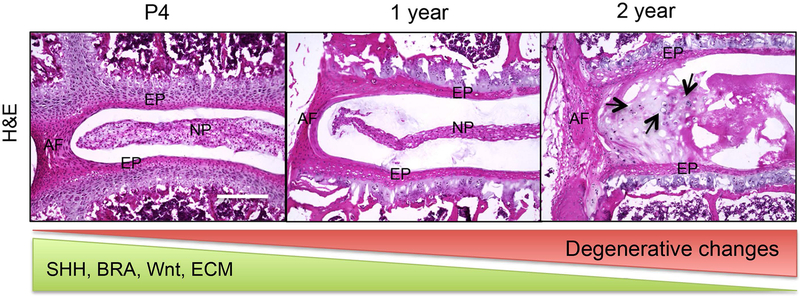
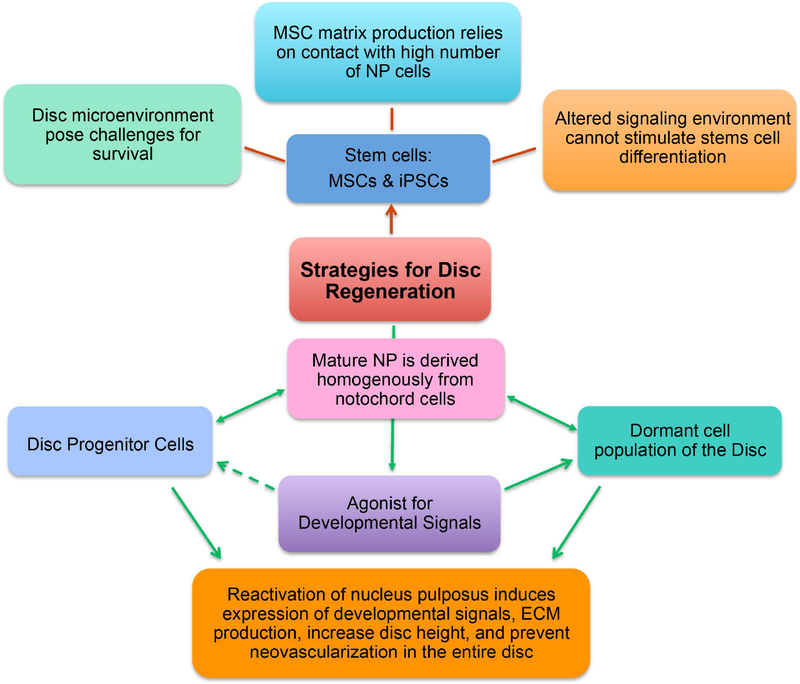
Abstract
Intervertebral discs are cartilaginous joints present between vertebrae. The centers of the intervertebral discs consist of a gelatinous nucleus pulposus derived from the embryonic notochord. With age or injury, intervertebral discs may degenerate, causing neurological symptoms including back pain, which affects millions of people worldwide. Back pain is a multifactorial disorder, and disc degeneration is one of the primary contributing factors. Recent studies in mice have identified the key molecules involved in the formation of intervertebral discs. Several of these key molecules including sonic hedgehog and Brachyury are not only expressed by notochord during development, but are also expressed by neonatal mouse nucleus pulposus cells, and are crucial for postnatal disc maintenance. These findings suggest that intrinsic signals in each disc may maintain the nucleus pulposus microenvironment. However, since expression of these developmental signals declines with age and degeneration, disc degeneration may be related to the loss of these intrinsic signals. In addition, findings from mouse and other mammalian models have identified similarities between the patterning capabilities of the embryonic notochord and young nucleus pulposus cells, suggesting that mouse is a suitable model system to understand disc development and aging. Future research aimed at understanding the upstream regulators of these developmental signals and the modes by which they regulate disc growth and maintenance will likely provide mechanistic insights into disc growth and aging. Further, such findings will likely provide insights relevant to the development of effective therapies for treatment of back pain and reversing the disc degenerative process.
Keywords: lower back pain, disc degeneration, Shh, Brachyury, regeneration
Graphical Abstract
Degeneration of the disc is a major cause of lower back pain. In mice, the molecular profile of the disc changes with age including decline in expression of crucial developmental markers including sonic hedgehog (SHH), Wnts, and Brachyury (BRA) along with their downstream targets like extracellular matrix (ECM). These molecular changes accompany age-associated degenerative changes in the disc cells (indicated by black arrows in 2-year-old mouse lumbar disc). NP, nucleus pulposus; AF, annulus fibrosus; EP, endplate.
INTRODUCTION
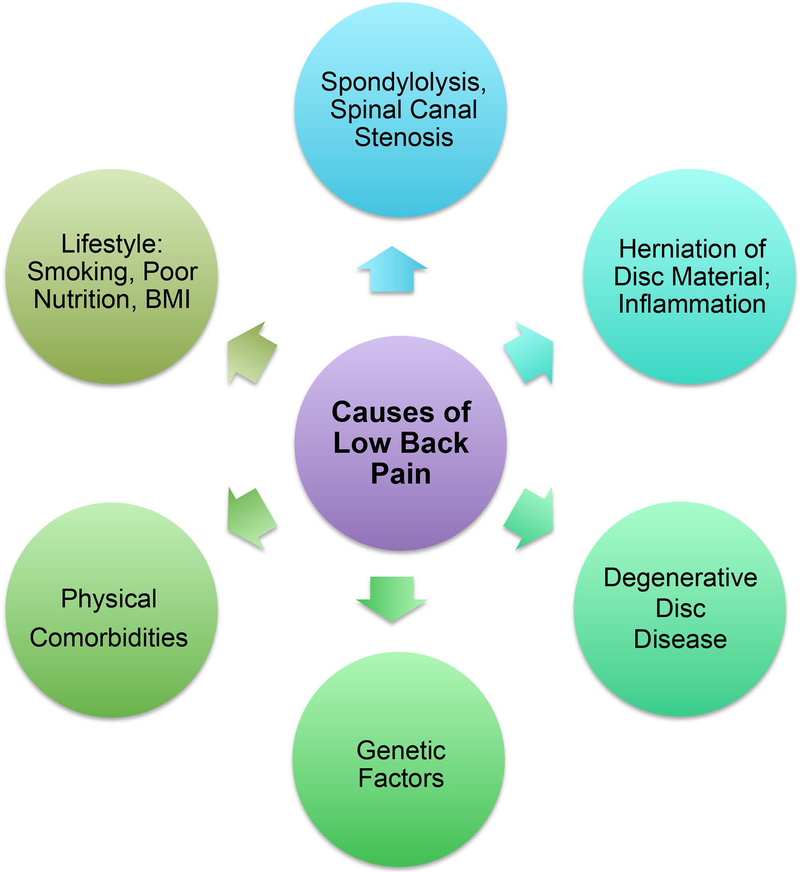
Lower back pain (LBP) is a major neurological disorder that affects more than 540 million people worldwide (Hartvigsen et al., 2018). Although lower back pain affects all age groups, its prevalence increases with age, and is higher in women than in men (Hoy et al., 2012). Due to the growing world population and expanding aged population, the percentage of people likely to be affected by chronic back pain is also increasing. In fact, the prevalence of chronic back pain increased by 54% percent from 1999 to 2015 and is considered a major global burden (HALE Collaborators, 2015; Hartvigsen et al., 2018). Though several causes contribute to lower back pain, some of the most prominent are structural and functional defects in the intervertebral disc in the spine that lead to its degeneration (Fig. 1). Hence, this review will specifically focus on the intervertebral disc and spine. While degenerated discs may be asymptomatic, back pain associated with degenerative disc disease may occur due to damage to the nerve roots (radiculopathy), damage to the peripheral nerves (neuropathy), or inflammation.
Figure 1.
Back pain is a multifactorial disorder. Degeneration of the disc and defects in the spine are significant contributors to back pain.
Structure of the Intervertebral Disc
All chordates have a segmented vertebral column with alternating levels of vertebral bone and intervertebral disc. The notochord and somite, which are mesodermal in origin, are embryonic structures that form the intervertebral disc and spine, respectively. The intervertebral discs are the largest avascular and aneural structures in the body. The disc has three main components: nucleus pulposus, annulus fibrosus, and cartilaginous end plates (Fig. 2). The nucleus pulposus, located in the center of each disc, is filled with a gelatinous proteoglycan and glycosaminoglycan (GAG) rich extracellular matrix (ECM) that keeps it hydrated. The nucleus pulposus is surrounded by orthogonal layers of collagen-rich annulus fibrosus. The top and bottom of each disc is connected to the adjacent vertebrae by cartilaginous endplates. The intervertebral disc performs three primary functions: 1) resists compressive and tension forces during movement; 2) provides flexibility to the spine; and 3) maintains space between vertebral bodies thereby preventing compression or injury to the spinal nerves. Disc degeneration is a multifactorial disorder, and genetics, smoking, physical and mental comorbidities, and poor nutrition are all risk factors (Hartvigsen et al., 2018; Mok et al., 2016). Much of what we know about intervertebral disc development and biology is from the mouse model system. Consequently, we focus mainly on findings from mouse models. We note findings made in other species including humans as appropriate.
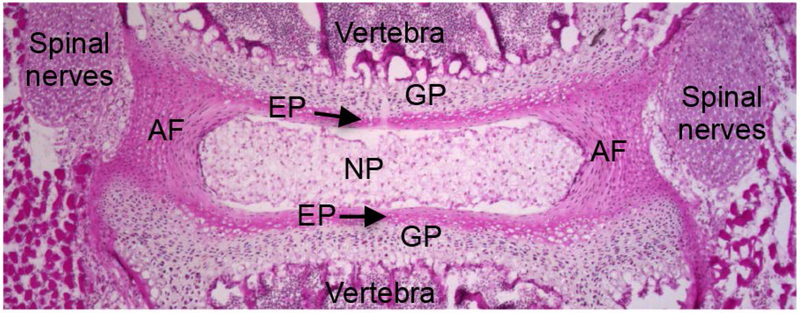
Figure 2.
Hematoxylin and eosin stained mid-coronal section of a two-week-old mouse lumbar spine. The center of the disc has gelatinous nucleus pulposus (NP) cells, surrounded by annulus fibrosus (AF) and located between cartilaginous endplate (EP). EP is adjacent to the growth plate (GP) of the vertebra. Spinal nerves are on the lateral side of the disc.
Spinal Defects Leading to Back Pain
The degenerated disc is marked by hypo-cellularity, loss of hydrated ECM, and reduced disc height (Urban et al., 2003; Vergroesen et al., 2015). These changes result in inadequate transport of metabolites into the nucleus pulposus, altering the metabolic and enzymatic activity of nucleus pulposus cells (Urban et al., 2007). Further, with aging and degeneration, the phenotype of nucleus pulposus cells changes from large, vacuolated notochord cells, known as reticular nucleus pulposus cells, to smaller cells embedded in lacunae (Trout et al., 1982). The latter have been described as “chondrocyte-like cells” and their presence is indicative of disc pathology (Risbud et al., 2015; Trout et al., 1982). In addition, the function of the disc may be negatively affected by herniation (a bulging or protrusion) of the nucleus pulposus, which may lead to spinal canal stenosis, a reduction of the space around the spinal cord, or may result in fissures in the annulus fibrosus, or in displacement of the disc in the spine leading to spondylolysis, a crack or stress fracture in vertebrae, which, if it shifts or slips out of place, leads to spondylolisthesis, another form of spinal canal stenosis (Arnoldi et al., 1976; Hartvigsen et al., 2018). Disc degeneration may also produce fractures in facet joints or in adjacent end plates. Structural defects caused by disc degeneration are associated with increased innervation and vascularization (Johnson et al., 2005; Kauppila, 1995; Nerlich et al., 2007), inflammatory responses triggered by herniation of disc material into the spinal canal (Burke et al., 2002), and compression of the nerve roots and the dorsal horn of the spinal cord leading to back pain. Together, these structural defects alter the biomechanics of spine, which in turn, accelerates the degenerative processes in the discs (Natarajan et al., 2017).
Although disc degeneration and associated lower back pain are leading causes of disability, they are rarely treated (HALE Collaborators, 2015). Available treatments are largely palliative and manage painful symptoms, rather than addressing the underlying causes. For example, to alleviate the symptoms in severe cases, spinal fusion of the vertebral bodies is the accepted treatment. However, due to limited spinal motion, altered mechanics, and increasing the load on neighboring intervertebral discs, spinal fusion often accelerates degeneration of adjacent discs, and may require reoperations (Ghiselli et al., 2004; Hanley et al., 2010; Martin et al., 2007; Zhang et al., 2016). More recently, disc arthroplasty, which is replacement of the intervertebral disc, has been used in efforts to restore disc mobility and function. However, disc arthroplasty is also largely ineffective. As the implants do not fully restore disc biomechanics, involve complicated spinal surgery, and have a propensity to fail over time, their long-term application is limited (Hanley et al., 2010). There is a broad consensus that our limited understanding of the etiology and progression of disc diseases at the cellular and molecular level is an important impediment to the development of effective treatments for disc disorders and associated back pain (Kepler et al., 2013; Mwale, 2013; Seguin et al., 2018). An ideal therapy would both alleviate the painful symptoms, and would restore disc structure and function by directly addressing the underlying molecular causes of disc degeneration. One potential approach to address both issues might be to use the same signals that regulate disc formation during development to induce disc regeneration in patients. Theoretically, this might be accomplished by stimulating the dormant or progenitor disc cells with developmental signals that once regulated disc formation and growth. Alternatively, it might be possible to introduce or transplant exogenous progenitor cells. In either case, the degenerating and hostile microenvironment of the disc is likely to pose significant challenges to efforts aimed at restoring disc function. These efforts will likely require further exploration of the basic biology of the intervertebral disc during healthy and degenerated stages.
Embryonic Origin and Formation of the Disc
Origin and Formation of Nucleus Pulposus
E. H. Weber first described the gelatinous part of the intervertebral disc (Weber, 1827), and later, Luschka referred to it as the “notochord” (Luschka, 1856). Balfour suggested that during development the notochord constricts between the vertebrae and disappears as the vertebrae ossify, but that the unconstricted notochord persists as nucleus pulposus within the intervertebral discs (Balfour, 1881). While Balfour and others (Peacock, 1951, 1952) suggested that notochord persisted as nucleus pulposus throughout the life of the animal, many groups argued that the notochord cells were lost during early postnatal life, and that the intervertebral disc contained only degenerated remnants of the notochord, especially in humans (Gray, 2008; Walmsley, 1953). However, almost 130 years after the first description of the nucleus pulposus, results of fate-mapping studies by Choi et al. employing a Shh-driver mouse line (ShhCre; R26YFP) showed that the nucleus pulposus in an adult mouse is derived from descendants of Shh-expressing cells (Choi et al., 2008) (also shown in Fig. 3a and b). In developmental biology, fate mapping establishes an association between developmentally distinct embryonic cells and their progeny at later stages using cell specific Cre and stop-floxed reporter alleles. Thus, the derivation of the nucleus pulposis from Shh-expressing lineage traced cells suggests their possible origin from the notochord. However, in addition to its expression by the notochord, Shh is also expressed by the floorplate before the formation of notochord sheath (Ribes et al., 2010; Teillet et al., 1998). Later, McCann et al. (McCann et al., 2012), using a NotoCre; R26LacZ mouse line, where LacZ reporter expression is specifically turned “on” in the node, the progenitor of notochord, showed that nucleus pulposus cells are descendants of Spemann and Mangold’s organizer or “node” (Mangold, 1924) and its descendent notochord.
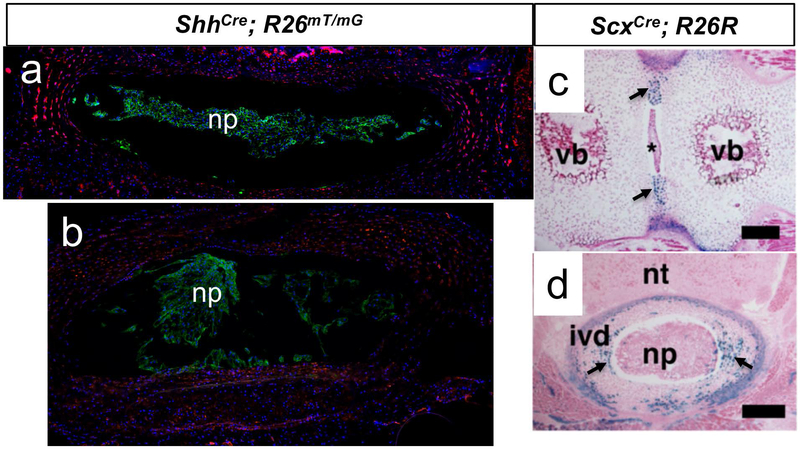
Figure 3.
Embryonic origin of the disc. a. Mid-coronal section, b. Transverse section- through a four-month-old ShhCre; R26mTmG mouse lumbar intervertebral disc. The nucleus pulposus (np) is a homogenous population of descendants of Shh-expressing cells. Panels c and d show intervertebral disc from ScxCre; R26R neonatal mouse where the annulus fibrosus (pointed by arrows) stained positive for X-gal staining. c. Frontal section through a lumbar. d Transverse section through a thoracic intervertebral disc. The asterisk in c shows nucleus pulposus cells. np- nucleus pulposus, vb- vertebral body, nt- neural tube, ivd- intervertebral disc. Panels c and d are adapted from Sugimoto et al., 2013 Genesis, DOI: (10.1002/dvg.22372), with permission from Wiley.
Notochord is not only the precursor of nucleus pulposus cells, it also plays crucial role in patterning of the developing intervertebral disc, which is discussed below. The notochord, a characteristic structure of all Chordates and Urochordates, has two crucial functions — to act as a signaling center and patterning regulator of surrounding embryonic structures, and to provide mechanical and structural support to the embryo [reviewed by (Stemple, 2005)]. The notochord is a midline structure that provides dorsal-ventral and left-right symmetry to the body. The notochord is derived from the node, which is a temporary structure that in mice appears in the dorsal lip at E7.5 and disappears by E9.0 [reviewed by (Stemple, 2005)]. Notochord has large vacuolated cells that contribute to its structural and mechanical properties facilitating anterior-posterior elongation (Adams et al., 1990). The notochord sheath forms around the notochord at E10.0 (Paavola et al., 1980), and is composed of laminin and collagen fibrils (Adams et al., 1990; Parsons et al., 2002). During formation of the nucleus pulposus, the notochord sheath functions to array notochord cells along the anterior-posterior axis (Adams et al., 1990). As the vertebrae undergo chondrogenesis, the notochord migrates and expands in the intervertebral disc forming the nucleus pulposus. Concurrent with this process, the developing annulus fibrosus surrounds the nucleus pulposus. Type II collagen a1 (COL2A1) is synthesized by the notochord and deposited on the notochord sheath (Cheah et al., 1991). Homozygous Col2a1-null mice lack the notochord sheath and the notochord persists in the vertebral bodies of these mutants at birth (Aszodi et al., 1998). The Col2a1-null mice had defects in regional expansion of the notochord to from the nucleus pulposus and formed only the inner part of annulus fibrosus (Aszodi et al., 1998). Results from this study suggest that absence of COL2A1 results in a failure to constrain the internal osmotic swelling pressure required for notochord compression and therefore resulted in failure to form the intervertebral discs (Aszodi et al., 1998). In addition, results from studies using chick embryo showed that the vertebral segmentation involves reciprocal signals between the notochord and sclerotome, both of which are required for proper segmentation (Ward et al., 2018). Results from studies using loss of function alleles for Sox5, Sox6, and Sox9 suggest these HMG-box family members play essential role in notochord survival and maintenance (Barrionuevo et al., 2006; Smits et al., 2001). Results from studies using null allele of both Sox5 and Sox6 (Sox5−/−; Sox6−/−) show defects in the formation of the basement membrane of the notochord sheath as well as skeletal defects (Smits et al., 2001). In the Sox5−/−; Sox6−/− mouse embryos, after formation of the notochord a drastic reduction of notochord cells was observed compared to controls (Smits et al., 2001). Conditional deletion of Sox9 in notochord cells results in the disintegration of the notochord and its loss (Barrionuevo et al., 2006).
Studies using animal models including mouse, Xenopus, fowl, and zebrafish have shown that SHH secreted by notochord is essential for patterning of surrounding structures including the floor plate, neural tube, and somitocoele (Placzek et al., 1996; Stemple, 2005; Teillet et al., 1998). SHH is necessary for induction of sclerotome from the somite by upregulation of Pax1 in the ventral sclerotome (Borycki et al., 1998; Dockter et al., 2000; Marcelle et al., 1999; Murtaugh et al., 1999) and by Pax9 in dorsal sclerotome (Neubuser et al., 1995). SHH also regulates expression of Nkx3.2 and Sox9 (shown in Fig. 4a). BMP signaling antagonizes SHH action, but Noggin and Gremlin1, in turn, antagonize BMP activity (Rider et al., 2010; Stafford et al., 2011). Mutations in Pax1 or Pax9 result in skeletal defects, suggesting the importance of these genes in the development of axial skeleton (Koseki et al., 1993; Wallin et al., 1994). Loss of SHH results in defective notochord structure and function (Chiang et al., 1996). SHH also regulates Laminin alpha1 expression necessary for initiation of basement membrane formation in the paraxial mesoderm (Anderson et al., 2009). However, knocking out the response to SHH in notochord by targeting its receptor (using ShhCre; Smof/f), results in minor changes in laminin expression in the rostral discs, but absence of laminin in caudal discs (Choi et al., 2012). Results from this study shows that SHH is required for notochord sheath formation. Col2a1 expression has not been analyzed in these mutants, although reduced notochord cell proliferation was observed. In addition, spatial variation in rostral and caudal notochord was not evaluated in this study. Thus, the mechanism by which SHH regulates the notochordal sheath and its components is somewhat uncertain. Interestingly, patterning of either the vertebral column or intervertebral discs after E11.5 was not dependent on SHH (Choi et al., 2012). Nonetheless, there remains uncertainty with respect to the molecular and structural integrity and maintenance of discs during the postnatal stage in these Shh mutants.
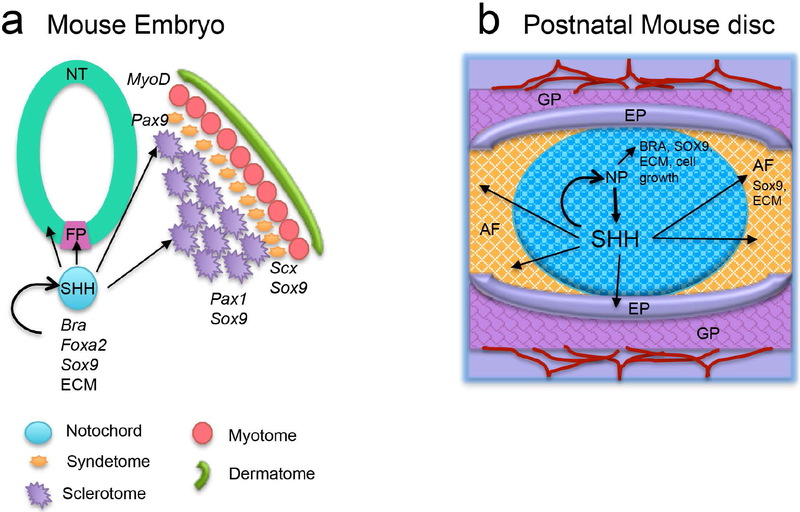
Figure 4.
Similarities between embryonic notochord (a) and postnatal nucleus pulposus (b). Both notochord and nucleus pulposus act as signaling center, and both express SHH which is crucial for maintenance and patterning of themselves and the surrounding structure. The young and healthy disc is vascularized, and blood vessels (red lines) are restricted at the growth plate (GP) in the vertebrae. FP- floor plate, NT- Neural tube, NP- nucleus pulposus, AF- annulus fibrosus, EP- endplate.
Bra or T is a marker of early mesodermal and primitive streak, but as development progresses, its expression is restricted to notochord. Brachyury, meaning “short tail” in Greek, was first identified by Nadine Dobrovolskaia-Zavadskaia in 1927 while screening for loss-of-function mutants during embryonic development (Dobrovolskaia-Zavadskaia, 1927). These mice were called “T” mutants due to their short and kinky tail phenotype. Later, in 1935, Chesley et al. determined that T homozygotes had abnormalities in the mesoderm (Chesley, 1935), and died by mid-gestation due to patterning defects. These mutants failed to form a node, lacked all trunk notochord, and had severe defects in the neural tube, and somites. In contrast, the heterozygous T mutants formed the notochord but failed to maintain it, which resulted in patterning defects of the axial skeleton. These findings suggested that the maintenance and further differentiation of the notochord was dependent on higher levels of Bra (Herrmann et al., 1994). It was not until 1990 that the gene responsible for developmental defects in T mutants was identified as Brachyury (Herrmann et al., 1990). Bra is the founding member of T-box family of transcription factors. T-box proteins act at different points and in different tissues during development (Papaioannou, 2014). Various Bra heterozygotes generated thus far have varying degrees of skeletal defects, which include defects in the pre-sacral vertebrae due to improper notochord formation and elongation (Concepcion et al., 2014; Pennimpede et al., 2012; Zhu et al., 2016). BRA regulates key developmental genes including Foxa2, Sox6, and Sox9, which are crucial for notochord development and function (Lolas et al., 2014). Recent findings have shown that loss of BRA expression at skeletal maturity is associated with the collapse of the sacral intervertebral discs (Bonavita et al., 2018), indicating that Bra may play a critical role in the maintenance of postnatal disc structure and function. Bra in turn is regulated by SHH in the embryonic notochord (Maier et al., 2013) and postnatal nucleus pulposus cells (Bonavita et al., 2018; Dahia et al., 2012).
Origin of Annulus Fibrosus
W. Carlier (Carlier, 1890) first described the cells surrounding the notochord while analyzing the embryonic development of the sheep intervertebral disc. The annulus fibrosus is derived from the somites. The dermomyotome originates from the dorsal epithelium, while the sclerotome originates from the ventral region of the somite starting at stage III of somitogenesis (Christ et al., 1995). Early attempts to fate-map the sclerotome derivatives using a Tbx18Cre driven reporter allele (Tbx18Cre; R26LacZ) suggested that the annulus fibrosus originates from the sclerotome region (Bruggeman et al., 2012). Since the Tbx18Cre driver line is constitutively active, which means the Cre recombinase under Tbx18 promoter is expressed in all Tbx18-expressing cells regardless of developmental stage. Also, since expression of Tbx18, a transcription factor, is not restricted to the sclerotome, Tbx18Cre also drives recombination of the R26LacZ reporter in several embryonic structures including myocardial cells at E9.5 (Cai et al., 2008). The tamoxifen-inducible CreERT or CreERT2 alleles on the other hand are inducible and offer the advantage of time and tissue-specificity. Besides, a few nucleus pulposus cells were observed to be Tbx18+ (Bruggeman et al., 2012), indicating that the Tbx18Cre allele used in this study is not sclerotome-specific. More recently, a new compartment of the somite located between the sclerotome and myotome was identified. This compartment, which was termed the “syndetome,” originates from the dorsolateral domain of early sclerotome (Brent et al., 2003; Schweitzer et al., 2001). Since the Tbx18Cre driver line is not inducible rather it is constitutively active, Tbx18Cre mediated R26LacZ may also recombine in syndetome precursor cells. Hence, it is not clear whether the Tbx18+ cells in the annulus fibrosus are derivatives of the syndetome or of the sclerotome. This uncertainty was resolved by analysis of scleraxis (Scx) expression. Scx is a basic helix-loop-helix transcription factor, and serves as a characteristic marker of the syndetome (Brent et al., 2003; Schweitzer et al., 2001). Lineage tracing of syndetome-derived cells using a ScxCre; R26LacZ reporter line convincingly showed that the entire annulus fibrosus was derived from an Scx-expressing cell lineage (Sugimoto, Takimoto, Hiraki, et al., 2013) (Fig. 3b and c). In this regard, ScxGFP expression was detected as early as E12.5 in the annulus fibrosus of the mouse embryo but was absent in vertebral bodies (Sugimoto, Takimoto, Akiyama, et al., 2013). The absence of Scx+ cells in vertebral bodies demonstrated the specificity of the Scx reporter, effectively tracing the origin of the annulus fibrosus cells to the syndetome, which is a subdivision of sclerotome. In addition, ScxGFP expression was also detected in postnatal mouse annulus fibrosus (Torre et al., 2018), these findings suggest that Scx continues to be a molecular marker for annulus fibrosus.
Origin of End Plate
The embryonic origin of cartilaginous endplate is not well studied. However, the endplate is not formed by the descendants of Shh- or Noto-expressing populations (Choi et al., 2008; McCann et al., 2012), indicating that endplate does not originate from the notochord. Also, descendants of Scx-expressing cells were not found in the endplate, indicating that it is not a derivative of the syndetome (Sugimoto, Takimoto, Hiraki, et al., 2013). The best evidence for the origin of endplate is provided by analysis of Tbx18- lineage tracing, as it was found that cellular descendants of Tbx18-expressing cells were present in endplate, indicating that it is derived from somite and possibly the sclerotome (Bruggeman et al., 2012).
Nucleus Pulposus as a Signaling Center in Postnatal Disc
The neonatal mouse nucleus pulposus synthesizes and responds to SHH and Wnt ligands, retains an “organizer” function, and acts as a signaling center by regulating the expression of BRA, SOX9, and ECM genes (Fig. 4b) (Dahia et al., 2009a, 2009b; Dahia et al., 2012; Winkler et al., 2014). In addition to SHH and Wnt, the disc also responds to other developmental signals including BMPs, TGFβ, and PTHrP during neonatal stages (Dahia et al., 2009a). Blockade of hedgehog signaling using cyclopamine in vitro or conditional targeting in vivo resulted in the loss of GLI1 and PTCH1 in both nucleus pulposus and annulus fibrosus cells indicating that both these compartments of the disc are targets of hedgehog signaling (Dahia et al., 2012). Blockade of Shh signaling deregulated the response to canonical Wnt, BMP, and TGFβ signaling in the neonatal mouse disc. In addition, SHH blockade resulted in a reduction in BRA expression, clumping and loss of proliferation of nucleus pulposus cells, and disorganization of the annulus fibrosus layers. These structural changes were associated with a decline in expression of SOX9 and ECM proteins COL1A1, COL2A1, ACAN, and GAGs including chondroitin sulfate in both, the nucleus pulposus and annulus fibrosus cells. These effects were rescued by expression of recombinant SHH in in vitro studies, suggesting that the observed changes could be specifically attributed to loss of hedgehog signaling. In addition, the fact that discs cultured up to five days in serum-free medium were capable of maintaining their differentiated state suggests that endogenous signals, which are mainly secreted by nucleus pulposus cells, are sufficient for maintenance of the disc (Fig. 4b).
Results from recent study showed that Shh signaling antagonizes canonical Wnt signaling in neonatal mouse disc (Dahia et al., 2012), but Wnt signaling positively regulates targets of Shh signaling (Winkler et al., 2014). Moreover, expression of Shh and it’s downstream targets decrease naturally from postnatal day four (P4) to one year of age in mouse lumbar discs (Winkler et al., 2014). In mice, this process is physiologically accelerated in the sacrum, where loss of SHH is associated with the collapse of the sacral discs during formation of the sacrum at around 12 to 14 weeks of age (Bonavita et al., 2018). Interestingly, conditional reactivation of hedgehog signaling, downstream of the ligand, in a subset of nucleus pulposus cells by turning “on” a constitutively active allele of Smoothened (Jeong et al., 2004) not only increased expression of SHH, but also stimulated the expression of its downstream targets including PTCH1 and ECM proteins (Bonavita et al., 2018). This was achieved using an inducible driver line, Ck19CreERT2 (Means et al., 2008) that was observed to be specific for nucleus pulposus cells in the spine (Bonavita et al., 2018). In this model, activation of hedgehog signaling restored the healthy reticular shape of nucleus pulposus cells and organized lamellae of annulus fibrosus, while also reducing vascularization of the annulus fibrosus (Bonavita et al., 2018). This observation indicates that SHH or more broadly hedgehog signaling, has the potential to therapeutically reactivate dormant nucleus pulposus cells, which in turn could increase expression of SHH and ECM proteins. Therefore, at least in this mouse model, targeting a developmentally crucial signaling pathway has the potential to reactivate or regenerate the entire disc. The potential of this approach will most certainly require testing in larger animal models in which the intervertebral disc is more comparable to that of humans.
Molecular and Pathological Changes in the Disc with Aging and Degeneration
In the mouse model, the expression of SHH and Wnt ligands by nucleus pulposus cells declines from P4 to one year of age (Dahia et al., 2009a; Winkler et al., 2014). In this same time frame, the responses to key developmental signaling pathways including Shh, Wnt, BMP, TGFβ, and PTHrP in all components of the disc are also reduced (Dahia et al., 2009a). Expression of BRA, a downstream target of SHH, in the mouse lumbar and sacral disc is also reduced with age (Bonavita et al., 2018; Dahia et al., 2012; Winkler et al., 2014). Interestingly, expression of BRA is not uniform among nucleus pulposus cells, which suggests cellular heterogeneity amongst the nucleus pulposus cells during postnatal growth and aging (Dahia et al., 2009b). It is possible that some nucleus pulposus cells turn Bra “off” as they age, or alternatively, it is possible that nucleus pulposus cells transition between Bra-expressing and non-expressing states. Examination of fixed tissue does not rule out either of these alternatives (Dahia et al., 2009b). A conclusive determination would require lineage analysis. Expression of Bra was reported in postnatal human nucleus pulposus cells (Minogue et al., 2010; Risbud et al., 2010). Results of some studies showed that Bra expression does not change with degeneration in the human nucleus pulposus (Risbud et al., 2010), while others showed that it declines with both age and degeneration (Richardson et al., 2017). Based on these observations, it is possible that Bra serves an essential role in postnatal disc maintenance and requires further investigation.
With age, the mouse intervertebral disc undergoes pathological changes in the phenotype of cells occupying the nucleus pulposus space accompanied by disorganization and loss of the annulus fibrosus layers [Fig. 5, adapted from (Winkler et al., 2014)]. Loss of the notochordal population in the disc is associated with the onset of disc degeneration during the life of many mammalian species, suggesting that these cells are involved in maintenance and repair of the disc [reviewed in (Hunter et al., 2003)]. Histological analysis of degenerating discs has delineated a progression from large reticular notochord cells into smaller chondrocyte-like cells located in lacunae, and subsequently, progression to fibrocartilaginous cells (F. Yang et al., 2009). While some investigators maintain that all nucleus pulposus cells including chondrocyte-like cells are derivatives of the notochord, others argue that with aging and degeneration, cells of non-notochordal origin infiltrate the disc [reviewed by (Mwale, 2013; Risbud et al., 2015)]. These conflicting views center on the disappearance of cells containing characteristic large vacuoles from the nucleus pulposus compartment during postnatal growth and aging, and the appearance in this compartment of cells within the lacunae that have the appearance of hypertrophic chondrocytes of the growth plate. Although notochord cells express specific markers of chondrocyte including Sox9, Col2a1, and Acan [reviewed by (Stemple, 2005)], the change in cellular phenotype is intriguing. In rat and bovine model system, gene profiling studies comparing the nucleus pulposus to cartilage or chondrocytes have identified common markers, but have not identified markers that are exclusive to chondrocytes and absent in the nucleus pulposus (Cui et al., 2010; Lee et al., 2007). The similarity in gene expression between nucleus pulposus and cartilage indicates the cartilaginous nature of nucleus pulposus, and is also characteristic of its precursor, the notochord [reviewed in (Stemple, 2005)]. Notochord markers CK-8, CK-18, CK-19, and Galectin-3 are expressed in human lumbar intervertebral discs of all ages, and to a variable extent during disc degeneration (Weiler et al., 2010). The subset of human disc cells that express these markers decreases with age (Richardson et al., 2017), suggesting either that the notochord derived nucleus pulposus cells are lost during aging, or that nucleus pulposus cells turn “off” expression of notochord markers as they differentiate and degenerate during aging. It is possible that as the disc ages and cell number decreases, the consequent reduction in cell-cell contact and signaling may result in insufficient signaling for the maintenance of nucleus pulposus cells. Moreover, as nucleus pulposus cells become isolated within lacunae, the lacunar microenvironment may undergo changes leading to loss of maintenance. It is also possible that notochord cells acquire alternate phenotypes while maintaining some aspects of their prior molecular profile and inductive properties, which requires further investigation. For the success of disc therapy, it will be important to accurately ascertain the origin and identity of the cells observed in degenerate discs. One way to address this is by lineage tracing. These studies would have the capacity to discriminate between the differentiation model and the lineage replacement model of nucleus pulposus degeneration. The mouse model is well suited for these genetic studies. In mice, chondrocyte-like cells are reported by two years of age (Fig. 5) (Winkler et al., 2014), suggesting similarity with age-related degeneration observed in human discs.
Figure 5.
Morphological changes in the mouse disc with normal aging. With aging, the expression of developmental signals and their targets like extracellular matrix (ECM) are reduced. Fewer nucleus pulposus cells that appear phenotypically different from the young nucleus pulposus cells are seen. Arrows point to smaller, “chondrocyte-like cells” encased within lacunae. The surrounding annulus fibrosus, which is regulated by signals from nucleus pulposus, also becomes thinner and disorganized with aging. NP- nucleus pulposus, AF- annulus fibrosus, EP- endplate. Adapted from Winkler et al., 2014, https://doi.org/10.1371/journal.pone.0098444.g004, with permission from PlosOne.
Potential Therapies for Disc Regeneration and Treatment of Back Pain
Stem cells
Because of their potential to differentiate into adipocytes, osteoblasts, and chondrocytes, it has been proposed that mesenchymal stem cells (MSCs) might be used to repair the intervertebral disc (Pittenger et al., 1999). However, due to its avascular structure, the disc has a harsh microenvironment characterized by anaerobic cellular metabolism, high lactic acid, low pH, hypoxia, and nutrient deprivation. Moreover, degraded ECM, and changing intervertebral disc conditions would be likely to alter the fate of nucleus pulposus progenitors (Mizrahi et al., 2013). The success of disc therapy with MSCs relies on the capacity of these cells to survive and to repopulate the disc with appropriately differentiated cells under harsh conditions (Fig. 6). The mechanism by which MSCs might regenerate the disc requires further analysis. Theoretically, MSCs may not only differentiate into nucleus pulposus cells, but they may also facilitate generation of an appropriate microenvironment through secretion of signaling molecules or by enhancing cell-cell contact, which stimulates regeneration from the remaining endogenous disc cells. Results from studies using noncontact and contact co-culture systems showed that contact between MSCs and a large numbers of nucleus pulposus cells is required for expression of ECM markers COL2A1 and ACAN (S. H. Yang et al., 2008). Because of the hypo-cellularity of degenerated discs (Maroudas et al., 1975), the effectiveness of this approach may be limited. The advantage of induced pluripotent stem cells (iPSCs) from a patient’s somatic cells is their pluripotency and patient-specificity. However, for their successful transition into notochord marker-expressing nucleus pulposus cells, iPSCs also rely on the disc microenvironment including the availability of FGF, EGF, VEGF, IGF-1, and the like, along with abundant ECM (Liu et al., 2015). While the differentiation of human iPSCs (hiPSCs) into nucleus pulposus cells that express notochord markers is undoubtedly impressive, their application to a degenerated state is currently limited, and numerous hurdles remain. For example, with aging, changes in the local microenvironment of the disc or changes in endogenous signals may limit regeneration from stem cells.
Figure 6.
Potential strategies for regeneration of the disc and cure for back pain. NP- nucleus pulposus, MSCs- Mesenchymal Stem Cells, iPSCs- induced pluripotent stem cells.
Progenitor cells
An alternative approach for disc therapy might make use of the few persistent disc progenitor cells. A recent study subdivided the nucleus pulposus cell population based on expression of disialoganglioside 2 (GD2) and tyrosine kinase receptor (Tie2) (Sakai et al., 2012). Using these two markers, nucleus pulposus cells were classified as belonging to one of four subtypes: dormant stem cells, self-renewing stem cells, committed nucleus pulposus progenitor cells, and mature nucleus pulposus cells (Sakai et al., 2012). As disc degeneration progresses, the population of dormant and self-renewing stem cells substantially declines (Sakai et al., 2012). The presence of progenitor cells was also reported in human disc samples (Risbud et al., 2007). In a study of skeletally mature 12-week-old mice, it was found that only a subset of nucleus pulposus cells expressed the notochord marker CK19 in the sacral disc (Bonavita et al., 2018), supplying further evidence of increasing molecular heterogeneity in nucleus pulposus cells with aging. It is not yet known if this age-related increase in molecular heterogeneity among sacral disc nucleus pulposus cells is a feature of other regions of the spine. With respect to disc therapy, it will be important to determine whether the nucleus pulposus cells that maintain expression of key notochord markers have the capacity to act as disc progenitor cells.
Circulating and Local Developmental Signals
Efforts toward development of therapeutic approaches for disc regeneration have begun shifting toward recapitulating the microenvironment of the young nucleus pulposus, stimulating further studies of disc development. Aging is a physiological phenomenon that affects the entire body at an almost synchronized temporal pace. One of the approaches to using developmental processes to combat aging has been to test the potential of adult nucleus pulposus cells to respond to the secretory factors or signaling molecules in notochord cell-conditioned medium. Results have shown that notochord cell-conditioned medium has anabolic effects on matrix synthesis by human MSCs and chondrocyte-like cells from bovine and canine discs (Bach et al., 2016; Purmessur et al., 2011). An increase in production of SOX9, COL2A1, and ACAN was observed among nucleus pulposus cells cultured in notochord cell-conditioned medium under micro mass culture induced, hypoxic conditions (Purmessur et al., 2011). Though promising, the specific soluble factor(s) in the notochord cell-conditioned medium responsible for these effects are unknown. A plausible hypothesis stemming from these data is that developmental signals secreted by the notochord cells under these culture conditions stimulate matrix production.
Stimulation of the dormant native cells of the disc using developmental signals like SHH is one potential direction for disc therapy (Fig. 6). As an example, in vitro stimulation of adult mouse disc with the small molecule Wnt agonist BIO activated hedgehog signaling and its downstream targets BRA and CK19 in nucleus pulposus cells, and stimulated ECM production throughout the disc (Winkler et al., 2014). In other studies it was found that reactivation of hedgehog signaling in only a sub-set of nucleus pulposus cells of the collapsed sacral disc reactivated the entire sacral disc (Bonavita et al., 2018), suggesting that the same development signals that were important for patterning at embryonic stages and during disc formation have potential for disc rejuvenation.
CONCLUSION AND PERSPECTIVE
The embryonic notochord contributes to the core of each intervertebral disc in the spine (Choi et al., 2008; McCann et al., 2012). In postnatal stages, the presence of identifiable large and vacuolated notochord cells in the nucleus pulposus is associated with a young and healthy phenotype (Hunter et al., 2003). However, loss of these identifiable notochord cells during aging is associated with degeneration of the disc, a major cause of back pain. Past and recent studies using mouse, xenopus, zebrafish, and chicken models have contributed significantly to our understanding of the formation of the notochord and its role in embryogenesis. Further, results from recent lineage-tracing studies in mice have solved a long-standing mystery regarding the origin of the different components of the disc (Choi et al., 2008; McCann et al., 2012; Schweitzer et al., 2001; Sugimoto, Takimoto, Akiyama, et al., 2013; Sugimoto, Takimoto, Hiraki, et al., 2013). However, we still face a conundrum regarding the source of small “chondrocyte-like cells” observed with disc aging and degeneration (Hunter et al., 2003; Mwale, 2013; Risbud et al., 2015; Trout et al., 1982). The nucleus pulposus, like its predecessor notochord, also continues to express critical molecules Shh and Bra and others that are crucial for both embryonic development and neonatal disc growth and maintenance (Bonavita et al., 2018; Dahia et al., 2009a, 2009b; Dahia et al., 2012). With age, nucleus pulposus cells also develop cellular heterogeneity, and maybe only a subset of them are capable of maintaining the disc microenvironment and health (Bonavita et al., 2018; Dahia et al., 2009b; Risbud et al., 2007; Sakai et al., 2012). Under this hypothesis, stimulating the progenitor nucleus pulposus cells without introducing exogenously sourced cells may yield better outcomes (Fig. 6), as was shown in mouse sacral discs (Bonavita et al., 2018). However, critical questions going forward are: Why does the expression of these developmental signals decline with age; Does their decline cause the disc to age? In addition, the fact that intervertebral discs are separated by vertebral bodies indicates that circulating or systemic signals are responsible for synchronized growth and aging of every disc in the spine and requires further investigation. Developing a successful therapy for back pain may require a multi-disciplinary approach that involves altering the disc microenvironment to facilitate activation of dormant or progenitor disc cells, as well as de-differentiation of aged cells to restore disc function.
The postnatal nucleus pulposus in each disc acts as an embryonic signaling center similar to notochord. The notochord expresses Sonic hedgehog (Shh) at E7.5 in the mouse embryo. Postnatal and up to one-year-old nucleus pulposus cells from mouse lumbar discs continue to express molecular markers of the notochord including Shh (Dahia et al., 2009a, 2009b; Dahia et al., 2012; DiPaola et al., 2005; Winkler et al., 2014) and Bra (or T) (Dahia et al., 2009b; Dahia et al., 2012; Winkler et al., 2014), suggesting a potential role of these developmental signals in the maintenance of the postnatal disc. BRA was later also identified in the human nucleus pulposus (Minogue et al., 2010; Richardson et al., 2017). Shh is a key regulator of embryonic patterning, and continues to regulate the components of the postnatal disc (Fig. 4). Point mutations in SHH and BRA cause vertebral defects in humans including defects in the formation of caudal vertebrae and sacral agenesis (Fontanella et al., 2016; Ghebranious et al., 2008; Horn et al., 2004; Linhares et al., 2014; Postma et al., 2014; Vargas et al., 1998). This indicates the translational importance of SHH and BRA in disc formation and maintenance. Understanding the formation and function of the postnatal nucleus pulposus in animal models may provide critical insights on whether it continues to act as a “signaling center” and maintains the same patterning properties as its precursor, the notochord.
Acknowledgments
FUNDING SOURCES
The authors’ research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R01AR065530 (to CLD), research awards from the Gerstner Family Foundation (to CLD), and from S & L Marx Foundation award (to CLD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
No conflicts to declare
REFERENCES
- Adams DS, Keller R, & Koehl MA (1990). The mechanics of notochord elongation, straightening and stiffening in the embryo of Xenopus laevis. Development, 110(1), 115–130. [DOI] [PubMed] [Google Scholar]
- Anderson C, Thorsteinsdottir S, & Borycki AG (2009). Sonic hedgehog-dependent synthesis of laminin alpha1 controls basement membrane assembly in the myotome. Development, 136(20), 3495–3504. doi: 10.1242/dev.036087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnoldi CC, Brodsky AE, Cauchoix J, Crock HV, Dommisse GF, Edgar MA, . . . Wiltse LL (1976). Lumbar spinal stenosis and nerve root entrapment syndromes. Definition and classification. Clin Orthop Relat Res(115), 4–5. [PubMed] [Google Scholar]
- Aszodi A, Chan D, Hunziker E, Bateman JF, & Fassler R (1998). Collagen II is essential for the removal of the notochord and the formation of intervertebral discs. J Cell Biol, 143(5), 1399–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach FC, de Vries SA, Riemers FM, Boere J, van Heel FW, van Doeselaar M, . . . Tryfonidou MA (2016). Soluble and pelletable factors in porcine, canine and human notochordal cell-conditioned medium: implications for IVD regeneration. Eur Cell Mater, 32, 163–180. [DOI] [PubMed] [Google Scholar]
- Balfour FM (1881). A treatise on comparative embryology (Vol. II). London: Macmillan. [Google Scholar]
- Barrionuevo F, Taketo MM, Scherer G, & Kispert A (2006). Sox9 is required for notochord maintenance in mice. Dev Biol, 295(1), 128–140. doi: 10.1016/j.ydbio.2006.03.014 [DOI] [PubMed] [Google Scholar]
- Bonavita R, Vincent K, Pinelli R, & Dahia CL (2018). Formation of the sacrum requires down-regulation of sonic hedgehog signaling in the sacral intervertebral discs. Biol Open. doi: 10.1242/bio.035592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borycki AG, Mendham L, & Emerson CP Jr. (1998). Control of somite patterning by Sonic hedgehog and its downstream signal response genes. Development, 125(4), 777–790. [DOI] [PubMed] [Google Scholar]
- Brent AE, Schweitzer R, & Tabin CJ (2003). A somitic compartment of tendon progenitors. Cell, 113(2), 235–248. [DOI] [PubMed] [Google Scholar]
- Bruggeman BJ, Maier JA, Mohiuddin YS, Powers R, Lo Y, Guimaraes-Camboa N, . . . Harfe BD (2012). Avian intervertebral disc arises from rostral sclerotome and lacks a nucleus pulposus: implications for evolution of the vertebrate disc. Dev Dyn, 241(4), 675–683. doi: 10.1002/dvdy.23750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke JG, Watson RW, McCormack D, Dowling FE, Walsh MG, & Fitzpatrick JM (2002). Intervertebral discs which cause low back pain secrete high levels of proinflammatory mediators. J Bone Joint Surg Br, 84(2), 196–201. [DOI] [PubMed] [Google Scholar]
- Cai CL, Martin JC, Sun Y, Cui L, Wang L, Ouyang K, . . . Evans SM (2008). A myocardial lineage derives from Tbx18 epicardial cells. Nature, 454(7200), 104–108. doi: 10.1038/nature06969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier EW (1890). Fate of the Notochord and Development of the Intervertebral Disc in the Sheep, with Observations on the Structure of the Adult Disc in these Animals. J Anat Physiol, 24(Pt 4), 573–584 571. [PMC free article] [PubMed] [Google Scholar]
- Cheah KS, Lau ET, Au PK, & Tam PP (1991). Expression of the mouse alpha 1(II) collagen gene is not restricted to cartilage during development. Development, 111(4), 945–953. [DOI] [PubMed] [Google Scholar]
- Chesley P (1935). Development of the short-tailed mutant in the house mouse. J.Exp.Zol, 70, 429–435. [Google Scholar]
- Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, & Beachy PA (1996). Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature, 383(6599), 407–413. doi: 10.1038/383407a0 [DOI] [PubMed] [Google Scholar]
- Choi KS, Cohn MJ, & Harfe BD (2008). Identification of nucleus pulposus precursor cells and notochordal remnants in the mouse: implications for disk degeneration and chordoma formation. Dev Dyn, 237(12), 3953–3958. doi: 10.1002/dvdy.21805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KS, Lee C, & Harfe BD (2012). Sonic hedgehog in the notochord is sufficient for patterning of the intervertebral discs. Mech Dev, 129(9–12), 255–262. doi: 10.1016/j.mod.2012.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ B, & Ordahl CP (1995). Early stages of chick somite development. Anat Embryol (Berl), 191(5), 381–396. [DOI] [PubMed] [Google Scholar]
- Concepcion D, & Papaioannou VE (2014). Nature and extent of left/right axis defects in T(Wis) /T(Wis) mutant mouse embryos. Dev Dyn, 243(8), 1046–1053. doi: 10.1002/dvdy.24144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Yu J, Urban JP, & Young DA (2010). Differential gene expression profiling of metalloproteinases and their inhibitors: a comparison between bovine intervertebral disc nucleus pulposus cells and articular chondrocytes. Spine (Phila Pa 1976), 35(11), 1101–1108. doi: 10.1097/BRS.0b013e3181c0c727 [DOI] [PubMed] [Google Scholar]
- Dahia CL, Mahoney EJ, Durrani AA, & Wylie C (2009a). Intercellular signaling pathways active during intervertebral disc growth, differentiation, and aging. Spine (Phila Pa 1976), 34(5), 456–462. doi: 10.1097/BRS.0b013e3181913e98 [DOI] [PubMed] [Google Scholar]
- Dahia CL, Mahoney EJ, Durrani AA, & Wylie C (2009b). Postnatal growth, differentiation, and aging of the mouse intervertebral disc. Spine (Phila Pa 1976), 34(5), 447–455. doi: 10.1097/BRS.0b013e3181990c64 [DOI] [PubMed] [Google Scholar]
- Dahia CL, Mahoney E, & Wylie C (2012). Shh signaling from the nucleus pulposus is required for the postnatal growth and differentiation of the mouse intervertebral disc. PLoS One, 7(4), e35944. doi: 10.1371/journal.pone.0035944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPaola CP, Farmer JC, Manova K, & Niswander LA (2005). Molecular signaling in intervertebral disk development. J Orthop Res, 23(5), 1112–1119. doi: 10.1016/j.orthres.2005.03.008 [DOI] [PubMed] [Google Scholar]
- Dobrovolskaia-Zavadskaia N (1927). Sur la mortification spontanee de la queue chez la souris nouveau-nee et sur l’existence d’un caractere hereditaire ‘non viable’. C.R. Hebd. Seanc. Soc. Biol, 97, 114–116. [Google Scholar]
- Dockter J, & Ordahl CP (2000). Dorsoventral axis determination in the somite: a re-examination. Development, 127(10), 2201–2206. [DOI] [PubMed] [Google Scholar]
- Fontanella F, van Maarle MC, Robles de Medina P, Oostra RJ, van Rijn RR, Pajkrt E, & Bilardo CM (2016). Prenatal Evidence of Persistent Notochord and Absent Sacrum Caused by a Mutation in the T (Brachyury) Gene. Case Rep Obstet Gynecol, 2016, 7625341. doi: 10.1155/2016/7625341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghebranious N, Blank RD, Raggio CL, Staubli J, McPherson E, Ivacic L, . . . Giampietro PF (2008). A missense T (Brachyury) mutation contributes to vertebral malformations. J Bone Miner Res, 23(10), 1576–1583. doi: 10.1359/jbmr.080503 [DOI] [PubMed] [Google Scholar]
- Ghiselli G, Wang JC, Bhatia NN, Hsu WK, & Dawson EG (2004). Adjacent segment degeneration in the lumbar spine. J Bone Joint Surg Am, 86-A(7), 1497–1503. [DOI] [PubMed] [Google Scholar]
- Gray H (2008). Gray’s anatomy (FORTIETH EDITION ed.): Churchill Livingstone ELSEVIER. [Google Scholar]
- HALE Collaborators, G. B. D. DALYs (2015). Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: quantifying the epidemiological transition. Lancet, 386(10009), 2145–2191. doi: 10.1016/S0140-6736(15)61340-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley EN Jr., Herkowitz HN, Kirkpatrick JS, Wang JC, Chen MN, & Kang JD (2010). Debating the value of spine surgery. J Bone Joint Surg Am, 92(5), 1293–1304. doi: 10.2106/JBJS.I.01439 [DOI] [PubMed] [Google Scholar]
- Hartvigsen J, Hancock MJ, Kongsted A, Louw Q, Ferreira ML, Genevay S, . . . Lancet Low Back Pain Series Working, Group. (2018). What low back pain is and why we need to pay attention. Lancet. doi: 10.1016/S0140-6736(18)30480-X [DOI] [PubMed] [Google Scholar]
- Herrmann BG, & Kispert A (1994). The T genes in embryogenesis. Trends Genet, 10(8), 280–286. [DOI] [PubMed] [Google Scholar]
- Herrmann BG, Labeit S, Poustka A, King TR, & Lehrach H (1990). Cloning of the T gene required in mesoderm formation in the mouse. Nature, 343(6259), 617–622. doi: 10.1038/343617a0 [DOI] [PubMed] [Google Scholar]
- Horn D, Tonnies H, Neitzel H, Wahl D, Hinkel GK, von Moers A, & Bartsch O (2004). Minimal clinical expression of the holoprosencephaly spectrum and of Currarino syndrome due to different cytogenetic rearrangements deleting the Sonic Hedgehog gene and the HLXB9 gene at 7q36.3. Am J Med Genet A, 128A(1), 85–92. doi: 10.1002/ajmg.a.30031 [DOI] [PubMed] [Google Scholar]
- Hoy D, Bain C, Williams G, March L, Brooks P, Blyth F, . . . Buchbinder R (2012). A systematic review of the global prevalence of low back pain. Arthritis Rheum, 64(6), 2028–2037. doi: 10.1002/art.34347 [DOI] [PubMed] [Google Scholar]
- Hunter CJ, Matyas JR, & Duncan NA (2003). The notochordal cell in the nucleus pulposus: a review in the context of tissue engineering. Tissue Eng, 9(4), 667–677. doi: 10.1089/107632703768247368 [DOI] [PubMed] [Google Scholar]
- Jeong J, Mao J, Tenzen T, Kottmann AH, & McMahon AP (2004). Hedgehog signaling in the neural crest cells regulates the patterning and growth of facial primordia. Genes Dev, 18(8), 937–951. doi: 10.1101/gad.1190304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson WE, Caterson B, Eisenstein SM, & Roberts S (2005). Human intervertebral disc aggrecan inhibits endothelial cell adhesion and cell migration in vitro. Spine (Phila Pa 1976), 30(10), 1139–1147. [DOI] [PubMed] [Google Scholar]
- Kauppila LI (1995). Ingrowth of blood vessels in disc degeneration. Angiographic and histological studies of cadaveric spines. J Bone Joint Surg Am, 77(1), 26–31. [DOI] [PubMed] [Google Scholar]
- Kepler CK, Ponnappan RK, Tannoury CA, Risbud MV, & Anderson DG (2013). The molecular basis of intervertebral disc degeneration. Spine J, 13(3), 318–330. doi: 10.1016/j.spinee.2012.12.003 [DOI] [PubMed] [Google Scholar]
- Koseki H, Wallin J, Wilting J, Mizutani Y, Kispert A, Ebensperger C, . . . Balling R (1993). A role for Pax-1 as a mediator of notochordal signals during the dorsoventral specification of vertebrae. Development, 119(3), 649–660. [DOI] [PubMed] [Google Scholar]
- Lee CR, Sakai D, Nakai T, Toyama K, Mochida J, Alini M, & Grad S (2007). A phenotypic comparison of intervertebral disc and articular cartilage cells in the rat. Eur Spine J, 16(12), 2174–2185. doi: 10.1007/s00586-007-0475-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linhares ND, Svartman M, Salgado MI, Rodrigues TC, da Costa SS, Rosenberg C, & Valadares ER (2014). Dental developmental abnormalities in a patient with subtelomeric 7q36 deletion syndrome may confirm a novel role for the SHH gene. Meta Gene, 2, 16–24. doi: 10.1016/j.mgene.2013.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Fu S, Rahaman MN, Mao JJ, & Bal BS (2015). Native nucleus pulposus tissue matrix promotes notochordal differentiation of human induced pluripotent stem cells with potential for treating intervertebral disc degeneration. J Biomed Mater Res A, 103(3), 1053–1059. doi: 10.1002/jbm.a.35243 [DOI] [PubMed] [Google Scholar]
- Lolas M, Valenzuela PD, Tjian R, & Liu Z (2014). Charting Brachyury-mediated developmental pathways during early mouse embryogenesis. Proc Natl Acad Sci U S A, 111(12), 4478–4483. doi: 10.1073/pnas.1402612111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luschka. (1856). Die Altersveranderungen des Zwischenwirbelknorpel. Virchow’s Arch, t. ix. [Google Scholar]
- Maier JA, Lo Y, & Harfe BD (2013). Foxa1 and Foxa2 are required for formation of the intervertebral discs. PLoS One, 8(1), e55528. doi: 10.1371/journal.pone.0055528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangold Hilde.; Spemann Hans. (1924). Über Induktion von Embryonalanlagen durch Implantation artfremder Organisatoren. Roux’s Archiv für Entwicklungsmechanik, 100, 599–638. [Google Scholar]
- Marcelle C, Ahlgren S, & Bronner-Fraser M (1999). In vivo regulation of somite differentiation and proliferation by Sonic Hedgehog. Dev Biol, 214(2), 277–287. doi: 10.1006/dbio.1999.9389 [DOI] [PubMed] [Google Scholar]
- Maroudas A, Stockwell RA, Nachemson A, & Urban J (1975). Factors involved in the nutrition of the human lumbar intervertebral disc: cellularity and diffusion of glucose in vitro. J Anat, 120(Pt 1), 113–130. [PMC free article] [PubMed] [Google Scholar]
- Martin BI, Mirza SK, Comstock BA, Gray DT, Kreuter W, & Deyo RA (2007). Are lumbar spine reoperation rates falling with greater use of fusion surgery and new surgical technology? Spine (Phila Pa 1976), 32(19), 2119–2126. doi: 10.1097/BRS.0b013e318145a56a [DOI] [PubMed] [Google Scholar]
- McCann MR, Tamplin OJ, Rossant J, & Seguin CA (2012). Tracing notochord-derived cells using a Noto-cre mouse: implications for intervertebral disc development. Dis Model Mech, 5(1), 73–82. doi: 10.1242/dmm.008128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means AL, Xu Y, Zhao A, Ray KC, & Gu G (2008). A CK19(CreERT) knockin mouse line allows for conditional DNA recombination in epithelial cells in multiple endodermal organs. Genesis, 46(6), 318–323. doi: 10.1002/dvg.20397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minogue BM, Richardson SM, Zeef LA, Freemont AJ, & Hoyland JA (2010). Transcriptional profiling of bovine intervertebral disc cells: implications for identification of normal and degenerate human intervertebral disc cell phenotypes. Arthritis Res Ther, 12(1), R22. doi: 10.1186/ar2929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizrahi O, Sheyn D, Tawackoli W, Ben-David S, Su S, Li N, . . . Gazit Z (2013). Nucleus pulposus degeneration alters properties of resident progenitor cells. Spine J, 13(7), 803–814. doi: 10.1016/j.spinee.2013.02.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok FP, Samartzis D, Karppinen J, Fong DY, Luk KD, & Cheung KM (2016). Modic changes of the lumbar spine: prevalence, risk factors, and association with disc degeneration and low back pain in a large-scale population-based cohort. Spine J, 16(1), 32–41. doi: 10.1016/j.spinee.2015.09.060 [DOI] [PubMed] [Google Scholar]
- Murtaugh LC, Chyung JH, & Lassar AB (1999). Sonic hedgehog promotes somitic chondrogenesis by altering the cellular response to BMP signaling. Genes Dev, 13(2), 225–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwale F (2013). Molecular therapy for disk degeneration and pain. Global Spine J, 3(3), 185–192. doi: 10.1055/s-0033-1349400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan RN, & Andersson GB (2017). Lumbar disc degeneration is an equally important risk factor as lumbar fusion for causing adjacent segment disc disease. J Orthop Res, 35(1), 123–130. doi: 10.1002/jor.23283 [DOI] [PubMed] [Google Scholar]
- Nerlich AG, Schaaf R, Walchli B, & Boos N (2007). Temporo-spatial distribution of blood vessels in human lumbar intervertebral discs. Eur Spine J, 16(4), 547–555. doi: 10.1007/s00586-006-0213-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubuser A, Koseki H, & Balling R (1995). Characterization and developmental expression of Pax9, a paired-box-containing gene related to Pax1. Dev Biol, 170(2), 701–716. doi: 10.1006/dbio.1995.1248 [DOI] [PubMed] [Google Scholar]
- Paavola LG, Wilson DB, & Center EM (1980). Histochemistry of the developing notochord, perichordal sheath and vertebrae in Danforth’s short-tail (sd) and normal C57BL/6 mice. J Embryol Exp Morphol, 55, 227–245. [PubMed] [Google Scholar]
- Papaioannou VE (2014). The T-box gene family: emerging roles in development, stem cells and cancer. Development, 141(20), 3819–3833. doi: 10.1242/dev.104471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons MJ, Pollard SM, Saude L, Feldman B, Coutinho P, Hirst EM, & Stemple DL (2002). Zebrafish mutants identify an essential role for laminins in notochord formation. Development, 129(13), 3137–3146. [DOI] [PubMed] [Google Scholar]
- Peacock A (1951). Observations on the prenatal development of the intervertebral disc in man. J Anat, 85(3), 260–274. [PMC free article] [PubMed] [Google Scholar]
- Peacock A (1952). Observations on the postnatal structure of the intervertebral disc in man. J Anat, 86(2), 162–179. [PMC free article] [PubMed] [Google Scholar]
- Pennimpede T, Proske J, Konig A, Vidigal JA, Morkel M, Bramsen JB, . . . Wittler L (2012). In vivo knockdown of Brachyury results in skeletal defects and urorectal malformations resembling caudal regression syndrome. Dev Biol, 372(1), 55–67. doi: 10.1016/j.ydbio.2012.09.003 [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, . . . Marshak DR (1999). Multilineage potential of adult human mesenchymal stem cells. Science, 284(5411), 143–147. [DOI] [PubMed] [Google Scholar]
- Placzek M, & Furley A (1996). Patterning cascades in the neural tube. Neural development. Curr Biol, 6(5), 526–529. [DOI] [PubMed] [Google Scholar]
- Postma AV, Alders M, Sylva M, Bilardo CM, Pajkrt E, van Rijn RR, . . . van Maarle MC (2014). Mutations in the T (brachyury) gene cause a novel syndrome consisting of sacral agenesis, abnormal ossification of the vertebral bodies and a persistent notochordal canal. J Med Genet, 51(2), 90–97. doi: 10.1136/jmedgenet-2013-102001 [DOI] [PubMed] [Google Scholar]
- Purmessur D, Schek RM, Abbott RD, Ballif BA, Godburn KE, & Iatridis JC (2011). Notochordal conditioned media from tissue increases proteoglycan accumulation and promotes a healthy nucleus pulposus phenotype in human mesenchymal stem cells. Arthritis Res Ther, 13(3), R81. doi: 10.1186/ar3344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribes V, Balaskas N, Sasai N, Cruz C, Dessaud E, Cayuso J, . . . Briscoe J (2010). Distinct Sonic Hedgehog signaling dynamics specify floor plate and ventral neuronal progenitors in the vertebrate neural tube. Genes Dev, 24(11), 1186–1200. doi: 10.1101/gad.559910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson SM, Ludwinski FE, Gnanalingham KK, Atkinson RA, Freemont AJ, & Hoyland JA (2017). Notochordal and nucleus pulposus marker expression is maintained by sub-populations of adult human nucleus pulposus cells through aging and degeneration. Sci Rep, 7(1), 1501. doi: 10.1038/s41598-017-01567-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rider CC, & Mulloy B (2010). Bone morphogenetic protein and growth differentiation factor cytokine families and their protein antagonists. Biochem J, 429(1), 1–12. doi: 10.1042/BJ20100305 [DOI] [PubMed] [Google Scholar]
- Risbud MV, Guttapalli A, Tsai TT, Lee JY, Danielson KG, Vaccaro AR, . . . Shapiro IM (2007). Evidence for skeletal progenitor cells in the degenerate human intervertebral disc. Spine (Phila Pa 1976), 32(23), 2537–2544. doi: 10.1097/BRS.0b013e318158dea6 [DOI] [PubMed] [Google Scholar]
- Risbud MV, Schaer TP, & Shapiro IM (2010). Toward an understanding of the role of notochordal cells in the adult intervertebral disc: from discord to accord. Dev Dyn, 239(8), 2141–2148. doi: 10.1002/dvdy.22350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbud MV, Schoepflin ZR, Mwale F, Kandel RA, Grad S, Iatridis JC, . . . Hoyland JA (2015). Defining the phenotype of young healthy nucleus pulposus cells: recommendations of the Spine Research Interest Group at the 2014 annual ORS meeting. J Orthop Res, 33(3), 283–293. doi: 10.1002/jor.22789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai D, Nakamura Y, Nakai T, Mishima T, Kato S, Grad S, . . . Mochida J (2012). Exhaustion of nucleus pulposus progenitor cells with ageing and degeneration of the intervertebral disc. Nat Commun, 3, 1264. doi: 10.1038/ncomms2226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer R, Chyung JH, Murtaugh LC, Brent AE, Rosen V, Olson EN, . . . Tabin CJ (2001). Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development, 128(19), 3855–3866. [DOI] [PubMed] [Google Scholar]
- Seguin CA, Chan D, Dahia CL, & Gazit Z . (2018). Latest advances in intervertebral disc development and progenitor cells. JOR Spine, 1(3), e1030. doi: 10.1002/jsp2.1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits P, Li P, Mandel J, Zhang Z, Deng JM, Behringer RR, . . . Lefebvre V (2001). The transcription factors L-Sox5 and Sox6 are essential for cartilage formation. Dev Cell, 1(2), 277–290. [DOI] [PubMed] [Google Scholar]
- Stafford DA, Brunet LJ, Khokha MK, Economides AN, & Harland RM (2011). Cooperative activity of noggin and gremlin 1 in axial skeleton development. Development, 138(5), 1005–1014. doi: 10.1242/dev.051938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemple DL (2005). Structure and function of the notochord: an essential organ for chordate development. Development, 132(11), 2503–2512. doi: 10.1242/dev.01812 [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Takimoto A, Akiyama H, Kist R, Scherer G, Nakamura T, . . . Shukunami C (2013). Scx+/Sox9+ progenitors contribute to the establishment of the junction between cartilage and tendon/ligament. Development, 140(11), 2280–2288. doi: 10.1242/dev.096354 [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Takimoto A, Hiraki Y, & Shukunami C (2013). Generation and characterization of ScxCre transgenic mice. Genesis, 51(4), 275–283. doi: 10.1002/dvg.22372 [DOI] [PubMed] [Google Scholar]
- Teillet MA, Lapointe F, & Le Douarin NM (1998). The relationships between notochord and floor plate in vertebrate development revisited. Proc Natl Acad Sci U S A, 95(20), 11733–11738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre OM, Das R, Berenblum RE, Huang AH, & Iatridis JC (2018). Neonatal mouse intervertebral discs heal with restored function following herniation injury. FASEB J, 32(9), 4753–4762. doi: 10.1096/fj.201701492R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trout JJ, Buckwalter JA, & Moore KC (1982). Ultrastructure of the human intervertebral disc: II. Cells of the nucleus pulposus. Anat Rec, 204(4), 307–314. doi: 10.1002/ar.1092040403 [DOI] [PubMed] [Google Scholar]
- Urban JP, & Roberts S (2003). Degeneration of the intervertebral disc. Arthritis Res Ther, 5(3), 120–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban JP, & Winlove CP (2007). Pathophysiology of the intervertebral disc and the challenges for MRI. J Magn Reson Imaging, 25(2), 419–432. doi: 10.1002/jmri.20874 [DOI] [PubMed] [Google Scholar]
- Vargas FR, Roessler E, Gaudenz K, Belloni E, Whitehead AS, Kirke PN, . . . Muenke M (1998). Analysis of the human Sonic Hedgehog coding and promoter regions in sacral agenesis, triphalangeal thumb, and mirror polydactyly. Hum Genet, 102(4), 387–392. [DOI] [PubMed] [Google Scholar]
- Vergroesen PP, Kingma I, Emanuel KS, Hoogendoorn RJ, Welting TJ, van Royen BJ, . . . Smit TH (2015). Mechanics and biology in intervertebral disc degeneration: a vicious circle. Osteoarthritis Cartilage, 23(7), 1057–1070. doi: 10.1016/j.joca.2015.03.028 [DOI] [PubMed] [Google Scholar]
- Wallin J, Wilting J, Koseki H, Fritsch R, Christ B, & Balling R (1994). The role of Pax-1 in axial skeleton development. Development, 120(5), 1109–1121. [DOI] [PubMed] [Google Scholar]
- Walmsley R (1953). The development and growth of the intervertebral disc. Edinb Med J, 60(8), 341–364. [PMC free article] [PubMed] [Google Scholar]
- Ward L, Pang ASW, Evans SE, & Stern CD (2018). The role of the notochord in amniote vertebral column segmentation. Dev Biol, 439(1), 3–18. doi: 10.1016/j.ydbio.2018.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber EH (1827). Meckel’s Archiv. [Google Scholar]
- Weiler C, Nerlich AG, Schaaf R, Bachmeier BE, Wuertz K, & Boos N (2010). Immunohistochemical identification of notochordal markers in cells in the aging human lumbar intervertebral disc. Eur Spine J, 19(10), 1761–1770. doi: 10.1007/s00586-010-1392-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler T, Mahoney EJ, Sinner D, Wylie CC, & Dahia CL (2014). Wnt signaling activates Shh signaling in early postnatal intervertebral discs, and re-activates Shh signaling in old discs in the mouse. PLoS One, 9(6), e98444. doi: 10.1371/journal.pone.0098444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Leung VY, Luk KD, Chan D, & Cheung KM (2009). Injury-induced sequential transformation of notochordal nucleus pulposus to chondrogenic and fibrocartilaginous phenotype in the mouse. J Pathol, 218(1), 113–121. doi: 10.1002/path.2519 [DOI] [PubMed] [Google Scholar]
- Yang SH, Wu CC, Shih TT, Sun YH, & Lin FH (2008). In vitro study on interaction between human nucleus pulposus cells and mesenchymal stem cells through paracrine stimulation. Spine (Phila Pa 1976), 33(18), 1951–1957. doi: 10.1097/BRS.0b013e31817e6974 [DOI] [PubMed] [Google Scholar]
- Zhang C, Berven SH, Fortin M, & Weber MH (2016). Adjacent Segment Degeneration Versus Disease After Lumbar Spine Fusion for Degenerative Pathology: A Systematic Review With Meta-Analysis of the Literature. Clin Spine Surg, 29(1), 21–29. doi: 10.1097/BSD.0000000000000328 [DOI] [PubMed] [Google Scholar]
- Zhu J, Kwan KM, & Mackem S (2016). Putative oncogene Brachyury (T) is essential to specify cell fate but dispensable for notochord progenitor proliferation and EMT. Proc Natl Acad Sci U S A, 113(14), 3820–3825. doi: 10.1073/pnas.1601252113 [DOI] [PMC free article] [PubMed] [Google Scholar]