Abstract
Background:
Metabolic perturbations in HIV-exposed uninfected (HEU) obese youth may differ from those in the general obese pediatric population.
Methods:
Metabolic parameters of obese (Body Mass Index Z-score >95th percentile) HEU youth in the Pediatric HIV/AIDS Cohort Study (PHACS) Surveillance Monitoring of ART Toxicities (SMARTT) study were compared with a matched sample of obese youth from the U.S. National Health and Nutrition Examination Survey (NHANES). We evaluated systolic and diastolic hypertension [blood pressure (BP) ≥90th percentile for age, sex, and height], total cholesterol (TC) >200 mg/dL, high-density lipoprotein cholesterol (HDL) <35 mg/dL, low-density lipoprotein cholesterol (LDL) >130 mg/dL, triglycerides (TG) >150 mg/dL, and Homeostatic Model Assessment-Insulin Resistance (HOMA-IR) >4.0. Modified Poisson regression models were fit to quantify the prevalence ratio (PR) of each outcome comparing the two cohorts, adjusting for confounders.
Results:
The BP outcome analytic subgroup included 1096 participants (n=304 HEU), the TC and HDL subgroup 1301 participants (n=385 HEU), and the LDL, TG, and HOMA-IR subgroup 271 (n=83 HEU). After adjustment, obese HEU youth had a higher prevalence of systolic and diastolic hypertension [PR=3.34, 95% Confidence Interval (CI): 2.48–4.50; PR=2.04, 95% CI: 1.18–3.52, respectively], but lower prevalence of insulin resistance (PR=0.67, 95% CI: 0.54–0.85) and hypercholesterolemia (PR=0.67, 95% CI: 0.44–1.01) compared to obese NHANES youth.
Conclusions:
In the U.S., obese HEU youth appear to have increased risk for hypertension, but lower risk for insulin resistance and hypercholesterolemia, compared to a general obese pediatric population. Monitoring for cardiovascular morbidity in adulthood may be warranted in HEU children.
Keywords: HIV-exposed uninfected children, Obesity, Insulin Resistance, Hypertension, Lipids, Cholesterol, Metabolic
Introduction:
Immense strides have been made in the reduction of mother-to-child transmission of HIV such that current rates of transmission are now <2%, and the overwhelming majority of HIV-exposed infants/children worldwide are uninfected, giving rise to an increasing population of HIV-exposed uninfected (HEU) children and a decreasing population of HIV-infected children.1 Another growing population is obese children and youth, particularly in high income countries. Since 1970, rates of obesity in the United States (U.S.) have nearly tripled in children ages 2–19,2 raising concern for poor future cardio-metabolic outcomes in these children and youth.
Despite the progress in eliminating mother to child transmission of HIV, long-term monitoring of HEU children into adolescence and adulthood remains important given the potential for long-term effects from in utero HIV/antiretroviral (ARV) exposure in these children.3–5 Metabolic disturbances from in utero HIV/ARV exposure have been reported in HEU infants and young children.6–10 These include mitochondrial toxicity,9–11 lipid disturbances,12 alterations in insulin sensitivity,8 and dysregulated intermediary metabolism.7,8,13,14 As many of these metabolic effects are intertwined and may be influenced by both fetal metabolic programming15 as well as postnatal environmental factors during the life course of an individual,16 robust studies with rigorous control for confounders have been necessary to disentangle the in utero HIV/ARV effects from other factors. HIV and antiretroviral therapy (ART) are known to be associated with metabolic complications in children living with HIV,17–19 but whether in utero HIV/ARV exposure has the potential to be associated with long-term metabolic complications among uninfected children as well remains unclear. Few published studies exist on metabolic outcomes in HEU youth with an appropriate comparison group of HIV-unexposed youth. The objective of our study was to assess whether obese HEU youth have a higher prevalence of cardio-metabolic risk factors such as hypertension, dyslipidemia, and insulin resistance compared to a matched group of obese HIV-unexposed youth in the general U.S. pediatric population.
Subjects and Methods:
HEU children enrolled in the Surveillance Monitoring of ART Toxicities (SMARTT) protocol of the Pediatric HIV/AIDS Cohort Study (PHACS) network constituted the study population, while selected children who participated in the 2005, 2007, 2009, or 2011 National Health and Nutrition Examination Survey (NHANES) study constituted the comparison control population.
SMARTT Study
SMARTT is a large prospective cohort study of HEU children born to pregnant women living with HIV designed to assess the safety of antenatal ART exposure on childhood and long-term outcomes. Enrollment has been ongoing since 2007 at 22 clinical sites in the U.S., including Puerto Rico. Children in the Dynamic SMARTT cohort were enrolled within 1 week of birth, and those in the Static SMARTT cohort were enrolled at <12 years of age with early life data collected through other co-enrolled studies.20 Children had height, weight,21 and blood pressure measurements performed yearly beginning at 1 year of age and then every other year after 5 years of age. Body Mass Index (BMI) was calculated as kg/m2. Z-scores for weight (WTZ), height (HTZ), and BMI (BMIZ) were calculated from CDC 2000 Growth Charts.22 At ≥3 years of age, children who met a pre-determined metabolic outcome trigger of obesity (BMIZ >95th percentile) underwent fasting laboratory testing for lipid sub-fractions [Total Cholesterol (TC), Triglycerides (TG), Low-Density Lipoprotein Cholesterol (LDL), and High-Density Lipoprotein Cholesterol (HDL)], and insulin resistance [Homeostatic Model of Assessment-Insulin Resistance (HOMA-IR)]. Information on potential confounders including age, sex, race/ethnicity, and anthropometrics was collected. In utero ARV exposures were also collected and summarized. If more than one ART regimen was used during pregnancy, the most potent ART was chosen. ART was classified in the following manner from most potent to least potent: ART consisting of ≥3 classes of ARVs, integrase strand transfer inhibitor (INSTI)-based ART, protease inhibitor (PI)-based ART, non-nucleoside reverse transcriptase inhibitor (NNRTI)-based ART, nucleoside reverse transcriptase inhibitor (NRTI)-based ART consisting of ≥3 NRTIs, non-combination ART, or no ART. If the lipid or blood pressure (BP) measurement was more than 6 months from the date of the BMI measurement meeting the >95th percentile criteria, they were excluded from the analysis.
NHANES
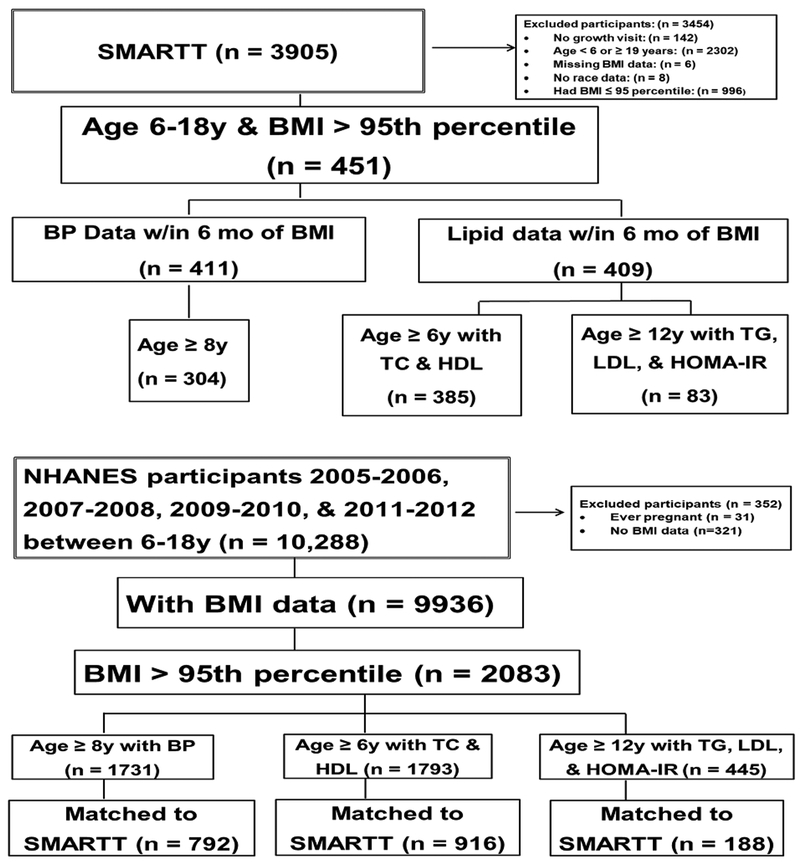
NHANES is a study designed to assess the health and nutrition of children and adults amongst the general population in the U.S. using a combination of survey and physical examination methods.21 Children participating in the NHANES study were 6–18 years of age, and for this analysis, participated in NHANES between 2005 and 2012. Those ≥8 years of age had their blood pressure measured. In addition, those ≥6 years of age had non-fasting lipid sub-fractions measured (TC and HDL), while only those ≥12 years had fasting measurements of LDL, TG, and HOMA-IR. Weight and height were measured and Z-scores calculated as described above for the SMARTTparticipants. Because only obese SMARTT children (BMI >95th percentile) had lipid and insulin resistance testing, only NHANES children with a BMI >95th percentile were selected for inclusion. (Figure 1)
Figure 1. Study Population Derivation.
BMI=Body Mass Index; BP=Blood Pressure; HOMA=Homeostatic Model of Assessment-Insulin Resistance; LDL=Low Density Lipoprotein Cholesterol; NHANES=National Health and Nutrition Examination Survey; SMARTT=Surveillance Monitoring of ART Toxicities; TC=Total Cholesterol; TG=Triglycerides
Primary Outcomes
Primary outcomes for this analysis included systolic and diastolic BP, insulin resistance as measured using the HOMA-IR,23 and the following fasting lipid sub-fractions: TC, LDL, HDL, and TG. Hypertension was defined as a systolic or diastolic BP ≥90th percentile according to age, sex, and height standards,24 insulin resistance as a HOMA-IR >4.025, hypercholesterolemia as TC >200 mg/dL, high LDL as LDL >130 mg/dL, low HDL as HDL <35 mg/dL, and hypertriglyceridemia as TG >150 mg/dL.26 Secondary analyses were conducted using the continuous measures corresponding to each primary outcome, with HOMA-IR log-transformed to more closely approximate a normal distribution and BP Z-scores calculated using U.S. standards.24
Analytic Sub-groups
For all analyses, non-pregnant SMARTT and NHANES participants 6–18 years of age were selected for inclusion. Because available NHANES data on different metabolic outcomes were limited to particular age groups, analyses were restricted to older cohorts in both SMARTT and NHANES for outcomes requiring a fasting blood specimen (LDL, TG, and HOMA-IR). Therefore, three analytic sub-groups were created for analyzing different outcomes with the following ages: 1) Systolic and diastolic BP outcomes, ≥8 years of age; 2) TC and HDL outcomes, ≥6 years of age; and 3) TG, LDL, and HOMA-IR outcomes, >12 years of age. For the first two subgroups above, the NHANES cohort was randomly sampled and individually matched by age (<10 vs. ≥10 years for females, <12 vs. ≥12 years for males), sex, and race/ethnicity (Non-Hispanic Black vs. Not Non-Hispanic Black) with up to 3 NHANES youth matched to each SMARTT HEU participant. For the third analytic subgroup, the NHANES cohort was randomly sampled and up to 3 youth were individually matched to each HEU youth on sex and race/ethnicity only.
Statistical Analysis
Baseline characteristics were compared between SMARTT HEU and NHANES children using Fisher’s exact test or a t-test with unequal variances, as appropriate. For the primary dichotomous outcomes (hypertension, insulin resistance, hypercholesterolemia, etc.), modified Poisson regression models with a robust error variance were fit to estimate the prevalence ratio (PR) and 95% confidence intervals (95% CI) of having each outcome as a function of cohort, adjusted for potential confounders of age (years), sex, race/ethnicity (Non-Hispanic Black vs. not non-Hispanic Black), and BMIZ. For the underlying continuous measures, generalized estimating equation (GEE) linear regression models were fit to obtain robust variances, specifying the distribution as normal and the identity link to estimate mean differences of continuous outcomes comparing the two cohorts (SMARTT HEU vs NHANES), unadjusted and adjusted for the same potential confounders. The GEE approach was utilized due to potential non-normality of our outcome measures. Although subjects were already matched on age category, race, and gender, the above variables were added to the models to account for any residual confounding. Additional models adjusting for income were fit due to the inability to match on income as there were too few non-Hispanic Blacks in NHANES who were at the same income level as SMARTT participants. Statistical analyses were performed using SAS® 9.4 (SAS Institute, Cary, NC), and two-sided p-values less than 0.05 were considered statistically significant.
Results
Number of participants in each analytic subgroup
For the BP analytic sample, 1731 NHANES participants were available to be matched to 304 SMARTT participants by age, sex, and race. For the TC/HDL sample, 1793 NHANES participants were eligible to be matched to 385 SMARTT participants. For the TG/LDL/HOMA-IR sample, 445 NHANES participants were eligible to be matched to 83 SMARTT participants. (Figure 1) After matching up to 3 NHANES youth (depending on availability) for each SMARTT HEU participant on age, sex, and race/ethnicity, 1096 participants (n=304 from SMARTT, n=792 from NHANES) were included in the BP outcome analytic subgroup, 1301 participants (n=385 from SMARTT, n=916 from NHANES) in the TC/HDL outcomes analytic subgroup, and 271 (n=83 from SMARTT, n=188 from NHANES) in the TG/LDL/HOMA-IR outcomes analytic subgroup.
Characteristics
Table 1 shows characteristics of each cohort group by outcome analysis. In the BP outcome analytic subgroup, SMARTT HEU youth had higher median HTZ (0.82 vs. 0.65) and WTZ scores (2.24 vs. 2.15), but similar BMIZ compared to NHANES participants. Fifty-two percent of the HEU youth in this analytic group were exposed to in utero PI-based ART, with a median in utero ART exposure duration of the most potent ART of 21.6 weeks. In the TC/HDL outcome analytic subgroup, HEU youth were younger (median of 9.9 vs. 10.6 years), more often non-Hispanic Black (58% vs. 47%), more likely to report an annual household income <$20,000 (64% vs. 30%) and have a higher median HTZ (0.85 vs. 0.68) compared to NHANES participants. Fifty-five percent of the HEU youth were exposed to in utero PI-based ART with a median in utero ART exposure duration of 21.4 weeks. In the TG/LDL/HOMA-IR outcome analytic subgroup, SMARTT HEU youth were younger (median of 14.8 vs. 15.4 years) and more likely to report an annual household income <$20,000 (60% vs. 33%) than those in NHANES. In utero ART exposure for HEU youth in this analytic group consisted primarily of PI-based ART (31%), NNRTI-based ART (15%), and non-combination ART (38%).
Table 1.
Characteristics of Children in Each Analytic Outcome Subgroup Comparing Obese PHACS SMARTT vs. NHANES Participants
| BLOOD PRESSURE OUTCOME ANALYTIC SUBGROUP | |||
|---|---|---|---|
| Cohort | |||
| Characteristic | SMARTT HEU (n=304) |
NHANES (n=792) |
p-value |
| Age (yr)/sex groups | |||
| Female age < 10 | 46 (15%) | 98 (12%) | 0.22 |
| Female age ≥ 10 | 107 (35%) | 317 (40%) | |
| Male age < 12 | 108 (36%) | 248 (31%) | |
| Male age ≥12 | 43 (14%) | 129 (16%) | |
| Age (years) | 11.1 (9.3, 13.2) | 11.8 (9.8, 14.9) | < 0.01 |
| Non-Hispanic Black | 187 (62%) | 441 (56%) | 0.09 |
| Female | 153 (50%) | 415 (52%) | 0.54 |
| Annual household income ($) | |||
| < 20,000 | 206 (68%) | 221 (29%) | < 0.01 |
| 20,000 – 50,000 | 82 (27%) | 357 (46%) | |
| > 50,000 | 16 (5%) | 190 (25%) | |
| BMIZ | 2.07 (1.87, 2.40) | 2.07 (1.85, 2.33) | 0.17 |
| HTZ | 0.82 (0.02, 1.59) | 0.65 (−0.02, 1.28) | <0.01 |
| WTZ | 2.24 (1.79, 2.66) | 2.15 (1.81, 2.49) | 0.03 |
| Duration of most potent in utero ART regimen (wk) | 21.6 (15.6, 28.9) | -- | |
| In utero ART regimen | |||
| No ARVs | 11 (4%) | -- | |
| Non-combination ART | 49 (16%) | -- | |
| ≥ 3 NRTIs | 35 (12%) | -- | |
| NNRTI-based ART | 36 (12%) | -- | |
| PI-based ART | 158 (52%) | -- | |
| ≥ 3 ARV classes | 1 (0%) | -- | |
| Missing | 14 (4%) | -- | |
| In utero exposure to AZT, d4T, ddI, or ddC | 261 (86%) | -- | |
| TC AND HDL OUTCOME ANALYTIC SUBGROUP | |||
| Cohort | |||
| Characteristic | SMARTT HEU (n=385) |
NHANES (n=916) |
p-value |
| Age (yr)/sex groups | |||
| Female age < 10 | 98 (25%) | 213 (23%) | 0.05 |
| Female age ≥ 10 | 98 (25%) | 287 (31%) | |
| Male age < 12 | 154 (40%) | 311 (34%) | |
| Male age ≥ 12 | 35 (9%) | 105 (11%) | |
| Age (yr) | 9.9 (7.4, 11.4) | 10.6 (8.5, 14.0) | <0.01 |
| Non-Hispanic Black | 224 (58%) | 433 (47%) | <0.01 |
| Female | 196 (51%) | 500 (55%) | 0.25 |
| Annual household income ($) | |||
| < 20,000 | 247 (64%) | 261 (30%) | <0.01 |
| 20,000 – 50,000 | 118 (31%) | 415 (47%) | |
| > 50,000 | 20 (5%) | 203 (23%) | |
| BMIZ | 2.08 (1.88, 2.41) | 2.09 (1.86, 2.38) | 0.53 |
| HTZ | 0.85 (0.15, 1.61) | 0.68 (0.03, 1.28) | <0.01 |
| WTZ | 2.20 (1.82, 2.63) | 2.15 (1.82, 2.54) | 0.24 |
| Duration of most potent in utero ART regimen (wk) | 21.4 (15.3, 29.0) | -- | |
| In utero ART regimen | |||
| No ARVs in pregnancy | 13 (3%) | -- | |
| Non-combination ART | 61 (16%) | -- | |
| ≥ 3 NRTIs | 39 (10%) | -- | |
| NNRTI-based ART | 41 (10%) | -- | |
| PI-based ART | 210 (55%) | -- | |
| INSTI-based ART | 3 (1%) | -- | |
| ≥ 3 ARV classes | 2 (1%) | -- | |
| Missing | 16 (4%) | -- | |
| In utero exposure to AZT, d4T, ddI, or ddC | 335 (87%) | -- | |
| TG, LDL, AND HOMA-IR OUTCOME ANALYTIC SUBGROUP | |||
| Cohort | |||
| Characteristic | SMARTT HEU (n=83) |
NHANES (n=188) |
p-value |
| Age (yr) | 14.8 (13.1, 15.3) | 15.4 (13.2, 17.3) | <0.01 |
| Non-Hispanic Black | 60 (72%) | 119 (63%) | 0.17 |
| Female | 53 (64%) | 98 (52%) | 0.09 |
| Annual household income ($) | |||
| < 20,000 | 50 (60%) | 60 (33%) | <0.01 |
| 20,000 – 50,000 | 26 (31%) | 76 (42%) | |
| > 50,000 | 7 (8%) | 45 (25%) | |
| BMIZ | 2.09 (1.86, 2.43) | 2.09 (1.85, 2.42) | 0.87 |
| HTZ | 0.41 (−0.16, 1.07) | 0.42 (−0.40, 0.99) | 0.30 |
| WTZ | 2.20 (1.90, 2.61) | 2.15 (1.88, 2.63) | 0.72 |
| Duration of most potent in utero ART regimen (wk) | 19.6 (11.6, 31.7) | -- | |
| In utero ART regimen | |||
| No ARVs | 6 (7%) | -- | |
| Non-combination ART | 31 (38%) | -- | |
| ≥ 3 NRTIs | 5 (6%) | -- | |
| NNRTI-based ART | 12 (15%) | -- | |
| PI-based ART | 26 (31%) | -- | |
| ≥ 3 ARV classes | 1 (1%) | -- | |
| Missing | 2 (2%) | -- | |
| In utero exposure to AZT, d4T, ddI, or ddC | 75 (90%) | -- | |
All continuous variables expressed as median (interquartile range) and categorical variables as n (%). ART=antiretroviral therapy; ARVs=antiretrovirals; BMIZ=Body Mass Index Z-score; HEU=HIV- exposed uninfected; HTZ=Height Z-score; INSTI=Integrase Strand Transfer Inhibitor; NHANES=National Health and Nutrition Examination Survey; NRTI=Nucleoside Reverse Transcriptase Inhibitor; NNRTI=Non-Nucleoside Reverse Transcriptase Inhibitor; PHACS=Pediatric HIV/AIDS Cohort Study; PI=Protease Inhibitor; SMARTT=Surveillance Monitoring of ART Toxicities; WTZ=Weight Z-score; wk=weeks; yr=years
Comparison of blood pressure between SMARTT HEU and NHANES youth
Overall, both median systolic and diastolic BP Z-scores were higher in HEU vs. NHANES participants (0.75 vs. 0.11, p<0.01 and 0.26 vs. −0.44, p<0.01 respectively), with HEU participants exhibiting higher rates of systolic and diastolic hypertension (28% vs. 9%, p<0.01 and 7% vs. 3%, p=0.02 respectively) (Table 2). These differences persisted even after adjustment for age, BMIZ, sex, and non-Hispanic Black race/ethnicity [adjusted mean difference = 0.64 in HEU vs. NHANES youth, p<0.01 for systolic BP; adjusted PR (aPR) =3.34, 95% CI: 2.48–4.50 for systolic hypertension; adjusted mean difference = 0.72 in HEU vs. NHANES youth, p<0.01 for diastolic BP; aPR = 2.04, 95% CI: 1.18–3.52 for diastolic hypertension]. (Table 3) Additional models adjusting for income as well as the above confounders showed similar results. (Supplemental Table 1)
Table 2.
Metabolic Outcome Measures in Each Analytic Outcome Subgroup Comparing Obese PHACS SMARTT vs. NHANES Participants
| Metabolic Outcome | Cohort | p-value | |
|---|---|---|---|
| SMARTT HEU | NHANES | ||
| Blood Pressure | n=304 | n=792 | |
| Systolic BP Z-score | 0.75 (0.12, 1.34) | 0.11 (−0.43, 0.68) | <0.01 |
| Abnormal Systolic BP (≥90th percentile) | 84 (28%) | 70 (9%) | <0.01 |
| Diastolic BP Z-score | 0.26 (−0.18, 0.74) | −0.44 (−1.13, 0.26) | <0.01 |
| Abnormal Diastolic BP (≥90th percentile) | 21 (7%) | 27 (3%) | 0.02 |
| Abnormal Systolic or Diastolic BP (Systolic or Diastolic BP >90th percentile) | 92 (30%) | 89 (11%) | <0.01 |
| TC and HDL | n=385 | n=916 | |
| TC (mg/dL) | 155 (138, 178) | 162 (145, 182) | <0.01 |
| Hypercholesterolemia (> 200 mg/dL) | 31 (8%) | 104 (11%) | 0.09 |
| HDL (mg/dL) | 47 (40, 55) | 46 (40, 53) | 0.18 |
| Low HDL (< 35 mg/dL) | 32 (8%) | 72 (8%) | 0.82 |
| TG, LDL, and HOMA-IR | n=83 | n=188 | |
| TG (mg/dL) | 66 (54, 100) | 77 (55, 117) | 0.22 |
| Hypertriglyceridemia (> 150 mg/dL) | 8 (10%) | 23 (12%) | 0.68 |
| LDL (mg/dL) | 88 (73, 104) | 94 (73, 110) | 0.43 |
| High LDL (> 130 mg/dL) | 8 (10%) | 18 (10%) | 1.00 |
| HOMA-IR | 4.05 (2.50, 5.93) | 5.47 (3.96, 7.51) | <0.01 |
| Insulin Resistant (> 4.0) | 42 (51%) | 140 (74%) | <0.01 |
| Systolic BP Z-score | 0.75(0.12, 1.34) | 0.11 (−0.43,0.68) | <0.01 |
| Abnormal Systolic BP (≥ 90th percentile) | 84 (28%) | 70 (9%) | <0.01 |
| Diastolic BP Z-score | 0.26 (−0.18, 0.74) | −0.44 (−1.13, 0.26) | <0.01 |
| Abnormal Diastolic BP (≥ 90th percentile) | 21 (7%) | 27 (3%) | 0.02 |
| Abnormal Systolic or Diastolic BP (Systolic or Diastolic BP ≥ 90th percentile) | 92 (30%) | 89(11%) | <0.01 |
| TC and HDL | n=385 | n=916 | |
All continuous variables expressed as median (interquartile range) and categorical variables as n (%). BP=Blood Pressure; HDL=High-Density Lipoprotein cholesterol; HEU=HIV-exposed uninfected; HOMA-IR=Homeostatic Model of Assessment-Insulin Resistance; LDL=Low-Density Lipoprotein cholesterol; PHACS=Pediatric HIV/AIDS Cohort Study; NHANES=National Health and Nutrition Examination Survey; SMARTT= Surveillance Monitoring of ART Toxicities study; TC=Total Cholesterol; TG=Triglycerides.
Table 3.
Models of Mean Differences and Prevalence Ratio Estimates Comparing SMARTT vs. NHANES for Each Metabolic Outcome*
| Model | Adjusted Mean Difference (95% CI) |
p-value | Adjusted Prevalence Ratio (95% CI) |
p-value |
|---|---|---|---|---|
| Systolic BP z-score | 0.64 (0.52, 0.75) | <0.01 | 3.34 (2.48, 4.50) | <0.01 |
| Diastolic BP z-score | 0.72 (0.61, 0.83) | <0.01 | 2.04 (1.18, 3.52) | 0.01 |
| TC | −5.49 (−8.98, −1.99) | <0.01 | 0.67 (0.44, 1.01) | 0.06 |
| HDL | −0.41 (−1.66, 0.85) | 0.52 | 1.27 (0.85, 1.88) | 0.25 |
| TG | −2.14 (−12.12, 7.85) | 0.67 | 1.00 (0.40, 2.49) | 1.00 |
| LDL | −1.60 (−8.90, 5.71) | 0.67 | 0.98 (0.38, 2.54) | 0.96 |
| Log HOMA-IR | −0.37 (−0.52, −0.21) | <0.01 | 0.67 (0.54, 0.85)^ | <0.01 |
All models adjusted for age, body mass index Z-score, sex, and non-Hispanic Black race/ethnicity.
Outcome is insulin resistance defined as HOMA-IR >4.0
BP=Blood Pressure; CI=Confidence Interval; HDL=High-Density Lipoprotein Cholesterol; HOMA-IR=Homeostatic Model Assessment-Insulin Resistance; LDL=Low-Density Lipoprotein Cholesterol; TC=Total Cholesterol; TG=Triglycerides
Comparison of TC and HDL between SMARTT HEU and NHANES youth
Though no differences in HDL were observed between HEU and NHANES youth, median TC was lower (155 mg/dL vs. 162 mg/dL, p<0.01). However, no differences were observed in prevalence of high TC between groups or in any of the other lipid sub-fractions. This difference in TC persisted even after adjustment for age, BMIZ, sex, and non-Hispanic Black race/ethnicity (adjusted mean difference = −5.49, p<0.01; aPR = 0.67, 95% CI: 0.44–1. 01). (Table 3) Models additionally adjusting for income showed similar results. (Supplemental Table 1)
Comparison of TG, LDL, and insulin resistance between SMARTT HEU and NHANES youth
Overall rates of insulin resistance was high in both HEU and NHANES youth combined (67%), and median HOMA-IR was >4.0 in both groups. Median HOMA-IR was lower in HEU vs. NHANES youth (4.05 vs. 5.47, p<0.01) with lower rates of insulin resistance amongst SMARTT participants (51% vs 74%, p<0.01) in univariate analyses. No differences in TG or LDL levels were observed between groups. After adjustment for sex and non-Hispanic Black race/ethnicity, there remained a significantly lower prevalence of insulin resistance in HEU youth as compared to NHANES (aPR = 0.67, 95% CI: 0.54–0.85), with a corresponding lower log-HOMA-IR (adjusted mean difference = −0.37 in HEU vs. NHANES participants, p<0.01) (Table 3). Supplemental Table 1 shows models additionally adjusting for income which demonstrated similar results.
Discussion
We found that obese HEU children in the PHACS SMARTT study had higher systolic and diastolic BP, but lower HOMA-IR and TC compared with a general obese pediatric population represented in NHANES. Few studies have evaluated long-term metabolic outcomes in HEU vs. HIV-unexposed uninfected (HUU) children.12,27 Our study is the largest to date to investigate these outcomes in older HEU, increasing the generalizability of our findings.
Overall, 14% of the entire study population met the definition of systolic hypertension, reflecting the obese nature of both cohorts. Our findings regarding BP outcomes in HEU children are similar to a study in Zambia of 111 HEU and 279 HUU children where systolic BP trended toward being significantly higher in HEU vs. HUU children.27 Another smaller U.S. study reported no differences in BP outcomes between HEU and HUU children.28 The increased risk for hypertension in our HEU population will be important to monitor as these HEU youth mature into adulthood since hypertension in childhood is known to predict hypertension in adulthood.29,30 A recent study estimated that adolescents with pre-hypertension progress to frank hypertension at a rate of approximately 7% per year.30 In addition, hypertension in children is known to be associated with several markers of cardiovascular morbidity including left ventricular hypertrophy31 and subclinical atherosclerosis as measured by carotid intima-media thickness.31–34 Compared to HUU children, HEU children have been shown to have left ventricular dysfunction,35 but the long-term significance of this and its relationship with childhood hypertension remain unclear.
While SMARTT HEU youth had lower HOMA-IR than NHANES youth, median HOMA-IR in both groups was >4.0, and both had high rates of insulin resistance, likely due to the fact that this was an obese population. The lower HOMA-IR that we observed in SMARTT HEU children compared to NHANES children may be explained by differences in pubertal stage, but data on Tanner staging was not available in the NHANES cohort. In addition, this phenomenon of lower HOMA-IR in HEU children has been described in HEU infants in Africa,8 while smaller studies have shown no differences in HOMA-IR between younger HEU and HUU children.12 Similar data of this type have not been published in HEU children above the age of 10. In Cameroon, HEU infants at 6 weeks of age had lower HOMA-IR than HUU infants, with HEU infants receiving zidovudine (AZT) infant prophylaxis exhibiting the lowest HOMA-IR values compared to HEU infants receiving nevirapine (NVP) prophylaxis and to HUU infants.8 In addition, mitochondrial DNA content was decreased11 and mitochondrial fuel utilization was altered, raising the notion that perhaps mechanisms involving mitochondrial toxicity from AZT may be at the center of association of in utero HIV/ARV and postnatal AZT exposure with lower HOMA-IR and altered fuel utilization. The vast majority of the SMARTT HEU cohort received AZT infant prophylaxis after birth for 4–6 weeks as per U.S. guidelines.36 Of note, 87% of the HDL and TC analytic subgroup and 90% of the LDL, TG, and HOMA-IR analytic subgroup were exposed to in utero dideoxy analogue NRTIs such as AZT, didanosine (ddI), stavudine (d4T), and zalcitabine (ddC) which are known to cause mitochondrial toxicity through inhibition of mitochondrial DNA polymerase-ɣ.37 Findings in our present study of older SMARTT HEU children would suggest that perhaps these same observations persist at least through childhood and early adolescence among youth who are obese. Long-term effects of these alterations in glucose and fuel utilization are still unknown.
While the finding of lower TC levels that we observed in SMARTT HEU compared to NHANES children is contradictory to other studies where HEU children have shown higher12 as well as no differences27, our results likely reflect the lower HOMA-IR levels observed in our cohort. Insulin resistance is associated with increased cholesterol synthesis and decreased cholesterol absorption38,39 and, conversely, insulin sensitivity (lower HOMA-IR) would be associated with lower cholesterol synthesis.
Our study comparison was limited due to the cross-sectional nature and lack of information on pubertal status in the NHANES study. However, the SMARTT HEU cohort was followed longitudinally, and in utero HIV/ARV exposures were well-documented to allow prospective evaluation of subsequent outcomes. In addition, though we had information on small-for-gestational-age (SGA), pre-term birth, and perinatal HIV exposure in SMARTT, these data were not collected in NHANES; both SGA and pre-term birth may be confounders. There is the possibility in NHANES that a child may have been HEU without our knowledge, though this would likely be rare in this U.S. cohort. We also did not have information on diet, physical activity, and family history of hypertension and cardio-metabolic outcomes in the NHANES study. Finally, we could not match on income because there were too few non-Hispanic blacks in NHANES who were at the same income level as SMARTT participants. However, when we adjusted for income in the analysis, results did not change.
In summary, obese HEU youth in the U.S. may have an increased risk for systolic and diastolic hypertension, but lower risks for insulin resistance and hypercholesterolemia compared to a general obese U.S. pediatric population in NHANES. The long-term significance of these findings remains unclear, but monitoring for cardiovascular morbidity in adulthood may be warranted in HEU children.
Supplementary Material
Acknowledgments:
We thank the children and families for their participation in PHACS, and the individuals and institutions involved in the conduct of PHACS. This manuscript is dedicated to the memory of Tracie Miller, MD, who passed away before its completion.
JJ was supported by NICHD K23HD070760 during part of the preparation of this manuscript. MG serves as a consultant for AbbVie, BioBridge, Daiichi Sankyo, Diurnal, Endo, Nutritional Growth Solutions, Novo Nordisk, Pfizer, and Sandoz; is a member of data safety monitoring boards for Ascendis and Tolmar; receives royalties from McGraw-Hill and UpToDate. EJM receives institutional support for conducting clinical trials investigating pediatric antiretrovirals sponsored by Gilead, Inc. For the remaining authors, no other funding sources or conflicts of interests were declared.
TM and DJ conceptualized this manuscript. JJ wrote the first draft of the manuscript and had primary responsibility for the final content and approval of the paper. DJ and WY analyzed the data. DJ, WY, and PLW made significant edits to the Methods section. JJ, TM, DJ, WY, WB, MEG, EJM, KP, and PLW edited and revised the entire manuscript.
The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development with co-funding from the National Institute on Drug Abuse, the National Institute of Allergy and Infectious Diseases, the Office of AIDS Research, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institute on Deafness and Other Communication Disorders, the National Institute of Dental and Craniofacial Research, and the National Institute on Alcohol Abuse and Alcoholism, through cooperative agreements with the Harvard T.H. Chan School of Public Health (HD052102) (Principal Investigator: George Seage; Program Director: Julie Alperen) and the Tulane University School of Medicine (HD052104) (Principal Investigator: Russell Van Dyke; Co-Principal Investigator: Ellen Chadwick; Project Director: Patrick Davis). Data management services were provided by Frontier Science and Technology Research Foundation (PI: Suzanne Siminski), and regulatory services and logistical support were provided by Westat, Inc (PI: Julie Davidson).
The following institutions, clinical site investigators and staff participated in conducting PHACS SMARTT in 2017, in alphabetical order: Ann & Robert H. Lurie Children’s Hospital of Chicago: Ellen Chadwick, Margaret Ann Sanders, Kathleen Malee, Scott Hunter; Baylor College of Medicine: William Shearer, Mary Paul, Norma Cooper, Lynnette Harris; Bronx Lebanon Hospital Center: Murli Purswani, Emma Stuard, Mahboobullah Mirza Baig, Alma Villegas; Children’s Diagnostic & Treatment Center: Ana Puga, Dia Cooley, Patricia A. Garvie, James Blood; New York University School of Medicine: William Borkowsky, Sandra Deygoo, Marsha Vasserman; Rutgers - New Jersey Medical School: Arry Dieudonne, Linda Bettica, Juliette Johnson; St. Jude Children’s Research Hospital: Katherine Knapp, Kim Allison, Megan Wilkins, Jamie Russell-Bell; San Juan Hospital/Department of Pediatrics: Nicolas Rosario, Lourdes Angeli-Nieves, Vivian Olivera; SUNY Downstate Medical Center: Stephan Kohlhoff, Ava Dennie, Ady Ben-Israel, Jean Kaye; Tulane University School of Medicine: Russell Van Dyke, Karen Craig, Patricia Sirois; University of Alabama, Birmingham: Marilyn Crain, Paige Hickman, Dan Marullo; University of California, San Diego: Stephen A. Spector, Kim Norris, Sharon Nichols; University of Colorado, Denver: Elizabeth McFarland, Emily Barr, Christine Kwon, Carrie Chambers; University of Florida, Center for HIV/AIDS Research, Education and Service: Mobeen Rathore, Kristi Stowers, Saniyyah Mahmoudi, Nizar Maraqa, Laurie Kirkland; University of Illinois, Chicago: Karen Hayani, Lourdes Richardson, Renee Smith, Alina Miller; University of Miami: Gwendolyn Scott, Sady Dominguez, Jenniffer Jimenez, Anai Cuadra; Keck Medicine of the University of Southern California: Toni Frederick, Mariam Davtyan, Guadalupe Morales-Avendano, Janielle Jackson-Alvarez; University of Puerto Rico School of Medicine, Medical Science Campus: Zoe M. Rodriguez, Ibet Heyer, Nydia Scalley Trifilio.
Note: The conclusions and opinions expressed in this article are those of the authors and do not necessarily reflect those of the National Institutes of Health or U.S. Department of Health and Human Services.
Footnotes
There are no disclaimers.
REFERENCES
- 1.UNAIDS 2013 AIDS by the numbers. Accessed at http://www.unaids.org/sites/default/files/media_asset/JC2571_AIDS_by_the_numbers_en_1.pdf on October 29, 2016. In. Geneva: UNAIDS. [Google Scholar]
- 2.Fryar CD CM, Ogden CL. . National Center for Health Statistics. Division of Health and Nutrition Examination Surveys. Prevalence of Overweight and Obesity Among Children and Adolescents Aged 2–19 Years: United States, 1963–1965 Through 2013–2014. Accessed at https://www.cdc.gov/nchs/data/hestat/obesity_child_13_14/obesity_child_13_14.pdf on June 2, 2018. . 2016.
- 3.Hleyhel M, Goujon S, Delteil C, et al. Risk of cancer in children exposed to didanosine in utero. AIDS. 2016. [DOI] [PubMed] [Google Scholar]
- 4.Blanche S, Tardieu M, Rustin P, et al. Persistent mitochondrial dysfunction and perinatal exposure to antiretroviral nucleoside analogues. Lancet. 1999;354(9184):1084–1089. [DOI] [PubMed] [Google Scholar]
- 5.Afran L, Garcia Knight M, Nduati E, Urban BC, Heyderman RS, Rowland-Jones SL. HIV-exposed uninfected children: a growing population with a vulnerable immune system? Clinical and experimental immunology. 2014;176(1):11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jao J, Abrams EJ. Metabolic complications of in utero maternal HIV and antiretroviral exposure in HIV-exposed infants. Pediatr Infect Dis J. 2014;33(7):734–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirmse B, Hobbs CV, Peter I, et al. Abnormal newborn screens and acylcarnitines in HIV-exposed and ARV-exposed infants. Pediatr Infect Dis J. 2013;32(2):146–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jao J, Kirmse B, Yu C, et al. Lower Preprandial Insulin and Altered Fuel Use in HIV/Antiretroviral-Exposed Infants in Cameroon. J Clin Endocrinol Metab. 2015;100(9):3260–3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aldrovandi GM, Chu C, Shearer WT, et al. Antiretroviral exposure and lymphocyte mtDNA content among uninfected infants of HIV-1-infected women. Pediatrics. 2009;124(6):e1189–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barret B, Tardieu M, Rustin P, et al. Persistent mitochondrial dysfunction in HIV-1-exposed but uninfected infants: clinical screening in a large prospective cohort. AIDS. 2003;17(12):1769–1785. [DOI] [PubMed] [Google Scholar]
- 11.Jao J, Powis KM, Kirmse B, et al. Lower mitochondrial DNA and altered mitochondrial fuel metabolism in HIV-exposed uninfected infants in Cameroon. AIDS. 2017;31(18):2475–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Claudio CC, Patin RV, Palchetti CZ, Machado DM, Succi RC, Oliveira FL. Nutritional status and metabolic disorders in HIV-exposed uninfected prepubertal children. Nutrition. 2013;29(7–8):1020–1023. [DOI] [PubMed] [Google Scholar]
- 13.Kirmse B, Yao TJ, Hofherr S, et al. Acylcarnitine Profiles in HIV-Exposed, Uninfected Neonates in the United States. AIDS Res Hum Retroviruses. 2016;32(4):339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schoeman JC, Moutloatse GP, Harms AC, et al. Fetal Metabolic Stress Disrupts Immune Homeostasis and Induces Proinflammatory Responses in Human Immunodeficiency Virus Type 1- and Combination Antiretroviral Therapy-Exposed Infants. J Infect Dis. 2017;216(4):436–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barker DJ. The developmental origins of insulin resistance. Horm Res. 2005;64 Suppl 3:2–7. [DOI] [PubMed] [Google Scholar]
- 16.Ben-Shlomo Y, Kuh D. A life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives. Int J Epidemiol. 2002;31(2):285–293. [PubMed] [Google Scholar]
- 17.Dimock D, Thomas V, Cushing A, et al. Longitudinal assessment of metabolic abnormalities in adolescents and young adults with HIV-infection acquired perinatally or in early childhood. Metabolism. 2011;60(6):874–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dirajlal-Fargo S, Musiime V, Cook A, et al. Insulin Resistance and Markers of Inflammation in HIV-infected Ugandan Children in the CHAPAS-3 Trial. Pediatr Infect Dis J. 2017;36(8):761–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aldrovandi GM, Lindsey JC, Jacobson DL, et al. Morphologic and metabolic abnormalities in vertically HIV-infected children and youth. AIDS. 2009;23(6):661–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Dyke RB, Chadwick EG, Hazra R, Williams PL, Seage GR, 3rd. The PHACS SMARTT Study: Assessment of the Safety of In Utero Exposure to Antiretroviral Drugs. Front Immunol. 2016;7:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Health National and Nutrition Examination Survey III Body Measurements (anthropometry) 2010. Available at https://www.cdc.gov/nchs/data/nhanes/nhanes3/cdrom/nchs/manuals/anthro.pdf (Accessed April 25, 2017).
- 22.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Advance data. 2000(314):1–27. [PubMed] [Google Scholar]
- 23.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. [DOI] [PubMed] [Google Scholar]
- 24.US Department of Health and Human Resources,National Institute of Health, National Heart, Lung, and Blood Institute. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. NIH Publication No. 05–5267. Bethesda, MD: National Heart, Lung, and Blood Institute; September 1996, Revised May 2005. Accessed at https://www.nhlbi.nih.gov/files/docs/resources/heart/hbp_ped.pdf on June 18, 2017.. [Google Scholar]
- 25.Valerio G, Licenziati MR, Iannuzzi A, et al. Insulin resistance and impaired glucose tolerance in obese children and adolescents from Southern Italy. Nutr Metab Cardiovasc Dis. 2006;16(4):279–284. [DOI] [PubMed] [Google Scholar]
- 26.Daniels SR, Greer FR. Lipid screening and cardiovascular health in childhood. Pediatrics. 2008;122(1):198–208. [DOI] [PubMed] [Google Scholar]
- 27.Nicholson L, Chisenga M, Siame J, Kasonka L, Filteau S. Growth and health outcomes at school age in HIV-exposed, uninfected Zambian children: follow-up of two cohorts studied in infancy. BMC Pediatr. 2015;15:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cade WT, Waggoner AD, Hubert S, Krauss MJ, Singh GK, Overton ET. Reduced diastolic function and left ventricular mass in HIV-negative preadolescent children exposed to antiretroviral therapy in utero. AIDS. 2012;26(16):2053–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bao W, Threefoot SA, Srinivasan SR, Berenson GS. Essential hypertension predicted by tracking of elevated blood pressure from childhood to adulthood: the Bogalusa Heart Study. American journal of hypertension. 1995;8(7):657–665. [DOI] [PubMed] [Google Scholar]
- 30.Falkner B, Gidding SS, Portman R, Rosner B. Blood pressure variability and classification of prehypertension and hypertension in adolescence. Pediatrics. 2008;122(2):238–242. [DOI] [PubMed] [Google Scholar]
- 31.Urbina EM, Khoury PR, McCoy C, Daniels SR, Kimball TR, Dolan LM. Cardiac and vascular consequences of pre-hypertension in youth. Journal of clinical hypertension. 2011;13(5):332–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lande MB, Carson NL, Roy J, Meagher CC. Effects of childhood primary hypertension on carotid intima media thickness: a matched controlled study. Hypertension. 2006;48(1):40–44. [DOI] [PubMed] [Google Scholar]
- 33.Sorof JM, Alexandrov AV, Garami Z, et al. Carotid ultrasonography for detection of vascular abnormalities in hypertensive children. Pediatr Nephrol. 2003;18(10):1020–1024. [DOI] [PubMed] [Google Scholar]
- 34.Lamotte C, Iliescu C, Libersa C, Gottrand F. Increased intima-media thickness of the carotid artery in childhood: a systematic review of observational studies. Eur J Pediatr. 2011;170(6):719–729. [DOI] [PubMed] [Google Scholar]
- 35.Lipshultz SE, Easley KA, Orav EJ, et al. Cardiovascular status of infants and children of women infected with HIV-1 (P(2)C(2) HIV): a cohort study. Lancet. 2002;360(9330):368–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams PL, Huo Y, Rutstein R, et al. Trends in Neonatal Prophylaxis and Predictors of Combination Antiretroviral Prophylaxis in US Infants from 1990 to 2015. AIDS Patient Care STDS. 2018;32(2):48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Konig H, Behr E, Lower J, Kurth R. Azidothymidine triphosphate is an inhibitor of both human immunodeficiency virus type 1 reverse transcriptase and DNA polymerase gamma. Antimicrob Agents Chemother. 1989;33(12):2109–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pihlajamaki J, Gylling H, Miettinen TA, Laakso M. Insulin resistance is associated with increased cholesterol synthesis and decreased cholesterol absorption in normoglycemic men. Journal of lipid research. 2004;45(3):507–512. [DOI] [PubMed] [Google Scholar]
- 39.Miettinen TA, Gylling H. Cholesterol absorption efficiency and sterol metabolism in obesity. Atherosclerosis. 2000;153(1):241–248. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.