Elevated intracranial pressure in patients with cryptococcal meningitis is common and associated with poor outcomes. This study evaluated factors at the time of diagnosis of cryptococcal meningitis that were associated with need ventriculo-peritoneal shunting.
Keywords: cryptococcosis, Cryptococcus neoformans, ventriculoperitoneal shunt
Abstract
Objective
Increased intracranial pressure (ICP) is an important complication of cryptococcal meningitis (CM) and impacts morbidity and mortality. Factors associated with permanent ventriculoperitoneal (VP) shunt placement are poorly characterized.
Method
We conducted a retrospective cohort study of patients with CM at the University of Alabama at Birmingham from 1996 through 2015. Characteristics of patients at time of CM diagnosis who did and did not receive a VP shunt were compared with use of the 2-group chi-square test or Fisher exact test for categorical variables and the 2-group t test for continuous variables. Stepwise logistic regression analysis was used to determine predictors of shunt placement.
Results
Of 422 patients with cryptococcosis, 257 (60.9%) had CM. Mean age was 47.7 years, 71.6% were male, and 44.4% were African American. The most common underlying conditions were HIV (42.4%), solid organ transplantation (29.6%), and corticosteroid use (34.2%). Forty-four (17.1%) received a VP shunt a median of 17 days (range, 1–320 days) post-diagnosis. By multivariable analysis, baseline opening pressure >30 cm H2O (OR, 9.4; 95% CI, 3.0, 28.8; P < .0001), being a normal host (OR, 6.3; 95% CI, 1.5, 26.1; P = .011) and hydrocephalus (OR, 4.9, 95% CI, 1.3, 17.9); P = .017) were associated with increased odds of shunting (Table 2). In contrast, age (OR, 0.96; 95% CI, 0.92, 0.99; P = .037) and male gender (OR, 0.18; 95% CI, 0.06, 0.55; P = .023) were associated with decreased odds of shunting.
Conclusions
Identification of factors at time of CM diagnosis associated with need for permanent VP shunt placement may allow for earlier, more aggressive treatment and potentially improve outcomes associated with increased ICP from cryptococcal meningitis.
INTRODUCTION
Cryptococcus species are encapsulated yeasts responsible for a broad range of infections. Pulmonary and central nervous system (CNS) disease are well-known presentations of invasive cryptococcosis, with meningoencephalitis the most frequent and serious manifestation [1]. One of the more important complications of cryptococcal meningoencephalitis (CM) is increased intracranial pressure (ICP), sometimes resulting in hydrocephalus. An opening cerebrospinal fluid (CSF) pressure of >25 centimeters (cm) of water is found in approximately half of patients with cryptococcosis and AIDS [2–4]. A high yeast burden is felt to be a contributing factor to increased ICP, in part through the pro-inflammatory effects of cryptococcal antigen in these patients [4]. Cryptococci may cause outflow obstruction by blocking passage of CSF across arachnoid villi and, similarly, soluble cryptococcal capsular polysaccharide may accumulate in arachnoid villi, leading to alterations in CSF drainage [2].
Elevated ICP in the setting of CM is associated with increased mortality and poor neurologic outcomes among immunosuppressed patients, especially among patients with AIDS [2, 5–7]. To alleviate poor outcomes, management of elevated ICP requires frequent lumbar punctures until pressure and symptoms have stabilized [1, 5]. Medications to reduce ICP, such as corticosteroids, mannitol, or acetazolamide, are typically not helpful and may lead to worse outcomes [2, 8, 9]. In a minority of patients, more aggressive measures such as lumbar drainage or ventriculoperitoneal (VP) shunt placement may be required to alleviate increased ICP [1, 4, 10–15]. However, it is important to note that in resource-limited settings, where the majority of HIV-related CM cases are encountered, availability of these procedures is lacking. It is difficult to predict for whom aggressive measures such as VP shunting should be prescribed, especially at time of diagnosis of CM [15]. Herein, we investigate factors at the time CM diagnosis associated with permanent VP shunt placement in a large cohort of patients with CM, in an effort to better identify those patients who may benefit from this procedure to alleviate increased ICP and improve outcomes.
METHODS
We conducted a retrospective observational cohort study of patients with incident CM. Included cases were identified ante-mortem at the University of Alabama at Birmingham (UAB) from January 1, 1996, through December 31, 2015. Cases were identified by review of microbiology, serology and histopathology reports, diagnosis codes, and referral for infectious diseases consultations. We utilized a standardized case report form to collect demographic data, site of involvement, underlying diseases, clinical presentation, diagnostic laboratory and radiographic findings, and outcomes.
Definitions
A case of CM was defined by a positive culture for Cryptococcus species or a positive cryptococcal antigen (CrAg) assay from CSF, in a patient with a syndrome consistent with CM. Patients were excluded if a VP shunt had been placed prior to the diagnosis of cryptococcosis. Length of symptoms was defined as day of symptom onset until date meeting the case definition. A patient was classified as a “normal host” if we found no evidence of HIV, transplantation, malignancy, neutropenia, chronic organ dysfunction, diabetes, corticosteroid use, other recent (12 months) immunosuppressant use, or other immunodeficiency.
Statistical Analysis
For analysis of the relationship of variables among patients who received VP shunts or not, the chi-square test or Fisher test exact was used for categorical variables and the 2-group t test or Wilcoxon rank sum test was used for continuous variables, as indicated. Multivariable analysis of baseline (at time of CM diagnosis) factors associated with VP shunt placement was performed with stepwise logistic regression analysis. We chose to analyze factors at time of diagnosis of CM that were associated with shunting or not to better minimize confounding by indication associated with physicians’ preferences of performing lumbar puncture or shunting procedures. First, a multivariable model was constructed for each of the following categories: demographics or underlying diseases; clinical presentation factors; and baseline diagnostic testing (labs, radiography). Covariates from each category with the strongest associations from univariate analyses were chosen for these models. A final multivariable logistic regression model containing the best predictor variable(s) from the individual multivariable models was performed. This model used all available data and was chosen in order to obtain more robust estimates of the odds ratios, confidence intervals, and P values given the small number of outcome events (44 shunted patients). The criterion for model entry was significance at α = 0.20, while the criterion for remaining in the model was significance at α = 0.05. Odds ratios and 95% confidence intervals (95% CI) were calculated. Model fit by the Hosmer-Lemeshow goodness-of-fit test was assessed and all models fit the data well (P > .05). All statistical tests were 2-tailed and utilized a 5% significance level. Analysis was performed using SAS software version 9.4 (SAS Institute, Inc., Cary, NC).
RESULTS
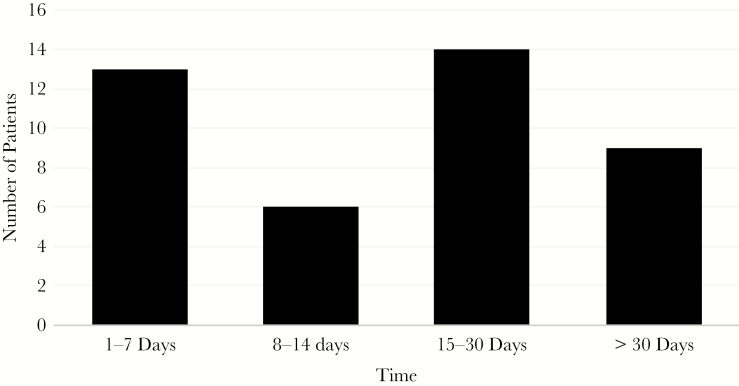
During the study period, there were 422 patients identified with incident cryptococcosis, 257 (60.9%) of whom had CM. Mean age of the cohort was 47.7 years, 71.6% were male, and 44.4% of black race (Table 1). The most common underlying conditions were HIV (42.4%), solid organ transplantation (29.6%), and corticosteroid use (34.2%). Forty-four (17.1%) received a VP shunt a median of 17 days (range, 1–320 days) following diagnosis. Forty-five percent of shunting occurred within 14 days of diagnosis (Figure 1). On univariate analysis, patients who received a VP shunt were significantly more likely to be younger, male, and be normal hosts (Table 1). Overall 1-year crude mortality for all patients with CM was 48.2% and lower in patients who received VP shunting (29.6 vs 52.1; P = .006). Among patients with opening pressure >25 mmHg, 1-year mortality was increased in patients who were not shunted compared with those who were (55.7% vs 32.1%; P = .035). This was more pronounced in patients with opening pressure >30 mmHg (63.6% vs 28.0%; P = .004).
Table 1.
Characteristics of Patients With Cryptococcal Meningitis
| Characteristics | Total N = 257 (%) |
Shunt N = 44 (%) |
No Shunt N = 213 (%) |
P value |
|---|---|---|---|---|
| Mean age, years (SD) | 47.7 (14.4) | 43.1 (10.0) | 48.6 (15.0) | .02 |
| Age <50 years (%) | 151 (58.8) | 34 (77.3) | 117 (54.9) | .006 |
| Male gender (%) | 184 (71.6) | 25 (56.8) | 159 (74.7) | .02 |
| Black race (%) | 114 (44.4) | 19 (43.2) | 95 (44.6) | .86 |
| Mortality (1 year) (%) | 124 (48.2) | 13 (29.6) | 11 (52.1) | .006 |
| Underlying disease (%) | ||||
| HIV | 109 (42.4) | 18 (40.9) | 91 (42.7) | .82 |
| Solid organ transplant | 76 (29.6) | 7 (15.9) | 69 (32.4) | .03 |
| Non-HIV, non-SOT | 72 (28.0) | 19 (43.2) | 53 (24.9) | .014 |
| Normal host | 29 (11.3) | 13 (29.6) | 16 (7.5) | <.001 |
| Renal insufficiency | 29 (11.3) | 1 (2.3) | 28 (13.2) | .04 |
| Diabetes | 39 (15.2) | 7 (15.9) | 32 (15.0) | .88 |
| Cirrhosis | 15 (5.8) | 0 (0) | 15 (7.0) | .07 |
| Corticosteroids | 88 (34.2) | 9 (20.5) | 79 (37.1) | .03 |
| Cancer | 22 (8.6) | 1 (2.3) | 21 (9.9) | .13 |
| Mean length of symptoms, days (SD) | 31.3 (49.2) | 46.4 (69.6) | 28.2 (43.4) | .02 |
| Clinical presentation (%) | ||||
| Symptoms present >14 days | 124 (48.3) | 25 (56.8) | 99 (46.5) | .21 |
| Malaise | 60 (23.4) | 7 (15.9) | 53 (24.9) | .20 |
| Weight loss | 56 (21.8) | 10 (22.7) | 46 (21.6) | .87 |
| Fever | 97 (37.7) | 13 (29.6) | 84 (39.4) | .22 |
| Headache | 181 (70.4) | 37 (84.1) | 144 (67.6) | .03 |
| Nausea and vomiting | 84 (32.7) | 19 (43.2) | 65 (30.5) | .10 |
| Cranial nerve palsy | 23 (9.0) | 9 (20.5) | 14 (6.6) | .003 |
| Altered mental status | 113 (44.0) | 19 (43.2) | 94 (44.1) | .90 |
| Seizures | 28 (10.9) | 4 (9.1) | 24 (11.3) | .80 |
| Visual changes | 59 (23.0) | 21 (47.7) | 38 (17.8) | <.0001 |
| Baseline diagnostic testing | ||||
| CSF protein, mean (SD) | 135.2 (219.1) | 159.2 (208.1) | 130.1 (221.6) | .44 |
| CSF protein > 125 mg/dl | 66 (25.7) | 12 (29.6) | 53 (24.9) | .51 |
| CSF glucose, mean (SD) | 47.1 (32.2) | 43.0 (27.5) | 48.0 (33.1) | .35 |
| CSF glucose < 20 mg/dl | 55 (21.4) | 10 (22.7) | 45 (21.1) | .81 |
| CSF WBC count, mean (SD) | 117.6 (297.3) | 122.6 (238) | 116.5 (308) | .90 |
| CSF WBC > 100 cells | 59 (23.0) | 11 (25.0) | 48 (22.5) | .72 |
| Opening pressure, mean mm H2O (SD) | 28.4 (16.0) | 41.3 (15.7) | 25.6 (14.6) | <.0001 |
| CSF OP > 25 cm H2O | 98/178 (55.1) | 28/32 (87.5) | 70/146 (48.0) | <.0001 |
| CSF OP > 30 cm H2O | 69/178 (38.8) | 25/32 (78.1) | 44/146 (30.1) | <.0001 |
| Repeated lumbar puncture for increased pressure | 100/251 (39.8) | 28/43 (65.1) | 72/211 (34.1) | .0001 |
| Serum CrAG ≥ 1024 | 70 (27.2) | 10 (22.7) | 60 (28.2) | .46 |
| Serum CrAG, median | 512 | 1024 | 512 | .30 |
| CSF CrAG ≥ 1024 | 71 (27.6) | 12 (27.3) | 59 (27.7) | .95 |
| CSF CrAG, median | 256 | 512 | 256 | .48 |
| Positive baseline CSF culture | 197/244 (80.7) | 35/43 (81.4) | 162/201 (80.6) | .90 |
| Hydrocephalus on baseline imaging | 30/223 (14.3) | 14/41 (34.1) | 16/182 (8.8) | <.0001 |
Abbreviations: CRAG, cryptococcal antigen; CSF, cerebrospinal fluid; OP, opening pressure; SD, standard deviation; SOT, solid organ transplant; WBC, white blood cell.
Figure 1.
Time to Shunting From Diagnosis in 42 Patients With Cryptococcal Meningitis
Clinical Presentation
As listed in Table 1, the mean length of symptoms in the cohort was 31.3 days and longer for patients who were shunted (46.4 vs 28.2 days; P = .02). Headache was present in 70.4% of patients and frequency was greater among patients who were later shunted (84.1% vs 67.6%; P = .03). The symptoms or signs most strongly associated with shunting were visual changes (47.7% vs 17.8%; P < .0001) and cranial nerve palsy (20.5% vs 6.6%; P < .003). The remainder of clinical symptoms and signs were similar (Table 1).
Laboratory and Radiographic Findings
Among patients with CM, the median serum CrAg was 1:512 and median CSF CrAg was 1:256; values were similar between patients who were shunted and those who were not (Table 1). The proportion of patients with positive CSF cultures was similar across groups, 80.7% to 81.4% (P = .90). Among the 210 patients who had opening pressure measured on baseline lumbar puncture, the mean pressure was 28.4 cmH20, and greater in patients who were later shunted (41.3 cm H20 vs 25.6 cm H20; P < .0001). Among shunted patients, 88% had an opening pressure of >30 cm H2O at baseline. Other CSF studies, including protein, glucose,, and white blood cell counts were similar among patients. Among 223 patients with baseline head computed tomography or magnetic resonance imaging, hydrocephalus was present in 30 (14.3%) patients. The frequency of hydrocephalus was greater in patients who were later shunted compared to those not shunted (34.1% vs 8.8%; P < .0001).
Multivariable Analysis
In multivariable logistic regression analysis, baseline opening pressure >30 cm H2O (OR, 9.4; 95% CI, 3.0, 28.8; P < .0001), being a normal host (OR, 6.3; 95% CI, 1.5, 26.1; P = .011) and hydrocephalus (OR, 4.9; 95% CI, 1.3, 17.9; P = .017) were associated with increased odds of shunting (Table 2). In contrast, increasing age (OR, 0.96; 95% CI, 0.92, 0.99; P = .037) and male gender (OR, 0.18; 95% CI, 0.06, 0.55; P = .023) were associated with decreased odds of shunting.
Table 2.
Multivariable Analysis of Baseline Factors Associated With Shunting
| Variable | Univariatea | Multivariableb | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age | 0.97 (0.95, 0.99) | .022 | 0.96 (0.92, 0.99) | .037 |
| Male | 0.45 (0.23, 0.88) | .019 | 0.18 (0.06, 0.55) | .023 |
| Normal host | 5.2 (2.27, 11.77) | <.0001 | 6.3 (1.5, 26.1) | .011 |
| OP > 30 cm H2O | 8.3 (3.3, 20.6) | <.0001 | 9.4 (3.0, 28.8) | <.0001 |
| Hydrocephalus | 5.4 (2.4, 12.3) | <.0001 | 4.9 (1.3, 17.9) | .017 |
a Logistic regression analysis. P values obtained are 2-tailed.
b Final logistic regression model containing the covariates with the strongest associations from the individual multivariable models of demographics or underlying diseases, clinical presentation factors, and baseline diagnostic testing.
DISCUSSION
Cryptococcal meningitis remains an important complication of patients worldwide and is especially common among immunocompromised patients [16]. Intracranial hypertension, reported in over half of patients with CM, is associated with significant morbidity and mortality [2, 3, 5]. Management of elevated intracranial pressure is of paramount importance and is typically achieved via serial lumbar punctures. In refractory cases, temporary or permanent solutions such as lumbar drains or ventriculoperitoneal shunting may be required; however, it is important to note that in resource limited areas, where the majority of HIV-associated CM cases are managed, these more aggressive procedures may not be available. To date, few data are available evaluating predictors of shunting in patients with CM [14, 15].
Our study investigated a large cohort of patients with CM and elevated ICP, with many receiving VP shunting for control of ICP. We observed that at time of presentation and first diagnosis of CM that there were several demographic, host, or laboratory or radiographic parameters independently associated with subsequent VP shunting. Moreover, shunted patients had significantly improved mortality that was more pronounced with increasing baseline opening pressures. Identifying patients at time of CM diagnosis who may require aggressive reduction of ICP and perhaps shunting may aid in improving cryptococcal outcomes.
Demographics such as age and gender were associated significantly with VP shunting. As age increased, the odds of receiving a shunt was lower. First, younger patients theoretically may induce a more robust inflammatory response, potentially leading to increased brain inflammation and elevated ICP. Second, this finding may be related to the ability to undergo a procedure. For example, in older patients, shunting procedures may have been deferred due to other comorbidities or goals of care. This may have impacted mortality in patients who were not shunted. Male patients were also significantly less likely to receive a shunt. This is likely related to the large number of HIV-infected patients, also more commonly younger, who were male in our cohort. Recent studies also have reported a predominance of younger male and female patients who receive shunting [12, 14, 15, 17].
The characteristic of being a phenotypically normal host was strongly associated with shunting. This is intuitive, as a more robust immune response to Cryptococcus may be associated with increased inflammation in the brain, potentially resulting in increased ICP. In addition, these patients typically have more prolonged symptoms and a greater frequency of hydrocephalus on baseline imaging [18, 19]. Indeed, in this cohort, when comparing normal hosts to other groups, hydrocephalus and greater length of symptoms were more common, but normal host type remained as an associated variable after adjustment in the final model. In our clinical experience, the normal host often is the most complicated CM case to manage due to recurrent problems with elevated ICP.
As expected, elevated opening pressure at baseline was strongly associated with VP shunting, when evaluated as either a continuous or categorical variable. This supports previous studies [14, 15]. For this analysis, we classified elevated ICP as greater than 30 cm H2O, which occurred in approximately 88% of shunted patients at baseline. It is possible a threshold of elevated ICP exists at the time of initial presentation that will not be alleviated with serial lumbar punctures and medical management (antifungals) alone. However, there were several patients in our cohort with opening pressures >35 cm H2O who were successfully treated without shunting. But, in the overall cohort and in those with elevated pressures (>25 mm H2O or >30 mm H2O), all-cause mortality was significantly greater in those patients who were not shunted.
An important aspect of ICP and outcomes is related to the frequency of serial lumbar punctures. Cherian and colleagues compared patients who had serial lumbar punctures and later shunting versus serial lumbar punctures alone. On univariate analysis, mean number of lumbar punctures was not associated with shunting; yet female gender and elevated CSF CrAG were [14]. For our cohort, there was no standard protocol for lumbar puncture frequency or timing. We did not collect data on total number of lumbar punctures and so we did not choose to analyze this variable due to potential for confounding by indication. However, patients who had repeated lumbar punctures for the indication of elevated pressure with symptoms were significantly more likely to be shunted.
Hydrocephalus on baseline imaging was uncommon (14%) in our cohort but was strongly associated with shunting. As many studies have documented in patients with CM, despite elevated opening pressures, hydrocephalus may not be present on imaging [2, 14, 15]. We suggest early neurosurgical consultation for persistent elevated ICP in patients with or without hydrocephalus, as we observed better outcomes in shunted patients.
Our study has limitations. Although this is a large series of patients with CM managed with VP shunting, we were limited in our multivariable analyses due to only 44 shunt events. It is likely that we were unable to include potentially important variables into the final multivariable model. We felt it was most appropriate to analyze factors related to VP shunt at baseline (time of presentation) in order to minimize confounding by indication. For example, the timing and choice of VP shunt placement may be related to preferences of neurosurgery or infectious diseases physicians. In addition, this strategy did not allow us to determine impact of antifungal therapy, role of serial or number of lumbar punctures, or value of quantitative cryptococcal CSF colony forming units as covariates. During the study, the indication or frequency of lumbar punctures was not standardized, nor was there a protocol for a specific treatment regimen. Our data may not be applicable in resource-limited settings where lumbar drainage procedures or shunting are not available.
In summary, we identified in our cohort of patients with CM independent baseline factors related to permanent VP shunting. As increased and persistent ICP in CM is associated with poor outcomes, early identification of patients who may require aggressive measures, such as shunting, could influence management decisions and mortality. Investigation of the role of antifungal therapies and serial lumbar punctures on the relationship to permanent VP shunting is warranted.
Acknowledgments
We would like to thank former Infectious Diseases Fellows Benjamin Klausing, MD, Kyle Brizendine, MD, and David Dougherty, MD, for assistance with data collection.
Financial support. None reported.
Potential conflicts of interest. J.W.B. is a consultant for Merck and Eli Lilly; he is also a data safety monitoring board or adjudication committee member for R-Pharm and Pfizer. P.G.P. is a scientific consultant for Cidara, IMMY, Scynexis, Amplyx, Astellas, Merck, and Mayne Pharma; he is provides research support for Cidara, IMMY, Scynexis, Gilead, Amplyx, Astellas, and Mayne Pharma. All other authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Perfect JR, Dismukes WE, Dromer F, et al. . Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 2010; 50:291–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Graybill JR, Sobel J, Saag M, et al. . Diagnosis and management of increased intracranial pressure in patients with AIDS and cryptococcal meningitis. The NIAID Mycoses Study Group and AIDS Cooperative Treatment Groups. Clin Infect Dis 2000; 30:47–54. [DOI] [PubMed] [Google Scholar]
- 3. Pappas PG. Managing cryptococcal meningitis is about handling the pressure. Clin Infect Dis 2005; 40:480–2. [DOI] [PubMed] [Google Scholar]
- 4. Bicanic T, Brouwer AE, Meintjes G, et al. . Relationship of cerebrospinal fluid pressure, fungal burden and outcome in patients with cryptococcal meningitis undergoing serial lumbar punctures. AIDS 2009; 23:701–6. [DOI] [PubMed] [Google Scholar]
- 5. Rolfes MA, Hullsiek KH, Rhein J, et al. . The effect of therapeutic lumbar punctures on acute mortality from cryptococcal meningitis. Clin Infect Dis 2014; 59:1607–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jarvis JN, Bicanic T, Loyse A, et al. . Determinants of mortality in a combined cohort of 501 patients with HIV-associated cryptococcal meningitis: implications for improving outcomes. Clin Infect Dis 2014; 58:736–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jarvis JN, Dromer F, Harrison TS, Lortholary O. Managing cryptococcosis in the immunocompromised host. Curr Opin Infect Dis 2008; 21:596–603. [DOI] [PubMed] [Google Scholar]
- 8. Newton PN, Thai le H, Tip NQ, et al. . A randomized, double-blind, placebo-controlled trial of acetazolamide for the treatment of elevated intracranial pressure in cryptococcal meningitis. Clin Infect Dis 2002; 35:769–72. [DOI] [PubMed] [Google Scholar]
- 9. Beardsley J, Wolbers M, Day JN; CryptoDex Investigators Dexamethasone in cryptococcal meningitis. N Engl J Med 2016; 375:189–90. [DOI] [PubMed] [Google Scholar]
- 10. Macsween KF, Bicanic T, Brouwer AE, et al. . Lumbar drainage for control of raised cerebrospinal fluid pressure in cryptococcal meningitis: case report and review. J Infect 2005; 51:e221–4. [DOI] [PubMed] [Google Scholar]
- 11. Liliang PC, Liang CL, Chang WN, Lu K, Lu CH. Use of ventriculoperitoneal shunts to treat uncontrollable intracranial hypertension in patients who have cryptococcal meningitis without hydrocephalus. Clin Infect Dis 2002; 34:E64–8. [DOI] [PubMed] [Google Scholar]
- 12. Liu L, Zhang R, Tang Y, Lu H. The use of ventriculoperitoneal shunts for uncontrollable intracranial hypertension in patients with HIV-associated cryptococcal meningitis with or without hydrocephalus. Biosci Trends 2014; 8:327–32. [DOI] [PubMed] [Google Scholar]
- 13. Corti M, Priarone M, Negroni R, et al. . Ventriculoperitoneal shunts for treating increased intracranial pressure in cryptococcal meningitis with or without ventriculomegaly. Rev Soc Bras Med Trop 2014; 47:524–7. [DOI] [PubMed] [Google Scholar]
- 14. Cherian J, Atmar RL, Gopinath SP. Shunting in cryptococcal meningitis. J Neurosurg 2016; 125:177–86. [DOI] [PubMed] [Google Scholar]
- 15. Phusoongnern W, Anunnatsiri S, Sawanyawisuth K, Kitkhuandee A. Predictive model for permanent shunting in cryptococcal meningitis. Am J Trop Med Hyg 2017; 97:1451–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Park BJ, Wannemuehler KA, Marston BJ, et al. . Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 2009; 23:525–30. [DOI] [PubMed] [Google Scholar]
- 17. Bian F, Wu Y, Yu S, et al. . Study on genotype and virulence of Cryptococcus neoformans and Cryptococcus gattii clinical isolates in Guigang, Guangxi Zhuang autonomous region. Zhonghua Liu Xing Bing Xue Za Zhi 2015; 36:491–5. [PubMed] [Google Scholar]
- 18. Brizendine KD, Baddley JW, Pappas PG. Predictors of mortality and differences in clinical features among patients with cryptococcosis according to immune status. PLOS ONE 2013; 8:e60431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bratton EW, El Husseini N, Chastain CA, et al. . Comparison and temporal trends of three groups with cryptococcosis: HIV-infected, solid organ transplant, and HIV-negative/non-transplant. PLOS ONE 2012; 7:e43582. [DOI] [PMC free article] [PubMed] [Google Scholar]