Abstract
The sulfur (VI) fluoride exchange (SuFEx) is a new family of click chemistry reactions that relies on readily available sulfuryl fluoride (SO2F2) and ethenesulfonyl fluoride (ESF) to build diverse chemical structures bearing the SVI-F motif, such as -OSO2F (fluorosulfate) and -SO2F (sulfonyl fluoride). These motifs could be useful functional groups and connective linkers in organic synthesis.[] This article describes two protocols for performing SuFEx. The first protocol describes an in situ method for rapid generation of arylfluorosulfates in 96-well plates for high-throughput screening (HTS). The second protocol outlines a shelf-stable fluorosulfuryl imidazolium salt for generating arylfluorosulfates and sulfamoyl fluorides.
Keywords: SuFEx, click chemistry, fluorosulfurylation, arylfluorosulfate, sulfamonyl fluoride
INTRODUCTION
The synthesis of arylfluorosulfates was first reported in the early 20th century, but the values of this class of molecules were barely recognized until the recent introduction of the concept of the sulfur (VI) fluoride exchange (SuFEx) as a new class of click chemistry (Dong et al., 2014). SuFEx click chemistry has presented a set of functional groups, e.g. sulfuryl fluoride, iminosulfur fluoride and arylfluorosulfate, reactive with variety of molecules as robust connective linkers in various chemical synthesis.
Arylfluorosulfates are very stable in aqueous solutions near neutral conditions even at 100 °C but may become reactive with nucleophilic amino acid side chain under certain conditions. We believe that this enhanced reactivity is contingent on the following factors: (1) an oil-water interface-like corridor in/around the transition state complex that stabilizes the exit route for fluoride; (2) the presence of one or more side chains in the binding site that can stabilize the departing fluoride ion, such as the positively charged side chains of Lys [-NH3]+, Arg [-(C=NH2)-NH2]+, and His [-imidazole-H]+ or the carboxylic acid side chains of Asp and Glu; (Brik et al., 2003) and (3) a proximal and properly oriented nucleophilic amino acid side chain in the binding site. Owing to these unique features, the FSO2-O motif may serve as a surrogate for F3C-O providing that it does not undergo any covalent reactions with biomolecules in vivo. When it does react, a covalent inhibitor or activator may be formed to modulate the biological functions of the specific protein targets in vivo. In sum, arylfluorosulfate would be a valuable vault for exploring novel bioactive molecules with strong therapeutic potency.
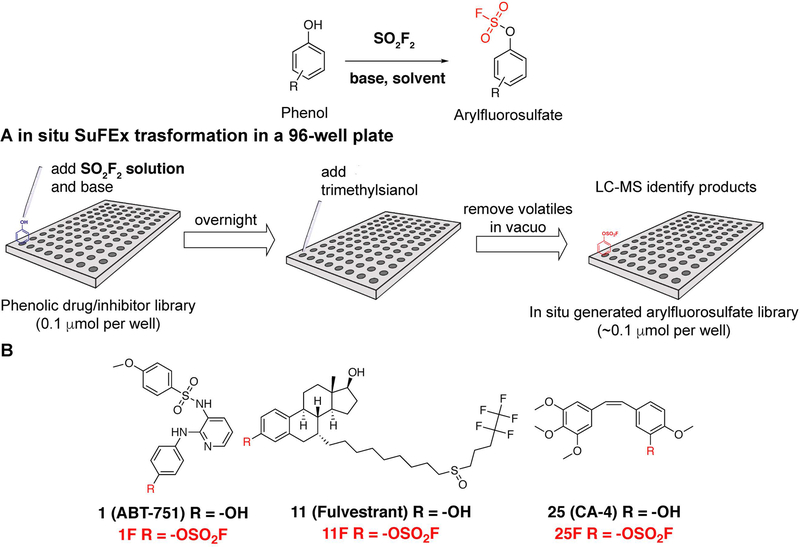
The transformation of phenols into arylfluorosulfates is normally conducted at the gas-liquid interface by subjecting phenol compounds dissolved in organic solvents to the SO2F2 gas in a sealed reaction vessel in the presence of an organic base. This procedure works well for high concentration (>100 mM) reactions but is difficult to perform in a high throughput fashion. This article outlines two protocols of arylfluorosulfate synthesis that are compatible with automated synthesis and high throughput screening to quickly assess the biological activities of the in situ generated, crude products. The first protocol below describes the in situ transformation of a phenol library into the corresponding arylfluorosulfate library using a saturated solution of SO2F2 in CH3CN (Figure 1).
Figure 1.
(A) Fluorosulfurylation of phenols using saturated SO2F2 solution in a 96-well plate. (B) Fluorosulfurylation of phenols, secondary amines and primary amines with the crystalline salt 1-(Fluorosulfuryl)-2,3-dimethyl-1H-imidazol-3-ium trifluoromethanesulfonate. Arylfluorosulfates, sulfamoyl fluorides and NH-sulfamoyl fluorides (or bis(fluorosulfuryl)imide, with different reaction conditions) are obtained respectively.
Despite the ideal reactivity and selectivity of SO2F2 towards phenols, sluggish or even unsuccessful reactions have been encountered in the reactions between SO2F2 and secondary or primary amines. Furthermore, due to limited availability of gaseous SO2F2 to research laboratories, a solid surrogate to SO2F2 would be of great value. Recently the preparation of a shelf-stable fluorosulfuryl imidazolium triflate salt was developed as a surrogate to SO2F2 (Dong et al., Patent CN107857730A, March 30, 2018). In addition to its reaction towards phenols, the salt was found to possess unprecedented reactivity in the fluorosulfurylation of primary and secondary amines (Guo et al., 2018). In the second protocol, we describe the use of this shelf-stable fluorosulfurylating reagent in the preparation of arylfluorosulfates from phenols and the preparation of different types of sulfamoyl fluorides from secondary amines, primary aliphatic amines, and anilines.
BASIC PROTOCOL 1
IN SITU METHOD FOR RAPIDLY GENERATING ARYLFLUOROSULFATES IN 96-WELL PLATES
This protocol describes the construction of a library of arylfluorosulfates from phenol precursors in micro molar scale in a 96-well plate. A solution of SO2F2 in acetonitrile is used. After the reaction, trimethylsilanol (TMSOH) is added to each well as a fluoride ‘scavenger’ to convert the fluoride byproducts into volatile fluorotrimethylsilane (TMSF) that can be easily removed in vacuo from the reaction system together with other volatile components (i.e. TMSOH and trimethylamine). The crude arylfluorosulfates produced in this transformation can be directly subjected to preliminary biological screenings to compare their bioactivities with the phenol precursors. Once hits with better activities than the phenol precursors are identified, they may be synthesized in larger quantities and purified for further biological assays.
Materials
Sulfuryl Fluoride (SO2F2) gas (Dow AgroSciences™)
Phenolic compound library (10 mM in DMSO, Selleck Chemicals)
Trimethylsilanol (TMSOH, Sigma-Aldrich)
Acetonitrile (CH3CN, Sigma-Aldrich)
Triethylamine (Sigma-Aldrich)
Polypropylene U shape 96-well plate (Corning®, Product NO. 3367)
Solvent resistant sealing mat (Corning® 96 well storage system, Product NO. 3346)
Liquid chromatography–mass spectrometry (LC-MS) was performed on an Agilent 1260 LC/MSD with an Agilent 6120 quadrupole mass spectrometer (electrospray ionization, ES) eluting with 0.05% trifluoroacetic acid in H2O and 0.05% trifluoroacetic acid in acetonitrile.
Preparation of SO2F2 solution in acetonitrile
Add acetonitrile (5 mL) to a 10 mL glass vial containing a magnetic stir bar, then remove air in the vial under reduced pressure for 30 sec. Connect a balloon containing SO2F2 to the glass vial to fill it with the gas. Stir vigorously for 30 min to form the sulfuryl fluoride stock solution with an approximate concentration of 4 mg/mL. The solution can be kept in a tightly sealed glass vial at −20 °C for one day before use.
The approximate concentration of SO2F2 in CH3CN is calculated according to the difference of solvent weight before and after stirring with SO2F2 gas. SO2F2 gas should be handled wearing gloves in fume hood to avoid over-exposure. For the toxicity of SO2F2 gas see https://pubchem.ncbi.nlm.nih.gov/compound/Sulfonyl_fluoride#section=FIFRA-Requirements1.
The in situ SuFEx transformation of arylfluorosulfate library from phenol library
A solution of SO2F2 in acetonitrile (~4 mg/mL, 100 μL) and triethylamine (1 μmol in 10 μL) are added into each well of a polypropylene U shaped 96-well plate containing phenol compounds (0.1 μmol in 10 μL DMSO).
All liquid transferring is done with a pipettor or a multichannel pipettor in fume hood. The process should be done as quick as possible to avoid the decrease in SO2F2 concentration in solvent. Normally 3 to 5 min for one plate does not affect reaction yields.
The plate is left tightly covered by a solvent resistant sealing mat at room temperature for 12 h with slight shaking on a shaker (Lab-line instrument Inc.).
The products and yields are monitored by LC-MS. 2 μL of reaction mixture is drawn from each well and diluted with 18 μL methanol. Then 8 μL of resulting solution is injected into LC-MS. This procedure is compatible with automation LC-MS system.
Trimethylsilanol (2.25 μL (2 μmol) in 10 μL acetonitrile) is added to each well and left for 0.5 h at room temperature before the plate is centrifuged at 400 × g for 3 mins. Then the plate is left within a vaccum dessicator connected with vacuum line overnight to remove all volatiles.
The resulting crude products are dissolved in 10 μL DMSO. The approximate concentration of the product is regarded as 10 mM. The crude products may then be connected to the pipeline of downstream biological evaluations.
BASIC PROTOCOL 2
THE PREPARATION OF ARYLFLUOROSULFATES AND SULFAMOYL FLUORIDES USING A SHELF-STABLE FLUOROSULFURYL IMIDAZOLIUM SALT
This protocol describes the facile preparation of arylfluorosulfates from phenols, and sulfamoyl fluorides from secondary amines, primary amines aliphatic or anilines. Bis(fluorosulfuryl)imide could also be obtained from primary aliphatic amines or anilines with a different procedure as described below.
Materials
1-(Fluorosulfuryl)-2,3-dimethyl-1H-imidazol-3-ium trifluoromethanesulfonate (CAS 2179072-33-2, now available from Sigma-Aldrich, product number 903132; prepared following the procedure described in Guo et al., 2018)
Phenols (Aladdin, TCI, Macklin, Energy, Alfa Aesar, Adamas, Tianlian, Shuya, and Bide)
Amines, including aliphatic primary amines, secondary amines and anilines (Aladdin, TCI, Macklin, Energy, Alfa Aesar, Adamas, Tianlian, Shuya, and Bide)
Triethylamine (Macklin)
Sodium hydroxide (DaHe)
Sodium Carbonate (DaHe)
Acetonitrile (Tianlian)
Dichloromethane (Tianlian)
Thin layer chromatography (TLC) was performed using TLC silica gel plates HSG F254 (Jiangyou) and visualized using UV light, iodine, ninhydrin or potassium permanganate. Silica gel column chromatography was carried out using 300–400 mesh silica gel (Jiangyou).
Analytical LC-MS data were recorded on a Waters ACQUITY UPLC H-Class system with a Waters ACQUITY QDa system operating in the electrospray ionization (ESI) mode eluting with H2O containing 0.1% trifluoroacetic acid and CH3CN. Method: 7000 psi, flow rate = 0.6 mL/min, eluent: t = 0, 95% H2O; t = 0.10, 95% H2O; t = 1.20, 5% H2O; t = 2.00, 5% H2O; t = 2.50, 95% H2O. Total acquisition time = 2.5 min.
GC-MS data were recorded on a Shimadzu GC-2010 Plus system with a Shimadzu GCMS-QP2010 Ultra system operating in the electron impact (EI+) mode.
Fluorosulfurylation of phenols
In a 4-milliliter cylindrical glass vial, stir a mixture of triethylamine (1.6 mmol) and the phenol (1.0 mmol) in acetonitrile (1 mL) at room temperature for 10 min.
In cases where multiple phenolic -OH functionalities are present in the substrate, the amount of the substrate used contains a total of 1.0 mmol of phenolic -OH.
To this stirring mixture, add a solution of 1-(Fluorosulfuryl)-2,3-dimethyl-1H-imidazol-3-ium trifluoromethanesulfonate (1.3 mmol) in acetonitrile (1 mL), and continue stirring at room temperature for 1 h, while monitoring by TLC.
After completion, purification by silica gel chromatography affords the pure arylfluorosulfate product. A number of example reaction products can be seen in Figure 2A.
Figure 2.
(A) Twelve phenols for in situ SuFEx transformation. (B) Removal of fluoride ions in situ after reaction. Adapted from J. Am. Chem. Soc. 140, 8, 2919-2925. Copyright American Chemical Society.
Fluorosulfurylation of secondary amines
Add the amine (1.0 mmol) to acetonitrile or dichloromethane (5 mL), in a 4-milliliter cylindrical glass vial.
In cases where multiple secondary amino NH functionalities are present in the substrate, the amount of the substrate used contains a total of 1.0 mmol of secondary amino NH.
Add 1-(fluorosulfuryl)-2,3-dimethyl-1H-imidazol-3-ium trifluoromethanesulfonate (1.0 mmol).
In cases where the amine substrates used are in the form of hydrochloride salts, add triethylamine (1.0 mmol) drop-wise to the stirred mixture following the addition of the 1-(fluorosulfuryl)-2,3-dimethyl-1H-imidazol-3-ium trifluoromethanesulfonate.
Stir the mixture at room temperature for 0.5–2 h, with the reaction monitored by TLC, LC-MS or GC-MS.
After completion, the product is purified by either water/organic solvent extraction or by silica gel chromatography (for details of the purification methods, see Guo et al., 2018). A number of example reaction products can be seen in Figure 2B.
Fluorosulfurylation of aliphatic primary amines
Add the amine (1.0 mmol) in acetonitrile or dichloromethane (3 mL) in a 4-milliliter cylindrical glass vial.
For amines as hydrochloride salt, the amine -NH2 is freed by stirring this mixture with Na2CO3 (2.0 mmol) for 16 h at room temperature, followed by filtration to remove the insoluble solid.
In cases where multiple primary amino -NH2 functionalities are present in the substrate, the amount of the substrate used contains a total of 1.0 mmol of primary amino -NH2.
Cool the filtrate containing free based amine solution from step 8 to 0 °C and add 1-(Fluorosulfuryl)-2,3-dimethyl-1H-imidazol-3-ium trifluoromethanesulfonate (1.0 mmol).
Warm the mixture to room temperature and stir for 0.2–4 h, with the reaction monitored by TLC, LC-MS or GC-MS.
After completion, the product is purified by water/organic solvent extraction or by silica gel chromatography (for details of the purification methods, see Guo et al., 2018). A number of example reaction products can be seen in Figure 2C.
Fluorosulfurylation of anilines
In a 20-milliliter cylindrical glass vial, add the aniline (1.0 mmol) to a suspension of 1-(Fluorosulfuryl)-2,3-dimethyl-1H-imidazole-3-ium trifluoromethanesulfonate (1.1 mmol) in dichloromethane (10 mL) at 0 °C.
In cases where multiple aniline -NH2 functionalities are present in the substrate, the amount of the substrate used contains a total of 1.0 mmol of aniline -NH2.
Warm the mixture to room temperature and stir for 4 h, with the reaction monitored by TLC, LC-MS or GC-MS.
After completion, the product is purified by water/organic solvent extraction or by silica gel chromatography (for details of the purification methods, see Guo et al., 2018). A number of example reaction products can be seen in Figure 2C.
Bisfluorosulfurylation of primary amines
In a 20-milliliter cylindrical glass vial, add 1-(fluorosulfuryl)-2,3-dimethyl-1H-imidazol-3-ium trifluoromethanesulfonate (2.5 mmol) to a solution of the amine (1.0 mmol) in acetonitrile (5 mL) at 0 °C. Stir the mixture at 0 °C for 10 min.
In cases where multiple amino -NH2 functionalities are present in the substrate, the amount of the substrate used contains a total of 1.0 mmol of amino -NH2.
To this stirring mixture, add triethylamine (0.5 equiv. to the amine) drop-wise at 0 °C. Warm the mixture to room temperature and stir for 1 h, while monitoring by TLC and LC-MS.
The product RN(SO2F)2 is purified by silica gel chromatography. A number of example reaction products can be seen in Figure 2D.
REAGENT AND SOLUTIONS
For Basic Protocol 1, use deionized, distilled water in all recipes and protocol steps.
For Basic Protocol 2, it is advised to store 1-(Fluorosulfuryl)-2,3-dimethyl-1H-imidazol-3-ium trifluoromethanesulfonate under a dry, cool condition.
COMMENTARY
Background Information
The synthesis of fluorosulfates was first reported at the beginning of the 20th century. Early efforts include using ClSO2F + SOF4 or SO2F2 as fluorosulfurylating reagents at high temperatures (Cramer et al., 1961) and the substitution of chlorosulfates (ROSO2Cl) with KF (Penney et al., 1981). Both procedures gave poor yields. Alternatively, the reaction of SO2F2 with oxygen nucleophiles in the presence of base to form fluorosulfates has long been known but attracted little attention. Inspired by these precedents and their recent new findings, Sharpless and coworkers introduced the concept of SuFEx, in which the synthesis of arylfluorosulfates from phenols in the presence of gaseous SO2F2 and an organic base serves a core transformation (Dong et al., 2014). Since then, the applications of SuFEx to polymer synthesis and chemical biology have been increasingly explored. However, there are notable limitations in certain scenarios.
First, the bi-phase based arylfluorosulfate synthesis is difficult to perform in a high-throughput fashion (Liu et al., 2018). Second, SO2F2 is a toxic gas that is strictly regulated. Therefore, it is not accessible to many laboratories that are interested in using it. Third, compared with its high reactivity and selectivity towards phenols, SO2F2 reacts sluggishly with secondary amines (Dong et al., 2014). Furthermore, the reaction between SO2F2 and primary amines fails to give the corresponding NH-sulfamoyl fluorides due to the facile elimination of HF under the basic reaction conditions (Edwards, D. R., Wolfenden, R., 2012). For these reasons, the high-throughput compatible arylfluorosulfate synthesis and a shelf-stable reagent that enables efficient preparation of R1R2N-SO2F or even RNH-SO2F described here are welcome advances for the field.
Critical Parameters
Basic Protocol 1
LC-MS is used to determine the formation and yields of arylfluorosulfate products. This method can only indicate the number of fluorosulfates formed, but not their location on the products. In addition, -SO2F could also be installed to secondary amines in certain cases. Therefore, the structural confirmation of arylfluorosulfate products needs to be performed by NMR analysis after the preliminary biological screening as described in Basic Protocol 1.
Fluoride containing byproducts formed in the reaction may have a synergistic or detrimental effect on arylfluorosulfates in the downstream biological assays. Excessive amount of trimethylsilanol is used to trap fluoride generated in the reaction for at least 0.5 h. The subsequent in vacuo treatment should ensure the removal of all the volatiles. 19F NMR can be used to detect the presence of untrapped fluoride.
Trace amount of triethylamine has no effect in various biological assays, but it should be considered if abnormal biological results are encountered. In addition, small amount of phenolic starting material is expected in each well considering not all reactions have quantitively yields. As a result, identified hits from the down-stream biological assays are recommended to synthesized and purified to perform assay again to confirm the results.
Heating is not recommended since high temperature will lower the concentration of SO2F2 in organic solvents.
Basic Protocol 2
It is advised that the reactions between primary amines (including aliphatic and aromatic) and 1-(Fluorosulfuryl)-2,3-dimethyl-1H-imidazol-3-ium trifluoromethanesulfonate start from 0 °C and then warm up to room temperature. More impurities may be formed when the reaction is started directly at room temperature.
It is recommended that only distilled water and brine are used as the aqueous phase during the purification of the NH-sulfamoyl fluorides (product prepared from primary aliphatic amines or anilines) by extraction. Lower isolated yield may be encountered when basic or acidic aqueous phase is used, as well as commonly used aqueous buffer solutions at neutral pH.
The isolated NH-sulfamoyl fluorides (prepared from primary aliphatic amines or anilines) should be stored in polypropylene containers under dry atmosphere. Some NH-sulfamoyl fluorides, especially when they are liquid, have been observed to decompose more rapidly when stored in glass containers.
Troubleshooting
Anticipated Results
Expected results for Basic Protocol 1 can be seen in Figure 2. Twelve phenols 1 – 12 were subjected to the in situ SuFEx transformation in a 96-well plate as described above. Good to quantitative yields of arylfluorosulfates were obtained. Molecules bearing multiple phenols can be fully transformed to the corresponding multi-arylfluorosulfates except in the case of phenols with a γ-carbonyl moiety due to their lower reactivity (compound 2 and 5). In Figure 2B, 19F NMR revealed that the anionic fluorine in the crude product 3F was removed by adding TMSOH followed by in vacuo treatment.
Expected results for Basic Protocol 2 are listed below in Figure 3. The products were obtained in good to excellent yields.
Figure 3.
Fluorosulfurylation products prepared with 1-(Fluorosulfuryl)-2,3-dimethyl-1H-imidazol-3-iumtrifluoromethanesulfonate at mmol-scale. The isolated yields were recorded. The red -SO2F in each molecular structure indicates the newly added fluorosulfuryl group via the reaction. (A) Fluorosulfates prepared from their corresponding phenols. (B) Sulfamoyl fluorides prepared from secondary amines. (C) NH-Sulfamoyl fluorides prepared from primary amines, including aliphatic amines and anilines. (D) Bis(fluorosulfuryl)imides prepared from primary aliphatic amines and anilines.
Time Considerations
Basic Protocol 1: The whole procedure in the 96-well plate can be completed in two days. The preparation of the SO2F2 solution requires at least 30 min. Overnight reaction (12 h) is sufficient to afford full conversion. The time of LC-MS analysis depends on the type of machine. Normally 2 hours is enough for one 96-well plate. Then it is followed by 30 min TMSOH treatment and overnight in vacuo treatment (12 h) for the completion of the whole process.
Basic Protocol 2: The whole procedure, including the reaction and the subsequent purification, takes no more than 6 h to complete for each phenol or amine, except for primary amine hydrochloride salts which takes up to 2 days.
Table 1.
Troubleshooting Commonly Encountered Problems
| Problem | Possible cause | Solution |
|---|---|---|
| Basic Protocol 1 | ||
| No reaction or low yield |
|
|
| Cannot find either phenol reactant or product | not detectable by LC-MS | Use NMR, including 19F NMR, to analyze product |
| Abnormal results in the downstream biological assays | Base reactant or fluoride byproduct are not completely removed before assays |
|
| Basic Protocol 2 | ||
| Signal for the product not found in the 19F NMR spectrum | The signal of the product is outside the chemical shift range of the NMR spectrum | The δF of ROSO2F, RNHSO2F or R1R2NSO2F are usually in the range between +30 ppm and +60 ppm. We recommend setting the test range at −100 ~ +100 ppm. |
Significant Statement.
The use of sulfur (VI) fluoride exchange (SuFEx) chemistry in chemical biology has increased significantly, but requires more cost-effective synthetic methods to access SuFEx molecular libraries for biological screenings. This manuscript describes two innovative protocols to conduct SuFEx reactions: (1) the first method of SuFEx chemistry for the conversion of phenolic compounds to their respective arylfluorosulfate derivatives in situ in 96-well plates, and (2) the preparation of arylfluorosulfates and sulfamonyl fluorides using a shelf-stable fluorosulfuryl imidazolium salt instead of the toxic SO2F2 gas employed in the traditional SuFEx method. Both methods are suitable to conduct SuFEx reactions in a cost-effective and environmental-friendly manner and will provide a useful toolbox for researchers to explore the applications of SuFEx chemistry in chemical biology.
Acknowledgements
P.W. and Z.L. contributed to BASIC PROTOCOL 1 and the work is supported by the NIH (R01GM117145 to K. B. Sharpless). J.D., G.M. and T.G. contribute to BASIC PROTOCOL 2 and the work is supported by the National Natural Science Foundation of China (NSFC 21672240), Strategic Priority Research Program of the Chinese Academy of Sciences (XDB200203), Shanghai Pujiang Program (16PJ1410800), and supported by Key Research Program of Frontier Sciences, CAS, Grant No. QYZDB-SSWSLH-028. We thank Professor Sharpless for his critical comments of the draft.
Footnotes
Conflicts of Interest
Basic protocol 1: Li, J.; Liu, Z.; Li, S.; Wu, P.; Sharpless, K. B., High-throughput construction of arylfluorosulfate library, Provisional patent application Serial Number 62/615,328, filed on Jan/9/2018.
Basic protocol 2: Shanghai Institute of Organic Chemistry, CAS, has filed patent applications on the reagent and its applications.
Literature Cited
- Brik A, & Wong CH (2003). HIV-1 protease: mechanism and drug discovery. Organic & Biomolecular Chemistry, 1(1), 5–14. [DOI] [PubMed] [Google Scholar]
- Cramer R, & Coffman D (1961). Notes-New Synthesis of Aryl Fluorides and Aryl Fluorosulfonates from Oxyfluorides of Sulfur. The Journal of Organic Chemistry, 26(10), 4164–4165. doi: 10.1021/jo01068a641 [DOI] [Google Scholar]
- Dong J, Krasnova L, Finn MG, & Sharpless KB (2014). Sulfur(VI) Fluoride Exchange (SuFEx): Another Good Reaction for Click Chemistry. Angewandte Chemie International Edition, 53(36), 9430–9448. doi: 10.1002/anie.201309399 [DOI] [PubMed] [Google Scholar]
- Liu Z, Li J, Li S, Li G, Sharpless KB, & Wu P (2018). SuFEx Click Chemistry Enabled Late-Stage Drug Functionalization. Journal of the American Chemical Society, 140(8), 2919–2925. doi: 10.1021/jacs.7b12788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penney CL, & Perlin AS (1981). A method for the sulfation of sugars, employing a stable, aryl sulfate intermediate. Carbohydrate Research, 93(2), 241–246. doi: 10.1016/S0008-6215(00)80853-8 [DOI] [Google Scholar]
- Guo T, Meng G, Zhan X, Yang Q, Ma T, Xu L, Sharpless KB, Dong J (2018). A New Portal to SuFEx Click Chemistry: A Stable Fluorosulfuryl Imidazolium Salt Emerging as an “F−SO2+” Donor of Unprecedented Reactivity, Selectivity, and Scope. Angewandte Chemie International Edition, 57, 2605–2610. doi: 10.1002/anie.201712429 [DOI] [PubMed] [Google Scholar]
- Dong J, Yang Q, Guo T, Zhan X, Meng G (2017). Fluorine-containing sulfonyl compound as intermediate, preparation method and application thereof. Patent CN107857730A, March 30, 2018.
- Edwards DR, Wolfenden R (2012). Proton-in-Flight Mechanism for the Spontaneous Hydrolysis of N-Methyl O-Phenyl Sulfamate: Implications for the Design of Steroid Sulfatase Inhibitors. Journal of Organic Chemistry, 77, 4450–4453. doi: 10.1021/jo300386u [DOI] [PMC free article] [PubMed] [Google Scholar]