Abstract
Background
The risk for developing a periprosthetic joint infection (PJI) during bacteremia is unclear, except for Staphylococcus aureus bacteremia. The aim of this study was to examine the risk for developing a PJI during bacteremia and to identify possible risk factors leading to it.
Methods
Patients with a primary knee or hip joint replacement performed in a tertiary care hospital between September 2002 and December 2013 were identified (n = 14 378) and followed up until December 2014. Positive blood culture results during the study period and PJIs were recorded. PJIs associated with an episode of bacteremia were identified and confirmed from patient records. Potential risk factors for PJI among those with bacteremia were examined using univariate logistic regression.
Results
A total of 542 (3.8%) patients had at least 1 episode of bacteremia. Seven percent (47/643) of the bacteremias resulted in a PJI. Development of a PJI was most common for Staphylococcus aureus (21% of bacteremias led to a PJI) and beta-hemolytic streptococci (21%), whereas it was rare for gram-negative bacteria (1.3%). Having ≥2 bacteremias during the study period increased the risk for developing a PJI (odds ratio, 2.29; 95% confidence interval, 1.17–4.50). The risk for developing a PJI was highest for bacteremias occurring within a year of previous surgery. Chronic comorbidities did not affect the risk for PJI during bacteremia.
Conclusions
The development of a PJI during bacteremia depends on the pathogen causing the bacteremia and the timing of bacteremia with respect to previous joint replacement surgery. However, significant patient-related risk factors for PJI during bacteremia could not be found.
Keywords: bacteremia, prosthetic joint infection, risk factors, Staphylococcus aureus, streptococci
Hematogenous periprosthetic joint infections (PJIs) are due to hematogenous spread from another infection site and can occur at any time after joint replacement surgery [1, 2]. Late PJIs are usually hematogenous, but there is no consensus on the definition of late PJI. According to the traditional definition by Coventry et al., they occur 2 years after surgery or later [3]. However, other studies have used time limits from 3 [4] to 12 months [5]. Late acute PJIs are also characterized by an asymptomatic postoperative period before the onset of symptoms of infection. The proportion of late hematogenous PJIs has been estimated to be 13%–27% of all PJIs [6–10], whereas the overall incidence of late PJIs is around 0.25%–0.7% [5, 6, 8, 9, 11, 12] or 0.069% per prosthesis-year [10].
The origin of hematogenous PJIs can be from the skin and soft tissues, urinary tract, dental sources [4, 5], cardiovascular system [13], lungs [11], or the gastrointestinal tract [14]. Although most PJIs are caused by either coagulase-negative staphylococci or Staphylococcus aureus, hematogenous PJIs are mostly caused by S. aureus, followed by streptococci and gram-negative bacteria [1, 2, 15–17].
According to previous studies, 25%–40% of patients with Staphylococcus aureus bacteremia (SAB) and a joint replacement develop a hematogenous PJI [18–22]. The presence of 3 or more joint replacements increased the risk for PJI considerably during SAB in 1 study [21], but other studies have not been able to show patient-related risk factors for developing a PJI during SAB [20]. The risk for developing a PJI during bacteremia caused by other pathogens is unclear, as most reports on PJIs during bacteremia have been case studies [23–26]. Uçkay et al. reported a PJI rate of 6% in patients with any bacteremia [14]. Risk factors for developing a PJI during any bacteremia have not been studied.
The aim of this study was to examine the risk for developing a PJI during bacteremia caused by different pathogens and to identify possible risk factors leading to it.
METHODS
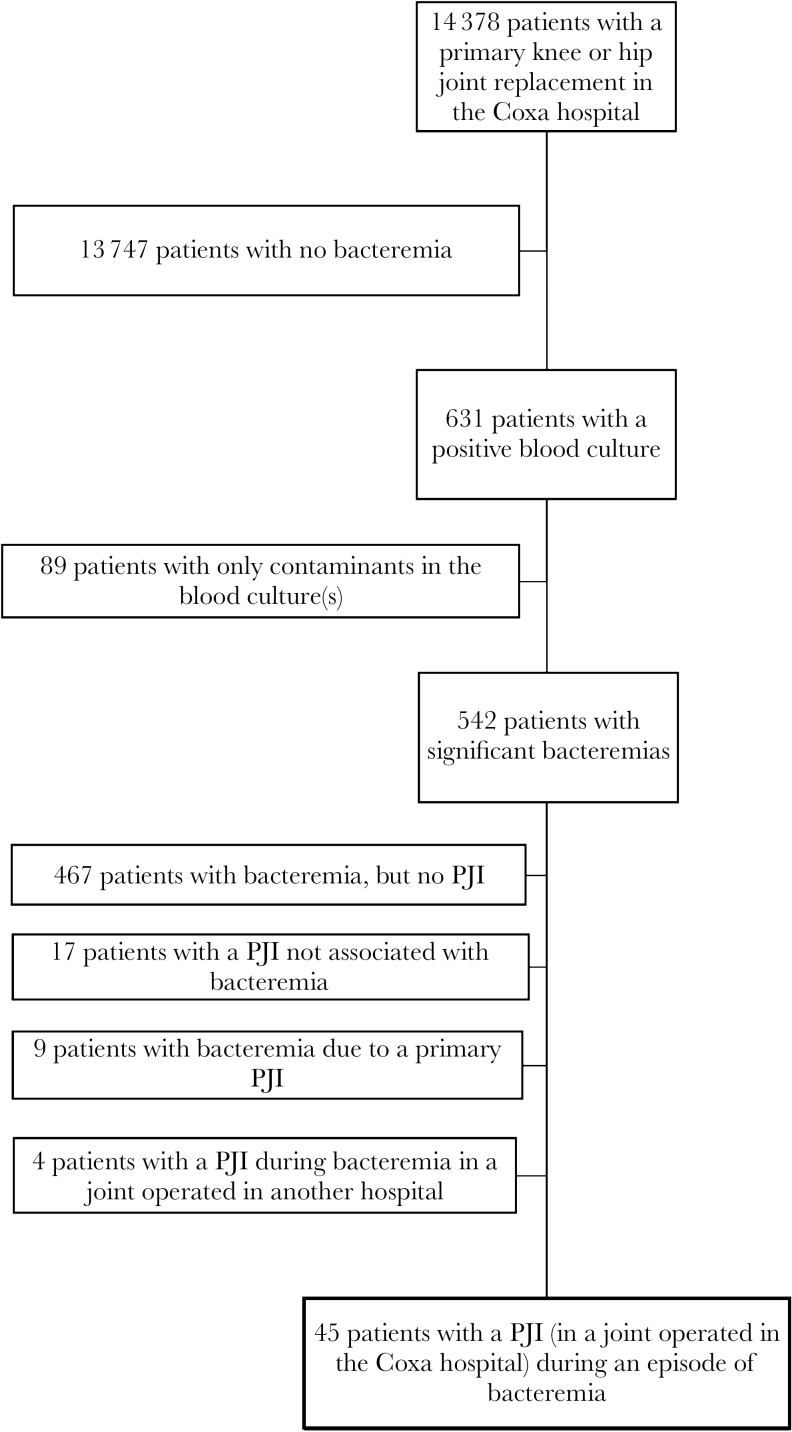
This is a retrospective study that was performed at the Coxa Hospital for Joint Replacement, Tampere, Finland. Patients from Pirkanmaa Hospital District (population ca. 500 000 inhabitants) who had a primary knee or hip replacement performed between September 2002 and December 2013 at the hospital were identified from the hospital database (see Figure 1 for a description of the study patients). Patients could have had additional primary hip and knee replacements (performed elsewhere or before the study period), but information on these was not available. The follow-up period for each patient was from the date of the first joint replacement surgery during the study period to the date of death or December 31, 2014, whichever occurred first. According to national legislation, patient informed consent was not required due to the retrospective design of the study. Institutional permission to conduct this study was granted by the authorities responsible for the patient records.
Figure 1.
Description of the study patients. Abbreviation: PJI, periprosthetic joint infection.
All positive blood culture results of the study patients occurring after the primary surgery until December 31, 2014, were obtained from the electronic records of the accredited microbiology laboratory of Tampere University Hospital. Positive blood cultures caused by coagulase-negative staphylococci, corynebacteria, micrococci, or Cutibacterium species were considered significant only if there was growth on 2 blood culture bottles (n = 28); otherwise, they were defined as contaminants (n = 89). Positive blood cultures by other pathogens were considered significant regardless of the number of positive culture bottles. All consecutive positive blood cultures with the same organism taken within 7 days of the first positive sample were considered part of the same episode of bacteremia.
PJI cases were identified from 6 different data sources: (1) prospective surveillance data from the national Finnish Hospital Infection Program of the National Institute of Health and Welfare, (2) postdischarge surveillance data gathered by an infection control nurse, (3) positive bacterial cultures of joint aspirates or tissue cultures from samples taken at the Coxa Hospital for Joint Replacement, (4) hospital discharge records with a diagnosis code of PJI (ICD-10 diagnosis code T84.5 or T81.4), (5) local joint replacement database records on revision arthroplasties performed due to PJI, and (6) Coxa’s own infection register. A detailed description of these data sources can be found in a previous study of the patient population [27]. If the PJI occurred in a joint that was replaced outside of the study period (n = 4), it was not included in the analyses (but the patients remained in the study cohort, as they had other joints replaced at the Coxa hospital during the study period).
Patients with the same organism cultured from blood and from the affected joint were considered to have a PJI as a consequence of bacteremia. Cases where the PJI was determined to be the source of the bacteremia, based on the timing of symptom onset, were identified from the patient charts (n = 9) by 1 of the authors (M.H.) and were not included in the analyses. If the patient had a culture-negative PJI and bacteremia, patient charts were also reviewed. If the treating clinicians considered the PJI to be caused by the pathogen identified in the blood culture, it was recorded as such. Also, patient charts of patients who had a long duration (>7 days) between the positive blood culture and identification of the PJI were checked to verify the association.
Information on patients’ chronic diseases (diabetes, rheumatic diseases, chronic heart failure, chronic coronary disease, arrhythmias, and chronic lung disease) was gathered from the drug reimbursement register of the Social Insurance Institution of Finland. For the statistical analyses, chronic heart failure, chronic coronary disease, and arrhythmias were grouped together (chronic heart disease). Information on body mass index (BMI), indication for joint replacement surgery, use of (antibiotic-impregnated) cement in the surgery, and date of death was gathered from Coxa’s electronic database.
Statistical Analysis
All data analyses and management were performed using the SPSS for Windows 25.0 statistical software package.
The incidence of bacteremia and PJI was calculated as the incidence rate per 1000 person-years. Incidence of PJI as a consequence of bacteremias caused by different pathogens was calculated. Means or medians were calculated for continuous variables with a normal or skewed distribution, respectively.
The association between different potential risk factors (number of bacteremias, BMI, male gender, knee location, time since previous joint replacement surgery, age, indication for joint replacement surgery, use of cement in the operation, and chronic diseases) and the development of a PJI during bacteremia were examined for each joint separately. If the patient had multiple bacteremias during the study period, each was included in the analyses separately when analyzing the effect of age and time since previous joint replacement surgery on the risk for PJI. Potential risk factors were analyzed using binary logistic regression with univariate analysis, and odds ratios and 95% confidence intervals were calculated.
RESULTS
There were 14 378 patients with a primary knee or hip joint replacement performed during the study period. Of these, 4475 patients had more than 1 primary joint replacement. A total of 1346 patients had a revision arthroplasty performed during the study period. The mean follow-up time (range) was 6.0 (0–12) years.
During the study period, 542 (3.8%) patients had at least 1 episode of bacteremia after the primary joint replacement surgery, and 85 (0.6%) patients had more than 1 bacteremia. The maximum number of separate episodes of bacteremia per patient was 8 (1 patient). In total, there were 643 episodes of bacteremia. The incidence rate of bacteremia was 7.4 per 1000 person-years. The bacteremias occurred 3–4285 days after the first joint replacement operation (median, 1460 days); 13% occurred within 1 year (85/643) and 4% within 3 months (27/643). Escherichia coli was the most common causative pathogen (241/643, 37% of bacteremias). The distribution of pathogens causing the bacteremias is shown in Table 1.
Table 1.
Distribution of Pathogens Causing Bacteremia and PJIs Developed During Bacteremias
| Total No. of Bacteremias (n = 643) | No. of Bacteremias With a PJI (n = 46) | |||
|---|---|---|---|---|
| Pathogen | No. | % | No. | % |
| Escherichia coli | 241 | 37 | 3 | 1 |
| Staphylococcus aureus | 105 | 16 | 21a | 20 |
| Beta-hemolytic streptococci | 58 | 9 | 12 | 21 |
| Streptococcus pneumoniae | 43 | 7 | 2 | 5 |
| Coagulase-negative staphylococci | 28 | 4 | 0 | 0 |
| Enterococci | 28 | 4 | 1 | 4 |
| Klebsiella species | 27 | 4 | 1 | 4 |
| Viridans group streptococci | 25 | 4 | 4 | 16 |
| Other gram-negative bacteriab | 46 | 7 | 0 | 0 |
| Anaerobes | 20 | 3 | 2 | 10 |
| Yeasts | 3 | 0.5 | 0 | 0 |
| Otherc | 12 | 2 | 0 | 0 |
| Polymicrobial | 7 | 1 | 0 | 0 |
Abbreviation: PJI, periprosthetic joint infection.
aIncludes 1 bilateral infection, which is counted as 1.
bIncludes enterobacteriae, Pseudomonas species, Proteus species, Morganella morganii, Citrobacter species, Haemophilus influenzae, Serratia marcescens.
cIncludes lactobacilli, Listeria monocytogenes, Actinobaculum schaalii, Aerococcus urinae, Arcanobacterium pyogenes, Granulicatella adiacens.
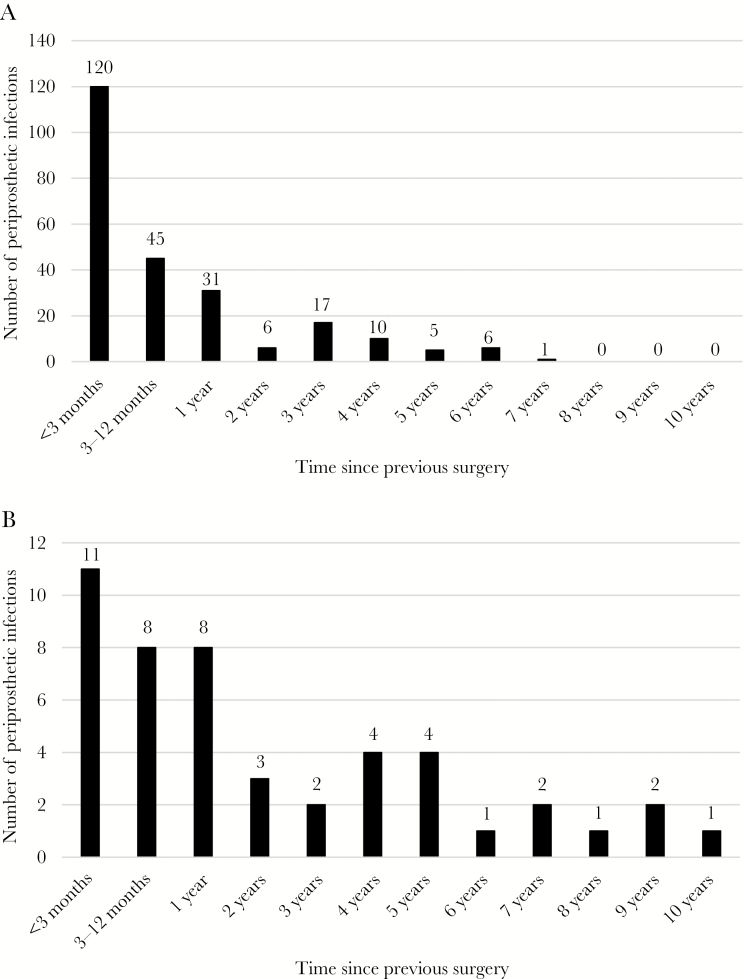
There were in total 288 PJIs, and the incidence rate was 3.3 per 1000 person-years. Of the infections, 131 (45%) were early infections occurring within 90 days of the previous surgery, 53 (18%) occurred within 3–12 months, and 104 (36%) occurred after 1 year. The distributions of the PJIs over time for patients with and without bacteremia are shown in Figure 2A and B.
Figure 2.
A, The distribution of periprosthetic infections over time in patients without bacteremia. B, The distribution over time of periprosthetic joint infections as a consequence of bacteremia.
Of the episodes of bacteremia, 7% (46/643) resulted in a PJI (Table 1). One of these was a bilateral knee infection. Of the PJIs during bacteremia, 29 (62%) were in the knees and 18 (38%) in the hips. Seven PJIs occurred after revision joint replacement. One patient had 2 separate PJIs associated with different episodes of bacteremia. Thus, 45/542 (8%) patients with 1 or more episodes of bacteremia developed a PJI. The duration between the first positive blood culture and identification of the PJI ranged from 0 to 512 days (median, 2 days). There were 10 (21%) PJIs where the pathogen could not be identified from the affected joint, but these were considered to be caused by the pathogen identified in the blood culture. In most cases, these patients had received antibiotics before taking the samples for bacterial culture from the joint.
The development of a PJI during bacteremia varied between different pathogens (Table 1). It was most common for Staphylococcus aureus, beta-hemolytic streptococci, and viridans group streptococci, but rare for gram-negative bacteria and coagulase-negative staphylococci; 1.3% of bacteremias caused by gram-negative bacteria resulted in a PJI. There were 11 PJIs as a consequence of bacteremia that occurred within 3 months of the previous surgery. These infections were caused by Staphylococcus aureus (n = 3), viridans group streptococci (n = 2), Streptococcus agalactiae (n = 2), Clostridium perfringens (n = 2), Klebsiella terrigena (n = 1), and group G streptococcus (n = 1).
Having more than 1 bacteremia during the study period increased the risk for developing a PJI (Table 2). Also, the risk for developing a PJI was higher for bacteremias occurring less than a year after the previous surgery than for bacteremias occurring later. Gender, obesity (BMI ≥ 25), operated joint (hip, knee), indication for primary surgery, use of antibiotic-impregnated cement for prosthesis fixation, and chronic diseases did not affect the risk of developing a PJI as a consequence of bacteremia (Table 2). Older age was associated with a lower risk of developing a PJI, but when the effect of bacteremias caused by E. coli was taken into account in a multivariable analysis, the effect of patients’ age was no longer statistically significant (odds ratio, 0.97; 95% confidence interval, 0.95–1.00).
Table 2.
Potential Risk Factors for Developing a Periprosthetic Joint Infection During Bacteremia
| Joints With PJI (n = 47) | Joints Without PJI (n = 672) | |||||
|---|---|---|---|---|---|---|
| Risk Factor | No. | % | No. | % | Odds Ratio | 95% Confidence Interval |
| No. of bacteremias ≥2 | 13 | 28 | 96 | 14 | 2.29 | 1.17–4.50 |
| BMI ≥25 kg/m2 | 38 | 81 | 473 | 70 | 1.82 | 0.70–4.72 |
| Chronic lung disease | 3 | 6 | 33 | 5 | 1.32 | 0.39–4.48 |
| Chronic heart disease | 6 | 13 | 72 | 11 | 1.22 | 0.50–2.97 |
| Male gender | 21 | 45 | 276 | 41 | 1.16 | 0.64–2.10 |
| Knee joint | 29 | 62 | 398 | 59 | 1.11 | 0.60–2.04 |
| Time since previous joint replacement, ya | ||||||
| <1 | 17 | 36 | 94 | 12 | 1.00 | |
| 1–10 | 28 | 60 | 672 | 85 | 0.23 | 0.12–0.44 |
| >10 | 2 | 4 | 24 | 3 | 0.46 | 0.10–2.13 |
| Age at the time of bacteremia, mean (SD),a y | 71 | (11) | 76 | (10) | 0.97 | 0.94–0.99 |
| Rheumatic disease | 3 | 6 | 59 | 9 | 0.71 | 0.21–2.35 |
| Diabetes | 5 | 11 | 103 | 15 | 0.66 | 0.25–1.70 |
| Primary osteoarthritis as indication for primary joint replacement | 43 | 92 | 588 | 88 | 0.65 | 0.23–1.86 |
| Use of antibiotic-loaded cement in the primary operation | 35 | 74 | 555 | 83 | 0.59 | 0.30–1.17 |
Abbreviations: BMI, body mass index; PJI, periprosthetic joint infection.
aCalculated for each bacteremia and joint (n = 837) separately.
DISCUSSION
This study shows that development of a PJI as a consequence of bacteremia is highly dependent on the type of pathogen causing the bacteremia. The risk was the highest in bacteremias caused by Staphylococcus aureus, beta-hemolytic streptococci, and viridans group streptococci and was associated with repeated episodes of bacteremia. On the other hand, the development of a PJI during bacteremia caused by gram-negative bacteria, especially E. coli, and coagulase-negative staphylococci, was rare. Patients with a bacteremia occurring within 1 year of previous surgery had a higher risk of developing a PJI than those with bacteremias occurring later.
In the current study, 7% of the episodes of bacteremia resulted in the development of a PJI, corresponding to the rate described by Uçkay et al. [14]. In their study, 6% (5/81) of bacteremias resulted in a PJI. Other studies examining the risk of developing a PJI during SAB [18–22] have reported slightly higher rates than in the current study. Uçkay et al. reported a rate of 2.9% for PJI during bacteremia caused by E. coli, 14% for SAB, and 40% for anaerobic bacteremia, but the number of PJIs as a consequence of bacteremia was so small (n = 5) that it is difficult to make meaningful conclusions from the results. No other studies have examined the risk of developing a PJI during bacteremia caused by pathogens other than S. aureus.
Interestingly, the risk of PJI was similar for bacteremias caused by Staphylococcus aureus and beta-hemolytic streptococci. However, it is not surprising, as streptococci have been reported to cause a considerable proportion of late hematogenous PJIs [1, 2, 16]. On the other hand, there were no PJIs during bacteremia caused by coagulase-negative staphylococci, even though they are significant pathogens that cause PJIs [1]. Coagulase-negative staphylococcal bacteremias are mostly nosocomial [28], and it has been shown that nosocomial SABs are not associated with PJIs [20, 21], making it likely that this is the case for coagulase-negative staphylococci as well. Unfortunately, the differentiation between community-acquired and nosocomial bacteremias was not possible in this study. Despite the commonness of urosepsis caused by E. coli, especially in the oldest age groups, it rarely leads to the development of a PJI. Thus, patients with bacteremia caused by gram-negative bacteria do not warrant the special attention with respect to the development of a PJI paid to those with bacteremias caused by S. aureus or beta-hemolytic streptococci.
This study demonstrates that viridans group streptococci can lead to the development of a hematogenous PJI, even though the absolute number was low. These bacteria are associated with dental or gastrointestinal sources [29, 30]. Unfortunately, due to the retrospective nature of the current study, patients’ dental status or previous dental procedures with or without antibiotic prophylaxis could not be evaluated, and thus conclusions on the risk for PJI after dental procedures could not be made.
No patient-related risk factors for the development of a PJI during bacteremia, such as chronic diseases, could be identified, and this has been the case in previous studies as well [14, 20]. However, having more than 2 bacteremias during the study period increased the risk of developing a PJI during bacteremia. This possibly reflects some unidentifiable factor that increased patients’ susceptibility to infection in general. Patients’ use of immunosuppressive medication was not evaluated, but rheumatic diseases did not increase the risk for developing a PJI during bacteremia, nor did diabetes. An important observation, instead, is that the risk for developing a PJI as a consequence of bacteremia was highest for bacteremias occurring within 1 year of previous surgery. Large studies have shown that the overall risk of developing a PJI is highest for the first 2 years after surgery [7–9], and this is probably partly due to the increased risk for hematogenous PJIs. On the other hand, in a study by Rakow et al., most of the hematogenous PJIs occurred after 2 years from primary surgery [13].
There are some limitations to this study. First, some of the patients could have had joint replacement surgery performed elsewhere, before the study period began, and thus all prosthetic joints at risk for infection could not be identified. In addition, there might have been other patients in the Pirkanmaa hospital district with joint replacements inserted at other hospitals who developed PJIs during bacteremia, but they could not be identified, and this might have resulted in lower incidence numbers. However, as the number of patients with surgery performed elsewhere was probably not very high, its effect can be assumed to be insignificant. Second, due to the retrospective nature of this study, some of the data, such as dental procedures, were not available. To avoid other limitations related to retrospective data collection, such as missing PJI cases, multiple data sources were used. In addition, the source of bacteremia could not be investigated, and differentiation between recurrent and relapsing bacteremia was not possible in cases with multiple episodes of bacteremia. Third, it is impossible to say with absolute certainty whether all PJIs attributed to bacteremia were truly so, but patient charts were reviewed carefully to minimize this error. The pathogens causing the early PJIs during bacteremia were not typical pathogens causing primary PJIs, thus supporting the fact that these PJIs were truly consequent to the bacteremia. Finally, as there was a limited follow-up period for each joint (maximum 12 years), PJIs as a consequence of bacteremia occurring after the study period were missed, thus potentially affecting the incidence numbers.
In conclusion, this large study shows that the type of pathogen, history of infections, and timing of bacteremia should be taken into account when evaluating the risk of PJI in a patient with bacteremia. Developing a PJI during an episode of bacteremia caused by Stapylococcus aureus or beta-hemolytic streptococci is fairly common, and viridans group streptococci can lead to PJIs during bacteremia. This should be taken into account when a patient with a joint replacement presents with bacteremia caused by these agents, especially during the first postoperative year. Although diabetes, rheumatoid arthritis, and chronic heart and lung diseases did not affect the risk of PJI, there is possibly some unknown factor that increases patients’ susceptibility to infection in general and thus also to developing a PJI during bacteremia.
Acknowledgments
The authors wish to thank infection control nurse Jaana Sinkkonen for her help in gathering the data.
Financial support. This work was supported by the Competitive State Research Financing of the Expert Responsibility area of Tampere University Hospital (to M.H.).
Potential conflicts of interest. Dr. Eskelinen reports grants from DePuy Synthes, grants from Zimmer Biomet, and personal fees from Zimmer Biomet outside the submitted work. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Stefánsdóttir A, Johansson D, Knutson K, et al. Microbiology of the infected knee arthroplasty: report from the Swedish Knee Arthroplasty Register on 426 surgically revised cases. Scand J Infect Dis 2009; 41:831–40. [DOI] [PubMed] [Google Scholar]
- 2. Rodríguez D, Pigrau C, Euba G, et al. ; REIPI Group (Spanish Network for Research in Infectious Disease) Acute haematogenous prosthetic joint infection: prospective evaluation of medical and surgical management. Clin Microbiol Infect 2010; 16:1789–95. [DOI] [PubMed] [Google Scholar]
- 3. Coventry MB. Treatment of infections occurring in total hip surgery. Orthop Clin North Am 1975; 6:991–1003. [PubMed] [Google Scholar]
- 4. Wouthuyzen-Bakker M, Sebillotte M, Lomas J, et al. ESCMID Study Group for Implant-Associated Infections (ESGIAI) Clinical outcome and risk factors for failure in late acute prosthetic joint infections treated with debridement and implant retention. J Infect 2019; 78:40–7. [DOI] [PubMed] [Google Scholar]
- 5. Maderazo EG, Judson S, Pasternak H. Late infections of total joint prostheses. A review and recommendations for prevention. Clin Orthop Relat Res 1988; 131–42. [PubMed] [Google Scholar]
- 6. Ainscow DA, Denham RA. The risk of haematogenous infection in total joint replacements. J Bone Joint Surg Br 1984; 66:580–2. [DOI] [PubMed] [Google Scholar]
- 7. Pulido L, Ghanem E, Joshi A, et al. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res 2008; 466:1710–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ong KL, Kurtz SM, Lau E, et al. Prosthetic joint infection risk after total hip arthroplasty in the Medicare population. J Arthroplasty 2009; 24:105–9. [DOI] [PubMed] [Google Scholar]
- 9. Kurtz SM, Ong KL, Lau E, et al. Prosthetic joint infection risk after TKA in the Medicare population. Clin Orthop Relat Res 2010; 468:52–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huotari K, Peltola M, Jämsen E. The incidence of late prosthetic joint infections: a registry-based study of 112,708 primary hip and knee replacements. Acta Orthop 2015; 86:321–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cook JL, Scott RD, Long WJ. Late hematogenous infections after total knee arthroplasty: experience with 3013 consecutive total knees. J Knee Surg 2007; 20:27–33. [DOI] [PubMed] [Google Scholar]
- 12. Tsaras G, Osmon DR, Mabry T, et al. Incidence, secular trends, and outcomes of prosthetic joint infection: a population-based study, Olmsted county, Minnesota, 1969–2007. Infect Control Hosp Epidemiol 2012; 33:1207–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rakow A, Perka C, Trampuz A, Renz N. Origin and characteristics of haematogenous periprosthetic joint infection. Clin Microbiol Infect. In press. [DOI] [PubMed] [Google Scholar]
- 14. Uçkay I, Lübbeke A, Emonet S, et al. Low incidence of haematogenous seeding to total hip and knee prostheses in patients with remote infections. J Infect 2009; 59:337–45. [DOI] [PubMed] [Google Scholar]
- 15. Swan J, Dowsey M, Babazadeh S, et al. Significance of sentinel infective events in haematogenous prosthetic knee infections. ANZ J Surg 2011; 81:40–5. [DOI] [PubMed] [Google Scholar]
- 16. Zeller V, Kerroumi Y, Meyssonnier V, et al. Analysis of postoperative and hematogenous prosthetic joint-infection microbiological patterns in a large cohort. J Infect 2018; 76:328–34. [DOI] [PubMed] [Google Scholar]
- 17. Triffault-Fillit C, Ferry T, Laurent F, et al. Lyon BJI Study Group Microbiologic epidemiology depending on time to occurrence of prosthetic joint infection: a prospective cohort study. Clin Microbiol Infect 2019; 25:353–8. [DOI] [PubMed] [Google Scholar]
- 18. Murdoch DR, Roberts SA, Fowler VG Jr, et al. Infection of orthopedic prostheses after Staphylococcus aureus bacteremia. Clin Infect Dis 2001; 32:647–9. [DOI] [PubMed] [Google Scholar]
- 19. Lalani T, Chu VH, Grussemeyer CA, et al. Clinical outcomes and costs among patients with Staphylococcus aureus bacteremia and orthopedic device infections. Scand J Infect Dis 2008; 40:973–7. [DOI] [PubMed] [Google Scholar]
- 20. Sendi P, Banderet F, Graber P, Zimmerli W. Periprosthetic joint infection following Staphylococcus aureus bacteremia. J Infect 2011; 63:17–22. [DOI] [PubMed] [Google Scholar]
- 21. Tande AJ, Palraj BR, Osmon DR, et al. Clinical presentation, risk factors, and outcomes of hematogenous prosthetic joint infection in patients with Staphylococcus aureus bacteremia. Am J Med 2016; 129:221.e20. [DOI] [PubMed] [Google Scholar]
- 22. Makki D, Elgamal T, Evans P, et al. The orthopaedic manifestation and outcomes of methicillin-sensitive Staphylococcus aureus septicaemia. Bone Joint J 2017; 99-B:1545–51. [DOI] [PubMed] [Google Scholar]
- 23. Reboli AC, Bryan CS, Farrar WE. Bacteremia and infection of a hip prosthesis caused by Bacillus alvei. J Clin Microbiol 1989; 27:1395–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chodos MD, Johnson CA. Hematogenous infection of a total knee arthroplasty with Klebsiella pneumoniae in association with occult adenocarcinoma of the cecum. J Arthroplasty 2009; 24:158.e13. [DOI] [PubMed] [Google Scholar]
- 25. Pepke W, Lehner B, Bekeredjian-Ding I, Egermann M. Haematogenous infection of a total knee arthroplasty with Klebsiella pneumoniae. BMJ Case Rep 2013; 2013:008588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Law GW, Wijaya L, Tan AHC. Group B streptococcal prosthetic knee joint infection linked to the consumption of raw fish. J Orthop Case Rep 2017; 7:54–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Honkanen M, Jämsen E, Karppelin M, et al. Concordance between the old and new diagnostic criteria for periprosthetic joint infection. Infection 2017; 45:637–43. [DOI] [PubMed] [Google Scholar]
- 28. Hitzenbichler F, Simon M, Salzberger B, Hanses F. Clinical significance of coagulase-negative staphylococci other than S. epidermidis blood stream isolates at a tertiary care hospital. Infection 2017; 45:179–86. [DOI] [PubMed] [Google Scholar]
- 29. Shenep JL. Viridans-group streptococcal infections in immunocompromised hosts. Int J Antimicrob Agents 2000; 14:129–35. [DOI] [PubMed] [Google Scholar]
- 30. Aas JA, Paster BJ, Stokes LN, et al. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol 2005; 43:5721–32. [DOI] [PMC free article] [PubMed] [Google Scholar]