Abstract
Aggressiveness has a high prevalence in psychiatric patients and is a major health problem. Two brain areas involved in the neural network of aggressive behavior are the amygdala and the hypothalamus. While pharmacological treatments are effective in most patients, some do not properly respond to conventional therapies and are considered medically refractory. In this population, surgical procedures (ie, stereotactic lesions and deep brain stimulation) have been performed in an attempt to improve symptomatology and quality of life. Clinical results obtained after surgery are difficult to interpret, and the mechanisms responsible for postoperative reductions in aggressive behavior are unknown. We review the rationale and neurobiological characteristics that may help to explain why functional neurosurgery has been proposed to control aggressive behavior.
Keywords: Aggression, Amygdala, Deep brain simulation, Hypothalamus, Stereotactic neurosurgery, Review
ABBREVIATIONS
- 5-HT
serotonin
- ASD
autism spectrum disorder
- DA
dopamine
- DBS
deep brain stimulation
- GABA
gamma-aminobutyric acid
- HFS
high-frequency stimulation
- MRI
magnetic resonance imaging
- PAG
periaqueductal gray
- PTSD
posttraumatic stress disorder
- VMH
ventromedial hypothalamic nucleus
Aggressive behavior is a primitive social conduct that is essential for individuals to compete for food, territory, and mating. In this regard, one may say that it is crucial for the maintenance of the species.1 In the case of humans, the presence of complex emotions makes understanding the neurobiological mechanisms underlying human aggressive behavior a challenging task.2,3 Violent crimes are often committed, and the costs required to address the consequences of these acts are high. Victims require physical and emotional care, and offenders are incarcerated and consequently became a burden on the government as a result of their loss of productivity.4
One strategy used to study human aggression is to simplify the behavior in a dichotomy model including premeditated (proactive or cold aggression) and impulsive (reactive or hot-headed aggression) aggression. Premeditated aggression involves a planned behavior that is intended to achieve a specific goal and is not accompanied by autonomic arousal or anger. Impulsive aggression is unrelated to a specific goal and usually involves frustration, provocation, or stress; this type of aggression is associated with high levels of autonomic arousal and impulsivity.3 Impulsive aggression is the core symptom of intermittent explosive disorder and presents as a feature of several psychiatric disorders, including schizophrenia, personality disorders (in particular, borderline and antisocial personality disorders), autism spectrum disorder (ASD), posttraumatic stress disorder (PTSD), and bipolar disorder.4-8 In addition, aggression in psychiatric patients is frequently associated with other comorbidities, such as anxiety, mood disorders, and sleep disturbances, as described in ASD patients.6 The association between mental disorders and violent behavior is a common reason for patient institutionalization.2
Studies in humans and other mammals indicate that the amygdala is a key component of a broader neural circuit that modulates aggressive behavior and also includes the hypothalamus, hippocampus, orbitofrontal cortex, and periaqueductal gray (PAG) matter.9,10 The amygdala presents reciprocal connections with the hypothalamus (mainly through the fornix and stria terminalis) and with the PAG (through the ventral amygdalofugal pathway), and receives massive projections from the prefrontal cortex through the uncinate fasciculus.11-14 The hypothalamus projects to the PAG via the dorsal longitudinal fasciculus and receives projections from the prefrontal cortex through the medial forebrain bundle.12,15,16 It is believed that impulsive forms of aggressive behavior occur when there is a hyperactivation of the limbic system, with insufficient top–down control from the prefrontal cortex.3 Figure 1 shows a schematic representation of the main neurocircuitry underlying aggressive behavior.
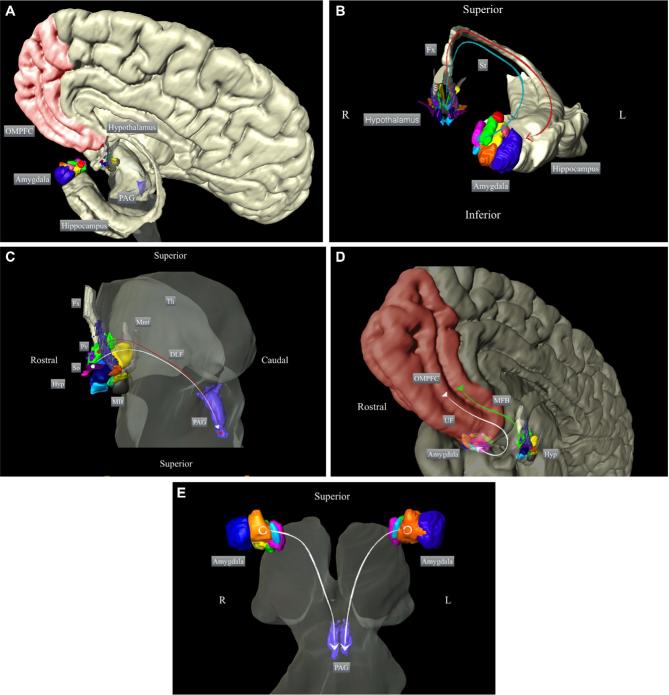
Figure 1.
Schematic representation of the participation of the amygdala and hypothalamus in the neurocircuitry underlying aggressive behavior. Overview of A, the main structures implicated in the control of aggressive behavior and B, the main connections between the hypothalamus and amygdala; C, between the hypothalamus and PAG; D, among the amygdala, hypothalamus, and frontal cortex; and E, between the amygdala and PAG. The 3-dimensional reconstructions are based on histological segmentations of the depicted structures (methods described in Alho et al144). OMPFC: orbitomedial prefrontal cortex; PAG: periaqueductal gray; Fx: fornix; St: stria terminalis; Hyp: hypothalamus; So: supraoptic nucleus; Pv: paraventricular hypothalamic nucleus; MB: mammillary body; Mmt: mammillothalamic tract; Th: thalamus; DLF: dorsal longitudinal fasciculus; MFB: medial forebrain bundle; UF: uncinate fasciculus.
The dysregulation of the serotonin (5-HT), dopamine (DA), and norepinephrine systems has been implicated in the overexpression of aggression. The impairment of receptor subunits and other neuronal elements, including the serotonin transporter (5-HT transporter), 5-HT1B receptor, gamma-aminobutyric acid A and B (GABA-A and GABA-B) receptors, glutamate (N-methyl D-aspartate) receptor, monoamine oxidase A, nitric oxide synthase, and neuroactive steroids, has been reported in aggressive subjects.17-19 It is necessary to integrate and understand these complex neurochemical interactions to effectively treat an aggressive patient.2
The primary treatment for aggressive behavior involves the use of medications and/or nonpharmacological treatments.20,21 Nonpharmacological treatments such as cognitive behavioral therapy and applied behavior analysis have an overall intervention effect that is considered low to medium and are sometimes ineffective.21,22 Electroconvulsive therapy is more efficacious but is associated with side effects.23,24
As impulsive aggression is often a symptom of associated disorders, first-line treatments are initially chosen to address the primary underlying conditions.25 As shown in Table 1, pharmacological treatment of aggressive behavior may involve the use of different classes of medications, such as typical and atypical antipsychotics, antidepressants, benzodiazepines, alpha 2 agonists, mood stabilizers, and anticonvulsants.6,26-49
Table 1.
Pharmacological Treatment of Aggressive Behavior
| Drug | Neurotransmitters involved | Target population | Observations |
|---|---|---|---|
| Typical antipsychotics26-28 | Dopaminergic antagonists (mainly D2) | ID, DB, psychotic, schizophrenia, bipolar disorders | Extrapyramidal side effects when receptor occupancy exceeds 80% |
| Atypical antipsychotics6,29-31 | Multiple: dopaminergic and serotonergic antagonists | ID, DB, ASD, dementia; psychotic | Risperidone and aripiprazole are FDA approved in ASD patients. Clozapine use is related to lower mortality in schizophrenia |
| Antidepressants32-35 | Selective serotonin reuptake inhibitors | ASD, ID, PTSD, unipolar depression, Alzheimer's disease, psychosis | The use of this class of drugs has been limited due to the side effects that occur at higher doses |
| Alpha 2 agonists36-38 | Alpha-2 adrenergic receptor agonists | ASD, DB | Changes in blood pressure, decreased activity, sedation |
| Mood stabilizers (lithium)39-42 | Unknown. Possibly by interaction with glutamate receptors and/or with K+, Na+, Ca2+ channels | ID, DB, ADHD, bipolar aggressive patients, prison inmates | High risk for adverse drug reactions |
| Psychostimulants (methylphenidate)43-46 | Dopamine and norepinephrine agonists | DB, ADHD, ODD | Delay in weight gain and growth; cardiovascular risk |
| Anticonvulsants (divalproex sodium)47-49 | Increases GABA concentration and/or inhibition of voltage-sensitive sodium channels | ADHD, ODD, DB, schizophrenia | Low-quality evidence to support the use of this drug |
ADHD = attention deficit/hyperactivity disorder; ASD = autism spectrum disorder; DB = disruptive behavior; ID = intellectual disability; ODD = oppositional defiant disorder; PTSD = posttraumatic stress disorder.
Typical antipsychotics include dopaminergic antagonists and are effective in treating psychotic patients, children with conduct disorders, and cognitively impaired individuals.27 Atypical antipsychotics, particularly risperidone and aripiprazole, act on multiple neurotransmitter systems (eg, antagonists of the DA and 5-HT2A receptors) and are effective in the patient populations described above.27 Their effectiveness is particularly notable in ASD patients for which they are FDA approved.6,29
Antidepressants, primarily selective serotonin reuptake inhibitors, are effective in reducing irritability and aggressive behavior in patients with unipolar depression, Alzheimer's disease, autism, mental retardation, psychosis, PTSD, and personality disorders.32 Mood stabilizers, such as lithium, have been shown to be effective in individuals with intellectual disabilities and physical handicaps, children with conduct-disordered and explosive behavior, and bipolar patients with excessive irritability and outbursts of rage.31,50
When patients fail to respond to an adequate dose and duration of a standard monotherapy, a high-dose monotherapy or a polypharmacy strategy may be used.20,27 These include the use of typical antipsychotics (2 or more), atypical antipsychotics (2 or more), or a combination of both classes of drugs. However, this type of polypharmacy can increase the burden of side effects, including sedation, akathisia, and dystonia.27
Despite the variety of drugs and doses used to treat aggression, there is a subset of individuals who do not respond adequately to medical treatment and are considered to be treatment refractory.29 For this limited population of nonresponsive impulsive aggressive patients, surgical interventions targeting the amygdala or hypothalamus have been proposed. We review the rationale behind and neurobiological mechanisms underlying these interventions and discuss some of the reported outcomes.
A review search was conducted in PubMed, Medline, and Scopus for original research articles. As this study aims to review a great number of published articles on the theme, there were no restrictions placed on the publication date for the search. Thus, we opted not to conduct a formal systematic review or meta-analysis. The studies were required to meet the terms “amygdalotomy,” “amygdala,” “hypothalamotomy,” “hypothalamus,” “lesion,” “aggressive behavior,” “aggression,” “deep brain stimulation, ” “DBS.” The selection criteria included studies that (1) were performed in humans, (2) performed amygdalotomy or hypothalamotomy, (3) were focused on aggressive behavioral disorders. Only English language articles were considered. Studies of all sample sizes were included in the analysis. Studies were excluded if they (1) were reviews of the literature and (2) present repeated data from previous included studies. Figure 2 shows a PRISMA flow diagram describing the study selection performed in Tables 2 and 3. In order to evaluate the risk of bias/quality assessment of an individual study, the quality was assessed based on Cochrane risk-of-bias tool (see Table 4).51
Figure 2.
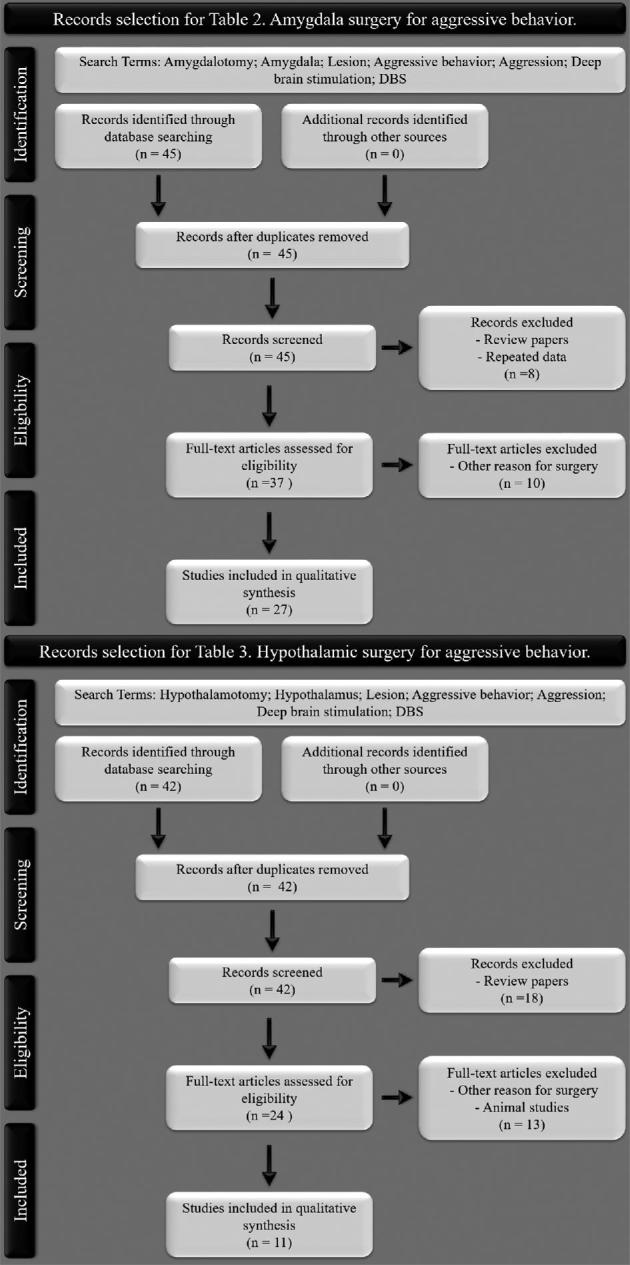
PRISMA flow diagram describing study selection in Tables 2 and 3.
Table 2.
Surgery Targeting the Amygdala for Aggressive Behavior
| Ref. and year | No. Gender Age | Population | Behavior disturbance | Surgical target and laterality | Imaging guidance | Electro physiological recordings | Surgical technique | Associated surgery | Improvement and form of evaluation | Side effects | Follow-up (mo) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 66/1963 | N: 60M: 38F: 225-35 yr | ID; IN; CI; hyperactivity; psychopath | Disruptive behavior with or without seizures; assaultive behavior; violent aggressiveness | Lateral nucleus of the amygdalaBilateral: 21 Unilateral: 39 | PEG; head X-rays | DR en route and at target with olfactory stimulation (ether-inhalation) | Oil-wax-lipiodol (surgical wax) | No other surgery | 85%Clinical observations | 1.5% Transient capsular palsy1.5% Transient hypersexuality | Up to 24 |
| 101/2012 | N: 7M: 5F: 9<53 yr | Schizophrenia PTPD; OCD; IN | Olfactory seizures and psychiatric disorders with olfactory hallucination | Medial amygdalaBilateral: 1Unilateral: 6 | PCV; head X-rays | EEG; DR of the amygdala with different stimuli (electric, olfactory, reading, calculation, anticonvulsant) | Olive oil + white bee wax + Iodized oil (surgical wax) | No other surgery | 100%Clinical observations | No side effects reported | 3-15 |
| 102/2017 | N: 25M: 14F: 117-61 yr | ID; IN; hyperactivity; In.patients | Hostile, aggressive, and destructive behavior; epilepsy and depression; refractory to drug therapy | Posterior half of the amygdalaBilateral: 8Unilateral: 16 | PEG; PCV | NR | Cryolesion (–120°C, 5 min cooling and 3 min place)2 lesions in each nucleus | Subsequent temporal lobectomy (1) | 80%Grading scale developed by the authors | 4% Worse behavior after surgery | 12-36 |
| 84/1966 | N: 40 | Follow-up of patients from previous paper (01/1963) | 67,5%Interview with authors and patient doctor; Family questionnaire | 2.5% Worse behavior after surgery1% Transient partial Kluver and Bucy syndrome | 36-72 | ||||||
| 67/1968 | N: 44N/G0-40 yr | CI; schizophrenia | Violent and destructive acts; pyromania; episodic attacks of behavior disorders | Amygdala nucleus not specifiedBilateral: 39Unilateral: 5 | PCV | DR of the amygdala with and without olfactory and electric stimulations | Thermal coagulation; mechanic methods | No other surgery | 62%Grading scale developed by the authors | 12% Worse behavior after surgery or died | 12-48 |
| 68/1969 | 1 Male 33 yr | IN; CI | Violent aggressive behavior with seizures; verbal and physical aggression | Lateral amygdalaBilateral | Head X-rays | EEG; DR of the amygdala with electric stimulation (implanted electrodes for 6 mo) | Thermal coagulation (insulated multi-lead deep electrodes) | No other surgery | 100%Clinical observation; psychological tests | No side effects or discomfort reported | 12 |
| 69/1970 | N: 100M: 82F: 180-50 yr | CI; schizophrenia; hyperactivity | Assaultive, destructive and self-destructive behavior; pyromania; hyper-oral | Whole amygdalaBilateral: 87Unilateral: 13 | PEG; PCV | DR of the amygdala with electric stimulation | Thermal coagulation; mechanic methods; oil-wax-lipiodol (surgical wax) | No other surgery | 75%Grading scale developed by the authors | 9% mortality | 24-72 |
| 70/1970 | N: 12 All female23-69 yr | ID; PD; schizophrenia; In.patients | Aggressive and destructive behavior with or without seizures; self-mutilation | Amygdala nucleus not specifiedAll bilateral | PEG; PCV | DR of the amygdala | Thermal coagulation (65°C, 45 s)2 lesions in each nucleus | Previous frontal lobotomy (5)Cingulectomy (2)Subsequent basofrontal tractotomy (3) | 75% Psychological tests | No side effects reported | Up to 36 |
| 82/1973 | N: 18M: 13F: 58-43 yr | ID; PD; AuD | Behavioral disturbances with seizures; abnormal aggressive behavior | Medial amygdalaBilateral: 17Unilateral: 1 | AngiographCerebral isotope scan. | DR en route and at target | Thermal coagulation3 × 1.8 mm probe | Previous unilateral amygdalotomy (1) | 55%Several questionnaires | 11% Hemiplegia with disability in one arm22% Deficit in face recognition | Up to 60 |
| 85/1966 | N: 18M: 14F: 413-37 yr | ID; PD; schizophrenia | Aggressive and self-mutilation behavior; refractory to ECT, drug therapy, and psychotherapy | Amygdala nucleus not specifiedBilateral: 15Unilateral: 3 | PEG; PCV | EEG; DR of the amygdala with electric stimulation | Thermal coagulation (60-65°C, 30 s)Cryoprobe (–70°C, 3 min/–120°, 3 min) | Previous leucotomy (1)Subsequent bimedial leucotomy (1) | 39%-50%Several questionnaires | 22% Convulsions5.5% Persistent mild hemiparesis | 12-72 |
| 86/1966 | 235N/GN/A | CI; schizophrenia | Aggression, violent and destructive behavior; low rage threshold; self-mutilation | Amygdala nucleus not specifiedBilateral: 207Unilateral: 28 | NR | EEG; DR of the amygdala with electric stimulation | Thermal coagulation; mechanic methods; surgical wax | Subsequent hypothalamotomy (33) | 75%Grading scale developed by the authors | 2.5% Transient hemiplegia1% Permanent hemiplegia1% Temporary ballistic movement4% Mortality | Up to 108 |
| 87/1974 | N: 10M: 8F: 210-20 yr | IH | Aggressive, assaultive and destructive behavior; low rage threshold; refractory to drug therapy | Amygdala nucleus not specifiedAll unilateral | PEG; PCV | DR of the amygdala with electric stimulation | Thermal coagulation; mechanic methods; surgical wax | Simultaneous thalamotomy (2) | 100%Grading scale developed by the authors | No side effects reported | 24-108 |
| 88/1975 | N: 8M: 6F: 212-26 yr | ID; psychotic; In.patients | Aggressive and impulsive behavior with seizures; dangerous outbursts of rage | Centre of the amygdalaBilateral: 6Unilateral: 2 | Head X-rays | EEG; DR of the amygdala with electric and olfactory stimulations (ether) | Thermal coagulation (70°, 80°, 90°C, 60 s. Mono and bipolar) 1 lesion at target and 1 above it (12 mm range) | Previous temporal lobectomy (1)Simultaneous fornicotomy (3) | 62.5%Observation scale and annotations of the staff members | 12.5% Behavior worse than before 25% Transient hemiparesis50% Rise in temperature12.5% Rise in blood pressure | NR |
| 89/1975 | N: 58M:39F: 198-61 yr | CI; In.patients | Aggressive and destructive behavior with or without seizures; refractory to therapies | Antero-medial of the amygdalaBilateral: 28Unilateral: 30 | PCV | NR | Cryolesion; mechanical methods | Previous frontal lobotomies (11) | 30%-40%Structured psychiatric interviews; neuropsychological tests | 2% Permanent hemiparesis2% Transient hyper sexuality5% Temporary visual field defects9% Memory loss12% Others mild2.5% Behavior | 12-132Mean: 72 |
| 65/1978 | N: 44N/G8-61 yr | ID; In.patients | Aggressive behavior with or without seizures | Anteromedial amygdalaBilateral: 14Unilateral: 30 | PCV | NR | NR | No other surgery | 30%-50%Grading scale developed by the authors | 12% Decrease in recent memory9% Temporary loss of peripheral vision5% Transient increase in sex drive2% Permanent hemiparesis2% Permanent speech difficulties | 12-132 |
| 90/1976 | N: 70M: 39F: 31N/A | Schizophrenia; suicidal tendencies; depression | Attacks of anger; verbal or physical aggression, with epilepsy; refractory to drug therapy | Medial nucleus of the amygdalaBilateral: 33Unilateral: 34 | NR | EEG; DR of the amygdala and hippocampus with electrical stimulation | NR | Previous temporal lobectomy (10)Simultaneous anterior hippocampotomy (29) | 75-84%Clinical observations | No side effects reported | 24-156 |
| 91/1977 | 1 Female 34 yr | ID; In.patients | Uncontrollable aggressive; refractory to ECT and drug therapy | Amygdala nucleus not specifiedBilateral | NR | NR | NR | No other surgery | 100%Clinical observations | No side effects reported | 12 |
| 92/1980 | N: 4All male17-57 yr | NR | Aggressive behavior with epilepsy | Amygdala nucleus not specified.All unilateral | NR | SEG | NR | No other surgery | 50%Clinical observations | 25% Occasional depression | 36-72 |
| 93/1981 | 1 Female 37 yr | PD; normal to superior IQ | Self-mutilation, depression and overdose; refractory to ECT, drug therapy, and psychotherapy | Amygdala nucleus not specifiedBilateral | PEG | NR | Thermal coagulation2 lesions in each nucleus (3 mm apart) | Previous bifrontal tractotomy | 100%Clinical observations | Disorders of facial recognition; social behavior; elements of Kluver and Bucy syndrome | 120 |
| 83/1988 | N: 481N/G<15 yr | ID; CI; hyperactivity | Aggressive, destructive, and self-destructive behavior; refractory to drug therapy | Amygdala nucleus not specifiedBilateral: 402 (at 1-stage surgery)Unilateral: NR | PCV | DR of the amygdala with electric stimulation | Thermal coagulation; surgical wax | Previous hypothalamotomy (47)Subsequenthypothalamotomy (73) | 70%Clinical observations.Psychological assessments in 60 patients | 6% Transient hemiplegia | 36 |
| 94/1983 | N: 11N/GN/A | ID | Automutilation and aggressive behavior with seizures | Medially in the amygdala.Bilateral: 7Unilateral: 4 | PEG; CT head scan | NR | NR | Simultaneous Unilateral fornicotomy (3)Temporal lobectomy (1) | 45.5%Clinical observations | No side effects reported | Up to 120 |
| 95/1986 | 2 Male 30 and 35 yr | CI; psychotic | Rage and aggression with seizures; refractory to drug therapy | Amygdala nucleus not specifiedAll unilateral | NR | Corticography | NR | SimultaneousLesion in Hippocampus and Uncus | 100%Clinical observations | Right hemiparesis and swallowing difficulty (surgical accident 1 patient) | 12-72 |
| 96/1988 | 2 Male 19 and 21 yr | CI; psychotic | Medically intractable aggressive behavior | Whole amygdalaAll bilateral | Brain MRI; stereotactic X-rays | NR | Thermal coagulation (80°, 90°C, 60 s. 2.1 × 5 mm uninsulated tip)3 lesions in each nucleus (4 mm apart) | No other surgery | 50%Clinical observations. | No side effects reported | 96 |
| 97/1992 | N: 2N/GN/A | NR | Medically intractable aggressive behavior | Amygdala nucleus not specifiedAll bilateral | PCV | NR | Thermal coagulation | SimultaneousSubcaudateTractotomy | 100%Several questionnaires | No side effects reported | 84 |
| 98/1998 | 1 Female 38 yr | SMPD | Aggressive behavior and self-inflicted injuries; refractory to drug and behavioral therapies | Whole amygdalaBilateral | Brain MRI; head CT scan; surgiplan workstation; fluoroscopy | NR | Thermal coagulation (90°C, 60 s. 2 × 4 mm, monopolar)3 lesions in each nucleus | No other surgery | 100% Clinical observations | No side effects reported | 18 |
| 99/2002 | 1 Male 13 yr | Severe Kanner's autism | Life-threatening self-injurious behavior; refractory to drug therapy | Basolateral nucleus of amygdalaBilateral | Brain MRI; stereotactic head CT scan; human brain atlas | NR | DBS2 quadripolar non-insulated electrodes 120 μs; 130 Hz; 2-6.5 V | No other surgery | 100%Father rating scale; clinical observation; questionnaires | No side effects reported | 24 |
| 100/2007 | 1 Female 19 yr | ID | Refractory aggressive behavior | Whole amygdalaBilateral | Brain MRI; stereotactic MRI; surgiplan workstation | NR | Thermal coagulation (75°C, 60 s) Multiple lesions | SimultaneousBilateral AnteriorCapsulotomy | 100%Several questionnairesPsychological tests | No side effects reported | 36 |
| SummaryTotal: 27 | N: 1217M: 268F: 139N/G: 8100-69 yr | ID: 12IN: 4CI: 20PTPD: 1OCD: 1PD: 4AuD: 1IH: 1SMPD: 1Hyperactivity: 4Psychopath: 5Schizophrenia: 6Suicidal: 1Depression: 1Autism: 1In.patients: 6NR: 2 | Refractory: 14With seizures: 13 | Lateral n: 2Medial n: 3Posterior: 1Centre: 1Anteromedial: 3Whole: 4NR: 12Bilateral: 907Unilateral: 227 | 1960s: PEG; PCV; X-rays1970s: PEG; PCV; ACIS1980s: PEG; PCV; CT1990s: MRI; stereotactic X-rays>2000: MRI; stereotactic CT; surgiplan workstation; brain atlas | DR en route: 2DR local: 13Olfactory stimulation: 4Electric stimulation: 10Other stimulation: 1EEG: 6SEG: 1Corticography: 1NR: 10 | Surgical wax: 6Cryolesion: 3Mechanic: 5Thermal: 15DBS:1NR: 6 | No other surgery: 12Previous: 8Simultaneous: 7Subsequent: 4 | Total: 69.5% | No side effects: 12Transient: 10Permanent: 9Worse behavior: 5 | NR: 10-12: 213-24: 425-36: 437: 17 |
ACIS = angiograph cerebral isotope scan; AuD = alcohol use disorder; CI = cerebral insults; CT = computed tomography; DBS = deep brain stimulation. DR = depth recording; ECT = electro-convulsive therapy; EEG = electroencephalogram; F = female; ID = intellectual disabilities; IH = infantile hemiplegia; IN = intellectual normal; In. patients. = institutionalized patients; M = male; MRI = magnetic resonance imaging; N/A = no age specified in the article; N/G = no gender specified in the article; NR = not reported; OCD = obsessive compulsive disorder; PCV = positive contrast ventriculography; PD = personality disorder; PEG = pneumoencephalography; PTPD: posttraumatic personality disorder; SEG = stereoelectroencephalography; SMPD = self-mutilation psychiatric disorder.
Table 3.
Hypothalamic Surgery for Aggressive Behavior
| Ref. and year | No.GenderAge | Population | Behavior disturbance | Surgical target and laterality | Imaging guidance | Electro physiological recordings | Surgical technique | Associated surgery | Improvement and form of evaluation | Side effects | Follow-up (mo) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 121/1972 | N: 11N/GN/A | CI; ID; psychopathic personality; schizophrenia | Hetero and auto-aggressiveness, violent and destructive behavior | Posteromedial hypothalamusBilateral: 10Unilateral: 1 | PEG | Electrical stimulation of the target | Thermal coagulation | Not reported | 90%Clinical observations | 18% Transient hypersomnia9% Transient tachycardia | Up to 48 |
| 86/1966 | N: 49N/GN/A | CI; schizophrenia | Aggression, violent and destructive behavior; low rage threshold; self-mutilation | Hypothalamus nucleus not specifiedBilateral: 21Unilateral: 28 | Not reported | DR and electrical stimulation of the target | Thermal coagulation; surgical wax | Previous amygdalotomy (33) | 75%Grading scale developed by the authors | 4% Transient diabetes insipidus2% Ballistic movement4.1% Mortality | Up to 108 |
| 83/1988 | N: 122N/GN/A | CI, ID | Refractory physical aggression, hyperkinesis, wandering tendency, destructive and self-destructive tendencies | Posteromedial hypothalamusLaterality not reported | PCV | Electrical stimulation of the target | Thermal coagulation | Amygdalotomy | 60%Clinical observations | No side effects reported | Up to 36 |
| 125/2008 | N: 60M: 44F: 16N/A | CI; ID | Refractory aggressive behavior, rage attacks, restless behavior | Posteromedial hypothalamusLaterality not reported | Ventriculo-graphy | EEG; electrical stimulation of the target | Thermal coagulation | Not reported | 78%Clinical observations | No side effects reported | Up to 300 |
| 126/2008 | 1 Male18 yr | Hypothalamic hamartoma | Refractory aggressive behavior | Hypothalamus: hamartomaUnilateral | Brain MRI; stereotactic head CT scan; Schaltenbrand digital brain atlas | EEG; DR en route and at target; electrical stimulation of target | Thermal coagulation | No other surgery | 100%Clinical observations | No surgical complications, no side effects reported | 24 |
| SummaryLesionsTotal: 5 | N: 243M:45F: 16N/G:182N/A | CI: 5ID: 3Psychopathic personality: 1 Schizophrenia: 2 | Refractory: 5With seizures:5 | Posteromedial hypothalamusBilateral: 31Unilateral: 30 | <2000: PEG; PCV; ventriculography>2000: brain MRI; stereotactic head CT scan; brain atlas | DR en route: 1DR target: 1Electrical stimulation of target: 5 | Thermal coagulation: 5Surgical wax: 1 | No other surgery: 3Associated surgery: 2 | Total: 80.6% | No side effects: 3Transient: 2Permanent: 1 | 0-24: 125-36: 155-48: 1>49: 2 |
| 127/2008 | 1 Male22 yr | ID | Drug-resistant aggressiveness | Posteromedial hypothalamusBilateral | Brain MRI; ventriculography | Scalp EEG; DR, and electrical stimulation of the target | DBSInitial parameters: left 0.4 V, right 0.1 V, 450 μs, 15 Hz | No other surgery | 100%ICAP | No surgical complications, worsening of unilateral headaches | 18 |
| 128/2010 | 1 Female22 yr | CI; ID | Drug-resistant self-mutilating behavior | Posterior hypothalamusBilateral | Not reported | Not reported | DBSInitial parameters: 1.5 V, 90 μs, 130 Hz | No other surgery | 100%Clinical observations | No surgical complications, no side effects of stimulation | 4 |
| 129/2013 | 1 Female19 yr | IED; ID | Severe violent attacks against family | Orbitofrontal projections to the hypothalamusUnilateral | Brain MRI; stereotactic head CT scan; Schaltenbrand-Wahren atlas | Not reported | DBSInitial parameters: 2.5 V, 360 μs,40 Hz, 1 min “on”/1 min “off” | No other surgery | 100%Clinical observations | No surgical complications, no side effects of stimulation | 24 |
| 130/2013 | N: 7M: 6F: 120-68 yr | CI; ID | Refractory aggressive behavior | Posterior hypothalamusAll bilateral | Brain MRI; stereotactic head CT scan Framelink 4 software | Scalp EEG; DR en route and at target; electrical stimulation of target | DBSInitial parameters:1-3 V, 60-90 μs, 185 Hz | No other surgery | 85%OAS | No surgical complications, no side effects of stimulation | Up to 118 |
| 131/2015 | N: 6M:4F: 217-488 yr | CI; ID | Uncontrollable refractory aggressiveness | Posteromedial hypothalamusLaterality not reported | Brain MRI; stereotactic head CT scan; BrainLAB wokstation. | Scalp EEG; DR and electrical stimulation of the target | DBSInitial parameters: 0.1-0.9 V, 15-60 Hz, 180-450 μs | 1 patient lesionST, AC, ICPMH, DmTN, IlTN | 83%ICAP | No surgical complications, worsening of unilateral headaches in 1 patient | Up to 82 |
| 124/1988 | N: 5M: 4F: 116-33 yr | ID | Intractable aggressive behavior | Posteromedial hypothalamusAll bilateral | Brain MRI; stereotactic head CT scan; Praezis 3.1 workstation | DR en route and at target | DBSInitial parameters: 2.4-3 V, 185 Hz, 90 μs1 min “on”/5 min “off” | No other surgery | 80%OAS | No surgical complications | Up to 48 |
| SummaryDBSTotal: 6 | N: 21M:15F: 616-68 yr | CI: 3ID: 6IED: 1 | Refractory: 6With seizures: 4 | Posteromedial: 3Posterior: 2Other: 1Bilateral: 31Unilateral: 30 | Brain MRI; stereotactic head CT scan; surgical planning workstations; brain atlas | EEG: 3DR en route: 2DR target: 4Electrical stimulation of target: 4 | DBS Parameters: 0.1-3 V, 60-450 μs, 15-185 Hz | No other surgery: 5Associated surgery: 1 | Total: 91.3% | No side effects: 4Permanent: 2 | 0-24: 325-48: 1>49: 2 |
AC = anterior cingulum, CI = cerebral Insults; CT = computed tomography; DBS = deep brain stimulation; DmTN = dorsomedial thalamic nuclei, DR = depth recording; EEG = electroencephalogram; F = female; IC = internal capsule; ICAP = Inventory for Client and Agency Planning; ID = intellectual disabilities; IED = intermittent explosive disorder; IlTN = intralaminar thalamic nuclei; M = male; MRI = magnetic resonance imaging; N/A = no age specified in the article; N/G = no gender specified in the article; OAS = Overt Aggression Scale; PCV = positive contrast ventriculography; PEG = pneumoencephalography; PMH = postermedial hypothalamus; ST = stria terminalis.
Table 4.
Risk of Bias According to the Cochrane Risk-of-Bias Tool
| Ref. and year | Random sequence generation | Allocation concealment | Blinding participants and investigators | Incomplete outcome data | Selective reporting bias |
|---|---|---|---|---|---|
| Amygdalotomy studies | |||||
| 66/1963 | High | High | High | High | High |
| 101/2012 | High | High | High | High | High |
| 102/2017 | High | High | High | High | High |
| 84/1966 | High | High | High | High | High |
| 67/1968 | High | High | High | High | High |
| 68/1969 | High | High | High | High | High |
| 69/1970 | High | High | High | High | High |
| 70/1970 | High | High | High | High | High |
| 82/1973 | High | High | High | High | High |
| 85/1966 | High | High | High | High | High |
| 86/1966 | High | High | High | High | High |
| 87/1974 | High | High | High | High | High |
| 88/1975 | High | High | High | High | High |
| 89/1975 | High | High | High | High | High |
| 65/1978 | High | High | High | High | High |
| 90/1976 | High | High | High | High | High |
| 91/1977 | High | High | High | High | High |
| 92/1980 | High | High | High | High | High |
| 93/1981 | High | High | High | Low | Low |
| 83/1988 | High | High | High | High | High |
| 94/1983 | High | High | High | High | High |
| 95/1986 | High | High | High | High | High |
| 96/1988 | High | High | High | Low | Low |
| 97/1992 | High | High | High | Low | Low |
| 98/1998 | High | High | High | Low | Low |
| 99/2002 | High | High | High | Low | Low |
| 100/2007 | High | High | High | Low | Low |
| Low | 0% | 0% | 0% | 22.2% | 22.2% |
| Unclear | 0% | 0% | 0% | 0% | 0% |
| High | 100% | 100% | 100% | 77.8% | 77.% |
| Hypothalamotomy studies | |||||
| 121/1972 | High | High | High | High | High |
| 86/1966 | High | High | High | High | High |
| 83/1988 | High | High | High | High | High |
| 125/2008 | High | High | High | High | High |
| 126/2008 | High | High | High | High | High |
| 127/2008 | High | High | High | Low | Low |
| 128/2010 | High | High | High | High | High |
| 129/2013 | High | High | High | High | High |
| 130/2013 | High | High | High | Low | Low |
| 131/2015 | High | High | High | Low | Low |
| 124/1988 | High | High | High | Low | Low |
| Low | 0% | 0% | 0% | 36.7% | 36.7% |
| Unclear | 0% | 0% | 0% | 0% | 0% |
| High | 100% | 100% | 100% | 63.3% | 63.3% |
The risk of bias is the percentage of bias items reported considering all included studies.
AMYGDALA
The amygdala is an almond-shaped structure located bilaterally in the temporal lobes. Its average size in humans ranges from 1.24 to 1.63 cm³.52 The amygdala plays a critical role in processing threatening stimuli and mediating autonomic, neuroendocrine, and behavioral responses that enable an organism to adapt to social and environmental challenges.14,52,53
In 1923, J. B. Johnston introduced a fundamental description of the amygdala based on a detailed analysis of comparative vertebrate species.54 He proposed subdividing the structure into a primitive group of nuclei associated with the olfactory system (the central, medial, and cortical nuclei and the nucleus of the lateral olfactory tract) and a phylogenetically newer group (the lateral and basal nuclei). More recently, a greater heterogeneity of regions within the amygdala has been unraveled, with one portion viewed as a ventromedial extension of the striatum, a second part comprising the caudal olfactory cortex, and a third region representing the ventromedial extension of the claustrum.53,55 Furthermore, the amygdala has been subdivided based on its histological characteristics into 2 major areas (anterior amygdaloid area and corticoamygdaloid transition area), 6 nuclei (central, medial, cortical, accessory basal, basal, and lateral), and 1 intercalated cell group. As the subdivision of the human amygdala proposed by Sims and Williams presents good homology with experimental animals, it will be used in this review.56
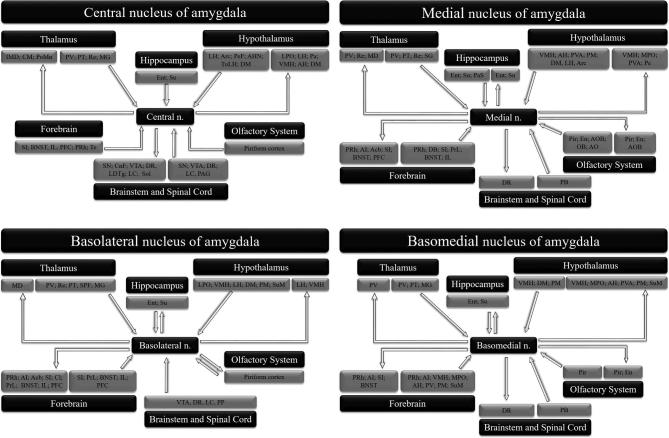
The lateral nucleus is viewed as the gatekeeper because it receives inputs from sensory systems (ie, visual, auditory, somatosensory, pain, olfactory, and taste) and enables the concurrent processing of multiple types of information.53,55 The central nucleus is considered a prominent output region for the expression of innate emotional responses and their associated physiological processes, projecting mainly to hypothalamic and brainstem regions.53 Another important set of output projections from the amygdala arises from the basal nucleus, which directly innervates the central nucleus and striatal areas involved in the control of instrumental behaviors, such as avoidance and escape.53,57 A schematic representation of the main projections, inputs, and outputs from the central, basolateral, basomedial, and medial amygdala nuclei is provided in Figure 3.
Figure 3.
Schematic representation of the main connections of the central, medial, basolateral, and basomedial amygdala nuclei. Acb: nucleus accumbens; AH: anterior hypothalamic area; AHN: anterior hypothalamic nucleus; AI: agranular insular cortex; AO: anterior olfactory nucleus; AOB: accessory olfactory bulb; Arc: arcuate nucleus of the hypothalamus; BNST: bed nucleus of the stria terminalis; Cl: claustrum; CM: central medial thalamic nucleus; CnF: cuneiform nucleus; DB: nucleus of the diagonal band; DM: dorsomedial hypothalamic nucleus; DR: dorsal raphe nucleus; En: endopiriform nucleus; Ent: entorhinal cortex; GP: globus pallidus; IL: infralimbic cortex; IMD: intermediodorsal thalamic nucleus; LC: locus coeruleus; LDTg: laterodorsal tegmental nucleus; LH: lateral hypothalamic area; LPO: lateral preoptic area; MD: mediodorsal thalamic nucleus; MG: medial geniculate nucleus; MPO: medial preoptic area; OB: olfactory bulb; Pa: paraventricular hypothalamic nucleus; PAG: periaqueductal gray; PaS: parasubiculum; PB: parabrachial nucleus; Pe: periventricular hypothalamic nucleus; PeF: perifornical nucleus; PFC: prefrontal cortex; Pir: piriform cortex; PM: premammillary nucleus; PoMn: posteromedial thalamic nucleus; PP: peripeduncular nucleus; PRh: perirhinal cortex; PrL: prelimbic cortex; PT: paratenial thalamic nucleus; PV: paraventricular nucleus of the thalamus; PVA: paraventricular nucleus of the hypothalamus; Re: reuniens thalamic nucleus; SG: suprageniculate thalamic nucleus; SI: substantia innominate; SN: substantia nigra; Sol: nucleus of the solitary tract; SPF: subparafascicular thalamic nucleus; Su: subiculum; SuM: supramammillary nucleus; Te: temporal cortex; TuLH: tuberal region of lateral hypothalamus; VMH: ventromedial hypothalamic nucleus; VTA: ventral tegmental area.
Since the beginning of the last century, several studies have been performed with the aim of understanding the role of the amygdala in social and emotional functions. As a result, the amygdala has been considered a key structure in a wide range of conditions from mood disorders to autism and schizophrenia.58,59 Likewise, the amygdala is a component of the neural network that regulates aggressive behavior and also includes the hypothalamus, hippocampus, orbitofrontal cortex, and PAG.3,10,17
Studies performed in dogs have shown that the bilateral removal of the temporal lobes has a taming effect.60 Similarly, bilateral lesions damaging the temporal lobe in nonhuman primates can produce dramatic changes in social and emotional behaviors, including aggressiveness.61-64 In a milestone article, Kluver and Bucy62,63 demonstrated that bilateral temporal lesions in rhesus monkeys markedly reduced aggressive behavior.
Thereafter, Rosvold and colleagues64 designed a study to evaluate changes in the social behavior of rhesus monkeys following damage to the amygdala. The researchers established artificial social groups of male rhesus monkeys and identified the dominant animal. A common finding after bilateral lesions of the amygdala was a decrease in social dominance, with the lesioned animals assuming a subordinate position within the group.64 It is well established that the stimulation or ablation of various amygdalar nuclei in animals produces not only reductions in aggressive behavior but also changes in autonomic functions, such as the heart rate, respiration, and skin conductance.65-69
In humans, amygdala stimulation increases aggression.70 Neuroimaging studies using functional magnetic resonance imaging (MRI) in humans have revealed pronounced amygdala activation when subjects are shown angry or fearful facial expressions.71,72 Similar results have been described in patients with antisocial behavior, intermittent explosive disorder, and other psychopathologies, revealing that the amygdala is a core structure involved in the processing of aggressive information, regardless of an individual's psychiatric status.2,73 In addition, recent reports have shown that subjective experiences may influence amygdala volume and connectivity. Veterans with aggressive behavior disorders have a more intense brain response to external stimuli, including the amygdala, and have lower connectivity between the amygdala and prefrontal cortex.74,75 Similarly, adolescents exposed to family aggression show larger amygdala volume and altered patterns of connections with cortical regions.74,76
In contrast, other studies have reported that the level of amygdala activation is lower in criminal psychopaths during processing of negative affective stimuli, fear conditioning paradigms, and emotional moral decision making.77-79 These apparently opposite effects could be explained by differences in data processing methods. Some studies have investigated the nucleus as a single compact structure, while others have subdivided it into a few regions. Ablating or stimulating distinct regions within the amygdala may cause different or opposing effects on aggressiveness in both animals and humans.80,81
Taken together, these results suggest a relationship between aggressive behavior and amygdala hyperactivity and that the removal of the amygdala may be sufficient to reduce aggressiveness. Although the exact mechanism responsible for the marked reduction in aggressive behavior observed after amygdala lesion remains unknown, it has been suggested that this effect is related to an increase in tolerance to provocation and a decline in the level of autonomic arousal.82,83 Taking this into account, investigators proposed the use of amygdalotomy in humans to control extreme aggressive behavior. Table 2 summarizes the published literature on the use of amygdalotomy in humans.
Over the last 60 yr, more than 1000 such surgeries have been reported. Their results have indicated that beneficial effects can be achieved, including reductions in the severity and frequency of aggressive behaviors.68-73,84-105 As shown in Table 2, nearly 70% of patients treated with amygdalotomy show good or excellent improvement in behavioral disorders. In patients with concomitant epilepsy, improvements in seizure frequency and intensity have also been reported. There were 6 case reports of only one patient and most studies comprised case series, summing up a total of 1217 patients included in the studies pooled in our review. Although many studies do not reported details of patient psychiatric status, the ones that present this information mostly reports cerebral insults, severe intellectual disabilities, or schizophrenia as cause for the behavioral disturbance. Moreover, several patients had other ablation surgeries performed previously, during or after the amygdalotomy (eg, frontal lobotomy, leucotomy, subcaudate tractotomy, cingulectomy, hypothalamotomy, thalamotomy, fornicotomy, hippocampotomy, fornicotomy, or hypothalamotomy). Thus, a conclusion based on intervention by diagnosis is not possible in those cases.
We note, however, that patients treated with amygdalotomy were often cognitively impaired and nonverbal prior to surgery. Tests to assess other emotional and cognitive aspects (ie, threat processing, avoidance, and approach)103,104 were usually not performed. Nevertheless, in most cases, authors reported transient or no postoperative side effects and no impairment in overall measures of intelligence and global memory. However, permanent side effects and worsened behavioral problems have been reported, including movement disorders, depression, and cognitive disturbances involving memory, language, and nonverbal visual stimuli.
It is also worth noting that studies published to date have numerous confounders, including differences in age, pathologies underlying the behavioral disturbances, heterogeneity of the behaviors, and most importantly, the use of different surgical ablation procedures before, after, or concomitant to the amygdalotomy. In addition, the methods used to lesion the amygdala, the lateralization of the lesion, and the precise targets that were lesioned varied among surgical centers. Some of the techniques used are now considered obsolete, and modern imaging guidance (eg, high-resolution computed tomography, multiplanar 1.5- and 3-Tesla magnetic resonance imaging, and neuronavigational devices) was not available when most of the studies were conducted.
In recent decades, deep brain stimulation (DBS) has emerged as an attractive alternative for treating neurological and psychiatric disorders.105-108 This technique involves the insertion of electrodes into specific brain targets and the subsequent local delivery of an electrical current, commonly at high frequencies (HFS; ie, 130-185 Hz). Though DBS and lesions are 2 different therapeutic modalities, common mechanisms of HFS include axonal depolarization and the inhibition of cell bodies in the vicinity of the electrodes.108-111 In patients with movement disorders, similar outcomes have been observed with the use of these 2 approaches.112 The fact that stimulation-induced effects are reversible and adjustable (ie, the current can be reduced or the systems turned off upon the occurrence of side effects) has helped to rekindle interest in the notion that psychiatric diseases can be treated with surgery.105,106 In a recent study, DBS was successfully used to treat an autistic teenager with life-threatening self-injurious behavior refractory to medications.101
Notwithstanding these promising results of lesions and DBS studies, the vast majority consist of open-label trials in which subjective measures of behavior were used, resulting in a low level of evidence and a high risk of bias, as presented in Table 4. Ideal surgical targets, the optimal localization within respective nuclei, and the extension/size of the lesions remain to be established. In addition, no detailed information has been provided on postoperative changes in personality and emotions, an issue that will need to be addressed by multidisciplinary teams. Further research is certainly necessary to evaluate the safety of chronic temporal lobe stimulation and to improve our understanding of the mechanisms underlying amygdala DBS.
HYPOTHALAMUS
The hypothalamus is a small diencephalic structure located under the thalamus. It lies on the wall and floor of the third ventricle, extends a few millimeters laterally, and is positioned above the optic chiasm anteriorly and adjacent to the mammillary bodies posteriorly.113 It is composed of several distinct nuclei with widespread connections throughout the nervous system.114 The hypothalamus is largely known for its role in controlling homeostasis and motivated behaviors.114
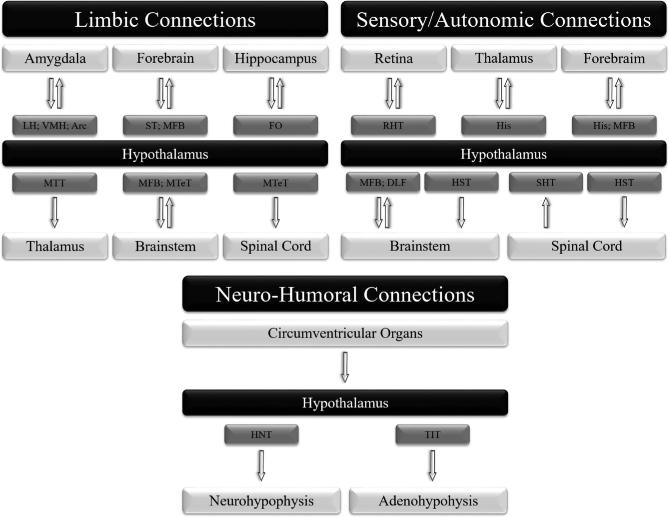
Based on nuclear landmarks, the hypothalamus can be divided into 3 areas along its rostro-caudal axis: anterior, medial, and posterior.15 Alternatively, based on the anatomical localization of cells projecting to the pituitary gland, it can be subdivided along its medial-lateral axis into periventricular, medial, and lateral areas.15,113,114 The anterior region is primarily responsible for producing oxytocin and vasopressin and for controlling the circadian cycle; the medial region is associated with producing hypothalamic-releasing hormones and controlling numerous motivated behaviors; and the posterior region is involved in thermoregulation, memory, and emotions.114,115 Figure 4 shows the main hypothalamic connections based on functions.
Figure 4.
Main hypothalamic connections based on functions. Arc: arcuate nucleus of the hypothalamus; DLF: dorsal longitudinal fasciculus; FO: fornix; His: histamine projection; HNT: hypothalamo-neurohypophyseal tract; HST: hypothalamo-spinal tract; LH: lateral hypothalamic area; MFB: medial forebrain bundle; MTeT: mammillo-tegmental tract; MTT: mammillo-thalamic tract; RHT: retino-hypothalamic tract; SHT: spino-hypothalamic tract; ST: stria terminalis; TIT: tubero-infundibular tract; VMH: ventromedial hypothalamic nucleus.
Studies performed in animals indicate the presence of specific hypothalamic areas (eg, the ventromedial nucleus of the hypothalamus [VMH] and lateral hypothalamus) that, when electrically stimulated, result in the expression of aggressive behavior.116,117 The VMH projects to the anteromedial hypothalamus and the dorsolateral aspect of the PAG. The neurons in the latter region project to other brainstem areas and the spinal cord, and induce autonomic and motor responses when excited. In terms of afferents, the VMH receives massive inputs from the lateral hypothalamus as well as the cortical and basolateral amygdala, which modulate the expression and duration of aggressive behaviors.118,119 Similarly, the lateral hypothalamus projects to the midbrain tegmentum, trigeminal motor nucleus, and locus coeruleus, and has reciprocal connections with the PAG. While the latter connections are important for controlling the duration of aggressive episodes, projections from the central, lateral, and basal nuclei of the amygdala facilitate aggressive attacks.116,119
In humans, studies suggest that there is a hypothalamic area related to the control of aggressive behavior located in the posteromedial region, an area that includes the midpoint of the anterior commissure/posterior commissure line, the anterior border of the mammillary bodies and the beginning of the aqueduct, and that forms a triangular zone, now called the “Triangle of Sano.”83,88,120,121 Likewise, neuroimaging studies show that the hypothalamus is more activated in individuals with aggressive features and that domestic violence offenders present lower metabolism in this region.122,123
Although these results seem conflicting, they reaffirm that the hypothalamus is a component of the neurocircuitry involved in human aggressive behaviors and corroborate the idea that different regions of the hypothalamus are associated with the expression or suppression of these behaviors.116,119 Furthermore subthalamic DBS induced acute transient aggressiveness when regions near the hypothalamus were stimulated,120 suggesting that it may be possible to modulate aggressive behavior by electrically stimulating the hypothalamic region in humans.
In the past century, extremely aggressive patients have been treated with hypothalamic lesions with encouraging results.83,88,121,124-130,131 Table 3 presents the studies using hypothalamic surgery to control aggressive behavior in humans.
When making a hypothalamic lesion, the choice of target is of major importance due to the potential for surgical complications, such as seizures, hyponatremia, cardiovascular changes (including hypertension and tachycardia), disturbances in food and water intake, and thermoregulatory disruption.132-134 Transient and permanent side effects have been observed after hypothalamic lesions, with one study reporting a 4% mortality rate.88,121 It is important to note that some patients had previous amygdalotomy surgery and patient psychiatric status is not carefully detailed, but overall is similar to that observed in the amygdalotomy studies (eg, severe intellectual disabilities, cerebral insults, and schizophrenia). Nevertheless, in the 243 published cases, the average rate of improvement in aggressive behavior is approximately 80%. This suggests that the hypothalamus may be a very attractive target for modulating aggressive behavior in humans.
Based on these data, DBS hypothalamic surgery has been performed to control aggressive behavior in a few centers around the world. Surgeries have been performed with the aid of modern imaging and surgical planning workstations that merge MRI and stereotactic computed tomography with brain atlases for optimal target localization. DBS studies include patients who suffered cerebral insults, with severe intellectual disabilities, or diagnosed with intermittent explosive disorder, and the average improvement in aggressive behavior after hypothalamic DBS is 91%. Side effects were observed in only a few cases and mainly included headaches that could be easily treated with medication. The most adequate hypothalamic target remains to be determined, as different studies have reported good results following the application of DBS to the posteromedial hypothalamus or the projections from the orbital frontal cortex to the hypothalamus.100,135
Even though there are few published reports on this technique, the results so far indicate that long-lasting reductions in violent outbursts, improved control over emotions, and higher quality of life can be achieved following surgery, with minor side effects. Despite these promising results, when viewed from a modern perspective, some studies lacked specific endpoints, specific measuring instruments, and multidisciplinary evaluation.136 Moreover, the bias analysis shows a high risk of bias for those studies (see Table 4) and a low level of evidence; thus, it is not possible to present any formal treatment recommendation. However, this literature undoubtedly has merit and needs to be analyzed according to the time and conditions in which it was published.
SURGICAL PERSPECTIVE
After a promising start, surgery for psychiatric indications was indiscriminately used with poor patient selection and a high incidence of serious side effects, which led to public disbelief.137-139 In the 1950s, new pharmacological and nonpharmacological treatments became available, limiting the need for surgical interventions even more. Since its peak, the use of ablative stereotactic surgery for psychiatric disorders has stagnated at a low level and is currently only conducted in a few centers around the world.140 The reasons for this decrease are multifactorial and include the development of psychopharmacology and the growing skepticism of the international community regarding the benefits of these surgical interventions.140
Several questions need to be addressed before considering surgery, including indications, patient selection, and criteria for treatment refractoriness. In addition, treating physicians and organizations need to follow regional/federal rules and mandates for conducting psychiatric surgery. If investigational procedures are to be conducted, these should be performed carefully and in a well-documented manner following approval by a research ethics board. The use of psychosurgery should be restricted to extremely severe cases that do not respond to standard/available treatment when no other means of relieving patient suffering is available.137-139,141 To manage the patients, the center is required to have an experienced multidisciplinary team that may provide optimal clinical care and follow-up support. Additionally, such surgeries should be considered as part of a clinical trial in which outcome measures are objective and reproducible. Modern neuroimaging and refined functional neurosurgery techniques are to be used to ensure optimal targeting.
Technically, stereotactic surgery has become widely available, and frameless stereotaxic approaches can now be applied with great precision.142 Should improvements in targeting translate into ameliorations in surgical outcomes, one may expect a revival of interest in psychiatric surgery, including surgeries used to treat certain cases of medically refractory aggressive behavior. Indeed, ablation in other targets have been previous reported for the control of aggressive behavior (eg, frontal lobotomy, leucotomy, subcaudate tractotomy, cingulectomy, thalamotomy, fornicotomy, hippocampotomy, anterior cingulotomy, anterior capsulotomy) and more recently, nucleus accumbens DBS was performed, with good results.143 Moreover, patient self-aggressive characteristic and cognitive performance can be a determinant factor when deciding the best surgical technique (ablative/neuromodulatory). More severe patients or those who present aggressive behavior toward face/head may not be eligible for DBS due to a greater risk of complications such as infection, skin erosion, and lead fracture. Thus, future research is certainly necessary for the determination of optimal target and technique.
CONCLUSION
Aggressive behavior is generally managed with medication and/or behavioral approaches. In a small number of well-selected refractory cases, surgery has been proposed with promising results. Due to the potential for side effects, the use of hypothalamotomy and amygdalotomy has been fairly restricted. The reversibility of DBS makes it an attractive alternative for treating these disorders. For all applications of the technique, however, we stress the need for multidisciplinary teams who are experienced in managing aggressive patients. In addition, the treatments must be performed with high ethical standards and in accordance with local legislation.
Disclosures
Dr Martinez and Dr Gouveia are the recipients of grants from FAPESP (#11/08575-7, #13/20602-5, #17/10466-8) from the government of Brazil. Dr Brentani is the recipient of grants from FAPESP and CNPQ. The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Acknowledgments
The authors wish to thank all the research assistants and staff of Hospital Sirio-Libanes and Hospital das Clinicas - HCFM-USP.
COMMENT
Does free will exist? Do humans have the ability to choose between what we consider good and evil, between moral and immoral behavior? The great majority of people in the world believe this to be the case. But until not very long ago we believed that infections were a divine punishment and that epilepsy was a sign of demonic possession. Are we on the cusp of viewing criminal or just plain nasty behavior as being biologically determined?
We have lesioning or DBS for movement disorders, well-established. We don’t understand exactly how it works but we more or less know the targets. Brain surgery for pain? Doesn’t work as well but what else do we have to offer some patients? Psychiatric surgery? Now you’re getting controversial…but ok, it seems to work for OCD and it's worth working on for patients who are suicidally depressed. But surgery for aggression? This is the third rail of stereotactic and functional neurosurgery. Mind control, turning rambunctious free-thinkers into zombies, political repression…surely neurosurgeons aren’t going to go near any of that again!
Read this paper, and open your minds. Consider the patients who are referred for this surgery. Amygdalotomy and hypothalamotomy, or DBS in those regions, are not for the jerk who takes your parking spot. These procedures are reserved for very rare patients, who cannot be managed anywhere without deep sedation, who have lost any quality of life by any reasonable measure, and whose families suffer tremendously. The authors bring together case reports and small, single center studies. As they point out, these all provide a “weak level of evidence”, but one that is tantalizing nonetheless. Surgery for severe and medically refractory aggression should be studied and perfected in a small number of centers where there are proper multidisciplinary teams who can be trusted to select the rare patient candidates, and who will advance our knowledge in this area by carefully designed and ethically proper trials.
Michael Schulder
Manhasset, New York
REFERENCES
- 1. Batrinos ML. Testosterone and aggressive behavior in man. Int J Endocrinol Metab. 2012;10(3):563–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Comai S, Tau M, Gobbi G. The psychopharmacology of aggressive behavior. J Clin Psychopharmacol. 2012;32(1):83–94. [DOI] [PubMed] [Google Scholar]
- 3. Siever LJ. Neurobiology of aggression and violence. Am J Psychiatry. 2008;165(4):429–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dorfman HM, Meyer-Lindenberg A, Buckholtz JW. Neurobiological Mechanisms for Impulsive-Aggression: The Role of MAOA. In: Miczek K, Meyer-Lindenberg A (eds). Neuroscience of Aggression. Current Topics in Behavioral Neurosciences. Vol. 17. Springer, Berlin, Heidelberg; 2013. doi:10.1007/7854_2013_272. [DOI] [PubMed] [Google Scholar]
- 5. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Vol. 64 Arlington, VA, American Psychiatric Association; 2013. doi:10.1176/appi.books.9780890425596. [Google Scholar]
- 6. Brentani H, de Paula CS, Bordini D et al.. Autism spectrum disorders: an overview on diagnosis and treatment. Rev Bras Psiquiatr. 2013;35(suppl 1):S62–S72. [DOI] [PubMed] [Google Scholar]
- 7. Comai S, Tau M, Pavlovic Z, Gobbi G. The psychopharmacology of aggressive behavior. J Clin Psychopharmacol. 2012;32(2):237–260. [DOI] [PubMed] [Google Scholar]
- 8. Arseneault L, Moffitt TE, Caspi A, Taylor PJ, Silva PA. Mental disorders and violence in a total birth cohort. Arch Gen Psychiatry. 2000;57(10):979. [DOI] [PubMed] [Google Scholar]
- 9. Blair R. The neurobiology of impulsive aggression. J Child Adolesc Psychopharmacol. 2016;26(1):4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rosell DR, Siever LJ. The neurobiology of aggression and violence. CNS Spectr. 2015;20(3):254–279. [DOI] [PubMed] [Google Scholar]
- 11. Rizvi TA, Ennis M, Behbehani MM, Shipley MT. Connections between the central nucleus of the amygdala and the midbrain periaqueductal gray: topography and reciprocity. J Comp Neurol. 1991;303(1):121–131. [DOI] [PubMed] [Google Scholar]
- 12. Reppucci CJ, Petrovich GD. Organization of connections between the amygdala, medial prefrontal cortex, and lateral hypothalamus: a single and double retrograde tracing study in rats. Brain Struct Funct. 2016;221(6):2937–2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Swanson LW. What is the brain? Trends Neurosci. 2000;23(11):519–527. [DOI] [PubMed] [Google Scholar]
- 14. LeDoux J. The amygdala. Curr Biol. 2007;17(20):R868–R874. [DOI] [PubMed] [Google Scholar]
- 15. Swanson LW. The hypothalamus. In: Björklund A, Hökfelt T, Swanson L, eds. Handbook of Chemical Neuroanatomy, Vol. 5: Integrated Systems off the CNS, Part I Amsterda: Elsevier Sciences; 1987:1–124. [Google Scholar]
- 16. Buijs RM, Van Eden CG. The integration of stress by the hypothalamus, amygdala and prefrontal cortex: balance between the autonomic nervous system and the neuroendocrine system. Prog Brain Res. 2000;126:117–132. doi:10.1016/S0079-6123(00)26011-1. [DOI] [PubMed] [Google Scholar]
- 17. Miczek KA, de Almeida RMM, Kravitz EA, Rissman EF, de Boer SF, Raine A. Neurobiology of escalated aggression and violence. J Neurosci. 2007;27(44):11803–11806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nelson RJ, Trainor BC, Chiavegatto S, Demas GE. Pleiotropic contributions of nitric oxide to aggressive behavior. Neurosci Biobehav Rev. 2006;30(3):346–355. [DOI] [PubMed] [Google Scholar]
- 19. Godar SC, Fite PJ, McFarlin KM, Bortolato M. The role of monoamine oxidase A in aggression: current translational developments and future challenges. Prog Neuropsychopharmacol Biol Psychiatry. 2016;69:90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Citrome L, Volavka J. The psychopharmacology of violence: making sensible decisions. CNS Spectr. 2014;19(5):411–418. [DOI] [PubMed] [Google Scholar]
- 21. Hassiotis A, Robotham D, Canagasabey A, Marston L, Thomas B, King M. Brief report: Impact of applied behaviour analysis (ABA) on carer burden and community participation in challenging behaviour: results from a randomised controlled trial. J Intellect Disabil Res. 2012;56(3):285–290. [DOI] [PubMed] [Google Scholar]
- 22. Smeets KC, Leeijen AAM, van der Molen MJ, Scheepers FE, Buitelaar JK, Rommelse NNJ. Treatment moderators of cognitive behavior therapy to reduce aggressive behavior: a meta-analysis. Eur Child Adolesc Psychiatry. 2015;24(3):255–264. [DOI] [PubMed] [Google Scholar]
- 23. Selvadurai MI, Waxman R, Ghaffar O, Fischler I. Efficacy and safety of maintenance electroconvulsive therapy for sustaining resolution of severe aggression in a major neurocognitive disorder. BMJ Case Rep. 2018;2018:bcr-2017-222100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van den Berg JF, Kruithof HC, Kok RM, Verwijk E, Spaans H-P. Electroconvulsive therapy for agitation and aggression in dementia: a systematic review. Am J Geriatr Psychiatry. 2018;26(4):419–434. [DOI] [PubMed] [Google Scholar]
- 25. Prado-Lima PAS do. Tratamento farmacológico da impulsividade e do comportamento agressivo. Rev Bras Psiquiatr. 2009;31(suppl 2):S58–S65. [DOI] [PubMed] [Google Scholar]
- 26. Uchida H, Takeuchi H, Graff-Guerrero A, Suzuki T, Watanabe K, Mamo DC. Dopamine D2 receptor occupancy and clinical effects. J Clin Psychopharmacol. 2011;31(4):497–502. [DOI] [PubMed] [Google Scholar]
- 27. Morrissette DA, Stahl SM. Treating the violent patient with psychosis or impulsivity utilizing antipsychotic polypharmacy and high-dose monotherapy. CNS Spectr. 2014;19(5):439–448. [DOI] [PubMed] [Google Scholar]
- 28. Correll CU, Yu X, Xiang Y, Kane JM, Masand P. Biological treatment of acute agitation or aggression with schizophrenia or bipolar disorder in the inpatient setting. Ann Clin Psychiatry. 2017;29(2):92–107. [PubMed] [Google Scholar]
- 29. Adler BA, Wink LK, Early M et al.. Drug-refractory aggression, self-injurious behavior, and severe tantrums in autism spectrum disorders: a chart review study. Autism. 2015;19(1):102–106. [DOI] [PubMed] [Google Scholar]
- 30. Yu X, Correll CU, Xiang Y et al.. Efficacy of atypical antipsychotics in the management of acute agitation and aggression in hospitalized patients with schizophrenia or bipolar disorder: results from a systematic review. Shanghai Arch Psychiatry. 2016;28(5):241–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Taskiran PS, Coffey DBJ. Unremitting impulsive aggression in a child with childhood onset schizophrenia and pervasive development disorder-not otherwise specified: the role of stimulants, atypical antipsychotics and mood stabilizers. J Child Adolesc Psychopharmacol. 2013;23(5):363–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fava M. Psychopharmacologic treatment of pathologic aggression. Psychiatr Clin North Am. 1997;20(2):427–451. [DOI] [PubMed] [Google Scholar]
- 33. Baribeau DA, Anagnostou E. An update on medication management of behavioral disorders in autism. Curr Psychiatry Rep 2014;16(3):437. [DOI] [PubMed] [Google Scholar]
- 34. Albrecht B, Staiger PK, Hall K, Miller P, Best D, Lubman DI. Benzodiazepine use and aggressive behaviour: a systematic review. Aust N Z J Psychiatry. 2014;48(12):1096–1114. [DOI] [PubMed] [Google Scholar]
- 35. Willner P. The neurobiology of aggression: Implications for the pharmacotherapy of aggressive challenging behaviour by people with intellectual disabilities. J Intellect Disabil Res. 2015;59(1):82–92. [DOI] [PubMed] [Google Scholar]
- 36. Farmer CA, Arnold LE, Bukstein OG et al.. The treatment of severe child aggression (TOSCA) study: design challenges. Child Adolesc Psychiatry Ment Health. 2011;5(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stigler KA. Psychopharmacologic management of serious behavioral disturbance in ASD. Child Adolesc Psychiatr Clin N Am. 2014;23(1):73–82. [DOI] [PubMed] [Google Scholar]
- 38. Giovannitti JA, Thoms SM, Crawford JJ. Alpha-2 adrenergic receptor agonists: a review of current clinical applications. Anesth Prog. 2015;62(1):31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Corrigan PW, Yudofsky SC, Silver JM. Pharmacological and behavioral treatments for aggressive psychiatric inpatients. Hosp Community Psychiatry. 1993;44(2):125–133. http://www.ncbi.nlm.nih.gov/pubmed/8432495. [DOI] [PubMed] [Google Scholar]
- 40. Malone RP, Delaney MA, Luebbert JF, Cater J, Campbell M. A double-blind placebo-controlled study of lithium in hospitalized aggressive children and adolescents with conduct disorder. Arch Gen Psychiatry. 2000;57(7):649. [DOI] [PubMed] [Google Scholar]
- 41. List BA, Barzman DH. Evidence-based recommendations for the treatment of aggression in pediatric patients with attention deficit hyperactivity disorder. Psychiatr Q. 2011;82(1):33–42. [DOI] [PubMed] [Google Scholar]
- 42. Hayes JF, Pitman A, Marston L et al.. Self-harm, unintentional injury, and suicide in bipolar disorder during maintenance mood stabilizer treatment. JAMA Psychiatry. 2016;73(6):630–637. [DOI] [PubMed] [Google Scholar]
- 43. Hannestad J, Gallezot J-D, Planeta-Wilson B et al.. Clinically relevant doses of methylphenidate significantly occupy norepinephrine transporters in humans in vivo. Biol Psychiatry. 2010;68(9):854–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schmeichel BE, Berridge CW. Neurocircuitry underlying the preferential sensitivity of prefrontal catecholamines to low-dose psychostimulants. Neuropsychopharmacol. 2013;38(6):1078–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gurnani T, Ivanov I, Newcorn JH. Pharmacotherapy of aggression in child and adolescent psychiatric disorders. J Child Adolesc Psychopharmacol. 2016;26(1):65–73. [DOI] [PubMed] [Google Scholar]
- 46. Balia C, Carucci S, Coghill D, Zuddas A. The pharmacological treatment of aggression in children and adolescents with conduct disorder. Do callous-unemotional traits modulate the efficacy of medication? Neurosci Biobehav Rev. 2018;91:218–238. doi:10.1016/j.neubiorev.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 47. Blader J, Schooler N, Jensen P, Pliszka S, Kafantaris V. New research. Child Adolesc Psychopharmacol News. 2009;14(4):8–9. [Google Scholar]
- 48. Victoroff J, Coburn K, Reeve A, Sampson S, Shillcutt S. Pharmacological management of persistent hostility and aggression in persons with schizophrenia spectrum disorders: a systematic review. J Neuropsychiatry Clin Neurosci. 2014;26(4):283–312. [DOI] [PubMed] [Google Scholar]
- 49. Pringsheim T, Hirsch L, Gardner D, Gorman DA. The pharmacological management of oppositional behaviour, conduct problems, and aggression in children and adolescents with attention-deficit hyperactivity disorder, oppositional defiant disorder, and conduct disorder: a systematic review and meta-analysis. Part 2: antipsychotics and traditional mood stabilizers. Can J Psychiatry. 2015;60(2):52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Toro-Martinez E. Pharmacological treatment of aggressive impulsive behavior. Vertex. 2012;23(104):281–286. [PubMed] [Google Scholar]
- 51. Viswanathan M, Ansari MT, Berkman ND et al.. Assessing the Risk of Bias of Individual Studies in Systematic Reviews of Health Care Interventions; 2008. http://www.ncbi.nlm.nih.gov/pubmed/22479713. Accessed August 3, 2018. [PubMed] [Google Scholar]
- 52. Brabec J, Rulseh A, Hoyt B et al.. Volumetry of the human amygdala—an anatomical study. Psychiatry Res. 2010;182(1):67–72. [DOI] [PubMed] [Google Scholar]
- 53. Swanson LW, Petrovich GD. What is the amygdala? Trends Neurosci. 1998;21(8):323–331. [DOI] [PubMed] [Google Scholar]
- 54. Johnston JB. Further contributions to the study of the evolution of the forebrain. J Comp Neurol. 1923;35(5):337–481. [Google Scholar]
- 55. Canteras NS, Simerly RB, Swanson LW. Organization of projections from the medial nucleus of the amygdala: a PHAL study in the rat. J Comp Neurol. 1995;360(2):213–245. [DOI] [PubMed] [Google Scholar]
- 56. Sims KS, Williams RS. The human amygdaloid complex: a cytologic and histochemical atlas using Nissl, myelin, acetylcholinesterase and nicotinamide adenine dinucleotide phosphate diaphorase staining. Neuroscience. 1990;36(2):449–472. [DOI] [PubMed] [Google Scholar]
- 57. Martinez RCR, Gupta N, Lazaro-Munoz G et al.. Active vs. reactive threat responding is associated with differential c-Fos expression in specific regions of amygdala and prefrontal cortex. Learn Mem. 2013;20(8):446–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biol Psychiatry. 2003;54(5):504–514. [DOI] [PubMed] [Google Scholar]
- 59. Pinkham AE, Hopfinger JB, Pelphrey KA, Piven J, Penn DL. Neural bases for impaired social cognition in schizophrenia and autism spectrum disorders. Schizophr Res. 2008;99(1-3):164–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Goltz F. Der Hund ohne Grosshirn. Siebente Abhandlung über die Verrichtung des Grosshirns. Pflügers Arch. 1892;51 (11-12):570–614. [Google Scholar]
- 61. Brown S, Shafer E. An investigation into the functions of the occipital an temporal lobes of the monkey's brain. Philos Trans R Soc London Biol Sci. 1888;179:303–327. [Google Scholar]
- 62. Kluver H, Bucy PC.. “Psychic blindness” and other symptoms following bilateral temporal lobectomy. Am J Physiol. 1937;119:254–284. [Google Scholar]
- 63. Kluver H, Bucy PC. Preliminary analysis of functions of the temporal lobes in monkeys. Arch NeurPsych. 1939;42(6):979–1000. [DOI] [PubMed] [Google Scholar]
- 64. Rosvold HE, Mirsky AF, Pribram KH. Influence of amygdalectomy on social behavior in monkeys. J Comp Physiol Psychol. 1954;47(3):173–178. [DOI] [PubMed] [Google Scholar]
- 65. Heimburger R, Small IF, Small JG, Milstein V, Moore D. Stereotactic amygdalotomy for convulsive and behavioral disorders. Long-term follow-up study. Appl Neurophysiol. 1978;41:43–51. [DOI] [PubMed] [Google Scholar]
- 66. Narabayashi H, Nagao T, Saito Y, Yoshida M, Nagahata M. Stereotaxic amygdalotomy for behavior disorders. Arch Neurol. 1963;9 (1):1–16. [DOI] [PubMed] [Google Scholar]
- 67. Balasubramaniam V, Ramamurthi B. Stereotaxic amygdalotomy. Proc Aust Assoc Neurol. 1968;5(2):277–278. [PubMed] [Google Scholar]
- 68. Stevens JR, Mark VH, Erwin F, Pacheco P, Suematsu K. Deep temporal stimulation in man. Arch Neurol. 1969;21(2):157–169. [DOI] [PubMed] [Google Scholar]
- 69. Balasubramaniam V, Ramamurthi B. Stereotaxic amygdalotomy in behavior disorders. Stereotact Funct Neurosurg. 1970;32(2-5):367–373. [DOI] [PubMed] [Google Scholar]
- 70. Vaernet K, Madsen A. Stereotaxic amygdalotomy and basofrontal tractotomy in psychotics with aggressive behaviour. J Neurol Neurosurg Psychiatry. 1970;33(6):858–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Putman P, Hermans E, Van Honk J. Emotional stroop performance for masked angry faces: it's BAS, not BIS. Emotion. 2004;4(3):305–311. [DOI] [PubMed] [Google Scholar]
- 72. da Cunha-Bang S, Fisher PM, Hjordt L V., Holst K, Knudsen GM. Amygdala reactivity to fearful faces correlates positively with impulsive aggression. Soc Neurosci. 2018:1–11. doi:10.1080/17470919.2017.1421262. [DOI] [PubMed] [Google Scholar]
- 73. Schneider F, Habel U, Kessler C, Posse S, Grodd W, Müller-Gärtner HW. Functional imaging of conditioned aversive emotional responses in antisocial personality disorder. Neuropsychobiology. 2000;42(4):192–201. [DOI] [PubMed] [Google Scholar]
- 74. Heesink L, Gladwin TE, Vink M, van Honk J, Kleber R, Geuze E. Neural activity during the viewing of emotional pictures in veterans with pathological anger and aggression. Eur Psychiatry. 2018;47:1–8. [DOI] [PubMed] [Google Scholar]
- 75. Varkevisser T, Gladwin TE, Heesink L, van Honk J, Geuze E. Resting-state functional connectivity in combat veterans suffering from impulsive aggression. Soc Cogn Affect Neurosci. 2017;12(12):1881–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Saxbe D, Lyden H, Gimbel SI et al.. Longitudinal associations between family aggression, externalizing behavior, and the structure and function of the amygdala. J Res Adolesc. 2018;28(1):134–149. [DOI] [PubMed] [Google Scholar]
- 77. Birbaumer N, Veit R, Lotze M et al.. Deficient fear conditioning in psychopathy. Arch Gen Psychiatry. 2005;62(7):799. [DOI] [PubMed] [Google Scholar]
- 78. Kiehl KA, Smith AM, Hare RD et al.. Limbic abnormalities in affective processing by criminal psychopaths as revealed by functional magnetic resonance imaging. Biol Psychiatry. 2001;50(9):677–684. [DOI] [PubMed] [Google Scholar]
- 79. Glenn AL, Raine A, Schug RA. The neural correlates of moral decision-making in psychopathy. Mol Psychiatry. 2009;14(1):5–6. [DOI] [PubMed] [Google Scholar]
- 80. Heath R, Monroe R, Mickle W. Stimulation of the amygdaloid nucleus in a schizophrenic patient. Am J Psychiatry. 1955;111(11):862–863. [DOI] [PubMed] [Google Scholar]
- 81. Reznikov R, Binko M, Nobrega JN, Hamani C. Deep brain stimulation in animal models of fear, anxiety and posttraumatic stress disorder. Neuropsychopharmacol. 2016;41(12):2810–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hitchcock E, Cairns V. Amygdalotomy. Postgrad Med J. 1973;49(578):894–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ramamurthi B. Stereotactic operation in behaviour disorders. Amygdalotomy and hypothalamotomy. Acta Neurochir Suppl. 1988;44:152–157. [DOI] [PubMed] [Google Scholar]
- 84. Chitanondh H. Stereotaxic amygdalotomy in the treatment of olfactory seizures and psychiatric disorders with olfactory hallucination. Stereotact Funct Neurosurg. 1966;27(1-3):181–196. [DOI] [PubMed] [Google Scholar]
- 85. Heimburger RF, Whitlock CC, Kalsbeck JE. Stereotaxic amygdalotomy for epilepsy with aggressive behavior. JAMA. 1966;198(7):741–745. [PubMed] [Google Scholar]
- 86. Narabayashi H, Uno M. Long range results of stereotaxic amygdalotomy for behavior disorders. Stereotact Funct Neurosurg. 1966;27(1-3):168–171. [DOI] [PubMed] [Google Scholar]
- 87. Kiloh LG, Gye RS, Rushworth RG, Bell DS, White RT. Stereotactic amygdaloidotomy for aggressive behaviour. J Neurol Neurosurg Psychiatry. 1974;37(4):437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Balasubramaniam V, Kanaka TS. Amygdalotomy and hypothalamotomy. A comparative study. ConfinNeurol. 1975;37(1-3):195–201. [DOI] [PubMed] [Google Scholar]
- 89. Balasubramaniam V, Kanaka TS. Why hemispherectomy? Appl Neurophysiol. 1975;38(3):197–205. [DOI] [PubMed] [Google Scholar]
- 90. Sonnen AE, Manen J V., van Dijk B. Results of amygdalotomy and fornicotomy in temporal lobe epilepsy and behaviour disorders. Acta Neurochir (Wien). 1976;219(23 suppl):215–219. [DOI] [PubMed] [Google Scholar]
- 91. Small IF, Heimburger RF, Small JG, Milstein V, Moore DF. Follow-up of stereotaxic amygdalotomy for seizure and behavior disorders. Biol Psychiatry. 1977;12(3):401–411. [PubMed] [Google Scholar]
- 92. Mempel E, Witkiewicz B, Stadnicki R et al.. The effect of medial amygdalotomy and anterior hippocampotomy on behavior and seizures in epileptic patients. Acta Neurochir Suppl. 1980;30:161–167. [DOI] [PubMed] [Google Scholar]
- 93. Bernasconi SA, Lynch ME, Holt C. Bilateral stereotactic amygdalotomy. Nurs Times. 1981;77(45):1928–1930. [PubMed] [Google Scholar]
- 94. Hood T, Siegfried J, Wieser HG. The role of stereotactic amygdalotomy in the treatment of temporal lobe epilepsy associated with behavioral disorders. Appl Neurophysiol. 1983;46(1-4):19–25. [DOI] [PubMed] [Google Scholar]
- 95. Jacobson R. Disorders of facial recognition, social-behavior and affect after combined bilateral amygdalotomy and subcaudate tractotomy—a clinical and experimental-study. Psychol Med. 1986;16(2):439–450. [DOI] [PubMed] [Google Scholar]
- 96. van Manen J, van Veelen CW. Experiences in psycho-surgery in The Netherlands. Acta Neurochir Suppl (Wien). 1988;44:167–169. [DOI] [PubMed] [Google Scholar]
- 97. Sachdev P, Smith JS, Matheson J, Last P, Blumbergs P. Amygdalo-hippocampectomy for pathological aggression. Aust N Z J Psychiatry. 1992;26(4):671–676. [DOI] [PubMed] [Google Scholar]
- 98. Lee GP, Bechara A, Adolphs R et al.. Clinical and physiological effects of stereotaxic bilateral amygdalotomy for intractable aggression. J Neuropsychiatry Clin Neurosci. 1998;10(4):413–420. [DOI] [PubMed] [Google Scholar]
- 99. Kim M-C, Lee T-K, Choi C-R. Review of long-term results of stereotactic psychosurgery. Neurol Med Chir (Tokyo). 2002;42(9):365–371. [DOI] [PubMed] [Google Scholar]
- 100. Fountas KN, Smith JR, Lee GP. Bilateral stereotactic amygdalotomy for self-mutilation disorder. Stereotact Funct Neurosurg. 2007;85(2-3):121–128. [DOI] [PubMed] [Google Scholar]
- 101. Sturm V, Fricke O, Bührle CP et al.. DBS in the basolateral amygdala improves symptoms of autism and related self-injurious behavior: a case report and hypothesis on the pathogenesis of the disorder. Front Hum Neurosci. 2012;6:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Zhang S, Zhou P, Jiang S, Li P, Wang W. Bilateral anterior capsulotomy and amygdalotomy for mental retardation with psychiatric symptoms and aggression. Medicine. 2017;96(1):10–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Harrison LA, Hurlemann R, Adolphs R. An enhanced default approach bias following amygdala lesions in humans. Psychol Sci. 2015;26(10):1543–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Hortensius R, Terburg D, Morgan B, Stein DJ, van Honk J, de Gelder B. The dynamic consequences of amygdala damage on threat processing in Urbach–Wiethe Disease. A commentary on Pishnamazi et al. (2016). Cortex. 2017;88:192–197. [DOI] [PubMed] [Google Scholar]
- 105. Greenberg BD, Askland KD, Carpenter LL. The evolution of deep brain stimulation for neuropsychiatric disorders. Front Biosci. 2008;13(13):4638–4648. [DOI] [PubMed] [Google Scholar]
- 106. Hamani C, Hodaie M, Lozano AM. Present and future of deep brain stimulation for refractory epilepsy. Acta Neurochir (Wien). 2005;147(3):227–229. [DOI] [PubMed] [Google Scholar]
- 107. Awan NR, Lozano A, Hamani C. Deep brain stimulation: current and future perspectives. Neurosurg Focus. 2009;27(1):E2. [DOI] [PubMed] [Google Scholar]
- 108. Hamani C, Temel Y. Deep brain stimulation for psychiatric disease: contributions and validity of animal models. Sci Transl Med. 2012;4(142):142rv8–142rv8. [DOI] [PubMed] [Google Scholar]
- 109. Hamani C, Florence G, Heinsen H et al.. Subthalamic nucleus deep brain stimulation: basic concepts and novel perspectives. eNeuro. 2017;4(5). doi:10.1523/ENEURO.0140-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Reznikov R, Binko M, Nobrega JN, Hamani C. Deep brain stimulation in animal models of fear, anxiety and posttraumatic stress disorder. Neuropsychopharmacol. 2016;41(12):2810–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Florence G, Sameshima K, Fonoff ET, Hamani C. Deep brain stimulation. Neuroscientist. 2016;22(4):332–345. [DOI] [PubMed] [Google Scholar]
- 112. Hamani C, Nobrega JN, Lozano AM. Deep brain stimulation in clinical practice and in animal models. Clin Pharmacol Ther. 2010;88(4):559–562. [DOI] [PubMed] [Google Scholar]
- 113. Swaab DF. The human hypothalamus. basic and clinical aspects. Part II: neuropathology of the hypothalamus and adjacent brain structures. In: Swaab Dick. (ed). Handbook of Clinical Neurology, Vol. 80 Elsevier, Amsterdam, The Netherlands; 2004:597. [Google Scholar]
- 114. Dudas B. The Human Hypothalamus Anatomy, Functions and Disorders. Nova Science Publishers; 2012. [Google Scholar]
- 115. Tyree SM, de Lecea L. Lateral hypothalamic control of the ventral tegmental area: reward evaluation and the driving of motivated behavior. Front Syst Neurosci. 2017;11:50 doi:10.3389/fnsys.2017.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Siegel A, Brutus M. Neural substrates of aggression and rage in the cat. In: Epstein AN, Morrison AR (eds). Progress in Psychobiology and Physiological Psychology. New York, NY, Academic Press; 1990:135–233. [Google Scholar]
- 117. Lammers JHCM, Kruk MR, Meelis W, van der Poel AM. Hypothalamic substrates for brain stimulation-induced attack, teeth-chattering and social grooming in the rat. Brain Res. 1988;449(1-2):311–327.3 [DOI] [PubMed] [Google Scholar]
- 118. Falkner AL, Lin D. Recent advances in understanding the role of the hypothalamic circuit during aggression. Front Syst Neurosci. 2014;8:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Gregg TR, Siegel A. Brain structures and neurotansmitters regulating aggression in cats: implications for human aggression. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25(1):91–140. [DOI] [PubMed] [Google Scholar]
- 120. Bejjani B, Houeto J, Hariz M et al.. Aggressive behavior induced by intraoperative stimulation in the triangle of Sano. Neurology. 2002;59(9):1425–1427. [DOI] [PubMed] [Google Scholar]
- 121. Schvarcz JR, Driollet R, Rios E, Betti O. Stereotactic hypothalamotomy for behaviour disorders. J Neurol Neurosurg Psychiatry. 1972;35(3):356–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. George DT, Rawlings RR, Williams WA et al.. A select group of perpetrators of domestic violence: evidence of decreased metabolism in the right hypothalamus and reduced relationships between cortical/subcortical brain structures in position emission tomography. Psychiatry Res. 2004;130(1):11–25. [DOI] [PubMed] [Google Scholar]
- 123. Van den Stock J, Hortensius R, Sinke C, Goebel R, de Gelder B. Personality traits predict brain activation and connectivity when witnessing a violent conflict. Sci Rep. 2015;5(1):13779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Sano K, Mayanagi Y. Posteromedial hypothalamotomy in the treatment of violent, aggressive behaviour. Acta Neurochir Suppl (Wien). 1988;44:145–151. [DOI] [PubMed] [Google Scholar]
- 125. De Almeida AN, Fonoff ET, Ballester G, Teixeira MJ, Marino R. Stereotactic disconnection of hypothalamic hamartoma to control seizure and behavior disturbance: case report and literature review. Neurosurg Rev. 2008;31(3):343–349. [DOI] [PubMed] [Google Scholar]
- 126. Hernando V, Pastor J, Pedrosa M, Peña E, Sola RG. Low-frequency bilateral hypothalamic stimulation for treatment of drug-resistant aggressiveness in a young man with mental retardation. Stereotact Funct Neurosurg. 2008;86(4):219–223. [DOI] [PubMed] [Google Scholar]
- 127. Kuhn J, Lenartz D, Mai JK, Huff W, Klosterkoetter J, Sturm V. Disappearance of self-aggressive behavior in a brain-injured patient after deep brain stimulation of the hypothalamus: technical case report. Neurosurgery. 2008;62(5):E1182; discussion E1182. doi:10.1227/01.neu.0000325889.84785.69. [DOI] [PubMed] [Google Scholar]
- 128. Maley JH, Alvernia JE, Valle EP, Richardson D. Deep brain stimulation of the orbitofrontal projections for the treatment of intermittent explosive disorder. Neurosurg Focus. 2010;29(2):E11 doi:10.3171/2010.5.FOCUS10102. [DOI] [PubMed] [Google Scholar]
- 129. Franzini A, Broggi G, Cordella R, Dones I, Messina G. Deep-brain stimulation for aggressive and disruptive behavior. World Neurosurg. 2013;80(3-4):S29.e11–S29.e14. [DOI] [PubMed] [Google Scholar]
- 130. Torres C V, Sola RG, Pastor J et al.. Long-term results of posteromedial hypothalamic deep brain stimulation for patients with resistant aggressiveness. J Neurosurg. 2013;119(2):277–287. [DOI] [PubMed] [Google Scholar]
- 131. Benedetti-Isaac JC, Torres-Zambrano M, Vargas-Toscano A et al.. Seizure frequency reduction after posteromedial hypothalamus deep brain stimulation in drug-resistant epilepsy associated with intractable aggressive behavior. Epilepsia. 2015;56(7):1152–1161. [DOI] [PubMed] [Google Scholar]
- 132. Anand B, Brobek J. Hypothalamic control of food intake in rats and cats. Yale J Biol Med. 1951;24(2):123–140. [PMC free article] [PubMed] [Google Scholar]
- 133. Sabatino JJ, Werner JK, Newsome SD. A rare case of hyponatremia from a hypothalamic lesion in a patient with multiple sclerosis. Mult Scler. 2015;21(5):662–665. [DOI] [PubMed] [Google Scholar]
- 134. Ranson S, Fisher C, Ingram W. Hypothalamic regulation of temperature in the monkey. Arch NeurPsych. 1937;38(3):445–466. [Google Scholar]
- 135. Franzini A, Messina G, Cordella R, Marras C, Broggi G. Deep brain stimulation of the posteromedial hypothalamus: indications, long-term results, and neurophysiological considerations. Neurosurg Focus. 2010;29(2):E13 doi:10.3171/2010.5.FOCUS1094. [DOI] [PubMed] [Google Scholar]
- 136. Lapchak PA. Scientific rigor recommendations for optimizing the clinical applicability of translational research. J Neurol Neurophysiol. 2012;3(5):e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Ballantine HT. Historical overview of psychosurgery and its problematic. Acta Neurochir Suppl (Wien). 1988;44:125–128. [DOI] [PubMed] [Google Scholar]
- 138. Neumaier F, Paterno M, Alpdogan S et al.. Surgical approaches in psychiatry: a survey of the world literature on psychosurgery. World Neurosurg. 2017;97:603–634.e8. [DOI] [PubMed] [Google Scholar]
- 139. Older J. Psychosurgery: ethical issues and a proposal for control. Am J Orthopsychiatry. 1974;44(5):661–674. [DOI] [PubMed] [Google Scholar]
- 140. Mpakopoulou M, Gatos H, Brotis A, Paterakis KN, Fountas KN. Stereotactic amygdalotomy in the management of severe aggressive behavioral disorders. Neurosurg Focus. 2008;25(1):E6 doi:10.3171/FOC/2008/25/7/E6 [DOI] [PubMed] [Google Scholar]
- 141. Earp JD. Psychosurgery: the position of the Canadian Psychiatric Association. Can J Psychiatry. 1979;24(4):353–365. [PubMed] [Google Scholar]
- 142. Woerdeman PA, Willems PWA, Noordmans HJ, Berkelbach van der Sprenkel JW, van Rijen PC. Frameless stereotactic subcaudate tractotomy for intractable obsessive–compulsive disorder. Acta Neurochir (Wien). 2006;148(6):633–637; discussion 637. [DOI] [PubMed] [Google Scholar]
- 143. Park HR, Kim IH, Kang H et al.. Nucleus accumbens deep brain stimulation for a patient with self-injurious behavior and autism spectrum disorder: functional and structural changes of the brain: report of a case and review of literature. Acta Neurochir. 2017;159(1):137–143. [DOI] [PubMed] [Google Scholar]
- 144. Alho EJL, Alho ATDL, Grinberg L et al.. High thickness histological sections as alternative to study the three-dimensional microscopic human sub-cortical neuroanatomy. Brain Struct Funct. 2018;223(3):1121–1132. doi: 10.1007/s00429-017-1548-2. [DOI] [PMC free article] [PubMed] [Google Scholar]