Summary:
Chronic Atypical Neutrophilic Dermatosis with Lipodystrophy and Elevated temperature (CANDLE) is a rare autoinflammatory interferonopathy caused by additive loss-of-function mutations in proteasome genes. Mutations in the proteasome chaperone, PSMG2/PAC2 are a novel cause of CANDLE.
Keywords: Chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature, Proteasome Associated Autoinflammatory Syndrome, Type I IFN, Interferonopathy, Whole exome sequencing, Peditatrics, Autoinflammatory diseases
To the Editor:
Chronic Atypical Neutrophilic Dermatosis with Lipodystrophy and Elevated temperature (CANDLE) or proteaseome associated autoinflammatory syndrome (PRAAS) is a rare autoinflammatory interferonopathy1 caused by additive loss-of-function (LOF) mutations in proteasome genes, [PRAAS1 MIM#256040 (recessive PSMB8 and digenic with PSMB4 and PSMA3,2,3,E1-E3 PRAAS2 MIM#618048 (POMP haploinsufficiency)3,4 and PRAAS3 MIM#617591 (recessive PSMB4 and digenic with PSMB9)].3 We report a patient with clinical features of CANDLE and an episode of autoimmune hemolytic anemia (AIHA), who has two novel mutations in the proteasome assembly gene PSMG2, identifying the earliest proteaseome assembly chaperone PAC2, as critical in human proteaseome assembly and as a novel gene causing CANDLE, PRAAS4.
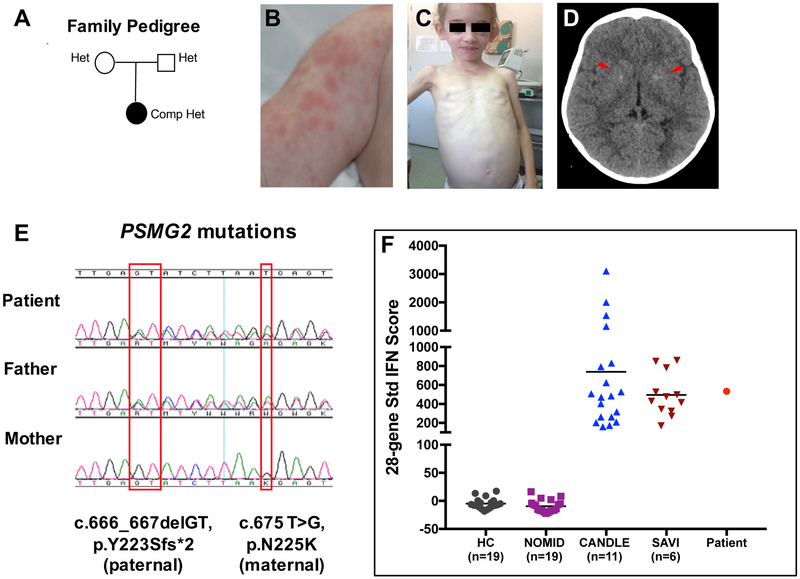
The patient, an 8-year, 10-months-old Caucasian girl at diagnosis, presented since 4-months of age with edema of the extremities (Fig 1, A, B). At 7-months she developed erythematous, violaceous nodules on the upper and lower extremities and was diagnosed with panniculitis. During a hospitalization for recurrent fever flares at 2 yrs of age, generalized lipodystrophy (Fig 1, C), mild gross motor and speech delay, in the context of elevated acute phase reactants, hepatosplenomegaly and diffuse lymphadenopathy were noted; height and weight were normal and an immunodeficiency work up was unremarkable. At 5 years of age, she developed severe direct antigen test (DAT) (or Coombs) positive autoimmune hemolytic anemia (AIHA), with a hemoglobin of 3.1 g/dL, requiring 3 blood transfusions, pulse doses of corticosteroids, 4 infusions of rituximab, and a 19-month-course of azathioprine plus IVIG every 4 weeks. Prednisolone, which was started at the age of 5 years, improved panniculitis and hepatosplenomegaly but lipoatrophy and joint contractures progressed. An evaluation at 7 yrs of age revealed active myositis on MRI (Fig E1), and significant muscle atrophy in her upper and lower extremities with marked fatty infiltration of thigh muscles. A brain CT showed basal ganglia calcifications (Fig 1, D) and a clinical diagnosis of possible CANDLE was made. A clinical genetic panel for known autoinflammatory diseases (including then known CANDLE genes) was negative. WES performed on a research protocol revealed 2 novel variants in conserved amino acids in PSMG2 (Chromosome 18p11.21, NM_020232) encoding the proteasome assembly protein, PAC2, a frameshift mutation, c.666_667delGT, p.Y223Sfs*2, inherited from her father and a missense mutation c.675 T>G, p.N225K, from her mother; both were confirmed by Sanger sequencing (Fig 1, E and see Fig E2, A,B). The variants were not found in public (gnomAD, ESP6500, 1000G) or local (308 exomes) databases. An interferon signature assessed at the time of active disease was elevated comparable to patients with genetically confirmed CANDLE and the interferonopathy SAVI (Fig 1, F).
FIG 1. Clinical presentation and genetic findings in CANDLE/PRAAS4.
A, Family tree depicting that both patient’s parents are heterozygous for a different PSMG2 mutation. B, Nodular skin rash at the age of 7months. C, Generalized lipoatrophy involving the face and upper extremities at the age of 7 years. D, A brain CT showing faint bilateral basal ganglia calcifications (arrow heads). E, Sanger sequencing electropherogram from patient and parents showing that the frameshift deletion is on the paternal allele and the missense variant is on the maternal allele. F, 28-gene interferon (IFN) score performed with a customized Nanostring assay demonstrated that our patient has a high IFN score, similar to the observed in patients with the interferonopathies CANDLE and SAVI9. Mean ± SD of 28-gene IFN score: healthy controls (HC): −4.8 ± 9.53; neonatal onset multisystem inflammatory disease (NOMID): −9.37 ± 10.97; CANDLE: 738.2 ± 754.4; SAVI: 495.6 ± 225.9; Patient: 533.6
The ubiquitin-proteasome system (UPS) is the primary homeostatic degradation system for ubiquitinated proteins; and proteasome dysfunction is associated with several human diseases including CANDLE /PRAAS.5, E4, E5 PAC2 is a proteaseome assembly chaperone that dimerizes with PAC1, encoded by PSMG1, to form the PAC1/PAC2 complex.E6 Studies in different model systems showed a pivotal role of the PAC1/PAC2-complex in incorporating the proteasome α-subunits into the full 20S proteasome (Fig E3).E7-E10 To assess whether the novel mutations confer a loss-of-function, we compared the proteolytic activity (chymotrypsin-, trypsin- and caspase-like activities) of the proteasome in the patient’s fibroblast lysates to lysates from disease controls of genetically confirmed CANDLE patients (DC) and healthy controls (HC). The patient had decreased proteolytic activities similar to the two DCs (Fig 2, A) which leads to similar accumulation of ubiquitin conjugates as in the 2 DCs (Fig 2, B). Proteasome-subunit expression showed a strong reduction of PAC1 and PAC2 protein subunits in the patient’s sample and no apparent changes in the expression of other proteasome subunits (Fig 2, C). However, the amount of assembled proteasome complexes was decreased (α6 staining), even when compared to the 2 DCs. As only small amounts of mutant PSMG2/PAC2-variants were incorporated into nascent proteasome complexes, the impaired proteasome assembly (Fig 2, D), is conferred by reduced binding of the mutant PAC1/PAC2 heterodimer to the α-subunit of the proteasome core (Fig 2, D) that results in reduced proteasome assembly and activity (Fig 2, E). To exclude extensive non-sense mediated RNA decay, we assessed messenger RNA (mRNA) expression of the mutant alleles (frameshift and missense) in construct-transfected HeLa cells (Fig 2, F). Compared to wildtype constructs, the proteasome-incorporation of each mutant protein (shown for α6) was reduced with more severe impairment for the frameshift mutation (Fig 2, G), thus confirming an independent contribution from each mutant allele.
FIG 2. Fibroblasts from the patient with mutant PAC2 had reduced proteolytic activity and proteasome amounts with accumulation of ubiquitin aggregates.
A, Fibroblasts lysates from the patient (Pt.) and CANDLE/PRAAS disease controls (DC) had approximately 75% of proteasome activity compared to healthy controls (HCs). Means±SD estimated from triplicates and normalized against HC1+2 (n=3). B, PRAAS patients accumulated insoluble ubiquitin aggregates. C, Reduced PAC1 and PAC2 expression in patient’s sample compared to HCs and DCs. Expression of subunits POMP/POMP, α6/PSMA1, β5i/PSMB8, PA28b/PSME2, RPT6/PSMC5, PA200/PSME4 was normal. β–Tubulin: loading control. D, Native PAGE-immunoblot-analyses shows a reduced PAC1 and PAC2 incorporation into the proteasome complexes. Reduced α6 staining indicates less proteasome complexes in all CANDLE/PRAAS patients E, Overlay of chymotrypsin-like activity shows reduced proteolytic activity in the main proteasome complexes for CANDLE/PRAAS patients.. F, The epitope–tagged PAC2 variants (wildtype (WT); fs (frameshift mutation); NK (N225K mutation) were expressed in HeLa cells and detected by a Flag-specific antibody. MOCK, empty vector backbone; HeLa, non-transfected control. β–Tubulin: loading control. Neomycin-Phosphatase (Neo-P): transfection efficiency control. All PAC2-variant mRNAs were equally expressed in HeLa cells (RT-PCR). GAPDH: loading control. Panels A-F: representative results from n=3. G, Density gradient fractionation (panel F) shows incorporation of both mutant Pac2 variants into proteasome complexes, without impacting total proteasome formation (α6 staining).
Most CANDLE-causing mutations reported so far occur in genes encoding α- or β- subunits of the 20S core particle (CP) proteasome which is composed of 2 α-rings, and 2 β-rings. Each α-ring is composed of 7 α-subunits (encoded by PSMA1-7) each β-ring is composed of 7 β-subunits (encoded by PSMB1-7).E11 The mutations are hypomorphic and inherited in a recessive or digenic mode, conferring additive LOF. PAC2, is one of 3 assembly chaperones including POMP (“human Ump1”)E8-E10 and the PAC3/PAC4 heterodimer.E7 The 3 reported hypomorphic disease-causing POMP mutations are dominant due to haploinsufficiency (Fig E4),3,4 whereas the hypomorphic, disease-causing PSMG2 mutations are recessive conferring additive LOF. As both parents are asymptomatic, the wildtype allele in the heterozygous state can rescue function.
In contrast to patients with IL-1-mediated autoinflammatory diseases, patients with interferonopathies have elevated autoantibody titers, including ANA and ENA, c-ANCA, anti-thyroid antibodies, lupus anticoagulant, anti-cardiolipin and beta-2-glycoprotein I [β2GPI] antibodies which is consistent with the prominent role of Type-1 IFN in driving autoantibody formation. In many instances the antibodies are transient and not associated with clinical pathology.E12-E14 However, our patient developed DAT (or Coombs) positive AIHA caused by antibodies against self-erythrocyte antigens. Most AIHA patients have IgG warm antibodies that trigger erythocyte phagocytosis, a smaller subset has IgM antibodies that are cold activated, fix complement and cause cold-type AIHA.6,E15, E16 AIHA has been reported in two CANDLE patients,7,8 and is frequently seen in a spectrum of patients with immune dysregulatory diseases (eg, systemic lupus erythematosus, lymphoproliferative diseases including B-cell malignancies, primary immunodeficiencies and infectionsE17 and can present as hemolytic anemia and thrombocytopenia (Evans syndrome), which suggests that common genetic variants influence the development of AIHA.E15
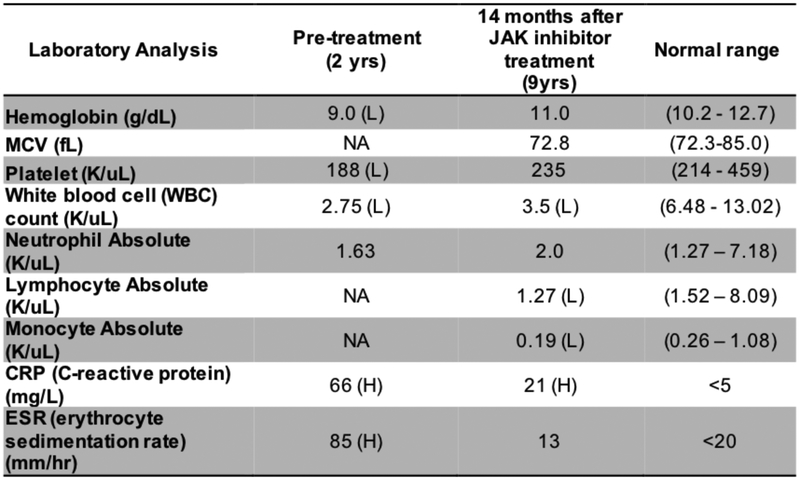
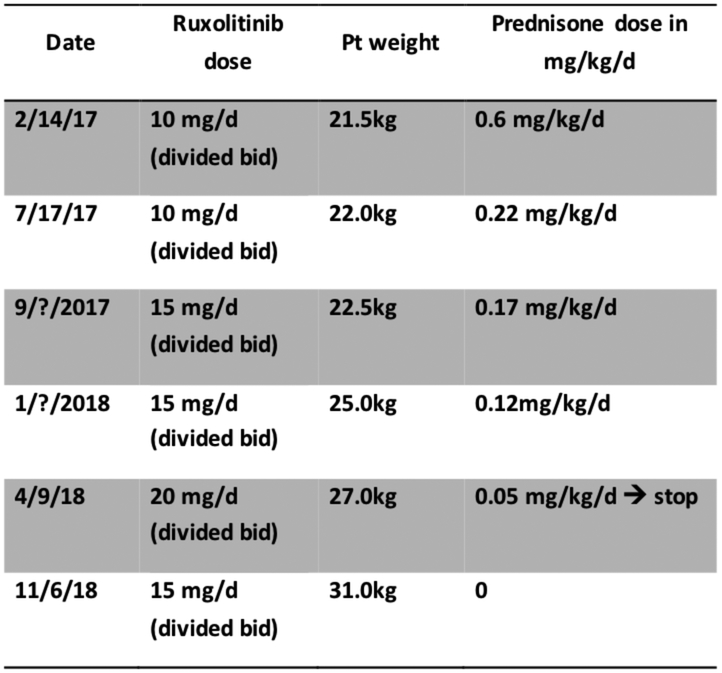
Lastly, the diangosis of CANDLE/PRAAS led to treatment with the JAK inhibitor ruxolitinib, which inhibits the transduction of IFN-mediated signals on the IFN-receptors and of other class I and class II cytokine receptors.E18 JAK inhibitors have shown benefit in reducing the symptoms and the IFN score in CANDLE patients,9 and treatment resulted in marked improvement and halted progressive lipoatrophy (Fig E5). Ruxolitinib was started at 8 years at 5mg twice a day and increased with weight gain. Ruxolitinib treatment enabled a rapid corticosteroid taper from 0.6 mg/kg/d to discontinuation after 14 months on JAK inhibition without a need to restart corticopsteroid treatment (Fig E6). Hepatosplenomegaly and diffuse lymphadenopathy disappeared, joint contractures improved dramatically (with the exception of the left ankle) and inflammatory markers normalized.
In summary, we describe two novel compound heterozygous, loss-of-function mutations in PSMG2 that encodes the proteasome assembly chaperone, PAC2, as a novel genetic cause of CANDLE/PRAAS. Given the advance of emerging treatments targeting Type-I interferon signaling for CANDLE, the recognition of new genetic causes of CANDLE/PRAAS will facilitate a rapid clinical diagnosis and early treatment.
Acknowledgements
We would like to acknowledge Daniela Ludwig for excellent technical assistance. This work was supported by the Intramural Research Program of the NIH, NIAID and NIAMS and by grants of the DFG (SFB740, SFBTR186, SFBTR167) to EK and the Fritz-Thyssen-Foundation (10.16.2.022MN) to EK and AB.
Funding: This research was supported by the Intramural Research Program of the NIH, NIAID and NIAMS. EK and AB were supported by the Fritz-Thyssen-Foundation and the DFG.
Abbreviations:
- CANDLE
Chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature
- PRAAS
Proteasome Associated Autoinflammatory Syndrome
- PAC2
Proteasome Assembly Chaperone 2
- PSMG2
Gene encoding PAC2
Supplementary Appendix
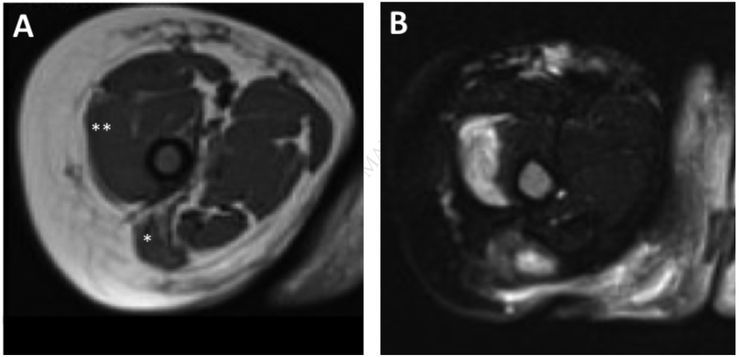
FIG E1. Magnetic resonance imaging (MRI) of upper thighs showing muscular atrophy and patchy myositis.
(A) T1 weighted axial MRI of right and left thigh shows atrophy and fatty replacement of the gluteus maximus and vastus medialis muscles. (B) The corresponding T2 weighted fat suppressed axial image shows patchy enhancement in these areas suggestive of active inflammation.
FIG E2. Whole Exome Sequencing (WES) and PSMG2 Multispecies Alignment.
(A) Visualization of the binary alignment map (bam) using Integrative Genomics Viewer (IGV) revealed that the two variants are in trans configuration. The sequencing reads containing the 2 base-pair deletion lack the missense mutation (red dashed box), whereas the sequencing reads containing the missense mutation lack the 2 base-pair deletion (blue dashed box), thus suggesting that each variant was inherited from a different parent which was confirmed by Sanger sequencing. (B) Multispecies alignment of the PSMG2 protein region affected by the compound heterozygous variants confirms conservation across species for both variants.
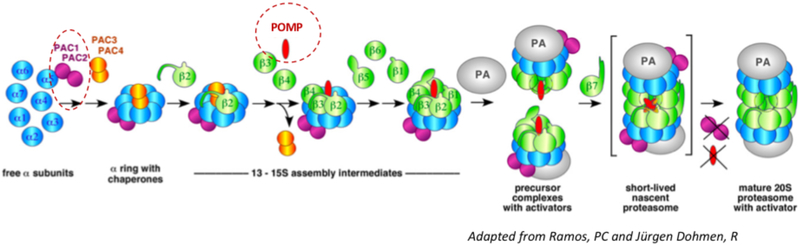
FIG E3. Model of chaperone-aided proteasome assembly.
The PAC1/PAC2 heterodimer initiates α-subunit-ring assembly by binding to the20S core protein subunits, α5/PSMA5 and α4/PSMA7E6 and prevents dimerization of the α-rings and promotes the assembly of the hetero-heptameric αβ-subunit-ring (Le Tallec et al 2007). The PAC3/PAC4 complex binds later and dissociates from the maturing proteasome complex, at the “half-mer (−β7) intermediate” stage when only the β7-subunit is lacking. At that point, POMP is recruited to mediate incorporation of either standard β5/PSMB5 or the β5i/LMP7/PSMB8 immunoproteasome subunit and dimerization of two “half-proteasome precursor complexes” to form the short-lived “nascent proteasome).E6 This conformational change triggers the release of the PAC1/PAC2 complex from the nascent proteasome and enables binding of the 19S regulatory particle.E6,E7,E11 In the red circles: Loss of function mutations in PSMG2/PAC2 (compound heterozygous, disease-causing mutations lead to additive loss of function) and POMP/POMP ((alias UMP1), heterozygous, disease-causing mutations cause haploinsufficiency), mutations in both genes cause CANDLE/PRAAS.
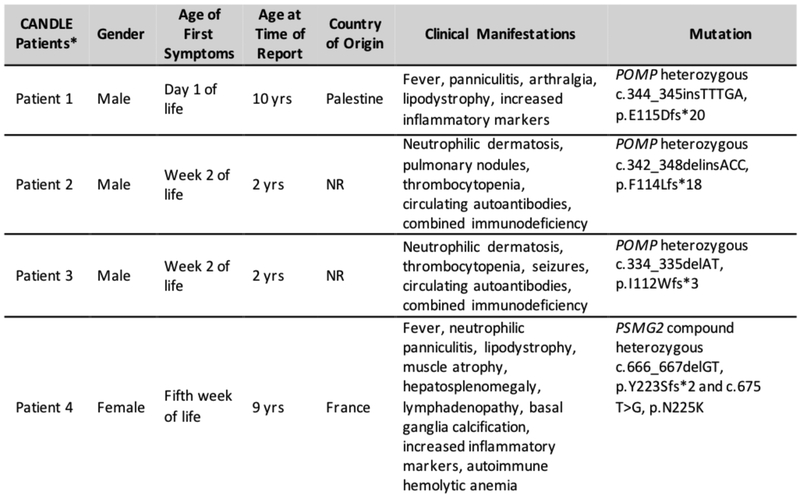
FIG E4. Reports of patients with CANDLE/PRAAS who have mutations in proteasome assembly chaperones4,5.
*denotes CANDLE patients with currently known chaperone mutations in POMP and including our patient with PSMG2 mutations. NR: not reported (in this figure, cases from refs. 4 and 5 were combined)
FIG E5. Laboratory parameters before and 14 months after treatment with the Jak inhibitor ruxolitinib.
H: high; L: low; NA: not available
FIG E6.
Ruxolitinib dose, weight changes and steroid taper on JAK inhibitor baricitinib.
METHODS
Patient
The parents provided consent and the patient was enrolled into an IRB approved NIH natural history protocol, “Studies of the Natural History, Pathogenesis, and Outcome of Autoinflammatory Diseases (NOMID/CAPS, DIRA, CANDLE, SAVI, NLRC4-MAS, Still’s-like Diseases, and other Undifferentiated Autoinflammatory Diseases”, 17-I-0016, NCT02974595).
Genetic Analysis
Whole exome sequencing (WES) was performed on Illumina® HiSeq sequencers as previously reported (Liu et al. 2014). In brief, exomes were captured with the Agilent SureSelect® Human 51 Mbp All Exon Kit (Agilent Technologies, Santa Clara, CA). Variants were called using a pipeline with the Genome Analysis Toolkit (GATK). Variants were annotated with functional impact and allele frequency from public and local datasets. Potential disease-causing mutations were selected and prioritized based on quality score, allele frequency, functional impact, probable inheritance model (de novo, autosomal recessive or autosomal dominant), and expert evaluation. The PSMG2 variants detected by WES in the patient were confirmed by Sanger sequencing of exon 6 and the reference sequence used for primer design and variant annotation was PSMG2 NM_020232.
Cell lines
Primary fibroblasts cell lines from patients and healthy controls were generated from superficial skin biopsies after obtaining consent. Standard protocols were used to generate the cell lines. In brief, cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum (FCS), 1× penicillin/streptomycin and stable glutamine.
Proteasome assay, immunoblot
The proteolytic activity assays were performed as described. Equal amounts of protein extracts from patients’ fibroblasts, or transfected HeLa cells were separated on SDS-Laemmli gels (15%) or native PAGE (3%–12%, Invitrogen) and immunoblotted for proteasome subunits using published protocols3.The following primary antibodies were used: β35i (LMP7), and POMP are from laboratory stocks (1, 2); PAC1 (Cell Signaling, 13378S); PAC2 (Enzo, EX-6); α6 (Enzo, MCP20); PA28β (Cell Signaling, 2409); RPT6 (Enzo, p45-110); PA200 (Novus, NBP2-22236); Flag (Sigma, M2 F1804); β-tubulin (Covance, MMS-410P); Neomycin-Phosphotransferase (Millipore, AC113). Ubiquitin blots were performed with cell lysates from fibroblasts in native buffer. The insoluble fraction was lysed in urea lysis buffer (7 M urea, 4 M thiourea, 4% m/v chaps) and separated on SDS-Laemmli gels (10%) and immunoblotted for ubiquitin (Dako, Z0458).
Transfection
HeLa cells (ATCC) were transiently transfected with expression vectors for wt Flag-Pac2 and mutant-Pac2 variants using Lipofectamine2000 (Invitrogen) for 24h. Transfection efficiency was monitored by immunoblot analyses of Neomycin-Phosphatase expression, encoded by the pcDNA3.1 vector backbone. Ectopic expression of the Flag-PAC2 mRNA from the expression vector in HeLa cells was monitored by semi qRT-PCR as described3 using the following primers:
Gapdh_for: (cagacaccatggggaaggtg);
Gapdh_rev: (CAGCAGTGAGGGTCTCTCTC);
T7_for: (TAATACgACTCACTATggg);
PAC2_rev: (gAAAAgTgCAgggggAAgAC),
Density gradient fractionation
A linear 10%-40% Glycerol gradient (BIOCOMP) was generated in ultracentrifugation tubes and loaded with 1 mg protein; gradient ultracentrifugation was performed at 4°C at 30 000 rpm for 18 hrs. Gradients were fractionated in 0.5 ml aliquots from the top of the gradient; TCA precipitated pellets were resuspended in 1.5x sDs-PAGE loading buffer for immunoblot analyses.
BACKGROUND
Proteasome defects have also been associated with neurologic diseases (Zheng Q et al. 2016). Copy-number variant (CNV) deletions or single-nucleotide variants (SNVs) in PSMD12, encoding the RPN5/PSMD12 subunit of the 19S regulatory protein cause an autosomal dominant syndromic form of intellectual disability. In murine models, loss of Psmd12 led to development of microcephaly, renal tubular atrophy, and aberrant rostrocaudal patterning of the branchial arches, suggesting a role of some proteasome components in organ development (Kury S et al. 2017). Whether the mild cognitive delay that is reported in some CANDLE/PRAAS patients is due to proteasome effects on neurological development or multifactorial remains to be assessed.E12
REFERENCES
- E1.Kitamura A, Maekawa Y, Uehara H, Izumi K, Kawachi I, Nishizawa M, et al. A mutation in the immunoproteasome subunit PSMB8 causes autoinflammation and lipodystrophy in humans. J Clin Invest 2011;121(10):4150–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E2.Arima K, Kinoshita A, Mishima H, Kanazawa N, Kaneko T, Mizushima T, et al. Proteasome assembly defect due to a proteasome subunit beta type 8 (PSMB8) mutation causes the autoinflammatory disorder, Nakajo-Nishimura syndrome. Proc Natl Acad Sci U S A 2011; 108(36):14914–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E3.Liu Y, Ramot Y, Torrelo A, Paller AS, Si N, Babay S, et al. Mutations in proteasome subunit beta type 8 cause chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature with evidence of genetic and phenotypic heterogeneity. Arthritis Rheum 2012;64(3):895–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E4.Ciechanover A. The ubiquitin-proteasome proteolytic pathway. Cell 1994;79(1):13–21. [DOI] [PubMed] [Google Scholar]
- E5.Seifert U, Bialy LP, Ebstein F, Bech-Otschir D, Voigt A, Schroter F, et al. Immunoproteasomes preserve protein homeostasis upon interferon-induced oxidative stress. Cell 2010;142(4):613–24. [DOI] [PubMed] [Google Scholar]
- E6.Hirano Y, Hendil KB, Yashiroda H, Iemura S, Nagane R, Hioki Y, et al. A heterodimeric complex that promotes the assembly of mammalian 20S proteasomes. Nature 2005;437(7063):1381–5. [DOI] [PubMed] [Google Scholar]
- E7.Ramos PC1, Dohmen RJ. PACemakers of proteasome core particle assembly. Structure 2008;16(9):1296–304. [DOI] [PubMed] [Google Scholar]
- E8.Fricke B, Heink S, Steffen J, Kloetzel PM, Kruger E. The proteasome maturation protein POMP facilitates major steps of 20S proteasome formation at the endoplasmic reticulum. EMBO Rep 2007;8(12):1170–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E9.Li X, Kusmierczyk AR, Wong P, Emili A, Hochstrasser M. beta-Subunit appendages promote 20S proteasome assembly by overcoming an Ump1-dependent checkpoint. EMBO J 2007;26(9):2339–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E10.Yashiroda H, Mizushima T, Okamoto K, Kameyama T, Hayashi H, Kishimoto T, et al. Crystal structure of a chaperone complex that contributes to the assembly of yeast 20S proteasomes. Nat Struct Mol Biol 2008;15(3):228–36. [DOI] [PubMed] [Google Scholar]
- E11.Tomko RJ Jr., Funakoshi M, Schneider K, Wang J, Hochstrasser M. Heterohexameric ring arrangement of the eukaryotic proteasomal ATPases: implications for proteasome structure and assembly. Mol Cell 2010;38(3):393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E12.Kim H, Sanchez GA, Goldbach-Mansky R. Insights from Mendelian Interferonopathies: Comparison of CANDLE, SAVI with AGS, Monogenic Lupus. J Mol Med (Berl) 2016;94(10):1111–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E13.Kiefer K, Oropallo MA, Cancro MP, Marshak-Rothstein A. Role of type I interferons in the activation of autoreactive B cells. Immunol Cell Biol 2012;90(5):498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E14.Tough DF. Modulation of T-cell function by type I interferon. Immunol Cell Biol 2012;90(5):492–7. [DOI] [PubMed] [Google Scholar]
- E15.Kochi Y. Genetics of autoimmune diseases: perspectives from genome-wide association studies. Int Immunol 2016;28(4):155–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E16.Abramson N, Gelfand EW, Jandl JH, Rosen FS. The interaction between human monocytes and red cells. Specificity for IgG subclasses and IgG fragments. J Exp Med 1970;132(6):1207–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E17.Go RS, Winters JL, Kay NE. How I treat autoimmune hemolytic anemia. Blood 2017;129(22):2971–9. [DOI] [PubMed] [Google Scholar]
- E18.O’Sullivan LA, Liongue C, Lewis RS, Stephenson SE, Ward AC. Cytokine receptor signaling through the Jak–Stat–Socs pathway in disease. Mol mmunol 2007; 44(10):2497–2506. [DOI] [PubMed] [Google Scholar]
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ClinicalTrials.gov identifier: NCT02974595
Conflicts of Interest
The authors declare no relevant conflict of interest. Dr. Goldbach-Mansky has received grant support from SOBI, Regeneron, Novartis and Eli Lilly.
REFERENCES
- 1.de Jesus AA, Canna SW, Liu Y, Goldbach-Mansky R. Molecular mechanisms in genetically defined autoinflammatory diseases: disorders of amplified danger signaling. Annu Rev Immunol 2015;33:823–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agarwal AK, Xing C, DeMartino GN, Mizrachi D, Hernandez MD, Sousa AB, et al. PSMB8 encoding the beta5i proteasome subunit is mutated in joint contractures, muscle atrophy, microcytic anemia, and panniculitis-induced lipodystrophy syndrome. Am J Hum Genet 2010;87(6):866–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brehm A, Liu Y, Sheikh A, Marrero B, Omoyinmi E, Zhou Q, et al. Additive loss-of-function proteasome subunit mutations in CANDLE/PRAAS patients promote type I IFN production. J Clin Invest 2015;125(11):4196–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poli MC, Ebstein F, Nicholas SK, de Guzman MM, Forbes LR, Chinn IK, et al. Heterozygous Truncating Variants in POMP Escape Nonsense-Mediated Decay and Cause a Unique Immune Dysregulatory Syndrome. Am J Hum Genet 2018;102(6):1126–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brehm A, Krüger E. Dysfunction in protein clearance by the proteasome: impact on autoinflammatory diseases. Semin Immunopathol 2015. July;37(4):323–33. [DOI] [PubMed] [Google Scholar]
- 6.Gehrs BC, Friedberg RC. Autoimmune hemolytic anemia. Am J Hematol 2002;69(4):258–71. [DOI] [PubMed] [Google Scholar]
- 7.Torrelo A, Patel S, Colmenero I, Gurbindo D, Lendínez F, Hernández A, et al. Chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature (CANDLE) syndrome. J Am Acad Dermatol 2010;62(3):489–95. [DOI] [PubMed] [Google Scholar]
- 8.Tüfekçi Ö, Bengoa Ş, Karapinar TH, Ataseven EB, İrken G, Ören H. CANDLE syndrome: a recently described autoinflammatory syndrome. J Pediatr Hematol Oncol. 2015;37(4):296–9. [DOI] [PubMed] [Google Scholar]
- 9.Sanchez GAM, Reinhardt A, Ramsey S, Wittkowski H, Hashkes PJ, Berkun Y, et al. JAK1/2 inhibition with baricitinib in the treatment of autoinflammatory interferonopathies. J Clin Invest 2018. July 2;128(7):3041–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]