Abstract
Objective:
We tested three models to determine how improvements in emotion regulation (ER) and cognitive skills (CS) as a result of intervention operate to affect reductions in diabetes distress DD.
Methods:
Change data were drawn from the baseline and 9-month T1-REDEEM trial. Adults with type 1 diabetes were recruited from several U.S. states and Toronto, Canada. A primary and two alternative structural equation models were tested to explore the directionality of effect: primary model – changes in ER and CS drive changes in DD; reverse model – changes in DD drive changes in ER and CS; and bidirectional model – changes in ER, CS and DD occur together with no directionality.
Results:
All three models displayed a good fit to the data. The primary model indicated 7 significant directional pathways: improvements in ER and CS operate together to drive reductions in DD. The reverse model only indicated that reductions in DD affected changes in one CS variable; and the bidirectional model indicated only that these results were bidirectional. Reductions in all tested domains of DD occurred together.
Conclusions:
Improvements in ER and CS drive reductions in DD.
Practice implications:
Interventions to reduce high DD should focus on improving ER and CS.
Keywords: diabetes, diabetes distress, emotion regulation, cognition
1. Introduction
Diabetes distress (DD) has received increased clinical attention in recent years in part because of its significant associations with poor self-management [1], problematic medication adherence [2], poor glycemic control [3] and impaired quality of life [4]. Distinct from depression, DD is highly prevalent [5, 6] and tends to be chronic, rather than episodic over time [7]. Recent reviews of interventions to reduce high DD among type 1 (T1D) and type 2 (T2D) adults with diabetes have indicated that DD is responsive to clinical efforts [8, 9] and that reductions in DD following intervention persist over time with minimal relapse [10, 11]. Given the impressive results of interventions to reduce high DD, we now focus on the identification of the underlying mechanisms and processes through which interventions drive reductions in DD.
T1-REDEEM (Reducing Distress and Enhancing Effective Management) was a randomized control trial to identify which of two interventions, one focused on diabetes education/management and the other on the emotional side of DD, most effectively reduced DD among highly distressed, poorly controlled adults with T1D [11]. Both interventions led to striking reductions in DD, with no between-group differences, which were sustained at nine months.
Using pre-intervention, cross-sectional T1-REDEEM data, we recently reported the results of a structural equation model that mapped the relationships that linked poor emotion regulation and cognition with high DD, which, in turn, was linked to problematic self-management and, subsequently, to poor glycemic control and more frequent hypoglycemic episodes among adults with T1D [12]. Recognizing that most current theories of health behavior and coping in chronic illness include both affective and cognitive-behavioral components [13, 14], our findings highlighted how both affect (emotion regulation) and cognition (appraisal, problem solving) were associated with high DD, with subsequent linkages to poor self-management and glycemic control.
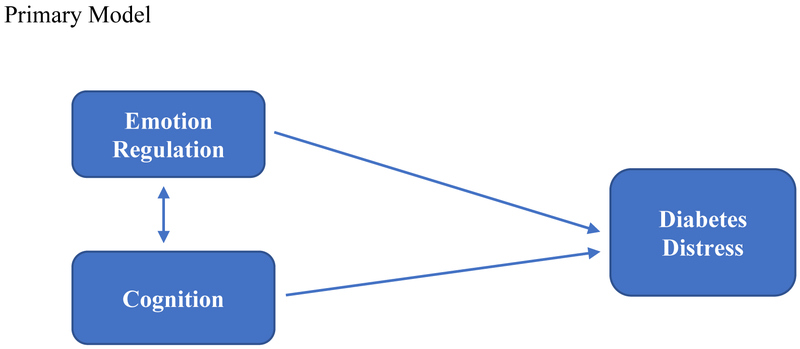
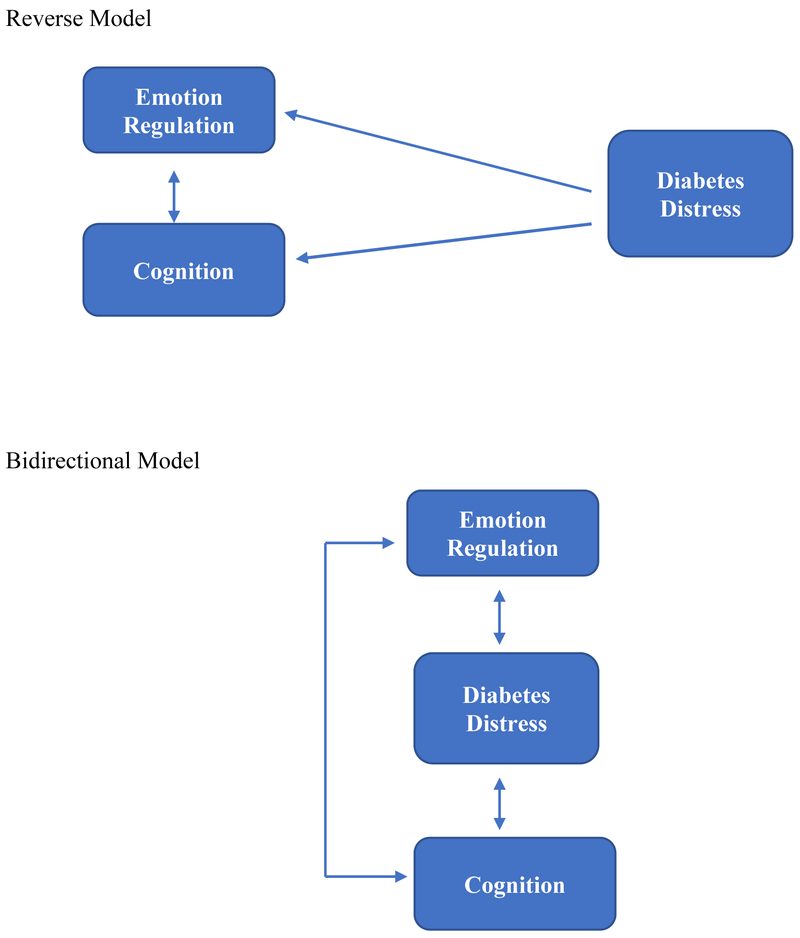
In this report we expand upon these findings by moving beyond an analysis of the static relationships among variables assessed at one time point to focus instead on the dynamics of the change process itself using 9-month longitudinal data from T1-REDEEM. We sought to determine how change in emotion regulation and cognitive skills as a result of intervention operates to affect change in DD. Based on previous literature [15, 16] and the results of our prior structural equation model, in this report we tested a primary, directional model of change (Figure 1) that asked: do changes in emotion regulation and changes in cognitive skills as a result of intervention drive changes in DD? This model, if supported, would suggest that interventions to reduce high DD should focus on improving emotion regulation (e.g., more self-awareness of affect, less self-blame) and/or cognitive skills (e.g., improved problem solving, reappraisal of what it means to be in glycemic control), since improvements in these areas drive reductions in DD. As checks on the primary model, we also tested two alternative models. The first, called the reverse model, asks if the direction of influence posited in the primary model, from emotion regulation and cognitive skills to DD, operates in the reverse direction; that is, whether reductions in DD drive changes in emotion regulation and cognitive skills. If supported, this model would suggest that DD itself should be targeted for intervention, for example, by alleviating specific sources of distress, and that reductions in DD drive improvements in emotion regulation and cognitive skill. The second alternative model, called the bidirectional model, asks if all three constructs (emotion regulation, cognitive skill, DD) change together with intervention, with no ordering or sequencing effects among the constructs. If supported, this model would suggest that interventions to reduce high DD should be broadly based to address all three targets at the same time. Evaluating the dynamics of the change process will assist in understanding how change takes place so that more effective strategies of intervention to reduce high DD can be identified.
Figure 1.
Schematic Diagrams of the Primary, Reverse, and Bidirectional Path Models Tested. Primary Model
2. Methods
2.1. Patients
Details of recruitment and methods have been presented previously [11]. Briefly, adults with T1D who scored ≥ 2.0 on the T1-Diabetes Distress Scale (T1-DDS) and whose most recent HbA1C result was ≥ 7.5% (58 mmol/mol) were recruited from multiple community and academic diabetes centers across several western U.S. states and Toronto, Canada to assure a highly diverse sample. Participants were ≥ 19 years of age, were diagnosed with T1D ≥ 12 months and displayed no severe diabetes complications or other major health problems that were functionally limiting. Human subjects approval was received from the appropriate institutional review board (IRB) and recruitment followed a combination of opt-in and opt-out procedures. Using opt-out procedures, the research team mailed letters to patients informing them of the study and telling them that a project representative would contact them by phone within 2 weeks unless they opted out of the call by returning an enclosed postcard or calling a toll-free telephone number. Using opt-in procedures, individuals receiving letters were encouraged to call our toll-free number or send an e-mail expressing interest. During initial contact with those identified by both recruitment procedures, the project was explained, informed consent was obtained, and initial screening commenced, including administration of the T1-DDS and permission to obtain their latest clinic-recorded HbA1c. The overall project was approved by the IRB at the University of California, San Francisco.
2.2. Procedure
Once recruited, participants completed an online, baseline assessment and either provided consent to obtain their most recent clinic HbA1C result or were provided with a form to obtain an HbA1C test at a local laboratory. Participants then were randomized to one of the two arms of the trial: KnowIt or OnTrack. Both study arms required the same time commitment: a one-day workshop with a trained leader and participation in four, one-hour, online video meetings with the group leader over the succeeding three months. Follow-up online survey occurred at 9 months after the workshop. Data were collected between 2015 and 2017, and analyzed in 2018.
KnowIt was an educational/management program that provided an intensive diabetes update on the causes and management of T1D. Each of the four subsequent online meetings reviewed participant action plans for management change and addressed a specific information-only topic: continuous glucose monitoring, islet and pancreas transplantation, hypoglycemia, and travel. In contrast, OnTrack focused on ways to deal with the emotional side of diabetes and to develop a personalized action plan that addressed DD directly to help get “unstuck” about management change. The four post-workshop online group meetings included information concerning: dealing with T1D 24 hours a day, coping with frustrating blood glucose numbers, dealing with family and friends, and addressing fears of hypoglycemia. Thus, the two arms directly compared an educational/behavioral change approach to reducing DD (KnowIt) to reducing DD with a program that addressed high DD and its management directly (OnTrack). Upon completion of each of the three assessments, participants received a $25 gift card.
2.3. Measures
T1-DDS is a 28-item scale (alpha = .91) [17] that assesses overall level of DD with items scored using six response options, from “not a problem” to a “very serious problem.” The scale also yields seven subscales. Because the models focused on change over time, we selected the T1-DDS subscales that demonstrated the largest reduction in DD as a result of intervention: powerlessness (5 items: “Feeling that no matter how hard I try with my diabetes it will never be good enough;” alpha = .87), eating distress (3 items: “Feeling that my eating is out of control;” alpha = .78), management distress (4 items: “Feeling that I am not as skilled in managing my diabetes as I should;” alpha = .76), and hypoglycemia distress (4 items: “Feeling that I can never be safe from the possibility of a serious hypoglycemic event;” alpha = .79). Mean item scores were calculated for each subscale.
We adopted a basic affective and instrumental coping model [18] that included important aspects of two major diabetes stress-related constructs: emotion regulation and cognition [13, 19–22]. Variable selection for each was based on theoretically sound, validated and widely used measures that represented each construct, and that have been used as high-value targets of intervention in T1-REDEEM and other programs [23]. Within the emotion regulation domain, Emotional Processing is a 4-item subscale (alpha = .87) from the Emotional Approach and Coping Scale [24]. Items are scored on a 4-point scale from “I haven’t been doing this at all” to “I have been doing this a lot,” and include “I take time to figure out what I am really feeling” and “I realize that my feelings are valid and important.” Non Judging of Inner Experience Scale (Non Judging) is an 8-item subscale (alpha = .95) from the Five Facet Mindfulness Scale [25]. Items are scored on a 5-point scale from “Never or very rarely true” to “Very often or always true, and include “I tell myself that I shouldn’t be feeling the way that I am feeling” and “I disapprove of myself when I have irrational ideas.”
Within the cognitive domain, Personal Control is a 10-item subscale from the Revised Illness Perception Questionnaire [26]. It uses five response options, from “Strongly disagree” to “Strongly agree” (alpha = 0.90), with items like, “I have the power to influence my illness,” and “The course of my disease depends on me.” Effective Problem Solving (Problem Solving) is a 10-item subscale of the Health Problem Solving Survey (HPSS) [27]. Items are scored on a 5-point scale from “Not at all true of me” to “Extremely true of me” (alpha = .90) and include items such as, “I am able to figure out when problems are making my health condition(s) worse” and “I have no trouble putting plans for dealing with my health condition(s) into actions.”
2.4. Data Analysis
We used two-group structural equation path models to examine hypothesized unidirectional and bidirectional relations among the emotion regulation, cognition, and T1-DDS variables. Primary, reverse, and bidirectional path models were separately estimated using Mplus software (version 6.1). Mplus uses an Expectation Maximization algorithm that allows for the handling of missing data, enabling the models to include all participants having at least one data point. Patient-level covariates of gender, age, and number of health complications, which have been related previously to the primary outcomes [12]were initially included; however, these covariates had little or no significant effects on other variables and were excluded to avoid confounding results and to achieve parsimonious final models. To allow for examining change, 9-month values of the outcome variables were regressed on their respective baseline values; regression parameters, covariances, means and variances were estimated to determine relations among the 9-month variables while accounting for change in these variables from baseline. The four T1-DDS subscales were covaried with each other. Regression coefficients (B) were standardized based on the variance of other observed variables.
Parameter estimates across the two intervention groups (OnTrack and KnowIt) were initially constrained to be equal, but a few variances were allowed to be freely estimated across the two groups when Wald test results indicated they significantly differed. These minor group differences had no effect on model fit or on significant pathways and were excluded from presentation.
3. Results
A total of 347 adults with T1D were eligible to participate. Of these, 301 (86%) (n=149 KnowIt and n=152 OnTrack) completed baseline assessment, attended the workshop, and were included in the present analyses (Table 1). The two groups did not differ significantly at baseline, except that the KnowIt group was significantly older than the OnTrack group (mean age = 47.3 years SD=14.5 vs. 42.8 SD=15.1, p = .009). There were no significant differences between eligible adults who participated and those who declined on gender, ethnicity, education, and insulin use. Those who participated, however, reported higher baseline T1-DDS scores than those who did not (mean = 2.9, SD = 0.6 vs. mean = 2.7, SD = 0.9; t = 3.20, p = .001). Average age was 45.05 (15.0) years, education was 15.3 (3.6) years, percent female was 69.1%, mean DD score was 2.9 (0.6), and mean HbA1C result was 8.8 (1.1) (206 mmol/mol). Table 2 shows a significant time effect for all T1-DDS, emotion regulation and cognitive variables, with the exception of Personal Control (p = 0.64). No time by intervention group effect occurred for any variable.
Table 1.
Participant Characteristics by Treatment Group (N=301)
| Variable | KnowIt Mean (SD) n=149 |
OnTrack Mean (SD) n=152 |
p Value |
|---|---|---|---|
| Age | 47.3 (14.5) | 42.8 (15.1) | .009 |
| Education (years) | 15.7 (3.6) | 15.2 (3.6) | .32 |
| Number of children | 1.1 (1.3) | .93 (1.0) | .20 |
| Age at diagnosis | 21.2 (14.4) | 19.5 (13.7) | .28 |
| Years with diabetes | 26.12 (13.97) | 23.17 (13.26) | .06 |
| Number of complications | 2.84 (2.57) | 2.65 (2.47) | .51 |
| % Female | 70.5% | 67.8% | .61 |
| % White | 82.6% | 77.6% | .29 |
| % with partner | 61.7% | 67.5% | .29 |
| % with insulin pump | 63.8% | 67.8% | .46 |
| % with CGM | 37.6% | 38.88% | .83 |
| HbAlc (mmol/mol) | 8.77 (1.13) | 8.83 (1.11) | .65 |
Note. t test or chi-square test, as appropriate.
Table 2.
Means (SD) of Model Variables at Baseline and 9-Months (N=301)
| Variable | Baseline Mean (SD) | 9 Months Mean (SD) | p Value |
|---|---|---|---|
| T1-DDS Total | 2.88 (.61) | 2.17 (.59) | .000 |
| Powerlessness | 4.09 (.98) | 2.91 (1.02) | .000 |
| Management | 3.38 (1.13) | 2.39 (.95) | .000 |
| Hypoglycemia | 2.66 (1.17) | 2.02 (.86) | .000 |
| Eating | 3.45 (1.23) | 2.63 (1.11) | .000 |
| Emotional Processing | 2.46 (.78) | 2.78 (.82) | .000 |
| Non Judging | 3.52 (1.03) | 3.96 (.92) | .000 |
| Personal Control | 24.82 (3.43) | 24.71 (4.06) | .640 |
| Problem Solving | 27.16 (5.9) | 29.12 (6.27) | .000 |
Note. Based on MANOVA models.
Attrition at 9 months was 12%, which did not differ by study arm; those who dropped out had significantly higher HbA1C and DD, more complications, and were younger at baseline than those who remained in the study.
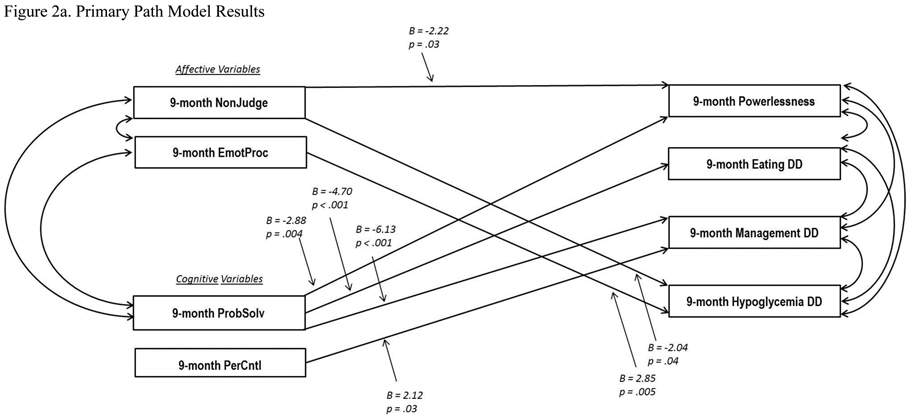
3.1. Testing the Primary Model
Model fitting procedures for the primary model, testing whether changes in emotion regulation and cognitive skills drive changes in DD (Figure 2a), indicated a good fit to the data: Comparative Fit Index = .959, Tucker Lewis Index = .957, and Root Mean Square Error of Approximation = .039. Generally acceptable fit indices are Comparative Fit Index and Tucker Lewis Index > 0.95, and Root Mean Square Error of Approximation < 0.06 [28].
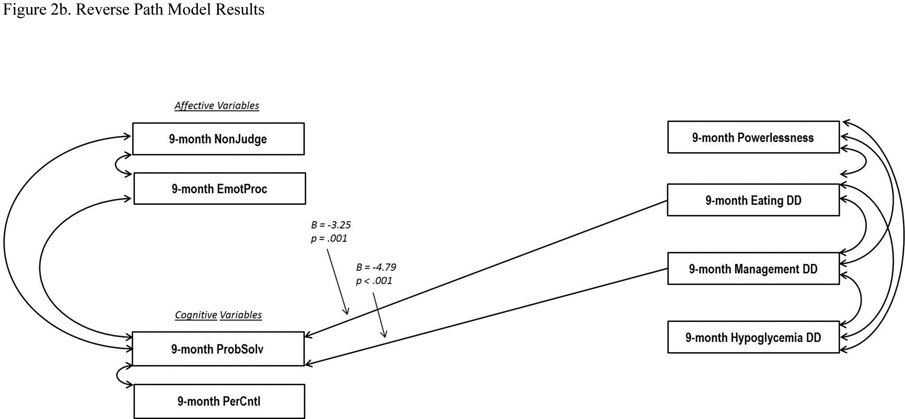
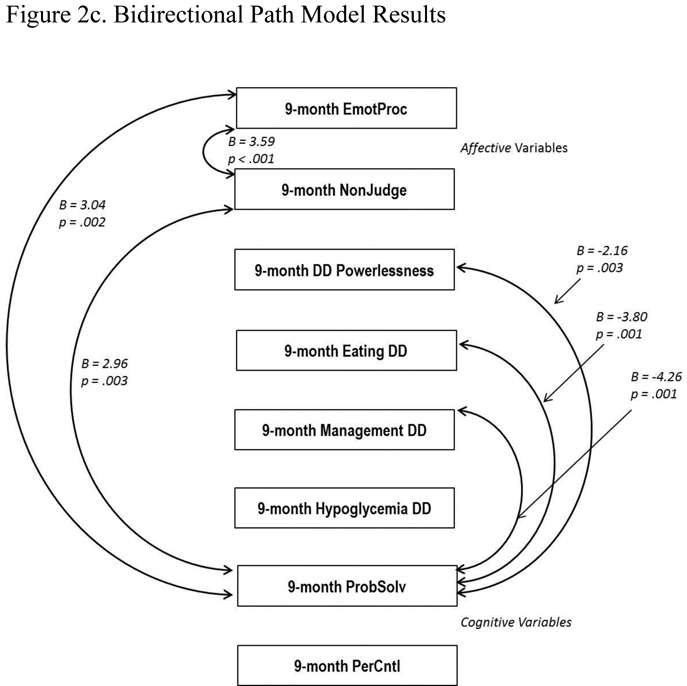
Figure 2.
Path Models (Two-Group). All 9-month values were regressed on baseline values (not shown). Standardized regression coefficients are presented. For clarity, only significant pathways are displayed. EmotProc = Emotional Processing; NonJudge = Non Judging of Inner Experience; PerContl = Personal Control; PribSolv = Effective Problem Solving.
Primary (2a): CFI = .959, TLI = .957, RMSEA = .039.
Reverse (2b): CFI = .953, TLI = 951, RMSEA = .041
Bidirectional (2c): CFI = .994, TLI = .991, RMSEA = .016.
Significant covariation was found among all four T1DDS change variables (correlation range = .40 to .54 at 9 months). Furthermore, multiple DD variables significantly regressed on emotion regulation variables: powerlessness on Non Judging, B = −2.22, p = .03; hypoglycemia DD on Non Judging, B = −.204, p = .04; and hypoglycemia DD on Emotional Processing, B = 2.85, p = .005. Likewise, multiple DD variables significantly regressed on cognition variables: powerlessness on Problem Solving, B = −2.88, p = .004; eating DD on Problem Solving, B = −4.70, p <.001; management DD on Problem Solving, B = −6.13, p < .001; and management DD on Personal Control, B = 2.12, p = .03. In addition, Problem Solving significantly covaried with the two emotion regulation variables, Non Judging (B = 3.04, p = .002) and Emotional Processing (B = 2.78, p = .005).
Overall, the findings suggest that changes in both emotion regulation and illness-related cognition as a result of intervention drive changes in DD, with significant, bidirectional relationships among the variables within the emotion regulation and DD domains.
3.2. Testing the Reverse Model
The reverse model, which tested the extent to which changes in DD drive changes in emotion regulation and cognitive skill, also indicated a generally good fit to the data (Figure 2b): Comparative Fit Index = .953, Tucker Lewis Index = 951, and Root Mean Square Error of Approximation = .041, but with far fewer significant pathways among the constructs. Problem Solving significantly regressed on only two DD constructs: eating DD (B = −3.25, p = .001) and management DD (B = −4.79, p < .001). No significant directional pathways occurred between emotion regulation and any of the four DD areas, nor between Personal Control and any DD domain. Significant covariances were found among all four T1-DDS variables, and between Problem Solving and the two emotion regulation variables: Non Judging (B = 2.45, p = .01) and Emotional Processing (B = 2.98, p = .003). Overall, the reverse model provided very limited new information: only changes in Problem Solving were linked with changes in eating and management DD.
3.3. Testing the Bidirectional Model
The bidirectional model, which tested linkages between changes in emotion regulation, cognition and DD without directionality, also showed good statistical fit to the data (Figure 2c): Comparative Fit Index = .994, Tucker Lewis Index = .991, and Root Mean Square Error of Approximation = .016. But, like the reverse model, it indicated only a few significant pathways among the three constructs: between Emotional Processing and Non Judging (B = 3.59, p < .001), Emotional Processing and Problem Solving (B = 3.04, p = .002), and Non Judging and Problem Solving (B = 2.96, p = .003). All four DD areas significantly covaried with each other (correlation range = .40 to .54 at 9 months), and three DD areas significantly covaried with Problem Solving (powerlessness B = −2.16, p = .03; eating DD B = −3.80, p < .001; management DD B = −4.26, p < .001). Thus, emotion regulation, cognition, and DD were linked through relationships among Problem Solving, Non Judging, and Emotional Processing, but not Personal Control. This more global model underscored the important bidirectional relationship between Problem Solving and DD shown in the other models, but did not provide additional insight into the linkages among the other variables found in the primary model.
4. Discussion and conclusion
4.1. Discussion
The analyses yielded several important results. First, the findings suggest that the primary model provides the most articulated information about a direction of influence among the constructs: there are seven significant directional pathways showing that change in all four emotion regulation and cognitive variables drive change in at least one area of DD. This directional effect suggests, for example, that reducing self-blame, increasing awareness of underlying emotions related to diabetes and its management, improving diabetes problem solving ability, and enhancing the experience of personal control of diabetes decreases multiple areas of DD. Thus, these may be considered primary targets of interventions since they lead to reduced DD among T1D adults.
The two alternative models also indicate a good fit to the data. The reverse model, however, shows only two significant pathways: from management DD and eating DD to Problem Solving, the opposite direction of influence found in the primary model. The bidirectional model confirms the bidirectionality between Problem Solving and management/eating DD shown in the primary and reverse models. Thus, all of the significant pathways between emotion regulation/cognition and DD are directional with the exception of two involving Problem Solving. How are we to interpret this finding? The bidirectional relationship involving Problem Solving may reflect the fact that changes in both problem solving and diet/management DD involve changes in specific, concrete behaviors: for example, developing new strategies to reduce carbohydrates or changing the timing of insulin bolusing. We suspect an iterative relationship between these sets of behaviors over time as the effects of intervention play out: intervention enhances problem-solving behavior, which leads to reductions in DD, which, in turn, leads to further improvements in problem-solving skills as additional management changes are explored, and so on. Future research should explore this process: that is, how do the bidirectional influences between problem solving and DD operate to affect changes in subsequent disease management behaviors that lead to improvements in glycemic control.
Second, changes in the four emotion regulation and cognitive skill variables with intervention occur together: the significant pathways among them are bidirectional in all three models. This simultaneity of change and their joint effect on change in DD runs counter to the frequent separation of these constructs in clinical research, with each having a separate and somewhat distinct research literature. Changes in emotion regulation and cognitive skill as a result of intervention appear to be intimately interrelated within the person as a whole, reminding us that these processes involve the simultaneous interplay of several interrelated constructs acting in concert. This finding fits well with our earlier, cross-sectional structural equation model [12]. Furthermore, in qualitative interviews, participants in T1-REDEEM [11] reported that the intervention helped them to gain emotional perspective about the realities of managing T1D, that self-blame was dispelled when, through intervention, they realized that it was the diabetes and not themselves that was the problem. Intermixed with these realizations was a re-appraising of their previous approaches to management based on input from others, thus gaining an increased personal sense of purpose and control in the process. Thus, changes in both emotion regulation and cognition likely co-occur, and do not operate separately or sequentially to drive changes in DD.
Third, in most models the four areas of DD tested (powerlessness, eating DD, management DD, hypoglycemia DD) changed together (bidirectional) over time. (Supplementary, exploratory analyses to explore potential ordering effects among them were nonsignificant [data not shown].) These findings highlight the need for interventions to adopt a broad DD perspective, addressing various areas of DD at the same time and understanding that reductions in one area of DD may affect changes in others.
All three of the models tested displayed good model fit [29], with each model providing a different window into the data [30]. Our goal was to test models that showed different views on the directionality of influence during intervention among emotion regulation, cognition and DD. In combination, the three well-fitting models provided complementary information to help achieve this goal.
This study had several limitations. First, mean age of participants was in the 40s, most were female and almost all were well educated. Replication with other samples of T1D adults is needed. Second, to reduce the complexity of model testing, only two aspects of emotion regulation and two aspects of cognition were included. Inclusion of other aspects of these constructs might demonstrate different relationships with changes in DD. Third, the models of change reviewed in this report were based on two interventions to reduce high DD, as part of T1-REDEEM, allowing for examination of a dynamic set of relationships under a period of dramatic change. Even though we find minimal between-group effects, other types of interventions may foster different relationships among changes in DD, emotion regulation and cognition.
5. Conclusion
Although linkages among DD, emotion regulation and cognition have been documented in numerous studies, efforts to articulate the mechanisms of change in ways that will enhance interventions have been limited. Our findings indicate that interventions to reduce high DD among adults with T1D should focus on specific aspects of both emotion regulation and cognition and that changes in these targets drive changes in DD. We also find that changes in emotion regulation and cognition operate together to drive reductions in DD, and that, likewise, reductions in different areas of DD occur together. In combination, these findings provide a template for developing effective interventions to reduce DD among adults with T1D.
Practice implications
DD is a pressing clinical concern: it is common and persistent, and it is linked to disease management, glycemic control and quality of life. Unfortunately, there are few guidelines to suggest how to develop effective interventions. In a previous paper we suggested that DD serves as a barrier, making patients with T1D less responsive to education and other interventions to improve specific self-management behaviors [11]. Emotional distress narrows perspective, reduces energy and bandwidth, and limits effective problem solving, creating a stagnant pattern that often locks patients into repeating the same closed strategies that produced the distress in the first place [31]. Our findings suggest that simultaneously addressing both the emotion management mechanisms that underlie the distress and the currently operational problem-solving strategies that continue to be ineffective are important targets for distress-reduction interventions. In both individual and group-focused programs, specific exercises, scenarios and discussions might focus, for example, on: labeling feelings and identifying beliefs (e.g., hopelessness, frustration, self-blame) that often go unrecognized, summarizing and reflecting the discussion back to patients to enhance awareness, normalizing the diabetes experience (e.g., reduce isolation, “Most people with T1D feel the same way”), illustrating how DD limits their actions (e.g., fatalism: “No matter what I do, it won’t matter anyway”), identifying different strategies to address a specific management problem (e.g., why morning blood glucose levels are high), enhancing information acquisition (e.g., explore new technology to assist management), and learning how to deal better with the health-care system. These examples focus on the emotional and cognitive sides of diabetes and address ways to reduce the impact of DD on management and quality of life among adults with T1D.
Highlights.
Interventions to reduce distress should target emotion regulation and cognition.
Improvements in emotion regulation and cognitive skills occur together.
Improvements in different domains of diabetes distress occur together.
These findings provide a template for developing effective interventions.
Acknowledgements
We greatfully acknowledge the assistance of the following site collaborators: Andrew Almanns MD, Marina Basina MD, Ian Blumer MD, Charles Chioe MD, Sara Kim MD, Ann Peters MD, Karen Weiss MD, and Patricia Wu MD. We also acknowledge the contributions of our project associates: Meredithy Craven, Britnee Ochabski, and Hannah Martin.
Funding sources
This work was supported by the National Institutes of Health RO1 DK094863.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Delahanty LM, Grant RW, Wittenberg E, Bosch JL, Wexler DJ, Cagliero E, Meigs JB, Association of diabetes-related emotional distress with diabetes treatment in primary care patients with type 2 diabetes., Diabetic Medicine 24 (2007) 48–54. [DOI] [PubMed] [Google Scholar]
- [2].Gonzalez J, Shreck E, Psaros C, Safren S, Distress and type 2 diabetes-treatment adherence: a mediating role for peerceived control., Health Psychology 34 (2015) 505–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Fisher L, Glasgow RE, Stryker LA, The relationship between diabetes distress and clinical depression among patients with type 2 diabetes., Diabetes Care 33 (2010) 1034–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fisher L, Hessler DH, Masharani U, Stryker LA, The impact of baseline patient characteristics on interventions to reduce diabetes distress: the role of personal conscientiousness and diabetes self-efficacy., Diabetic Medicine 31 (2014) 739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Snoek FJ, Bremmer MA, Hermanns N, Constructs of depression and distress in diabetes: time for an appraisal., Lancet Diabetes Endocrinology 858 (2015) 135–137. [DOI] [PubMed] [Google Scholar]
- [6].Perrin N, Davies M, Robertson N, Snoek FJ, Khunti K, The prevalence of diabetes-specific emotional distress in people with Type 2 diabetes: a systematic review and meta-analysis., Diabetic Medicine 34 (2017) 1508–1520. [DOI] [PubMed] [Google Scholar]
- [7].Fisher L, Hessler DH, Polonsky W, Strycker L, Masharani U, Diabetes distress in adults with type 1 diabetes: prevalence, incidence and change over time., J Diabetes Complications 30(1123–1128) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sturt J, Dennick K, Hessler DH, Hunter BM, Oliver J, Fisher L, Effective interventions for redusing diabetes distress: systematic review and meta-analysis., Int Diabetes Nursing 12 (2016) 40–55. [Google Scholar]
- [9].Schmidt CB, van Loon BJ, Vergouwen AC, Snoek FJ, Honig A, Systematic review and meta-analysis of psychological interventions in people with diabetes and elevated diabetes-distress., Diabetic Medicine 35(1157–1172) (2018). [DOI] [PubMed] [Google Scholar]
- [10].Fisher L, Hessler DH, Glasgow RE, Arean PA, Masharani U, Naranjo D, Stryker LA, REDEEM: a practical trial to reduce diabetes distress., Diabetes Care 36 (2014) 2551–2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fisher L, Hessler DH, Polonsky W, Masharani U, Guzman S, Bowyer V, Stryker L, Ahmann A, Basina M, Blumer I, Chloe C, Kim S, Peters A, Shumway M, Weihs K, Wu P, T1-REDEEM: A randomized controlled trial to reduce diabetes distress among adults with type 1 diabetes., Diabetes Care 41 (2018) 1862–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Fisher L, Hessler DH, Polonsky W, Guzman S, Bowyer V, Blumer I, Masharani U, Emotion regulation contributes to the development of diabetes distress among adults with type 1 diabetes., Patient Educ Counsel 101 (2018) 124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Leventhal H, Brissette I, Leventhasl EA, The common-sense model of self-regulation of health and illness, in: Cameron LD, Levelthal H (Eds.), The self-regulation of health and illness behavior, Routledge Press, New York, NY, 2003, pp. 42–65. [Google Scholar]
- [14].Moulton CD, Piuckupo JC, Ismail K, The link between depression and diabetes: the search for shared mechanisms., Lancet Diabetes and Endo 3(461–471) (2015). [DOI] [PubMed] [Google Scholar]
- [15].Joorman J, Stanton CH, Examining emotion regulation in depression: a review and future directions., Behav Res Ther 86 (2016) 35–49. [DOI] [PubMed] [Google Scholar]
- [16].Leventhal H, Nerenz DR, The assessment of illness cognition, in: Karoly P (Ed.), Measurement strategies in health psychology, John Wiley & Sons, New York, 1985, pp. 517–554. [Google Scholar]
- [17].Fisher L, Polonsky WH, Hessler DH, Masharani U, Blumer I, Peters AL, Strycker LA, Bowyer V, Understanding the sources of diabetes distress in adults with type 1 diabetes., J Diabetes Complications 29 (2015) 572–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Folkman S, Positive psychological states and coping with severe stress., Soc Sci Med 45 (1997) 1207–1221. [DOI] [PubMed] [Google Scholar]
- [19].Hudson JL, Bundy C, Coventry PA, Dickens CO, G., Exploring the relationship between cognitive illness representations and poor emotional health and their combined association with diabetes self-care. a systematic review with meta-analysis., J Psychosom Res 76 (2014) 265–274. [DOI] [PubMed] [Google Scholar]
- [20].Nigg JT, On the relations among self-regulation, self-control, executive functioning, effortful control, cognitive control, impulsivity, risk taking and inhibition for developmental psychopathology., J Child Psychol Psychiat 58 (2016) 361–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sagui SJ, Levens M, Cognitive reappraisal ability buffers against the indirect effects of perceived stress reactivity on type 2 diabetes., Health Psychology 35 (2016) 1154–1158. [DOI] [PubMed] [Google Scholar]
- [22].Wiebe DJ, Berg CA, Mello D, Kelly CS, Self- and social-regulation in type 1 diabetes management during late adolescence and emerging adulthood., Current Diabetes Reports 18 (2018) 892–918. [DOI] [PubMed] [Google Scholar]
- [23].Friis AM, Johnson MH, Cutfield RG, Consedine NS, Kindness matters: a randomized controlled trial of a mindful self-compassion intervention improves depression, distress and HbA1C among patients with diabetes., Diabetes Care 39 (2016) 1963–1971. [DOI] [PubMed] [Google Scholar]
- [24].Stanton AL, Kirk SB, Cameron CL, Danoff-Burg S, Coping through emotional approach: scale construction and validation., J Personality Soc Psychol 78 (2000) 1150–1169. [DOI] [PubMed] [Google Scholar]
- [25].Baer RA, Smith GT, Lykins E, Button D, Kreitemeyer J, Sauer S, Walsh E, Duggan D, Williams JM, Construct validity of the five facet mindfulness questionnaire in meditating and nonmeditating samples., Assessment 15 (2008) 329–342. [DOI] [PubMed] [Google Scholar]
- [26].Moss-Morris R, Weinman J, Petrie KJ, Horne R, Cameron LD, Buick D, The revised illness perception questionnaire (IPQ-R), Psychol and Health 17 (2002) 1–16. [Google Scholar]
- [27].Hill-Briggs F, Yeh HC, Gary TL, Batts-Turner M, D’Zurilla T, Brancati F.l., Diabetes Problem-Solving Scale development in an adult, African-American sample., Diabetes Educ 33 (2007) 291–299. [DOI] [PubMed] [Google Scholar]
- [28].Hu L, Bentler PM, Cutroff criteria for fit indices in covariance structure analysis: conventional criteria versus new alternatives., Structural Equation Modeling: A Multidisciplinary Journal 6 (1999) 1–55. [Google Scholar]
- [29].Cangur S, Ercan I, Comparison of model fit indices used in structural equation modeling under multivariate normality., J Modern Appl Stat Methods 14 (2015) 35–80. [Google Scholar]
- [30].Schermelleh-Engel K, Moosbrugger H, Muller H, Evaluating the fit of structural equation models: tests of significance and descriptive goodness-of-fit measures., Methods of Psychol Research Online 8 (2003) 23–74. [Google Scholar]
- [31].Nezu A, Nezu R, Social problem solving as a moderator of stress-related depression symptoms: A prospective analysis., J Couns Psychol 35 (1988) 134–138. [Google Scholar]