Abstract
Background
Tenofovir-diphosphate (TFV-DP) in dried blood spots (DBS) is an objective long-term adherence measure but data are limited on its ability to predict virologic suppression (VS) in people on antiretroviral (ARV) treatment. There are also no data comparing DBS TFV-DP with plasma ARV concentrations as predictors of VS.
Methods
Women who were on a first-line regimen of tenofovir, emtricitabine, and efavirenz (EFV) were enrolled in a cross-sectional study. Plasma EFV and tenofovir (TFV), DBS TFV-DP assays, and 30-day self-reported adherence were evaluated as predictors of VS (<50 copies/mL) with area under the curve (AUC) of receiver operating characteristics (ROC) and logistic regression.
Results
We enrolled 137 women; mean age 33 years; median 4 years on ART; 88 (64%) had VS. In ROC analyses: DBS TFV-DP (0.926 [95%CI 0.876–0.976]) had a higher AUC than plasma TFV (0.864 [0.797–0.932]; p=0.006), while plasma EFV (0.903 [0.839–0.967]) was not significantly different from DBS TFV-DP (p=0.138) or plasma TFV (p=0.140); all ARV assays performed better than self-report. The association of TFV-DP in DBS with VS strengthened with increasing concentrations (reference <350 fmol/punch: 350–699 fmol/punch aOR 37 [8–178]; 700–1249 fmol/punch aOR 47 [13–175]; ≥1250 fmol/punch aOR 175 [20–1539]). “White coat adherence” (defined as DBS TFV-DP <350 fmol/punch with detectable plasma TFV) was only detected in 4 women.
Conclusions
Plasma EFV, TFV and DBS TFV-DP were all strong predictors of VS. EFV or TFV assays have potential for development as point-of-care assays for use as objective adherence measures in resource-limited settings.
Keywords: antiretroviral therapy, adherence, efavirenz, tenofovir, tenofovir-diphosphate in dried blood spots, virologic suppression
Introduction
Adherence to antiretroviral therapy (ART) is required to achieve and sustain virologic suppression [1]. A multitude of adherence measures exist but there is no gold standard and measuring ART adherence in both routine care and research settings is a major challenge [2]. Self-reported adherence frequently overestimates actual treatment adherence [3] while HIV viral load, which is often used as a marker of ART adherence, does not capture patterns of adherence or discriminate between poor adherence and resistance as causes of virologic failure [2].
Measuring antiretroviral (ARV) drug concentrations has limitations, but is an objective way to assess ART adherence [2]. Plasma concentrations of the ARVs tenofovir (TFV) and efavirenz (EFV), which are used in first line ART regimens, are only informative about dosing in the past 4–5 days [4,5]. Plasma TFV has a half-life of approximately 14 hours [6] while EFV concentrations and half-life vary with CYP2B6 metaboliser genotype [4,7,8]. The half-life of EFV 600 mg (the current standard dose) ranges from approximately 16 hours in people considered extensive metabolisers, to 49 hours among slow metabolisers [4], complicating the interpretation of plasma EFV concentrations as an adherence measure. An assay has been developed to measure tenofovir-diphosphate (TFV-DP) concentrations in dried blood spots (DBS) [9]. TFV-DP in DBS has a half-life of 17 days and can be detected for up to 12 weeks after stopping, a major benefit for assessing long-term adherence [10–12]. Both plasma and DBS ARV concentrations are measured using complex and expensive laboratory assays and neither are likely to be feasible outside of the context of clinical trials.
To date, most data on DBS TFV-DP as an adherence measure have been obtained from HIV-negative individuals in pre-exposure prophylaxis (PrEP) studies. There are very few studies reporting TFV-DP DBS concentrations or therapeutic concentration thresholds for detection of viral suppression and thus ART adherence in PLWH. Two small studies compared DBS TFV-DP with other adherence measures: pharmacy refill adherence among 35 women in the United States [10] and electronic drug monitoring in South Africa (n=29) [13]. Only one study has reported on the ability of TFV-DP in DBS to predict virologic suppression [11]. In addition, little is known about how TFV-DP in DBS compares with less expensive plasma ARV drug concentrations or other more affordable adherence measures such as self-reported adherence that could be used in low-resource settings. Existing data on TFV-DP concentrations in DBS indicate that concentrations are up to 19% lower in males than females [12] and that there appear to be differences across populations with lower concentrations among Black compared with White or Hispanic individuals [11]. African women bear the largest burden of the HIV epidemic but to date there is just one unpublished report on TFV-DP in DBS in an African cohort on ART [13] and there are no data on the ability of TFV-DP in DBS to predict virologic suppression among African women. Here we describe plasma EFV, plasma TFV and DBS TFV-DP concentrations, and self-reported adherence, in a cohort of women living with HIV who are on ART in Cape Town, South Africa, and assess their relationship with HIV viral load.
Methods
Design, participants and setting
Women who were enrolled during pregnancy in a large implementation science study (the MCH-ART study) in Gugulethu, Cape Town were approached between 36 and 60 months postpartum and invited to participate in this cross-sectional sub-study. The MCH-ART study methods and results have been described previously [14,15]. All women had initiated the first line regimen of tenofovir disoproxil fumarate (TDF) 300mg, emtricitabine 200mg or lamivudine 300mg (XTC), and EFV 600mg, provided as a once daily fixed-dose combination. For this sub study, the first 150 women who agreed to participate were recruited and had blood drawn for ARV assays. Women who were pregnant or had switched to second line ART were excluded.
Procedures
During the study visit, all women completed structured face-to-face interviews in the predominant local language, isiXhosa. Self-reported medication adherence in the past 30 days was measured using a simple, three-item scale that has been described previously and is presented in supplementary (S) Table 1 [16,17]. Women reported on their current ART use and which regimens they were taking. ART regimen history was reviewed using the provincial electronic pharmacy records. Women were asked to report their current pregnancy status and their height and weight were measured by study staff who were trained in anthropometric techniques. Weight was measured using a standing scale and height using a stadiometer with equipment calibrated regularly.
Antiretroviral drug assays and viral loads
Venous EDTA blood samples were drawn from each participant and kept below 4°C until they were processed for storage. Within six hours of collection, samples were centrifuged at 3500 rpm and plasma decanted into cryovials, before being frozen at −80°C. The majority of specimens were collected in the mid-dose interval (12–18 hours post-dose). For the TFV-DP DBS assay, 50 μL of whole blood was pipetted onto Whatman 903 Proteinsaver® cards, allowed to dry overnight at room temperature and then stored desiccated in airtight freezer-safe bags at −80°C.
EFV, TFV and TFV-DP were analysed with validated liquid chromatography tandem mass spectrometry assays by the Clinical PK Laboratory, Division of Clinical Pharmacology, University of Cape Town. EFV plasma concentrations were determined as described by Bienczak et al [18]. TFV concentrations were determined using a protein precipitation extraction method, with tenofovir-d6 as internal standard, followed by high performance liquid chromatography with MS/MS detection using an AB SCIEX API 3000 instrument. Gradient chromatography was performed on a Waters Atlantis T3 (C18, 3μm, 100 X 2.1 mm) analytical column. The mobile phase consisted of 0.1% formic acid in water and 0.1% formic acid in acetonitrile and was delivered at a flow-rate of 300 μl per minute. The analyte and internal standard were monitored at mass transitions of the protonated precursor ions m/z 288.1 and m/z 294.1 to the product ions m/z 176.2 and m/z 182.2 for tenofovir and tenofovir-d6, respectively. The calibration curve fitted a quadratic (weighted by 1/concentration) regression over the ranges 10 to 1600 ng/ml. TFV-DP in DBS was indirectly measured using a slightly modified LC-MS/MS assay as described by Castillo-Mancilla et al [9].
An additional venous sample was sent to the National Health Laboratory Services (NHLS) for viral load testing (Roche COBAS AmpliPrep/COBAS TaqMan HIV-1 assay; Roche Diagnostics, Branchburg, New Jersey, US).
Routine medical records
In addition to data collected from participants during the study visit, routine electronic health records were requested from the Western Cape Provincial Health Data Centre. This included data from the NHLS (for serum creatine and CD4 cell count measures) and pharmacy databases (to validate ARV regimens), all of which are linked by a unique patient identifier and include all public health facilities in the Western Cape Province. Kidney function is an important consideration when measuring drug concentrations while CD4 cell count is known to be independently associated with viral load, thus serum creatinine concentrations and CD4 cell counts measured within six months before or after the study visit were abstracted from the laboratory records [11,19,20]. Serum creatine and age were used to calculate creatine clearance using the CKD-EPI formula for Black women [21]. Less than 25% of women had recent CD4 cell counts available so these data were not included. Anaemia is also associated with HIV disease and can impact drug concentration measures, particularly in DBS [22], however measures of haemoglobin or haematocrit were not available in this study.
Analysis
All analyses were performed in Stata (Stata Corporation, College Station, TX). Means with standard deviations, medians with interquartile ranges, or proportions were used to describe the characteristics of the cohort and the adherence measures. Drug concentrations were also log-transformed and back-transformed to report geometric means with 95% confidence intervals (CI). Concentrations below the lower limit of quantification for each assay (LLOQ; 0.0195 μg/mL for plasma EFV, 10 ng/mL for plasma TFV and 16.6 fmol/punch for DBS TFV-DP) were assigned a value half that of the LLOQ (0.00975 μg/mL for plasma EFV, 5 ng/mL for plasma TFV and 8.3 fmol/punch for DBS TFV-DP) [10].
Logistic regression models were used to evaluate the relationship between continuous adherence measures and virologic suppression. Age, body mass index (BMI) and duration on ART were included as a priori covariates; a sub analysis was conducted among women with creatinine clearance available. Virologic suppression was defined as viral load <50 copies/mL with sensitivity analyses using thresholds of <400 and <1000 copies/mL. Area under the curves (AUC) of receiver operating characteristics (ROC) were used to assess discrimination of the adherence measures to predict virologic suppression. ROC AUCs for each drug concentration and self-reported adherence were compared using chi-squared tests for equality of AUCs proposed by De Long et al [23]. We estimated sensitivity, specificity and positive and negative predictive values (PPV and NPV) using established drug concentration thresholds: ≥0.7μg/mL for plasma EFV [24,25], ≥35.5ng/mL for plasma TFV [26], and five adherence thresholds (<350, 350–699, 700–1249, 1250–1849 and ≥1850 fmol/punch) for TFV-DP from DBS that were determined in a healthy volunteer study [11,12]. We also examined which thresholds of each of the continuous drug concentrations would maximise both sensitivity and specificity to detect women with and without virologic suppression. Sensitivity analyses were conducted excluding women who reported taking no ART in the past 30 days, a group in whom drug concentrations may not be measured in a routine care setting. Women who were pregnant at the time of the study visit [27] or who had switched to second line ART regimens were excluded from all analyses.
Lastly, we examined whether there was improved short-term ART adherence related to the study visit among participants (so called “white coat” adherence [2,28]), by comparing plasma TFV concentrations, with a relatively short half-life of approximately 14 hours [6] indicating recent dosing, with TFV-DP concentrations in DBS, with a half-life of up to 17 days [12] indicating consistent dosing over the recent weeks.
Ethics
All participants completed written informed consent, including consent for specimen storage and drug assays, prior to completing any study procedures. This study was reviewed and approved by the University of Cape Town Human Research Ethics Committee and the Columbia University Institutional Review Board.
Results
Thirteen of the 150 consecutive women screened were excluded (seven were pregnant and six had switched to second line ART regimens). The characteristics of 137 women enrolled into the study are displayed in Table 1. At the study visit the median time on ART was 3.9 years (IQR 3.7–4.0) and 88 women (64%) were virologically suppressed (<50 copies/mL).
Table 1.
Characteristics of 137 non-pregnant women on first line ART at the time of the study visit.
| All women | |
|---|---|
| Mean age (SD) | 33 (5) |
| Median (IQR) years on ART | 3.9 (3.7–4.0) |
| Median (IQR) weight (kg) | 81 (65–93) |
| Median (IQR) BMI (kg/m2) | 32 (26–36) |
| BMI <18.5 kg/m2 | 3 (2) |
| BMI ≥18.5 and <25 kg/m2 | 26 (19) |
| BMI ≥25 and <30 kg/m2 | 33 (24) |
| BMI ≥30 kg/m2 | 75 (56) |
| Creatinine clearance available, n (%) | 74 (54) |
| Median (IQR) creatinine clearance1 mL/min/1.73m² (n=74) | 137.7 (129.5–143.7) |
| Median (IQR) log10 viral load | 1.30 (1.30–3.56) |
| Viral load <50 copies/mL, n (%) | 88 (64) |
| Viral load ≥50 and <400 copies/mL, n (%) | 8 (6) |
| Viral load ≥400 and <1000 copies/mL, n (%) | 2 (1) |
| Viral load ≥1000 copies/mL, n (%) | 39 (28) |
Serum creatine measures were abstracted from routine laboratory records within six months before or after the study visit; creatinine clearance was calculated using the CKD-EPI formula.
The distribution of plasma EFV, TFV and DBS TFV-DP concentrations are presented in Table 2 and S Figure 1. Specimens were collected at a median of 15 hours after last dosing (IQR 14–16). In total, 84%, 76% and 86% of women had any detectable plasma EFV, plasma TFV and DBS TFV-DP, respectively. There were 38 women (28%) who had drug concentrations below the LLOQ for all three ARV assays; two of these 38 women were virologically suppressed. These two women reported that they were no longer taking ART and this was confirmed in medical records. Both women were virologically suppressed from the time of ART initiation and their HIV diagnosis was confirmed on ELISA. Overall, most women had high self-reported adherence scores with 56% of women (n=77) reporting 100% adherence in the last 30 days on the three-item scale; 62 of whom (81%) were virologically suppressed.
Table 2.
Concentrations of TFV-DP in DBS, TFV plasma and EFV plasma as well as self-reported adherence among 137 women, grouped by viral load <50, <400 and <1000 copies/mL. Results presented as n (%) unless specified.
| Viral load <50 copies/mL | Viral load ≥50 copies/mL | Viral load <400 copies/mL | Viral load ≥400 copies/mL | Viral load <1000 copies/mL | Viral load ≥1000 copies/mL | All women | |
|---|---|---|---|---|---|---|---|
| Number of women | 88 (64) | 49 (36) | 96 (70) | 41 (30) | 98 (72) | 39 (28) | 137 |
| EFV plasma (μg/mL) | |||||||
| Median (range) | 1.9 (0.00975, 19.5) | 0.00975 (0.00975, 4.8) | 1.91 (0.00975, 19.5) | 0.00975 (0.00975, 4.8) | 1.87 (0.00975, 19.5) | 0.00975 (0.00975, 4.8) | 1.52 (0.00975, 19.5) |
| Geometric mean (95% CI) | 1.8 (1.4–2.3) | 0.03 (0.02–0.06) | 1.6 (1.2–2.1) | 0.02 (0.01–0.03) | 1.5 (1.1–2.0) | 0.02 (0.01–0.03) | 0.4 (0.3–0.6) |
| LLOQ to <0.7 | 9 (10) | 39 (80) | 11 (11) | 37 (90) | 13 (13) | 35 (90) | 48 (35) |
| 0.7 to <4 | 66 (75) | 8 (16) | 71 (74) | 3 (7) | 71 (72) | 3 (8) | 74 (54) |
| ≥4 | 13 (15) | 2 (4) | 14 (15) | 1 (2) | 14 (14) | 1 (3) | 15 (11) |
| TFV plasma (ng/mL) | |||||||
| Median (range) | 44.3 (5.0, 120) | 5.0 (5.0, 90.1) | 44.2 (5.0, 120) | 5.0 (5.0, 90.1) | 44.0 (5.0, 120) | 5.0 (5.0, 90.1) | 32.90 (5.0, 120) |
| Geometric mean (95% CI) | 35 (30–42) | 8 (6–11) | 34 (29–41) | 7 (5–8) | 33 (28–39) | 7 (5–9) | 21 (17–25) |
| LLOQ to <35.5 | 31 (35) | 42 (86) | 35 (36) | 38 (93) | 37 (38) | 36 (92) | 73 (53) |
| ≥35.5 | 57 (65) | 7 (14) | 61 (64) | 3 (7) | 61 (62) | 3 (8) | 64 (47) |
| TFV-DP DBS (fmol/punch) | |||||||
| Median (range) | 961.5 (8.3, 2402) | 8.3 (8.3, 1359) | 952.50 (8.3, 2402) | 8.3 (8.3, 930) | 940.5 (8.3, 2402) | 8.3 (8.3, 930) | 701 (8.3, 2402) |
| Geometric mean (95% CI) | 815 (670–992) | 25 (14–43) | 740 (591–926) | 16 (10–25) | 700 (551–889) | 15 (9–24) | 241 (163–336) |
| LLOQ to <350 | 7 (8) | 40 (81) | 10 (10) | 37 (90) | 12 (12) | 35 (90) | 47 (35) |
| 350 to <700 | 16 (18) | 3 (6) | 17 (18) | 2 (5) | 17 (17) | 2 (5) | 19 (14) |
| 700 to <1250 | 36 (41) | 5 (10) | 39 (41) | 2 (5) | 39 (40) | 2 (5) | 41 (30) |
| 1250 to <1850 | 23 (26) | 1 (3) | 24 (25) | 0 (0) | 24 (24) | 0 (0) | 24 (18) |
| ≥1850 | 6 (7) | 0 (0) | 6 (6) | 0 (0) | 6 (6) | 0 (0) | 6 (4) |
| Self-reported adherence in past 30 days | |||||||
| Median(range) | 100 (0–100) | 92 (0–100) | 100 (0–100) | 83 (0–100) | 100 (0–100) | 83 (0–100) | 100 (0–100) |
| Score ≤80 | 3 (3) | 20 (41) | 4 (4) | 19 (46) | 5 (5) | 18 (46) | 23 (17) |
| Score >80 and ≤100 | 23 (26) | 14 (29) | 27 (28) | 10 (24) | 28 (29) | 9 (23) | 37 (27) |
| Score=100 | 62 (70) | 15 (31) | 65 (68) | 12 (29) | 65 (66) | 12 (31) | 77 (56) |
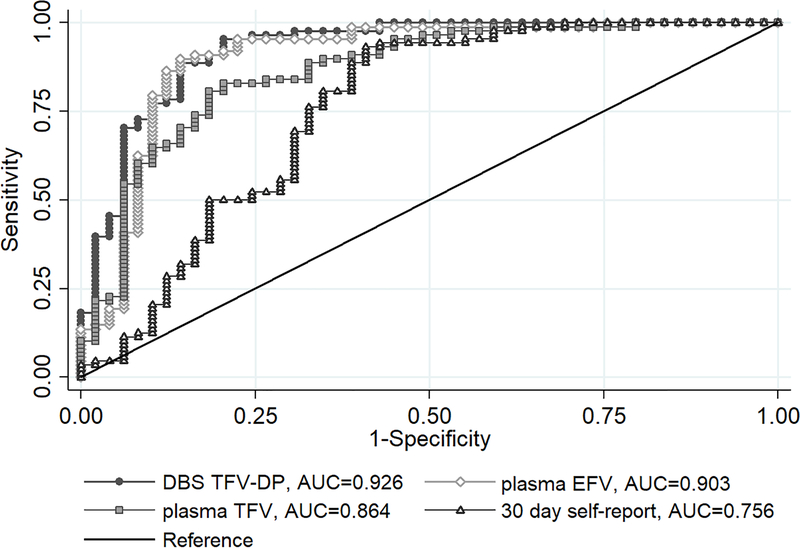
In ROC analyses to evaluate how well each adherence marker predicted virologic suppression, DBS TFV-DP had the highest AUC of 0.926 (95% CI 0.876–0.976) after adjusting for age and duration on ART (Figure 1; unadjusted ROC curves shown in S Figure 2). This was significantly better than plasma TFV (AUC=0.864 95% CI 0.797–0.932, p=0.006) but not significantly different from plasma EFV (AUC=0.903 95% CI 0.839–0.967, p=0.138). All drug concentrations performed better than 30-day self-reported adherence (AUC=0.756 95% CI 0.660–0.852, p<0.05 for each assay).
Figure 1.
Adjusted area under the receiver operating characteristics (ROC) curves of DBS TFV-DP (grey), plasma EFV (green), plasma TFV (maroon), and self-reported adherence (blue) to predict viral suppression; n=137 (adjusted for age and duration on ART)
Using drug concentrations to predict virologic suppression, we used ROC analyses to examine the diagnostic characteristics of different drug concentration cut-off points, including established thresholds for TFV-DP in DBS [11], plasma TFV [26] and plasma EFV [24,25] (Table 3). Having any detectable drug concentration on any of the three assays was highly predictive of virologic suppression. Almost 90% of the cohort were correctly classified as virologically suppressed or not suppressed based on any detectable TFV-DP in DBS or any plasma EFV; 85% were correctly classified as virologically suppressed or not by any detectable plasma TFV. Higher drug concentration cut-offs resulted in higher positive predictive values.
Table 3.
Diagnostic characteristics of different binary drug concentration thresholds to predict virological suppression (<50 copies/mL) using both established drug concentration cut-off values and alternative cut-off values (indicated by *) that maximised sensitivity and specificity in ROC analyses.
| Tenofovir-diphosphate (TFV-DP) in DBS | ||||||
|---|---|---|---|---|---|---|
| Select TFV-DP cut-off values | Probability of virologic suppression1 | Sensitivity2 (%) | Specificity3 (%) | PPV4 (%) | NPV5 (%) | Correctly classified6 (%) |
| >LLOQ | >0.190 | 97.7 | 73.5 | 86.9 | 94.7 | 89.1 |
| ≥350 | ≥0.464 | 92.1 | 81.6 | 90.0 | 85.1 | 88.3 |
| ≥399* | ≥0.515 | 92.1 | 83.7 | 91.0 | 85.4 | 89.1 |
| ≥700 | ≥0.787 | 73.9 | 87.8 | 91.5 | 65.2 | 78.8 |
| ≥1250 | ≥0.975 | 33.0 | 98.0 | 96.7 | 44.9 | 56.2 |
| ≥1850 | ≥0.998 | 6.8 | 100.0 | 100.0 | 37.4 | 40.2 |
| Plasma tenofovir (TFV) | ||||||
| Select plasma TFV cut-off values | Probability of virologic suppression1 | Sensitivity2 (%) | Specificity3 (%) | PPV4 (%) | NPV5 (%) | Correctly classified6 (%) |
| >LLOQ | ≥0.411 | 88.6 | 77.6 | 87.6 | 79.2 | 84.7 |
| ≥23.5* | ≥0.586 | 78.4 | 79.6 | 87.3 | 67.2 | 78.8 |
| ≥35.5 | ≥0.750 | 64.8 | 85.7 | 89.1 | 57.5 | 72.3 |
| Plasma efavirenz (EFV) | ||||||
| Select plasma EFV cut-off values | Probability of virologic suppression1 | Sensitivity2 (%) | Specificity3 (%) | PPV4 (%) | NPV5 (%) | Correctly classified6 (%) |
| >LLOQ | ≥0.253 | 97.7 | 75.5 | 87.8 | 94.9 | 89.8 |
| ≥0.70 | ≥0.483 | 89.8 | 79.6 | 88.8 | 81.3 | 86.1 |
| ≥1.00 | ≥0.605 | 85.2 | 81.6 | 89.3 | 75.5 | 83.9 |
| ≥1.13* | ≥0.636 | 81.8 | 83.7 | 90.0 | 71.9 | 82.5 |
| ≥4.00 | ≥0.995 | 14.8 | 95.9 | 86.7 | 38.5 | 43.8 |
Probability estimated from logistic regression models.
Proportion of subjects with a viral load <50 copies/mL who were detected using this cut-off
Proportion of subjects with a viral load ≥50 copies/mL who were detected using this cut-off
Positive Predictive Value: proportion of those who were detected with this drug concentration cut-off who had a viral load <50 copies/mL
Negative Predictive Value: proportion of those who were not detected with this drug concentration cut-off who had a viral load ≥50 copies/mL
Overall proportion of those above and below the cut-off who were correctly classified. Note that this proportion is always biased towards the larger group.
Thresholds of DBS TFV-DP have been shown to relate back to doses taken per week in healthy volunteers [11]. Table 4 examines in more detail the viral load distribution and association between virologic suppression and DBS TFV-DP in our cohort within these established thresholds. In our cohort 97% of women (n=29) who had a DBS TFV-DP concentration ≥1250 fmol/punch were virologically suppressed (defined as <50 copies/mL). This threshold also perfectly predicted viral loads <400 and <1000 copies/mL. Seven of the 47 women (15%) in the lowest TFV-DP category, <350 fmol/punch, remained suppressed despite none or very low drug concentrations. A dose-response relationship was observed between virologic suppression and increasing concentrations of TFV-DP in DBS; this was not observed for increasing concentrations of plasma EFV or TFV (data not shown). Similar associations were observed in sensitivity analyses excluding women who self-reported not taking any ART in the past 30 days (S Table 2). Increasing years of age (aOR 1.12 95% CI 1.01–1.25) was associated with virologic suppression, while the association between suppression and increasing duration on ART had very wide confidence intervals (aOR 1.56 95% CI 0.13–18.07) and was not statistically significant (crude models in S Table 3). Creatinine clearance was not measured at the time of the drug concentration testing and only 74 women had available serum creatinine concentrations in the six months before or after the study visit. In this group, creatinine clearance was not associated with virologic suppression. Neither creatinine clearance nor BMI changed the associations between drug concentrations and viral load and were therefore not included in adjusted models.
Table 4.
Viral load characteristics within established DBS TFV-DP thresholds among 137 women. Odds ratios (OR) predicting viral load <50, <400 and <1000 copies/mL are presented.
| TFV-DP threshold (approximate doses per week1) | <350 (<2) | 350–699 (2–3) | 700–1249 (4–6) | ≥1250 (7) |
|---|---|---|---|---|
| Total number of women | 47 | 19 | 41 | 30 |
| Median viral load log10 copies/mL (IQR) | 3.7 (2.8–4.6) | 1.3 (1.3–1.3) | 1.3 (1.3–1.3) | 1.3 (1.3–1.3) |
| Median viral load copies/mL (IQR) | 12200 (618–40027) | 20 (20–20) | 20 (20–20) | 20 (20–20) |
| Viral load <50 copies/mL | ||||
| Viral load <50 copies/mL, n (%) | 7 (15) | 16 (84) | 36 (88) | 29 (97) |
| OR (95% CI) | Ref | 30 (7–133) | 41 (12–141) | 166 (19–1421) |
| aOR2 (95% CI) | Ref | 37 (8–178) | 47 (13–175) | 175 (20–1539) |
| Viral load <400 copies/mL | ||||
| Viral load <400 copies/mL, n (%) | 12 (26) | 17 (89) | 39 (95) | 30 (100) |
| OR (95% CI) | Ref | 31 (6–159) | 72 (15–352) | Omitted |
| aOR2 (95% CI) | Ref | 47 (8–287) | 100 (17–579) | Omitted |
| Viral load <1000 copies/mL | ||||
| Viral load <1000 copies/mL, n (%) | 10 (21) | 17 (89) | 39 (95) | 30 (100) |
| OR (95% CI) | Ref | 25 (5–123) | 57 (12–272) | Omitted |
| aOR2 (95% CI) | Ref | 31 (6–167) | 64 (12–330) | Omitted |
As previously described by Castillo-Mancilla et al (CID, 2018)
Adjusted for age and duration on ART
To examine improved short-term ART adherence related to the study visit among participants, or “white coat” adherence [2,28], we compared plasma TFV concentrations (indicating recent dosing) with concentrations of TFV-DP in DBS (indicating consistent dosing in recent weeks). Concentrations of TFV-DP in DBS correlated well with plasma TFV concentrations (r=0.700, S figure 3). There were four women with any detectable plasma TFV but DBS TFV-DP concentrations below 350 fmol/punch. All four women also had detectable plasma EFV concentrations and only two women had viral loads above 50 copies/mL (S table 4).
Discussion
Our findings from a cohort of South African women show that concentrations of TFV-DP in DBS, plasma EFV and plasma TFV were all strongly associated with virologic suppression and all performed better than self-reported adherence. The strength of association between DBS TFV-DP and virologic suppression increased with increasing drug concentrations and remained consistent after adjustment for covariates and in sensitivity analyses, confirming recent findings in a US cohort [11]. This study is the first to compare TFV-DP in DBS with plasma ARV concentrations and HIV viral load for measuring ART adherence in an African population and adds novel insights for the potential use of TFV-DP in DBS and plasma EFV or TFV to measure adherence among PLWH.
Plasma EFV and DBS TFV-DP concentrations above the LLOQ both had sensitivities approaching 100% and correctly identified 89% of women with virologic suppression. We found a stronger association between plasma EFV concentrations and virologic suppression than previously reported in a similar population up to one year on ART [7]. Adherence is known to change over time and there may be less intermittent adherence in our cohort at 3–4 years after ART start compared with the first year on ART. However, there are mixed results in the literature regarding the association between increasing duration on ART and adherence [29]. Although we were not able to measure the genes relevant to EFV metabolism in this study, it is likely that women with EFV concentrations above 4 μg/mL (11% of our cohort) possess the CYP2B6 slow metaboliser genotype resulting in a longer EFV half-life [8]. The longer DBS TFV-DP half-life and possibly longer EFV half-life compared to plasma TFV may explain why, even when administered in a fixed dose combination, plasma TFV concentrations performed slightly worse than plasma EFV and TFV-DP in DBS at predicting virologic suppression. There were two women in the cohort with no detectable drug concentrations and suppressed viral loads and both were thought to be elite controllers.
TFV-DP concentrations in DBS among virologically suppressed women in our cohort (geometric mean 815 fmol/punch, median 961 fmol/punch) were similar to values reported in another South African cohort of predominantly women (median 939 fmol/punch) [13]. However, they were lower than observed among suppressed Black PLWH in the US (geometric mean 1453 fmol/punch) [11] and higher than observed among non-pregnant HIV-negative African women on PrEP in Uganda and Kenya (mean 637 fmol/punch) [27]. This supports recent findings that virologically suppressed PLWH have higher DBS TFV-DP concentrations than HIV-negative individuals on PrEP [11]. This comparison of our findings with existing studies also suggests that therapeutic thresholds of DBS TFV-DP may be lower among Black African PLWH compared to PLWH of other ethnicities and further research is needed to establish therapeutic adherence thresholds for DBS TFV-DP concentrations in this population. Although we observed lower TFV-DP concentrations in DBS than Castillo-Mancilla and colleagues [11], we found a similar dose-response relationship between virologic suppression and increasing TFV-DP concentrations in DBS. In contrast to previous findings [11], we found no association between TFV-DP in DBS and BMI which may be due to most women in our cohort having BMI ≥25kg/m2. Other characteristics such as chronic kidney disease, anaemia or use of concomitant medication (for example ritonavir or cobicistat) may also influence TFV-DP concentrations in DBS among PLWH [11,19,20,22,30] but we were not able to evaluate these in our study. We were also unable to assess variability in TFV-DP concentrations in DBS due to ARV regimen, race or gender as our cohort was restricted to non-pregnant women on a first line FDC regimen of TDF+XTC+EFV. Pregnancy status has also recently been shown to influence TFV-DP concentrations in DBS among African women on PrEP [27] and this requires further evaluation in the context of HIV infection.
Our findings should be interpreted with the following additional limitations in mind. Previous studies have accounted for CD4 cell count, kidney function and anaemia in their analyses [10, 11], however these data were not collected by the study and, although effort was made to abstract information from routine medical records, few women had available measures within six months before or after the study visit. We ascertained ART regimen by self-report and through triangulation with routine pharmacy dispensing data, but errors may have occurred. In this cross-sectional study, we were also unable to assess daily dosing or to match drug concentration thresholds to actual ART dosing prior to the study visit as the last dose was not observed. Given the observed differences in TFV-DP concentrations in DBS among people living with and without HIV, as well as the lower concentrations reported among Black individuals, further research is needed to evaluate adherence thresholds in African populations living with HIV.
In both research and routine care there is often concern about improved adherence shortly before a scheduled research or clinical visit, or “white coat” adherence [2,28]. We found little evidence of this in our cohort with only four women with therapeutic plasma TFV but TFV-DP in DBS below 350 fmol/punch. The long half-life of TFV-DP in DBS and the dose-response relationship with virologic suppression show that measuring TFV-DP in DBS provides a more nuanced measure of adherence than plasma EFV or TFV concentrations. However, our findings suggest that plasma EFV and TFV can also be strong predictors of virologic suppression and support the use of both plasma and DBS drug concentrations for measuring ARV adherence among PLWH in research settings. Both plasma and DBS assays were superior to self-reported adherence in this analysis, but both are costly and require laboratory testing, with the associated logistical challenges of transport, storage and laboratory capacity. These practical considerations prohibit their use in resource-limited settings and the development of non-invasive, point-of-care drug concentration measures for the measurement of adherence to PrEP and HIV treatment, such as urine testing [31,32], should remain a research priority.
In summary, our data add to the evidence on the use of TFV-DP in DBS for monitoring ART adherence in PLWH, providing new insights from a cohort of African women. TFV-DP in DBS and plasma EFV and TFV concentrations were strongly associated with virologic suppression and are superior to self-reported adherence. Further research is needed into less expensive and point of care technologies as well as to assess the added value of drug concentration assays in the context of viral load monitoring and screening for ART resistance.
Supplementary Material
S figure 1. Histograms of a) plasma efavirenz, b) plasma tenofovir, c) tenofovir-diphosphate (TFV-DP) concentrations in dried blood spots (DBS), d) self-reported adherence in the past 30 days (three-item scale score)
S figure 2. Unadjusted area under the receiver operating characteristics (ROC) curves of DBS TFV-DP (grey), plasma EFV (green), plasma TFV (maroon), and self-reported adherence (blue) to predict viral suppression; n=137
S figure 3: Scatter plot of tenofovir-diphosphate (TFV-DP) in DBS and plasma tenofovir (TFV) concentrations.
S table 1. Three-item self-reported adherence scale.
S table 2. Characteristics of DBS TFV-DP thresholds restricted to women who report taking ART in the last 30 days (n=87). Odds ratios (OR) predicting viral load <50, <400 and <1000 copies/mL are presented.
S table 3. Univariable logistic regression models predicting viral load <50, <400 and <1000 copies/mL among 137 women.
S table 4. Women with detectable plasma tenofovir (TFV) and efavirenz (EFV) concentrations but very low tenofovir-diphosphate concentration in dried blood spots (DBS TFV-DP)
Acknowledgments
The authors would like to thank the women who participated in this study, as well as the study staff in Gugulethu for their support of this research.
Conflicts of interest and sources of funding:
This research was supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the National Institute of Child Health and Human Development (NICHD), grant number 1R01HD074558 and 1R01HD080465. The University of Cape Town (UCT) Clinical PK Laboratory is supported by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health under award numbers UM1 AI068634, UM1 AI068636, and UM1 AI106701. Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) at UCT was provided by the National Institute of Allergy and Infectious Diseases (U01 AI068632), The Eunice Kennedy Shriver National Institute of Child Health and Human Development, and National Institute of Mental Health grant AI068632. Ms Phillips receives partial funding from the South African Department of Science and Technology/National Research Foundation (DST-NRF), Centre of Excellence in Epidemiological Modelling and Analysis (SACEMA), Stellenbosch University, Stellenbosch, South Africa. Dr Orrell is partially supported through DAIDS grants (1R01AI122300 – 01, 1R34MH108393-01 and 2UM1AI0695-08).
References
- 1.World Health Organization; Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Geneva, Switzerland; 2016 [PubMed] [Google Scholar]
- 2.Castillo-mancilla JR, Haberer JE. Adherence Measurements in HIV : New Advancements in Pharmacologic Methods and Real-Time Monitoring. Curr HIV/AIDS Rep 2018; 15:49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stirratt MJ, Dunbar-Jacob J, Crane HM, Simoni JM, Czajkowski S, Hilliard ME, et al. Self-report measures of medication adherence behavior: recommendations on optimal use. Transl Behav Med 2015; 5:470–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dickinson L, Amin J, Else L, Boffito M, Egan D, Owen A, et al. Pharmacokinetic and Pharmacodynamic Comparison of Once-Daily Efavirenz (400 mg vs. 600 mg) in Treatment-Naive HIV-Infected Patients: Results of the ENCORE1 Study. Clin Pharmacol Ther 2015; 98:406–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jackson A, Moyle G, Watson V, Tjia J, Ammara A, Back D, et al. Tenofovir, emtricitabine intracellular and plasma, and efavirenz plasma concentration decay following drug intake cessation: Implications for HIV treatment and prevention. J Acquir Immune Defic Syndr 2013; 62:275–281. [DOI] [PubMed] [Google Scholar]
- 6.Anderson PL, Kiser JJ, Gardner EM, Rower JE, Meditz A, Grant RM. Pharmacological considerations for tenofovir and emtricitabine to prevent HIV infection. J Antimicrob Chemother 2011; 66:240–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orrell C, Cohen K, Leisegang R, Bangsberg DR, Wood R, Maartens G. Comparison of six methods to estimate adherence in an ART-naïve cohort in a resource-poor setting: Which best predicts virological and resistance outcomes? AIDS Res Ther 2017; 14:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sinxadi PZ, Leger PD, McIlleron HM, Smith PJ, Dave JA, Levitt NS, et al. Pharmacogenetics of plasma efavirenz exposure in HIV-infected adults and children in South Africa. Br J Clin Pharmacol 2015; 80:146–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castillo-Mancilla JR, Zheng J-H, Rower JE, Meditz A, Gardner EM, Predhomme J, et al. Tenofovir, Emtricitabine, and Tenofovir Diphosphate in Dried Blood Spots for Determining Recent and Cumulative Drug Exposure. AIDS Res Hum Retroviruses 2013; 29:384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castillo-mancilla JR, Searls K, Caraway P, Zheng J, Gardner EM, Predhomme J, et al. Short Communication : Tenofovir Diphosphate in Dried Blood Spots As an Objective Measure of Adherence in HIV-infected Women. AIDS Res Hum Retroviruses 2015; 31:428–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castillo-Mancilla JR, Morrow M, Coyle RP, Coleman SS, Gardner EM, Zheng J, et al. Tenofovir Diphosphate in Dried Blood Spots Is Strongly Associated With Viral Suppression in Individuals With Human Immunodeficiency Virus Infections. Clin Infect Dis 2018; Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson PL, Liu AY, Castillo-Mancilla JR, Gardner EM, Seifert SM, McHugh C, et al. Intracellular Tenofovir-Diphosphate and Emtricitabine-Triphosphate in Dried Blood Spots following Directly Observed Therapy. Antimicrob Agents Chemother 2017; 62:e01710–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warne P, Robbins RN, Anderson PL, Gouse H, Joska J, Leu C-S, et al. Utility of Dried Blood Spot-Derived ARV Biomarkers as an Objective Measure of Treatment Adherence in South Africa. In: 10th International Conference on HIV Treatment and Prevention Adherence Miami, FL, USA; 2015. [Google Scholar]
- 14.Myer L, Phillips TK, Zerbe A, Ronan A, Hsiao N, Mellins CA, et al. Optimizing Antiretroviral Therapy ( ART ) for Maternal and Child Health ( MCH ): Rationale and design of the MCH-ART study. J Acquir Immune Defic Syndr 2016; 72:189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Myer L, Phillips TK, Zerbe A, Brittain K, Lesosky M, Hsiao N-Y, et al. Integration of postpartum healthcare services for HIV-infected women and their infants in South Africa: A randomised controlled trial. PLOS Med 2018; 15:e1002547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson IB, Lee Y, Michaud J, Fowler FJ, Rogers WH. Validation of a New Three-Item Self-Report Measure for Medication Adherence. AIDS Behav 2016; 20:2700–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phillips T, Brittain K, Mellins CA, Zerbe A, Remien RH, Abrams EJ, et al. A Self-Reported Adherence Measure to Screen for Elevated HIV Viral Load in Pregnant and Postpartum Women on Antiretroviral Therapy. AIDS Behav 2017; 21:450–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bienczak A, Cook A, Wiesner L, Olagunju A, Mulenga V, Kityo C, et al. The impact of genetic polymorphisms on the pharmacokinetics of efavirenz in African children. Br J Clin Pharmacol 2016; 82:185–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baxi SM, Greenblatt RM, Bacchetti P, Scherzer R, Minkoff H, Huang Y, et al. Common clinical conditions-age, low BMI, ritonavir use, mild renal impairment-affect tenofovir pharmacokinetics in a large cohort of HIV-infected women. Aids 2014; 28:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu Y, Goti V, Chaturvedula A, Haberer JE, Fossler MJ, Sale ME, et al. Population Pharmacokinetics of Tenofovir in HIV-1-Uninfected Members of Serodiscordant Couples and Effect of Dose Reporting Methods. Antimicrob Agents Chemother 2016; 60:5379–5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michels WM, Grootendorst DC, Verduijn M, Elliott EG, Dekker FW, Krediet RT. Performance of the Cockcroft-Gault, MDRD, and new CKD-EPI formulas in relation to GFR, age, and body size. Clin J Am Soc Nephrol 2010; 5:1003–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilhelm AJ, den Burger JCG, Swart EL . Therapeutic Drug Monitoring by Dried Blood Spot: Progress to Date and Future Directions. Clin Pharmacokinet 2014; 53:961–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeLong E, DeLong D, Clarke-Pearson D. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988; 44:837–845. [PubMed] [Google Scholar]
- 24.Orrell C, Bienczak A, Cohen K, Bangsberg D, Wood R, Maartens G, et al. Effect of mid-dose efavirenz concentrations and CYP2B6 genotype on viral suppression in patients on first-line antiretroviral therapy. Int J Antimicrob Agents 2016; 47:466–472. [DOI] [PubMed] [Google Scholar]
- 25.Dickinson L, Amin J, Else L, Boffito M, Egan D, Owen A, et al. Comprehensive Pharmacokinetic, Pharmacodynamic and Pharmacogenetic Evaluation of Once-Daily Efavirenz 400 and 600 mg in Treatment-Naïve HIV-Infected Patients at 96 Weeks: Results of the ENCORE1 Study. Clin Pharmacokinet 2016; 55:861–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hendrix CW, Andrade A, Bumpus NN, Kashuba AD, Marzinke MA, Moore A, et al. Dose Frequency Ranging Pharmacokinetic Study of Tenofovir-Emtricitabine After Directly Observed Dosing in Healthy Volunteers to Establish Adherence Benchmarks (HPTN 066). AIDS Res Hum Retroviruses 2016; 32:32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pyra M, Anderson PL, Hendrix CW, Heffron R, Mugwanya K, Haberer JE, et al. Tenofovir and tenofovir-diphosphate concentrations during pregnancy among HIV-uninfected women using oral preexposure prophylaxis. AIDS 2018; 32:1891–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Podsadecki TJ, Vrijens BC, Tousset EP, Rode RA, Hanna GJ. “White Coat Compliance” Limits the Reliability of Therapeutic Drug Monitoring in HIV-1—Infected Patients. HIV Clin Trials 2008; 9:238–246. [DOI] [PubMed] [Google Scholar]
- 29.Wilson IB, Bangsberg DR, Shen J, Simoni JM, Reynolds NR, Goggin K, et al. Heterogeneity Among Studies in Rates of Decline of Antiretroviral Therapy Adherence Over Time. JAIDS J Acquir Immune Defic Syndr 2013; 64:448–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cattaneo D, Minisci D, Baldelli S, Mazzali C, Giacomelli A, Milazzo L, et al. Effect of cobicistat on tenofovir disoproxil fumarate (TDF). JAIDS J Acquir Immune Defic Syndr 2017; 77:1. [DOI] [PubMed] [Google Scholar]
- 31.Haaland RE, Martin A, Holder A, Fountain JJ, Hall L, Pescatore NA, et al. Urine tenofovir and emtricitabine concentrations provide biomarker for exposure to HIV preexposure prophylaxis. AIDS 2017; 31:1647–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koenig HC, Mounzer K, Daughtridge GW, Sloan CE, Lalley-Chareczko L, Moorthy GS, et al. Urine assay for tenofovir to monitor adherence in real time to tenofovir disoproxil fumarate/emtricitabine as pre-exposure prophylaxis. HIV Med 2017; 18:412–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S figure 1. Histograms of a) plasma efavirenz, b) plasma tenofovir, c) tenofovir-diphosphate (TFV-DP) concentrations in dried blood spots (DBS), d) self-reported adherence in the past 30 days (three-item scale score)
S figure 2. Unadjusted area under the receiver operating characteristics (ROC) curves of DBS TFV-DP (grey), plasma EFV (green), plasma TFV (maroon), and self-reported adherence (blue) to predict viral suppression; n=137
S figure 3: Scatter plot of tenofovir-diphosphate (TFV-DP) in DBS and plasma tenofovir (TFV) concentrations.
S table 1. Three-item self-reported adherence scale.
S table 2. Characteristics of DBS TFV-DP thresholds restricted to women who report taking ART in the last 30 days (n=87). Odds ratios (OR) predicting viral load <50, <400 and <1000 copies/mL are presented.
S table 3. Univariable logistic regression models predicting viral load <50, <400 and <1000 copies/mL among 137 women.
S table 4. Women with detectable plasma tenofovir (TFV) and efavirenz (EFV) concentrations but very low tenofovir-diphosphate concentration in dried blood spots (DBS TFV-DP)