Abstract
Background/Purpose:
Blood type has become an increasingly recognized risk factor for coagulopathy. We explored the association between blood type and hematoma expansion (HE) after intracerebral hemorrhage (ICH).
Methods:
Spontaneous ICH patients prospectively enrolled in an ongoing ICH cohort study at Columbia University Irving Medical Center from 2009 to 2016 were evaluated. Primary ICH patients with admission blood type testing were evaluated for HE differences, defined as > 33% relative HE. The association of blood type with radiographic HE outcomes was assessed using multivariable logistic regression models. The association of blood type and poor clinical outcomes using modified Rankin Scale (mRS 4–6) was additionally explored.
Results:
Of 272 ICH patients with blood type data and neuroimaging available to determine HE, there were 146 (54%) type-O, 82 (30%) type-A, 34 (13%) type-B, and 10 (3%) type-AB patients. No significant baseline demographic, clinical, or radiographic differences were noted between blood types. Type-B blood was associated with more HE compared to other blood types (OR 2.82; 95% CI 1.23–6.45) after adjusting for known covariates of HE (anticoagulant use, time to admission computed tomography scan, and baseline hematoma volume). No associations with blood type and poor 3 month mRS were identified, but these analyses were limited secondary to our smaller cohort.
Conclusions:
There may be differences in HE after ICH in patients with different blood types. Further work is required to replicate these findings and identify the pathophysiologic mechanisms behind coagulopathy between blood types after ICH.
Keywords: Intracerebral hemorrhage, Coagulopathy, ABO blood type, Hematoma expansion
Introduction
Hematoma expansion (HE) is associated with worse outcomes after intracerebral hemorrhage (ICH) [1]. HE prevention is difficult given the heterogeneity of ICH and paucity of risk factors associated with HE. Traditional risk factors for HE include larger hematoma size, anticoagulant use, and time from symptom onset to admission computed tomography (CT) scan [2]. Current paradigms of coagulopathy treatment after ICH focus on rapid correction of medication-related coagulopathy in efforts to improve outcome [3] given the absence of other modifiable risk factors.
Increasing evidence has revealed associations of ABO blood type with cardiovascular disease and thrombosis [4]. These findings have been thought to be driven by higher levels of von Willebrand factor (vWF) and factor VIII (fVIII) in patients with non-O (A, B, AB) blood types [5] making them more prone to thrombosis. However, it is unclear if there are associations of ABO blood type and coagulopathy in diseases of active bleeding. Though vWF deficiency may be associated with HE after ICH [6], it is unclear if ICH patients with type-O blood will be at higher risk of HE as ABO blood type may have a multifaceted role in coagulopathy beyond vWF/fVIII variations in patients with active disease.
Though a prior study failed to show an association of blood type on hospital discharge outcomes after ICH, it is unclear what role blood type has on HE and long-term follow-up [7]. Subsequently, we sought to explore the association of blood type with HE after ICH, in addition to exploring the association of blood type with discharge and 3-month outcome.
Methods
Data were evaluated from an Institutional Review Board approved, prospective cohort of spontaneous ICH patients consecutively admitted to Columbia University Irving Medical Center called the ICH Outcomes Project. Baseline characteristics, medication history, neuroimaging, laboratory results, interventions, and outcomes were analyzed for patients enrolled between 2009 and 2016. Patients under 18 years were excluded. Consent was provided by the patient or family as appropriate. Patients were managed according to American Heart Association guidelines [3] with treatment protocols described previously [8].
Patient Selection
Primary ICH patients with admission blood type testing, baseline and follow-up CT were included. Patients presenting after 24 h from symptom onset were excluded. Patients with known or suspected secondary ICH (ischemic stroke with hemorrhagic transformation, vascular malformation, aneurysm, malignancy), primary intraventricular hemorrhage, and those receiving neurosurgery prior to follow-up CT were also excluded (extraventricular drain [EVD] placement was not excluded) (Fig. 1).
Fig. 1.
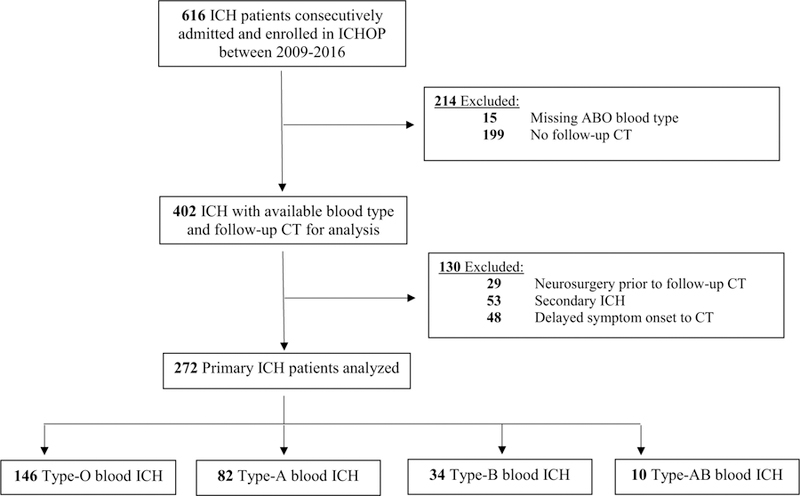
Patient selection and screening. CT computed tomography, ICH intracerebral hemorrhage, ICHOP intracerebral hemorrhage outcomes project,IVH intraventricular hemorrhage
Blood Type
Per clinical protocol, admission ABO blood typing was obtained for all ICH patients admitted when possible in anticipation for hemorrhage reversal transfusion treatment compatibility. Blood type was evaluated both as a dichotomized (O vs. non-O) and categorical variable (type O as reference).
Neuroimaging and Outcome Assessment
Semi-automatic hematoma size measurements (MIPAV: Medical Imaging Processing, Analysis and Visualization software, NIH) were obtained for all CTs using previously described techniques [8, 9]. Symptom onset to admission CT times was recorded. Given the possibility of differing baseline hematoma volumes or ICH location distributions between different blood types, HE was primarily defined using the commonly referenced relative HE threshold: > 33% growth [10]. This was done primarily to avoid limitations that would arise from using absolute HE thresholds (> 6 mL) when comparing groups with different baseline hematoma volumes. Clinical outcomes included hospital mortality and poor modified Rankin Scale (mRS: 4–6) at discharge and 3-month follow-up. Three-month outcomes were obtained via standardized phone interviews by trained research staff with method-logical details described previously [8].
Statistical Analysis
Intergroup differences were determined applying ANOVA or Kruskal–Wallis tests for continuous variables and χ2 for categorical variables. The association of blood type with HE was assessed using adjusted multivariable logistic regression. HE models adjusted for previously identified covariates of HE (anticoagulant use, hematoma volume and time to admission CT) [2] and other intergroup differences thought to affect HE. Exploratory analysis was performed using multivariable logistic regression models to assess the association of blood type with clinical outcomes after adjusting for ICH score [11] and other intergroup differences thought to affect outcome. Statistical significance was judged at p value < 0.05. Analyses were performed using SPSS (ver23).
Results
Of 272 ICH patients meeting inclusion criteria, there were 146 (54%) type-O, 82 (30%) type-A, 34 (13%) type-B, and 10 (3%) type-AB patients. Intergroup differences are shown in Table 1. There were no significant differences in medical interventions (hemorrhage reversal/ hyperosmolar treatments), EVD, do-not-resuscitate or withdrawal-of-care across groups. No differences in admission functional coagulation tests or hematoma volumes were seen across blood groups. There were 21 (8%) patients lost to 3-month follow-up. There were no significant differences in blood groups, baseline demographics, baseline hematoma size, or clinical severity (ICH score or Glasgow Coma Score) between inclusion and exclusion cohorts (Fig. 1). There were expectedly longer times to baseline CT in the exclusion cohort.
Table 1.
Baseline intracerebral hemorrhage characteristics by blood type
| All ICH N = 272 | Type-O N =146 | Type-A N =82 | Type-B N =34 | Type-AB N =10 | p value | |
|---|---|---|---|---|---|---|
| Age: mean (SD) | 66 (16) | 65 (16) | 67 (14) | 66 (15) | 59 (18) | 0.42 |
| Female: N (%) | 125 (46) | 66 (45) | 32 (39) | 21 (62) | 6 (60) | 0.12 |
| Race: N (%) | ||||||
| White | 66 (24) | 37 (25) | 22 (27) | 6 (18) | 1 (10) | 0.05 |
| Black | 79 (29) | 42 (29) | 22 (27) | 10 (29) | 5 (50) | |
| Hispanic | 111 (41) | 60 (41) | 35 (43) | 14 (41) | 2 (20) | |
| Other/unknown | 16 (6) | 7 (5) | 3 (4) | 4 (12) | 2 (20) | |
| Medical history: N (%) | ||||||
| Dyslipidemia | 69 (25) | 34 (23) | 22 (27) | 11 (32) | 2 (20) | 0.69 |
| Coronary artery disease | 39 (14) | 19 (13) | 12 (15) | 7 (21) | 1 (10) | 0.71 |
| Atrial fibrillation | 31 (11) | 17 (12) | 9 (11) | 4 (12) | 1 (10) | 0.99 |
| Hypertension | 217 (80) | 117 (80) | 62 (76) | 29 (85) | 9 (90) | 0.54 |
| Diabetes | 82 (30) | 50 (34) | 23 (28) | 8 (24) | 1 (10) | 0.27 |
| Prior ischemic stroke | 30 (11) | 17 (12) | 8 (10) | 3 (9) | 2 (20) | 0.79 |
| Prior ICH | 15 (6) | 10 (7) | 2 (2) | 3 (9) | 0 (0) | 0.25 |
| Medication history: N (%) | ||||||
| Antiplatelet | 104 (38) | 56 (38) | 33 (40) | 12 (35) | 3 (30) | 0.91 |
| Anticoagulation | 38 (14) | 19 (13) | 13 (16) | 5 (15) | 1 (10) | 0.92 |
| Statin | 55 (20) | 30 (21) | 15 (18) | 7 (21) | 3 (30) | 0.85 |
| Clinical/radiographic | ||||||
| GCS: median (IQR) | 11 (7–15) | 10 (7–14) | 11 (7.5–15) | 10 (6–14) | 14 (4–15) | 0.67 |
| ICH Score: median (IQR) | 2 (1–3) | 2 (1–3) | 2 (1–3) | 2 (1–3) | 2 (1–3) | 0.53 |
| ICH volume (mL): median (IQR) | 13 (4.2–33.8) | 12.3 (4–33) | 14 (4.9–35) | 13.8 (5.2–29) | 16.7 (7.8–69.8) | 0.87 |
| DNR: N (%) | 71 (26) | 43 (29) | 18 (22) | 7 (21) | 3 (30) | 0.55 |
| Deep location: N (%) | 168 (62) | 89 (61) | 49 (60) | 24 (71) | 6 (60) | 0.73 |
| Lobar location: N (%) | 75 (28) | 39 (27) | 26 (32) | 7 (21) | 3 (30) | 0.66 |
| Brainstem location: N (%) | 15 (6) | 9 (6) | 3 (4) | 2 (6) | 1 (10) | 0.79 |
| Infratentorial location: N (%) | 14 (5) | 9 (6) | 4 (5) | 1 (3) | 0 (0) | 0.62 |
| IVH: N (%) | 134 (51) | 70 (50) | 41 (52) | 17 (50) | 6 (60) | 0.94 |
| EVD placement: N (%) | 81 (30) | 48 (33) | 20 (25) | 10 (29) | 3 (30) | 0.65 |
| Time to baseline CT (hours): median (IQR) | 4.9 (1.6–10.7) | 5.5 (1.8–11) | 5.9 (1.4–11.7) | 4.5 (1.5–7.3) | 3.8 (1.8–8.6) | 0.62 |
| Admission SBP: mean (SD) | 187 (40) | 185 (40) | 184 (38) | 193 (40) | 212 (38) | 0.14 |
| Admission DBP: mean (SD) | 101 (25) | 100 (27) | 99 (23) | 103 (23) | 111 (27) | 0.57 |
| Laboratory testing: mean (SD) | ||||||
| Hemoglobin (g/dL) | 13.4 (1.9) | 13.3 (2.0) | 13.4 (1.8) | 13.6 (1.9) | 13.5 (2.1) | 0.89 |
| PT (sec) | 15.3 (5.9) | 15.2 (5.3) | 15.4 (6.6) | 15.6 (7.3) | 15.9 (6.3) | 0.97 |
| PTT (sec) | 30.5 (6.9) | 30.5 (6.6) | 30.6 (7.3) | 30.8 (8.2) | 28.4 (4.9) | 0.88 |
| INR | 1.3 (0.7) | 1.2 (0.6) | 1.3 (0.7) | 1.3 (0.8) | 1.3 (0.7) | 0.96 |
| Platelet count (103/uL) | 218 (71) | 215 (68) | 218 (71) | 229 (88) | 215 (72) | 0.79 |
| Rhesus positive: N (%) | 244 (90) | 128 (88) | 75 (92) | 31 (91) | 10 (100) | 0.37 |
CI confidence interval, DBP diastolic blood pressure, DNR do-not-resuscitate, EVD extraventricular drain, GCS Glasgow coma scale, HE hematoma expansion, ICH intracerebral hemorrhage, INR international normalized ratio, IQR interquartile range, IVH intraventricular hemorrhage, mRS modified Rankin Score, OR odds ratio, PT prothrombin time, PTT partial thromboplastin time, SD standard deviation, SBP systolic blood pressure
There were no associations of dichotomized blood type (O vs. non-O) with HE or clinical outcomes. However, when evaluating blood type categorically, type-B blood was associated with increased odds of HE (adjusted OR 2.82; 95% CI 1.23–6.45; p = 0.01) in multivariable logistic regression models after adjusting for admission hematoma size, anticoagulation medication history and time from symptom onset to admission CT (Table 2). Though higher admission systolic blood pressure in patients with type B was non-significant, sensitivity analysis were performed with this covariate in addition to sex, race, age and ICH location (lobar vs. deep) which did not change our overall result. Because of the low numbers in the AB blood type group and the potential confounding effect of patients with AB blood type having both A and B antigens present, additional HE models were investigated with patients that had AB blood type excluded. This did not lead to a change in the association of type-B blood with HE (adjusted OR 2.84; 95% CI 1.25–6.49; p = 0.01).
Table 2.
Association of ABO blood type with outcomes
| Multivariable-adjusted analysis assessing association of type-O blood (vs. non-O) with hematoma expansion and neurological outcomes | ||||||||
|---|---|---|---|---|---|---|---|---|
| HE > 33%a |
Hospital-mortalityb |
Discharge mRS 4–6b |
3 month mRS 4–6b,c |
|||||
| ABO type | N (%) | OR (95% CI); p value | N (%) | OR (95% CI); p value | N (%) | OR (95% CI); p value | N (%) | OR (95% CI); p value |
| Type-O (n = 146) | 28 (19) | 0.69 (0.38–1.26); p = 0.23 | 30 (21) | 1.34 (0.67–2.67); p = 0.41 | 119 (82) | 1.22 (0.61–2.46); p = 0.58 | 95 (65) | 1.12 (0.59–2.09); p = 0.73 |
| Non-O (n = 126) | 32 (25) | 22 (17) | 100 (79) | 82 (65) | ||||
|
Multivariable adjusted analysis assessing association of ABO blood type (categorical variable) with hematoma expansion and neurological outcomes | ||||||||
| HE > 33%a |
Hospital-mortalityb |
Discharge mRS 4–6b |
3 month mRS 4–6b,c |
|||||
| ABO type | N (%) | OR (95% CI); p value | N (%) | OR (95% CI); p value | N (%) | OR (95% CI); p value | N (%) | OR (95% CI); p value |
| Type-O (n = 146) | 28 (19) | Reference | 30 (21) | Reference | 119 (82) | Reference | 95 (71) | Reference |
| Type-A (n = 82) | 17 (21) | 1.11 (0.56–2.24); p = 0.76 | 11 (13) | 0.49 (0.21–1.17); p = 0.11 | 64 (78) | 0.92 (0.40–2.09); p = 0.92 | 52 (68) | 0.87 (0.42–1.80); p = 0.71 |
| Type-B (n = 34) | 14 (41) | 2.82 (1.23–6.45); p = 0.01 | 8 (24) | 1.39 (0.51–3.78); p = 0.52 | 28 (82) | 0.97 (0.31–3.03); p = 0.96 | 25 (76) | 1.30 (0.47–3.58); p = 0.61 |
| Type-AB (n = 10) | 1 (10) | 0.48 (0.06–4.13); p = 0.50 | 3(30) | 1.23 (0.23–6.52); p = 0.81 | 8 (80) | 0.76 (0.11–5.19); p = 0.78 | 5 (71) | 0.42 (0.04–4.14); p = 0.46 |
CI confidence interval, HE hematoma expansion, ICH intracerebral hemorrhage, OR odds ratio, mRS modified Rankin Scale
Model adjusted for anticoagulant medication history, time from symptom onset to admission CT, admission ICH volume
Model adjusted for ICH score
3 month loss to follow-up was 8% (n = 21); percentages of 3 month mRS 4–6 from 251 patients: 146 type O, 82 type A, 34 type B, 10 type AB
Logistic regression revealed that HE was associated with increased hospital mortality after adjusting for ICH score (adjusted OR 2.41; 95% CI 1.14–5.12; p = 0.02). However, estimations of HE’s association with poor mRS at discharge (adjusted OR 2.55; 95% CI 0.85–7.64; p = 0.09) and 3-month follow-up (adjusted OR 1.83; 95% CI 0.79–4.23; p = 0.16) were imprecise. There were no associations of blood type with mortality or functional outcomes (Table 2); however, these exploratory outcome models were limited secondary to the small, underpowered sample size.
Discussion
In our exploratory analysis, when modeling blood type as a categorical variable, we were able to identify an association of primary ICH patients with type-B blood with an increased odds of HE. While HE is a well-known driver of poor outcome after ICH, exploratory models did not identify a relationship between blood type and clinical outcomes. These findings were largely hindered by our underpowered sample size to explore blood type’s association with outcome, specifically with the small amount of patients with type-B blood in our cohort. However, it is possible that this may also support prior work that did not identify an association of blood type with hospital discharge outcome after ICH [7].
In our ICH cohort, type-O blood was identified most commonly (54%). While blood type O is the most frequent blood type in the USA, our cohort’s type-O predominance was higher than national population data findings (54 vs. 47%). Additionally, type-A blood was less represented in our cohort compared to national data (30 vs. 37%) [12]. Though this requires further study in a population-based cohort, our ICH cohort’s higher proportion of type-O individuals may reflect the initial hemorrhage risk that this blood type confers secondary to lower levels of vWF/fVIII.
However, having type-O blood did not translate to coagulopathy and increased HE after initial hemorrhage. Rather, we found ICH patients with type-B blood to have significant associations with increased odds of HE. There were no differences in baseline characteristics, medication use, or coagulation testing to explain these findings. It is possible that there may be detectable functional coagulation differences not identifiable using traditional plasma-based testing which removes erythrocytes from their cascade-based coagulation assessments.
While these findings are preliminary, exploratory and require replication in a separate cohort, our findings may highlight the inherent limitations of dichotomizing blood group simply as O versus non-O when analyzing ABO blood type effect on ICH. Prior studies evaluating blood type and association with thrombotic events and vWF/ fVIII did so in healthy patients and excluded patients with prior incident events and those with active coagulopathy or bleeding. VWF and fVIII are acute phase reactants and can dynamically change in the face of active physiologic derangement, and may not be the primary driving factors in associations found with blood type in active disease processes.
There is a growing literature that blood type has a multifaceted role in coagulopathy beyond merely vWF/fVIII variations as blood group phenotype may affect endothelial leukocyte interactions and recruitment via adhesions molecules in directions opposite to those found with vWF [13]. These may play a role in platelet–fibrinogen and platelet–vWF interactions in dynamic disease processes. Additionally, erythrocytes themselves may be implicated in hemostasis through their adhesion to the injured vessel wall in addition to its interaction with platelets and fibrinogen leading to blood clot contraction [14]. This may suggest that although vWF levels may be higher in patients with type-B blood placing them at lower risk for initial hemorrhage, once endothelial disruption occurs leading to bleeding, these erythrocytes may fail to adhere to the endothelial wall or activate platelets as well as other blood types leading to worsening HE. However, this is speculative and requires study directly evaluating vWF and fVIII after ICH.
Our findings are speculative and hypothesis generating, but if there are indeed differences in coagulopathy between blood types, this may open opportunities to individualized, tailored coagulopathy treatment approaches in the future. Further investigation is required to validate our findings in addition to elucidating whether blood group phenotype affects dynamic coagulopathy through endothelial recruitment mechanisms, platelet function, erythrocyte function, or vWF/fVIII variations. The use of plasma-based coagulation tests may be limited in providing appropriate assessment of erythrocyte contribution to coagulopathy as these tests remove erythrocytes from testing. Whole blood viscoelastic hemostatic assays may be better equipped to evaluate this in the future. Our study strengths include the prospective collection of data, relative protocolization of ICH treatment limiting clinical heterogeneity, and the multidisciplinary consensus adjudication of ICH characteristics. Inherent limitations were its smaller, single-center cohort, inability to adjust for other potential confounders, exploratory nature of the analysis, use of relative thresholds for HE which may have different impacts on clinical outcome, and the absence of other coagulation-based tests of interest (vWF, fVIII, P-selectin, E-selectin, intracellular adhesion molecule-1).
Conclusion
Further investigation is warranted to confirm our findings of more HE after ICH in patients with type-B blood and investigate potential pathophysiologic mechanisms for blood type influence on coagulopathy in the acute injured state.
Acknowledgments
Source of Support
Dr. Roh is supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number UL1TR001873. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Compliance with Ethical Standards
Conflict of interest
None.
Ethical Approval
The Institutional Review Board approved the study. Written informed consent was obtained from patients, orfamily members when appropriate.
References
- 1.Davis SM, et al. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology 2006;66:1175–81. [DOI] [PubMed] [Google Scholar]
- 2.Brouwers HB, et al. Predicting hematoma expansion after primary intracerebral hemorrhage. JAMA Neurol 2014;71:158–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hemphill JC, et al. Guidelines for the management of spontaneous intracerebral hemorrhage a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2015. 10.1161/str.0000000000000069. [DOI] [PubMed]
- 4.Vasan SK, et al. ABO blood group and risk of thromboembolic and arterial disease: a study of 1.5 million blood donors. Circulation 2016;133:1449–57. [DOI] [PubMed] [Google Scholar]
- 5.Zakai NA, et al. ABO blood type and stroke risk: the reasons for geographic and racial differences in stroke study. J Thromb Haemost JTH 2014;12:564–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Appelboom G, et al. von Willebrand factor genetic variant associated with hematoma expansion after intracerebral hemorrhage. J Stroke Cerebrovasc Dis Off J Natl Stroke Assoc 2013;22:713–7. [DOI] [PubMed] [Google Scholar]
- 7.Dentali F, et al. Role of ABO blood group as a prognostic factor in patients with spontaneous intracerebral hemorrhage. J Thromb Haemost JTH 2013;11:187–9. [DOI] [PubMed] [Google Scholar]
- 8.Witsch J, et al. Intraventricular hemorrhage expansion in patients with spontaneous intracerebral hemorrhage. Neurology 2015;84:989–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Appelboom G, et al. Volume-dependent effect of perihaematomal edema on outcome for spontaneous intracerebral haemorrhages. J Neurol Neurosurg Psychiatry 2013;84:488–93. [DOI] [PubMed] [Google Scholar]
- 10.Dowlatshahi D, et al. Defining hematoma expansion in intracerebral hemorrhage: relationship with patient outcomes. Neurology 2011;76:1238–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hemphill JC, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke J Cereb Circ 2001;32:891–7. [DOI] [PubMed] [Google Scholar]
- 12.Garratty G, Glynn SA, McEntire R, Retrovirus Epidemiology Donor Study. ABO and Rh(D) phenotype frequencies of different racial/ethnic groups in the United States. Transfusion (Paris) 2004;44:703–6. [DOI] [PubMed] [Google Scholar]
- 13.Zhong M, et al. ABO blood group as a model for platelet glycan modification in arterial thrombosis significance. Arterioscler Thromb Vasc Biol 2015;35:1570–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cines DB, et al. Clot contraction: compression of erythrocytes into tightly packed polyhedra and redistribution of platelets and fibrin. Blood 2014;123:1596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]


