Abstract
Background:
Wernicke’s encephalopathy (WE) is a neurological condition resulting from thiamine deficiency. Although commonly associated with alcoholism, non-alcoholic WE has been described in individuals with Human Immunodeficiency Virus (HIV) infection, but subclinical WE may be underdiagnosed. The current study questioned whether presence of subclinical WE signs underlies cognitive and motor deficits in HIV individuals as observed in alcoholism.
Setting:
56 HIV positive individuals (HIV+) and 53 HIV negative controls (HIV−) were assessed on 6 cognitive and motor domains: attention/working memory, production, immediate and delayed episodic memory, visuospatial abilities, and upper limb motor function.
Methods:
Based on a rating scheme by Caine et al, HIV+ individuals were categorized by subclinical WE risk factors (dietary deficiency, oculomotor abnormality, cerebellar dysfunction, and altered mental state). Performance was expressed as age- and education-corrected Z-scores standardized on controls.
Results:
Sorting by Caine criteria yielded 20 HIV+ as Caine 0 (i.e., meeting no criteria), 22 as Caine 1 (i.e., meeting one criterion), and 14 as Caine 2 (i.e., meeting two criteria). Comparison among HIV+ Caine subgroups revealed a graded effect: Caine 0 performed at control levels, Caine 1 showed mild to moderate deficits on some domains, and Caine 2 showed the most severe deficits on each domain.
Conclusion:
This graded severity pattern of performance among Caine subgroups suggests that signs of subclinical WE can partly explain the heterogeneity in HIV-related cognitive and motor impairment. This study highlights the utility of Caine criteria in identifying potential causes of HIV-related neurocognitive disorders and has implications for disease management.
Keywords: Human Immunodeficiency Virus, Caine criteria, Wernicke’s encephalopathy, neurocognition, heterogeneity
INTRODUCTION
Treatment of HIV infection with highly active antiretroviral therapy (HAART) and combination antiretroviral therapy (cART) has been successful in reducing viral load and improving immune system functioning. As a consequence, the prevalence of central nervous system (CNS) opportunistic infections, acquired immunodeficiency syndrome (AIDS), and premature death in people living with HIV has decreased 1-4. However, despite pharmacological treatment, HIV-associated neurocognitive disorder (HAND) occurs in upwards of 50% of HIV-infected patients 5-12 and is marked by heterogeneity in the severity of cognitive impairment, ranging from asymptomatic neuropsychological impairment (ANI) to mild neurocognitive disorder (MND) and HIV-associated dementia (HAD) 13-17.
Antiretroviral therapy (ART) has been instrumental in preventing the development of the most severe stage of HAND, i.e., HAD 18, but even in this era of improved pharmacological therapy, mild and moderate cognitive and motor dysfunctions endure in HIV-infected individuals19,20. HIV infection is associated with deficits in selective cognitive domains including attention/working memory and executive functioning 7,21-27, episodic memory 26,28,29, psychomotor speed 26, and visuospatial abilities 23,30. Motor domains are also affected and include deficits in balance, gait, and manual dexterity 31-34.
Wernicke’s encephalopathy (WE), an acute neurological condition resulting from severe thiamine depletion or deficiency, has been historically diagnosed by a clinical triad of symptoms: oculomotor abnormalities, gait and balance instability, and altered mental state 35. Although this neurological syndrome is commonly associated with alcoholism, non-alcoholic Wernicke’s encephalopathy can occur in individuals with HIV infection with or without AIDS and subclinical WE could be underdiagnosed in clinical practice due to confounding HIV-related etiologies, notably infectious and neoplastic etiologies due to the immunosuppressed state 36-39. Indeed, the diagnosis of WE is often made only postmortem and could be missed in as many as 75-80% in patients with alcoholism and AIDS 40,41. Thiamine deficiency was observed in a large proportion of HIV-positive individuals in advanced stages of HIV infection and in clinically asymptomatic patients in the pre- and post-HAART era 41-43. A recent review reported that genetic studies enabled determination of selective proteins linking thiamine to HIV pathology, although thiamine also affects HIV through non-genomic factors (for review 44).
By conducting chart reviews of clinical histories of alcoholic patients with definitive postmortem neuropathological WE diagnosis not identified during their lifetime, Caine et al (1997) 45 proposed operational criteria enabling the antemortem identification of subclinical WE with a high degree of specificity. Postmortem WE diagnosis required the historical in vivo presence of just two of the following criteria: (1) dietary deficiency, (2) oculomotor abnormality, (3) cerebellar dysfunction, or (4) an altered mental state or cognitive impairment. In vivo studies have identified graded effects in neuropsychological performance when alcoholic individuals were classified by number of Caine criteria met 46,47. Those meeting two or more criteria demonstrated the most severe cognitive impairment compared with those meeting none or just one criterion. These observations raised the question whether the presence of subclinical WE signs as defined by Caine et al could contribute to the heterogeneity of cognitive and motor deficits observed in HIV-infected patients 14-16.
The present study aimed to determine whether the heterogeneity in the pattern and severity of cognitive and motor performance deficits observed in HIV infection could be partially explained by risk factors for WE. We tested the following hypotheses: (1) categorizing the HIV+ group by Caine criteria would reveal a stepwise deficit severity, where HIV+ participants meeting two or more criteria would show the greatest and most widespread cognitive and motor deficits as similarly observed in alcoholic individuals and (2) given the prolonged life expectancy of ART-treated HIV-infected individuals, aging, disease-related clinical variables (e.g., CD4 cell count, log viral load, and length of time with HIV), comorbid infections such as hepatitis C virus, and AIDS status represent additional factors of the heterogeneity of HIV-associated neurocognitive impairment. Therefore, we hypothesized that risk factors for WE would predict cognitive and motor performance above and beyond these other clinical variables.
METHODS
Participants
Study participants included 56 HIV seropositive individuals (HIV+; 40 men and 16 women) and 53 individuals seronegative for HIV infection (HIV−; 29 men and 24 women). HIV+ individuals were included between 2008 and 2017, with 36 HIV+ participants between 2008-2013 and 20 HIV+ participants between 2014-2017. Data from these HIV− control participants were included in earlier studies that examined cognitive impairment in alcoholism related to Caine criteria 46,47. The current study was conducted in accordance with protocols approved by the Institutional Review Boards of Stanford University and SRI International. Written informed consent was obtained from all participants in accordance with the Declaration of Helsinki.
All participants were screened using the Structured Clinical Interview for DSM-IV (SCID) 48 and structured health questionnaires. Upon initial assessment, subjects were excluded if they had fewer than 8 years of education, a significant history of medical (e.g., epilepsy, stroke, multiple sclerosis, uncontrolled diabetes or loss of consciousness >30 minutes), psychiatric (i.e., schizophrenia or bipolar I disorder), or neurological disorder (e.g., neurodegenerative disease). All participants completed the Ohio State University Traumatic Brain Injury Identification Method-short form49 and none of them reported signs of lifetime history of TBI. Other exclusionary criteria were recent (i.e., past month) substance abuse or dependence other than nicotine in the HIV+ group according to the DSM-IV criteria, HIV-related opportunistic infection in the HIV+ group, or any DSM-IV Axis I disorder in the HIV− control group. No HIV− control participant met DSM-IV criteria for substance abuse or dependence. Of the 56 HIV positive participants, 28 (50%) had a lifetime history of DSM-IV substance abuse/dependence. Of the 28 participants with a lifetime history of DSM-IV substance abuse/dependence, 18 (64.3%) had Cocaine Abuse/Dependence, 16 (57.1%) had Cannabis Abuse/Dependence, 9 (32.1%) had Amphetamine Abuse/Dependence, 2 (7.1%) had Opioid Abuse/Dependence, and 1 (3.6%) had Hallucinogen Abuse/Dependence. Of the 28 HIV positive participants with a lifetime history of substance abuse/dependence, all were in remission (mean remission time = 622.7 ± 498.7 weeks; median = 516 weeks; range = 9 to 1395 weeks). For nicotine, 11 (19.6 percent) of the 56 HIV+ participants were current smokers and 10 HIV+ (17.9 percent) were past smokers, whereas 1 of 53 (1.4 percent) HIV− controls were current smokers, and 2 (2.8 percent) were past smokers. In the group of 56 HIV+ subjects, 51 participants had no history of alcohol abuse or dependence, four had a remote history of alcohol abuse lasting 4 months to 6 years and one participant had a remote history of alcohol dependence lasting 2 years. Regarding DSM-IV alcohol dependence/abuse remission criteria at the time of visit, these five HIV+ individuals were in full sustained remission with length of abstinence from 5 years to 34 years for the four HIV+ subjects with alcohol abuse history and 22 years for the HIV+ individual with alcohol dependence history.
Blood assays confirmed self-report of HIV status via antibody testing, determined plasma viral load (log10 transformed), and CD4 T-lymphocyte count in the HIV+ group. Hepatitis C status was also tested. Twenty-eight of the 55 HIV+ participants met CDC criteria for AIDS (CD4 cell count <200 or an AIDS-defining event, e.g., coccidioidomycosis) sometime during the course of their illness. At the time of testing, only 2 of these 28 HIV+ participants had CD4 T-lymphocyte count <200. Most of the HIV+ participants (50 HIV, 90.9%) were on HAART (highly active antiretroviral therapy). In addition, 16 of the 55 (29.1%) HIV+ subjects tested positive for the hepatitis C virus (HCV). Severity of depressive symptoms was assessed with the Beck Depression Inventory-II 50.
Demographics for the HIV+ and HIV− groups are presented in Table 1. HIV+ were older, had fewer years of education, and endorsed more depressive symptoms than HIV− controls.
Table 1.
Demographic and clinical characteristics of participants
| Demographic Variables | HIV− controls | HIV+ | p value |
|---|---|---|---|
| n = | 53 | 56 | |
| BACKGROUND DEMOGRAPHICS | |||
| Sex (Men/Women) | 29/24 | 40/16 | ns |
| Age | 47.8 ± 13.2 | 52.5 ± 7.9 | .03 [HIV+ > HIV−] |
| Education (years) | 15.7 ± 2.5 | 13.7 ± 2.5 | <.001 [HIV+ < HIV−] |
| Hepatitis C (%) | 0% | 29.1% | / |
| Beck Depression Inventory-II (BDI-II) Score * | 2.4 ± 3.5; 1.0 | 9.8 ± 8.4; 8.5 | <.001 [HIV+ > HIV−] |
| HIV-RELATED DEMOGRAPHICS | |||
| HIV Approx. Age of Onset | / | 35.9 ± 9.5 | / |
| Length of time with HIV (years) | / | 16.6 ± 8.2 | / |
| Log Viral Load (n = 51) | / | 2.1 ± 1.1 | / |
| CD4 Count* (n = 54) | / | 611.3 ± 286.0; 571.0 | / |
| AIDS Status (%) (n = 55) | / | 50.9% | / |
| HAART Medications (%) (n = 55) | / | 90.9% | / |
Mean ± SD; median
ns=not significant
Neuropsychological test measures
Six cognitive and motor domains were assessed: attention/working memory, production (ability to initiate, plan, and strategize), immediate episodic memory, delayed episodic memory, visuospatial construction, and upper limb motor speed/dexterity.
Attention/working memory
The Trail Making Test (Trails A and B) 51 required participants to sequence numbers from 1 to 25 and then sequence numbers and letters in alternating fashion (1–A–2–B–3–C…). Time to completion for each condition was the dependent measure.
Production ability
The Controlled Oral Word Association Test 52 required participants to say aloud as many words as they could beginning with the letters F, A and S. Each trial was 60 seconds. The total number of correct words produced across the three trials was calculated.
Ruff Figural Fluency Test 53, often considered a visual analog of the Controlled Oral Word Association Test, required participants to generate as many different designs as possible by using straight lines to connect at least two of the five dots in each array. The test consists of five conditions, some with distractors and each with a 1-minute time limit. The total number of correct unique designs was summed across the five conditions.
Immediate memory
Logical Memory-I (WMS-R) 54 required participants to recall two short narratives immediately after each story was read to them. Number of details recalled from both stories was summed (max = 50).
Rey–Osterrieth Complex Figure—immediate recall 55 required participants to draw a complex figure from memory immediately after having copied it. The number of details recalled according to standardized scoring instructions was calculated (max = 36).
Delayed memory
Logical Memory-II (WMS-R) 54 had participants recall the short narratives presented after a 30-minute delay.
Rey–Osterrieth Complex Figure —delayed recall 55 required participants to draw from memory the complex figure after a 30-minute delay.
Visuospatial construction
Rey–Osterrieth Complex Figure—copy condition required participants to copy the complex figure as accurately as possible. Scoring was according to standardized criteria.
Upper limb motor performance
Fine finger movement 56 required participants to turn a knurled rod with their forefinger and thumb, unimanually and then bimanually. Three, 30-second trials for each condition were administered with the unimanual and bimanual scores reflecting the average number of rotations across trials.
Grooved Pegboard 57 required participants to insert keyed pegs into a 5 × 5 grooved pegboard. The times to completion for right and left hand trials were averaged.
Caine criteria
The four Caine criteria were operationalized in this study as follows: (1) Dietary Deficiency—showing abnormal low serum levels of prealbumin assessing current nutritional status (Adult Men: <21 mg/dL, normal range 21-43 mg/dL; Adult Women: <17 mg/dL, normal range 17-34 mg/dL), (2) Oculomotor Disturbance—exhibiting nystagmus (i.e., rapid and uncontrollable eyes movements) on neurological examination, determined by the observation of saccades and smooth pursuit during frontal eye gaze and fixed head position while subjects tracked the examiner’s index finger as it moved to the extreme right, extreme left, upward, and downward; an oculomotor abnormality is scored for participants exhibiting moderate (i.e., with fast phase to left or right, looking straight ahead) or severe (i.e., with fast phase to left when looking toward the right or to the right when looking toward the left) nystagmus, (3) Cerebellar Dysfunction—scoring at least 1.5 standard deviations below the mean for age-matched and sex-matched controls on at least two of the four balance and ataxia measures with eyes open [standing heel-to-toe on a line for 60 seconds (Romberg), walking 10 steps on a line, balancing on the right leg for 30 seconds and balancing on the left leg for 30 seconds] 58, and (4) Altered Mental State or Mild Cognitive Impairment—scoring below 137 out of 144 points on the Dementia Rating Scale 59,60.
HIV+ participants were assessed for Caine criteria: 20 did not meet any criteria (36%, Caine 0), 22 met one criterion (39%, Caine 1), 14 met two criteria (25%, Caine 2), and no one met three or four criteria. In the HIV+ Caine 1 subgroup, one patient met the dietary deficiency criterion, one had oculomotor disturbance, 12 had cerebellar dysfunction, and 8 had mild cognitive impairment. In the HIV+ Caine 2 subgroup, one patient had dietary deficiency and mild cognitive impairment criteria, 2 had dietary deficiency and cerebellar dysfunction criteria, and 11 met cerebellar dysfunction and mild cognitive impairment criteria.
Statistical analyses
Test scores were statistically corrected for age and education and standardized on the HIV− control group [mean and standard deviation: Z = 0 ± 1], allowing direct comparisons across groups and tests. Where higher raw scores indicated worse performance, scores were multiplied by −1, so that lower Z-scores always indicated worse performance. Theoretically- derived composite scores were then calculated as the mean of all Z-scores of tests comprising each of six cognitive and motor domains: attention/working memory, production, immediate memory, delayed memory, visuospatial construction and upper limb motor function. First, we conducted initial confirmatory analyses to attest that HIV+ individuals had mild to moderate deficits on cognitive (i.e., executive functions, episodic memory and visuospatial construction) and motor (i.e., upper limb motor speed and dexterity) measures compared with HIV− healthy control participants as observed in previous studies. Two sample t-tests tested group differences on the composite scores and individual neuropsychological test scores between HIV+ and HIV− controls. Second, one-tailed nonparametric Spearman rank-order correlations were conducted within the HIV+ group to test relations between age, depression (BDI-II score), and disease-related variables (length of time with HIV infection, log plasma viral load, and CD4 T-lymphocyte count) and neuropsychological composite scores, with the following directional hypotheses: poorer cognitive performance was predicted to be related to older age, a higher current BDI-II score, longer disease duration, higher viral load, and lower CD4 T-lymphocyte count. Furthermore, nonparametric Mann-Whitney U analyses explored subgroup differences within the HIV+ group to assess whether individuals with hepatitis C or AIDS demonstrated greater cognitive impairment than those without hepatitis C or AIDS. Third, analysis of variance (ANOVA) tested group differences on the composite scores among the HIV− controls and the three Caine subgroups in the HIV+ group (i.e., HIV+ individuals with no Caine criteria, those meeting one Caine criterion, and those meeting two or more Caine criteria), with follow-up post-hoc analyses examining two group comparisons for significant omnibus results. Finally, when correlations were observed between age, depression (BDI-II score), disease-related variables and specific cognitive and motor composite scores, supplementary analyses of covariance (ANCOVAs) were conducted to control for these additional factors potentially explaining the heterogeneity of HIV-associated neurocognitive impairment.
RESULTS
Comparisons between HIV− controls and HIV+ participants on cognitive and motor performance: confirmatory analyses
Neuropsychological test composite scores
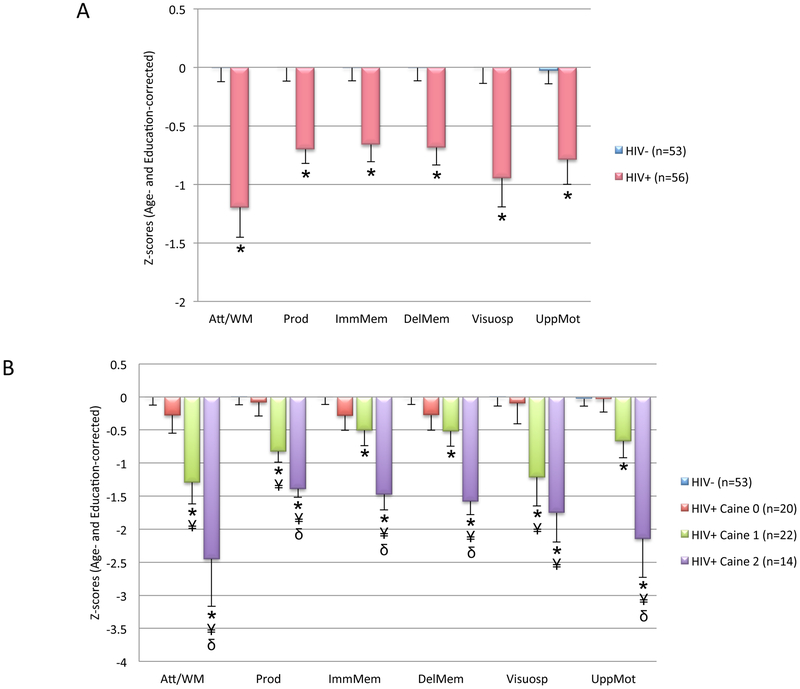
Two sample t-tests conducted on the six cognitive and motor domains revealed that the HIV+ group scored lower than the HIV− group on all age-corrected and education-corrected cognitive and motor composite scores: attention/working memory, production, immediate episodic memory, delayed episodic memory, visuospatial construction and upper limb motor function (Table 2 and Figure 1A). HIV+ subgroups divided into individuals with and those without lifetime drug history did not differ on any composite score.
Table 2.
Age- and education-corrected Z-scores for neuropsychological composite scores and individual tests (mean and standard deviation)
| HIV+ | HIV− controls |
t | Cohen’s d | p value | |
|---|---|---|---|---|---|
| Neuropsychological composite scores a | |||||
| Attention/Working Memory | −1.20 (1.90) | 0.00 (0.88) | −4.16 | 0.81 | <.001 |
| Production | −0.70 (0.91) | 0.00 (0.85) | −4.10 | 0.79 | <.001 |
| Immediate Episodic Memory | −0.66 (1.08) | 0.00 (0.82) | −3.52 | 0.69 | <.001 |
| Delayed Episodic Memory | −0.68 (1.09) | 0.00 (0.82) | −3.66 | 0.71 | <.001 |
| Visuospatial Construction | −0.95 (1.83) | 0.00 (1.01) | −3.32 | 0.64 | .001 |
| Upper Limb Motor Function | −0.79 (1.53) | −0.03 (0.83) | −3.14 | 0.62 | .002 |
| Neuropsychological individual tests b | |||||
| Trails Ac | −0.80 (1.40) | 0.00 (1.01) | −3.15 | 0.66 | .002 |
| Trails Bc | −1.69 (3.05) | 0.00 (1.01) | −3.82 | 0.74 | <.001 |
| FAS total | −0.97 (1.24) | 0.00 (1.02) | −4.41 | 0.85 | <.001 |
| Ruff Figural Fluency total | −0.43 (0.95) | 0.00 (1.02) | −2.23 | 0.45 | .03 |
| Logical Memory-immediate | −0.79 (1.32) | 0.00 (1.01) | −3.47 | 0.67 | <.001 |
| Rey-Osterrieth-immediate | −0.50 (1.28) | 0.00 (1.01) | −2.26 | 0.43 | .03 |
| Logical Memory-delayed | −0.90 (1.38) | 0.00 (1.02) | −3.83 | 0.74 | <.001 |
| Rey-Osterrieth-delayed | −0.43 (1.18) | 0.00 (1.01) | −2.04 | 0.39 | .04 |
| Rey-Osterrieth-copy | −0.95 (1.83) | 0.00 (1.01) | −3.32 | 0.64 | .001 |
| Grooved Pegboardc | −1.27 (2.37) | 0.00 (0.93) | −3.57 | 0.71 | <.001 |
| Fine Finger Movement | −0.32 (0.96) | 0.00 (0.93) | −1.69 | 0.34 | ns |
ns=not significant
Collected on a range of 52 and 56 HIV individuals and on a range of 51 and 53 controls
Collected on a range of 54 and 56 HIV individuals and on a range of 52 and 53 controls
Test scores were inverted to have higher scores reflect better performance
Figure 1.
Neuropsychological performance: (A) Age- and education-corrected Z-scores on the cognitive and motor composites in the HIV− controls (HIV−) and HIV seropositive participants (HIV+) (mean and standard error of the mean). * Significant difference compared with HIV− (p<.05), (B) Neuropsychological performance in the HIV− group and three Caine subgroups of HIV+ patients (mean and standard error of the mean). * Significant difference compared with HIV− (p<.05). ¥ Significant difference compared with HIV+ participants who did not meet any criteria (HIV+ CAINE 0, p<.05). δ Significant difference compared with HIV+ participants at risk of WE (one criterion, HIV+ CAINE 1, p<.05). Neuropsychological data collected on a range of 18 and 20 HIV+ Caine 0, 22 HIV+ Caine 1, and a range of 12 and 14 HIV+ Caine 2.
Scores on individual neuropsychological tests
Two sample t-tests conducted on the age- and education-corrected individual test scores revealed that HIV+ scored significantly lower than HIV− on ten cognitive and motor measures: Trails A and B, FAS, Ruff Figural Fluency, Logical Memory (immediate and delayed memory), Rey-Osterrieth Complex Figure (copy, immediate, and delayed memory), and Grooved Pegboard (Table 2). Groups did not differ on Fine Finger Movement.
Within-group analyses in the HIV+ group
Relations between age and disease-related variables and composite scores in the HIV+ group
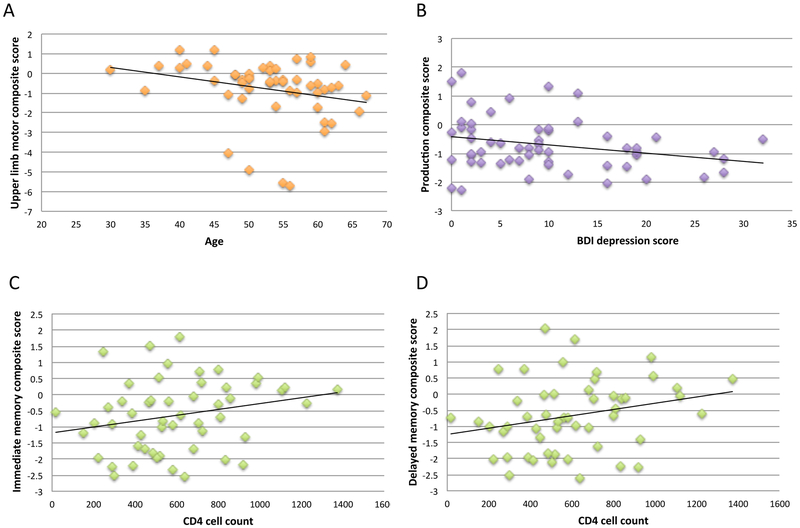
Poorer upper limb motor score correlated with older age (N=52; rho=−0.37, p=.003). Higher BDI-II score correlated with lower production composite score (N=56; rho=−0.23, p=.047. Lower CD4 cell counts correlated with lower immediate memory composite score (N=52; rho=0.25, p=.034) and lower delayed memory composite score (N=52; rho=0.27, p=.025) (Figure 2). By contrast, longer disease duration and higher viral load did not correlate with cognitive or motor composite scores in the HIV+ group.
Figure 2.
In the HIV+ group, scatterplots for significant correlations between (A) age and upper limb motor composite score, (B) depression and production composite score, (C) CD4 cell count and immediate memory composite score, (D) CD4 cell count and delayed memory composite score.
Hepatitis C, AIDS, and composite scores
The three HIV+ Caine subgroups were comparable in terms of hepatitis C and AIDS distribution (Table 3).
Table 3.
Demographic and clinical characteristics of the three HIV+ Caine subgroups
| HIV+ Caine 0 (n=20) |
HIV+ Caine 1 (n=22) |
HIV+ Caine 2 (n=14) |
p value | |
|---|---|---|---|---|
| Age | 49.7 ± 6.3 | 53.3 ± 9.1 | 55.2 ± 7.3 | ns |
| Education (years) | 14.8 ± 1.5 | 13.5 ± 3.2 | 12.6 ± 2.1 | <.05 [HIV+ Caine 2 <HIV+ Caine 0] |
| Beck Depression Inventory-II (BDI-II) Score * | 8.5 ± 7.0; 9.0 | 9.5 ± 8.8; 7.5 | 12.2 ± 9.5; 9.0 | ns |
| Approximate Age of HIV Onset | 33.6 ± 9.0 | 38.5 ± 9.0 | 34.9 ± 10.3 | ns |
| Disease Duration (years) | 16.0 ± 8.4 | 14.7 ± 8.2 | 20.4 ± 7.1 | ns |
| Log Viral Loada | 2.0 ± 1.0 | 2.1 ± 1.2 | 2.2 ± 1.3 | ns |
| CD4 Count*b | 701.7 ± 273.4; 711.0 | 582.7 ± 289.4; 538.5 | 527.3 ± 283.7; 519.0 | ns |
| Lifetime Alcohol Consumption (kg)* | 83.8 ± 84.3; 69.5 | 72.0 ± 75.8; 50.0 | 85.9 ± 80.8; 79.5 | ns |
| Hepatitis Cc | ||||
| Yes | 2 | 8 | 6 | .06, Χ2=5.65 |
| No | 18 | 13 | 8 | |
| AIDSc | ||||
| Yes | 8 | 14 | 6 | .30, Χ2=2.38 |
| No | 11 | 8 | 8 | |
| Drug History | ||||
| Yes | 11 | 10 | 7 | .83, Χ2=.38 |
| No | 9 | 12 | 7 | |
Mean ± SD; median
ns=not significant
Collected on 17 HIV+ Caine 0, 21 HIV+ Caine 1, and 13 HIV+ Caine 2
Collected on 19 HIV+ Caine 0, 22 HIV+ Caine 2, and 13 HIV+ Caine 2
Collected on 55 HIV+ individuals
Individuals with and without seropositive hepatitis C status were comparable in age and education. HIV+ patients seropositive for hepatitis C achieved lower scores on the attention/working memory composite score (mean±stdev: −2.28±2.51) than those without hepatitis C (mean±stdev: −0.75±1.41) (U=188.00, p=.029). Other differences related to hepatitis C were not significant.
Individuals with and without history of AIDS were comparable in age and education, as well as lifetime alcohol consumption, HIV disease duration, HIV age of onset, log viral load, CD4 count, and hepatitis C distribution. A history of AIDS was not associated with lower scores on any composite.
Comparisons between HIV− and Caine-determined HIV+ subgroups on cognitive and motor performance
The HIV+ Caine subgroups did not differ on age, Beck Depression Inventory-II (BDI-II) score, age of HIV infection onset, length of HIV infection, log viral load, or CD4 count (Table 3). Groups did differ on years of education, with Caine 2 having fewer years of education than Caine 0. Two of the four HIV+ participants with remote history of alcohol abuse were in the HIV+ Caine 0 group, one in the HIV+ Caine 1 group, and one in the HIV+ Caine 2 group. The HIV+ individual with remote history of alcohol dependence was in the HIV+ Caine 1 group. The HIV+ Caine subgroups did not differ on lifetime alcohol consumption (kg). The three HIV+ Caine subgroups were comparable in terms of drug history distribution (Table 3).
One-way ANOVAs revealed significant differences among the HIV+ Caine subgroups and HIV− controls on all six composite scores: attention/working memory [F(3,104) = 13.34, p <.001], production [F(3,103) = 14.20, p <.001], immediate memory [F(3,103) = 9.33, p <.001], delayed memory [F(3,103) = 10.90, p <.001], visuospatial construction [F(3,105) = 8.17, p <.001], and upper limb motor function [F(3,99) = 13.03, p <.001] (Figure 1B). Follow-up analyses indicated that for attention/working memory, production, immediate and delayed memory, and upper limb motor function, HIV+ Caine 2 demonstrated the most severe deficits, scoring lower than HIV+ Caine 1, HIV+ Caine 0, and HIV− controls. Further, for visuospatial construction composite score, HIV+ Caine 2 and HIV+ Caine 1 performed at the same level, but both scored lower than HIV+ Caine 0 and HIV− controls. Finally, HIV+ Caine 1 scored lower than HIV+ Caine 0 and HIV− controls on the attention/working memory, production, visuospatial construction composite scores, and lower than HIV− controls on the immediate and delayed memory, and motor composite scores.
Supplementary analyses reinforced these results when taking account confounding variables: (1) when age was included as a covariate for the analysis of the upper limb motor composite score, the significant difference endured among the HIV+ Caine subgroups [F(2,48)=7.52, p =.001], with HIV+ Caine 2 demonstrating the most severe deficits, scoring lower than HIV+ Caine 1 and HIV+ Caine 0; (2) when CD4 T-lymphocyte count was included as a covariate for the analyses of the immediate and delayed memory composite scores, the significant difference endured among the HIV+ Caine subgroups (immediate memory: [F(2,48) = 4.46, p =.02] and delayed memory [F(2,48) = 5.69, p =.006]), with HIV+ Caine 2 demonstrating the most severe deficits, scoring lower than HIV+ Caine 1 and HIV+ Caine 0; (3) when BDI-II score was included as a covariate for the analysis of the production composite score, significant difference was still observed among the HIV+ Caine subgroups [F(2,52) = 11.20, p <.001], with a graded effect where HIV+ Caine 2 demonstrated the most severe deficits, scoring lower than both HIV+ Caine 1 and HIV + Caine 0 and where HIV+ Caine 1 scored lower than HIV+ Caine 0.
DISCUSSION
Subclinical signs of WE and neurocognitive heterogeneity in HIV
Conducted on the entire HIV+ group, analyses on neurocognitive functions confirmed impairment in cognitive and motor domains previously shown to be affected by HIV infection: attention/working memory, production (i.e., verbal and visual fluency), immediate and delayed episodic memory, and motor dexterity skills 61,62. HIV+ patients also showed deficits in visuospatial abilities assessed by Rey–Osterrieth Complex Figure copy, which contrasts with other studies reporting relative sparing of this cognitive domain 63. Previous investigations report that cognitive and motor impairments are observed in upwards of 50% of HIV-infected patients despite ART treatment 5-7.
Classification by Caine criteria revealed that 25% of the individuals with HIV in this sample met two criteria of subclinical signs of WE, consistent with the assumption that WE may be underestimated in HIV infection. This study also shows that 39% of the HIV patients met one criterion and may, therefore, be considered at risk of subclinical WE. Significant heterogeneity in cognitive deficit severity 13 was unmasked by subgrouping HIV+ participants. Indeed, variability in HIV-related neurocognitive performance among the total group of HIV+ individuals was partially accounted for by the number of Caine criteria met. Comparison among HIV+ Caine subgroups revealed a graded effect, with those not meeting any criterion performing at control levels, those at risk for WE showing mild-to-moderate deficits on select cognitive and motor domains, and those with subclinical signs of WE showing the most severe deficits in each domain. This graded severity pattern of performance among Caine subgroups suggests that signs of subclinical WE can partly explain the heterogeneity in pattern and severity of HIV-related cognitive and motor impairments.
Age, hepatitis C, and neurocognitive heterogeneity in HIV
Older age was associated with poorer upper limb motor performance in HIV-infected individuals. Deficits in regional brain volumes of the caudate nucleus in HIV+ individuals is systematically reported in the literature and has been most consistently associated with cognitive impairment, particularly poor motor performance assessed with the Grooved Pegboard 64-66. Older age and HIV infection have been reported to be independently correlated with smaller volumes of the caudate, but some evidence for premature aging of the caudate has been noted in HIV-infection 67.
HIV-related cognitive disorder has also been related to comorbid conditions such as hepatitis C 68-70. Compromised attention, memory, and psychomotor speed 71-76, with less evidence for deficits in executive functioning 77,78 and fine motor coordination 79, have been reported in HCV mono-infection. A number of studies indicate that HIV/HCV co-infection results in higher level of cognitive impairment than HIV mono-infection 80-86. A recent meta-analysis indicated that co-infected patients are more likely to be impaired in information processing speed than HIV mono-infected patients 70. The current study consistently showed that co-infected individuals demonstrated worse performance on the attention/working memory composite score than those without HCV even if both groups were comparable with respect to age and education. In the present study, the attention/working memory composite score was calculated primarily from the mean of age- and education-corrected Z-scores from Trails A and B times. Trail making tests have been hypothesized to reflect a constellation of components processes including visual attention, sequencing and shifting, mental flexibility, working memory, and psychomotor speed 87. Therefore, worse attention/working memory performance in HIV/HCV co-infected individuals might be explained by their more severe processing speed deficit than HIV mono-infected individuals.
Immune system restoration, memory compromise, and clinical implications
These results suggest that Caine criteria assessment could be useful in clinical settings for the detection of subclinical WE in HIV infected individuals 88. Given the high prevalence of subclinical signs of WE in HIV+ participants and the report of thiamine deficiency in a large proportion of HIV-positive individuals in advanced stages of HIV infection as well as in clinically asymptomatic patients in the pre- and post-HAART era 41-43, thiamine supplementation could be considered as adjunctive therapy 44.
Similarly, the present study reported that low current CD4 cell count is associated with poor immediate and delayed episodic memory performance. Consistent relations between a low CD4 cell count in the past (i.e., CD4 nadir) and greater risk for HIV-related neurocognitive disorders have been reported in HIV+ individuals even after initiation of ART and immune recovery 6,89. Nevertheless, the association of sustained prevalent impairment with worse current immune status (low current CD4 cell count) was also observed, suggesting that restoring immunocompetence increases the likelihood of neurocognitive recovery 90. As suggested here, the cognitive domain of episodic memory may be particularly sensitive to a compromised immune system. Previous investigations have reported that micronutrient supplementation including thiamine can significantly improve CD4 cell counts in HIV-infected individuals on HAART 91. Thus, we speculate that thiamine supplementation is relevant for immune system restoration and prevention of memory decline in individuals with HIV infection.
Limitations and conclusion
An inherent limitation of this study is its retrospective nature restricting the choice to use prealbumin as a proxy for dietary deficiency or malnutrition. Serum proteins such as albumin and prealbumin have been widely used to determine nutritional status. Although prealbumin has been considered a more reliable indicator of acute changes in nutritional status than albumin 92, laboratory markers are not reliable by themselves. Rather, they should be supplemented by a comprehensive nutrition-focused physical examination including muscle wasting and subcutaneous fat loss in future research 92. Further, our analyses were based on a limited sample and require replication with a larger and more diverse group including HIV+ individuals meeting three or four Caine criteria. Nonetheless, the graded pattern of performance compromise among HIV+ Caine subgroups provides initial evidence that signs of subclinical WE contribute to the heterogeneity in HIV-related cognitive and motor impairment independent of other clinical variables such as age, depression level, disease-related variables (length of time with HIV infection, log plasma viral load, and CD4 T-lymphocyte count), hepatitis C, AIDS, alcohol and drug history. Even mild cognitive deficits challenge HIV disease management by compromising treatment adherence, daily life functioning, and quality of life 93-95. We conclude that Caine criteria can aid in determining cognitive and motor impairment severity and potentially in identifying HIV+ individuals at risk for WE, promoting thiamine as a supplement treatment to mitigate further cognitive and motor decline.
Acknowledgments
APLB, RF, EVS, NMZ, and AP were responsible for the study concept and design. SAS and NMZ contributed to the acquisition of data. APLB, RF, EVS, SAS and NMZ assisted with data analyses and collection. APLB, RF, EVS, SAS, AP and NMZ interpreted the findings. APLB and EVS drafted the manuscript. EVS, SAS, NMZ, RF and AP provided critical revision of the manuscript for important intellectual content. All authors have critically reviewed content and approved final version submitted for publication.
Conflicts of Interest and Source of Funding
The authors declare they have no conflict of interest to disclose.
The current analysis, writing, and manuscript preparation were supported by the U.S. National Institute on Alcohol Abuse and Alcoholism grants AA017347, AA017168, AA017923, AA010723, AA005965, and the Moldow Women’s Hope and Healing Fund.
References
- 1.Moore RD, Chaisson RE. Natural history of HIV infection in the era of combination antiretroviral therapy. Aids. 1999;13(14):1933–1942. [DOI] [PubMed] [Google Scholar]
- 2.Antiretroviral Therapy Cohort Collaboration. Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. The lancet HIV. 2017;4(8):e349–e356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwarcz L, Chen MJ, Vittinghoff E, Hsu L, Schwarcz S. Declining incidence of AIDS-defining opportunistic illnesses: results from 16 years of population-based AIDS surveillance. Aids. 2013;27(4):597–605. [DOI] [PubMed] [Google Scholar]
- 4.Maschke M, Kastrup O, Esser S, Ross B, Hengge U, Hufnagel A. Incidence and prevalence of neurological disorders associated with HIV since the introduction of highly active antiretroviral therapy (HAART). Journal of neurology, neurosurgery, and psychiatry. 2000;69(3):376–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schouten J, Cinque P, Gisslen M, Reiss P, Portegies P. HIV-1 infection and cognitive impairment in the cART era: a review. Aids. 2011;25(5):561–575. [DOI] [PubMed] [Google Scholar]
- 6.Heaton RK, Clifford DB, Franklin DR Jr., et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75(23):2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heaton RK, Franklin DR, Ellis RJ, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. Journal of neurovirology. 2011;17(1):3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sacktor N, McDermott MP, Marder K, et al. HIV-associated cognitive impairment before and after the advent of combination therapy. Journal of neurovirology. 2002;8(2):136–142. [DOI] [PubMed] [Google Scholar]
- 9.Clifford DB, Ances BM. HIV-associated neurocognitive disorder. Lancet Infectious Diseases. 2013;13(11):976–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clifford DB. HIV-associated neurocognitive disorder. Current opinion in infectious diseases. 2017;30(1):117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sacktor N, Robertson K. Evolving clinical phenotypes in HIV-associated neurocognitive disorders. Current Opinion HIV and AIDS. 2014;9:517–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saylor D, Dickens AM, Sacktor N, et al. HIV-associated neurocognitive disorder - pathogenesis and propects for treatment. Nature Reviews Neurology. 2016;12:234–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sacktor N, Skolasky RL, Seaberg E, et al. Prevalence of HIV-associated neurocognitive disorders in the Multicenter AIDS Cohort Study. Neurology. 2016;86(4):334–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dawes S, Suarez P, Casey CY, et al. Variable patterns of neuropsychological performance in HIV-1 infection. Journal of clinical and experimental neuropsychology. 2008;30(6):613–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vassallo M, Durant J, Lebrun-Frenay C, et al. Virologically suppressed patients with asymptomatic and symptomatic HIV-associated neurocognitive disorders do not display the same pattern of immune activation. HIV Med. 2015;16(7):431–440. [DOI] [PubMed] [Google Scholar]
- 16.Joseph J, Colosi DA, Rao VR. HIV-1 Induced CNS Dysfunction: Current Overview and Research Priorities. Current HIV research. 2016;14(5):389–399. [DOI] [PubMed] [Google Scholar]
- 17.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gates TM, Cysique LA. The Chronicity of HIV Infection Should Drive the Research Strategy of NeuroHIV Treatment Studies: A Critical Review. CNS drugs. 2016;30(1):53–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Letendre S Central nervous system complications in HIV disease: HIV-associated neurocognitive disorder. Topics in antiviral medicine. 2011;19(4):137–142. [PMC free article] [PubMed] [Google Scholar]
- 20.Simioni S, Cavassini M, Annoni JM, et al. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. Aids. 2010;24(9):1243–1250. [DOI] [PubMed] [Google Scholar]
- 21.Cattie JE, Doyle KL, Weber E, Grant I, Woods SP, Group THNRPH. Planning deficits in HIV-associated neurocognitive disorders: component processes, cognitive correlates, and implications for everyday functioning. Journal of clinical and experimental neuropsychology. 2012;34(9):906–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giesbrecht CJ, Thornton AE, Hall-Patch C, et al. Select neurocognitive impairment in HIV-infected women: associations with HIV load, Hepatitis C virus, and depression, but not leukocyte telomere length. PloS one. 2014;9(3):e89556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woods SP, Moore DJ, Weber E, Grant I. Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychology review. 2009;19(2):152–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hinkin CH, Castellon SA, Hardy DJ, Granholm E, Siegle G. Computerized and traditional stroop task dysfunction in HIV-1 infection. Neuropsychology. 1999;13(2):306–316. [DOI] [PubMed] [Google Scholar]
- 25.Martin EM, Pitrak DL, Rains N, et al. Delayed nonmatch-to-sample performance in HIV-seropositive and HIV-seronegative polydrug abusers. Neuropsychology. 2003;17(2):283–288. [DOI] [PubMed] [Google Scholar]
- 26.Fama R, Sullivan EV, Sassoon SA, Pfefferbaum A, Zahr NM. Impairments in Component Processes of Executive Function and Episodic Memory in Alcoholism, HIV Infection, and HIV Infection with Alcoholism Comorbidity. Alcoholism, clinical and experimental research. 2016;40(12):2656–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stout JC, Salmon DP, Butters N, et al. Decline in working memory associated with HIV infection. HNRC Group. Psychological medicine. 1995;25(6):1221–1232. [DOI] [PubMed] [Google Scholar]
- 28.Maki PM, Rubin LH, Valcour V, et al. Cogntive function in women with HIV: findings from the Women’s Interagency HIV study. Neurology. 2015;84:231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woods SP, Hoebel C, Pirogovsky E, et al. Visuospatial temporal order memory deficits in older adults with HIV infection. Cognitive and behavioral neurology : official journal of the Society for Behavioral and Cognitive Neurology. 2013;26(4):171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bogdanova Y, Neargarder S, Cronin-Golomb A. Mapping mental number line in physical space: vertical and horizontal visual number line orientation in asymptomatic individuals with HIV. Neuropsychologia. 2008;46(12):2914–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fama R, Eisen JC, Rosenbloom MJ, et al. Upper and lower limb motor impairments in alcoholism, HIV infection, and their comorbidity. Alcoholism, clinical and experimental research. 2007;31(6):1038–1044. [DOI] [PubMed] [Google Scholar]
- 32.Sullivan EV, Rosenbloom MJ, Rohlfing T, Kemper CA, Deresinski S, Pfefferbaum A. Pontocerebellar contribution to postural instability and psychomotor slowing in HIV infection without dementia. Brain imaging and behavior. 2011;5(1):12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson TW, Heinrichs-Graham E, Robertson KR, et al. Functional brain abnormalities during finger-tapping in HIV-infected older adults: a magnetoencephalography study. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2013;8(4):965–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prakash A, Hou J, Liu L, Gao Y, Kettering C, Ragin AB. Cognitive function in early HIV infection. Journal of neurovirology. 2017;23(2):273–282. [DOI] [PubMed] [Google Scholar]
- 35.Victor M, Adams RD, Collins GH. The Wernicke-Korsakoff Syndrome and Related Neurologic Disorders Due to Alcoholism and Malnutrition. 2nd edn Philadelphia, PA: F.A. Davis Company; 1989. [Google Scholar]
- 36.Larsen TR, Dragu D, Williams M. Wernicke’s Encephalopathy: An Unusual Consequence of the Acquired Immune Deficiency Syndrome-Case Report and Literature Review. Case reports in medicine. 2013;2013:709474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwenk J, Gosztonyi G, Thierauf P, Iglesias J, Langer E. Wernicke’s encephalopathy in two patients with acquired immunodeficiency syndrome. Journal of neurology. 1990;237(7):445–447. [DOI] [PubMed] [Google Scholar]
- 38.Alcaide ML, Jayaweera D, Espinoza L, Kolber M. Wernicke’s encephalopathy in AIDS: a preventable cause of fatal neurological deficit. International journal of STD & AIDS. 2003;14(10):712–713. [DOI] [PubMed] [Google Scholar]
- 39.Kamel E, Yerokhin O, Ionete C. A case of Wernicke’s encephalopathy responsive to thiamine as initial presentation of HIV seropositivity. Journal of Neurology, Neurosurgery and spine. 2017;2(1):1008. [Google Scholar]
- 40.Zuccoli G, Pipitone N. Neuroimaging findings in acute Wernicke’s encephalopathy: review of the literature. AJR American journal of roentgenology. 2009;192(2):501–508. [DOI] [PubMed] [Google Scholar]
- 41.Butterworth RF, Gaudreau C, Vincelette J, Bourgault AM, Lamothe F, Nutini AM. Thiamine deficiency and Wernicke’s encephalopathy in AIDS. Metabolic brain disease. 1991;6(4):207–212. [DOI] [PubMed] [Google Scholar]
- 42.Muri RM, Von Overbeck J, Furrer J, Ballmer PE. Thiamin deficiency in HIV-positive patients: evaluation by erythrocyte transketolase activity and thiamin pyrophosphate effect. Clinical nutrition (Edinburgh, Scotland). 1999;18(6):375–378. [DOI] [PubMed] [Google Scholar]
- 43.Shanina E, Gelman B, Smith RG. Thiamine deficiency in HIV associated NRTI neuropathy. Annals of neurology. 2008;64:S62. [Google Scholar]
- 44.Kv LN, Nguyen LT. The role of thiamine in HIV infection. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases. 2013;17(4):e221–227. [DOI] [PubMed] [Google Scholar]
- 45.Caine D, Halliday GM, Kril JJ, Harper CG. Operational criteria for the classification of chronic alcoholics: identification of Wernicke’s encephalopathy. Journal of neurology, neurosurgery, and psychiatry. 1997;62(1):51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pitel AL, Zahr NM, Jackson K, et al. Signs of preclinical Wernicke’s encephalopathy and thiamine levels as predictors of neuropsychological deficits in alcoholism without Korsakoff’s syndrome. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36(3):580–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fama R, Le Berre AP, Hardcastle C, et al. Neurological, nutritional and alcohol consumption factors underlie cognitive and motor deficits in chronic alcoholism. Addiction biology. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) Version 2.0. . New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1998. [Google Scholar]
- 49.Corrigan JD, Bogner J. Initial reliability and validity of the Ohio State University TBI Identification Method. J Head Trauma Rehabil. 2007;22(6):318–329. [DOI] [PubMed] [Google Scholar]
- 50.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- 51.Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual and Motor Skills. 1958;8(3):271–276. [Google Scholar]
- 52.Borkowski JG, Benton AL, Spreen O. Word fluency and brain damage. Neuropsychologia. 1967;5(2):135–140. [Google Scholar]
- 53.Ruff R Ruff Figural Fluency Test: Administration Manual. San Diego, CA: Neuropsychological Resources; 1988. [Google Scholar]
- 54.Wechsler D Wechsler Memory Scale-Revised. San Antonio, TX: The Psychological Corporation; 1987. [Google Scholar]
- 55.Rey A L’examen psychologique dans les cas d’encéphalopathie traumatique. Archives de Psychologie. 1942;28:286–340. [Google Scholar]
- 56.Corkin S, Growdon JH, Sullivan EV, Nissen MJ, Huff FJ. Assessing treatment effects from a neuropsychological perspective In: Poon L, ed. Handbook of Clinical Memory Assessment in Older Adults. Washington DC: American Psychological Association; 1986:156–167. [Google Scholar]
- 57.Matthews CG, Kløve H. Instruction Manual for the Adult Neuropsychology Test Battery. Madison, WI: University of Wisconsin Medical School; 1964. [Google Scholar]
- 58.Fregly AR, Smith MJ, Graybiel A. Revised normative standards of performance of men on a quantitative ataxia test battery. Acta oto-laryngologica. 1973;75(1):10–16. [DOI] [PubMed] [Google Scholar]
- 59.Springate BA, Tremont G, Papandonatos G, Ott BR. Screening for Mild Cognitive Impairment Using the Dementia Rating Scale-2. Journal of geriatric psychiatry and neurology. 2014;27(2):139–144. [DOI] [PubMed] [Google Scholar]
- 60.Mattis S Dementia Rating Scale (DRS) Professional Manual. Odessa, FL: Psychological Assessment Resources, Inc.; 2004. [Google Scholar]
- 61.Grant I Neurocognitive disturbances in HIV. International review of psychiatry (Abingdon, England). 2008;20(1):33–47. [DOI] [PubMed] [Google Scholar]
- 62.Woods SP, Moore DJ, Weber E, Grant I. Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychology review. 2009;19(2):152–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cysique LA, Maruff P, Brew BJ. The neuropsychological profile of symptomatic AIDS and ADC patients in the pre-HAART era: a meta-analysis. Journal of the International Neuropsychological Society : JINS. 2006;12(3):368–382. [DOI] [PubMed] [Google Scholar]
- 64.Kieburtz K, Ketonen L, Cox C, et al. Cognitive performance and regional brain volume in human immunodeficiency virus type 1 infection. Archives of neurology. 1996;53(2):155–158. [DOI] [PubMed] [Google Scholar]
- 65.Paul R, Cohen R, Navia B, Tashima K. Relationships between cognition and structural neuroimaging findings in adults with human immunodeficiency virus type-1. Neuroscience and biobehavioral reviews. 2002;26(3):353–359. [DOI] [PubMed] [Google Scholar]
- 66.Hestad K, McArthur JH, Dal Pan GJ, et al. Regional brain atrophy in HIV-1 infection: association with specific neuropsychological test performance. Acta neurologica Scandinavica. 1993;88(2):112–118. [DOI] [PubMed] [Google Scholar]
- 67.Ances BM, Ortega M, Vaida F, Heaps J, Paul R. Independent effects of HIV, aging, and HAART on brain volumetric measures. Journal of Acquire Immune Deficiency Syndromes. 2012;59(5):469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martin-Thormeyer EM, Paul RH. Drug abuse and hepatitis C infection as comorbid features of HIV associated neurocognitive disorder: neurocognitive and neuroimaging features. Neuropsychology review. 2009;19(2):215–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zahr NM. The Aging Brain With HIV Infection: Effects of Alcoholism or Hepatitis C Comorbidity. Frontiers in aging neuroscience. 2018;10:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fialho R, Pereira M, Bucur M, Fisher M, Whale R, Rusted J. Cognitive impairment in HIV and HCV co-infected patients: a systematic review and meta-analysis. AIDS care. 2016;28(12):1481–1494. [DOI] [PubMed] [Google Scholar]
- 71.Forton DM, Thomas HC, Murphy CA, et al. Hepatitis C and cognitive impairment in a cohort of patients with mild liver disease. Hepatology (Baltimore, Md). 2002;35(2):433–439. [DOI] [PubMed] [Google Scholar]
- 72.Hilsabeck RC, Perry W, Hassanein TI. Neuropsychological impairment in patients with chronic hepatitis C. Hepatology (Baltimore, Md). 2002;35(2):440–446. [DOI] [PubMed] [Google Scholar]
- 73.Capuron L, Pagnoni G, Demetrashvili M, et al. Anterior cingulate activation and error processing during interferon-alpha treatment. Biological psychiatry. 2005;58(3):190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Iriana S, Curry MP, Afdhal NH. Neurologic Manifestations of Hepatitis C Virus Infection. Clinics in liver disease. 2017;21(3):535–542. [DOI] [PubMed] [Google Scholar]
- 75.Perry W, Hilsabeck RC, Hassanein TI. Cognitive dysfunction in chronic hepatitis C: a review. Digestive diseases and sciences. 2008;53(2):307–321. [DOI] [PubMed] [Google Scholar]
- 76.Posada C, Morgan EE, Moore DJ, et al. Neurocognitive effects of the hepatitis C virus. Current Hepatitis Reports. 2009;8(S1):18–26. [Google Scholar]
- 77.Cordoba J, Flavia M, Jacas C, et al. Quality of life and cognitive function in hepatitis C at different stages of liver disease. Journal of hepatology. 2003;39(2):231–238. [DOI] [PubMed] [Google Scholar]
- 78.Weissenborn K, Krause J, Bokemeyer M, et al. Hepatitis C virus infection affects the brain-evidence from psychometric studies and magnetic resonance spectroscopy. Journal of hepatology. 2004;41(5):845–851. [DOI] [PubMed] [Google Scholar]
- 79.Vigil O, Posada C, Woods SP, et al. Impairments in fine-motor coordination and speed of information processing predict declines in everyday functioning in hepatitis C infection. Journal of clinical and experimental neuropsychology. 2008;30(7):805–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hilsabeck RC, Castellon SA, Hinkin CH. Neuropsychological aspects of coinfection with HIV and hepatitis C virus. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2005;41 Suppl 1:S38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.von Giesen HJ, Heintges T, Abbasi-Boroudjeni N, et al. Psychomotor slowing in hepatitis C and HIV infection. J Acquir Immune Defic Syndr. 2004;35(2):131–137. [DOI] [PubMed] [Google Scholar]
- 82.Cherner M, Letendre S, Heaton RK, et al. Hepatitis C augments cognitive deficits associated with HIV infection and methamphetamine. Neurology. 2005;64(8):1343–1347. [DOI] [PubMed] [Google Scholar]
- 83.Richardson JL, Nowicki M, Danley K, et al. Neuropsychological functioning in a cohort of HIV− and hepatitis C virus-infected women. Aids. 2005;19(15):1659–1667. [DOI] [PubMed] [Google Scholar]
- 84.Caldwell JZ, Gongvatana A, Navia BA, et al. Neural dysregulation during a working memory task in human immunodeficiency virus-seropositive and hepatitis C coinfected individuals. Journal of neurovirology. 2014;20(4):398–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hinkin CH, Castellon SA, Levine AJ, Barclay TR, Singer EJ. Neurocognition in individuals co-infected with HIV and hepatitis C. Journal of addictive diseases. 2008;27(2):11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ryan EL, Morgello S, Isaacs K, Naseer M, Gerits P. Neuropsychiatric impact of hepatitis C on advanced HIV. Neurology. 2004;62(6):957–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Strauss E, Sherman EMS, Spreen O. A compendium of neuropsychological tests: Administration, norms, and commentary (3rd ed.). New York, NY: Oxford University Press; 2006. [Google Scholar]
- 88.Scalzo SJ, Bowden SC, Ambrose ML, Whelan G, Cook MJ. Wernicke-Korsakoff syndrome not related to alcohol use: a systematic review. Journal of neurology, neurosurgery, and psychiatry. 2015;86(12):1362–1368. [DOI] [PubMed] [Google Scholar]
- 89.Ellis RJ, Badiee J, Vaida F, et al. CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. Aids. 2011;25(14):1747–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Robertson KR, Smurzynski M, Parsons TD, et al. The prevalence and incidence of neurocognitive impairment in the HAART era. Aids. 2007;21(14):1915–1921. [DOI] [PubMed] [Google Scholar]
- 91.Kaiser JD, Campa AM, Ondercin JP, Leoung GS, Pless RF, Baum MK. Micronutrient supplementation increases CD4 count in HIV-infected individuals on highly active antiretroviral therapy: a prospective, double-blinded, placebo-controlled trial. J Acquir Immune Defic Syndr. 2006;42(5):523–528. [DOI] [PubMed] [Google Scholar]
- 92.Bharadwaj S, Ginoya S, Tandon P, et al. Malnutrition: laboratory markers vs nutritional assessment. Gastroenterol Rep (Oxf). 2016;4(4):272–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Anand P, Springer SA, Copenhaver MM, Altice FL. Neurocognitive impairment and HIV risk factors: a reciprocal relationship. AIDS and behavior. 2010;14(6):1213–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Heaton RK, Marcotte TD, Mindt MR, et al. The impact of HIV-associated neuropsychological impairment on everyday functioning. Journal of the International Neuropsychological Society : JINS. 2004;10(3):317–331. [DOI] [PubMed] [Google Scholar]
- 95.Thames AD, Kim MS, Becker BW, et al. Medication and finance management among HIV-infected adults: the impact of age and cognition. Journal of clinical and experimental neuropsychology. 2011;33(2):200–209. [DOI] [PMC free article] [PubMed] [Google Scholar]