Abstract
Elevations of intracellular free Ca2+ concentration ([Ca2+]i) are a potent trigger for Weibel-Palade body (WPB) exocytosis and secretion of Von Willebrand factor (VWF) from endothelial cells, however, the identity of WPB-associated Ca2+-sensors involved in transducing acute increases in [Ca2+]i into granule exocytosis remain unknown. Here we show that synaptotagmin 5 (SYT5) is expressed in human umbilical vein endothelial cells (HUVEC) and is recruited to WPBs to regulate Ca2+-driven WPB exocytosis. Western blot analysis of HUVEC identified SYT5 protein, and exogenously expressed SYT5-mEGFP localized almost exclusively to WPBs. shRNA-mediated knockdown of endogenous SYT5 reduced the rate and extent of histamine-evoked WPB exocytosis and reduced secretion of the WPB cargo VWF-propeptide (VWFpp). The shSYT5-mediated reduction in histamine-evoked WPB exocytosis was prevented by expression of shRNA-resistant SYT5-mCherry. Overexpression of SYT5-EGFP increased the rate and extent of histamine-evoked WPB exocytosis, and increased secretion of VWFpp. Expression of a Ca2+-binding defective SYT5 mutant (SYT5-Asp197Ser-EGFP) mimicked depletion of endogenous SYT5. We identify SYT5 as a WPB-associated Ca2+ sensor regulating Ca2+-dependent secretion of stored mediators from vascular endothelial cells.
Keywords: Endothelial, Synaptotagmin, Weibel-Palade body, exocytosis, calcium, secretion
Introduction
Endothelial cells store von Willebrand factor (VWF) and a complex mixture of inflammatory mediators, vasoactive peptides and regulators of tissue growth in special secretory granules called Weibel-Palade bodies (WPBs) (Knipe et al., 2010; Schillemans et al., 2018a; van Breevoort et al., 2012). WPB cargo molecules act together at sites of vessel injury to reduce blood loss, control infection and aid in tissue repair, but have also been implicated in various disease states (see discussion). WPBs undergo different modes of exocytosis resulting in rapid (subsecond) cargo release (Babich et al., 2008; Conte et al., 2015), selective cargo secretion (Babich et al., 2008; Nightingale et al., 2018), compound/cumulative exocytosis (Kiskin et al., 2014; Valentijn et al., 2011) as well as slower forms of cargo release (2-10 seconds) requiring post-fusion recruitment of actomyosin to the WPB (Nightingale et al., 2011). Physiological and pathological mediators can trigger VWF secretion through several intracellular signalling pathways (Huang et al., 2012; Lowenstein et al., 2005; Schillemans et al., 2018a), however, sustained elevations of intracellular free Ca2+ concentration ([Ca2+]i) constitute a particularly potent trigger (Birch et al., 1994; Zupancic et al., 2002). Surprisingly, little is known about how increases in [Ca2+]i are sensed and transduced into WPB exocytosis. Early studies identified a role for calmodulin (CaM) in Ca2+-driven VWF secretion (Birch et al., 1992). Ca2+-CaM binds the guanine nucleotide exchange factor RalGDS which activates the small GTPase RalA (Rondaij et al., 2008). RalA binds components of the exocyst complex that is involved in vesicle-plasma membrane docking, but also stimulates phospholipase D1 (PLD1) activity through activation of ADP-ribosylation factor 6 (Arf6) (Vitale et al., 2005). The latter is important for Ca2+-driven VWF secretion (Disse et al., 2009), and together these processes provide a mechanism to generate domains at the plasma membrane that directly, or through recruitment of adapter proteins, promote WPB docking and fusion. Annexin A2 (AnxA2) in complex with the Ca2+ binding protein S100A10 may represent one such adapter complex (Brandherm et al., 2013; Chehab et al., 2017; Gerke, 2016). Cytosolic AnxA2 is recruited to the plasma membrane by acidic phospholipids, such as phosphatidic acid, where it promotes further phospholipid clustering (Gerke, 2016). Importantly, S100A10 can bind the WPB-Rab27A associated effector Munc13-4 (Chehab et al., 2017) providing a molecular scaffold linking the WPB to the plasma membrane. WPBs may also engage the plasma membrane through a Rab27A-Slp4a-syntaxin binding protein 1 (STXBP1) complex (Bierings et al., 2012; van Breevoort et al., 2014). Once close to the plasma membrane, WPB-fusion is driven by SNARE proteins (see (Schillemans et al., 2018b; van Breevoort et al., 2014) and references therein) and is, almost universally, regulated by one or more vesicle-associated Ca2+-sensors (Südhof, 2014). The nature of the WPB-associated Ca2+-sensors that regulate WPB fusion remain unknown. The best characterised family of vesicle-associated Ca2+-sensors are the syntaptotagmins (SYTs) (Chapman, 2008; Südhof, 2014).There are 17 mammalian SYT isoforms (Craxton, 2010) and all share a common basic structure consisting of a short highly-variable N-terminal region, a transmembrane domain, a linker region, and two C-terminal C2A and C2B domains that mediate Ca2+-dependent binding to phospholipids (Pang and Südhof, 2010). The properties of Ca2+-dependent phospholipid binding/dissociation and capacity to drive membrane fusion vary between the different SYT family members (Bai et al., 2004; Davis et al., 1999; Hui et al., 2005), and studies indicate that multiple SYT isoforms (both Ca2+-dependent and -independent (von Poser et al., 1997)) contribute to fine tuning vesicle fusion kinetics to the specific needs of the cell (Luo and Sudhof, 2017; Rao et al., 2017; Robinson et al., 2002). Here we show that SYT5 is expressed in human umbilical vein endothelial cells (HUVEC) and is recruited to WPBs where it regulates Ca2+-driven WPB exocytosis.
Results and Discussion
SYT5 is expressed in HUVEC and localises to WPBs
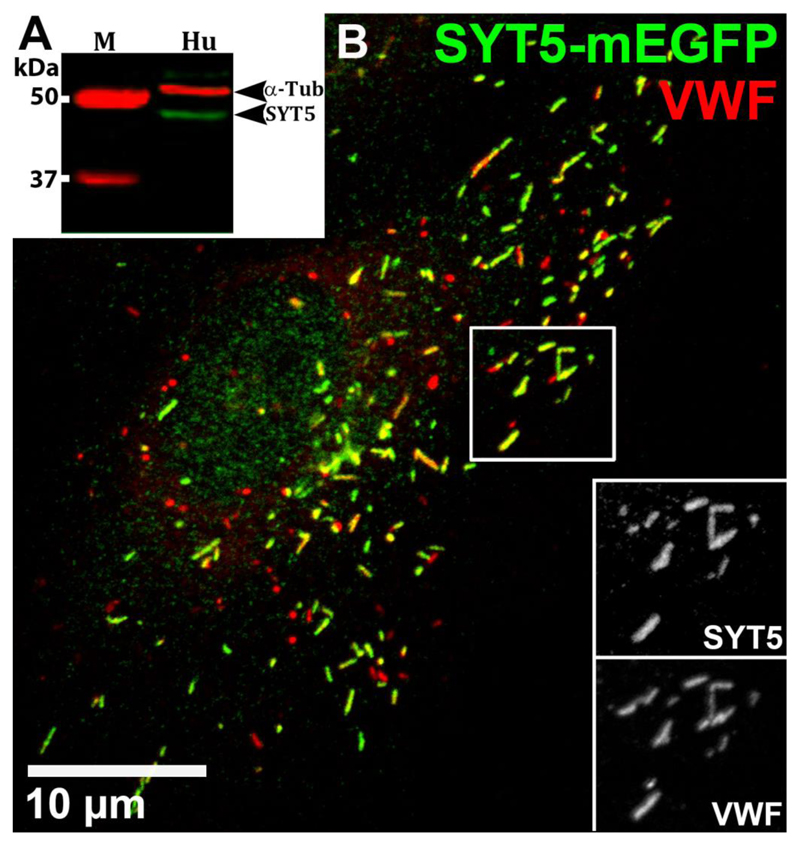
Western blot analysis showed SYT5 is expressed in HUVEC (Figure 1A). Our commercial antibody to SYT5 did not recognise SYT5 by immunocytochemistry (Supplemental Figure S1) so instead we expressed SYT5-mEGFP and analysed the subcellular localisation by counter-staining with antibodies to different subcellular compartments. SYT5-mEGFP co-localised almost exclusively with WPBs (Figure 1B and Figure S2) and was detected on perinuclear TGN46-positive WPBs indicating that SYT5 is incorporated into the WPB during its formation (Figure S2A). Overlap analysis of EGFP (green) and WPB-VWF (red) signals in dual labelled images gave Manders' colocalization Coefficients, M1 (green overlap with red) and M2 (red overlap with green) of 0.998±0.0007 (s.e.m.) and 0.838±0.025 respectively (n=10 cells). The molecular basis for SYT5 trafficking to secretory granules, remains unclear, although studies of other SYT’s (e.g. SYT1 and SYT7) show the N-terminal regions and palmitoylation of cysteine residues within the linker between the transmembrane and C2A domains play important roles in directing these SYTs to their target membranes (Han et al., 2004; Kang et al., 2004). Because SYT1 is reported to localise to pseudo-WPBs in AtT20 cells (Blagoveshchenskaya et al., 2002), we also analysed this SYT in HUVEC. Although SYT1 protein was detected (Figure S3A), SYT1-EGFP localised to the plasma membrane and not WPBs (Figure S3B-C). shRNA depletion of endogenous SYT1 mRNA had no significant effect on VWFpp secretion (Figure S3D) indicating SYT1 does not regulate WPB exocytosis. Having established that SYT5 can be recruited to WPBs we next determined if it might play a role in regulating Ca2+-dependent hormone-evoked WPB exocytosis.
Figure 1. SYT5 is expressed in HUVEC and recruited to WPBs.
(A) Representative western blot of HUVEC (Hu) lysate probed with the rabbit SYT5 primary antibody (abcam ab116452, 1:200). Marker sizes (M) are indicated. α-tubulin was used as loading control. The strong band at approximately 48 kDa represents SYT5 protein, confirmed by depletion after shSYT5 treatment (Figure 2). (B) Confocal fluorescence image of a HUVEC 48 hours after Nucleofection™ with SYT5-mEGFP. Cells were immuno-labelled with antibodies to GFP (sheep; green) and VWF (rabbit; red). Scale bar is 10μm. Inset panels (greyscale) here and below are from regions indicated by white boxes. Manders' Colocalization Coefficients for the fractional overlap of EGFP signal with that of the WPB-VWF signal (Manders' Coefficient M1) was 0.998±0.0007 (s.e.m.) and for WPB-VWF signal overlapping the EGFP signal (Manders' Coefficient M2) was 0.838±0.025 (n=10 cells). Confocal fluorescence images here and in all subsequent figures were taken at room temperature.
Depletion of SYT5 modulates histamine-evoked VWFpp secretion and WPB exocytosis
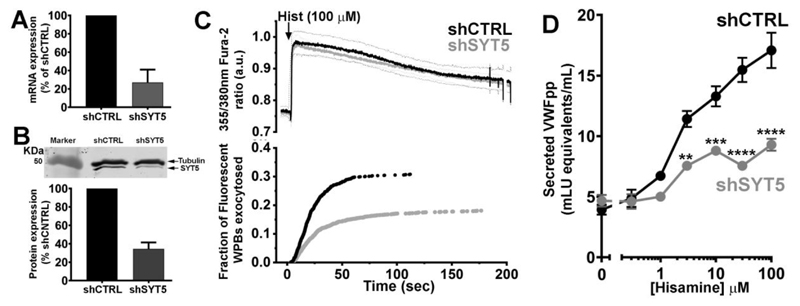
We depleted endogenous SYT5 by shRNA using a lentiviral vector with a puromycin selection cassette that allowed duel expression and selection of cells expressing both the shSYT5 (or shcontrol (shCTRL)) and VWFpp-EGFP, enabling fluorescent labelling of WPBs in transduced cells. Transduced cells were directly monitored for fluorescent WPB exocytosis evoked by the physiological Ca2+-dependent secretagogue histamine (Erent et al., 2007; Hamilton and Sims, 1987; Lorenzi et al., 2008). shSYT5 treatment reduced SYT5 mRNA by 73% (Figure 2A) and by >65% at the protein level (Figure 2B). Comparison of changes in [Ca2+]i during histamine (100 μM) stimulation of Fura-2 loaded HUVEC in shCTRL or shSYT5 transduced cells showed no effect of shSYT5 treatment (Figure 2C upper panel), however in the same experiments the kinetics and extent of fluorescent WPB exocytosis was significantly altered in shSYT5 transduced cells (Figure 2C lower panel). There was a significant reduction in the mean maximal rate of WPB exocytosis response to histamine (shCTRL; 2.2 ± 0.4 WPBs/second, n=35 cells, shSYT5; 1.3 ± 0.1 WPBs/second, n=46 cells, P=0.043, t-test) and a reduction in the fraction of fluorescent WPBs that underwent exocytosis (shCTRL; black trace, 30.8 ± 2.1%, 527 fusion events, n=35 cells, shSYT5; grey trace,18.1 ± 1.3%, 507 fusion events, n=46 cells, P<0.0001, t-test). SYT5 depletion had no effect on WPB movements close to the plasma membrane or on the fraction of WPBs showing restricted movements (Figure S4). Over expression of SYT5-mCherry containing 7 silent mutations in the region targeted by shSYT5 (SYT5-mCherry (7sm)), labelled WPBs and prevented inhibition of WPB exocytosis in shSYT5 treated cells (Figure S5). Consistent with direct analysis of WPB exocytosis we found that histamine-evoked VWFpp secretion was reduced in shSYT5 treated cells (Figure 2D). At 100 μM histamine the reduction in secretion was ~40% compared to shCTRL, similar to the ~40% reduction in WPB exocytosis observed directly by live cell imaging.
Figure 2. SYT5 depletion reduces WPB exocytosis and VWFpp secretion.
(A) Quantification of shRNA mediated SYT mRNA depletion after lentiviral transduction. Data is normalized to shCTRL (mean±s.e.m. of 4 independent experiments). (B) top; western blot showing SYT5 depletion by shRNA and bottom; quantification of SYT5 depletion (mean±s.e.m of 4 independent experiments). SYT5 was detected using a rabbit SYT5 primary antibody (abcam ab ab116452, 1:200), α-tubulin was used as loading control. (C) top panel shows the mean 355nm/380nm Fura-2 fluorescence ratio recorded in shCTRL (black, n=12 cells) or shSYT5 (grey, n=12 cells) treated HUVEC expressing VWFpp-EGFP and stimulated with histamine (100μM, arrow). Here and in subsequent Figures thin dashed lines show the ±95% confidence limits for the mean fluorescence ratios (black; shCTRL, grey; shSYT5). Lower panel in A shows the cumulative plot of histamine-evoked WPB fusion times scaled by the mean fraction of WPBs that underwent exocytosis. (D) Shows histamine (0.3-100μM)-evoked VWFpp secretion from HUVEC following lentiviral transduction with shCTRL (black) or shSYT5 (grey). Data are mean±s.e.m of 4 independent experiments, each carried out in triplicate. *P≤0.5, ** P≤ 0.01, *** P≤ 0.001, **** P≤ 0.0001, t-test.
SYT5 overexpression increases histamine-evoked VWFpp secretion and WPB exocytosis
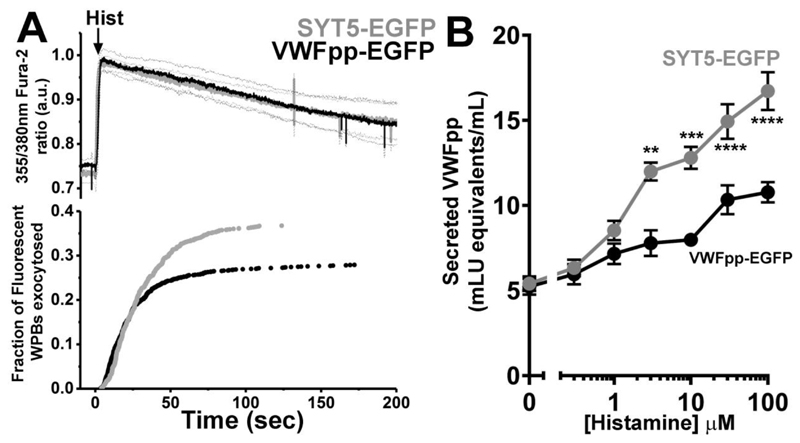
We next examined the effect of SYT5-mEGFP overexpression on WPB exocytosis and VWFpp secretion. Overexpressed SYT5-mEGFP labels WPBs exclusively allowing us to directly visualize the organelles and their exocytosis by monitoring changes in WPB morphology and the abrupt loss of WPB SYT5-EGFP fluorescence, as described previously for other WPB membrane proteins (Knipe et al., 2010). SYT5-mEGFP overexpression was compared to data from VWFpp-EGFP expressing HUVEC as control and the data (Figure 3) is presented in the same way as in Figure 2C. Histamine evoked identical increases in [Ca2+]i in SYT5-EGFP (grey) and the control VWFpp-EGFP (black) expressing HUVEC (Figure 3A upper panels). However, in SYT5-mEGFP expressing cells there was a significant increase in the mean maximal rate of WPB exocytosis from 2.1±0.31 WPBs/second (VWFpp-EGF; n=30) to 5.9±2.4 WPBs/second (SYT5-EGFP, n=18 cells, P=0.049 t-test) and a significant increase in the fraction of fluorescent WPBs that underwent exocytosis (VWFpp-EGFP; black trace, 28.0±1.5%, 517 fusion events, n=30 cells, SYT5-EGFP; grey trace, 36.7±1.63%, 512 fusion events, n=18 cells, P<0.0004, t-test). Consistent with live imaging data SYT5-mEGFP overexpression significantly increased histamine-evoked VWFpp secretion (Figure 3B). No effect of SYT5 overexpression was found on WPB movements or on the fraction of WPBs showing restricted motion close to the plasma membrane (Figure S6) indicating that SYT5 does not contribute to WPB immobilisation at the plasma membrane.
Figure 3. SYT5 overexpression increases WPB exocytosis and VWFpp secretion.
A. top panel shows the mean 355nm/380nm Fura-2 fluorescence ratio recorded in VWFpp-EGFP (black, n=12 cells) or SYT5-EGFP (grey, n=12 cells) treated HUVEC expressing VWFpp-EGFP and stimulated with histamine (100μM, arrow). Lower panel in B shows cumulative plots of histamine-evoked WPB fusion times scaled by the mean fraction of WPBs that underwent exocytosis. B. Histamine (0.3-100μM)-evoked VWFpp secretion from HUVEC expressing VWFpp-EGFP (black) or SYT5-EGFP (grey) after lentiviral transduction. Data is mean±s.e.m of 3 independent experiments, each carried out in triplicate. *P≤0.5, ** P≤ 0.01, *** P≤ 0.001, **** P≤ 0.0001, t-test.
Ca2+-independent SYT5 mutant decreases histamine-evoked WPB exocytosis
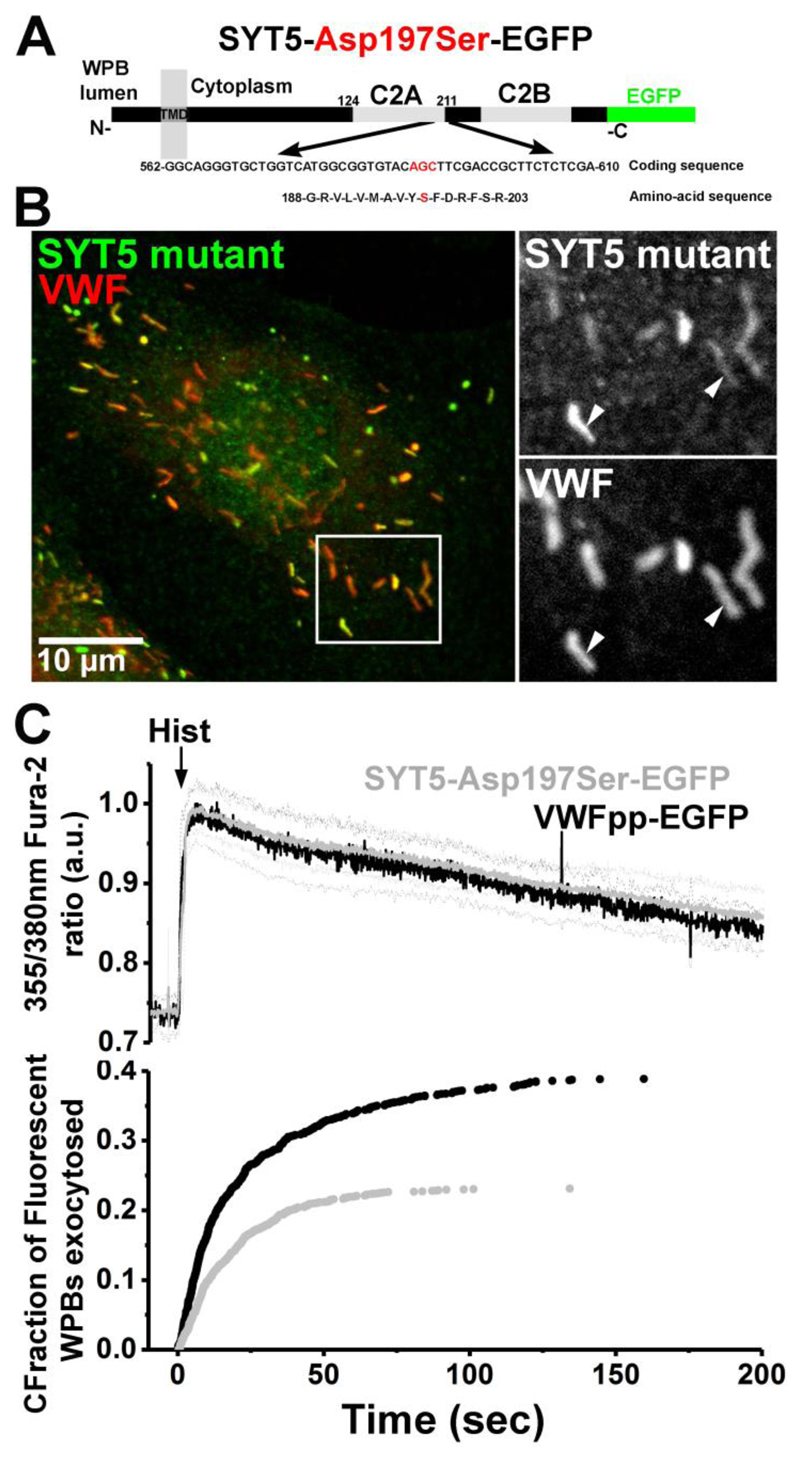
To confirm that SYT5 function depends on its ability to sense Ca2+ we mutated the third aspartate residue of the Ca2+-binding motif of the C2A domain of SYT5-mEGFP to a serine to generate the calcium-insensitive mutant SYT5-Asp197Ser-mEGFP (von Poser et al., 1997) (Figure 4A) and overexpressed this in HUVEC using Nucleofection™. SYT5-Asp197Ser-mEGFP localised to WPBs (Figure 4B; Manders' Colocalization Coefficients, M1 and M2 of 0.993±0.0007 and 0.920±0.013 (n=10 cells). Analysis of WPB exocytosis revealed a dominant negative effect of SYT5-Asp197Ser-mEGFP on a back ground of WT SYT5. SYT5-Asp197Ser-mEGFP significantly reduced both the mean maximal rate of WPB exocytosis from 3.1±0.53 WPBs/second (VWFpp-EGF; n=17) to 1.4±0.29 WPBs/second (SYT5-Asp197Ser-mEGFP, n= 25 cells) (P=0.0046, t-test) and the fraction of fluorescent WPBs that underwent exocytosis (VWFpp-EGFP; black trace, 38.9±3.5%, 532 fusion events, n=17 cells, SYT5-Asp197Ser-mEGFP; grey trace, 23.1±1.5%, 422 fusion events, n=27 cells, P<0.0001, t-test) (Figure 4C).
Figure 4. Ca2+-independent SYT5 mutant decreases WPB exocytosis.
(A) Cartoon showing the point mutation, Asp197Ser, in the C2A domain of SYT5-EGFP generating the calcium-insensitive mutant. (B) Fluorescence image of a HUVEC expressing SYT5-Asp197Ser-EGFP (48 hours post-transfection) and immuno-labelled for GFP (green; rabbit Ab) and endogenous VWF (red, sheep Ab). Arrows in grayscale images show the localization of SYT5-Asp197Ser-EGFP to WPBs. Manders' Colocalization Coefficients for the fractional overlap of EGFP signal with that of the WPB-VWF signal (Manders' Coefficient M1) were 0.993±0.0007, and for WPB-VWF signal overlapping the EGFP signal (Manders' Coefficient M2) 0.920±0.013 (n=10 cells). (C) top panel shows the mean 355nm/380nm Fura-2 fluorescence ratio recorded in HUVEC expressing VWFpp-EGFP (black, n=12 cells) or SYT5-Asp197Ser-EGFP (grey, n=12 cells) stimulated with histamine (100μM, arrow). Lower panel in Aiii shows the cumulative plot of histamine-evoked WPB fusion times scaled by the mean fraction of WPBs that underwent exocytosis.
Our results provide evidence for a role for SYT5 in regulating Ca2+-dependent WPB exocytosis. SYT5 was the first non-neuronal member of the SYT family to be described (Hudson and Birnbaum, 1995, Craxton and Geodert, 1995) and has been implicated in regulating Ca2+-driven exocytosis in neuronal, endocrine and neuroendocrine cell types (Birch et al., 1992; Fukuda et al., 2002; Gut et al., 2001; Iezzi et al., 2004; Lynch and Martin, 2007; Roper et al., 2015; Saegusa et al., 2002; Xu et al., 2007) as well as pH regulation of phagosomes and phagocytosis in macrophages (Vinet et al., 2008; Vinet et al., 2009). SYT5 has a lower Ca2+-affinity for phospholipid or SNARE protein interactions compared to the other main SYT reported to function in endocrine and neuroendocrine cell types, SYT7 (Chieregatti et al., 2004; Gustavsson et al., 2009; Hui et al., 2005; Iezzi et al., 2004; Schonn et al., 2008; Sugita et al., 2001). The higher Ca2+-affinity of SYT7 is thought to underlie its role in asynchronous neurotransmitter release at low [Ca2+]i (Bacaj et al., 2013; Luo and Sudhof, 2017; Weber et al., 2014), the sensitivity of SYT7-containing dense core vesicles of chromaffin cells to low [Ca2+]i and weak stimulation (Rao et al., 2017), and in vesicle replenishment and release during insulin secretion as [Ca2+]i declines to low levels (Dolai et al., 2016). However, the ability to sense low [Ca2+]i during weak stimulation is not a prominent feature of endothelial WPBs. The rate of WPB exocytosis under resting conditions is very low (Erent et al., 2007) and WPB exocytosis is largely insensitive to small increases in [Ca2+]i during weak stimulation (Birch et al., 1994; Erent et al., 2007). Studies in permeabilized or whole cell patch-clamped endothelial cells show a supra micromolar [Ca2+] threshold for activation of WPB exocytosis (Frearson et al., 1995; Zupancic et al., 2002), and a requirement for sustained high (5-30μM) [Ca2+]i to drive strong exocytosis and VWF secretion (Birch et al., 1994; Birch et al., 1992; Carter and Ogden, 1994). Such high [Ca2+]i are achieved during stimulation with physiological agonists or cell injury that occur at wound sites (Carter and Ogden, 1994; Zupancic et al., 2002). SYT5 with its lower Ca2+-affinity for phospholipid and SNARE protein interactions may help to limit WPB exocytosis during weak cell activation. This is potentially important because WPBs store high molecular weight forms of VWF that are potent at capturing platelets to the vessel wall, a process vital during primary haemostasis at wound sites (Sadler, 1998), but potentially hazardous if released inappropriately. Elevated VWF is a risk factor for coronary heart disease, ischemic stroke and sudden death (van Schie et al., 2011; Wieberdink et al., 2010). WPBs also contain and co-release inflammatory mediators (P-selectin and chemokines) and tissue growth regulators (IGFBP7, Ang2) many of which have been linked to the aetiology of vascular disease (Papadopoulou et al., 2008). Thus, the involvement of a lower affinity SYT and a requirement for larger prolonged increases in [Ca2+]i may minimise the risk of unwanted WPB exocytosis under normal conditions where endothelial cells may experience intermittent low level activation.
The nature of the molecular interactions between SYT5 and WPB SNAREs remain to be determined. Endothelial cells utilize at least two distinct SNARE complexes to regulate WPB exocytosis, one comprising syntaxin 4:SNAP23:VAMP3 and a second complex comprising syntaxin-3:SNAP23:VAMP8 (Fu et al., 2005; Matsushita et al., 2003; Schillemans et al., 2018b; van Breevoort et al., 2014; Zhu et al., 2015; Zhu et al., 2014), and each complex may play a role in specific modes of WPB exocytosis (Schillemans et al., 2018b). SYT-SNARE interactions have been extensively studied for neuronal SYT1, which binds both the neuronal SNAP (SNAP-25) and syntaxin (syntaxin 1) in heterodimers or fully assembled SNARE complexes (reviewed in (Chapman, 2008)). SYT5 binds poorly to the WPB SNAP, SNAP23 (Chieregatti et al., 2004), and although the SYT5 C2AB domains can bind syntaxin 1 (Li et al., 1995) it remains to be established if endothelial syntaxins implicated in WPB exocytosis bind SYT5. WPB exocytosis and VWFpp secretion was significantly reduced but not abolished upon depletion of endogenous SYT5. This is most likely due to the incomplete (~65%) depletion of SYT5 in these experiments, but may also reflect the involvement of other SYT isoforms in the Ca2+-sensing mechanism and/or of the cytosolic Ca2+-sensors CaM and the AnxA2/S100A10 complex described in the introduction. AnxA2-phospholipid interactions are typically low affinity and fit well with a general requirement for high [Ca2+]i for WPB exocytosis. The ability of S100A10 to bind WPB-associated Munc13-4 (Chehab et al., 2017) and of AnxA2 to bind SNAP-23 (Wang et al., 2007) indicate that WPB exocytosis is likely coordinated by a complex network of Ca2+-sensors ensuring that WPB exocytosis only occurs when needed.
Methods
Tissue culture, VWF and VWFpp ELISA assays, antibodies, and reagents
Primary HUVECs tested for contamination were purchased from PromoCell GmbH (Heidelberg, Germany) and cultured as previously described (Hannah et al., 2005). Human Embryonic Kidney-293 (HEK-293) cells were cultured in Minimal Essential Medium (MEM) Alpha Medium 1x (Invitrogen) supplemented with 10% fetal calf serum (Biosera, Ringmer, UK) and 50 μg/ml gentamycin (Invitrogen) at 37°C, 5% CO2 as previously described (Kiskin et al., 2010). Secreted VWF propolypeptide (VWFpp) was assayed by specific ELISA as previously described (Hewlett et al., 2011). Primary antibodies (Abs) along with the dilutions for immunofluorescence or western blotting are given in supplementary Table S1. All reagents were from Sigma-Aldrich unless otherwise stated. Fura-2/AM was from Invitrogen.
DNA Constructs, Site Directed Mutagenesis, Lentiviral Production and Transfection
The VWF propeptide fused to enhanced green fluorescent protein (VWFpp-EGFP) has been described previously (Hannah et al., 2005). A mEGFP fusion protein of human SYT5 was made using the ligation independent cloning (LIC) approach as previously described (Bierings et al., 2012) using the primers in Supplementary Table S2. Construction of SYT1-EGFP and site-Directed Mutagenesis of SYT5 is described in Supplemental Materials. Production and transfection of SYT5 specific shRNA or mEGFP tagged SYT5 constructs using lentiviral vectors are described in Supplementary Materials. SYT5 shRNA was obtained from the MISSION® shRNA library developed by TRC at the Broad Institute of MIT and Harvard and distributed by Sigma-Aldrich (Supplementary Table S3). For conventional transfection of HUVEC the Amaxa Nucleofection™ system was used with HUVEC OLD Nucleofector™ Solution containing 2-4 μg of target DNA and programme U-01 according to the manufacturer’s instructions (Lonza Biologics Plc, Slough, UK). Cells were used for experiments 48 hours following transfection. HEK cell transfection by Nucleofection™ was identical to that for HUVECs with the exceptions that MEM Alpha medium was used in place of HGM, Cell Line Nucleofector™ Solution V was used in place of HUVEC OLD Nucleofector™ Solution, Nucleofection programme was Q-01.
RT-PCR and quantitative PCR analysis
RNA was extracted using RNeasy Mini Kit (QIAGEN). The quantity and purity of extracted RNA was determined by measuring its absorbance at 280 and 260 nm using a Nandrop-1000® device (Thermo Scientific, Denmark). cDNA was synthesized using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, ThermoFisher Scientific). Briefly, 1 μg of RNA was added to the reaction mix, and cDNA was synthesized using one cycle of heating to 55°C for 20 minutes following by an increase to 94°C for two minutes. Subsequent PCR amplification of HUVEC cDNA was achieved using 40 cycles of denaturation (94°C for 15 seconds), followed by annealing (55°C for 30 seconds) and extension (72°C for 1 minute). PCR was performed using a Mastercycler® machine (eppendorf, Stevenage).The products of PCR were run on a 1.5% agarose gel and visualized by ethidium-bromide staining. All bands were sequenced verified (GATC Biotech, Cologne, Germany).
Immunocytochemistry and Immunoblotting
For immunocytochemistry HUVEC or HEK were grown on 9mm glass coverslips and immunostaining and confocal fluorescence imaging of fixed cells were performed as previously described3. The intensity measurements, exposures at each wavelength were first set to ensure no detector saturation on the brightest sample and then kept constant for all images. Images were prepared in Adobe Photoshop CS6. Immunoblotting was carried out as previously described (Bierings et al., 2012).
Live cell imaging, vesicle tracking, confocal imaging of fixed cells and fluorescence overlap analysis
Exocytosis of VWFpp-EGFP-containing and EGFP-SYT5 associated WPBs were determined as previously described (Erent et al., 2007; Knipe et al., 2010). The moment of fusion of EGFP-SYT5 containing WPBs was determined by an abrupt decrease in WPB fluorescence on fusion as previously described (Knipe et al., 2010). Automatic tracking of WPB movements was carried out as previously described (Conte et al., 2016). Image data were acquired at 10 frames/second in Winfluor (http://spider.science.strath.ac.uk/sipbs/software_imaging.htm), exported as raw format to GMimPro/Motility freeware software (Dr Gregory Mashanov, Francis Crick Institute Mill Hill Laboratory, London, www.mashanov.uk). The automatic single particle tracking (ASPT) module in GMimPro (Mashanov and Molloy, 2007) was used to track the X,Y position in time of individual WPBs expressing VWFpp-EGFP or SYT5-EGFP, yielding maximum velocities and maximum displacements. ASPT settings were FWHM500nm, R7, L20, Q25 and C5000. The time, X and Y positions for WPBs for individual cells were exported in text file format for subsequent analysis of mean squared displacement (MSD) in Matlab using custom written functions. MDS plots were fitted as previously described and the proportion of WPBs showing subdiffusive/restricted diffusion and their corresponding cage radii determined (Conte et al., 2016). Confocal images for fixed cells were taken at room temperature using either Leica SP2 or SP8 confocal microscopes (Mannheim, Germany) equipped with 40x, 63x and 100x objectives (HCX PL APO40x 1.2 NA, PLAPO 63x/1.40, PLAPO100x 1.4NA) or a BioRad Radiance 2100 confocal running LaserSharp 2000 software and equipped with a Nikon 60x and x100 PLAPO 1.40 NA objectives. Dual color images were acquired sequentially with pinhole setting Airy 1, image size 1024x1024 and frame averaging over 6-12 scans. To determine the fractional overlap of green (EGFP) with red (VWF) signals (Manders' Colocalization Coefficient M1) or vici versa (Manders' Colocalization Coefficient M2) in images of HUVEC expressing SYT5-GFP or SYT5 SYT5-Asp197Ser-mEGFP and stained for endogenous VWF, we used the ImageJ plugin JACoP that implements the Manders' Colocalization Coefficient with the Costes method for automatically estimating threshold values for identifying background levels (Costes et al., 2004), as reviewed in (Dunn et al., 2011).
Statistical analysis
Data were plotted in Origin 2017 or GraphPad Prism Version 7.02. Statistical analysis was by nonparametric t test (except where indicated) using GraphPad Prism Version 7.02. Significance values are shown on the Figures or in Figure legends. Data are shown as mean±SEM.
Supplementary Material
Summary statement.
How elevations of intracellular free Ca2+ concentration are detected by Weibel-Palade bodies (WPBs) remains unclear. Here we show that synaptotagamin 5 is a WPB-associated Ca2+-sensor regulating exocytosis.
Acknowledgements
Funding: TC was supported by an UK MRC grant MC_PC_13053. CL was supported by an SGUL PhD studentship. RB was supported by a European Hematology Association Research Fellowship.
Footnotes
Authorship
CL, JS, RB, TC performed research and analyzed data; MJH, RB and DO contributed vital reagents and expertise; TC designed the research; TC and CL wrote the paper.
Competing interests
The authors declare no competing interests.
References
- Babich V, Meli A, Knipe L, Dempster JE, Skehel P, Hannah MJ, Carter T. Selective release of molecules from Weibel Palade bodies during a lingering kiss. Blood. 2008;111:5282–90. doi: 10.1182/blood-2007-09-113746. [DOI] [PubMed] [Google Scholar]
- Bacaj T, Wu D, Yang X, Morishita W, Zhou P, Xu W, Malenka RC, Sudhof TC. Synaptotagmin-1 and synaptotagmin-7 trigger synchronous and asynchronous phases of neurotransmitter release. Neuron. 2013;80:947–59. doi: 10.1016/j.neuron.2013.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai J, Wang CT, Richards DA, Jackson MB, Chapman ER. Fusion pore dynamics are regulated by synaptotagmin*t-SNARE interactions. Neuron. 2004;41:929–42. doi: 10.1016/s0896-6273(04)00117-5. [DOI] [PubMed] [Google Scholar]
- Bierings R, Hellen N, Kiskin N, Knipe L, Fonseca AV, Patel B, Meli A, Rose M, Hannah MJ, Carter T. The interplay between the Rab27A effectors Slp4-a and MyRIP controls hormone-evoked Weibel-Palade body exocytosis. Blood. 2012;120:2757–67. doi: 10.1182/blood-2012-05-429936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch KA, Ewenstein BM, Golan DE, Pober JS. Prolonged peak elevations in cytoplasmic free calcium ions, derived from intracellular stores, correlate with the extent of thrombin-stimulated exocytosis in single human umbilical vein endothelial cells. J Cell Physiol. 1994;160:545–54. doi: 10.1002/jcp.1041600318. [DOI] [PubMed] [Google Scholar]
- Birch KA, Pober JS, Zavoico GB, Means AR, Ewenstein BM. Calcium/calmodulin transduces thrombin-stimulated secretion: studies in intact and minimally permeabilized human umbilical vein endothelial cells. J Cell Biol. 1992;118:1501–10. doi: 10.1083/jcb.118.6.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagoveshchenskaya AD, Hannah MJ, Allen S, Cutler DF. Selective and signal-dependent recruitment of membrane proteins to secretory granules formed by heterologously expressed von Willebrand factor. Molecular Biology of the Cell. 2002;13:1582–93. doi: 10.1091/mbc.01-09-0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandherm I, Disse J, Zeuschner D, Gerke V. cAMP-induced secretion of endothelial von Willebrand factor is regulated by a phosphorylation/dephosphorylation switch in annexin A2. Blood. 2013;122:1042–51. doi: 10.1182/blood-2012-12-475251. [DOI] [PubMed] [Google Scholar]
- Carter TD, Ogden D. Acetylcholine-stimulated changes of membrane potential and intracellular Ca2+ concentration recorded in endothelial cells in situ in the isolated rat aorta. Pflugers Arch. 1994;428:476–84. doi: 10.1007/BF00374568. [DOI] [PubMed] [Google Scholar]
- Chapman ER. How does synaptotagmin trigger neurotransmitter release? Annu Rev Biochem. 2008;77:615–41. doi: 10.1146/annurev.biochem.77.062005.101135. [DOI] [PubMed] [Google Scholar]
- Chehab T, Santos NC, Holthenrich A, Koerdt SN, Disse J, Schuberth C, Nazmi AR, Neeft M, Koch H, Man KNM, et al. A novel Munc13-4/S100A10/Annexin A2 complex promotes Weibel-Palade body exocytosis in endothelial cells. Molecular Biology of the Cell. 2017 doi: 10.1091/mbc.E17-02-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chieregatti E, Chicka MC, Chapman ER, Baldini G. SNAP-23 functions in docking/fusion of granules at low Ca2+ Molecular Biology of the Cell. 2004;15:1918–30. doi: 10.1091/mbc.E03-09-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte IL, Cookson E, Hellen N, Bierings R, Mashanov G, Carter T. Is there more than one way to unpack a Weibel-Palade body? Blood. 2015;126:2165–7. doi: 10.1182/blood-2015-08-664961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte IL, Hellen N, Bierings R, Mashanov GI, Manneville JB, Kiskin NI, Hannah MJ, Molloy JE, Carter T. Interaction between MyRIP and the actin cytoskeleton regulates Weibel-Palade body trafficking and exocytosis. J Cell Sci. 2016;129:592–603. doi: 10.1242/jcs.178285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costes SV, Daelemans D, Cho EH, Dobbin Z, Pavlakis G, Lockett S. Automatic and quantitative measurement of protein-protein colocalization in live cells. Biophys J. 2004;86:3993–4003. doi: 10.1529/biophysj.103.038422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craxton M. A manual collection of Syt, Esyt, Rph3a, Rph3al, Doc2, and Dblc2 genes from 46 metazoan genomes--an open access resource for neuroscience and evolutionary biology. BMC genomics. 2010;11:37. doi: 10.1186/1471-2164-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AF, Bai J, Fasshauer D, Wolowick MJ, Lewis JL, Chapman ER. Kinetics of synaptotagmin responses to Ca2+ and assembly with the core SNARE complex onto membranes. Neuron. 1999;24:363–76. doi: 10.1016/s0896-6273(00)80850-8. [DOI] [PubMed] [Google Scholar]
- Disse J, Vitale N, Bader MF, Gerke V. Phospholipase D1 is specifically required for regulated secretion of von Willebrand factor from endothelial cells. Blood. 2009;113:973–80. doi: 10.1182/blood-2008-06-165282. [DOI] [PubMed] [Google Scholar]
- Dolai S, Xie L, Zhu D, Liang T, Qin T, Xie H, Kang Y, Chapman ER, Gaisano HY. Synaptotagmin-7 Functions to Replenish Insulin Granules for Exocytosis in Human Islet β-Cells. Diabetes. 2016;65:1962–1976. doi: 10.2337/db15-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KW, Kamocka MM, McDonald JH. A practical guide to evaluating colocalization in biological microscopy. Am J Physiol Cell Physiol. 2011;300:C723–42. doi: 10.1152/ajpcell.00462.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erent M, Meli A, Moisoi N, Babich V, Hannah MJ, Skehel P, Knipe L, Zupancic G, Ogden D, Carter T. Rate, extent and concentration dependence of histamine-evoked Weibel-Palade body exocytosis determined from individual fusion events in human endothelial cells. The Journal of Physiology. 2007;583:195–212. doi: 10.1113/jphysiol.2007.132993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frearson JA, Harrison P, Scrutton MC, Pearson JD. Differential regulation of von Willebrand factor exocytosis and prostacyclin synthesis in electropermeabilized endothelial cell monolayers. Biochemical Journal. 1995;309(Pt 2):473–9. doi: 10.1042/bj3090473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Naren AP, Gao X, Ahmmed GU, Malik AB. Protease-activated receptor-1 activation of endothelial cells induces protein kinase Calpha-dependent phosphorylation of syntaxin 4 and Munc18c: role in signaling p-selectin expression. J Biol Chem. 2005;280:3178–84. doi: 10.1074/jbc.M410044200. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Kowalchyk JA, Zhang X, Martin TF, Mikoshiba K. Synaptotagmin IX regulates Ca2+-dependent secretion in PC12 cells. J Biol Chem. 2002;277:4601–4. doi: 10.1074/jbc.C100588200. [DOI] [PubMed] [Google Scholar]
- Gerke V. Annexins A2 and A8 in endothelial cell exocytosis and the control of vascular homeostasis. Biol Chem. 2016;397:995–1003. doi: 10.1515/hsz-2016-0207. [DOI] [PubMed] [Google Scholar]
- Gustavsson N, Wei SH, Hoang DN, Lao Y, Zhang Q, Radda GK, Rorsman P, Sudhof TC, Han W. Synaptotagmin-7 is a principal Ca2+ sensor for Ca2+ -induced glucagon exocytosis in pancreas. The Journal of Physiology. 2009;587:1169–78. doi: 10.1113/jphysiol.2008.168005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gut A, Kiraly CE, Fukuda M, Mikoshiba K, Wollheim CB, Lang J. Expression and localisation of synaptotagmin isoforms in endocrine (β)-cells: their function in insulin exocytosis. Journal of Cell Science. 2001;114:1709–1716. doi: 10.1242/jcs.114.9.1709. [DOI] [PubMed] [Google Scholar]
- Hamilton KK, Sims PJ. Changes in cytosolic Ca2+ associated with von Willebrand factor release in human endothelial cells exposed to histamine. Study of microcarrier cell monolayers using the fluorescent probe indo-1. J Clin Invest. 1987;79:600–8. doi: 10.1172/JCI112853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han W, Rhee JS, Maximov A, Lao Y, Mashimo T, Rosenmund C, Sudhof TC. N-glycosylation is essential for vesicular targeting of synaptotagmin 1. Neuron. 2004;41:85–99. doi: 10.1016/s0896-6273(03)00820-1. [DOI] [PubMed] [Google Scholar]
- Hannah MJ, Skehel P, Erent M, Knipe L, Ogden D, Carter T. Differential kinetics of cell surface loss of von Willebrand factor and its propolypeptide after secretion from Weibel-Palade bodies in living human endothelial cells. J Biol Chem. 2005;280:22827–30. doi: 10.1074/jbc.M412547200. [DOI] [PubMed] [Google Scholar]
- Hewlett L, Zupančič G, Mashanov G, Knipe L, Ogden D, Hannah MJ, Carter T. Temperature-Dependence of Weibel-Palade Body Exocytosis and Cell Surface Dispersal of von Willebrand Factor and Its Propolypeptide. PLOS ONE. 2011;6:e27314. doi: 10.1371/journal.pone.0027314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Haberichter SL, Sadler JE. The B subunits of Shiga-like toxins induce regulated VWF secretion in a phospholipase D1-dependent manner. Blood. 2012;120:1143–9. doi: 10.1182/blood-2012-01-408096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui E, Bai J, Wang P, Sugimori M, Llinas RR, Chapman ER. Three distinct kinetic groupings of the synaptotagmin family: candidate sensors for rapid and delayed exocytosis. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:5210–4. doi: 10.1073/pnas.0500941102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iezzi M, Kouri G, Fukuda M, Wollheim CB. Synaptotagmin V and IX isoforms control Ca2+ -dependent insulin exocytosis. Journal of Cell Science. 2004;117:3119–3127. doi: 10.1242/jcs.01179. [DOI] [PubMed] [Google Scholar]
- Kang R, Swayze R, Lise MF, Gerrow K, Mullard A, Honer WG, El-Husseini A. Presynaptic trafficking of synaptotagmin I is regulated by protein palmitoylation. J Biol Chem. 2004;279:50524–36. doi: 10.1074/jbc.M404981200. [DOI] [PubMed] [Google Scholar]
- Kiskin NI, Babich V, Knipe L, Hannah MJ, Carter T. Differential cargo mobilisation within Weibel-Palade bodies after transient fusion with the plasma membrane. PLoS One. 2014;9:e108093. doi: 10.1371/journal.pone.0108093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiskin NI, Hellen N, Babich V, Hewlett L, Knipe L, Hannah MJ, Carter T. Protein mobilities and P-selectin storage in Weibel-Palade bodies. J Cell Sci. 2010;123:2964–75. doi: 10.1242/jcs.073593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe L, Meli A, Hewlett L, Bierings R, Dempster J, Skehel P, Hannah MJ, Carter T. A revised model for the secretion of tPA and cytokines from cultured endothelial cells. Blood. 2010;116:2183–2191. doi: 10.1182/blood-2010-03-276170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Ullrich B, Zhang JZ, Anderson RGW, Brose N, Südhof TC. Ca2+-dependent and -independent activities of neural and non-neural synaptotagmins. Nature. 1995;375:594–599. doi: 10.1038/375594a0. [DOI] [PubMed] [Google Scholar]
- Lorenzi O, Frieden M, Villemin P, Fournier M, Foti M, Vischer UM. Protein kinase C-delta mediates von Willebrand factor secretion from endothelial cells in response to vascular endothelial growth factor (VEGF) but not histamine. Journal of Thrombosis and Haemostasis. 2008;6:1962–9. doi: 10.1111/j.1538-7836.2008.03138.x. [DOI] [PubMed] [Google Scholar]
- Lowenstein CJ, Morrell CN, Yamakuchi M. Regulation of Weibel-Palade body exocytosis. Trends Cardiovasc Med. 2005;15:302–8. doi: 10.1016/j.tcm.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Luo F, Sudhof TC. Synaptotagmin-7-Mediated Asynchronous Release Boosts High-Fidelity Synchronous Transmission at a Central Synapse. Neuron. 2017;94:826–839 e3. doi: 10.1016/j.neuron.2017.04.020. [DOI] [PubMed] [Google Scholar]
- Lynch KL, Martin TFJ. Synaptotagmins I and IX function redundantly in regulated exocytosis but not endocytosis in PC12 cells. Journal of Cell Science. 2007;120:617–627. doi: 10.1242/jcs.03375. [DOI] [PubMed] [Google Scholar]
- Mashanov GI, Molloy JE. Automatic detection of single fluorophores in live cells. Biophys J. 2007;92:2199–211. doi: 10.1529/biophysj.106.081117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita K, Morrell CN, Cambien B, Yang SX, Yamakuchi M, Bao C, Hara MR, Quick RA, Cao W, O'Rourke B, et al. Nitric oxide regulates exocytosis by S-nitrosylation of N-ethylmaleimide-sensitive factor. Cell. 2003;115:139–50. doi: 10.1016/s0092-8674(03)00803-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nightingale TD, McCormack JJ, Grimes W, Robinson C, Lopes da Silva M, White IJ, Vaughan A, Cramer LP, Cutler DF. Tuning the endothelial response: differential release of exocytic cargos from Weibel-Palade bodies. Journal of Thrombosis and Haemostasis. 2018 doi: 10.1111/jth.14218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nightingale TD, White IJ, Doyle EL, Turmaine M, Harrison-Lavoie KJ, Webb KF, Cramer LP, Cutler DF. Actomyosin II contractility expels von Willebrand factor from Weibel-Palade bodies during exocytosis. J Cell Biol. 2011;194:613–29. doi: 10.1083/jcb.201011119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang ZP, Südhof TC. Cell biology of Ca2+-triggered exocytosis. Current Opinion in Cell Biology. 2010;22:496–505. doi: 10.1016/j.ceb.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulou C, Corrigall V, Taylor PR, Poston RN. The role of the chemokines MCP-1, GRO-alpha, IL-8 and their receptors in the adhesion of monocytic cells to human atherosclerotic plaques. Cytokine. 2008;43:181–6. doi: 10.1016/j.cyto.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao TC, Santana Rodriguez Z, Bradberry MM, Ranski AH, Dahl PJ, Schmidtke MW, Jenkins PM, Axelrod D, Chapman ER, Giovannucci DR, et al. Synaptotagmin isoforms confer distinct activation kinetics and dynamics to chromaffin cell granules. J Gen Physiol. 2017;149:763–780. doi: 10.1085/jgp.201711757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson IM, Ranjan R, Schwarz TL. Synaptotagmins I and IV promote transmitter release independently of Ca(2+) binding in the C(2)A domain. Nature. 2002;418:336–40. doi: 10.1038/nature00915. [DOI] [PubMed] [Google Scholar]
- Rondaij MG, Bierings R, van Agtmaal EL, Gijzen KA, Sellink E, Kragt A, Ferguson SS, Mertens K, Hannah MJ, van Mourik JA, et al. Guanine exchange factor RalGDS mediates exocytosis of Weibel-Palade bodies from endothelial cells. Blood. 2008;112:56–63. doi: 10.1182/blood-2007-07-099309. [DOI] [PubMed] [Google Scholar]
- Roper LK, Briguglio JS, Evans CS, Jackson MB, Chapman ER. Sex-specific regulation of follicle-stimulating hormone secretion by synaptotagmin 9. Nature Communications. 2015;6 doi: 10.1038/ncomms9645. 8645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler JE. Biochemistry and genetics of von Willebrand factor. Annu Rev Biochem. 1998;67:395–424. doi: 10.1146/annurev.biochem.67.1.395. [DOI] [PubMed] [Google Scholar]
- Saegusa C, Fukuda M, Mikoshiba K. Synaptotagmin V Is Targeted to Dense-core Vesicles That Undergo Calcium-dependent Exocytosis in PC12 Cells. Journal of Biological Chemistry. 2002;277:24499–24505. doi: 10.1074/jbc.M202767200. [DOI] [PubMed] [Google Scholar]
- Schillemans M, Karampini E, Kat M, Bierings R. Exocytosis of Weibel-Palade bodies: how to unpack a vascular emergency kit. Journal of Thrombosis and Haemostasis. 2018a doi: 10.1111/jth.14322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schillemans M, Karampini E, van den Eshof B, Gangaev A, Hofman M, van Breevoort D, Meems H, Janssen H, Mulder AA, Jost CR, et al. The Weibel-Palade Body Localized SNARE (Soluble NSF Attachment Protein Receptor) Syntaxin-3 Modulates Von Willebrand Factor Secretion From Endothelial Cells. Arterioscler Thromb Vasc Biol. 2018b doi: 10.1161/ATVBAHA.117.310701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonn JS, Maximov A, Lao Y, Sudhof TC, Sorensen JB. Synaptotagmin-1 and -7 are functionally overlapping Ca2+ sensors for exocytosis in adrenal chromaffin cells. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:3998–4003. doi: 10.1073/pnas.0712373105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof TC. The Molecular Machinery of Neurotransmitter Release (Nobel Lecture) Angewandte Chemie International Edition. 2014;53:12696–12717. doi: 10.1002/anie.201406359. [DOI] [PubMed] [Google Scholar]
- Sugita S, Han W, Butz S, Liu X, Fernandez-Chacon R, Lao Y, Sudhof TC. Synaptotagmin VII as a Plasma Membrane Ca2+ Sensor in Exocytosis. Neuron. 2001 doi: 10.1016/s0896-6273(01)00290-2. [DOI] [PubMed] [Google Scholar]
- Valentijn KM, Sadler JE, Valentijn JA, Voorberg J, Eikenboom J. Functional architecture of Weibel-Palade bodies. Blood. 2011;117:5033–43. doi: 10.1182/blood-2010-09-267492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Breevoort D, Snijders AP, Hellen N, Weckhuysen S, van Hooren KW, Eikenboom J, Valentijn K, Fernandez-Borja M, Ceulemans B, De Jonghe P, et al. STXBP1 promotes Weibel-Palade body exocytosis through its interaction with the Rab27A effector Slp4-a. Blood. 2014;123:3185–94. doi: 10.1182/blood-2013-10-535831. [DOI] [PubMed] [Google Scholar]
- van Breevoort D, van Agtmaal EL, Dragt BS, Gebbinck JK, Dienava-Verdoold I, Kragt A, Bierings R, Horrevoets AJ, Valentijn KM, Eikenboom JC, et al. Proteomic screen identifies IGFBP7 as a novel component of endothelial cell-specific Weibel-Palade bodies. J Proteome Res. 2012;11:2925–36. doi: 10.1021/pr300010r. [DOI] [PubMed] [Google Scholar]
- van Schie MC, de Maat MP, Isaacs A, van Duijn CM, Deckers JW, Dippel DW, Leebeek FW. Variation in the von Willebrand factor gene is associated with von Willebrand factor levels and with the risk for cardiovascular disease. Blood. 2011;117:1393–9. doi: 10.1182/blood-2010-03-273961. [DOI] [PubMed] [Google Scholar]
- Vinet AF, Fukuda M, Descoteaux A. The Exocytosis Regulator Synaptotagmin V Controls Phagocytosis in Macrophages. The Journal of Immunology. 2008;181:5289–5295. doi: 10.4049/jimmunol.181.8.5289. [DOI] [PubMed] [Google Scholar]
- Vinet AF, Fukuda M, Turco SJ, Descoteaux A. The Leishmania donovani lipophosphoglycan excludes the vesicular proton-ATPase from phagosomes by impairing the recruitment of synaptotagmin V. PLoS Pathog. 2009;5:e1000628. doi: 10.1371/journal.ppat.1000628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale N, Mawet J, Camonis J, Regazzi R, Bader MF, Chasserot-Golaz S. The Small GTPase RalA controls exocytosis of large dense core secretory granules by interacting with ARF6-dependent phospholipase D1. J Biol Chem. 2005;280:29921–8. doi: 10.1074/jbc.M413748200. [DOI] [PubMed] [Google Scholar]
- von Poser C, Ichtchenko K, Shao X, Rizo J, Sudhof TC. The evolutionary pressure to inactivate. A subclass of synaptotagmins with an amino acid substitution that abolishes Ca2+ binding. J Biol Chem. 1997;272:14314–9. doi: 10.1074/jbc.272.22.14314. [DOI] [PubMed] [Google Scholar]
- Wang P, Chintagari NR, Gou D, Su L, Liu L. Physical and functional interactions of SNAP-23 with annexin A2. Am J Respir Cell Mol Biol. 2007;37:467–76. doi: 10.1165/rcmb.2006-0447OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber JP, Toft-Bertelsen TL, Mohrmann R, Delgado-Martinez I, Sorensen JB. Synaptotagmin-7 is an asynchronous calcium sensor for synaptic transmission in neurons expressing SNAP-23. PLOS ONE. 2014;9:e114033. doi: 10.1371/journal.pone.0114033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieberdink RG, van Schie MC, Koudstaal PJ, Hofman A, Witteman JC, de Maat MP, Leebeek FW, Breteler MM. High von Willebrand factor levels increase the risk of stroke: the Rotterdam study. Stroke. 2010;41:2151–6. doi: 10.1161/STROKEAHA.110.586289. [DOI] [PubMed] [Google Scholar]
- Xu J, Mashimo T, Sudhof TC. Synaptotagmin-1, -2, and -9: Ca(2+) sensors for fast release that specify distinct presynaptic properties in subsets of neurons. Neuron. 2007;54:567–81. doi: 10.1016/j.neuron.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Zhu Q, Yamakuchi M, Lowenstein CJ. SNAP23 Regulates Endothelial Exocytosis of von Willebrand Factor. PLOS ONE. 2015;10:e0118737. doi: 10.1371/journal.pone.0118737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q, Yamakuchi M, Ture S, de la Luz Garcia-Hernandez M, Ko KA, Modjeski KL, LoMonaco MB, Johnson AD, O'Donnell CJ, Takai Y, et al. Syntaxin-binding protein STXBP5 inhibits endothelial exocytosis and promotes platelet secretion. J Clin Invest. 2014;124:4503–16. doi: 10.1172/JCI71245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zupancic G, Ogden D, Magnus CJ, Wheeler-Jones C, Carter TD. Differential exocytosis from human endothelial cells evoked by high intracellular Ca(2+) concentration. The Journal of Physiology. 2002;544:741–55. doi: 10.1113/jphysiol.2002.027490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.