates of adverse surgical outcomes in several large ART-era studies. An ART-era study of 332 procedure-matched pairs in Kaiser Permanente Northern California found higher rates of post-operative pneumonia among PWH but no differences in short-term mortality[22]. A larger analysis of VACS patients compared 1,641 Veterans with HIV infection undergoing major surgery to procedure-matched uninfected comparators and found higher 30-day mortality associated with HIV infection after adjustment. In that study increasing age, very low CD4 count, comorbidity burden and albumin level were all independently associated with post-operative mortality[8]. Another large study evaluated colorectal surgery outcomes from a national sample (years 2000–2010) by HIV status and found similar results; patients with an AIDS diagnosis had significantly higher post-operative mortality and infectious and non-infectious surgical complications compared to uninfected persons.[9] Persons with HIV but without an AIDS diagnosis were not at higher risk for mortality or complications however.
Previous data on postoperative outcomes for PWH specific to lung cancer surgery are limited. An early ART-era review compiled published surgical series, finding no perioperative deaths associated with lung cancer surgery among 24 PWH (mostly cases from the pre-ART era) but 8% died within 90 days of surgery[23]. Additionally, a Baltimore-based single center series spanning both the pre-ART and ART-era compared 22 HIV+ patients (median age 47.5) who were treated with surgical resection to a matched cohort[24]. That study did not observe any perioperative deaths (0% at 30 days) for PWH but did find very high rates of postoperative complications, with nearly half of the PWH experiencing at least 2 major complications. Similarly, in our ART-era cohort, we found no differences in 30-day mortality by HIV status, but in contrast to the Baltimore cohort, we found no difference in the incidence of major postoperative complications associated with HIV status. The low rate of major complications likely reflects our more contemporary HIV+ cohort, with a low prevalence of uncontrolled viremia and immunosuppression.
Our study benefited from a national data source with detailed clinical data and prospectively collected short-term surgical complication information. VASQIP provides a unique opportunity to link high quality surgical data to detailed HIV clinical information that is not possible with the analogous non-VA program used by many hospitals to track 30-day surgical outcomes - American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) - that has no data on HIV status or severity. A major limitation of our study is that we could not fully account for potential selection regarding the use of surgery as treatment for the HIV+ patients and this could have biased the lack of association of HIV with adverse outcomes. However, we found no disparity in the use of surgery to treat early stage lung cancer patients by HIV status, suggesting that there may not be major selection bias present. Additionally we conducted adjusted analyses to account for potential differences between the PWH who underwent surgery and uninfected persons and still found no association between HIV and post-operative mortality or major complications. We also had no data on pack-year smoking quantity or recent smoking cessation for study subjects, factors that might have differed by HIV status and have been associated with lung cancer surgery complications.[25]
Conclusion
In a national ART-era cohort of PWH and uninfected lung cancer patients undergoing surgical lung cancer resection, short-term outcomes following surgery did not differ significantly by HIV status.
Concern for short-term surgical complications related to HIV infection should have limited influence on treatment decisions for PWH with lung cancer.
Table 2.
Frequency of Major Surgical Complications within 30 Days of Lung Cancer Surgery by HIV Status
| Complication | HIV Infected (N=137) N (%) |
Uninfected (N=8,234) N (%) |
P-value |
|---|---|---|---|
| 90-Day Mortality | 9 (6.6) | 423 (5.1) | 0.5 |
| 30-Day Mortality | 3 (2.2) | 202 (2.5) | 0.8 |
| Any Major Complication | 24 (17.5) | 1,596 (19.4) | 0.6 |
| Pneumonia | 15 (11.0) | 774 (9.4) | 0.5 |
| Reoperation | 13 (9.5) | 614 (7.5) | 0.4 |
| Reintubation | 10 (7.3) | 681 (8.3) | 0.7 |
| Urinary Tract Infection | 4 (2.9) | 201 (2.4) | 0.7 |
| Sepsis | 5 (3.7) | 345 (4.2) | 0.8 |
| Superficial Wound Infection | 2 (1.5) | 94 (1.1) | 0.7 |
| Pulmonary Embolism | 1 (0.7) | 58 (0.7) | 0.9 |
| Deep Wound Infection | 0 (0) | 24 (0.3) | 0.5 |
| Myocardial Infarction | 1 (0.7) | 37 (0.4) | 0.6 |
| Bleeding Requiring >4 Units Transfused | 0 (0) | 35 (0.4) | 0.4 |
| Cerebrovascular Accident | 0 (0) | 36 (0.4) | 0.4 |
Acknowledgement
KMS had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. KS, JPW, LSP, CYK, MJS, SB, MG, MCRB, CG, FS, RB, RW, JK and KC contributed substantially to the study design, data analysis and interpretation, and the writing of the manuscript.
Funding: This study was supported by the National Institutes of Health through R01CA210806, K07CA180782 to KS, R01CA173754 to KC, JW, and U01AA013566 to support the Veterans Aging Cohort Study. The funder played no role in the design, execution or reporting of the analysis.
Conflicts of Interest: Dr. Wisnivesky has received consulting honorarium from Quintiles, AstraZeneca, Merck and Sanofi and research grants from Sanofi and Quorum. Dr. Silverberg has received research grants from Gilead and Merck. Dr. Bedimo has received research grants from ViiV Healthcare and Merck and has served on advisory boards for ViiV Healthcare, Merck and Napo Pharmaceuticals. The other authors report no potential conflicts of interest.
Footnotes
Disclaimer: The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
References
- 1.Shiels MS, Pfeiffer RM, Gail MH, Hall HI, Li J, Chaturvedi AK, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst 2011; 103(9):753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Winstone TA, Man SF, Hull M, Montaner JS, Sin DD. Epidemic of lung cancer in patients with HIV infection. Chest 2013; 143(2):305–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sigel K, Wisnivesky J, Gordon K, Dubrow R, Justice A, Brown ST, et al. HIV as an independent risk factor for incident lung cancer. AIDS 2012; 26(8):1017–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sigel K, Makinson A, Thaler J. Lung cancer in persons with HIV. Curr Opin HIV AIDS 2017; 12(1):31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marcus JL, Chao C, Leyden WA, Xu L, Yu J, Horberg MA, et al. Survival among HIV-infected and HIV-uninfected individuals with common non-AIDS-defining cancers. Cancer Epidemiol Biomarkers Prev 2015; 24(8):1167–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suneja G, Shiels MS, Melville SK, Williams MA, Rengan R, Engels EA. Disparities in the treatment and outcomes of lung cancer among HIV-infected individuals. AIDS 2013; 27(3):459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suneja G, Shiels MS, Angulo R, Copeland GE, Gonsalves L, Hakenewerth AM, et al. Cancer treatment disparities in HIV-infected individuals in the United States. J Clin Oncol 2014; 32(22):2344–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.King JT Jr., Perkal MF, Rosenthal RA, Gordon AJ, Crystal S, Rodriguez-Barradas MC, et al. Thirty-day postoperative mortality among individuals with HIV infection receiving antiretroviral therapy and procedure-matched, uninfected comparators. JAMA Surg 2015; 150(4):343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gahagan JV, Halabi WJ, Nguyen VQ, Carmichael JC, Pigazzi A, Stamos MJ, et al. Colorectal Surgery in Patients with HIV and AIDS: Trends and Outcomes over a 10-Year Period in the USA. J Gastrointest Surg 2016; 20(6):1239–1246. [DOI] [PubMed] [Google Scholar]
- 10.Sigel K, Crothers K, Dubrow R, Krauskopf K, Jao J, Sigel C, et al. Prognosis in HIV-infected patients with non-small cell lung cancer. Br J Cancer 2013; 109(7):1974–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sigel K, Wisnivesky J, Shahrir S, Brown ST, Justice A, Kim J, et al. Findings in asymptomatic HIV-infected patients undergoing chest computed tomography testing: implications for lung cancer screening. AIDS 2014; 28(7):1007–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hulbert A, Hooker CM, Keruly JC, Brown T, Horton K, Fishman E, et al. Prospective CT screening for lung cancer in a high-risk population: HIV-positive smokers. J Thorac Oncol 2014; 9(6):752–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Makinson A, Eymard-Duvernay S, Raffi F, Abgrall S, Bommart S, Zucman D, et al. Feasibility and efficacy of early lung cancer diagnosis with chest computed tomography in HIV-infected smokers. AIDS 2016; 30(4):573–582. [DOI] [PubMed] [Google Scholar]
- 14.Aberle DR, Abtin F, Brown K. Computed tomography screening for lung cancer: has it finally arrived? Implications of the national lung screening trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2013; 31(8):1002–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kramer BS, Berg CD, Aberle DR, Prorok PC. Lung cancer screening with low-dose helical CT: results from the National Lung Screening Trial (NLST). Journal of medical screening 2011; 18(3):109–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tate JP, Justice AC, Hughes MD, Bonnet F, Reiss P, Mocroft A, et al. An internationally generalizable risk index for mortality after one year of antiretroviral therapy. AIDS 2013; 27(4):563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buchanan AL, Hudgens MG, Cole SR, Lau B, Adimora AA, Women’s Interagency HIVS. Worth the weight: using inverse probability weighted Cox models in AIDS research. AIDS Res Hum Retroviruses 2014; 30(12):1170–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shiels MS, Islam JY, Rosenberg PS, Hall H, Jacobson E, Engels EA. Projected cancer incidence rates and burden of incident cancer cases in hiv-infected adults in the united states through 2030. Annals of Internal Medicine 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kong CY, Sigel K, Criss SD, Sheehan DF, Triplette M, Silverberg MJ, et al. Benefits and harms of lung cancer screening in HIV-infected individuals with CD4+ cell count at least 500 cells/mul. AIDS 2018; 32(10):1333–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suneja G, Shiels MS, Melville SK, Williams MA, Rengan R, Engels EA. Disparities in the treatment and outcomes of lung cancer among HIV-infected people in Texas. AIDS 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suneja G, Boyer M, Yehia BR, Shiels MS, Engels EA, Bekelman JE, et al. Cancer Treatment in Patients With HIV Infection and Non-AIDS-Defining Cancers: A Survey of US Oncologists. J Oncol Pract 2015; 11(3):e380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horberg MA, Hurley LB, Klein DB, Follansbee SE, Quesenberry C, Flamm JA, et al. Surgical outcomes in human immunodeficiency virus-infected patients in the era of highly active antiretroviral therapy. Arch Surg 2006; 141(12):1238–1245. [DOI] [PubMed] [Google Scholar]
- 23.Cadranel J, Garfield D, Lavole A, Wislez M, Milleron B, Mayaud C. Lung cancer in HIV infected patients: facts, questions and challenges. Thorax 2006; 61(11):1000–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hooker CM, Meguid RA, Hulbert A, Taylor JT, Shin J, Wrangle J, et al. Human immunodeficiency virus infection as a prognostic factor in surgical patients with non-small cell lung cancer. Ann Thorac Surg 2012; 93(2):405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gagne S, McIsaac DI. Modifiable risk factors for patients undergoing lung cancer surgery and their optimization: a review. J Thorac Dis 2018; 10(Suppl 32):S3761–S3772. [DOI] [PMC free article] [PubMed] [Google Scholar]