Highlights
-
•
Chronic constriction injury of the infraorbital nerve produced ongoing hyperalgesia and allodynia.
-
•
Pharmacological conditioning with an opioid and contextual cues produce only a trend towards placebo analgesia in this chronic orofacial pain model.
-
•
A natural history group is essential to disentangle real placebo effects from non-specific analgesic responses.
Keywords: Pharmacological conditioning, Expectancy, Orofacial pain, Facial grimace, Allodynia, Hyperalgesia, Conditioning effects, Neuropathic pain, Fentanyl
Abstract
All treatments are given in a context, suggesting that conditioning cues may significantly influence therapeutic outcomes. We tested the hypothesis that context affects placebo analgesia in rodents. To produce neuropathic pain in rats, we performed chronic constriction injury of the infraorbital nerve. We then treated the rats daily, over a seven day period, with injections of either fentanyl or saline, with or without associated conditioning cues; a fourth group received no treatment. On the eighth day, we replaced fentanyl with saline to test for conditioned placebo analgesia.
We tested the effects of treatment by measuring sensitivity to mechanical stimuli and grimace scale scores. We found no significant differences in either of these outcomes among the four experimental groups. These findings suggest that chronic, neuropathic pain in rats may not be susceptible to placebo analgesia.
1. Introduction
Pain is a highly complex sensory and emotional phenomenon that can be substantially modulated by conditioning factors, such as expectation, attention, social factors and external cues (Carlino et al., 2014). Conditioning processes can produce analgesia in a variety of clinical and experimental pain conditions (Fanselow, 1998). Placebo analgesia, the most studied form of the placebo effect, is a prominent example of the phenomenon by which initially innocuous cues can acquire salience to cause a physiologically beneficial effect. Thus, placebo analgesia and related effects have emerged as a potential approach to reduce harmful drug effects by interspersing treatments with placebos (Colloca et al., 2016, Sandler et al., 2010). The characterization of placebo analgesia in a variety of models of chronic pain is essential to an understanding of its neurobiological mechanisms and an assessment of its therapeutic potentials. This approach could be especially relevant in orofacial neuropathic pain, which presents a particularly difficult form of chronic pain to treat (Colloca et al., 2017, Kitt et al., 2000, Koopman et al., 2009, Watson, 2004) and perhaps help mitigate the use of pharmacological treatments with side effects.
Placebo analgesia has primarily been investigated in clinical studies, and the handful of studies conducted in animals have mostly focused on models of acute pain (Keller et al., 2018). These studies have provided some evidence for the occurrence of conditioned placebo analgesia effects in rodents with acute pain. To our knowledge, only two published animal studies have attempted to examine placebo analgesia in chronic pain. McNabb et al (McNabb et al., 2014) conditioned rats with chronic pain after spinal nerve ligation by pairing analgesics with contextual cues for four days. On the fifth day, pairing saline administration with the contextual cues failed to affect pain withdrawal thresholds. The authors postulated that the failure to evoke placebo analgesia may reflect: (i) the severity of their injury model; (ii) inadequate temporal alignment of the conditioning cues to analgesics; (iii) the reliance on only a reflexive measure of tactile sensitivity. More recently, Zeng et al. (Zeng et al., 2018) reported success in producing pharmacologically conditioned placebo analgesia using a spinal nerve ligation model. However, this study did not include appropriate control groups to clearly disentangle placebo effects from non-specific responses that may be due to factors such as the natural history of the disease or regression to the mean. Here, we attempted to address these shortcomings in a rodent model of chronic, neuropathic orofacial pain by incorporating the fast-acting opioid, fentanyl, that may improve the temporal alignment of conditioning. We also monitored metrics of ongoing pain, in addition to reflexive pain measures, and included natural history and fentanyl control groups. Our hypothesis was that placebo affective and sensory effects can be elicited in a chronic, neuropathic orofacial pain model in rodents.
2. Methods
We adhered to accepted standards for rigorous study design and reporting to maximize the reproducibility and translational potential of our findings as described by Landis et al. (2012) and in ARRIVE (Animal Research: Reporting In Vivo Experiments). Keeping in line with NIH recommendations for scientific rigor, we performed an a priori power analysis to estimate required sample sizes (Landis et al., 2012).
2.1. Animals
All procedures were approved by the University of Maryland, Institutional Animal Care and Use Committee. Fifty male Sprague-Dawley rats (Envigo Laboratories, Frederick, MD) were obtained at 8 weeks of age. Rats were housed in a limited-access animal room at the University of Maryland, Baltimore animal facility. All animals were group-housed, with 3 male rats per cage, in polycarbonate cages at room temperature (23 ± 0.5 °C), kept on a 12 h light/dark cycle (lights on from 7:00am to 7:00 pm) and allowed access to standard chow and sterilized drinking water ad-libitum throughout the study. Rats were between the ages of 10–13wks and weighed 200–300 g at the start of the study.
2.2. Experimental approach
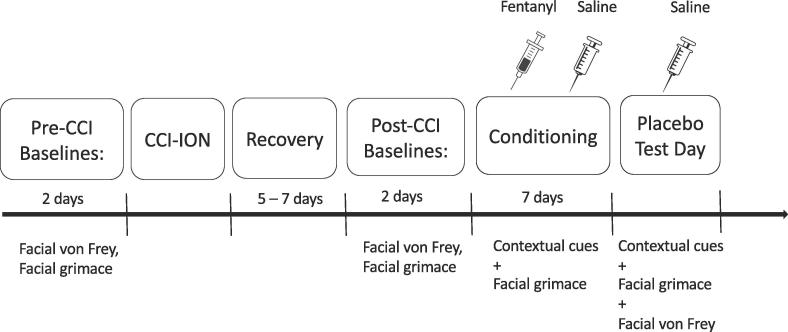
An outline of the time course of the experimental design is depicted in Fig. 1. To reduce anxiety or stress, we handled all animals and acclimatized them to the experimenters and all apparatuses for 3 days before testing. This involved daily, 5 min sessions where we held them gently and stroked their vibrissae pad area for habituation to von Frey filaments. We then placed them in the facial grimace apparatus for a 10-minute habituation period
Fig. 1.
Outline of the experimental paradigm. Following a habituation period, baseline mechanical sensitivity and facial grimace score readings were taken 2 days before injury. Post-CCI, animals were allowed to recover for 5–7 days in their home cage. After the recovery period, scores were recorded again. For conditioning, 10 days post CCI, animals were divided into four groups and treated with fentanyl, saline or no treatment respectively. Grimace scores alone were recorded on each of the 7 conditioning days. On test day (the day after conditioning day 7), scores were recorded from all four groups.
Two days before the surgical injury was induced, we obtained baseline mechanical sensitivity and facial grimace scores. Post-injury, animals recovered for 5–7 days in their home cage and were monitored daily, followed by the 7-day conditioning paradigm, discussed in detail below. During conditioning, we obtained only facial grimace recordings, to avoid the potential stress that repeated tests of mechanical sensitivity could cause. Two trained female experimenters handled and performed all behavioral manipulations on all animals.
2.3. Chronic constriction injury of the infra-orbital nerve
We used an established rodent model of neuropathic pain, evoked by unilateral chronic constriction of the infraorbital nerve (CCI-ION) (Benoist et al., 1999, Castro et al., 2017, Okubo et al., 2013, Vos et al., 1994). On the day of the surgery, all animals were first induced with 2% isoflurane and then injected intraperitoneally (i.p.) with ketamine (100 mg/kg)/xylazine (10 mg/kg). We made an 8 to10 mm long intraoral incision along the buccal vestibule next to left cheek, beginning distal to the first molar. After freeing the infraorbital nerve from surrounding connective tissue, we placed a loose ligature with silk thread (4–0), 1–2 mm from the nerve’s emergence from the infraorbital foramen. We used silk thread, rather than chromic gut as originally described by Benoist et al (Benoist et al., 1999), because silk ligatures demonstrate more stable neuropathic pain behaviors in mouse CCI-ION models (van der Wal et al., 2015).
We monitored the animals daily as they recovered for 5–7 days in their home cage.
2.4. Mechanical sensitivity
We held the rats gently, without restraint, while applying von Frey filaments (North Coast Medical, Gilroy, CA) of varying forces to the buccal region. A response to the filaments was defined as an active withdrawal of the head from the probing filament. We used the up-down method to determine withdrawal thresholds, as described previously (Chaplan et al., 1994, Dixon, 1965).
2.5. Facial grimace test
We placed rats in a Plexiglas chamber (20 × 20 cm) containing home-cage bedding and obtained video recordings of the rats for 20 min. These videos were processed to score the facial expressions, using the semi-automated “Face Finder” application (Sotocinal et al., 2011). Briefly, the application processed each 20-minute video recording to automatically select and collate representative still facial frames. To reduce selection bias, we included only every third image selected by the application, to obtain a total of ten frames for each animal. We scanned all images manually for eligibility, excluding images where the animal was either asleep or grooming. The experimenter, who was blinded to treatment, screened, labelled, scrambled and scored each image. The grimace scale quantifies changes in four “action units”: orbital tightening, nose-cheek bulge, whisker tightening and ear position (Sotocinal et al., 2011). For each selected frame, we assigned to each action unit a rat grimace scale (RGS) score of 0, 1, or 2, as previously described (Akintola et al., 2017, Langford et al., 2010, Sotocinal et al., 2011). Mean grimace scale scores represent the average score across all the action units for each animal.
2.6. Allocation and experimental groups
We randomly assigned animals to experimental groups (Table 1), as described in Kim and Shin (2014). Group 1 (fentanyl/context− group) received fentanyl and no exposure to conditioning cues on all days. Group 2 (fentanyl/placebo/context+) received fentanyl and were exposed to the conditioning cues on the 7 conditioning days and saline with conditioning cues on test day. Group 3 (saline/context+) received saline and were exposed to the conditioning cues on all 7 conditioning days as well as on test day. Rats in Group 4 (natural history) were tested on all 8 days without receiving any treatment or conditioning exposure to determine the natural history/time course of neuropathic orofacial pain.
Table 1.
The table depicts the four animal groups: Fentanyl/context- (n = 4), Fentanyl/placebo/context+ (n = 13), Saline/context+ (n = 11) and natural history group (n = 7), as well as the treatment they received during the 7-day conditioning period, and on test day.
| Group | Group Name | Conditioning | Test-day |
|---|---|---|---|
| 1 | Fentanyl/context- | Fentanyl, no cues | Fentanyl, no cues |
| 2 | Fentanyl/Placebo/Context+ | Fentanyl with cues | Saline with cues |
| 3 | Saline/Context+ | Saline with cues | Saline with cues |
| 4 | Natural History | No treatment, no cues | No treatment, no cues |
2.7. Blinding
The experimenters who performed all experimental tests, including drug administration, conditioning and behavioral testing, and data analysis, were blinded to the allocation of treatment for groups. Animals were randomly assigned to groups and all drugs were aliquoted, sterilized, and labelled by another colleague. A coded key of all specimens evaluated was kept and not shared with the investigators performing the experiments until data analysis was completed. Thus, allocation concealment, blinded conduct of the experiment and blinded assessment of the outcomes were performed.
2.8. Drug administration
We administered either fentanyl citrate (West-Ward Pharmaceuticals, Eatontown, NJ) or saline (0.9% NaCl; Pfizer, New York City, NY) to groups 1–3. Fentanyl dose, 25 μg/kg, was selected based on previous studies (Saine et al., 2016, van den Hoogen and Colpaert, 1987, Wong et al., 1994) and shown to not produce catalepsy in rats. To confirm that this dose effectively suppressed hyperalgesia in our model, we administered to a separate group of rats (n = 9) with CCI-ION the selected dose (25 μg/kg) and tested for tactile hypersensitivity (p = 0.0039) (data not shown).
2.9. Treatment & conditioning context
We designed the conditioning context to include distinct cues, which collectively served as the conditioned stimulus (CS): (1) a tactile cue, that is, swaddling and injection of either fentanyl or saline; (2) an olfactory cue consisting of 95% citral scent and home-cage bedding; (3) a visual cue of blue incandescent light (GE floodlight bulb, 1310 lm) over the chamber; (4) a gustatory cue consisting of a chocolate chip (Nestle, Toll House, semi-sweet morsels); and (5) an auditory cue consisting of a instrumental-only track (“Can’t stop the feeling” by Justin Timberlake), playing continuously on a loop at 67–72 dB. On each conditioning day, animals from Groups 2 & 3 were gently swaddled in a towel and given either fentanyl or saline. We then held the animal for 5 min before placing it in the facial grimace chamber for 20 min. With the exception of the tactile cues that were only present during the injection and for 5 min after, all other cues were present throughout the 20-min conditioning session.
During this session, we left the animals undisturbed and only recorded videos to be analyzed for facial grimace scoring. This conditioning paradigm was repeated for 7 consecutive days.
Animals from Group 1 were administered 25 μg/kg of fentanyl citrate i.p. and placed in the grimace chamber with home bedding but with no other conditioning cues. Animals from Group 4 received no treatment and were placed in the grimace chamber with home bedding for recording only. Recording sessions for all groups lasted 20 min.
2.10. Testing phase
On test day, one day following conditioning day 7, both conditioning Groups 2 and 3 received saline injections, were placed in the conditioning chamber, and recorded for facial grimace scoring. All conditioning cues described were presented as above. After this 20-minute session, we recorded von Frey thresholds before returning the animals to their home cage. On this day, animals from Groups 1 and Group 4 received fentanyl or no treatment, respectively, and were also tested on both von Frey and facial grimace tests.
2.11. Statistical analysis
Primary outcomes for this study were metrics recorded before and after injury (pre-CCI and post- CCI), and differences (pre minus post treatment measurements) in the mechanical sensitivity and RGS scores on the test day.
We first tested that the surgical procedure induced pain. To determine the efficacy of the surgical procedure, we used a repeated measures ANOVA with pre-CCI and post-CCI as within factors and groups as between factor for both mechanical sensitivity and RGS scores. To test the treatment main effect during conditioning (grimace only, seven measurements), we used ANOVA for repeated measurements with conditions (pre-treatment and post-treatment) as within factor, days as within factor (seven) and groups as between factor (four groups) controlling for post-CCI RGS scores. To determine the treatment and placebo effects, we calculated the post- and pre- treatment delta (normalization) of mechanical sensitivity and RGS scores across groups to account for the intergroup variability and compared them using univariate ANOVA. Normalization was performed by computing the difference between test day and the post-CCI injury measurements of both mechanical sensitivity and facial grimace scores. These post-injury measurements were those assayed after the 5–7 day recovery period and before the start of the conditioning period. Post-injury scores for each rat were calculated as the average mechanical sensitivity score of two, day-apart, facial von Frey test assessments and one 20-minute facial grimace assessment. Planned post-hoc comparisons were calculated using Least Significant Difference.
As is typical for CCI surgeries, not all animals developed allodynia after CCI-ION. Indeed, in some animals withdrawal thresholds increased after CCI-ION, suggesting that nerve ligation may have inadvertently severed nerve fibers. We started from a full cohort of 50, and based on this, excluded 15 rats whose mechanical threshold scores did not decrease post-injury. Therefore, analyses were performed only on those rats who developed allodynia (n = 35).
All statistical comparisons were calculated using SPSS software package (SPSS Inc., Chicago Illinois, USA).
3. Results
3.1. Effects of CCI
Because the goal of this study was to test drug and placebo effects on ongoing pain behaviors, we focused the analyses on only those rats that developed post-CCI mechanical sensitivity.
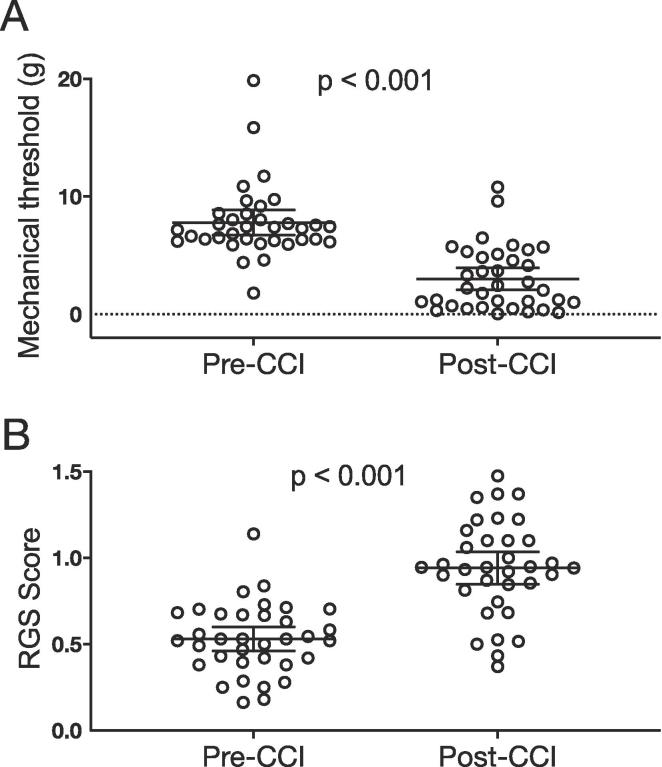
Thirty five (35) rats met the inclusion criterion and were included in all subsequent analyses. We used a repeated measures ANOVA to compare pre-CCI and post-CCI condition scores for each test. For allodynia assessed with the von Frey filaments there was a significant main effect of condition (F1,31 = 95.713; p < 0.001) (Fig. 2a), independent of group allocation (F3,31 = 0.897; p = 0.454). Similarly, for the facial grimace test, there was a significant main effect of condition (F1,31 = 26.561; p < 0.001) (Fig. 2b) with no significance for treatment group (F3,31 = 1.099; p = 0.364). Thus, we confirmed that CCI injury produced significant allodynia and signs of ongoing pain.
Fig. 2.
CCI increases mechanical sensitivity (allodynia) and facial grimace scores. A. Mechanical sensitivity thresholds pre & post injury. Repeated measures analysis showed that CCI-ION injury resulted in allodynia or mechanical hypersensitivity, shown by a significant decrease in von Frey thresholds (F1,31 = 95.713; p < 0.001). There was no difference across groups (F3,31 = 0.897; p = 0.454). B. The graph shows that CCI-ION injury resulted in a significant in increase in RGS (F1,31 = 26.561; p < 0.001). Similar to the test for allodynia, there was no significant difference across groups (F3,31 = 1.099; p = 0.364). Data are reported as scatterplots with means and 95% CI, showing individual animal scores. (n = 35).
3.2. Drug, placebo effects and placebo responses
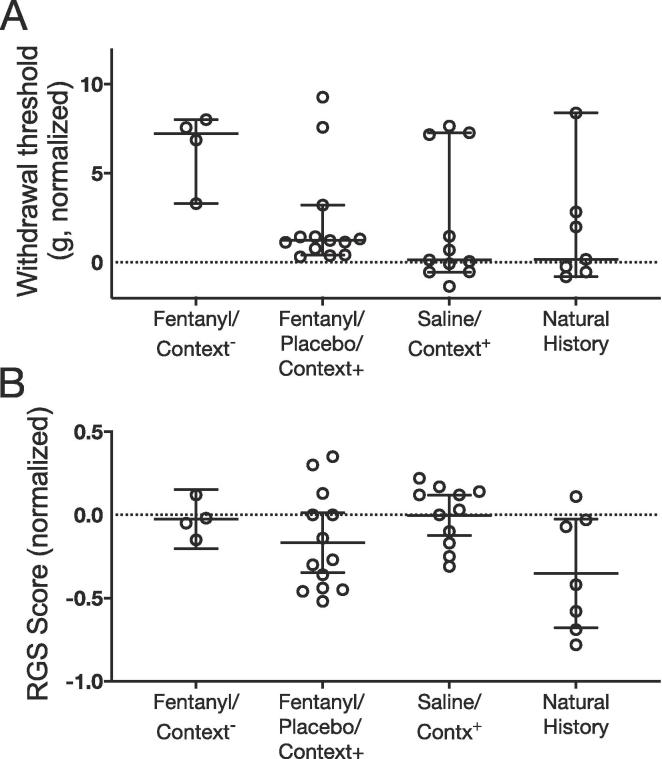
To test for modulatory drug and placebo effects we considered the differences between post- and pre-treatment outcomes for each animal across groups. The term placebo response is currently used to refer to changes in outcomes that can be due to factors outlined above, whereas the term placebo effect is used to indicate changes that are detected by virtue of inclusion of a no-treatment arm (Colloca, 2019). Based on this, the true placebo effect would be the difference between the placebo treatment arm and the no treatment arm. We operationally defined a placebo effect as the difference between the placebo group (Group 2) and the natural history group (Group 4), while a placebo response was defined as the difference between the placebo group and the fentanyl-control group (Group 1) (Colloca, 2019). For allodynia, univariate ANOVA revealed no significant treatment main effect across groups (F3,31 = 2.422; p = 0.085) (Fig. 3a) on test day. Similarly, RGS analysis revealed no significant main effect of treatment (F3, 31 = 2.751, p = 0.059) (Fig. 3b).
Fig. 3.
Treatment main effect on allodynia and grimace. A. Normalized mechanical sensitivity thresholds across groups on test day. Normalization was performed by computing the difference between threshold scores post injury and on test day (i.e., pre and post treatment). There was no treatment main effect across groups (F3,31 = 2.422; p = 0.085). B. For grimace, there was no significant treatment main effect across groups on test day (F3, 31 = 2.751, p = 0.059). Normalization was also performed by computing the difference between RGS scores post injury and on test day. Data are reported as scatterplots with means and 95% CI, showing individual animal scores.
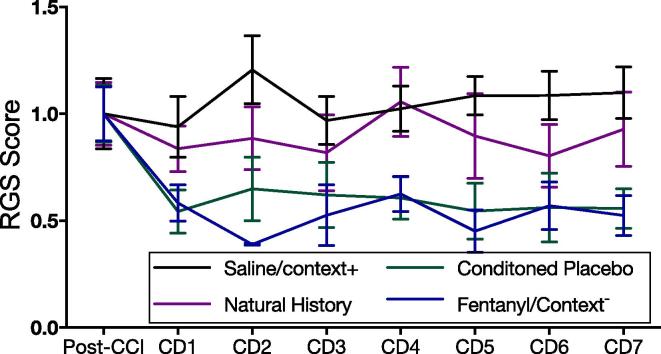
The negative RGS result was observed despite a significant main effect of treatment across groups (F3,40 = 5.020, p = 0.005) during the seven day conditioning phase, suggesting that fentanyl was effective in inducing pain reduction, an important prerequisite for associative learning and the development of conditioned responses. Post-hoc LSD comparisons indicated that RGS scores in Group 1 (fentanyl/context−) were significantly lower than in Group 3 (Saline/context+, p < 0.001) and Group 4 (natural history, p < 0.001). Similarly, Group 2 (fentanyl/placebo/context+) had lower RGS scores during the conditioning than both Groups 3 (p < 0.001) and Group 4 (p < 0.001) (Fig. 4).
Fig. 4.
Time-courses of treatment effects for grimace during the conditioning phase. Repeated measures ANOVA showed a main treatment effect of treatment group (F3,40 = 5.020, p = 0.005) during the conditioning phase. Controlling for post-CCI levels, RGS scores in group 1 (fentanyl/context-) were significantly lower than in Group 3 (saline/context+, (p < 0.001) and Group 4 (natural history, p < 0.001). Similarly, Group 2 (fentanyl/placebo/context+, reported in the figure label as ‘conditioned placebo’) had lower RGS scores during the conditioning than both Groups 3 (p < 0.001) and 4 (p < 0.001). Chart shows days normalized to post-CCI RGS scores. Data are reported showing means with 95% CI.
4. Discussion
Our study attempted to disentangle drug effects, placebo effects and responses in a rodent model of orofacial neuropathic pain, with allodynia and facial grimace scores as outcomes.
Ernst and Resch (1995) distinguished between general, perceived placebo effects and the true placebo effect. He emphasized that though the observed changes in the placebo arm of randomized clinical trials may be due to the placebo given, these responses could also be due to other factors, such as regression to the mean, the natural history of the disease and reporting biases. The overall physiological changes that take place after placebo administration comprise the placebo response (perceived placebo effects). To investigate true placebo effects, the inclusion of a no-treatment arm is essential to observe any non-specific changes. Ours is the first study to include a natural history group in animal models of placebo research (Keller et al., 2018) a sine qua non condition for separating drug from placebo effects and placebo responses (Colloca, 2019). Further, this is the first placebo study that combines a reflexive test for allodynia and the facial grimace test of ongoing neuropathic pain.
For allodynia, fentanyl alone (no conditioning cues) increased mechanical sensitivity thresholds (i.e. reduced allodynia) on test day, compared to saline given after fentanyl conditioning and saline given with conditioning cues or no treatment. Based on the analysis (Test Day scores – Post-injury scores), a higher normalized threshold indicates that animals had higher withdrawal thresholds on test day than they did post-injury. Therefore on test day, these animals exhibited more analgesia and reduced mechanical hypersensitivity, reflected by the higher withdrawal thresholds (Fig. 3a). This suggests the presence of drug effects but not conditioned placebo effects. For grimace, fentanyl was effective in reducing RGS scores during the conditioning phase (Fig. 4) but not on test day, indicating no placebo effects and responses in this model of orofacial neuropathic pain. In Fig. 3b, the lower normalized RGS scores (Test Day scores – Post-injury scores) in the placebo group suggest that test day RGS scores were lower than post-injury scores i.e. more analgesia. We note that although these differences did not meet our criterion for statistical significance (F3, 31 = 2.751, p = 0.059), they suggest a placebo analgesic effect. However, this effect seems to be masked by the lower RGS scores/unexpected improvement of the no-treatment group as well. Here, the fentanyl/context- group does not show a significant improvement which may reflect the smaller sample size in this group, or the slight variability in drug efficacy that could occur from day to day opioid treatment. These negative results for placebo effects and placebo responses are consistent with a previous study on neuropathic pain in female rats (McNabb et al., 2014), but conflict with a recent study indicating placebo responses in a neuropathic pain model (Zeng et al., 2018). Studies with larger sample sizes might be needed to draw conclusive remarks.
Previous studies used a spare nerve injury of L5 (McNabb et al., 2014) or nerve injury of L5 and L6 (Zeng et al., 2018). Here, we employed the CCI-ION model (Benoist et al., 1999, Castro et al., 2017, Okubo et al., 2013, Vos et al., 1994) of orofacial pain that causes persistent mechanical hyperalgesia, hypersensitivity (allodynia) and spontaneous pain lasting at least 28 days (Castro et al., 2017, Okubo et al., 2013). Both central and peripheral mechanisms are involved in the development of primary mechanical hyperalgesia at the site of injury, as well as secondary mechanical hyperalgesia (Okubo et al., 2013, Shibuta et al., 2012) and extra-territorial hypersensitivity (Tal and Bennett, 1994). The relevance of this model for the present study lies in its development of robust and long-lasting hypersensitivity. In addition, this model allowed us to assess the affective component of non-evoked pain non-invasively using the facial grimace scale (Langford et al., 2010, Sotocinal et al., 2011) which we have previously shown to reliably assess spontaneous pain in rats with CCI-ION (Akintola et al., 2017).
These negative findings of pharmacological conditioning and placebo effects contradict some studies, in both humans and animals, which have spurred the discussion of whether such processes can be replicated and further exploited with dose-extending placebos (Colloca et al., 2016, Sandler et al., 2010). Here, we showed no difference on the test day across treatments. It is possible that placebo effects may be different based on the severity and the nature of pain (e.g. neuropathic versus non-neuropathic pain) (Vase et al., 2015). For example, neuropathic pain is difficult to treat (Colloca et al., 2017) and less prone to be modulation by conditioning processes, therefore requiring larger sample size to detect significant treatment effects. This is also in line with the McNabb et al. study that suggested that placebo effects may be “elusive” in neuropathic pain due to its severity (McNabb et al., 2014). Nevertheless, our findings highlight the need for further investigation of different components of the pain experience in animal models.
Many clinical studies have investigated placebo responses in randomized clinical trials of neuropathic pain (Derry et al., 2016, Finnerup et al., 2015). However, none of these studies included the crucial no-treatment group, which we have described as essential to the establishment of true placebo analgesic effects. In addition, the increasing magnitude of placebo effects in clinical trials of neuropathic pain in the last decade emphasizes the need for additional research on the mechanistic underpinnings in these chronic pain states (Hauser et al., 2011, Tuttle et al., 2015) which would require appropriate control groups. To our knowledge, there have been only two well-controlled clinical studies specifically examining the occurrence placebo analgesia in neuropathic pain (Colloca et al., 2017, Vase et al., 2016). In both studies, large placebo analgesia effects were reported in patients who had developed neuropathic pain after a thoracotomy. These reports of significant placebo analgesic effects in controlled clinical studies (e.g. inclusion of the no-treatment group) further underscore the need for similar studies in animal models of neuropathic pain. Importantly, neither of the studies of placebo analgesia in chronic neuropathic pain used opioids. The utility of opioids for chronic neuropathic pain remains controversial due to modest efficacy reported, large placebo effects in clinical trials, as well as the short duration of studies (Derry et al., 2016, McNicol et al., 2013). Therefore, further longitudinal studies using opioid pharmacological conditioning paradigms need to be conducted.
We recognize some limitations of our study. We did not include a female group, making it difficult to draw conclusions about potential sex effects. We also did not include a saline-only group (i.e. saline with no conditioning cues), and, therefore, cannot disentangle the contextual effects (e.g. cues) from the effect related to saline administration. It is possible that the effect on Group 2 on test day may be due to long-lasting effects of fentanyl administration during the conditioning phase. However, due to its high lipophilicity, fentanyl rapidly crosses the blood-brain barrier, achieving brain plasma equilibrium in as little as 1.5 min and being gradually eliminated in under 45 min (Hug and Murphy, 1981, Janssen et al., 1963, Scott et al., 1991), making this unlikely. We also did not include a group receiving the opioid antagonist naloxone, preventing us from making inferences about the potential underlying endogenous opioid-based mechanisms. However, previous studies with inflammatory acute pain and neuropathic pain have already demonstrated that naloxone blocks the placebo analgesic effect (Guo et al., 2010, Zeng et al., 2018, Zhang et al., 2013).
Furthermore, using multiple cues versus a single cue may have influenced these findings. Relevant here is the distinction between implicit and explicit/contextual memory which tends to involve multiple factors that make up a context. These are thought to occur via different mechanisms (Hall, 1998). For this study, the combination of multiple factors (cues) making up a context was relevant because it is similar to human clinical encounters and represents a more holistic form of memory. Future studies investigating the salience of each individual cue for the strength of associative learning may further elucidate placebo analgesic mechanisms in this model.
We did not measure mechanical sensitivity on conditioning days, to avoid the repeated, daily stress associated with these measurements. As noted above, our dose–response studies in a separate cohort (n = 9) of rats with CCI-ION demonstrated that the same dose of fentanyl (25 μg/kg) significantly reduced hyperalgesia, as measured by facial von Frey scores. Further, animals in the fentanyl/context- group, which received fentanyl on test day, showed higher withdrawal thresholds and lower hypersensitivity than their post-injury scores. These findings, and the significant analgesic effects of fentanyl on facial grimace scores (Fig. 4), render it unlikely that the absence of placebo effects in our model is due to the ineffectiveness of fentanyl on mechanical sensitivity.
Despite these limitations, finding an animal model of placebo-induced analgesia could help explore alternatives to opioid treatments for non-cancer neuropathic pain, and rodent models might be especially relevant in disentangling drug versus placebo effects and responses, guiding optimization of clinical trial designs in difficult to treat neuropathic pain populations.
Acknowledgments
Acknowledgements
Research reported in this publication was supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health grant R01NS099245 (AK) and the National Institute of Dental and Craniofacial Research grant R01DE025946 (LC). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding sources has no role in study design; the collection, analysis and interpretation of data; the writing of the report; or in the decision to submit the article for publication.
Declaration of interest
The authors report no conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynpai.2019.100033.
Contributor Information
Luana Colloca, Email: colloca@umaryland.edu.
Asaf Keller, Email: akeller@som.umaryland.edu.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Akintola T., Raver C., Studlack P., Uddin O., Masri R., Keller A. The grimace scale reliably assesses chronic pain in a rodent model of trigeminal neuropathic pain. Neurobiol. Pain. 2017;2:13–17. doi: 10.1016/j.ynpai.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoist J.M., Gautron M., Guilbaud G. Experimental model of trigeminal pain in the rat by constriction of one infraorbital nerve: changes in neuronal activities in the somatosensory cortices corresponding to the infraorbital nerve. Exp. Brain Res. 1999;126(3):383–398. doi: 10.1007/s002210050745. [DOI] [PubMed] [Google Scholar]
- Carlino E., Frisaldi E., Benedetti F. Pain and the context. Nat. Rev. Rheumatol. 2014;10(6):348–355. doi: 10.1038/nrrheum.2014.17. [DOI] [PubMed] [Google Scholar]
- Castro A., Li Y., Raver C., Chandra R., Masri R., Lobo M.K., Keller A. Neuropathic pain after chronic nerve constriction may not correlate with chloride dysregulation in mouse trigeminal nucleus caudalis neurons. Pain. 2017;158(7):1366–1372. doi: 10.1097/j.pain.0000000000000926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplan S.R., Bach F.W., Pogrel J.W., Chung J.M., Yaksh T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods. 1994;53(1):55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Colloca L. The placebo effect in pain therapies. Annu. Rev. Pharmacol. Toxicol. 2019;59:191–211. doi: 10.1146/annurev-pharmtox-010818-021542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colloca L., Enck P., DeGrazia D. Relieving pain using dose-extending placebos: a scoping review. Pain. 2016;157(8):1590–1598. doi: 10.1097/j.pain.0000000000000566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colloca L., Ludman T., Bouhassira D., Baron R., Dickenson A.H., Yarnitsky D., Raja S.N. Neuropathic pain. Nat. Rev. Dis. Prim. 2017;3:17002. doi: 10.1038/nrdp.2017.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derry S., Stannard C., Cole P., Wiffen P.J., Knaggs R., Aldington D., Moore R.A. Fentanyl for neuropathic pain in adults. Cochr. Database Syst. Rev. 2016;10:CD011605. doi: 10.1002/14651858.CD011605.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon W.J. The up-and-down method For small samples. J. Am. Statist. Assoc. 1965;60(312):967–968. [Google Scholar]
- Ernst E., Resch K.L. Concept of true and perceived placebo effects. BMJ. 1995;311(7004):551–553. doi: 10.1136/bmj.311.7004.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow M.S. Pavlovian conditioning, negative feedback, and blocking: mechanisms that regulate association formation. Neuron. 1998;20(4):625–627. doi: 10.1016/s0896-6273(00)81002-8. [DOI] [PubMed] [Google Scholar]
- Finnerup N.B., Attal N., Haroutounian S., McNicol E., Baron R., Dworkin R.H., Wallace M. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162–173. doi: 10.1016/S1474-4422(14)70251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J.Y., Wang J.Y., Luo F. Dissection of placebo analgesia in mice: the conditions for activation of opioid and non-opioid systems. J. Psychopharmacol. 2010;24(10):1561–1567. doi: 10.1177/0269881109104848. [DOI] [PubMed] [Google Scholar]
- Hall, R. H. 1998. Contextual Memory.
- Hauser W., Bartram-Wunn E., Bartram C., Reinecke H., Tolle T. Systematic review: Placebo response in drug trials of fibromyalgia syndrome and painful peripheral diabetic neuropathy-magnitude and patient-related predictors. Pain. 2011;152(8):1709–1717. doi: 10.1016/j.pain.2011.01.050. [DOI] [PubMed] [Google Scholar]
- Hug C.C., Jr., Murphy M.R. Tissue redistribution of fentanyl and termination of its effects in rats. Anesthesiology. 1981;55(4):369–375. doi: 10.1097/00000542-198110000-00006. [DOI] [PubMed] [Google Scholar]
- Janssen P.A., Niemegeers C.J., Dony J.G. The inhibitory effect of fentanyl and other morphine-like analgesics on the warm water induced tail withdrawl reflex in rats. Arzneimittelforschung. 1963;13:502–507. [PubMed] [Google Scholar]
- Keller A., Akintola T., Colloca L. Placebo analgesia in rodents: current and future research. Int. Rev. Neurobiol. 2018;138:1–15. doi: 10.1016/bs.irn.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Shin W. How to do random allocation (randomization) Clin. Orthop. Surg. 2014;6(1):103–109. doi: 10.4055/cios.2014.6.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitt C.A., Gruber K., Davis M., Woolf C.J., Levine J.D. Trigeminal neuralgia: opportunities for research and treatment. Pain. 2000;85(1–2):3–7. doi: 10.1016/s0304-3959(99)00310-3. [DOI] [PubMed] [Google Scholar]
- Koopman J.S., Dieleman J.P., Huygen F.J., de Mos M., Martin C.G., Sturkenboom M.C. Incidence of facial pain in the general population. Pain. 2009;147(1–3):122–127. doi: 10.1016/j.pain.2009.08.023. [DOI] [PubMed] [Google Scholar]
- Landis S.C., Amara S.G., Asadullah K., Austin C.P., Blumenstein R., Bradley E.W., Silberberg S.D. A call for transparent reporting to optimize the predictive value of preclinical research. Nature. 2012;490(7419):187–191. doi: 10.1038/nature11556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford D.J., Bailey A.L., Chanda M.L., Clarke S.E., Drummond T.E., Echols S., Mogil J.S. Coding of facial expressions of pain in the laboratory mouse. Nat. Methods. 2010;7(6):447–449. doi: 10.1038/nmeth.1455. [DOI] [PubMed] [Google Scholar]
- McNabb C.T., White M.M., Harris A.L., Fuchs P.N. The elusive rat model of conditioned placebo analgesia. Pain. 2014;155(10):2022–2032. doi: 10.1016/j.pain.2014.07.004. [DOI] [PubMed] [Google Scholar]
- McNicol E.D., Midbari A., Eisenberg E. Opioids for neuropathic pain. Cochrane Database Syst. Rev. 2013;(8):CD006146. doi: 10.1002/14651858.CD006146.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo M., Castro A., Guo W., Zou S., Ren K., Wei F., Dubner R. Transition to persistent orofacial pain after nerve injury involves supraspinal serotonin mechanisms. J. Neurosci. 2013;33(12):5152–5161. doi: 10.1523/JNEUROSCI.3390-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saine L., Helie P., Vachon P. Effects of fentanyl on pain and motor behaviors following a collagenase-induced intracerebral hemorrhage in rats. J. Pain Res. 2016;9:1039–1048. doi: 10.2147/JPR.S121415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler A.D., Glesne C.E., Bodfish J.W. Conditioned placebo dose reduction: a new treatment in attention-deficit hyperactivity disorder? J. Dev. Behav. Pediatr. 2010;31(5):369–375. doi: 10.1097/DBP.0b013e3181e121ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J.C., Cooke J.E., Stanski D.R. Electroencephalographic quantitation of opioid effect: comparative pharmacodynamics of fentanyl and sufentanil. Anesthesiology. 1991;74(1):34–42. doi: 10.1097/00000542-199101000-00007. [DOI] [PubMed] [Google Scholar]
- Shibuta K., Suzuki I., Shinoda M., Tsuboi Y., Honda K., Shimizu N., Iwata K. Organization of hyperactive microglial cells in trigeminal spinal subnucleus caudalis and upper cervical spinal cord associated with orofacial neuropathic pain. Brain Res. 2012;1451:74–86. doi: 10.1016/j.brainres.2012.02.023. [DOI] [PubMed] [Google Scholar]
- Sotocinal S.G., Sorge R.E., Zaloum A., Tuttle A.H., Martin L.J., Wieskopf J.S., Mogil J.S. The Rat Grimace Scale: a partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol. Pain. 2011;7:55. doi: 10.1186/1744-8069-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tal M., Bennett G.J. Extra-territorial pain in rats with a peripheral mononeuropathy: mechano-hyperalgesia and mechano-allodynia in the territory of an uninjured nerve. Pain. 1994;57(3):375–382. doi: 10.1016/0304-3959(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Tuttle A.H., Tohyama S., Ramsay T., Kimmelman J., Schweinhardt P., Bennett G.J., Mogil J.S. Increasing placebo responses over time in U.S. clinical trials of neuropathic pain. Pain. 2015;156(12):2616–2626. doi: 10.1097/j.pain.0000000000000333. [DOI] [PubMed] [Google Scholar]
- van den Hoogen R.H., Colpaert F.C. Epidural and subcutaneous morphine, meperidine (pethidine), fentanyl and sufentanil in the rat: analgesia and other in vivo pharmacologic effects. Anesthesiology. 1987;66(2):186–194. doi: 10.1097/00000542-198702000-00013. [DOI] [PubMed] [Google Scholar]
- van der Wal S., Cornelissen L., Behet M., Vaneker M., Steegers M., Vissers K. Behavior of neuropathic pain in mice following chronic constriction injury comparing silk and catgut ligatures. Springerplus. 2015;4:225. doi: 10.1186/s40064-015-1009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vase L., Skyt I., Hall K.T. Placebo, nocebo, and neuropathic pain. Pain. 2016;157(Suppl 1):S98–105. doi: 10.1097/j.pain.0000000000000445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vase L., Vollert J., Finnerup N.B., Miao X., Atkinson G., Marshall S., Segerdahl M. Predictors of the placebo analgesia response in randomized controlled trials of chronic pain: a meta-analysis of the individual data from nine industrially sponsored trials. Pain. 2015;156(9):1795–1802. doi: 10.1097/j.pain.0000000000000217. [DOI] [PubMed] [Google Scholar]
- Vos B.P., Strassman A.M., Maciewicz R.J. Behavioral evidence of trigeminal neuropathic pain following chronic constriction injury to the rat's infraorbital nerve. J. Neurosci. 1994;14(5 Pt 1):2708–2723. doi: 10.1523/JNEUROSCI.14-05-02708.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson C.P. Management issues of neuropathic trigeminal pain from a medical perspective. J. Orofac. Pain. 2004;18(4):366–373. [PubMed] [Google Scholar]
- Wong J.O., Chang C.L., Cheng J.T. The effect of nifedipine on fentanyl-induced analgesia in rats. Acta Anaesthesiol. Sin. 1994;32(2):109–114. [PubMed] [Google Scholar]
- Zeng Y., Hu D., Yang W., Hayashinaka E., Wada Y., Watanabe Y., Cui Y. A voxel-based analysis of neurobiological mechanisms in placebo analgesia in rats. Neuroimage. 2018;178:602–612. doi: 10.1016/j.neuroimage.2018.06.009. [DOI] [PubMed] [Google Scholar]
- Zhang R.R., Zhang W.C., Wang J.Y., Guo J.Y. The opioid placebo analgesia is mediated exclusively through mu-opioid receptor in rat. Int. J. Neuropsychopharmacol. 2013;16(4):849–856. doi: 10.1017/S1461145712000673. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.