Abstract
Objective
To evaluate the histopathology of human temporal bones (HTBs) that exhibit cochlear injury secondary to translocation or displacement of the cochlear implant (CI).
Background
The gold standard to understand the complications of cochlear implantation are histopathological studies in patients.
Material and methods
13 HTBs from 12 patients with a history of CI were evaluated for the presence of translocation and intracochlear trauma. Six HTBs exhibited translocation with localized injury (Group 1) and seven HTBs exhibited translocation with significant lateral wall injury (Group 2). Not included are those without auditory or clinical history. 4 out of 6 of Group 1 had round window approach, while 7 out of 7 of Group 2 had cochleostomy approach. There were no significant differences between group 1 and group 2 with regard to age at death, age at implantation, and years with cochlear implant.
Results
Translocation injuries tended to occur near 180 degrees of angular insertion for both Groups 1 and 2 with a mean of 186.36 +/− 51.62 degrees. Longer electrodes were more prone to translocation, and none of the electrodes shorter than 10 mm had undergone translocation. Average insertion length for Group 1 was 18.50 ± 3.33 mm vs. Group 2: 21.86 ± 2.55 (p = .031). Group 1 had an average of 17300 ± 9415 spiral ganglia neuronal (SGN) number while Group 2 was 6714 ± 4269 which was significantly lower (p = 0.015), Average auditory performance was 66.55 ± 27.20 % in Group 1 vs. 39.86 ± 15.36 % for Group 2. In 6 out of the 7 cases in Group 2, there was osteoneogenesis, and all cases in Group 2 had infiltration of cells.
Conclusion
Translocation injuries tend to occur at an insertion angle of 180 degrees, corresponding to 9 to 10 mm. Lateral wall injury and damage to the organ of Corti appear to incite fibrosis, osteoneogenesis and infiltration, and Group 2 was associated with a significantly lower average spiral ganglia neuron (SGN) number, poorer auditory performance. Longer insertion length of electrodes was associated with higher chance of significant injury. Electrophysiological monitoring that is designed to alert surgeons in real-time when there is impending damage to the cochlea may be useful to minimize trauma.
Introduction
The aim of cochlear implantation surgery is to place the stimulating electrode arrays fully into scala tympani with the least degree of intracochlear damage. With the advancement of soft surgical techniques and the significant improvements to the CI electrode design, it is possible to preserve hearing in the low frequencies and provide substantial benefits (1, 2). We have previously conducted a study of the HTBs from patients who had received CI and demonstrated that the round window insertion of electrodes is preferred over cochleostomy to minimize the secondary intracochlear fibrosis and osteoneogenesis from the site of endosteal damage, which can lead to endolymphatic hydrops, possibly causing a delayed loss of residual hearing (3).
The gold standard for assessing cochlear trauma related to cochlear implantation remains the histopathological evaluations of human temporal bones from CI patients (4,5). Soft technique with slow and atraumatic insertion for CI surgery (6) was developed with the realization of the impact of intraoperative cochlear trauma on auditory outcomes (7).
We analyzed the histopathology of intracochlear injury related to CI electrode translocation or displacement. We also evaluated for differences between localized translocation injury (Group 1) and translocation with significant lateral wall injury (Group 2). We evaluated for osteoneogenesis related to CI intracochlear trauma and cochleostomy. Of note, these studies are of the first generation of CI electrodes, and the current models are designed to be slimmer and more flexible. The average length of electrode insertion was significantly longer for group 2 compared with group 1, and the average spiral ganglia neuronal count was significantly lower in group 2 compared with group 1. Given the detrimental effect of translocation injury, we believe that intraoperative monitoring designed to prevent intracochlear trauma may help in hearing preservation surgeries and to optimize audiological outcomes.
Material and methods
Subjects
Included in this study are 13 human temporal bones (HTBs) from 12 patients who had a history of cochlear implantation (CI). 1 of the patients had bilateral CI, and both sides were analyzed. Eight of the HTBs had been studied in a previous investigation: Cases 1–2, 4–8, and 13 had previously been studied for presence of endolymphatic hydrops in the setting of fibrosis (3). The Institutional Review Board (IRB) of UCLA approved this study (IRB protocol #10–001449). All methods used in this study are in accordance with NIH and IRB guidelines and regulations. The temporal bone donors were part of a National Institute of Health funded National Temporal Bone Laboratory at UCLA through the National Institute on Deafness and Other Communication Disorders.
Audiological Data
Clinical records were searched to obtain demographic and implant-related clinical information. Word test scores (percent correct) were documented in most cases. About half of the word scores were obtained using the Consonant Nucleus Consonant (CNC) words or the Central Institute for the Deaf (CID) Auditory test. Other audiological tests used included the Monosyllable, Trochee, Spondee Test (MTS), the Iowa test, and the House Ear Institute test (HEI).
Selection of HTBs:
CI cases with histopathological evidence of translocation with or without lateral wall injury were studied. Thirteen cases of translocation with or without lateral wall injury were found. Excluded from the analysis were subjects without associated clinical records or auditory performance. (3).
HTB processing:
The temporal bones had been removed postmortem and placed in 10% neutral buffered formalin for three weeks, decalcified in EDTA until shown by X-ray to be free of calcium. Embedding was done in increasingly concentrated celloidin to allow complete penetration. To minimize extraction movement, the electrode was removed just before the specimen was placed in hardening chloroform. The celloidin block was cut into 20-micron sections of which every tenth was mounted and stained with hematoxylin and eosin (H & E).
Assessment of the angle of insertion and length in mm at translocation site:
In each case, the entire set of HTB sections was evaluated to determine the angle of insertion where translocation occurs. In order to confirm the accuracy of the use of 2-D histopathology to estimate the angle of translocation, Amira 3-dimensional reconstruction was used in four HTBs. The H & E images were aligned using TrakEM2 plugin on Fiji. A hyper stack of images to account for the 10 slices was created. The desired structures were segmented on Amira software as described previously (9). In all 4 cases, the estimated angular site of translocation was confirmed by 3-dimensional reconstruction using Amira. In two cases (Case 2 and 7), there was an artifact which impeded analysis of the angle of translocation. In Case 11, the HTB had been sectioned parallel to the basal turn of the cochlea due to an electrode that could not be removed. Fortuitously, this specimen allowed for an accurate measurement of the mm of the length of insertion, as well as the corresponding angle of insertion, from the round window to the point of translocation. In case 10, measurements of the fibrous sheath on serial images could be made to determine the length of the insertion of the CI electrode at point of translocation.
Statistical analysis
Comparisons of age at death, age at implantation, years with the cochlear implant, electrode length of insertion, spiral ganglia number, and auditory performance were made between Group 1, comprised of the translocation localized group (Cases 1 – 6, n = 6) and Group 2, comprised of the translocation with significant lateral wall injury group (Cases 7 – 13, n = 7). A student t-test was obtained using the IBM SPSS statistics software program. A value of p < 0.05 was denoted as a statistically significant difference.
Results
Table 1 summarizes the demographic characteristics of the HTBs from CI recipients with translocation, the associated auditory performance, the electrode length as determined on histopathology, electrode localization and angular degree of translocation, and electrode type.
Table 1:
Summary of demographic data for group 1(case 1–6, T localized) and group 2(case 7–13, T + LW).
| Case # | Figure | Age | Age at Implant | F=1 M=2 | Years with the CI | 1= Right, 2=Left | Performance | Angular CI Insertion at Translocation | Electrode length (mm) | Electrode type |
|---|---|---|---|---|---|---|---|---|---|---|
| Group 1: TRANSLOCATION WITHOUT SIGNIFICANT LATERAL WALL INJURY | ||||||||||
| 1 | 1A1–3 | 59 | NA | 1 | N/A | 2 | MTS 42% | 180 | 13 | 3M/House |
| 2 | 1B | 61 | 60 | 1 | 1 | 2 | MTS 85% | Artifact | 20 | Nucleus 22 |
| 3 | 1C | 70 | 67 | 1 | 3 | 2 | CID 63% | 330 | 20 | Nucleus 22 |
| 4 | 1D | 87 | 77 | 1 | 10 | 1 | 98% accuracy | 180 | 20 | Nucleus 22 |
| 5 | 2A | 61 | 55 | 1 | 6 | 1 | MTS 83% | 190 | 16 | Alpha single |
| 6 | 2B | 76 | 74 | 1 | 2 | 1 | CNC 28% | 180 | 22 | Nucleus 24 |
| Group 2: TRANSLOCATION WITH SIGNIFICANT LATERAL WALL INJURY | ||||||||||
| 7 | 2C | 74 | 66 | 2 | 8 | 2 | Iowa 58% | Artifact | 21 | Nucleus 22 |
| 8 | 2D | 81 | 73 | 1 | 8 | 1 | CNC 60% | 150 | 25 | AB Clarion |
| 9 | 3A | 61 | 60 | 2 | 1. | 2 | CNC 28% | 120 | 22 | AB Clarion |
| 10 | 3B | 72 | N/A | 2 | N/A | 2 | CID 20% | 180 | 18 | Nucleus C-124 |
| 11 | 3C | 75 | 71 | 2 | 4 | 2 | HEI 47% | 180 | 22 | N/A |
| 12 | 3D | 73 | 70 | 1 | 3 | 2 | MTS 32% | 180 | 25 | Nucleus 22 |
| 13 | 3E | 89 | 73 | 2 | 16 | 2 | CID 34% | 180 | 20 | Nucleus 22 |
N/A = unknown; F= female; M = male; CI = cochlear implant; Electrode length = insertion length as determined on histopathology; see text for auditory tests e.g. HEI = House Ear Institute test.
Table 2 summarizes in tabular format the histopathological findings.
Table 2.
Summary of histopathological changes for temporal bones with translocation.
| Case # | Fig | Spiral Ganglia Number |
Insertion 1= RW 1a = Extended RW 2= Cochleostomy | Translocation Site | Endolymphatic Hydrops | Osteoneogenesis & Ossification | Spiral Ligament |
Organ of Corti | Stria Vascularis | Fibrosis & Infiltration |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 A A1–3 | 27972 | 1a | Through the osseous spiral lamina into scala vestibuli Mid-Apical is normal | Localized only Reissner’s membrane collapse at the base | Yes, New Bone formation near drilling of operculum | Mild Atrophy | Atrophy | Mild Atrophy | Mild fibrosis at the CI insertion hook- basal portion |
| 2 | 1B | 24398 | 1 | Through the basilar membrane into scala vestibuli /Mid-Apical is normal | No Hydrops | Yes, New Bone formation at site of translocation injury | Damaged by CI translocation | Atrophy | Atrophy | Mild fibrosis at CI insertion hook- basal portion |
| 3 | 1C | 10323 | 1a | Direct damage to the basilar membrane and spiral ligament | No Hydrops | Yes, New Bone formation at hook region only | Damage to spiral ligament | Complete loss of cells | Normal | Mild fibrosis at CI insertion hook-basal |
| 4 | 1D | 18486 | 2 | Rupture of basilar membrane | Distortion of Reissner’s membrane and Cochlear Hydrops | New Bone Formation extending from cochleostomy site | Atrophy | Atrophy, Disrupted due to CI translocation | Atrophy | Fibrosis basal cochlea extending to scala vestibuli |
| 5 | 2A | 20177 | 1 | Through basilar membrane to scala vestibuli | No hydrops | No new bone formation | Damage by the CI electrode at the base where spiral ligament is torn | Loss of hair cells at the basal region | Normal | No fibrosis/No infiltration |
| 6 | 2B | 2446 | 2 | Through basilar membrane to scala vestibuli | Yes, Severe hydrops at basal turn, mild hydrops in mid turn | Yes, Ossification extending from the basal including scala tympani and vestibuli | Atrophy | Loss of hair cells | Atrophy | Fibrosis basal / No Infiltration |
| 7 | 2C | 7767 | 2 | Ossified up to the mid turn | Yes, Severe hydrops throughout | Yes, Ossification extending from basal up to middle turn; Otosclerosis | Atrophy | Loss of cells hair cells and supporting cells | Absence | Fibrosis and Chronic Infiltration |
| 8 | 2D | 11520 | 2 | Ossified throughout | Yes, Severe hydrops throughout | Yes, New Bone formation from the base hook to mid apical and the mid-modiolus | Atrophy at the Scala tympani | Remnants at the middle upper segment | Mild atrophy | Fibrosis, Infiltration at the Scala tympani |
| 9 | 3A | 8506 | 2 | Disrupted at the basal region | Hydrops at the mid-base | No new bone formation | Occupies the spiral ligament, disruption of basilar lamina | Loss of hair cells | Atrophy and loss at the basal turn | Fibrosis in scala vestibuli and Infiltration |
| 10 | 3B | 9561 | 2 | Disrupted by the electrodeat the basal region; into scala vestibuli | No hydrops | No new bone formation | Defect in the basal spiral ligament due to CI damage | Loss of hair cells | Normal | Fibrosis in scala tympani, little fibrosis in scala vestibuli, Infiltration in basal Rosenthal’s canal |
| 11 | 3C | *Due to artifact, cannot be counted | 2 | Disrupted by the electrode at the basal region | Yes, pronounced hydrops throughout | Yes, New Bone formation | Atrophy | Loss of cells | Atrophy | Fibrosis and Infiltration in scala tympani and scala vestibuli |
| 12 | 3D | 2000 | 2 | Disrupted by the electrode; Ossification of scala vestibuli | Yes, pronounced hydrops throughout | Yes, New bone formation from hook to basal region | Spiral ligament destroyed in anterior basal turn due to CI electrode path | Loss of hair cells throughout, distortion of the organ of Corti in many parts | Absent in the basal turn | Fibrosis and Infiltration |
| 13 | 3E | 935 | 2 | Disrupted by the electrodefrom the hook- middle region; Ossification | Yes, pronounced hydrops throughout | Yes, New bone formation from hook to basal region, Severe Otosclerosis | Atrophy and hyalinization in some parts | Complete loss of cells | Atrophy | Fibrosis in scala tympani and scala vestibuli, Infiltration, fresh blood, Otospongiosis in Rosenthal’s canal |
Table 3 compares group 1 : translocation without significant lateral wall injury with group 2: translocation with significant lateral wall injury.
Table 3.
Comparison of group 1 (case 1–6, T localized) vs. group 2 (case 7–13, T + LW).
| Condition | G1 (Ave ± SD) | G2 (Ave ± SD) | t-test | p-value | p < 0.05 (significant) |
|---|---|---|---|---|---|
| Age at death | 69 ± 10.97 | 75 ± 6.53 | − 1.203 | 0.126 | No |
| Age at implant | 66.60 ± 9.23 | 68.83 ± 5.03 | −0.511 | 0.310 | No |
| Years with CI | 4.40 ± 3.64 | 6.67 ± 5.35 | −0.801 | 0.221 | No |
| Electrode length | 18.50 ± 3.33 | 21.86 ± 2.55 | −2.060 | 0.031 | Yes |
| Electrode degree | 212 ± 66 | 165 ± 25 | 1.621 | 0.069 | No |
| SGN number | 17300 ± 9415 | 6714 ± 4269 | 2.508 | 0.015 | Yes |
| Performance | 66.55 ± 27.20 | 39.86 ± 15.36 | 2.22 | 0.024 | Yes |
Statistical analysis. t-test and – values (one-tailed hypothesis).
p < 0.05 as significant.
G = group
Ave = average.
SD = standard deviation.
Electrode length = millimeters.
Age= years.
Statistical analysis: Comparisons of age at death, age at implant, years with the cochlea implant, electrode length, degree, performance and spiral ganglia number were made between the translocation localized group 1 (G1, n=6) and translocation with lateral wall group 2 (G2, n=7). A student t-test was obtained using and IBM SPSS statistics software program. A value p < 0.05 denotes statistically significant difference.
Group 1: Translocation without significant lateral wall injury (Cases 1 – 6)
Demographics: Six HTBs had localized translocation or localized intracochlear damage: Cases 1– 6. There were 6 females, and an average age at implant of 66.60 ± 9.23 years old. The average number of years with the CI was 4.4 ± 3.64 years. Age at death was 69 ± 10.97 years old. There were 3 out of 6 on the left side.
Length in mm of CI insertion:
In group 1, the average length of insertion of the CI electrodes was 18.5 ± 3.33 mm.
Angle of insertion of the CI at the point of translocation:
An artifact precluded an accurate analysis of the angle of translocation for Case 2, likely secondary due to removal of CI after hardening had occurred. Out of the remaining, four had translocation at or near 180 degrees, with translocation of Case 3 occurring at 330 degrees.
Electrode types:
included 3M/ House, Alpha single, Nucleus 22 and 24, which are the first generation models of the CI.
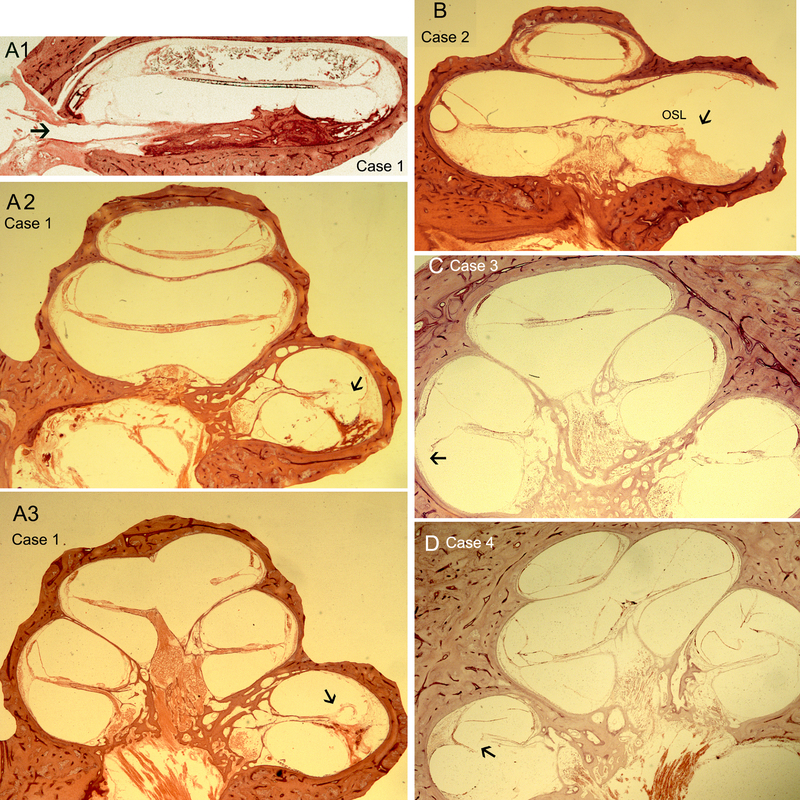
Table 2: Differential localized injury patterns: Figures 1A-D and Figures 2A-B are photomicrographs from Cases 1 through 6. The type of injury varied within these cases with two types of translocation or displacement injury: disruption of the basilar membrane (BM) and/ or the osseous spiral lamina (OSL), and / or dissection between the bone and spiral ligament. For example, Case 1 and Case 5 represent translocation into scala vestibuli with minimal damage to the lateral wall (Figure 1A2 and Figure 2B, respectively). In these cases, there is excellent preservation of the spiral ganglia neurons, with counts of 27, 972 in case 1 and 20,177 in Case 5. In Case 1, the translocation occurs through the osseous spiral lamina (OSL) and the basilar membrane (BM), causing localized damage at 180 degrees (Figure 1A2). In Figure 1A3: the electrode is lying near the cochlear wall within the scala vestibuli. In Case 2, the electrode initially translocates into the lateral wall (Figure 1B) and then damages the BM, dissecting between the bone and spiral ligament and translocates into the scala vestibuli (not shown). In Case 3, there is isolated translocation into the lateral wall with significant damage to the BM (Figure 1C). In Case 4, the translocation occurs through the organ of Corti (Figure 1D), and there was fibrosis and osteoneogenesis apparently extending from the site of intracochlear damage. There is also fibrosis and osteoneogenesis that involves the scala vestibuli extending from the cochleostomy site which was associated with significant endolymphatic hydrops. In Case 6, there is dissection between the bone and spiral ligament, through the basilar membrane, and both the cochleostomy and the CI translocation injury are associated with extensive osteoneogenesis, fibrosis, and endolymphatic hydrops (Figure 2B). In all of these cases, there is osteoneogenesis surrounding or near the site of cochleostomy and also near the site of endosteal disruption at the operculum for extended round window approaches.
Fig. 1.
Case 1. (A1) The electrode can be seen penetrating the round window into the scala tympani. The electrode traverses the scala tympani to the anterior part of the cochlea. (A2) At the anterior inferior basal turn, the electrode translocates through the osseous spiral lamina (OSL) and basilar membrane (BM) (A3). The electrode can be seen located in the scala vestibuli. The arrowhead points to the translocation site. B. Case 2. The electrode has damaged the OSL and BM and the lateral wall (arrowhead), dissecting between the bone and spiral ligament. Electrode fibrous sheath can be seen in the opposite side. The insertion angle of translocation cannot be accurately assessed due to artifact. C. Case 3. As the electrode passed ventrally in the posterior basal turn, it produces significant damage to the BM and spiral ligament. D. Case 4. The CI occupies and replaces the area of the spiral ligament and the area of the organ of Corti. The spiral ligament is partially destroyed by the CI. There is evidence of cochlear hydrops. There is rupture of the basilar membrane by translocation of the electrode. The arrowhead points to the translocation site. 200X magnification. Hematoxylin and eosin (H&E) staining.
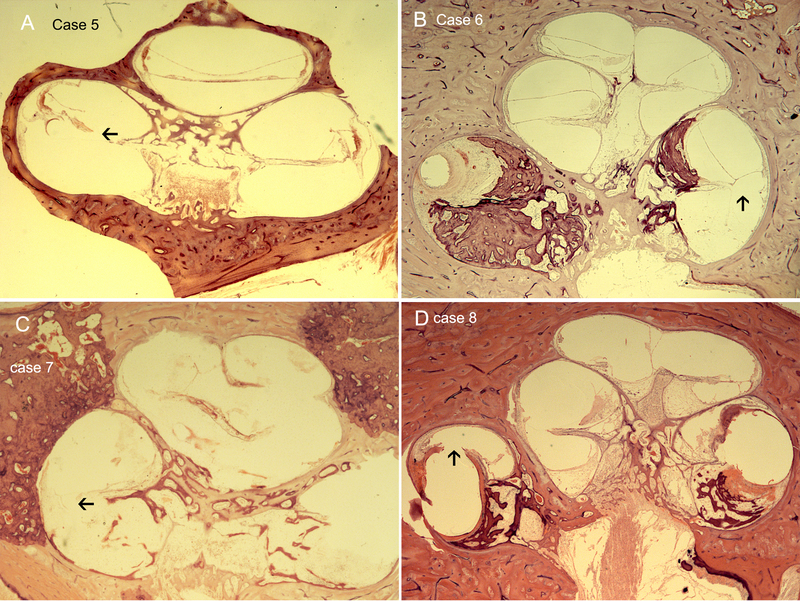
Fig 2.
A. Case 5. The electrode ruptures the BM and OSL and passes into the scala vestibuli. (arrowhead). It then hugs the cochlear wall along the insertion of the spiral ligament to the posterior superior basal portion of the cochlea. B. Case 6. The electrode is seen penetrating between the bone and spiral ligament to enter the scala vestibuli (arrowhead). There is severe endolymphatic hydrops at the base and moderate hydrops throughout cochlea. C. Case 7 The electrode translocation occurs within the basal portion and thenceforth the electrode occupies the space normally occupied by the organ of Corti. The arrowhead points to the approximate site where the translocated electrode can be seen however the site cannot be accurately assessed due to artifact. D. Case 8. The electrode translocates into the scala vestibuli (arrowhead). Most of the scala tympani is ossified. There is pronounced hydrops. Remnants of the organ of Corti are evident only within the middle upper segment.. The arrowhead points to the translocation site. 200X magnification, H&E staining.
Group 2: Translocation with Significant Lateral Wall Injury (Cases 7 – 13)
Demographics:
Seven HTBs exhibited translocation with significant lateral wall damage, Cases 7 – 13. There were 2 females and 5 males, and an average age at implant of 68.8 ± 5.03 years of age. The average number of years with the CI in this group was 6.67 ± 5.35 years. There were 6 out of 7 on the left side.
Length in mm of CI insertion:
The average length of insertion of the CI electrodes was 21.9 ± 2.55 mm. There were no electrode insertion lengths of less than 18 mm within this group and 6 out of the 7 cases had a CI insertion length of 20 mm or more.
Angle of insertion of the CI at the point of translocation:
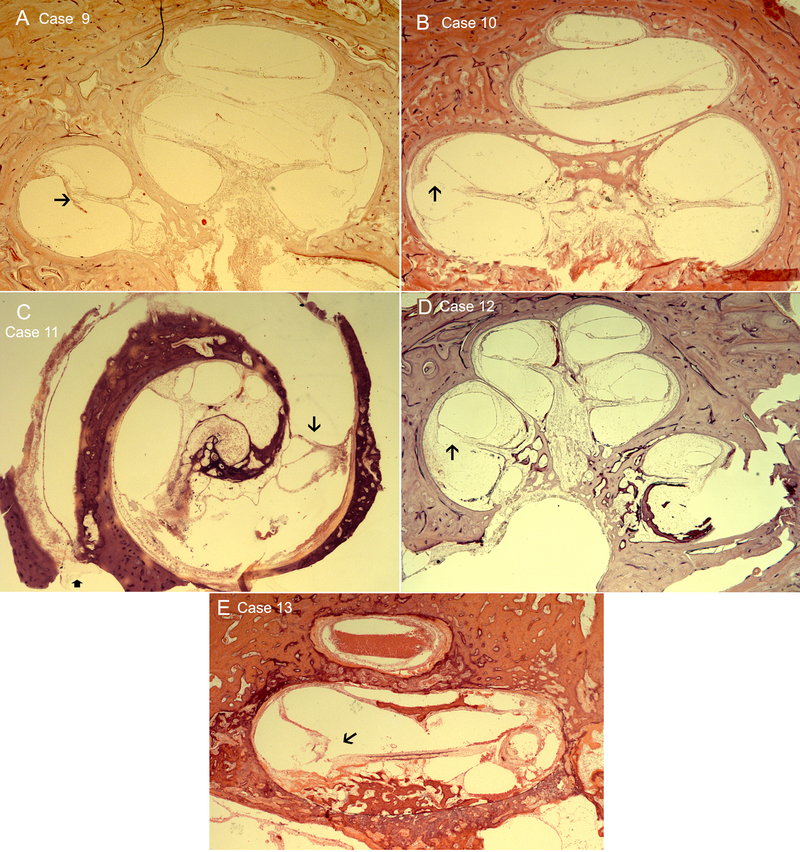
An artifact on histopathology precluded analysis of the angle of translocation injury in one case (Case 7). Of the remaining six HTBs, all had translocations near 180 degrees with Case 8 at 150 degrees, and case 9 at 120 degrees. In Case 10, the fibrous sheath was followed sequentially to measure a length of 9 mm from insertion to point of translocation. In Case 11, due to the cutting of the HTB in the horizontal plane, the length in mm of the electrode insertion as well as angle of insertion could be measured at 180 degrees, 9.55 mm (Figure 3C).
Fig 3.
A. Case 9. The electrode enters the scala vestibuli at the base (Arrowhead), and occupies the area of the spiral ligament as far as the middle turn of the cochlea. B. Case 10. The electrode enters the scala tympani just anterior to the round window and then translocates into the scala vestibuli at the basal portion of the cochlea (Arrowhead). Anterior to this is loose areolar type of fibrosis that extends about 9 mm into the basal turn. C. Case 11. This temporal bone was sectioned parallel to the basal turn of the cochlea because of the long wire that was inserted at least 22 mm and could not be removed using the usual approach. The electrode can be seen to transverse the scala tympani and translocates through the basilar membrane in the posterior basal coil (Arrowhead). The spiral ligament and the basilar membrane have been destroyed throughout the extent of the electrode insertion. D. Case 12. The electrode path destroys the spiral ligament in the anterior basal turn. There is pronounced hydrops. E. Case 13 The electrode translocates into the scala vestibuli at the mid-basal portion (Arrowhead). Rosenthal’s canal in the lower cochlea is obliterated by otospongiosis growing into new bone in the scala tympani and mid-modiolus. There is extensive endolymphatic hydrops. 200X magnification, H&E staining.
Electrode types:
included AB Clarion, Nucleus 22, and Nucleus C124, which are the older models of the CI.
Table 2: Differential injury patterns: Figures 2C-D and Figures 3A-E demonstrate varying degrees of lateral wall damage. In all cases, there was fibrosis and / or infiltration, which was more prominent in the basal region of the cochlea. All except Case 10 exhibited moderate to severe endolymphatic hydrops. In Case 7, the electrode path has translocated and replaced the organ of Corti space (Figure 2C). Cases 8 and 12 exhibited osteoneogenesis from the basal region to mid-apical, osteoneogenesis extending from the cochleostomy into both the scala vestibuli and the scala tympani, associated with severe hydrops. Cases 7, 11, and 13 represent cases of otosclerosis, and it appears that with otosclerosis the effect of translocation with lateral wall injury is magnified due to increased host-tissue reactions. In Case 7, there is ossification up to the middle turn, and infiltration throughout the cochlea (Figure 2C). Similarly, in Case 11 there was fibrous tissue throughout the middle turn of the scala tympani and extensive osteoneogenesis and ossification of both the scala tympani and of scala vestibuli (Figure 3C: limited view due to cut). In Case 13, there is extensive otosclerosis and otospongiosis with osteoneogenesis within the scala tympani and scala vestibuli, and active inflammation (Figure 3E). In this specimen, it is likely that other factors, apart from the translocation and lateral wall injury, play a role in the inciting of inflammatory factors given the extensive damage.
Table 3: Comparison of Group 1 with localized translocation injury alone with Group 2 with translocation injury with significant lateral wall injury. There were no significant differences between group 1 and group 2 with regard to age at death, age at implantation, and years with cochlear implant. Age at death for group 1 was 69 ± 10.97 and age at death for group 1 was 75 ± 6.53. Age at implantation for group 1 was 66.60 ± 9.23 and for group 2 was 68.83 ± 5.03. Years with cochlear implantation for group 1 was 4.40 ± 3.64 and for group 2 was 6.67 ± 5.35. There was no statistical difference between these parameters with p = 0.126; 0.310, 0.221 respectively.
There was a significant difference between Group 1 and Group 2 with respect to length of electrode insertion, with Group 2 associated with a significantly longer insertion length. The average insertion length for Group 1 was 18.50 ± 3.33 mm while the average insertion length for Group 2 was 21.86 ± 2.55 (p = .031). There was a significant difference between Group 1 and Group 2 with respect to spiral ganglia neuronal (SGN) number. Group 1 had an average of 17300 ± 9415 SGN number while Group 2 had an average of 6714 ± 4269 SGN number, which was significant for a lower SGN number associated with Group 2 (p = 0.015). Group 2 was also associated with poorer auditory performance: 66.55 ± 27.20 % in Group 1 vs. 39.86 ± 15.36 % for Group 2 (p = 0.024).
Discussion
Cochlear trauma at the time of CI surgery is associated with poorer audiological outcomes (7) and the translocation of the electrodes and associated intracochlear injury can cause permanent damage. We undertook this study to evaluate the histopathology of the HTBs of CI patients exhibiting intracochlear injury related to translocation. In a previous report, we studied the histopathology of the HTBs from CI patients to note the association of osteoneogenesis, fibrosis and/ or ossification from the cochleostomy site, blocking the ductus reuniens, resulting in endolymphatic hydrops (3). Within this study, we confirmed the association of endolymphatic hydrops with cochleostomy.
Studies have documented that the round window (RW) approach and the extended round window (extended RW) approaches are more likely to result in scala tympani insertion of the electrode than the cochleostomy approach (91% and 84% vs. cochleostomy 37%) (10). The present study is suggestive that the degree of intracochlear damage following translocation injury may be more severe when it occurs in the setting of cochleostomy, possibly due to the triggering of osteoneogenesis from the site of cochleostomy due to endosteal damage, but further studies would be needed to verify this finding. In all cases with significant lateral wall injury, the cochleostomy approach had been used. Out of the 6 cases with localized translocation damage, four were round window (RW) or extended RW approach (Cases 1, 2, 3, and 5). Thus, we advocate the round window approach, with a careful drilling of the operculum, using care to not enter the scala vestibuli for the placement of CI.
A classification scheme regarding translocation has been developed using fresh frozen human cadaveric temporal bones and three perimodiolar electrode designs which categorizes translocation injuries on a scale from 1 to 4 as follows: 1) elevation of the basilar membrane 2) rupture of basilar membrane 3) electrode in the scala vestibuli 4) severe trauma such as fracture of the osseous spiral lamina or modiolus or tear of stria vascularis (11). The histopathological evaluation of the translocation injuries is critical to understand the pathology of translocations. We propose that there are important host-tissue reactions which can be triggered which are not addressed by using these imaging-based classification schemes. This is due to the fact that the imaging-based schemes are evaluations of the pathology occurring at the time of surgery: translocation and / or intracochlear injury. However, the present study provides evidence that the host-tissue response can trigger reactions including osteoneogenesis which can be associated with endolymphatic hydrops, and possibly causing a secondary loss of spiral ganglia neurons given that Group 2 had significantly fewer spiral ganglia neuronal count than Group 1. Organ of Corti injury (Case 4) and rupture of the basilar membrane (Case 6) seem to be associated with osteoneogenesis and these responses may be more severe in otosclerosis (Cases 7, 11, and 13).
Even the higher resolution studies such as cone beam CT or micro CT, are not likely able to detect isolated lateral wall damage or organ of Corti damage, especially when the electrode remains close to the lateral wall. In Cases 1 and 2, translocation into the scala vestibuli occurs, but in both cases, the electrode remains close to the lateral wall. In Case 3, the CI has damaged the spiral ligament and basilar membrane without translocation into the scala vestibuli and thus may be radiographically occult. In Case 4, the electrode has damaged the organ of Corti and violated the scala media space. However, despite the degree of damage, this may also be radiographically occult due to the CI electrode pathway remaining within the scala media. Intraoperative real-time monitoring of hearing would be more advantageous over the post-surgical radiological assessment since irreversible damage will have already taken place if using post-operative radiologic assessment alone (12). Many surgeons adopt a soft technique by inserting the electrode array to the “point of first resistance” (13). However, the degree of force needed to cause intracochlear injury may be minimal and ideally intraoperative aids can be developed to help prevent intracochlear damage at the time of surgery.
To our knowledge, no prior studies have used histopathological analysis of HTBs from CI patients to localize the angular insertion at the level of the translocation. The translocation or lateral wall damage occurred at or near 180 degrees in 10 out of 11 of the cases in which assessment was possible. The one exception is Case 3, which is an isolated spiral ligament and basilar membrane injury which occurred at 330 degrees. Despite the wide variance in the type of injury, nearly all cases of electrode translocation or migration occurred near the same angular insertion. The length of CI insertion was 9.0 and 9.5 mm at the point of translocation in the two HTBs which could be measured (Cases 10 and 11). The junction between the descending and the ascending basal turn of the cochlea appears to be the area susceptible to translocation from scala tympani to scala vestibuli. In prior studies using fresh frozen HTBs, the tendency for scala translocations to occur at 180 degrees has also been reported (11, 14).
In the present study, Group 2 with significant lateral wall injury had a significantly lower SGN number than Group 1 without significant lateral wall injury. This appeared to correspond with poorer auditory performance scores in group 2 but statistical comparisons could not be made due to differences of testing modalities used. Other nnotable differences between the two groups include more extensive osteoneogenesis and a higher incidence of endolymphatic hydrops in Group 2. In a study using 3- dimensional Amira analysis, Kamakura and Nadol (15) noted that osteoneogenesis was associated with a lower spiral ganglion neuronal count and poorer auditory performance. Fayad and Linthicum studied the HTBs of patients with CI noting that there was little to no osteoneogenesis in the apical cochlea in all ten HTBs (9). These authors proposed that osteoneogenesis and fibrosis occur in the setting of cochlear trauma related to CI implantation.
There was a statistically significant association of a longer length of electrode insertion with group 2 that had a higher degree of intracochlear trauma. In the present study, the average length of electrode insertion was 19.6 mm for all translocation cases. At the extreme, none of the very short earlier electrodes less than 10 mm had translocation injuries and all four electrode insertion lengths of greater than 20 mm had translocation injury. It seems logical that translocation may occur with higher frequency with longer electrodes and that longer electrodes may risk injury to the apical cochlea. Several studies have demonstrated a higher incidence of CI translocation into the scala vestibuli if inserted beyond the recommended point with perimodiolar electrodes (16–17). Thus, it is critical that surgeons do not insert the electrode beyond the recommended point. In a recent study, the newer Cochlear 532 slim perimodiolar electrode was placed in all cases into the scala tympani without evidence of translocation in 45 CI patients. There was tip fold over in two cases, one related to over insertion (18). Because this electrode type can have the same degree of cochlea coverage with shorter electrode distances, the design of the electrode makes sense conceptually with the present study findings.
There are several limitations to the present study. All of the electrodes studied are the first generation CI models and there have been great advances in the design of the electrodes. The auditory performance studies used varied methods of testing, in some cases, non-standardized, making it difficult to make comparisons. There is no ability to control for the type of cochlear implant, nor insertion approach. Within this group, three of the lateral wall injury group had otosclerosis and it is possible that these subjects have the tendency to have translocation due to pathological processes, and at the same time have higher chance of host-tissue response and poor audiological outcomes. Future studies are warranted in order to further improve the outcomes of CI patients.
In conclusion, the current study reveals that translocation of the electrodes, when occurring with lateral wall damage, may be associated with detrimental effects on auditory function, compared with translocation alone. Direct damage to the organ of Corti or rupture through the scala media may tend to induce a high degree of host-tissue response. In contrast, isolated OSL and BM damage appears to incite less response. Histopathological studies in HTBs remain the gold standard as only these studies can reveal the long-term effects of intracochlear damage and translocations. Further studies are indicated to develop interventions to prevent intracochlear damage at surgery and to diminish the detrimental effects which may be magnified by the host-tissue response. Soft technique needs to be applied in every case of cochlear implantation to minimize intracochlear trauma. We believe that electrophysiological monitoring may be helpful for intraoperative use to alert surgeons of impending cochlear damage during implantation surgeries.
Acknowledgments
IRB# 10-001449 UCLA Supported by NIDCD/NIH: 1U24DC015910-01
NIDCD National Temporal Bone Laboratory at UCLA
Department of Head and Neck Surgery, David Geffen School of Medicine, University of California, Los Angeles.
Footnotes
The authors have no conflicts of interest to disclose
Presented at the American Otologic Society Meeting at the Combined Otolaryngology (COSM) in Washington D.C. on April 21st, 2018.
References
- 1.Von Ilberg CA, Baumann U, Kiefer J et al. Electric-acoustic stimulation of the auditory system: a review of the first decade. Audiol Neurotol 2011;16(2):1–30. [DOI] [PubMed] [Google Scholar]
- 2.Roland JT Jr, Gantz BJ, Waltzman SB et al. United States multicenter clinical trial of the cochlear nucleus hybrid implant system. Laryngoscope 2016;126(1):175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ishiyama A, Doherty J, Ishiyama G et al. Post Hybrid Cochlear Implant Hearing Loss and endolymphatic hydrops. Otol Neurotol. 2016; 37(10):1516–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Connell BP, Hunter JB, Wanna GB. The importance of electrode location in cochlear implantation. Laryngoscope Investigative Otolaryngology 2016;169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Connell BP., Hunter JB., Gifford RH., Rivas A., Haynes DS., Noble JH., Wanna GB. Electrode Location and Audiologic Performance after Cochlear Implantation: A Comparative Study between Nucleus CI422 and CI512 Electrode Arrays. Otol Neurotol, 2016; 37(8):1032–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lehnhardt E Intracochlear placement of cochlear implant electrodes in soft surgery technique HNO 1993; 41 (7):356–359. [PubMed] [Google Scholar]
- 7.Carlson ML, Driscoll CL, Gifford RH et al. Implications of minimizing trauma during conventional cochlear implantation. Otol Neurotol 2011; 32: 962–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doherty JK and Linthicum FH. Cochlear endosteal erosion with focal osteomyelitis induced by cochlear implantation. 2004. 25:1029–1030. [DOI] [PubMed] [Google Scholar]
- 9.Fayad JN, Makarem AO, Linthicum FH Jr. Histopathologic assessment of fibrosis and new bone formation in implanted human temporal bones using 3D reconstruction. Otolaryngol Head Neck Surg 2009; 141(2): 247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wanna GB, Noble JH, Gifford RH et al. Impact of intrascalar electrode location, electrode type, and angular insertion depth on residual hearing in cochlear implant patients: preliminary results. Otol Neurotol 2015;36:1343–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eshraghi A, Yang NW, Balkany TJ. Comparative study of cochlear damage with three perimodiolar electrode designs. Laryngoscope 2003; 113: 415–419. [DOI] [PubMed] [Google Scholar]
- 12.Hoth S, Dziemba OC. The Role of Auditory Evoked Potentials in the Context of Cochlear Implant Provision. Otol Neurotol 2017; 38(10), e522–530. [DOI] [PubMed] [Google Scholar]
- 13.Adunka O, Kiefer J, Unkelback MH et al. Development and evaluation of an improved cochlear implant electrode design for electric acoustic stimulation. Laryngoscope 2004; 114(7): 1237–1241. [DOI] [PubMed] [Google Scholar]
- 14.Dietz A, Iso-Mustajarvi M, Sipari S, Tervaniemi J, Gazibegovic D. Evaluation of a new slim lateral wall electrode for cochlear implantation: an imaging study in human temporal bones. Eur Arch Otorhinolaryngol 2018; 275(7): 1723–1729. [DOI] [PubMed] [Google Scholar]
- 15.Kamakura T and Nadol JB Jr. Correlation between word recognition score and intracochlear new bone and fibrous tissue after cochlear implantation in the human. Hearing Research 2016; 339: 132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boyer E, Karkas A, Attye A et al. Scalar localization by cone-beam computed tomography of cochlear implant carriers: a comparative study between straight and perimodiolar precurved electrode arrays. Otol Neurotol 2015;36(3): 422–429. [DOI] [PubMed] [Google Scholar]
- 17.Mittmann P, Todt I, Wesarg T, Arndt S, Ernst A, Hassepass F. Electrophysiological Detection of Intracochlear Scalar Changing Perimodiolar Cochlear Implant Electrodes: A Blinded Study. Otol Neurotol. 2015;36(7): 1166–71. [DOI] [PubMed] [Google Scholar]
- 18.Aschendorff A, Briggs R, Brademann G, Helbig S, Hornung J et al. Clinical investigation of the Nucleus Slim Modiolar Electrode. Audiol Neurotol. 2017; 22(3): 169–179. [DOI] [PubMed] [Google Scholar]