Abstract
Background
Wnt and Wnt‐associated pathways play an important role in the genetic etiology of oligodontia, a severe form of tooth agenesis. Loss‐of‐function mutations in LRP6 , encoding a transmembrane cell‐surface protein that functions as a coreceptor in the canonical Wnt/b‐catenin signaling cascade, also contribute to genetic oligodontia.
Methods and results
We describe a three‐generation family with hereditary thrombocytopenia and oligodontia. Genome wide array analysis was performed. The array results from the index patient revealed an interstitial loss of 150 kb in 8p23.1 (chr8:6,270,299–6,422,558; hg19) encompassing MCPH1 and ANGPT2 and an interstitial loss of 290 kb in 12p13.2 (chr12:12,005,720–12,295,290; hg19) encompassing ETV6, BCL2L14 and LRP6.
Conclusion
This case report shows a three‐generation family with hereditary thrombocytopenia and oligodontia with a heterozygous 290 kb novel contiguous gene deletion in band p13.2 of chromosome 12, encompassing LRP6 and ETV6. In this report we discuss the clinical relevance of the deletion of both genes and illustrate the importance of thorough examination of oligodontia patients. Comprising not only the oral status but also the medical history of the patients and their relatives.
Keywords: contiguous gene deletion, ETV6, LRP6, oligodontia, thrombocytopenia
1. INTRODUCTION
Tooth agenesis or hypodontia is a developmental anomaly, in which one or more permanent teeth fail to develop. It has a reported prevalence of 5.5% in Europe (Polder, Van't Hof, Van der Linden, Frans PGM, & Kuijpers‐Jagtman, 2004). Common forms affecting one or a few absent teeth represent the great majority of cases. Severe hypodontia or oligodontia is defined as the absence of six or more teeth excluding the third molars. These types are estimated to be present in 0.14% of the Caucasian population, with a higher incidence in women than in men (Polder et al., 2004). Congenital absence of teeth is seen as an isolated trait or as part of a syndrome in case of concurring nondental anomalies, and is caused by both (epi‐) genetic as well as environmental factors (van der Weide, Yvonne Schalk, Prahl‐Andersen, & Bosman, 1993).
Over the last few decades, an increasing number of genes involved in embryo‐ and tooth‐ development have been associated with non‐syndromic tooth agenesis (Yu, Wong, Han, & Cai, 2018). Most causal genes encode for components of three interacting signaling pathways (Wnt/β‐catenin, the TGF‐β/BMP and the Eda/Edar/NF‐κB pathways) contributing to a complex development signaling network orchestrating tooth morphogenesis. Mutations in only seven genes (i.e. AXIN2 [MIM: 604,025], EDA [MIM: 300,451], MSX1 [MIM: 142,983], PAX9 [MIM: 167,416], WNT10A [MIM: 606,268], WNT10B [MIM: 601,906], and LRP6 [MIM: 603,507]) are responsible for the majority of cases with non‐syndromic tooth agenesis (Yu et al., 2018).
LRP6 (low density lipoprotein receptor related protein 6) encodes a transmembrane cell‐surface protein. It functions as a WNT coreceptor with members from the Frizzled protein family in the canonical Wnt/β‐catenin signaling cascade. Heterozygous loss‐of‐function mutations in LRP6 were found to cause oligodontia in three patients (Massink et al., 2015).
ETV6 (MIM: 600,618) encodes an ETS family transcription factor, which binds DNA via a highly conserved C‐terminal DNA‐binding domain. ETV6 is known to be of interest in hematopoiesis and embryonic development and of major importance in regulating megakaryocytes and platelets (Hock & Shimamura, 2017) Zang et al reported on three families with dominantly inherited thrombocytopenia and a predisposition for hematological malignancies caused by heterozygous germline ETV6 mutations (Zhang et al., 2015). Additional studies confirm these findings (Duployez et al., 2018; Melazzini et al., 2016; Moriyama et al., 2015; Noetzli et al., 2015; Paulsson et al., 2010; Poggi et al., 2017; Topka et al., 2015). To date, more than 80 germline ETV6 mutation carriers from 22 families and one pedigree with an intragenic deletion of ETV6 are reported. (Duployez et al., 2018; Paulsson et al., 2010) Nevertheless, the exact understanding of the clinical impact of ETV6 mutations and the physiological role of ETV6 remains to be elucidated. Most individual germline ETV6 mutations have been identified in single families or patients. It is yet unclear whether different mutations result in different predispositions to develop specific types of malignancy or are associated with different risks of bleeding (Hock & Shimamura, 2017).
In this clinical report we describe two boys, their father and their paternal grandmother with resembling dental phenotypes caused by a 290 kb deletion in band p13.2 of chromosome 12. This deletion contains LRP6 but also ETV6, which could explain the characteristic phenotype described.
2. CLINICAL PRESENTATION
The index patient (proband III‐5, Figure 1a,b) was a 10‐year old boy born at 39 + 6 weeks after a troubled pregnancy with regular cardiotocogram abnormalities. At birth his weight was 3,210 g. There were no major deviations in growth and (cognitive) development. On physical examination he had sparse hair, mild sparseness of the eyebrows, agenesis of various permanent teeth, taurodontia, dysmorphic ears with an under folded helix, and slight underdevelopment of the distal phalanx of the thumbs (Figure 1g,h).
Figure 1.
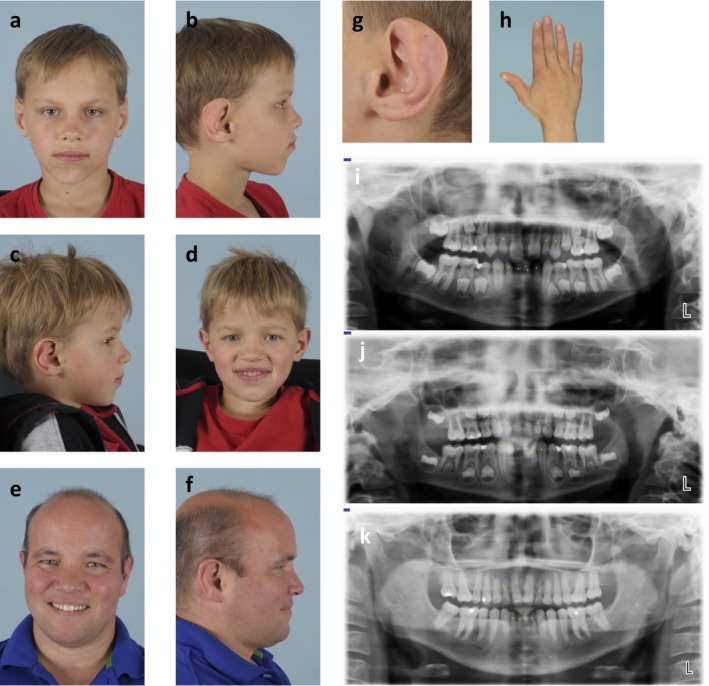
(a) Index patient (proband III‐5). (b) Index patient (proband III‐5). (c) Proband III‐6. (d) Proband III‐6. (e) Proband II‐3. (f) Proband II‐3. (g) Left ear of the index patient, note the mostly absent helix. (h) Slight underdevelopment of the thumb of the index patient. (i) Dental panoramic radiograph of the index patient. Yellow dots indicate deciduous teeth. (j) Dental panoramic radiograph proband III‐6. Yellow dots indicate deciduous teeth. (k) Dental panoramic radiograph proband II‐3. Yellow dots indicate deciduous teeth
Together with his 37‐year old father (proband II‐3, Figure 1e,f), his younger brother (proband III‐6: six years old, Figure 1c,d) and his paternal grandmother (proband I‐2)he consulted a clinical geneticist for a suspicion of hereditary thrombocytopenia. Figure 2a shows the corresponding three‐generation‐pedigree. In addition to the increased bleeding tendency, the index patient, his younger brother and their father had an almost identical tooth agenesis pattern. They all lack the following permanent teeth: 1.2, 1.3, 2.2, 2.3, 3.1, 3.2, 4.1, 4.2 and 4.3. Additionally, the father and his youngest son lack the lower left permanent canine (3.3). Figure 1i‐k show the dental panoramic radiographs. Corresponding Tooth Agenesis Codes (TAC) are 006.006.007.007 (probands II‐3 and III‐6) and 006.006.003.007 (proband III‐5). The TAC is a method based on the binary number system, used to describe patterns of missing teeth. Depending on the number and location of missing teeth, every possible pattern of tooth agenesis has a unique TAC (Van Wijk & Tan, 2006); (Creton, Cune, Verhoeven, & Meijer, 2007). Questions on family history revealed similar dental abnormalities in the paternal grandmother (proband I‐2), however she currently wears a full dental prosthesis and no documentation is available to confirm an oligodontia diagnosis.
Figure 2.
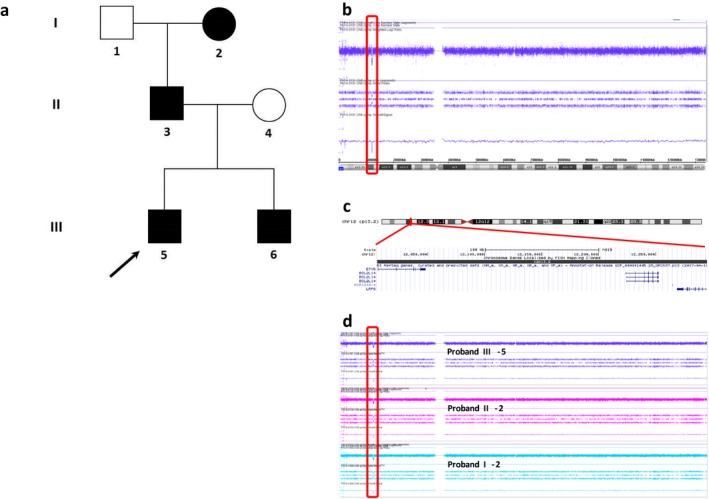
(a) A three‐generation‐pedigree of familial hypodontia and thrombocytopenia with the affected family members indicated by solid black squares (males) or circles (females). The index patient (proband III‐5) is indicated by an arrow. (b) Array plot of chromosome 12 of the index patient with an interstitial deletion of 290 kb in 12p13.2 (red rectangle). (c) Schematic representation of chromosome 12 with the p13.2 region enlarged in the lower part of the figure, showing a screen shot of the UCSC Genome Browser Build 37/hg19 (http://genome.ucsc.edu/). The genes located in the deleted 12p13.2 region are shown below the chromosome bands. (d) Array plot of chromosome 12 of the index patient (proband III‐5), his father (proband II‐3) and the paternal grandmother (proband I‐2), each showing an interstitial deletion of 290 kb in 12p13.2 (red rectangle).
3. MICRO ARRAY ANALYSIS
DNA was extracted from peripheral blood using standard procedures. Genome wide array analysis was performed on DNA using the Affymetrix CytoScan HD array platform (Affymetrix, Inc., Santa Clara, CA, USA) following the manufacturer's protocols. The array results from the index patient (proband III‐5) revealed an interstitial loss of 150 kb in 8p23.1 (chr8:6,270,299–6,422,558; hg19) encompassing MCPH1 (MIM: 607,117, NM_024596.5) and ANGPT2 (MIM: 601,922, NM_001147.2) and an interstitial loss of 290 kb in 12p13.2 (chr12:12,005,720–12,295,290; hg19) encompassing ETV6 (NM_001987.4), BCL2L14 (MIM: 606,126, NM_030766.1) and LRP6 (NM_002336.3). (according to ISCN 2016 nomenclature the genotype of the index patient is: arr[GRCh37] 8p23.1(6270299_6422558)x1,12p13.2(12005720_12295290)x1).
With subsequent carrier testing by array in the parents, both losses were also detected in the similarly affected father. Next, the same loss in 12p13.2 was also detected by array in proband III‐6 and the brothers’ paternal grandmother (proband I‐2), who were both similarly affected (Figure 2b‐d). They did not have a loss in 8p23.1.
4. DISCUSSION
We describe a three‐generation family affected by a deletion in 12p13.2. This deletion encompasses not only part ofLRP6 , but also BCL2L14 and part ofETV6 .ETV6 plays an important role in hematopoiesis and is established as a major intrinsic regulator of megakaryocytes and platelets (Hock & Shimamura, 2017). Individuals with a mutation in this gene have an increased susceptibility to thrombocytopenia, hematologic malignancies and possibly solid neoplasms. All, except one, of the germline ETV6 mutations, cluster within the highly conserved ETS domain responsible for binding to DNA (Hock & Shimamura, 2017). To our knowledge, there is only one pedigree reported with a deletion comprising exon 2 (including the PNT domain) (Paulsson et al., 2010). The deletion found in our proband and affected family members encompasses the last five exons (4, 5, 6, 7, 8) ofETV6 which includes the ETS domain (Exon 6, 7, 8). This is thus likely to explain their reported chronic thrombocytopenia and increased bleeding tendency. The family history does not reveal hematologic malignancies or solid neoplasms.
How germline ETV6 mutations predispose to malignancy remains poorly understood. Although, ETV6 has been identified as a fusion partner in different chromosomal translocation oncogenes and somatic ETV6‐RUNX1 fusions are common in childhood ALL, a somatic ETV6‐RUNX1 fusion is reported in just one case with a germline ETV6 variant (Moriyama et al., 2015). In our family a fusion of ETV6 and LRP6 is not expected, while the transcription of both genes are in opposite direction. However, the well‐defined association of ETV6 germline mutations and hematologic malignancies, would entail a predisposition to malignancies in this family.
The deletion of the last eight exons (exon 16–23) ofLRP6 explains the extensive agenesis of teeth (3). This is the first deletion reported forLRP6 as a cause of hypodontia. Noteworthy is the almost identical pattern of tooth agenesis shown in the father and his two sons. Generally, the presentation of the dentition in oligodontia is heterogeneous, with highly variable numbers and patterns of missing teeth between affected family members (Creton et al., 2007; Dreesen, Swinnen, Devriendt, & Carels, 2013). The strikingly similar pattern of tooth agenesis in this family seems to indicate an important role for genetics in determining the pattern of tooth agenesis.
The Database of Genomic Variants (DGV), which contains genomic variations observed in healthy individuals (http://genome.ucsc.edu and http://projects.tcag.ca/variation/) reports one additional contiguous deletion of LRP6 and ETV6, several separate small deletions of LRP6 and ETV6 and one extended deletion of LRP6. One might hypothesize that in these healthy individuals the associated tooth agenesis and thrombocytopenia are not identified as congenital malformations or the features are subclinical.
Previous reported families with tooth agenesis caused by a LRP6 mutation appeared to be nonsyndromic. Nevertheless, both Massink and Ockeloen independently reported cases showing minor anatomical variations of the ear and underdevelopment of the fingers or thumb (Massink et al., 2015; Ockeloen et al., 2016). In addition to a number of other subtle physical features our proband also displays these minor anatomical variations: dysmorphic ears with a severe under folded helix and slight underdevelopment of the distal phalanx of the thumbs. Paying specific attention to these characteristics, we noticed these minor dysmorphic features also in other patients carrying a LRP6 mutation. This might implicate that the ear and thumb could serve as a detection marker for a deletion or mutation involvingLRP6 . Further research is necessary to support this association.
Apart from parts ofLRP6 and the ETV6 , the deletion also encompassesBCL2L14 . The corresponding protein belongs to the BCL2 family of proteins, which comprises both pro‐ and antiapoptotic regulators of programmed cell death. To our knowledge, so far no disorders have been associated to BCL2L14 mutations or deletions.
In summary, this case report describes a family with oligodontia and thrombocytopenia due to a deletion encompassing bothLRP6 andETV6 illustrating the importance of examining apparent isolated hypodontia patients more extensively. Besides dental examination, thorough consultation should consist of extensive questioning on the medical history of the patient and his or her relatives. Hence, seeing these young patients with tooth agenesis in a multidisciplinary setting and/or referring for genetic counseling can aid in the early recognition of associated disorders. Seen in this light, genetic counseling of individuals with oligodontia should thereby not solely consist of mutation identification in possible causative genes, but also a contiguous gene deletion should be considered.
CONFLICT OF INTEREST
All authors declare that they do not have any conflict of interest.
ACKNOWLEDGMENTS
We would like to thank the family presented here for their contribution to this report.
Ross J, Fennis W, de Leeuw N, et al. Concurrent manifestation of oligodontia and thrombocytopenia caused by a contiguous gene deletion in 12p13.2: A three‐generation clinical report. Mol Genet Genomic Med. 2019;7:e679 10.1002/mgg3.679
REFERENCES
- Creton, M. A. , Cune, M. S. , Verhoeven, J. W. , & Meijer, G. J. (2007). Patterns of missing teeth in a population of oligodontia patients. International Journal of Prosthodontics, 20(4), 409–413. [PubMed] [Google Scholar]
- Dreesen, K. , Swinnen, S. , Devriendt, K. , & Carels, C. (2013). Tooth agenesis patterns and phenotype variation in a cohort of Belgian patients with hypodontia and oligodontia clustered in 79 families with their pedigrees. European Journal of Orthodontics, 36(1), 99–106. 10.1093/ejo/cjt021 [DOI] [PubMed] [Google Scholar]
- Duployez, N. , Abou Chahla, W. , Lejeune, S. , Marceau‐Renaut, A. , Letizia, G. , Boyer, T. , … Nelken, B. (2018). Detection of a new heterozygous germline ETV 6 mutation in a case with hyperdiploid acute lymphoblastic leukemia. European Journal of Haematology, 100(1), 104–107. [DOI] [PubMed] [Google Scholar]
- Hock, H. , & Shimamura, A. (2017). ETV6 in hematopoiesis and leukemia predisposition. Seminars in Hematology, 54(2), 98–104. S0037-1963(17)30046-X [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massink, M. P. G. , Créton, M. A. , Spanevello, F., Fennis, W. M. M. , Cune, M. S. , Savelberg, S. M. C. , … van Haaften, G. (2015). Loss‐of‐function mutations in the WNT co‐receptor LRP6 cause autosomal‐dominant oligodontia. The American Journal of Human Genetics, 97(4), 621–626. 10.1016/j.ajhg.2015.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melazzini, F. , Palombo, F. , Balduini, A. , De Rocco, D. , Marconi, C. , Noris, P. , … Savoia, A. (2016). Clinical and pathogenic features of ETV6‐related thrombocytopenia with predisposition to acute lymphoblastic leukemia. Haematologica, 101(11), 1333–1342. haematol.2016.147496 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama, T. , Metzger, M. L. , Wu, G. , Nishii, R. , Qian, M. , Devidas, M. , … Yang, J. J. (2015). Germline genetic variation in ETV6 and risk of childhood acute lymphoblastic leukaemia: A systematic genetic study. The Lancet Oncology, 16(16), 1659–1666. 10.1016/S1470-2045(15)00369-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noetzli, L. , Lo, R. W. , Lee‐Sherick, A. B. , Callaghan, M. , Noris, P. , Savoia, A. , … Di Paola, J. (2015). Germline mutations in ETV6 are associated with thrombocytopenia, red cell macrocytosis and predisposition to lymphoblastic leukemia. Nature Genetics, 47(5), 535–538. 10.1038/ng.3253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ockeloen, C. W. , Khandelwal, K. D. , Dreesen, K. , Ludwig, K. U. , Sullivan, R. , van Rooij, I. A. L. M. , … Carels, C. E. L. (2016). Novel mutations in LRP6 highlight the role of WNT signaling in tooth agenesis. Genetics in Medicine, 18(11), 1158 10.1038/gim.2016.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsson, K. , Forestier, E. , Lilljebjorn, H. , Heldrup, J. , Behrendtz, M. , Young, B. D. , & Johansson, B. (2010). Genetic landscape of high hyperdiploid childhood acute lymphoblastic leukemia. Proceedings of the National Academy of Sciences of the United States of America, 107(50), 21719–21724. 10.1073/pnas.1006981107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poggi, M. , Canault, M. , Favier, M. , Turro, E. , Saultier, P. , Ghalloussi, D. , … Alessi, M. C. (2017). Germline variants in ETV6 underlie reduced platelet formation, platelet dysfunction and increased levels of circulating CD34+ progenitors. Haematologica, 102(2), 282–294. 10.3324/haematol.2016.147694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polder, B. J. , Van't Hof, M. A. , Van der Linden, F. P. G. M. , & Kuijpers‐Jagtman, A. M. (2004). A meta‐analysis of the prevalence of dental agenesis of permanent teeth. Community Dentistry and Oral Epidemiology, 32(3), 217–226. 10.1111/j.1600-0528.2004.00158.x [DOI] [PubMed] [Google Scholar]
- Topka, S. , Vijai, J. , Walsh, M. F. , Jacobs, L. , Maria, A. , Villano, D. , … Offit, K. (2015). Germline ETV6 mutations confer susceptibility to acute lymphoblastic leukemia and thrombocytopenia. PLoS Genetics, 11(6), e1005262 10.1371/journal.pgen.1005262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Weide, Y. , Schalk, P.‐A. , & B., & Bosman, F., (1993). Tooth formation in patients with oligodontia. The Angle Orthodontist, 63(1), 31–37. [DOI] [PubMed] [Google Scholar]
- Van Wijk, A. J. , & Tan, S. P. (2006). A numeric code for identifying patterns of human tooth agenesis: A new approach. European Journal of Oral Sciences, 114(2), 97–101. 10.1111/j.1600-0722.2006.00340.x [DOI] [PubMed] [Google Scholar]
- Yu, M. , Wong, S. , Han, D. , & Cai, T. (2018). Genetic analysis: Wnt and other pathways in nonsyndromic tooth agenesis. Oral Diseases 25(3),646–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, M. Y. , Churpek, J. E. , Keel, S. B. , Walsh, T. , Lee, M. K. , Loeb, K. R. , … Shimamura, A. (2015). Germline ETV6 mutations in familial thrombocytopenia and hematologic malignancy. Nature Genetics, 47(2), 180–185. 10.1038/ng.3177 [DOI] [PMC free article] [PubMed] [Google Scholar]


