Summary
Objective
25-hydroxyvitamin D (25(OH)D) is critical for bone mineralization and may prevent fractures. Understanding vitamin D deficiency trends in midlife women is particularly important given their concurrent menopausal changes that increase risk for fracture. We aimed to evaluate changes in mean 25(OH)D over time and their determinants in a racially, ethnically, and socioeconomically diverse cohort of midlife women.
Design
A multi-center prospective cohort study.
Patients
1585 women ages 42-52 years at baseline.
Measurements
We measured serum 25(OH)D at 2 timepoints (1998-2000 and 2009-2011). Between-visit change was assessed in the whole cohort and in socioeconomic and demographic subgroups. Among those with vitamin D deficiency (25(OH)D <30 nmol/L) at baseline, we evaluated determinants of persistent deficiency at follow-up.
Results
Mean 25(OH)D increased from 53.8 to 70.0 nmol/L (p<0.001), and the prevalence of deficiency decreased from 20.4 to 9.7% (p<0.001). While baseline 25(OH)D differed among subgroups, the changes in 25(OH)D were similar among groups. The proportion of women reporting dietary supplement use increased from 40.8 to 67.1% (p<0.001), and the increase in 25(OH)D was significantly higher in supplement users. Among women with vitamin D deficiency at baseline, White women and supplement users were less likely to remain deficient at follow-up.
Conclusions
Among midlife women, temporal increases in 25(OH)D concentrations are driven largely by increases in supplement use. The proportion of women with 25(OH)D<30 nmol/L and thus at high risk for skeletal consequences remains substantial. Targeted screening for vitamin D deficiency in populations at risk for fragility fracture may be advisable.
Keywords: Humans, Female, Prospective Studies, Vitamin D deficiency, 25-hydroxyvitamin D, Menopause, Dietary Supplements
Introduction
Vitamin D is a critical determinant of calcium absorption and, by extension, bone mineralization1. Several researchers have proposed an additional role for vitamin D in extraskeletal health which remains an area of active investigation2, 3. Circulating 25-hydroxyvitamin D (25(OH)D), an index of vitamin D sufficiency, is a metabolite of both endogenous vitamin D produced in the skin following ultraviolet light exposure as well as vitamin D from food and supplements1.
The definition of “adequate” 25(OH)D for bone health is controversial. Imprecision in the standard antibody-based assays of 25(OH)D has also contributed to difficulty in defining requirements4. The 2010 Institute of Medicine report suggested that 25(OH)D ≥ 50 nmol/L is sufficient for optimal bone health for the majority of the population5; by contrast, recent reports suggest that supplemental vitamin D for fracture prevention benefits only those with 25(OH)D <30 nmol/L6, 7.
Population-based serial cross-sectional data from the United States NHANES demonstrated a concerning increase in the prevalence of 25(OH)D<30 nmol/L in community-dwelling adults, from 3 to 7% in men and from 7 to 11% in women, over the period spanning 1988-1994 to 2001-20028. Rates of deficiency were higher among Black and Mexican participants in NHANES when compared with White participants8; Asian Americans also have an elevated risk of deficiency9, 10. Subsequent NHANES surveys found a modest increase in the population mean 25(OH)D in the 2007-8 and 2009-10 samples, coincident with an increase in the use of vitamin-D containing dietary supplements11.
Women undergoing the menopausal transition have accelerated bone loss, and we have previously shown in the Study of Women’s Health Across the Nation (SWAN) that lower baseline 25(OH)D is associated with increased non-traumatic fracture risk over the next 9.5 years12. Improved understanding of population trends in circulating 25(OH)D may inform public health interventions targeting vitamin D status. We here report longitudinal changes in serum 25(OH)D in the SWAN cohort, spanning the period from 1998-2000 to 2009-2011, using precise and accurate mass spectrometry methodology, and we evaluate the association of socioeconomic and demographic factors with change in 25(OH)D over time.
Materials and Methods
Study participants
SWAN is a multi-site, longitudinal, community-based cohort study initiated in 1996-1997 that enrolled women aged 42-52 years who were pre- or early perimenopausal at baseline13. Participants were recruited at 7 sites; each site recruited White women, and women from one additional racial/ethnic group (Black, Chinese, Hispanic, or Japanese). Women who attended Visits 2 (1998-2000) and 12 (2009-2011) and who had serum samples collected for measurement of (25(OH)D) were included in the present analysis (n=1585) (Figure 1). The SWAN study was approved by the Institutional Review Boards at each site and the coordinating center, and all subjects provided written informed consent.
Figure 1.
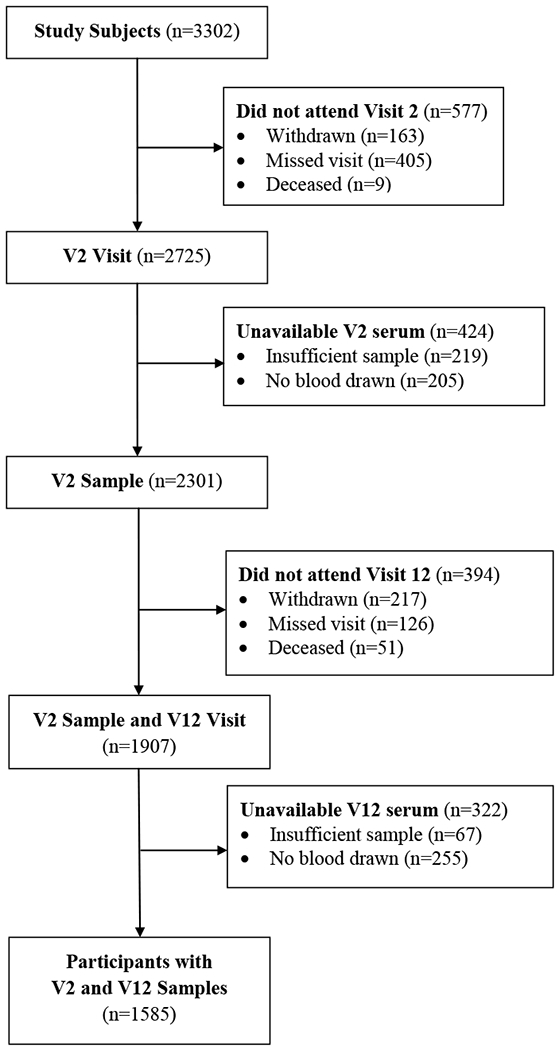
Flow chart of SWAN participants included in the present analysis.
Measurement of 25(OH)D
Blood was drawn in the fasting state prior to 10:00 am. Serum aliquots were stored at −80°C until measurement. 25(OH)D in samples from both Visits 2 and 12 were measured in a single batch by liquid chromatography/tandem mass spectrometry (LC-MS/MS). 25(OH)D was calculated as the sum of 25(OH)D2 and 25(OH)D3. As previously described, the limit of detection was 3 ng/mL, and the interassay CV was 7.5%12. Vitamin D insufficiency was defined as 25(OH)D<50 nmol/L5, and vitamin D deficiency was defined as 25(OH)D≤30 nmol/L6.
Ascertainment of multivitamin and vitamin D supplement use
At Visits 2 and 12, use of supplemental vitamins was assessed in standardized interviewer-administered questionnaires and on a worksheet recording medication use. Of note, the format of the worksheet changed between Visits 2 and 12. Vitamin use was coded as “yes” if subjects reported taking either a multivitamin or a vitamin D supplement at least one day per week.
Additional covariates
Age (Visits 2 and 12), race/ethnicity (baseline visit), country of origin (baseline visit), household income (Visits 2 and 12), language use (Visits 2 and 12), educational attainment (baseline visit), and health insurance status (Visits 2 and 12), were assessed by standardized interviewer-administered questionnaires. Height and weight were measured at each visit on calibrated scales and stadiometers, and BMI was calculated. BMI categories were defined as normal weight (BMI <25 kg/m2), overweight (25 kg/m2 ≤ BMI <30 kg/m2), and obese (BMI ≥ 30 kg/m2). The season of blood draw for each visit was defined as winter (October through March) and summer (April through September).
Statistical Analyses
All data were visually inspected for outliers. Baseline characteristics of the cohort were described with mean and standard deviation for continuous variables and number and percent for binary and categorical variables. The change in the proportion of subjects taking vitamin D supplements between visits was assessed with McNemar’s test.
Differences in 25(OH)D among subgroups at both Visits 2 and 12 were evaluated with ANOVA and were corrected using the Bonferroni method for multiple comparisons. Unadjusted comparisons of Visit 2 and Visit 12 25(OH)D in the whole cohort and in designated subgroups were evaluated with paired t-tests. Differences in Δ25(OH)D among subgroups was evaluated with ANOVA followed by Bonferroni correction. The association of continuous covariates with change in 25(OH)D was assessed with linear regression. Between-visit comparisons of the prevalence of 25(OH)D below the indicated thresholds was assessed with McNemar’s test.
A longitudinal mixed model was generated to evaluate the independent association of race/ethnicity group with change in 25(OH)D. The model included age, BMI, educational attainment, household income, language use, insurance status, season of blood draw, study site, and menopausal status.
Women with and without baseline 25(OH)D > 30 nmol/L were compared by t-test for continuous variables (age and BMI), or by χ2 for binary/categorical variables (country of birth, race/ethnicity group, household income, educational attainment and vitamin use). Logistic regression was used to determine predictors of remaining vitamin D deficient at Visit 12 among subjects who were deficient at Visit 2. Covariates in this model included age, BMI, change in BMI, race/ethnicity group, household income, vitamin use, educational attainment, and Visit 12 season of blood draw. Study site was not included in this model after regression diagnostics determined that it was collinear with race/ethnicity.
Differences in the proportion of women of different race/ethnicity groups taking vitamin supplements at Visit 12 were evaluated with ANOVA.
Results
Clinical and demographic characteristics of the cohort
1585 women were included in the current analysis, of whom 43.1% had vitamin D insufficiency (25(OH)D<50 nmol/L) and 20.4% had vitamin D deficiency (25(OH)D ≤30 nmol/L) at Visit 2 (1998-2000), the first visit at which 25(OH)D was measured (Table 1). The women represented a wide array of racial and ethnic groups, socioeconomic statuses, and levels of formal education. At visit 12 (2009-2011), average age had increased to 60.3 ± 2.7 years, and average BMI had increased to 29.1 ± 7.3 kg/m2. The distribution of household income was similar to that at Visit 2 (10%, 12%, 13%, 21%, and 44% at <$20,000, 20-35,000, 35-50,000, 50-75,000, and ≥75,000 respectively), and the percentage of subjects who were uninsured was also similar at 5.9%. The proportion of subjects who reported taking multivitamins or vitamin D at the follow-up visit increased from 40.8% to 67.1% (p<0.001). Of note, among all the women who had 25(OH)D measured at visit 2, we did not observe a difference among those who did (n=1585) or did not (n=716) have 25(OH)D measured at visit 12 (53.8 ± 24.3 vs 54.0 ± 24.5 nmol/L, p=0.92).
Table 1:
Clinical and Demographic Characteristics
| Variable | Mean (SD) or n (%) |
|---|---|
| Age at V2 (years), mean (SD) | 48.6 (2.7) |
| BMI at V2, kg/m2, mean (SD) | 28.0 (7.2) |
| Race/ethnicity, n (%) | |
| Black | 386 (24.4) |
| White | 778 (49.1) |
| Chinese | 172 (10.9) |
| Hispanic | 66 (4.2) |
| Japanese | 183 (11.6) |
| Country of origin, n (%) | |
| United States | 1225 (77.3) |
| Other | 311 (19.6) |
| Unknown | 49 (3.1) |
| Household income at V2, n (%) | |
| <$20,000 | 142 (9.0) |
| $20,000 to <$35,000 | 183 (11.5) |
| $35,000 to <$50,000 | 236 (14.9) |
| $50,000 to <$75,000 | 389 (24.5) |
| ≥$75,000 | 559 (35.3) |
| Unknown | 76 (4.8) |
| Language use at V2, n (%) | |
| Non-English language only | 78 (4.9) |
| English and non-English language | 181 (11.4) |
| English language only | 1299 (81.9) |
| Unknown | 27 (1.8) |
| Educational attainment, n (%) | |
| Less than high school | 69 (4.4) |
| Completed high school or GED | 252 (15.9) |
| Some college | 468 (29.5) |
| College graduate | 359 (22.6) |
| Post-graduate education | 425 (26.8) |
| Unknown | 12 (0.8) |
| Covered by health insurance at V2, n (%) | |
| Yes | 1489 (93.9) |
| No | 96 (6.1) |
| Taking MVI or vitamin D at V2, n (%) | 643 (40.8) |
| Vitamin use over observation period, n (%) | |
| Neither V2 nor V12 | 395 (24.9) |
| Discontinued after V2 | 124 (7.8) |
| Initiated after V2 | 539 (34.0) |
| Both V2 and V12 | 519 (32.8) |
| Unknown | 8 (0.5) |
| 25(OH)D at V2 (nmol/L), mean (SD) | 53.8 (24.3) |
| 25(OH)D <50 nmol/L at V2, n (%) | 683 (43.1) |
| 25(OH)D ≤30 nmol/L at V2, n (%) | 324 (20.4) |
V2, Visit 2, GED, general education diploma; MVI, multivitamin; 25(OH)D, 25-hydroxyvitamin D.
Change in serum 25(OH)D concentration over time
As shown in Table 2, the mean serum 25(OH)D concentration in the cohort increased by 16.2 nmol/L (p<0.001) between Visits 2 and 12. As expected, baseline 25(OH)D varied significantly by race/ethnicity, household income, educational attainment, vitamin use, season of blood draw, and BMI category at each visit. Specifically, Black women had significantly lower mean baseline 25(OH)D compared with Chinese and Hispanic women, who were significantly lower than Japanese and White women. Between visits, we observed a significant increase in 25(OH)D over time in every subgroup investigated with the exception of women who discontinued vitamin use after Visit 2. Within subgroups, we observed significant differences in the absolute change in 25(OH)D concentration (Δ25(OH)D) by race/ethnicity, vitamin use, and season of blood draw. We did not observe differences in Δ25(OH)D when comparing by country of origin, household income, language use, educational attainment, medical insurance status, or BMI category (Supplemental Figure 1). For continuous covariates, we did not observe an association of age or of baseline BMI with Δ25(OH)D (data not shown), but we did observe a small but significant inverse association of change in BMI with Δ25(OH)D, such that women who gained more weight between visits had a smaller increase in 25(OH)D (−0.7 nmol/L per additional kg/m2, R=−0.088, p<0.001).
Table 2:
Change in 25(OH)D between Visit 2 and Visit 12
| Visit 2 25(OH)D, Mean (SD) | Visit 12 25(OH)D, Mean (SD) | Δ25OHD Mean (SD) | p value for comparison of Δ25OHD | |
|---|---|---|---|---|
| Entire cohort (nmol/L) Site a,b | 53.8 (24.3) | 70.0c (28.7) | 16.2 (28.2) | -- |
| Ann Arbor | 41.9 (23.4) | 58.5c (31.5) | 16.6 (29.0) | 0.7701 |
| Boston | 54.7 (25.9) | 71.6c (27.8) | 16.9 (28.1) | |
| Chicago | 51.7 (27.1) | 67.0c (26.5) | 15.3 (28.0) | |
| UC Davis | 55.6 (20.7) | 72.7c (25.8) | 17.1 (26.0) | |
| UC Los Angeles | 63.4 (21.2) | 77.3c (29.2) | 13.9 (30.8) | |
| New Jersey | 52.5 (20.4) | 70.7c (27.4) | 18.2 (28.6) | |
| Pittsburgh | 53.7 (25.9) | 70.2c (27.0) | 16.5 (26.7) | |
| Race/ethnicitya,b | ||||
| Black | 34.9 (18.7) | 55.4c (28.8) | 20.5 (26.5) | <0.001 |
| White | 63.5 (23.9) | 76.6c (27.2) | 13.1 (29.1) | |
| Chinese | 49.3 (17.2) | 70.5c (24.0) | 21.1 (23.9) | |
| Hispanic | 45.7 (16.4) | 63.3c (25.8) | 17.7 (25.5) | |
| Japanese | 59.9 (18.9) | 74.8c (28.6) | 14.9 (31.1) | |
| Country of origin | ||||
| United States | 54.1 (25.3) | 70.3c (29.4) | 16.2 (28.6) | 0.800 |
| Other | 53.9 (19.9) | 70.6c (25.9) | 16.7 (26.6) | |
| Household income a,b | ||||
| <$20,000 | 44.5 (23.3) | 58.6c (28.2) | 14.1 (26.2) | 0.378 |
| $20,000 to <$35,000 | 50.5 (25.8) | 64.5c (29.2) | 14.0 (27.8) | |
| $35,000 to <$50,000 | 50.8 (25.0) | 66.9c (30.1) | 16.1 (28.1) | |
| $50,000 to <$75,000 | 53.8 (22.8) | 72.1c (26.5) | 18.2 (28.5) | |
| ≥$75,000 | 59.3 (23.9) | 74.9c (27.8) | 15.7 (28.0) | |
| Language use | ||||
| Non-English language only | 49.6 (17.4) | 67.8c (28.1) | 18.2 (25.8) | 0.696 |
| English and non-English language | 51.6 (18.8) | 68.9c (25.2) | 17.3 (26.9) | |
| English language only | 54.4 (25.4) | 70.4c (29.3) | 16.0 (28.6) | |
| Educational attainment a,b | ||||
| Less than high school | 49.6 (19.2) | 61.8c (24.4) | 12.2 (23.0) | 0.493 |
| Completed high school or GED | 47.6 (23.7) | 64.9c (29.5) | 17.3 (29.4) | |
| Some college | 51.4 (24.7) | 68.5c (31.1) | 17.1 (28.6) | |
| College graduate | 57.8 (24.8) | 72.5c (28.0) | 14.6 (28.5) | |
| Post-graduate education | 57.6 (23.6) | 74.3c (26.0) | 16.6 (27.9) | |
| Covered by health insurance at V2 | ||||
| Yes | 53.9 (24.4) | 70.2c (28.7) | 16.3 (28.5) | 0.541 |
| No | 52.0 (23.9) | 66.5c (28.3) | 14.5 (24.7) | |
| Taking MVI or vitamin D at V2 a,b | ||||
| Yes | 61.3 (24.1) | 77.2c (27.5) | 15.8 (28.2) | 0.657 |
| No | 48.7 (23.2) | 65.2c (28.6) | 16.5 (28.3) | |
| Vitamin use over observation period a,b | ||||
| Neither V2 nor V12 | 44.1 (22.8) | 48.4c (22.1) | 4.3 (22.5) | <0.001 |
| Discontinued after V2 | 53.4 (26.8) | 55.3 (24.3) | 1.9 (25.9) | |
| Initiated after V2 | 52.1 (22.9) | 77.5c (26.6) | 25.4 (28.8) | |
| Both V2 and V12 | 63.2 (23.1) | 82.4c (25.5) | 19.2 (27.8) | |
| Season of blood draw a | ||||
| Summer/winter | 56.3 (23.3) | 70.8c (14.5) | 14.5 (29.8) | <0.001 |
| Winter/summer | 49.4 (23.7) | 72.2c (27.6) | 22.8 (26.6) | |
| Same season | 54.1 (25.0) | 68.6c (27.7) | 14.5 (27.5) | |
| BMI category at V2 a,b | ||||
| Normal weight | 61.1 (23.7) | 77.7c (27.3) | 16.6 (29.0) | 0.693 |
| Overweight | 52.5 (24.1) | 68.0c (27.7) | 15.4 (27.7) | |
| Obese | 40.4 (20.4) | 57.3c (28.8) | 16.9 (27.1) |
p<0.001 among covariate levels at Visit 2,
p<0.001 among covariate levels at Visit 12,
p<0.001 at Visit 12 compared with Visit 2. 25(OH)D, 25-hydroxyvitamin D; Δ25(OH)D, change in 25-hydroxyvitamin D, GED, general education diploma
As shown in Figure 2A, in the overall cohort, the percentage of women with vitamin D insufficiency decreased from 43.1 to 23.8% between Visits 2 and 12. In addition, the percentage of women with vitamin D deficiency decreased from 20.4 to 9.7% between visits. As shown in Figures 2B-E, the observed right-shift in the population distribution of 25(OH)D was most evident in the women who either initiated vitamin use after Visit 2 or took vitamins throughout, with limited evident change among the women who never took vitamins or who discontinued vitamin use after Visit 2.
Figure 2.
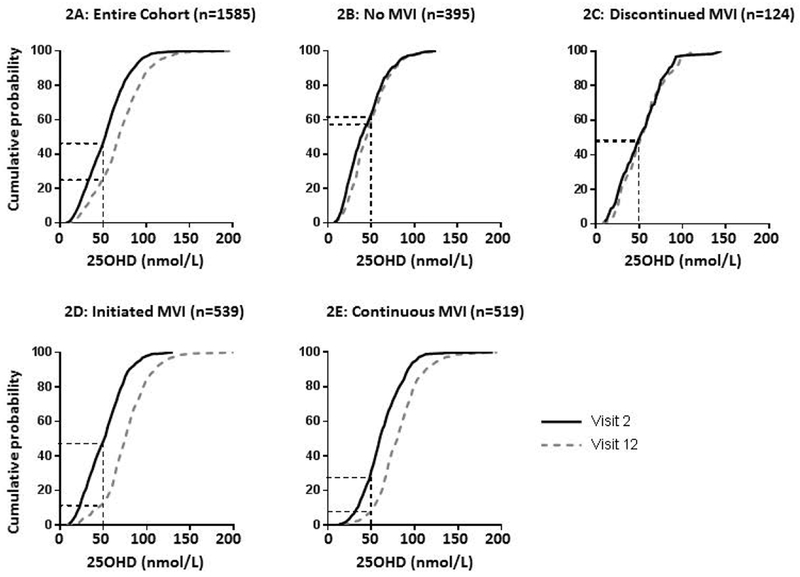
Cumulative distribution graph of 25-hydroxyvitamin D in the whole cohort (2A) and among women who did not use vitamins (2B), who discontinued vitamin use after Visit 2 (2C), who initiated vitamin use after Visit 12 (2D), and who took vitamins at both visits (2E). In the cumulative distribution graph, the cumulative frequency along the y-axis represents the proportion of the population with 25(OH)D less than or equal to the corresponding value on the x-axis. Visit 2 in black, Visit 12 in gray. Dotted lines indicate the proportion of subjects with 25-hydroxyvitamin D < 50 nmol/L at each visit. Note that vitamin use information was unavailable for 8 subjects.
While the cohort mean 25(OH)D increased with time, 25(OH)D decreased in 412 (26.0%) subjects and decreased by more than 25 nmol/L in 71 (4.5%) subjects. Notably, among subjects who were not vitamin D deficient at Visit 2 (n=1261), 64 (5.1%) were newly deficient at Visit 12.
Independent effect of race/ethnicity on Δ25(OH)D
As noted above, we observed substantial variation in baseline 25(OH)D by race/ethnicity group. Therefore, to investigate the effect of race/ethnicity group on Δ25(OH)D, we generated a longitudinal mixed model adjusting for the following: age, BMI, educational attainment, household income, language use, insurance status, and season of blood draw. Study site and menopausal status were included as additional covariates in the mixed model. Compared to Black women, White and Japanese women had a significantly smaller increase in 25(OH)D (8.0 and 6.7 nmol/L smaller increase per 11 years, p<0.001 and p=0.005, respectively). We did not observe significant differences in the rate of change of 25(OH)D between Black women and either Hispanic or Chinese women (3.0 and 2.7 nmol/L smaller increase per 11 years, p=0.444 and p=0.250 respectively).
Δ25(OH)D among women with vitamin D deficiency
To further evaluate the women at highest risk for consequences of low serum vitamin D, we assessed Δ25(OH)D among women with vitamin D deficiency at baseline (n=324, 20.4% of cohort, see Table 1). Compared with the women with baseline 25(OH)D>30 nmol/L, the women with deficiency were slightly younger (48.0 vs. 48.7 years, p<0.001), had a higher BMI (31.5 vs. 27.1 kg/m2, p<0.001), were more likely to have been born in the US (p<0.001), were more likely to be Black and less likely to be White, Chinese, or Japanese, and tended to have lower household income and educational attainment (data not shown). They were also less likely to report using a vitamin supplement (20.7 vs. 45.9%, p<0.001).
Of the 324 women with vitamin D deficiency at baseline, 89 (27.5%) remained deficient when measured again at Visit 12. In univariable analyses, predictors of remaining deficient at Visit 12 included higher baseline BMI (p=0.046), race/ethnicity (p=0.001), household income (p=0.049), and vitamin use (p<0.001). We generated a multivariable logistic regression model for remaining deficient at Visit 12, including all the parameters found to be significant in univariable regressions as well as age, educational attainment, change in BMI, and Visit 12 season of blood draw as other potentially important covariates. Of note, none of the Japanese women with 25(OH)D≤30 nmol/L at baseline (n=14) remained deficient at Visit 12, so these subjects could not be included in the logistic model. As seen in Table 3, race/ethnicity, vitamin use, and increase in BMI over time were independent predictors of remaining deficient at Visit 12. Specifically, compared with Black women, White women were 3.7 fold less likely to remain deficient at Visit 12. Compared with women who never used vitamin supplements, those who either started after Visit 2 or took vitamins throughout were 4.5 and 5.7 fold less likely to remain deficient at Visit 12.
Table 3:
Predictors of 25(OH)D≤30 nmol/L at Visit 12 among women with 25(OH)D≤30 nmol/L at Visit 2
| Variable | Overall p value for covariate | Odds ratio (95% CI) |
|---|---|---|
| Age (years) | 0.763 | 0.98 (0.87-1.11) |
| BMI, kg/m2, mean (SD) | 0.425 | 1.02 (0.97-1.06) |
| Change in BMI (kg/m2/year) | 0.033 | 1.11 (1.01-1.23) |
| Race/ethnicity | ||
| Black (n=196) | 0.017 | Reference |
| White (n=75) | 0.27 (0.11-0.66) | |
| Chinese (n=25) | 0.42 (0.11-1.59) | |
| Hispanic (n=14) | 0.25 (0.04-1.46) | |
| Japanese (n=14) | Unable to calculate | |
| Household income | ||
| <$20,000 (n=52) | 0.203 | Reference |
| $20,000 to <$35,000 (n=50) | 2.06 (0.68-6.25) | |
| $35,000 to <$50,000 (n=59) | 1.71 (0.56-5.27) | |
| $50,000 to <$75,000 (n=67) | 0.69 (0.22-2.16) | |
| ≥$75,000 (n=75) | 1.01 (0.30-3.41) | |
| Educational attainment | ||
| Less than high school (n=12) | 0.367 | Reference |
| Completed high school or GED (n=67) | 0.35 (0.06-2.17) | |
| Some college (n=117) | 0.35 (0.06-2.08) | |
| College graduate (n=52) | 0.15 (0.02-1.10) | |
| Post-graduate education (n=73) | 0.29 (0.04-1.99) | |
| Vitamin use over observation period, n (%) | ||
| Neither V2 nor V12 (n=142) | <0.001 | Reference |
| Discontinued after V2 (n=32) | 1.63 (0.62-4.31) | |
| Initiated after V2 (n=114) | 0.22 (0.10-0.49) | |
| Both V2 and V12 (n=35) | 0.17 (0.05-0.66) | |
| Visit 12 season | ||
| Winter | 0.007 | Reference |
| Summer | 0.40 (0.21-0.78) |
25(OH)D, 25-hydroxyvitamin D; BMI, body mass index; GED, general education diploma. Note that no Japanese women had 25(OH)D≤30 nmol/L at Visit 12.
Prevalence of low 25(OH)D at Visit 12 by race/ethnicity and vitamin use status
Given the strong associations of race/ethnicity and vitamin use with serum 25(OH)D, we evaluated the prevalence of low serum 25(OHD) at Visit 12 using a threshold of ≤ 30 nmol/L (deficient) after stratifying by these factors. Of note, the proportion of women who reported taking vitamins differed by race/ethnicity (70%, 56%, 72%, 58%, 77% among White, Black, Chinese, Hispanic, and Japanese women respectively, p<0.001).
As shown in Figure 3, the risk of low 25(OH)D varied substantially by these clinically available variables. Notably, the overall prevalence of deficiency was 23% among women who were not taking vitamin supplements at Visit 12, but the prevalence of deficiency varied dramatically by race/ethnicity, ranging from 5% of Japanese women to 46% of Black women. Among those who did report taking vitamins, the overall prevalence of severe deficiency was 3%, but rose to 9% among Black women taking vitamins.
Figure 3.
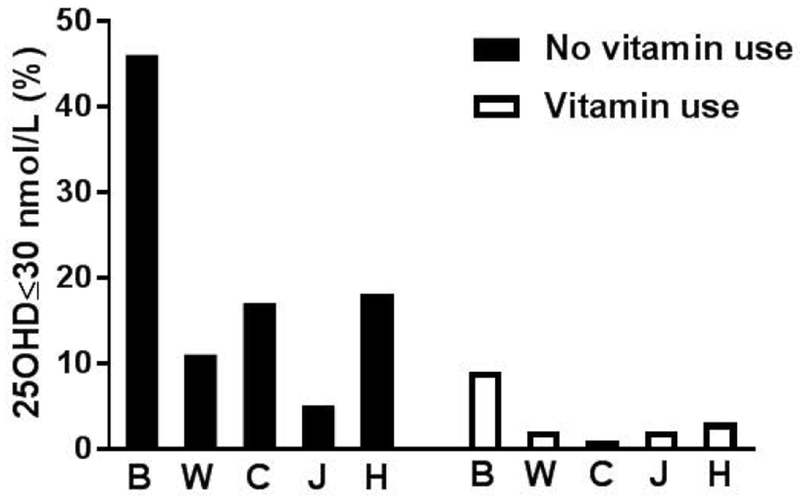
Bar chart showing the proportion of subjects meeting criteria for low serum 25-hydroxyvitamin D defined as ≤30 nmol/L when stratified by race/ethnicity and vitamin use. B, Black; W, White, C, Chinese; J, Japanese; H, Hispanic
Discussion
In the SWAN prospective cohort of women in midlife, we observed a significant increase in mean serum 25(OH)D concentrations as measured by LC-MS/MS between the periods 1998-2000 and 2009-2011. The observed increase was clinically meaningful, with approximately 50% reductions in the proportion of women with vitamin D insufficiency and deficiency. The absolute increase was higher in Black women compared to White and Japanese women, though women of all racial/ethnic groups demonstrated significant increases. The degree of increase did not depend on other social and demographic factors including household income, education, language use, or health insurance status. Use of vitamin D-containing supplements was a major driver of the increase in mean 25(OH)D, with higher increases in subjects who initiated or continued supplement use compared to those who discontinued or never used. Concerningly however, rates of significant deficiency remained high, with almost 10% of the cohort having 25(OH)D≤30 nmol/L at the 2009-2011 visit. Deficiency was particularly high among Black women at ~25% overall, and at 9% even among those using vitamin D-containing supplements.
Our finding of a temporal increase in 25(OH)D is consistent with data from the US population-based NHANES dataset11. Of note, in NHANES, the assay methodology was not consistent between samples, and the data are thus mathematically standardized; our data by contrast provide direct evidence of a temporal increase in mean 25(OH)D. Our results are also consistent with the Canadian Multicentre Osteoporosis Study (CaMos), but extend its findings from an almost exclusively White population to additional racial/ethnic groups14. Other longitudinal studies, by contrast, show stable or declining 25(OH)D with time15–17. The cause of the discrepancies in temporal trends in 25(OH)D between study cohorts is unclear, but may relate to regional differences in dietary patterns, sunlight exposure, and other health-related behaviors.
The period between 1998 and 2011 was marked by significant increases in press reports regarding the health benefits of vitamin D, aimed both at health care providers and at the general public. An emphasis on dietary supplements to achieve adequate body stores of vitamin D was a prominent theme in contemporaneous newspaper articles18. Consumer spending on vitamin D supplements in the United States rose from $40 million to $425 million between 2001 and 200919. NHANES data indicate that, while the overall proportion of people taking vitamin D-containing supplements in the US was relatively stable between 1999 and 2012 (37 to 40%), the proportion of people taking vitamin D as an individual supplement rather than as part of a multivitamin increased from 5 to 19%, which may indicate an increase in the absolute amount consumed20. Given the strong association of supplement intake with longitudinal change in 25(OH)D in SWAN, the similar increase in 25(OH)D across subgroups of SWAN subjects suggests that outreach regarding the benefits of vitamin D was both accessible and persuasive to women of diverse backgrounds and socioeconomic strata. As our data are from an observational study rather than a supplementation trial, they reflect “real-world” intake of supplements. Our findings of persistent low 25(OH)D among supplement users differ from the results of randomized controlled trials of supplementation21–23, and may reflect real-world usage. A portion of the increase in 25(OH)D among supplement users may also reflect engagement in other health-promoting behaviors including increased dietary vitamin D intake and/or increased sun exposure. A recent survey of supplement users in NHANES found that supplement users had better self-reported health, more physical activity, and less smoking than non-users, and similar findings have been reported in other populations24, 25.
Several trials have investigated the dose-response of 25(OH)D to vitamin D supplementation, establishing that White and Black women absorb and metabolize cholecalciferol equivalently, and that the rise in 25(OH)D with supplementation is independent of race and age21–23. Supplement-driven increases in 25(OH)D are inversely proportional to baseline 25(OH)D, meaning that, for a given supplement intake, the rise in 25(OH)D is greater among people with a lower baseline23, 26. Our observation of a larger increase in 25(OH)D among Black women compared with White and Japanese women, likely thus stems from differences in 25(OH)D at Visit 2.
Our study has several strengths. We used LC-MS/MS to obtain highly accurate and precise measures of 25(OH)D in a diverse cohort of women who were well-phenotyped regarding social and demographic variables. There are limitations as well. We enrolled women at midlife, so these results are not generalizable to men or to women of differing ages. However, women at midlife are quite vulnerable to health consequences of low vitamin D given perimenopausal bone loss with attendant risk of osteoporosis and fracture27–29. Our results are also not generalizable to women outside the United States, who may have different sunlight exposure, dietary patterns, and attitudes towards supplement use. For example, despite the Scientific Advisory Committee on Nutrition of the United Kingdom recommending supplementation of 400 IU daily in 2016, only 43% of adults in a recent survey were adhering to this recommendation30. We measured total 25(OH)D rather than free or bioavailable 25(OH)D, and we have not measured vitamin D binding protein (DBP). However, it remains unclear whether free or bioavailable 25(OH)D offers additional information beyond total 25(OH)D 21, 31, and most, but not all, studies do not support a significant difference in circulating DBP concentration among individuals of different racial and ethnic backgrounds32–36. In addition, the mass spectrometry assay did not specifically exclude 3-epi-25(OH)D, which has been shown to constitute on average 2-3% of total 25(OH)D37. We had no information on the dose or duration of use of vitamin D supplements, and the format of the worksheet recording vitamin use changed to include more detailed questions at the later visit, potentially affecting the precision of the measure. We do not have measurements of other factors which may influence serum 25(OH)D including time spent outdoors and sunlight exposure. In particular, differences in climate and/or latitude between sites may influence both these factors. Reassuringly however, we observed no difference in change in 25(OH)D by study site. Additionally, our analysis of the effect of race/ethnicity on change in 25(OH)D was adjusted for study site, suggesting that subgroup-specific differences were independent of climate and latitude. Finally, study participation may have changed SWAN subjects’ health-related behaviors38. Reassuringly, while BMD was an outcome that was measured at 5 sites, potentially heightening awareness among participants, the subjects enrolled in the 2 sites which did not assess BMD (Chicago and New Jersey) did not differ in their change in mean 25(OH)D over time compared with subjects whose bone density was evaluated (Table 2).
In conclusion, in this cohort at risk for osteoporosis and fragility fractures given their female sex and transition through the menopause, mean 25(OH)D rose significantly and substantially over an 11 year timespan. While we observed expected differences in absolute 25(OH)D, the temporal increase in 25(OH)D was similar in all demographic subgroups, suggesting that public health interventions to raise awareness about vitamin D and its health effects had equitable impact. However, a sizeable proportion of women continued to have 25(OH)D concentrations low enough to put them at risk for skeletal complications of vitamin D deficiency. While considerable controversy exists regarding the optimal 25(OH)D for skeletal health, data suggest that concentrations under 30 nmol/L are associated with osteomalacia39, 40 and that supplementation of individuals below this threshold improves bone mineral density6, 7. Current guidelines do not recommend screening asymptomatic individuals for low 25(OH)D, given the paucity of data indicating population-wide benefit and concern for over-treatment of low 25(OH)D in the absence of true disease41, 42. However, our data demonstrating that 9.6% of mid-life women and, in particular, 25.4% of mid-life Black women have 25OHD≤30 nmol/L suggest that more intensive case-finding may be warranted, in order to efficiently target those likely to benefit from supplementation both clinically and in the context of future research studies. Our data suggest that factors including race/ethnicity, socioeconomic status, years of formal education, and supplement use could be used to guide clinicians or health systems to identify women at higher risk of 25OHD<30 nmol/L. These data, if replicated in other cohorts, may prompt revision of current public health guidance and facilitate targeted supplementation strategies to improve bone health.
Supplementary Material
Supplemental Figure 1
Boxplot showing change in 25-hydroxyvitamin D when stratified by household income (Supp1A), country of origin (Supp1B), language use (Supp1C), educational attainment (Supp1D), insurance status (Supp1E), and BMI category (Supp1F). Δ25(OH)D, change in 25-hydroxyvitamin D.
Acknowledgements
The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495). The SWAN Repository was supported by U01AG017719. This publication was supported in part by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1 RR024131. Deborah Mitchell was supported by K23DK105350 (NIDDK). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
Clinical Centers: University of Michigan, Ann Arbor – Siobán Harlow, PI 2011 – present, MaryFran Sowers, PI 1994-2011; Massachusetts General Hospital, Boston, MA – Joel Finkelstein, PI 1999 – present; Robert Neer, PI 1994 – 1999; Rush University, Rush University Medical Center, Chicago, IL – Howard Kravitz, PI 2009 – present; Lynda Powell, PI 1994 – 2009; University of California, Davis/Kaiser – Ellen Gold, PI; University of California, Los Angeles – Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY – Carol Derby, PI 2011 – present, Rachel Wildman, PI 2010 – 2011; Nanette Santoro, PI 2004 – 2010; University of Medicine and Dentistry – New Jersey Medical School, Newark – Gerson Weiss, PI 1994 – 2004; and the University of Pittsburgh, Pittsburgh, PA – Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD – Chhanda Dutta 2016-present; Winifred Rossi 2012–2016; Sherry Sherman 1994 – 2012; Marcia Ory 1994 – 2001; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory: University of Michigan, Ann Arbor – Daniel McConnell (Central Ligand Assay Satellite Services).
SWAN Repository: University of Michigan, Ann Arbor – Siobán Harlow 2013 - Present; Dan McConnell 2011 – 2013; MaryFran Sowers 2000 – 2011.
Coordinating Center: University of Pittsburgh, Pittsburgh, PA – Maria Mori Brooks, PI 2012 - present; Kim Sutton-Tyrrell, PI 2001 – 2012; New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995 – 2001.
Steering Committee: Susan Johnson, Current Chair, Chris Gallagher, Former Chair
We thank the study staff at each site and all the women who participated in SWAN.
Footnotes
Conflict of interest statement: No authors have any disclosures
Data Sharing Statement: SWAN provides access to public use datasets that extend through the tenth annual follow-up visit. Some, but not all, of the data used for this manuscript are contained in the public use data sets. Members of the scientific community who are interested in working with the SWAN data that are not contained in the public use datasets may submit an application to become a SWAN Investigator. Links to each of the public use data sets, as well as instructions for how to apply for SWAN Investigator status, are located on the SWAN web site: http://www.swanstudy.org/swan-research/data-access/. Investigators who require assistance accessing the public use data set or applying for SWAN investigator status may contact the SWAN Coordinating Center at the following: swanaccess@edc.pitt.edu.
References
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. 2007; 357: 266–281. [DOI] [PubMed] [Google Scholar]
- 2.Manson JE, Bassuk SS, Lee IM, Cook NR, Albert MA, Gordon D, Zaharris E, Macfadyen JG, Danielson E, Lin J, et al. The VITamin D and OmegA-3 TriaL (VITAL): rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp Clin Trials. 2012; 33: 159–171.3253961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pittas AG, Dawson-Hughes B, Sheehan PR, Rosen CJ, Ware JH, Knowler WC, Staten MA & Group DdR. Rationale and design of the Vitamin D and Type 2 Diabetes (D2d) study: a diabetes prevention trial. Diabetes Care. 2014; 37: 3227–3234.4237973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Binkley N, Krueger D, Cowgill CS, Plum L, Lake E, Hansen KE, DeLuca HF & Drezner MK. Assay variation confounds the diagnosis of hypovitaminosis D: a call for standardization. J Clin Endocrinol Metab. 2004; 89: 3152–3157. [DOI] [PubMed] [Google Scholar]
- 5.Institute of Medicine (2011) Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC. [Google Scholar]
- 6.Macdonald HM, Reid IR, Gamble GD, Fraser WD, Tang JC & Wood AD. 25-Hydroxyvitamin D Threshold for the Effects of Vitamin D Supplements on Bone Density: Secondary Analysis of a Randomized Controlled Trial. J Bone Miner Res. 2018. [DOI] [PubMed] [Google Scholar]
- 7.Reid IR, Horne AM, Mihov B, Gamble GD, Al-Abuwsi F, Singh M, Taylor L, Fenwick S, Camargo CA, Stewart AW, et al. Effect of monthly high-dose vitamin D on bone density in community-dwelling older adults substudy of a randomized controlled trial. J Intern Med. 2017; 282: 452–460. [DOI] [PubMed] [Google Scholar]
- 8.Looker AC, Johnson CL, Lacher DA, Pfeiffer CM, Schleicher RL & Sempos CT. Vitamin d status: United States, 2001-2006. NCHS Data Brief. 2011: 1–8. [PubMed] [Google Scholar]
- 9.Mitchell DM, Henao MP, Finkelstein JS & Burnett-Bowie SA. Prevalence and predictors of vitamin D deficiency in healthy adults. Endocr Pract. 2012; 18: 914–923.3755751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levy MA, McKinnon T, Barker T, Dern A, Helland T, Robertson J, Cuomo J, Wood T & Dixon BM. Predictors of vitamin D status in subjects that consume a vitamin D supplement. Eur J Clin Nutr. 2015; 69: 84–89. [DOI] [PubMed] [Google Scholar]
- 11.Schleicher RL, Sternberg MR, Lacher DA, Sempos CT, Looker AC, Durazo-Arvizu RA, Yetley EA, Chaudhary-Webb M, Maw KL, Pfeiffer CM, et al. The vitamin D status of the US population from 1988 to 2010 using standardized serum concentrations of 25-hydroxyvitamin D shows recent modest increases. Am J Clin Nutr. 2016; 104: 454–461.4962157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cauley JA, Greendale GA, Ruppert K, Lian Y, Randolph JF Jr., Lo JC, Burnett-Bowie SA & Finkelstein JS. Serum 25 hydroxyvitamin D, bone mineral density and fracture risk across the menopause. J Clin Endocrinol Metab. 2015; 100: 2046–2054.4422899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sowers MF, Crawford SL, Sternfeld B, Morganstein D, Gold EB, Greendale GA, Evans D, Neer R, Matthews K, Sherman S, et al. (2000) SWAN: A multiethnic, community-based cohort study of women and the menopausal transition In Menopause: Biology and pathobiology eds. Lobo R, Kelsey J & Marcus R). Academic Press, San Diego. [Google Scholar]
- 14.Berger C, Greene-Finestone LS, Langsetmo L, Kreiger N, Joseph L, Kovacs CS, Richards JB, Hidiroglou N, Sarafin K, Davison KS, et al. Temporal trends and determinants of longitudinal change in 25-hydroxyvitamin D and parathyroid hormone levels. J Bone Miner Res. 2012; 27: 1381–1389.5101062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jorde R, Sneve M, Hutchinson M, Emaus N, Figenschau Y & Grimnes G. Tracking of serum 25-hydroxyvitamin D levels during 14 years in a population-based study and during 12 months in an intervention study. Am J Epidemiol. 2010; 171: 903–908. [DOI] [PubMed] [Google Scholar]
- 16.Mirfakhraee S, Ayers CR, McGuire DK & Maalouf NM. Longitudinal changes in serum 25-hydroxyvitamin D in the Dallas Heart Study. Clin Endocrinol (Oxf). 2017; 87: 242–248.5561481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park JH, Hong IY, Chung JW & Choi HS. Vitamin D status in South Korean population: Seven-year trend from the KNHANES. Medicine (Baltimore). 2018; 97: e11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caulfield T, Clark MI, McCormack JP, Rachul C & Field CJ. Representations of the health value of vitamin D supplementation in newspapers: media content analysis. BMJ Open. 2014; 4: e006395.4281532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maxmen A Nutrition advice: the vitamin D-lemma. Nature. 2011; 475: 23–25. [DOI] [PubMed] [Google Scholar]
- 20.Kantor ED, Rehm CD, Du M, White E & Giovannucci EL. Trends in Dietary Supplement Use Among US Adults From 1999-2012. JAMA. 2016; 316: 1464–1474.5540241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alzaman NS, Dawson-Hughes B, Nelson J, D’Alessio D & Pittas AG. Vitamin D status of black and white Americans and changes in vitamin D metabolites after varied doses of vitamin D supplementation. Am J Clin Nutr. 2016; 104: 205–214.4919528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gallagher JC, Jindal PS & Smith LM. Vitamin D supplementation in young White and African American women. J Bone Miner Res. 2014; 29: 173–181. [DOI] [PubMed] [Google Scholar]
- 23.Gallagher JC, Sai A, Templin T 2nd & Smith L. Dose response to vitamin D supplementation in postmenopausal women: a randomized trial. Ann Intern Med. 2012; 156: 425–437. [DOI] [PubMed] [Google Scholar]
- 24.Bailey RL, Gahche JJ, Miller PE, Thomas PR & Dwyer JT. Why US adults use dietary supplements. JAMA Intern Med. 2013; 173: 355–361. [DOI] [PubMed] [Google Scholar]
- 25.Dickinson A, Blatman J, El-Dash N & Franco JC. Consumer usage and reasons for using dietary supplements: report of a series of surveys. J Am Coll Nutr. 2014; 33: 176–182. [DOI] [PubMed] [Google Scholar]
- 26.Leidig-Bruckner G, Roth HJ, Bruckner T, Lorenz A, Raue F & Frank-Raue K. Are commonly recommended dosages for vitamin D supplementation too low? Vitamin D status and effects of supplementation on serum 25-hydroxyvitamin D levels--an observational study during clinical practice conditions. Osteoporos Int. 2011; 22: 231–240. [DOI] [PubMed] [Google Scholar]
- 27.Recker R, Lappe J, Davies K & Heaney R. Characterization of perimenopausal bone loss: a prospective study. J Bone Miner Res. 2000; 15: 1965–1973. [DOI] [PubMed] [Google Scholar]
- 28.Finkelstein JS, Brockwell SE, Mehta V, Greendale GA, Sowers MR, Ettinger B, Lo JC, Johnston JM, Cauley JA, Danielson ME, et al. Bone mineral density changes during the menopause transition in a multiethnic cohort of women. J Clin Endocrinol Metab. 2008; 93: 861–868.2266953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greendale GA, Sowers M, Han W, Huang MH, Finkelstein JS, Crandall CJ, Lee JS & Karlamangla AS. Bone mineral density loss in relation to the final menstrual period in a multiethnic cohort: results from the Study of Women’s Health Across the Nation (SWAN). J Bone Miner Res. 2012; 27: 111–118.3378821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Connor C, Glatt D, White L & Revuelta Iniesta R. Knowledge, Attitudes and Perceptions towards Vitamin D in a UK Adult Population: A Cross-Sectional Study. Int J Environ Res Public Health. 2018; 156267199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bouillon R Free or Total 25OHD as Marker for Vitamin D Status? J Bone Miner Res. 2016; 31: 1124–1127. [DOI] [PubMed] [Google Scholar]
- 32.Powe CE, Evans MK, Wenger J, Zonderman AB, Berg AH, Nalls M, Tamez H, Zhang D, Bhan I, Karumanchi SA, et al. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N Engl J Med. 2013; 369: 1991–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoofnagle AN, Eckfeldt JH & Lutsey PL. Vitamin D-Binding Protein Concentrations Quantified by Mass Spectrometry. N Engl J Med. 2015; 373: 1480–1482.4654614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Denburg MR, Hoofnagle AN, Sayed S, Gupta J, de Boer IH, Appel LJ, Durazo-Arvizu R, Whitehead K, Feldman HI, Leonard MB, et al. Comparison of Two ELISA Methods and Mass Spectrometry for Measurement of Vitamin D-Binding Protein: Implications for the Assessment of Bioavailable Vitamin D Concentrations Across Genotypes. J Bone Miner Res. 2016; 31: 1128–1136.4945118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nielson CM, Jones KS, Bouillon R, Osteoporotic Fractures in Men Research G, Chun RF, Jacobs J, Wang Y, Hewison M, Adams JS, Swanson CM, et al. Role of Assay Type in Determining Free 25-Hydroxyvitamin D Levels in Diverse Populations. N Engl J Med. 2016; 374: 1695–1696.4870041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nielson CM, Jones KS, Chun RF, Jacobs JM, Wang Y, Hewison M, Adams JS, Swanson CM, Lee CG, Vanderschueren D, et al. Free 25-Hydroxyvitamin D: Impact of Vitamin D Binding Protein Assays on Racial-Genotypic Associations. J Clin Endocrinol Metab. 2016; 101: 2226–2234.4870848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lutsey PL, Eckfeldt JH, Ogagarue ER, Folsom AR, Michos ED & Gross M. The 25-hydroxyvitamin D3 C-3 epimer: distribution, correlates, and reclassification of 25-hydroxyvitamin D status in the population-based Atherosclerosis Risk in Communities Study (ARIC). Clin Chim Acta. 2015; 442: 75–81.4339618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCambridge J, Witton J & Elbourne DR. Systematic review of the Hawthorne effect: new concepts are needed to study research participation effects. J Clin Epidemiol. 2014; 67: 267–277.3969247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parfitt AM (2005) Vitamin D and the pathogenesis of rickets and osteomalacia In Vitamin D eds. Feldman, Pike JW & Glorieux FH). Elsevier Academic Press, San Diego, pp. 1029–1048. [Google Scholar]
- 40.Priemel M, von Domarus C, Klatte TO, Kessler S, Schlie J, Meier S, Proksch N, Pastor F, Netter C, Streichert T, et al. Bone mineralization defects and vitamin D deficiency: histomorphometric analysis of iliac crest bone biopsies and circulating 25-hydroxyvitamin D in 675 patients. J Bone Miner Res. 2010; 25: 305–312. [DOI] [PubMed] [Google Scholar]
- 41.LeFevre ML & Force USPST. Screening for vitamin D deficiency in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015; 162: 133–140. [DOI] [PubMed] [Google Scholar]
- 42.Manson JE, Brannon PM, Rosen CJ & Taylor CL. Vitamin D Deficiency - Is There Really a Pandemic? N Engl J Med. 2016; 375: 1817–1820. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1
Boxplot showing change in 25-hydroxyvitamin D when stratified by household income (Supp1A), country of origin (Supp1B), language use (Supp1C), educational attainment (Supp1D), insurance status (Supp1E), and BMI category (Supp1F). Δ25(OH)D, change in 25-hydroxyvitamin D.


