Abstract
Introduction:
Transcranial direct current stimulation (tDCS) and transcranial magnetic stimulation (TMS) are noninvasive neuromodulation techniques used as therapeutic and research tools for several neuropsychiatric conditions. Given the exponential scientific growth of this field we aimed to systematically review the most cited clinical trials using TMS or tDCS.
Areas covered:
A de-novo keyword search strategy identified and characterized the 100 most-cited trials. Total citation count for the most cited trials was 13,204. Articles were published between 2008 and 2014 in 50 different journals with a median impact factor of 6.52 (IQR 3.37). Almost half of the top cited papers were investigating mechanisms of action in healthy subjects. Most studies were feasibility trials and only 5 were pivotal trials, including the ones used for recent FDA approval. Seven articles were interlinked with another article by at least 25 citations and eight authors had collaborated with at least one other author.
Expert Commentary:
Although there has been a significant increase in interest for rTMS and tDCS, most of the cited clinical trials are still small feasibility studies, what reinforced the need for more robust clinical trials (larger samples sizes and effects sizes) to better define clinical effectiveness.
Keywords: bibliometrics, citation analysis, neurosciences, transcranial magnetic stimulation, transcranial direct current stimulation, noninvasive brain stimulation, neuromodulation
1. Introduction
The use of noninvasive brain stimulation in both research and clinical settings has considerably grown over the last two decades. Among all noninvasive brain stimulation techniques, transcranial direct current stimulation (tDCS) and transcranial magnetic stimulation (TMS) have been broadly studied inside the field of neurorehabilitation and tested for several psychiatric and neurologic conditions such as depression, stroke and schizophrenia [1–3].
Most of the published research efforts in the field of non-invasive brain stimulation for neurorehabilitation have been concentrated on TMS and tDCS. These two techniques have overall the larger number of scientific publication and the higher published level of evidence type of research when compared to other NIBS. While TMS was the first non-invasive technique to induce large currents into cortical areas in a non-painful manner that could induce action potential, tDCS has been an attractive option given its neuromodulatory effect that can potentiate learning and rehabilitation strategies. In the past years, the use of these techniques grew exponentially from primarily being used as diagnostic and mapping tools to applications in research such as in neurosciences and as therapeutic treatments in psychiatric diseases such as depression [5]. In 2008, clinical research efforts led the US Food and Drug Administration (FDA) to approve prefrontal rTMS (repetitive transcranial magnetic stimulation) therapy for treating Major Depressive Disorder for adults which were antidepressant medications non-responders [4,5]. A few years later, TMS was also approved by FDA for treating Migraine with aura [6]. These new applications of old techniques have boosted the number of publications in the field.
Citation analysis has been used as a measure of quality and impact of scientific publications [7–9]. Although citation analyses have been made in the broad neuroscience field [10–12], including a recently published bibliometric analysis for invasive neuromodulation [13], there is limited information for the noninvasive neuromodulation field with few published studies only [14]. Therefore, this systematic review aimed to describe and characterize the available literature in neurorehabilitation neuromodulation with focus on clinical trials on both transcranial direct current stimulation (tDCS) and transcranial magnetic stimulation (TMS), over the past 10 years, according to citation analysis. We believe that a systematic selection of the most cited neuromodulation articles, together with a descriptive analysis of it, will offer information to the where the clinical research in this field is focusing its efforts hence adding to the understanding of its directions and trends.
2. Methods
2. 1. Search Strategy
The search strategy used to identify the articles relevant for the rank of the 100 most cited neuromodulation trials presented in this study was:
“TS=”TMS” OR TS=”transcranial magnetic stimulation” OR TS=”tDCS” OR TS=”transcranial direct current stimulation” OR TS=”transcranial stimulation” OR TS=”noninvasive brain stimulation” OR TI=”neuromodulation” OR TI=”transcranial electric stimulation” OR TI=”electrical direct current therapy” OR TI=”noninvasive brain therapy” OR TI=”magnetic brain stimulation” OR TI=”repeated brain stimulation” OR TI=”neuromodulatory interventions” OR TI=”neuromodulatory therapy” OR TI=”neuromodulatory treatment” OR TI=”brain modulation” OR TI=neurostimulation OR TI=”electrical neural stimulation” OR TI=”magnetic neural stimulation” OR TI=”transcranial DC stimulation” NOT TS=”deep brain stimulation”
This search strategy was adopted after several discussion and analysis of different strategies in Web of Science database. The goal was to balance between sensibility and specificity of the strategy in order to exclude off topic articles but not to exclude articles that could be of interest for our research.
2.2. Data Source and Extraction
A search for all articles relevant for the rank of the 100 most cited neuromodulation trials presented in this study was performed between December 2017 and February 2018 at the Web of Science Core Collection database which includes but is not limited to all papers indexed by the NIH’s MEDLINE database and dates back to 1900 with a curated collection of over 20,000 peer-reviewed journals. Web of Science was chosen as database for our research in a more conservative approach since it has been historically the standard tool for bibliometric analysis. Although other databases have been also used for bibliometrics analysis, there is no available comparison between databases. [15]. An English language filter was applied since most articles published were in English followed by a temporal filter that would select only articles from between 2008 and 2017. The temporal filter was chosen as one of our goals was to discuss recent trends in the field. The remaining articles were sorted by the number of times the article was cited. To confirm that the language filter did not create any bias to our search strategy, the search process was repeated without the filter and there was no change to the results. One of the authors reviewed articles starting from the most cited and only included clinical trials until the list of 100 most cited was completed. This reviewer has been certified in a 9-month collaborative distance-learning program on methodology of clinical trials. Clinical trials were defined as according to NIH’s definition of clinical trial which is “A research study in which one or more human subjects are prospectively assigned to one or more interventions (which may include placebo or other control) to evaluate the effects of those interventions on health-related biomedical or behavioral outcomes” [16]. To determine whether a trial study could be classified as a clinical trial, the authors made use of the Decision Tree for NIH Trial Definition [16], which consists in four key questions that define a study as a clinical trial if all answers are yes (see Supplementary Material). Also, the articles should report use of tDCS and/or TMS as intervention. When necessary, uncertainty on these aspects were cleared with up to three experienced investigators until a consensus between all of them was achieved. The final list was reviewed by all the authors and uncertainty regarding the classification of any of the articles was discussed before a final decision. We included in the final list clinical trials that used tDCS or TMS with clinical parameters and clinical objectives as intervention or both intervention and assessment.
The 100 cited clinical trials published between 2008 and 2017 were described and/or classified by one or more authors according to number of citations, year, location, and journal of publication, disease under study (e.g.: Depression, Stroke), primary outcome (e.g.: Motor function, Mood), type of outcome (Clinical × Surrogate), number of authors, number of subjects, if the technique used was tDCS, TMS or both and if the technique was also used as assessment, impact factor in 2017, impact factor in the last 5 years. Considering that the interventions of interest were delivered through medical devices, we also described and/or classified according to type of study followed FDA’s classification of either feasibility or pivotal trial.
2.3. Data Analysis
Data was entered in IBM SPSS Statistics for Windows, version 21 (IBM Corp., Armonk, N.Y., USA) for descriptive and quantitative analysis. We compared TMS with tDCS trials to evaluate differences in the publications of these two techniques. To take in account the differences in citations number due to differences in time since publications we also calculated average citation/year of each article (total of citations/years of publication). Besides that, as journals impact factor can influence the number of citations, we presumed that higher journals impact factor (in the last 5 years) could be leading to higher average citation/year. Therefore, to investigate this association we performed a Spearman correlation test between the adjusted citation count (average citation/year) and last 5 years journals impact factor.
For the citation network analysis, Web of Science information was exported in CSV format and used by the VOSviewer software (version 1.6.9) to construct the co-authorship bibliometric network [17]. To evaluate if the 100 most cited clinical trials were interlinked (citing one another), a citation network consisting of these interlinked articles was visualized by using VOSviewer (Van Eck and Waltman, 2009). Only articles cited at least 25 times by other articles in the list are presented. Besides that, to reveal co-authorship collaboration, we also used VOSviewer (Van Eck and Waltman, 2009), in this analysis each author was represented by a bubble. Two bubbles were connected by a line if either one of them published a paper together. Bubble size and color indicated the citation count received within the network.
3. Results
The final search strategy yielded 34,345 published articles, including reviews and original articles. After filtering for English language only, the number of articles was reduced to 33,384. A second temporal filter was set to only select articles published between 2008 and 2017 as the authors meant to focus in the past decade (Fig.1). After the language and temporal filters, 20,020 articles remained. The final list of 100 most cited articles is depicted in table 1.
Figure 1:
Flow diagram of study selection
Table 1.
Top 100 cited neuromodulation trials published between 2008 and 2017.
| RANK | REF. | AUTHORS | YEAR | TITLE | JOURNAL | TIMES CITED | AVERAGE CITATION/YEAR |
|---|---|---|---|---|---|---|---|
| 1 | [18] | Reis, J et al | 2009 | Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation | Proceeding of the National Academy of Sciences of the United States of America http://dx.doi.org/10.1073/pnas.0805413106 |
555 | 61.67 |
| 2 | [1] | George, MS et al | 2010 | Daily Left Prefrontal Transcranial Magnetic Stimulation Therapy for Major Depressive Disorder A Sham-Controlled Randomized Trial | Archives of General Psychiatry http://dx.doi.org/10.1001/archgenpsychiatry.2010.46 |
318 | 39.75 |
| 3 | [19] | Zanto, TP et al | 2011 | Causal role of the prefrontal cortex in top-down modulation of visual processing and working memory | Nature Neuroscience http://dx.doi.org/10.1038/nn.2773 |
269 | 38.43 |
| 4 | [20] | Figner, B et al | 2010 | Lateral prefrontal cortex and self-control in intertemporal choice | Nature Neuroscience http://dx.doi.org/10.1038/nn.2516 |
250 | 31.25 |
| 5 | [21] | Batsikadze, G et al | 2013 | Partially non-linear stimulation intensity-dependent effects of direct current stimulation on motor cortex excitability in humans | Journal of Physiology-London http://dx.doi.org/10.1113/jphysiol.2012.249730 |
244 | 48.8 |
| 6 | [22] | Lindenberg, R et al | 2010 | Bihemispheric brain stimulation facilitates motor recovery in chronic stroke patients | Neurology http://dx.doi.org/10.1212/WNL.0b013e318202013a |
239 | 29.88 |
| 7 | [23] | Boggio, PS et al | 2008 | A randomized, double-blind clinical trial on the efficacy of cortical direct current stimulation for the treatment of major depression | International Journal of Neuropsychopharmacology http://dx.doi.org/10.1017/S1461145707007833 |
236 | 23.6 |
| 8 | [24] | Baker, JM et al | 2010 | Using Transcranial Direct-Current Stimulation to Treat Stroke Patients With Aphasia | Stroke http://dx/doi/org/10.1161/STROKEAHA.109.576785 |
233 | 29.13 |
| 9 | [25] | Galea, JM et al | 2011 | Dissociating the Roles of the Cerebellum and Motor Cortex during Adaptive Learning: The Motor Cortex Retains What the Cerebellum Learns | Cerebral Cortex http://dx.doi.org/10.1093/cercor/bhq246 |
229 | 32.71 |
| 10 | [26] | Monti, A. et al et al | 2008 | Improved naming after transcranial direct current stimulation in aphasia | Journal of Neurology Neurosurgery and Psychiatry http://dx.doi.org/10.1136/jnnp.2007.135277 |
204 | 20.4 |
| 11 | [27] | Keeser, D et al | 2011 | Prefrontal Transcranial Direct Current Stimulation Changes Connectivity of Resting-State Networks during fMRI | Journal of Neuroscience http://dx.doi.org/10.1523/JNEUROSCI.0542-11.2011 |
194 | 27.71 |
| 12 | [28] | Dockery, CA. et al | 2009 | Enhancement of Planning Ability by Transcranial Direct Current Stimulation | Journal of Neuroscience http://dx.doi.org/10.1523/JNEUROSCI.0065-09.2009 |
193 | 21.44 |
| 13 | [29] | Floeel, A et al | 2008 | Noninvasive brain stimulation improves language learning | Journal of Cognitive Neuroscience http://dx.doi.org/10.1162/jocn.2008.20098 |
192 | 19.2 |
| 14 | [30] | Wiethoff, S et al | 2014 | Variability in Response to Transcranial Direct Current Stimulation of the Motor Cortex | Brain Stimulation http://dx.doi.org/10.1016/j.brs.2014.02.003 |
181 | 45.25 |
| 15 | [31] | Monte-Silva, K et al | 2013 | Induction of Late LTP-Like Plasticity in the Human Motor Cortex by Repeated Non-Invasive Brain Stimulation | Brain Stimulation http://dx.doi.org/10.1016/j.brs.2012.04.011 |
181 | 36.2 |
| 16 | [32] | Loo, Colleen K et al | 2012 | Transcranial direct current stimulation for depression: 3-week, randomised, sham-controlled trial | British Journal of Psychiatry http://dx.doi.org/10.1192/bjp.bp.111.097634 |
171 | 28.5 |
| 17 | [33] | Ohn, SH et al | 2008 | Time-dependent effect of transcranial direct current stimulation on the enhancement of working memory | Neuroreport http://dx.doi.org/10.1097/WNR.0b013e3282f2adfd |
169 | 16.9 |
| 18 | [34] | Ferrucci, R. et al | 2008 | Transcranial direct current stimulation improves recognition memory in Alzheimer disease | Neurology http://dx.doi.org/10.1212/01.wnl.0000317060.43722.a3 |
162 | 16.2 |
| 19 | [35] | Brunoni, AR. et al | 2013 | The Sertraline vs Electrical Current Therapy for Treating Depression Clinical Study Results From a Factorial, Randomized, Controlled Trial | JAMA Psychiatry http://dx.doi.org/10.1001/2013.jamapsychiatry.32 |
161 | 32.2 |
| 20 | [36] | Nowak, DA. et al | 2008 | Effects of Low-Frequency Repetitive Transcranial Magnetic Stimulation of the Contralesional Primary Motor Cortex on Movement Kinematics and Neural Activity in Subcortical Stroke | Archives of Neurology http://dx.doi.org/10.1001/archneur.65.6.741 |
160 | 16 |
| 21 | [37] | Stagg, CJ et al | 2009 | Polarity and timing-dependent effects of transcranial direct current stimulation in explicit motor learning | Neuropsychologia http://dx.doi.org/10.1016/j.neuropsychologia.2011.02.009 |
151 | 16.78 |
| 22 | [38] | Galea, JM et al | 2009 | Modulation of Cerebellar Excitability by Polarity-Specific Noninvasive Direct Current Stimulation | Journal of Neuroscience http://dx.doi.org/10.1523/JNEUROSCI.2184-09.2009 |
150 | 25 |
| 23 | [39] | Brunelin, J et al | 2012 | Examining Transcranial Direct-Current Stimulation (tDCS) as a Treatment for Hallucinations in Schizophrenia | American Journal of Psychiatry http://dx.doi.org/10.1176/appi.ajp.2012.11071091 |
150 | 16.67 |
| 24 | [40] | Sparing, R et al | 2009 | Bidirectional alterations of interhemispheric parietal balance by non-invasive cortical stimulation | Brain http://dx.doi.org/10.1093/brain/awp154 |
146 | 16.22 |
| 25 | [41] | Vines, B et al | 2008 | Dual-hemisphere tDCS facilitates greater improvements for healthy subjects’ non-dominant hand compared to uni-hemisphere stimulation | BMC Neuroscience http://dx.doi.org/10.1186/1471-2202-9-103 |
146 | 14.6 |
| 26 | [42] | Grefkes, C et al | 2010 | Modulating cortical connectivity in stroke patients by rTMS assessed with fMRI and dynamic causal modeling | NeuroImage http://dx.doi.org/10.1016/j.neuroimage.2009.12.029 |
142 | 17.75 |
| 27 | [43] | Fiori, V et al | 2011 | Transcranial Direct Current Stimulation Improves Word Retrieval in Healthy and Nonfluent Aphasic Subjects | Journal of Cognitive Neuroscience http://dx.doi.org/10.1162/jocn.2010.21579 |
139 | 19.86 |
| 28 | [44] | Zaehle, T et al | 2011 | Transcranial direct current stimulation of the prefrontal cortex modulates working memory and performance: combined behavioral and electrophysiological evidence | BMC Neuroscience http://dx.doi.org/10.1186/1471-2202-12-2 |
137 | 19.57 |
| 29 | [45] | Polania, R et al | 2011 | Modulating Functional Connectivity Patterns and Topological Functional Organization of the Human Brain with Transcranial Direct Current Stimulation | Human Brain Mapping http://dx.doi.org/10.1002/hbm.21104 |
133 | 19 |
| 30 | [46] | Holland, R et al | 2011 | Speech Facilitation by Left Inferior Frontal Cortex Stimulation | Current Biology http://dx.doi.org/10.1016/j.cub.2011.07.021 |
132 | 18.86 |
| 31 | [47] | Boggio, PS et al | 2008 | Prefrontal cortex stimulation using transcranial DC stimulation reduces alcohol craving: A double-blind, sham-controlled study | Drug and Alcohol Dependence http://dx.doi.org/10.1016/j.drugalcdep.2007.06.011 |
130 | 16.25 |
| 32 | [48] | Fridriksson, J et al | 2011 | Transcranial Direct Current Stimulation Improves Naming Reaction Time in Fluent Aphasia A Double-Blind, Sham-Controlled Study | Stroke http://dx.doi.org/10.1161/STROKEAHA.110.600288 |
130 | 13 |
| 33 | [6] | Lipton, RB et al | 2010 | Single-pulse transcranial magnetic stimulation for acute treatment of migraine with aura: a randomised, double-blind, parallel-group, sham-controlled trial | Lancet Neurology http://dx.doi.org/10.1016/S1474-4422(10)70054-5 |
130 | 18.57 |
| 34 | [49] | Boggio, PS et al | 2009 | Temporal cortex direct current stimulation enhances performance on a visual recognition memory task in Alzheimer disease | Journal of Neurology Neurosurgery and Psychiatry http://dx.doi.org/10.1136/jnnp.2007.141853 |
130 | 14.44 |
| 35 | [50] | Bolognini, N et al | 2011 | Neurophysiological and Behavioral Effects of tDCS Combined With Constraint-Induced Movement Therapy in Poststroke Patients | Neurorehabilitation and Neural Repair http://dx.doi.org/10.1177/1545968311411056 |
130 | 18.57 |
| 36 | [51] | Lisanby, SH et al | 2009 | Daily Left Prefrontal Repetitive Transcranial Magnetic Stimulation in the Acute Treatment of Major Depression: Clinical Predictors of Outcome in a Multisite, Randomized Controlled Trial | Neuropsychopharmacology http://dx.doi.org/10.1038/npp.2008.118 |
128 | 14.22 |
| 37 | [52] | Andrews, SC et al | 2011 | Improving working memory: the effect of combining cognitive activity and anodal transcranial direct current stimulation to the left dorsolateral prefrontal cortex | Brain Stimulation http://dx.doi.org/10.1016/j.brs.2010.06.004 |
127 | 14.11 |
| 38 | [53] | Loo, CK et al | 2010 | A double-blind, sham-controlled trial of transcranial direct current stimulation for the treatment of depression | International Journal of Neuropsychopharmacology http://dx.doi.org/10.1017/S1461145709990411 |
127 | 14.11 |
| 39 | [54] | Ameli, M et al | 2009 | Differential Effects of High-Frequency Repetitive Transcranial Magnetic Stimulation Over Ipsilesional Primary Motor Cortex in Cortical and Subcortical Middle Cerebral Artery Stroke | Annals of Neurology http://dx.doi.org/10.1002/ana.21725 |
127 | 18.14 |
| 40 | [55] | Cho, SS; Strafella, AP | 2009 | rTMS of the Left Dorsolateral Prefrontal Cortex Modulates Dopamine Release in the Ipsilateral Anterior Cingulate Cortex and Orbitofrontal Cortex | Plos One http://dx.doi.org/10.1371/journal.pone.0006725 |
127 | 15.88 |
| 41 | [56] | Sparing, R et al | 2008 | Enhancing language performance with non-invasive brain stimulation - A transcranial direct current stimulation study In healthy humans | Neuropsychologia http://dx.doi.org/10.1016/j.neuropsychologia.2007.07.009 |
123 | 12.3 |
| 42 | [57] | Pobric, G et al | 2010 | Category-Specific versus Category-General Semantic Impairment Induced by Transcranial Magnetic Stimulation | Current Biology http://dx.doi.org/10.1016/j.cub.2010.03.070 |
122 | 15.25 |
| 43 | [58] | Ferrucci, R. et al | 2008 | Cerebellar transcranial direct current stimulation impairs the practice-dependent proficiency increase in working memory | Journal of Cognitive Neuroscience http://dx.doi.org/10.1162/jocn.2008.20112 |
121 | 12.1 |
| 44 | [59] | Koch, G et al | 2008 | Hyperexcitability of parietal-motor functional connections in the intact left-hemisphere of patients with neglect | Brain http://dx.doi.org/10.1093/brain/awn273 |
119 | 11.9 |
| 45 | [60] | Antal, A et al | 2010 | Anodal Transcranial Direct Current Stimulation Of The Motor Cortex Ameliorates Chronic Pain And Reduces Short Intracortical Inhibition. | Journal of Pain and Symptom Management http://dx.doi.org/10.1016/j.jpainsymman.2009.09.023 |
118 | 14.75 |
| 46 | [61] | Fertonani, A et al | 2010 | Naming facilitation induced by transcranial direct current stimulation | Behavioral Brain Research http://dx.doi.org/10.1016/j.bbr.2009.10.030 |
118 | 14.75 |
| 47 | [62] | Boggio, PS et al | 2008 | Modulatory effects of anodal transcranial direct current stimulation on perception and pain thresholds in healthy volunteers | European Journal of Neurology http://dx.doi.org/10.1111/j.1468-1331.2008.02270.x |
117 | 11.7 |
| 48 | [63] | Kirton, A et al | 2008 | Contralesional repetitive transcranial magnetic stimulation for chronic hemiparesis in subcortical paediatric stroke: a randomised trial | Lancet Neurology http://dx.doi.org/10.1016/S1474-4422(08)70096-6 |
116 | 11.6 |
| 49 | [64] | Keeser, D et al | 2011 | Prefrontal direct current stimulation modulates resting EEG and event-related potentials in healthy subjects: A standardized low resolution tomography (sLORETA) study | NeuroImage http://dx.doi.org/10.1016/j.neuroimage.2010.12.004 |
115 | 16.43 |
| 50 | [65] | Fitzgerald, PB et al | 2009 | A Randomized Trial of rTMS Targeted with MRI Based Neuro-Navigation in Treatment-Resistant Depression | Neuropsychopharmacology http://dx.doi.org/10.1038/npp.2008.233 |
114 | 12.67 |
| 51 | [66] | Ferrucci, R. et al | 2009 | Transcranial direct current stimulation in severe, drug-resistant major depression | Journal of Affective Disorders http://dx.doi.org/10.1016/j.jad.2009.02.015 |
112 | 12.44 |
| 52 | [67] | Polania, R et al | 2012 | Modulating cortico-striatal and thalamo-cortical functional connectivity with transcranial direct current stimulation | Human Brain Mapping http://dx.doi.org/10.1097/PHM.0b013e3181a0e4cb |
111 | 18.5 |
| 53 | [68] | Jo, JM et al | 2009 | Enhancing the Working Memory of Stroke Patients Using tDCS | American Journal of Physical Medicine & Rehabilitation http://dx.doi.org/10.1097/PHM.0b013e3181a0e4cb |
111 | 12.33 |
| 54 | [69] | Fregni, F et al | 2008 | Cortical stimulation of the prefrontal cortex with transcranial direct current stimulation reduces cue-provoked smoking craving: A randomized, sham-controlled study | Journal of Clinical Psychiatry http://www.psychiatrist.com/jcp/article/pages/2008/v69n01/v69n0105.aspx |
110 | 11 |
| 55 | [70] | Amiaz, T et al | 2009 | Repeated high-frequency transcranial magnetic stimulation over the dorsolateral prefrontal cortex reduces cigarette craving and consumption | Addiction http://dx.doi.org/10.1111/j.1360-0443.2008.02448.x |
109 | 12.11 |
| 56 | [71] | Benningerm, DH et al | 2010 | Transcranial direct current stimulation for the treatment of Parkinson’s disease | Journal of Neurology Neurosurgery and Psychiatry http://dx.doi.org/10.1136/jnnp.2009.202556 |
107 | 13.38 |
| 57 | [72] | Fregni, F et al | 2008 | Transcranial direct current stimulation of the prefrontal cortex modulates the desire for specific foods | Appetite http://dx.doi.org/10.1016/j.appet.2007.09.016 |
107 | 10.7 |
| 58 | [73] | Takeuchi, N et al | 2008 | Inhibition of the unaffected motor cortex by 1 Hz repetitive transcranial magnetic stimulation enhances motor performance and training effect of the paretic hand in patients with chronic stroke | Journal of Rehabilitation Medicine http://dx.doi.org/10.2340/16501977-0181 |
106 | 10.6 |
| 59 | [74] | Meinzer, M et al | 2012 | Electrical Brain Stimulation Improves Cognitive Performance by Modulating Functional Connectivity and Task-Specific Activation | Journal of Neuroscience http://dx.doi.org/10.1523/JNEUROSCI.4812-11.2012 |
105 | 17.5 |
| 60 | [75] | Santiesteban, I et al | 2012 | Enhancing Social Ability by stimulating Right Temporoparietal Junction | Current Biology http://dx.doi.org/10.1016/j.cub.2012.10.018 |
103 | 17.17 |
| 61 | [76] | Boros, K et al | 2008 | Premotor transcranial direct current stimulation (tDCS) affects primary motor excitability in humans | European Journal of Neuroscience http://dx.doi.org/10.1111/j.1460-9568.2008.06090.x |
103 | 10.3 |
| 62 | [77] | Fertonani, A et al | 2011 | Random Noise Stimulation Improves Neuroplasticity in Perceptual Learning | Journal of Neuroscience http://dx.doi.org/10.1523/JNEUROSCI.2002-11.2011 |
102 | 14.57 |
| 63 | [78] | Kadosh, RC et al | 2010 | Modulating Neuronal Activity Produces Specific and Long-Lasting Changes in Numerical Competence | Current Biology http://dx.doi.org/10.1016/j.cub.2010.10.007 |
102 | 12.75 |
| 64 | [79] | Baugmgartner, T et al | 2011 | Dorsolateral and ventromedial prefrontal cortex orchestrate normative choice | Nature Neuroscience http://dx.doi.org/10.1038/nn.2933 |
101 | 14.43 |
| 65 | [80] | Pobric, G et al | 2010 | Amodal semantic representations depend on both anterior temporal lobes: Evidence from repetitive transcranial magnetic stimulation | Neuropsychologia http://dx.doi.org/10.1016/j.neuropsychologia.2009.12.036 |
101 | 12.63 |
| 66 | [81] | Nitsche, MA et al | 2009 | Serotonin Affects Transcranial Direct Current-Induced Neuroplasticity in Humans | Biological Psychiatry http://dx.doi.org/10.1016/j.biopsych.2009.03.022 |
98 | 10.89 |
| 67 | [82] | Hsu, TY et al | 2011 | Modulating inhibitory control with direct current stimulation of the superior medial frontal cortex | NeuroImage http://dx.doi.org/10.1016/j.neuroimage.2011.03.059 |
97 | 9.7 |
| 68 | [83] | Koch, G et al | 2008 | Changes in intracortical circuits of the human cortex following theta burst stimulation of the lateral cerebellum | Clinical Neurophysiology http://dx.doi.org/10.1016/j.clinph.2008.08.008 |
97 | 9.7 |
| 69 | [84] | Khedr, EM et al | 2008 | Effect of daily repetitive transcranial magnetic stimulation for treatment of tinnitus: a comparison of different stimulus frequencies | Journal of Neurology Neurosurgery and Psychiatry http://dx.doi.org/10.1136/jnnp.2007.127712 |
97 | 13.86 |
| 70 | [85] | Berryhill, ME et al | 2012 | tDCS selectively improves working memory in older adults with more education | Neuroscience Letters http://dx.doi.org/10.1016/j.neulet.2012.05.074 |
96 | 16 |
| 71 | [86] | Soler, MD et al | 2010 | Effectiveness of transcranial direct current stimulation and visual illusion on neuropathic pain in spinal cord injury | Brain http://dx.doi.org/10.1093/brain/awq184 |
96 | 12 |
| 72 | [87] | Tseng, P et al | 2012 | Unleashing Potential: Transcranial Direct Current Stimulation over the Right Posterior Parietal Cortex Improves Change Detection in Low-Performing Individuals | Journal of Neuroscience http://dx.doi.org/10.1523/JNEUROSCI.0362-12.2012 |
95 | 10.56 |
| 73 | [88] | Hesse, S et al | 2011 | Combined Transcranial Direct Current Stimulation and Robot-Assisted Arm Training in Subacute Stroke Patients: An Exploratory, Randomized Multicenter Trial | Neurorehabilitation and Neural Repair http://dx.doi.org/10.1177/1545968311413906 |
95 | 15.83 |
| 74 | [89] | Koch, G et al | 2009 | Cerebellar magnetic stimulation decreases levodopa-induced dyskinesias in Parkinson disease | Neurology http://dx.doi.org/10.1212/WNL.0b013e3181ad5387 |
95 | 13.57 |
| 75 | [90] | Boggio, PS et al | 2009 | Modulation of emotions associated with images of human pain using anodal transcranial direct current stimulation (tDCS) | Neuropsychologia http://dx.doi.org/10.1016/j.neuropsychologia.2008.07.022 |
95 | 10.56 |
| 76 | [91] | Kuo, MF et al | 2008 | Limited impact of homeostatic plasticity on motor learning in humans | Neuropsychologia http://dx.doi.org/10.1016/j.neuropsychologia.2008.02.023 |
95 | 9.5 |
| 77 | [92] | Jung, P; Ziemann, U | 2009 | Homeostatic and Nonhomeostatic Modulation of Learning in Human Motor Cortex | Journal of Neuroscience http://dx.doi.org/10.1523/JNEUROSCI.0222-09.2009 |
94 | 10.44 |
| 78 | [93] | Cotelli, M et al | 2008 | Transcranial magnetic stimulation improves naming in Alzheimer disease patients at different stages of cognitive decline | European Journal of Neurology http://dx.doi.org/10.1111/j.1468-1331.2008.02202.x |
94 | 9.4 |
| 79 | [94] | DaSilva, AF et al | 2012 | tDCS-Induced Analgesia and Electrical Fields in Pain-Related Neural Networks in Chronic Migraine | Headache http://dx.doi.org/10.1111/j.1526-4610.2012.02141.x |
92 | 15.33 |
| 80 | [95] | Celnik, P et al | 2009 | Effects of Combined Peripheral Nerve Stimulation and Brain Polarization on Performance of a Motor Sequence Task After Chronic Stroke | Stroke http://dx.doi.org/10.1161/STROKEAHA.108.54050 |
92 | 10.22 |
| 81 | [96] | Martin, PI et al | 2009 | Overt naming fMRI pre- and post-TMS: Two nonfluent aphasia patients, with and without improved naming post-TMS | Brain and Language http://dx.doi.org/10.1016/j.bandl.2009.07.007 |
91 | 10.11 |
| 81 | [97] | Pena, Gomez, C et al | 2012 | Modulation of large-scale brain networks by transcranial direct current stimulation evidenced by resting-state functional MRI | Brain Stimulation http://dx.doi.org/10.1016/j.brs.2011.08.006 |
91 | 15.17 |
| 83 | [98] | Galea, JM; Celnik, P | 2009 | Brain Polarization Enhances the Formation and Retention of Motor Memories | Journal of Neurophysiology http://dx.doi.org/10.1152/jn.00184.2009 |
91 | 10.11 |
| 84 | [99] | Zimerman, M et al | 2012 | Modulation of Training by Single-Session Transcranial Direct Current Stimulation to the Intact Motor Cortex Enhances Motor Skill Acquisition of the Paretic Hand | Stroke http://dx.doi.org/10.1161/STROKEAHA.111.645382 |
90 | 11.25 |
| 85 | [100] | Floel, A et al | 2011 | Short-Term Anomia and Electrical Brain Stimulation | Stroke http://dx.doi.org/10.1161/STROKEAHA.110.609032 |
90 | 12.86 |
| 86 | [101] | Khedr, EM et al | 2010 | Long-term effect of repetitive transcranial magnetic stimulation on motor function recovery after acute ischemic stroke | Acta Neurologica Scandinavica http://dx.doi.org/10.1111/j.1600-0404.2009.01195.x |
90 | 9 |
| 87 | [102] | Priori, A et al | 2008 | Lie-Specific Involvement of Dorsolateral Prefrontal Cortex in Deception | Cerebral Cortex http://dx.doi.org/10.1093/cercor/bhm088 |
90 | 15 |
| 88 | [103] | Mori, F et al | 2010 | Effects of Anodal Transcranial Direct Current Stimulation on Chronic Neuropathic Pain in Patients With Multiple Sclerosis | Journal of Pain http://dx.doi.org/10.1016/j.jpain.2009.08.011 |
89 | 9.89 |
| 89 | [104] | Khedr, EM et al | 2009 | Role of 1 and 3 Hz repetitive transcranial magnetic stimulation on motor function recovery after acute ischaemic stroke | European Journal of Neurology http://dx.doi.org/10.1111/j.1468-1331.2009.02746.x |
89 | 11.13 |
| 90 | [105] | Meinzer, M et al | 2013 | Anodal Transcranial Direct Current Stimulation Temporarily Reverses Age-Associated Cognitive Decline and Functional Brain Activity Changes | Journal of Neuroscience http://dx.doi.org/10.1523/JNEUROSCI.5743-12.2013 |
88 | 17.6 |
| 91 | [106] | Antal, A et al | 2008 | Transcranial direct current stimulation over somatosensory cortex decreases experimentally induced acute pain perception | Clinical Journal of Pain http://dx.doi.org/10.1097/AJP.0b013e318157233b |
88 | 8.8 |
| 92 | [107] | Nyffeler, T et al | 2009 | One Session of Repeated Parietal Theta Burst Stimulation Trains Induces Long-Lasting Improvement of Visual Neglect | Stroke http://dx.doi.org/10.1161/STROKEAHA.109.552323 |
87 | 9.67 |
| 93 | [108] | Jayaram, G et al | 2012 | Modulating locomotor adaptation with cerebellar stimulation | Journal of Neuphysiology http://dx.doi.org/10.1152/jn.00645.2011 |
86 | 14.33 |
| 94 | [109] | You, DS et al | 2011 | Cathodal transcranial direct current stimulation of the right Wernicke’s area improves comprehension in subacute stroke patients | Brain and Language http://dx.doi.org/10.1016/j.bandl.2011.05.002. |
86 | 12.29 |
| 95 | [110] | Brunoni, AR et al | 2011 | Transcranial direct current stimulation (tDCS) in unipolar vs. bipolar depressive disorder | Progress in Neuro-Psychopharmacology & Biological Psychiatry http://dx.doi.org/10.1016/j.pnpbp.2010.09.010 |
86 | 12.29 |
| 96 | [111] | Weiduschat, N et al | 2011 | Effects of Repetitive Transcranial Magnetic Stimulation in Aphasic Stroke A Randomized Controlled Pilot Study | Stroke http://dx.doi.org/10.1161/STROKEAHA.110.597864 |
85 | 12.14 |
| 97 | [112] | de Vries, MH et al | 2010 | Electrical Stimulation of Broca’s Area Enhances Implicit Learning of an Artificial Grammar | Journal of Cognitive Neuroscience http://dx.doi.org/10.1162/jocn.2009.21385 |
85 | 8.5 |
| 98 | [113] | Kleinjung, T et al | 2008 | Combined temporal and prefrontal transcranial magnetic stimulation for tinnitus treatment: A pilot study | Otolaryngology-Head and Neck Surgery http://dx.doi.org/10.1016/j.otohns.2007.12.022 |
85 | 10.63 |
| 99 | [114] | Avenanti, A et al | 2012 | Low-frequency rTMS promotes use-dependent motor plasticity in chronic stroke A randomized trial | Neurology http://dx.doi.org/10.1212/WNL.0b013e3182436558 |
82 | 13.67 |
| 100 | [115] | Cotelli, M et al | 2011 | Improved language performance in Alzheimer disease following brain stimulation | Journal of Neurology Neurosurgery and Psychiatry http://dx.doi.org/10.1136/jnnp.2009.197848 |
81 | 11.57 |
3.1. Number of Citations
The 100 most cited noninvasive neuromodulation clinical trials are responsible all together for 13,204 citations. These articles were cited a median of 113 times (range 81–555). The average number of citations for the top 10 cited articles was 278. The median adjusted number of citation (citation count/years from publication) was 14.4 (range 8.5–61.7). Moreover, the median citation count of tDCS clinical trials was higher (117; range 555–85) than the TMS trials (103; range 318–81).
The median impact factor for the journals that published the top 100 most cited clinical trial in TMS and tDCS was 6.52 (Interquartile Range 3.37). Moreover, we found a significant correlation between the last 5 years journal impact factor and the adjusted number of citation (r= 0.29892, p = 0.003).
By analysing the reference lists for the 100 cited neuromodulation papers, we have found out that seven articles were interlinked with one another with at least 25 citations. Table 2 shows the list of articles that have had at least 25 citations within the 100 most cited articles list. Nitsche MA article published in 2000 and named “Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation” had the greatest number of citations (45) within the 100 most cited articles list.
Table 2.
Citations network within the 100 most cited articles list
| Author | Year | Article | Citations within top 100 list | Reference number |
|---|---|---|---|---|
| Nitsche, MA | 2000 | Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation | 45 | [116] |
| Gandiga, PC | 2006 | Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation | 36 | [117] |
| Nitsche, MA | 2001 | Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans | 34 | [118] |
| Liebetanz, D | 2002 | Pharmacological approach to the mechanisms of transcranial DC‐stimulation‐induced after‐effects of human motor cortex excitability | 29 | [119] |
| Iyer, MB | 2005 | Safety and cognitive effect of frontal DC brain polarization in healthy individuals | 27 | [120] |
| Fregni, F | 2005 | Anodal transcranial direct current stimulation of prefrontal cortex enhances working memory | 25 | [121] |
| Nitsche, MA | 2003 | Pharmacological Modulation of Cortical Excitability Shifts Induced by Transcranial Direct Current Stimulation in Humans | 25 | [122] |
3.2. Journals
The 100 most cited clinical trials were published in 50 different journals of which, 29 had only one paper included in the list (Table 1 – Supplementary Material). The remaining 21 journals published two or more articles among the 100 most frequently cited neuromodulation clinical trials. The Journal of Neuroscience and Stroke Journal can be highlighted as the ones with most trials included in the list with eight and seven papers, respectively.
3.3. Year of Publication
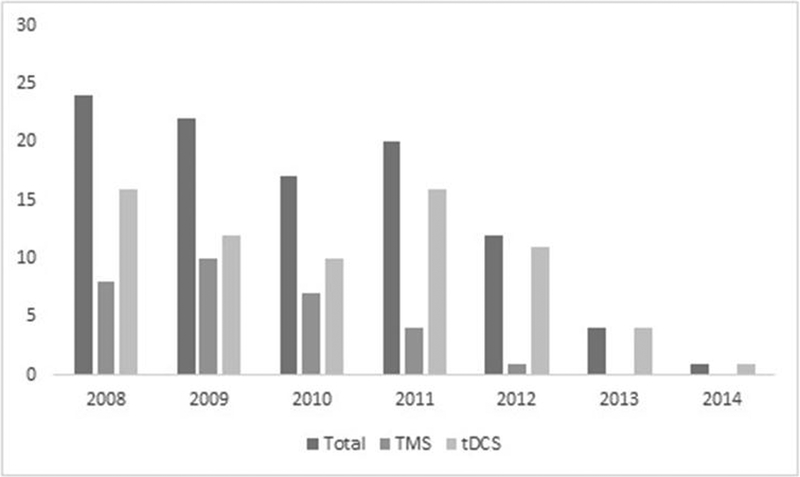
Although the timespan filter applied included articles from 2008 to 2017, the most cited neuromodulation trials from this list were published between 2008 and 2014. Most articles in the list were published in 2008 (24) and 2009 (22) and only one was published in 2014. It was expected that articles published earlier would have more citations as they have had more time to be cited. Table 1 we shows the average citation per year – number of citations divided by the number of years being published. Figure 2. Illustrates the number of articles within the 100 most cited neuromodulation trials by year of publication. Table 2 (Supplementary Material) shows the year of publication of the trials according to TMS vs tDCS.
Figure 2:
Number of articles within the 100 most cited neuromodulation trials by year of publication
3.4. Most Common Areas of Investigation
Among the 100 cited neuromodulation trials, Stroke was the most commonly studied neurological condition, representing one quarter of the papers, being followed by Depression with 9 papers. Other diseases include Alzheimer’s Disease (4), Smoking Addiction (2), Migraine (2), Parkinson’s Disease (2), Tinnitus (2), Alcohol dependence (1), Chronic Pain (1), Multiple Sclerosis (1), Schizophrenia (1) and Spinal Cord Injury (1). Three out of the 25 trials studying Stroke included both diseased patients and healthy subjects to compare the effects of either TMS or tDCS on health-related outcomes for this condition, such as comparing the effects or tDCS over word retrieval in healthy and aphasic stroke patients [43].
It is important to point out that almost half (49) of the publications were not investigating the therapeutic effects of tDCS or TMS for specific diseases. Instead, those clinical trials meant to gather information on behalf the effects of noninvasive brain stimulation on several different health related outcomes (e.g.: motor acquisition, language learning, cortical excitability) hence aiming to better understand its mechanisms. These mechanistic trials applied tDCS or TMS to healthy subjects and measured its effects.
Most trials (95) composing the rank were classified as feasibility trials, according to a FDA’s definition. Traditional feasibility study is a clinical investigation that is commonly used to capture preliminary safety and effectiveness information on a near-final or final device design to adequately plan an appropriate pivotal study” while a pivotal study can be defined as “a clinical investigation designed to collect definitive evidence of the safety and effectiveness of a device for a specified intended use, typically in a statistically justified number of subjects”. [123] Only 5 out of the total of trials were classified as pivotal trials, with 4 testing rTMS or tDCS for the treatment of Depression and 1 investigation single pulse TMS for the treatment of Migraine. Although most studies were classified as feasibility trials, 54 used a clinical outcome as primary outcome (e.g.: Visual Analog Scales for pain or craving or Montgomery-Åsberg Depression Rating Scale) while 46 used surrogate outcomes (e.g.: EEG, cortical excitability measurements with TMS or fMRI). The primary outcome chosen varied amongst motor related outcomes (21), mood (9), aphasia (8), working memory (8) and others (54).
Furthermore, most clinical trials were investigating the use of tDCS (70) as intervention, and some of them also included TMS as an assessment technique (4 papers). Out of the 100 most cited trials, 30 were using repetitive TMS (rTMS) as intervention. Table 3 depicts the most common focus of the 100 most cited trials according to TMS or tDCS studies.
Table 3.
Most common areas of investigation for TMS and tDCS studies
| tDCS | TMS | |||
|---|---|---|---|---|
| Disease/Condition under study | n | (%) | n | (%) |
| Healthy | 41 | 58.6 | 8 | 26.7 |
| Stroke | 13 | 18.6 | 12 | 40 |
| Depression | 6 | 8.6 | 3 | 10 |
| Alzheimer Disease | 2 | 2.9 | 2 | 6.7 |
| Addiction – Smoking | 1 | 1.4 | 1 | 3.3 |
| Alcohol dependence | 1 | 1.4 | - | - |
| Chronic Pain | 1 | 1.4 | - | - |
| Migraine | 1 | 1.4 | 1 | 3.3 |
| Multiple Sclerosis | 1 | 1.4 | - | - |
| Parkinson’s Disease | 1 | 1.4 | 1 | 3.3 |
| Schizophrenia | 1 | 1.4 | - | - |
| Spinal Cord Injury | 1 | 1.4 | - | - |
| Tinnitus | 0 | 0 | 2 | 6.7 |
| Total | 70 | 100 | 30 | 100 |
3.5. Location of Study
Regarding the geographic region in which the study was conducted, this information was not clear in the manuscript for about one third of the trials (32), but authors were not contacted to clarify the information nor trial registration was consulted. From the remaining 68 articles that reported this information, 31 publications were from Europe, 25 from North and South America - represented by the following countries: USA (15 publications), Brazil (8 publications) and Canada (2 publications). The other trials included in the rank were performed in Asia (5), Oceania (3) and Africa (3). A single multicenter trial was identified, and it was conducted in USA, Canada and Australia. Table 4 shows where trials using rTMS and tDCS took place accordingly.
Table 4.
Geographical location of trials
| TMS | tDCS | |||
|---|---|---|---|---|
| Trial Location (Country) | n | (%) | n | (%) |
| Unknown | 10 | 33.3 | 22 | 31.4 |
| Germany | 4 | 13.3 | 12 | 17.1 |
| USA | 3 | 10 | 12 | 17.1 |
| Brazil | - | - | 8 | 11.4 |
| Italy | 2 | 6.7 | 5 | 7.1 |
| Australia | 1 | 3.3 | 2 | 2.9 |
| Spain | - | - | 2 | 2.9 |
| Taiwan | - | - | 2 | 2.9 |
| UK | - | - | 1 | 1.4 |
| France | - | - | 1 | 1.4 |
| South Korea | - | - | 1 | 1.4 |
| Switzerland | 2 | 6.7 | 1 | 1.4 |
| Canada | 3 | 10 | - | - |
| Egypt | 3 | 10 | - | - |
| Israel | 1 | 3.3 | - | - |
| Japan | 1 | 3.3 | - | - |
| Total | 30 | 100 | 70 | 100 |
3.6. Authorship and Co-authorship Collaborations
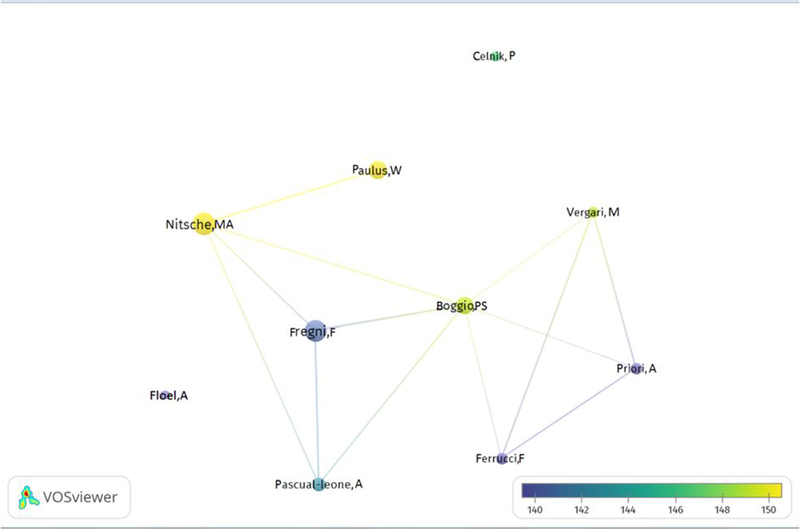
A total of 477 unique authors contributed to these 100-most cited articles. It is important to point out that most of these authors are co-authors and collaborated in several of these publications. Therefore, to better understand the co-authorship and collaborations in the noninvasive brain stimulation field, we also performed a co-authorship network map using the Vosviewer software.
The co-authorship network analysis illustrates the collaboration among the authors of the 100 most cited neuromodulation trials. Eight authors have at least one common author to whom they have collaborated. Figure 3 shows the patterns of collaboration among authors. Table 5 shows the co-authors and their respective number of articles and citations among the 100 most cited neuromodulation clinical trials.
Figure 3:
Co-authorship inter-citation diagram among the 100 most-cited Non-invasive brain stimulation articles. In the map, the color of a point in the visualization is determined by the density of items at that point, the co-authorship is classified with a link of strength (from 140 to 150 citations on average) based on the citations of the articles, higher the number more links and citations between the authors.
Table 5.
Co-authors number of articles and citations among the 100 most cited neuromodulation clinical trials.
| Author | Number of Articles | Citations | Average citations per article | h-index |
|---|---|---|---|---|
| Fregni, F | 12 | 1691 | 141 | 76 |
| Nitsche, MA | 11 | 1787 | 162 | 81 |
| Boggio, PS | 9 | 1340 | 149 | 47 |
| Paulus, W | 9 | 1361 | 151 | 109 |
| Pascual-Leone, A | 7 | 998 | 143 | 131 |
| Ferruci, R | 6 | 839 | 140 | 29 |
| Priori, A | 6 | 839 | 140 | 38 |
| Vergari, M | 5 | 743 | 149 | 14 |
| Celnik, P | 5 | 732 | 146 | 13 |
| Fink, GR | 5 | 758 | 152 | 155 |
| Floeel, A | 5 | 623 | 125 | 45 |
4. Discussion
This study classified the most cited clinical trials articles on neuromodulation with focus on both transcranial direct current stimulation (tDCS) and transcranial magnetic stimulation (TMS) over the past 10 years using a citation analysis. Although this type of analysis is available for the neurology field, including a recently published one for invasive neuromodulation [13] and on TMS [14], yet, citation analyses of noninvasive neuromodulation is still uncommon. The aim of this citation analysis was to understand further areas of highest impact of tDCS and TMS. Even though, citation counts are not a direct measure of scientific work quality and importance, the comparison of citation counts over periods of time has the potential to allow identification of how the main topics being discussed in the field change overtime, hence detecting trends in the field.
According to Bornmann et al., citations count analysis depends on several factors, not only scientific ones. There are, for example, journal-dependent factors, such as journal’s impact factor and frequency of publication. Findings from our citation analysis corroborate with their theory, as we showed that the most frequently cited articles in the list are among the journals considered to have high impact factors, we also showed a significant but weak correlation between adjusted number of citations with last 5 years journals impact factor. It is important to highlight that, although impact factor of the journal and citations count were correlated in this paper, we believe that, in concordance to the San Francisco’s Declaration of Research Assessment (DORA), impact factor should not be used as surrogate measure of quality of research and the 100 cited articles found in this paper are not ranked by quality [124]. Time-dependent factors were also observed in the present study as for example the article with more citations was published in 2009. According to the authors “citations become more probable from year to year” [7]. However, this article also has the biggest adjusted number of citations (61.67).
Although the number of articles being published has continued to increase, the most cited publications tend to be limited to the initial years of the series (2008–2012). Therefore, it is unlikely that a recently published article would be able to rapidly overthrow a highly cited one that has been published few years before.
The average citation count of the 100 most-cited clinical trials in noninvasive brain stimulation was 132 times (median 113 - range 81–555), a much lower citation than the one observed in general neuroscience articles (3,087) [125]. Even though this difference could be explained by the fact that basic science research and reviews (both included in the general neuroscience top 100 publications) often generates more citations than specific clinical trial publications [126], when we compared this field – NIBS - with clinical/surgical subfields such as neurosurgery (452.6) [12], neurointervention (363.5) [11], neurorehabilitation (317.0) [10] and invasive brain stimulation (median of 236 citations among the top 50 most cited) [13] we also observed a lower average/median citation count.
Interestingly, the most common topic of our analysis was related with motor or language outcomes for stroke recovery. This may be due to stroke’s large burden on health resources, since this condition still is a known leading cause of disability in the United States and worldwide. Even though previous clinical trials have shown promising results regarding the uses of TMS and tDCS [127] for stroke rehabilitation, these are still small clinical trials and it is still unclear whether results from these trials are clinically meaningful [128,129].
Our results revealed a predominance of mechanistic trials among the most cited ones. This suggests that the field of noninvasive neuromodulation is still under development. This means that several questions remain to be answered, and new questions emerge as the field moves towards a more clinical application, especially when translating knowledge from preclinical animal studies into clinical trials. Currently, key factors such as safety, dose-response, regimen of treatment, inter subject variability are still under investigation since they can change the induced physiological responses. According to our list of most frequent cited articles, some of the current topics in noninvasive brain stimulation are trials investigating inter individual variability, predictors of response and target engagement-network connectivity [130,131].
The authorship pattern analysis reveals the core authors collaboration network in the neuromodulation research field. This information can be relevant for clinical researchers and research institutions that are searching the network of research leaders in this field to explore potential collaborations. The number of authors (7) revealed in our authorship analysis is not as large as in other fields in clinical research. Azondekon et al, 2018 performed a scientific authorship and collaboration network analysis on malaria research and a much larger collaboration was compared to our study. Many variables can explain the differences of the size of our analysis to Azondekon et al, 2018 including time period of analysis, public health burden of the condition and field differences. It is likely that the neuromodulation authorship network will grow with time as the fields progress into more clinical applications [132,133].
Finally, another interesting result is that in our analysis, studies using tDCS were more frequent and had a higher median citation count when compared to rTMS studies. This is likely because tDCS has some practical advantages when compared with TMS since it is a much more affordable and as safe technique, besides that the device is portable and can be easily combined with other methods. These facts could explain why there is a higher number of studies involving such technique and hence a higher probability of citation; however, journal publishing TMS studies have an average higher impact factor (9.18) that the ones publishing tDCS (5.8). As previously discussed, the interpretation of citations count analysis and how it can be influenced by journals impact factor is not straightforward. Finally, it is important to address some limitations that are intrinsic of this type of analysis as for instance number of citations does not always reflect the impact of the publications. For example, some citations might be inappropriate, and the number of citations can also be manipulated by self-citations or induced citations. Besides that, actual geographic distance and the preference of citing articles in English can influence citation numbers.
5. Conclusion
This review provides an overview of the most cited clinical trials within the noninvasive neuromodulation field, showing that both TMS and tDCS have been extensively studied as potential therapies for several neurologic and psychiatric conditions. In the light of above, the noninvasive neuromodulation field prevails as a relevant research area. The increasing number of scientific productions is an objective measure of peer recognition and is also indicative of high need of efficacious interventions for the neuropsychiatric clinical practice. Even though several mechanistic questions remain topic of discussion, hence the high number of mechanistic trials being cited, the use of noninvasive brain stimulation as therapeutic tool continues growing. In fact, year of publication and journal related factors play an important role in this type of analysis, however the results presented highlighted major milestones in the field, corroborated the diversity of application of these techniques and shaded light in future challenges to be addressed.
6. Expert Opinion
The use of noninvasive brain stimulation techniques has exponentially grown in the last years as therapeutic and research tools for several neuro-psychiatric conditions. The neurophysiological mechanisms of action of both tDCS and TMS have been extensively investigated to support its applications in clinical scenarios. Even though most of the clinical trials still show small to moderate the effect size, there is significant variability within studies. Studies with more citations are mechanistic trials, which reinforces the need to better understanding the mechanisms underlying the effects of these interventions over neuroplasticity and current distribution through the stimulated brain areas. In addition, the growing search for surrogate markers, neuro-signatures and neurophysiological predictors that could be correlated with disease diagnostic, prognostic or tailor a more individualize intervention protocols. For example, the use of functional MRI, single and paired pulse TMS markers (motor threshold, motor evoked potential and paired pulse) and quantitative EEG.
The analysis of the most cited clinical articles may provide significant insights as the field of noninvasive brain stimulation moves towards more clinical applications. For instance, differences in research field interest when comparing studies using TMS with the ones using tDCS. Moreover, the present study provides a glance in where the studies are being conducted, identifying how collaboration in the field contributed to the science growth. This citation analysis in noninvasive brain stimulation also shows an historical perspective on tDCS and TMS research and allows the comprehensive identification of the most relevant research topics, hence being relevant for researchers and research institutions. Even though new devices and NIBS techniques are emerging, certain methodological issues seem to be common for both techniques independently on the condition to be treated.
For the near future, increasing effectiveness of these interventions seems to be critical for the field. For that, a better understanding of dosage and factors that might enhance or block the tDCS and TMS effects and the duration of the after-effects are essential.
Future clinical studies addressing the interaction of NIBS with drugs, behavioral therapies and other will continue to explore and identify which are the best ways to optimize the existent protocols. Moreover, there are potential strategies for home-based therapies (mainly for tDCS) and monitoring (eg. EEG, smart watches) that can lead to a more feasible assessments of the techniques specially in specific populations such as in spinal cord injury. The non-invasive brain stimulation field has developed quickly in the past 10 years, including FDA approval for the TMS use in major depression, migraine headaches and more recently for obsessive compulsive disorder. For the next 5 years it is expected more robust clinical trials (larger samples sizes and effects sizes) leading towards a tailored protocol intervention and, as consequence, to more definite clinical effectiveness.
Supplementary Material
Article Highlights.
Exponential growth of the number of publications and clinical applications of tDCS and TMS, mainly due to its potential of enhancing brain plasticity while having few side effects.
Citation analysis can measure the impact of scientific publications providing a better understanding of specific research field its directions and trends, however there is lack of such information for the noninvasive neuromodulation field.
The identification of the 100 most-cited clinical trials using TMS or tDCS by this review aimed to describe and characterize the available literature in neuromodulation over the past 10 years.
Citation counts might not be a direct measure of scientific quality; however, it is a valid method to identify trends in research. This review provides an overview of the neuromodulation field and its co-authorship network and collaborating authors.
Funding
This paper was funded by the National Institutes of Health, grant nos. 1 R01 AT009491-01A1 and 5R01HD082302-02.
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
REFERENCES
* Reference of importance due to the similarity with the current paper in subject, serves as comparison for our work.
** Reference of considerable importance since it was used as reference for definition of methodological approach for the present study.
- [1].George MS, Lisanby SH, Avery D et al. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Arch Gen Psychiatry. 2010. May;67(5):507–16. [DOI] [PubMed] [Google Scholar]
- [2].O’Brien AT, Bertolucci F, Torrealba-Acosta G et al. Non-invasive brain stimulation for fine motor improvement after stroke: a meta-analysis. Eur J Neurol. 2018. August;25(8):1017–1026 [DOI] [PubMed] [Google Scholar]
- [3].He H, Lu J, Yang L et al. Repetitive transcranial magnetic stimulation for treating the symptoms of schizophrenia: A PRISMA compliant meta-analysis. Clin Neurophysiol. 2017. May;128(5):716–724 [DOI] [PubMed] [Google Scholar]
- [4].Perera T, George MS, Grammer G et al. The Clinical TMS Society Consensus Review and Treatment Recommendations for TMS Therapy for Major Depressive Disorder. Brain Stimul. 2016. May-Jun;9(3):336–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Horvath JC, Mathews J, Demitrack MA et al. The NeuroStar TMD Device: Conducting the FDA Approved Protocol for Treatment of Depression. J Vis Exp. 2010;(45):2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lipton RB, Dodick DW, Silberstein SD et al. Single-pulse transcranial magnetic stimulation for acute treatment of migraine with aura: a randomised, double-blind, parallel-group, sham-controlled trial. Lancet Neurol. 2010. April;9(4):373–380. [DOI] [PubMed] [Google Scholar]
- [7].Bornmann L, Daniel HD. What do citation counts measure? A review of studies on citing behavior. J Doc. 2008;64(1):45–80 [Google Scholar]
- [8].Radicchi F, Fortunato S, Castellano C. Universality of citation distributions: toward an objective measure of scientific impact. Proc Natl Acad Sci U S A. 2008. November;105(45):17268–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Maggio LA, Meyer HS, Artino AR Jr. Beyond Citation Rates: A Real-Time Impact Analysis of Health Professions Education Research Using Altmetrics. Acad Med. 2017. October;92 (10):1449–1455 [DOI] [PubMed] [Google Scholar]
- [10].Kreutzer JS, Agyemang AA, Weedon D et al. The top 100 cited neurorehabilitation papers. NeuroRehabilitation. 2017;40(2):163–174** [DOI] [PubMed] [Google Scholar]
- [11].Kim ES, Yoon DY, Kim HJ et al. Citation classics in neurointerventional research: a bibliometric analysis of the 100 most cited articles. J Neurointerv Surg. 2017. May; 9(5): 508–511 [DOI] [PubMed] [Google Scholar]
- [12].Ponce FA, Lozano AM. Highly cited works in neurosurgery. Part I: the 100 top-cited papers in neurosurgical journals. J Neurosurg 2010. February;112(2):223–32 [DOI] [PubMed] [Google Scholar]
- [13].Ward M, Doran J et al. The 50 Most Cited Articles in Invasive Neuromodulation. World Neurosurg. 2018. March 14 pii: S1878–8750(18)30443–1 [DOI] [PubMed] [Google Scholar]
- [14].Lawson McLean A Publication trends in transcranial magnetic stimulation: a 30-year panorama. Brain Stimul. 2019. January 9 Pii. S1935-861X(19)30030-0* [DOI] [PubMed] [Google Scholar]
- [15].Kulkami AV, Aziz B, Shams I et al. Comparisons of citations in Web of Science [DOI] [PubMed]
- [16].NIH’s Definition of a Clinical Trial [Internet]. Bethesda (MD): National Institutes of Health; 2017. August 8 [cited 2019 Jan 13]. Available from: https://grants.nih.gov/policy/clinical-trials/definition.htm [Google Scholar]
- [17].van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. 2010;84(2):523–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Reis J, Schambra HM, Cohen LG et al. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. 2009. Proc Natl Acad Sci U S A. 2009. February 3;106(5):1590–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zante TP, Rubens MT, Thangavel A et al. Causal role of the prefrontal cortex in top-down modulation of visual processing and working memory. Nat Neurosci. 2011. May;14(5):656–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Finger B, Knoch D, Johnson EJ et al. Lateral prefrontal cortex and self-control in intertemporal choice. Nat Neurosci. 2010. May;13(5):538–9 [DOI] [PubMed] [Google Scholar]
- [21].Batsikadze G, Moliadze, Paulus W et al. Partially non-linear stimulation intensity-dependent effects of direct current stimulation on motor cortex excitability in humans.J Physiol. 2013. April 1;591(7):1987–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lindenberg R, Renga V, Zhu LL et al. Bihemispheric brain stimulation facilitates motor recovery in chronic stroke patients. Neurology. 2010. December 14;75(24):2176–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Boggio PS, Rigonatti SP, Ribeiro RB et al. A randomized, double-blind clinical trial on the efficacy of cortical direct current stimulation for the treatment of major depression. Int J Neuropsychopharmacol. 2008. March;11(2):249–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Baker JM, Rorden C, Fridiksson J. Using transcranial direct-current stimulation to treat stroke patients with aphasia. Stroke. 2010. June;41(6):1229–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Galea JM, Vazquez A, Pasricha N et al. Dissociating the roles of the cerebellum and motor cortex during adaptive learning: the motor cortex retains what the cerebellum learns. Cereb Cortex. 2011. August;21(8):1761–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Monti A, Cogiamanian F, Marceglia S et al. Improved naming after transcranial direct current stimulation in aphasia. J Neurol Neurosurg Psychiatry. 2008. April;79(4):451–3 [DOI] [PubMed] [Google Scholar]
- [27].Keeser D, Meindl T, Bor J et al. Prefrontal transcranial direct current stimulation changes connectivity of resting-state networks during fMRI. J Neurosci. 2011. October 26;31(43):15284–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Dockery CA, Hueckel-Weng R, Birbaumer N et al. Enhancement of planning ability by transcranial direct current stimulation. J Neurosci. 2009. June 3;29(22):7271–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Flöel A, Rösser N, Micka O et al. Noninvasive brain stimulation improves language learning. J Cogn Neurosci. 2008. August;20(8):1415–22 [DOI] [PubMed] [Google Scholar]
- [30].Wiethoff S, Hamada M, Rothwell JC. Variability in response to transcranial direct current stimulation of the motor cortex. Brain Stimul. 2014. May-Jun;7(3):468–75 [DOI] [PubMed] [Google Scholar]
- [31].Monte-Silva K, Kuo MF, Hessenthaler S et al. Induction of late LTP-like plasticity in the human motor cortex by repeated non-invasive brain stimulation. Brain Stimul. 2013. May;6(3):424–32 [DOI] [PubMed] [Google Scholar]
- [32].Loo CK, Alonzo A, Martin D et al. Transcranial direct current stimulation for depression: 3-week, randomised, sham-controlled trial. Br J Psychiatry. 2012. January;200(1):52–9 [DOI] [PubMed] [Google Scholar]
- [33].Ohn SH, Park CI, Yoo WK et al. Time-dependent effect of transcranial direct current stimulation on the enhancement of working memory. Neuroreport. 2008. January 8;19(1):43–7 [DOI] [PubMed] [Google Scholar]
- [34].Ferrucci R, Mameli F, Guidi I et al. Transcranial direct current stimulation improves recognition memory in Alzheimer disease. Neurology. 2008. August 12;71(7):493–8 [DOI] [PubMed] [Google Scholar]
- [35].Brunoni AR, Valiengo L, Baccaro A et al. The sertraline vs. electrical current therapy for treating depression clinical study: results from a factorial, randomized, controlled trial. JAMA Psychiatry. 2013. April;70(4):383–91 [DOI] [PubMed] [Google Scholar]
- [36].Nowak DA, Grefkes C, Dafotakis M et al. Effects of low-frequency repetitive transcranial magnetic stimulation of the contralesional primary motor cortex on movement kinematics and neural activity in subcortical stroke. Arch Neurol. 2008. June;65(6):741–7 [DOI] [PubMed] [Google Scholar]
- [37].Stagg CJ, Jayaram G, Pastor D et al. Polarity and timing-dependent effects of transcranial direct current stimulation in explicit motor learning. Neuropsychologia. 2011. April;49(5):800–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Galea JM, Jayaram G, Ajagbe L et al. Modulation of cerebellar excitability by polarity-specific noninvasive direct current stimulation. J Neurosci. 2009. July 15;29(28):9115–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Brunelin J, Mondino M, Gassab L et al. Examining transcranial direct-current stimulation (tDCS) as a treatment for hallucinations in schizophrenia. Am J Psychiatry. 2012. July;169(7):719–24 [DOI] [PubMed] [Google Scholar]
- [40].Sparing R, Thimm M, Hesse MD et al. Bidirectional alterations of interhemispheric parietal balance by non-invasive cortical stimulation. Brain. 2009. November;132(Pt 11):3011–20 [DOI] [PubMed] [Google Scholar]
- [41].Vines BW, Cerruti C, Schlaug G. Dual-hemisphere tDCS facilitates greater improvements for healthy subjects’ non-dominant hand compared to uni-hemisphere stimulation. BMC Neurosci. 2008. October 28;9:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Greeks C, Nowak DA, Wang LE et al. Modulating cortical connectivity in stroke patients by rTMS assessed with fMRI and dynamic causal modeling. Neuroimage. 2010. March;50(1):233–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Fiori V, Coccia M, Marinelli CV et al. Transcranial direct current stimulation improves word retrieval in healthy and nonfluent aphasic subjects [DOI] [PubMed]
- [44].Zaehle T, Sandmann P, Thorne JD et al. Transcranial direct current stimulation of the prefrontal cortex modulates working memory and performance: combined behavioral and electrophysiological evidence. BMC Neurosci. 2011. January 6;12:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Polania R, Nitsche MA, Paulus W. Modulating Functional Connectivity Patterns and Topological Functional Organization of the Human Brain with Transcranial Direct Current Stimulation. Hum Brain Mapp. 2011. August;32(8):1236–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Holland R, Leff AP, Josephs O et al. Speech facilitation by left inferior frontal cortex stimulation. Curr Biol. 2011. August 23;21(16):1403–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Boggio PS, Sultani N, Fecteau S et al. Prefrontal cortex stimulation using transcranial DC stimulation reduces alcohol craving: A double-blind, sham-controlled study. Drug Alcohol Depend. 2008. January 1;92(1–3):55–60 [DOI] [PubMed] [Google Scholar]
- [48].Fridriksson J, Richardson JD, Baker JM et al. Transcranial Direct Current Stimulation Improves Naming Reaction Time in Fluent Aphasia A Double-Blind, Sham-Controlled Study. Stroke. 2011. March;42(3):819–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Boggio PS, Khoury LP, Martins DC et al. Temporal cortex direct current stimulation enhances performance on a visual recognition memory task in Alzheimer disease. J Neurol Neurosurg Psychiatry. 2009. April;80(4):444–7 [DOI] [PubMed] [Google Scholar]
- [50].Bolognini N, Vallar G, Casati C et al. Neurophysiological and Behavioral Effects of tDCS Combined With Constraint-Induced Movement Therapy in Poststroke Patients. Neurorehabil Neural Repair. 2011. Nov-Dec;25(9):819–29 [DOI] [PubMed] [Google Scholar]
- [51].Lisanby SH, Husain MM, Rosenquist PB et al. Daily left prefrontal repetitive transcranial magnetic stimulation in the acute treatment of major depression: clinical predictors of outcome in a multisite, randomized controlled clinical trial. Neuropsychopharmacology. 2009. January;34(2):522–34 [DOI] [PubMed] [Google Scholar]
- [52].Andrews SC, Hoy KE, Enticott PG et al. Improving working memory: the effect of combining cognitive activity and anodal transcranial direct current stimulation to the left dorsolateral prefrontal cortex. Brain Stimul. 2011. April;4(2):84–9 [DOI] [PubMed] [Google Scholar]
- [53].Loo CK, Sachdev P, Martin D et al. A double-blind, sham-controlled trial of transcranial direct current stimulation for the treatment of depression. Int J Neuropsychopharmacol. 2010. February;13(1):61–9 [DOI] [PubMed] [Google Scholar]
- [54].Ameli M, Grefkes C, Kemper F et al. Differential effects of high-frequency repetitive transcranial magnetic stimulation over ipsilesional primary motor cortex in cortical and subcortical middle cerebral artery stroke. Ann Neurol. 2009. September;66(3):298–309 [DOI] [PubMed] [Google Scholar]
- [55].Cho SS, Strafella AP. rTMS of the left dorsolateral prefrontal cortex modulates dopamine release in the ipsilateral anterior cingulate cortex and orbitofrontal cortex. PLoS One. 2009. August 21;4(8):e6725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Sparing R, Dafotakis M, Meister IG et al. Enhancing language performance with non-invasive brain stimulation - A transcranial direct current stimulation study In healthy humans. Neuropsychologia. 2008. January 15;46(1):261–8 [DOI] [PubMed] [Google Scholar]
- [57].Pobric G, Jefferies E, Lambon Ralph MA. Category-specific versus category-general semantic impairment induced by transcranial magnetic stimulation. Curr Biol. 2010. May 25;20(10):964–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Ferruci R, Marceglia S, Vergari M et al. Cerebellar transcranial direct current stimulation impairs the practice-dependent proficiency increase in working memory. J Cogn Neurosci. 2008. September;20(9):1687–97 [DOI] [PubMed] [Google Scholar]
- [59].Koch G, Oliveri M, Cheeran B et al. Hyperexcitability of parietal-motor functional connections in the intact left-hemisphere of patients with neglect. Brain. 2008. December;131(Pt 12):3147–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Antal A, Terney D, Kuhnl S. Anodal transcranial direct current stimulation of the motor cortex ameliorates chronic pain and reduces short intracortical inhibition. J Pain Symptom Manage. 2010. May;39(5):890–903 [DOI] [PubMed] [Google Scholar]
- [61].Fertonani A, Rosini S, Cotelli M et al. Naming facilitation induced by transcranial direct current stimulation. Behav Brain Res. 2010. April 2;208(2):311–8 [DOI] [PubMed] [Google Scholar]
- [62].Boogio PS, Zaghi S, Lopes M et al. Modulatory effects of anodal transcranial direct current stimulation on perception and pain thresholds in healthy volunteers. Eur J Neurol. 2008. October;15(10):1124–30 [DOI] [PubMed] [Google Scholar]
- [63].Kirton A, Chen R, Friefeld S et al. Contralesional repetitive transcranial magnetic stimulation for chronic hemiparesis in subcortical paediatric stroke: a randomised trial. Lancet Neurol. 2008. June;7(6):507–13 [DOI] [PubMed] [Google Scholar]
- [64].Keeser D, Padberg F, Reisinger E et al. Prefrontal direct current stimulation modulates resting EEG and event-related potentials in healthy subjects: A standardized low resolution tomography (sLORETA) study. Neuroimage. 2011. March 15;55(2):644–57 [DOI] [PubMed] [Google Scholar]
- [65].Fitzgerald PB, Hoy K, McQueen S et al. A randomized trial of rTMS targeted with MRI based neuro-navigation in treatment-resistant depression. Neuropsychopharmacology. 2009. April;34(5):1255–62 [DOI] [PubMed] [Google Scholar]
- [66].Ferruci R, Bortolomasi M, Vergari M et al. Transcranial direct current stimulation in severe, drug-resistant major depression. J Affect Disord. 2009. November;118(1–3):215–9 [DOI] [PubMed] [Google Scholar]
- [67].Polania R, Paulus W, Nitsche MA. Modulating cortico-striatal and thalamo-cortical functional connectivity with transcranial direct current stimulation. Hum Brain Mapp. 2012. October;33(10):2499–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Jo JM, Kim YH, Ko MH et al. Enhancing the working memory of stroke patients using tDCS. Am J Phys Med Rehabil. 2009. May;88(5):404–9 [DOI] [PubMed] [Google Scholar]
- [69].Fregni F, Liguori P, Fecteau S et al. Cortical stimulation of the prefrontal cortex with transcranial direct current stimulation reduces cue-provoked smoking craving: a randomized, sham-controlled study. J Clin Psychiatry. 2008. January;69(1):32–40 [DOI] [PubMed] [Google Scholar]
- [70].Amiaz R, Levy D, Vainiger D et al. Repeated high-frequency transcranial magnetic stimulation over the dorsolateral prefrontal cortex reduces cigarette craving and consumption. Addiction. 2009. April;104(4):653–60 [DOI] [PubMed] [Google Scholar]
- [71].Benninger DH, Lomarev M, Lopez G, Wassermann EM et al. Transcranial direct current stimulation for the treatment of Parkinson’s disease J Neurol Neurosurg Psychiatry. 2010. October;81(10):1105–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Fregni F, Orsati F, Pedrosa W et al. Transcranial direct current stimulation of the prefrontal cortex modulates the desire for specific foods. Appetite. 2008. July;51(1):34–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Takeuchi N, Tada T, Toshiba M et al. Inhibition of the unaffected motor cortex by 1 Hz repetitive transcranial magnetic stimulation enhances motor performance and training effect of the paretic hand in patients with chronic stroke. J Rehabil Med. 2008. April;40(4):298–303 [DOI] [PubMed] [Google Scholar]
- [74].Meinzer M, Antonenko D, Lindenber R et al. Electrical brain stimulation improves cognitive performance by modulating functional connectivity and task-specific activation. J Neurosci. 2012. February 1;32(5):1859–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Santiesteban I, Banissy MJ, Catmur C et al. Enhancing social ability by stimulating right temporoparietal junction. Curr Biol. 2012. December 4;22(23):2274–7 [DOI] [PubMed] [Google Scholar]
- [76].Boros K, Poreisz C, Münchau A et al. Premotor transcranial direct current stimulation (tDCS) affects primary motor excitability in humans. Eur J Neurosci. 2008. March;27(5):1292–300 [DOI] [PubMed] [Google Scholar]
- [77].Fertonani A, Pirulli C, Miniussi C. Random noise stimulation improves neuroplasticity in perceptual learning. J Neurosci. 2011. October 26;31(43):15416–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Cohen Kadosh R, Soskic S, Iuculano T et al. Modulating neuronal activity produces specific and long-lasting changes in numerical competence. Curr Biol. 2010. November 23;20(22):2016–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Baumgartner T, Knoch D, Hotz P et al. Dorsolateral and ventromedial prefrontal cortex orchestrate normative choice. Nat Neurosci. 2011. October 2;14(11):1468–74 [DOI] [PubMed] [Google Scholar]
- [80].Pobric G, Jefferies E, Ralph MA. Amodal semantic representations depend on both anterior temporal lobes: Evidence from repetitive transcranial magnetic stimulation. Neuropsychologia. 2010. Apr;48(5):1336–42 [DOI] [PubMed] [Google Scholar]
- [81].Nitsche MA, Kuo MF, Karrasch R et al. Serotonin affects transcranial direct current-induced neuroplasticity in humans. Biol Psychiatry. 2009. September 1;66(5):503–8 [DOI] [PubMed] [Google Scholar]
- [82].Hz TY, Tseng LY, Yu JX et al. Modulating inhibitory control with direct current stimulation of the superior medial frontal cortex. Neuroimage. 2011. June 15;56(4):2249–57 [DOI] [PubMed] [Google Scholar]
- [83].Koch G, Mori F, Marconi B et al. Changes in intracortical circuits of the human cortex following theta burst stimulation of the lateral cerebellum. Clin Neurophysiol. 2008. November;119(11):2559–69 [DOI] [PubMed] [Google Scholar]
- [84].Khedr EM, Rothwell JC, Ahmed MA et al. Effect of daily repetitive transcranial magnetic stimulation for treatment of tinnitus: a comparison of different stimulus frequencies. J Neurol Neurosurg Psychiatry. 2008. February;79(2):212–5 [DOI] [PubMed] [Google Scholar]
- [85].Berryhill ME, Jones KT. tDCS selectively improves working memory in older adults with more education. Neurosci Lett. 2012. July 19;521(2):148–51 [DOI] [PubMed] [Google Scholar]
- [86].Soler MD, Kumru H, Pelayo R et al. Effectiveness of transcranial direct current stimulation and visual illusion on neuropathic pain in spinal cord injury. Brain. 2010. September;133(9):2565–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Tseng P, Hsu TY, Chang CF et al. Unleashing potential: transcranial direct current stimulation over the right posterior parietal cortex improves change detection in low-performing individuals. J Neurosci. 2012. August 1;32(31):10554–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Hesse S, Waldner A, Mehrholz J et al. Combined transcranial direct current stimulation and robot-assisted arm training in subacute stroke patients: an exploratory, randomized multicenter trial. Neurorehabil Neural Repair. 2011. Nov-Dec;25(9):838–46 [DOI] [PubMed] [Google Scholar]
- [89].Koch G, Brusa L, Carillo F et al. Cerebellar magnetic stimulation decreases levodopa-induced dyskinesias in Parkinson disease. Neurology. 2009. July 14;73(2):113–9 [DOI] [PubMed] [Google Scholar]
- [90].Boggio PS, Zaghi S, Fregni F. Modulation of emotions associated with images of human pain using anodal transcranial direct current stimulation (tDCS). Neuropsychologia. 2009. January;47(1):212–7 [DOI] [PubMed] [Google Scholar]
- [91].Kuo MF, Unger M, Liebetanz D et al. Limited impact of homeostatic plasticity on motor learning in humans. Neuropsychologia. 2008;46(8):2122–8 [DOI] [PubMed] [Google Scholar]
- [92].Jung P, Ziemann U. Homeostatic and Nonhomeostatic Modulation of Learning in Human Motor Cortex. J Neurosci. 2009. April 29;29(17):5597–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Cotelli M, Manenti R, Cappa SF et al. Transcranial magnetic stimulation improves naming in Alzheimer disease patients at different stages of cognitive decline. Eur J Neurol. 2008. December;15(12):1286–92 [DOI] [PubMed] [Google Scholar]
- [94].Dasilva AF, Mendonca ME, Zaghi S et al. tDCS-induced analgesia and electrical fields in pain-related neural networks in chronic migraine. Headache. 2012. September;52(8):1283–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Celnik P, Paik NJ, Vandermeeren Y et al. Effects of combined peripheral nerve stimulation and brain polarization on performance of a motor sequence task after chronic stroke. Stroke. 2009. May;40(5):1764–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Martin PI, Naeser MA, Ho M et al. Overt naming fMRI pre- and post-TMS: Two nonfluent aphasia patients, with and without improved naming post-TMS. Brain Lang. 2009. October;111(1):20–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Peña-Gómez C, Sala-Lonch R, Junqué C et al. Modulation of large-scale brain networks by transcranial direct current stimulation evidenced by resting-state functional MRI. Brain Stimul. 2012. July;5(3):252–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Galea JM, Celnik P. Brain polarization enhances the formation and retention of motor memories. J Neurophysiol. 2009. July;102(1):294–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Zimerman M, Heise KF, Hoppe J et al. Modulation of training by single-session transcranial direct current stimulation to the intact motor cortex enhances motor skill acquisition of the paretic hand. Stroke. 2012. August;43(8):2185–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Flöel A, Meinzer M, Kirstein R et al. Short-term anomia training and electrical brain stimulation. Stroke. 2011. July;42(7):2065–7 [DOI] [PubMed] [Google Scholar]
- [101].Khedr EM, Etraby AE, Hemeda M et al. Long-term effect of repetitive transcranial magnetic stimulation on motor function recovery after acute ischemic stroke. Acta Neurol Scand. 2010. January;121(1):30–7 [DOI] [PubMed] [Google Scholar]
- [102].Priori A, Mameli F, Cogiamanian F et al. Lie-specific involvement of dorsolateral prefrontal cortex in deception. Cereb Cortex. 2008. February;18(2):451–5 [DOI] [PubMed] [Google Scholar]
- [103].Mori F, Codecà C, Kusayanagi H et al. Effects of anodal transcranial direct current stimulation on chronic neuropathic pain in patients with multiple sclerosis. J Pain. 2010. May;11(5):436–42 [DOI] [PubMed] [Google Scholar]
- [104].Khedr EM, Abdel-Fadeil MR, Farghali A et al. Role of 1 and 3 Hz repetitive transcranial magnetic stimulation on motor function recovery after acute ischaemic stroke. Eur J Neurol. 2009. December;16(12):1323–30 [DOI] [PubMed] [Google Scholar]
- [105].Meinzer M, Lindenber R, Antonenko D et al. Anodal transcranial direct current stimulation temporarily reverses age-associated cognitive decline and functional brain activity changes. J Neurosci. 2013. July 24;33(30):12470–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Antal A, Brephohl N, Poreisz C et al. Transcranial direct current stimulation over somatosensory cortex decreases experimentally induced acute pain perception. Clin J Pain. 2008. January;24(1):56–63 [DOI] [PubMed] [Google Scholar]
- [107].Nyffeler T, Cazzoli D, Hess CW et al. One session of repeated parietal theta burst stimulation trains induces long-lasting improvement of visual neglect. Stroke. 2009. August;40(8):2791–6 [DOI] [PubMed] [Google Scholar]
- [108].Jayaram G, Tang B, Pallegadda R et al. Modulating locomotor adaptation with cerebellar stimulation. J Neurophysiol. 2012. June;107(11):2950–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].You DS, Kim DY, Chun MH et al. Cathodal transcranial direct current stimulation of the right Wernicke’s area improves comprehension in subacute stroke patients. Brain Lang. 2011. October;119(1):1–5 [DOI] [PubMed] [Google Scholar]
- [110].Brunoni AR, Ferruci R, Bortolomasi M et al. Transcranial direct current stimulation (tDCS) in unipolar vs. bipolar depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2011. January 15;35(1):96–101 [DOI] [PubMed] [Google Scholar]
- [111].Weiduschat N, Thiel A, Rubi-Fessen I et al. Effects of repetitive transcranial magnetic stimulation in aphasic stroke: a randomized controlled pilot study. Stroke. 2011. February;42(2):409–15 [DOI] [PubMed] [Google Scholar]
- [112].de Vries MH, Barth AC, Maiworm S et al. Electrical stimulation of Broca’s area enhances implicit learning of an artificial grammar. J Cogn Neurosci. 2010. November;22(11):2427–36 [DOI] [PubMed] [Google Scholar]
- [113].Kleinjung T, Eichhammer P, Landgrebe M et al. Combined temporal and prefrontal transcranial magnetic stimulation for tinnitus treatment: A pilot study. Otolaryngol Head Neck Surg. 2008. April;138(4):497–501 [DOI] [PubMed] [Google Scholar]
- [114].Avenanti A, Coccia M, Ladavas E et al. Low-frequency rTMS promotes use-dependent motor plasticity in chronic stroke: a randomized trial. Neurology. 2012. January 24;78(4):256–64 [DOI] [PubMed] [Google Scholar]
- [115].Cotelli M, Calabria M, Manenti R et al. Improved language performance in Alzheimer disease following brain stimulation. J Neurol Neurosurg Psychiatry. 2011. July;82(7):794–7 [DOI] [PubMed] [Google Scholar]
- [116].Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000. September 15;727 Pt 3:633–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol. 2006. April;117(4):845–50. [DOI] [PubMed] [Google Scholar]
- [118].Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001. November 27;5(10):1899–901 [DOI] [PubMed] [Google Scholar]
- [119].Liebetanz D, Nitsche MA, Tergau F, Paulus W. Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain. 2002. October; 125(Pt 10):2238–47 [DOI] [PubMed] [Google Scholar]
- [120].Iyer MB, Mattu U, Grafman J et al. Safety and cognitive effect of frontal DC brain polarization in healthy individuals. Neurology. 2005. March 8;64(5):872–5 [DOI] [PubMed] [Google Scholar]
- [121].Fregni F, Boggio PS, Nitsche MA et al. Anodal transcranial direct current stimulation of prefrontal cortex enhances working memory. Exp Brain Res. 2005. September; 166(1):23–30 [DOI] [PubMed] [Google Scholar]
- [122].Nitsche MA, Fricke K, Henschke U et al. Pharmacological modulation of cortical excitability shifts induced by Transcranial Direct Current Stimulation in Humans. J Physiol. 2003. November 15;553(Pt 1):293–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Investigational Device Exemptions (IDEs) for Early Feasibility Medical Device Clinical Studies, Including Certain First in Human (FIH) Studies. [Internet]. Silver Spring (MD): U.S. Food and Drug Administration; 2011. November 10 [cited 2019 April 10]. Available from: https://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm279103.pdf [Google Scholar]
- [124].San Francisco Declaration on Research Assessment. [Internet]. [cited 2019 April 7]. Available from: https://sfdora.org/read/
- [125].Yeung AWK, Goto TK, Leung WK. At the Leading Front of Neuroscience: A Bibliometric Study of the 100 Most-Cited Articles. Front Hum Neurosci. 2017. July; 11:363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].van Eck NJ, Waltman L, Van Raan AF et al. Citation Analysis May Severely Underestimate the Impact of Clinical Research as Compared to Basic Research. PLoS One. 2013. April 24; 8(4):e62395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Fregni F, Boggio PS, Mansur CG, Wagner T, Ferreira MJ, Lima MC et al. Transcranial direct current stimulation of the unaffected hemisphere in stroke patients. Neuroreport 2005. September 28; 16(14):1551–5 [DOI] [PubMed] [Google Scholar]
- [128].Elsner B, Kluger J, Pohi M, Mehrholz J. Transcranial direct current stimulation (tDCS) for improving function and activities of daily living in patients after stroke. Cochrane Database Syst Rev. 2013; (11) [DOI] [PubMed] [Google Scholar]
- [129].Hao Z, Wang D, Zeng Y, Liu M. Repetitive transcranial magnetic stimulation for improving function after stroke. Cochrane Databae Syst Rev. 2013;(5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Chew T, Ho KA, Loo CK. Inter- and intra-individual Variability in Response to Transcranial Direct Current Stimulation (tDCS) at Varying Current Intensities. Brain Stimul. 2015;8(6):1130–7 [DOI] [PubMed] [Google Scholar]
- [131].Lee Won Hee Kennedy NI, Bikson M, Frangou S A Computational Assessment of Target Engagement in the Treatment of Auditory Hallucinations with Transcranial Direct Current Stimulation. Front Psychiatry. 2018;9:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Azondekon R, Harper ZJ, Agossa FR et al. Scientific authorship and collaboration network analysis on malária research in Benin: papers indexed in the web of science (1996–2016). Global Health research and policy. 2018. April 6;3:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Anandhalli GB. Authorship Trend and Collaborative Research in Lung Cancer: A Time Series Analysis Study. Library Philosophy and Practice. 2018. e:1622. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.