Summary
Contractile to synthetic phenotypic switching of smooth muscle cells (SMCs) contributes to stenosis in vascular disease and vascular transplants. To generate more contractile SMCs, we performed a high-throughput differentiation screen using a MYH11-NLuc-tdTomato human embryonic stem cell reporter cell line. We identified RepSox as a factor that promotes differentiation of MYH11-positive cells by promoting NOTCH signaling. RepSox induces SMCs to exhibit a more contractile phenotype than SMCs generated using PDGF-BB and TGF-β1, two factors previously used for SMC differentiation but which also cause intimal hyperplasia. In addition, RepSox inhibited intimal hyperplasia caused by contractile to synthetic phenotypic switching of SMCs in a rat balloon injury model. Thus, in addition to providing more contractile SMCs that could prove useful for constructing artificial blood vessels, this study suggests a strategy for identifying drugs for inhibiting intimal hyperplasia that act by driving contractile differentiation rather than inhibiting proliferation non-specifically.
Keywords: pluripotent stem cells, contractile smooth muscle cells, differentiation, maturation, RepSox, NOTCH, intima hyperplasia, MYH11-NLuc-tdTomato reporter cell line, high-throughput screen, restenosis
Highlights
-
•
Fully defined differentiation of contractile (95% MYH11+) smooth muscle cells (SMCs)
-
•
RepSox-NOTCH signal promotes SMC differentiation and inhibits intimal hyperplasia
-
•
RepSox-SMCs could reduce the risk of intimal hyperplasia compared with PDGF/TGF-SMCs
-
•
Applying SMC differentiation for high-throughput screening of anti-restenosis drugs
Thomson, Zhang, and colleagues report a high-throughput screen that can be used for optimization of the fully defined differentiation of contractile smooth muscle cells and identification of intimal hyperplasia inhibitors. Both in vitro and in vivo evidence revealed that RepSox is better than PDGF-BB and TGF-β1 in inducing contractile phenotype of smooth muscle cells and reducing the risk of intimal hyperplasia.
Introduction
Balloon angioplasty, stents, and bypass surgery are commonly used for occlusive arterial disease, a leading cause of morbidity and mortality worldwide (de Vries et al., 2016). However, restenosis occurs in a significant number of treated patients because of intimal hyperplasia (Beamish et al., 2010, Dangas and Kuepper, 2002). During the development of intimal hyperplasia, contractile smooth muscle cells (SMCs) decrease contractile protein expression and increase proliferation, migration, and extracellular matrix (ECM) production; a process described as contractile to synthetic phenotypic switching (Beamish et al., 2010, Rensen et al., 2007). Small molecules that promote the differentiation of contractile SMCs at the expense of synthetic SMCs could minimize the development of intimal hyperplasia.
Transforming growth factor β1 (TGF-β1) and platelet-derived growth factor BB (PDGF-BB) are widely used for the differentiation of SMCs from human pluripotent stem cells (PSCs) (Bajpai et al., 2012, Cao et al., 2013, Cheung et al., 2012, Dash et al., 2015, Karamariti et al., 2013, Lin et al., 2019, Patsch et al., 2015, Wang et al., 2014, Wanjare et al., 2013, Yang et al., 2016, Zhang et al., 2011). However, upregulation of PDGF and TGF-β signaling has been shown to promote the switching of SMC phenotypes from contractile to synthetic, contributing to intimal hyperplasia (Muto et al., 2007, Nabel et al., 1993, Newby and Zaltsman, 2000, Raines, 2004, Suwanabol et al., 2011, Wolf et al., 1994). As a result, SMCs generated from PSCs using PDGF-BB and TGF-β1 carry a risk of intimal hyperplasia if used in tissue-engineered vascular constructs. One strategy to overcome this problem is to use high-throughput screening to identify small molecules that can promote contractile SMC differentiation. Since normal vascular differentiation and the dedifferentiation observed in vascular disease share common pathways (Beamish et al., 2010, Mack, 2011), this screening strategy using human PSC-based SMC differentiation might also identify drugs for preventing restenosis caused by intimal hyperplasia. Compared with primary cell lines used in previous screening (Goel et al., 2014), human PSCs provide an unlimited and more consistent cell source for screening. In addition, current Food and Drug Administration-approved anti-restenosis drugs (rapamycin and paclitaxel), as well as previous screening, focused on proliferation antagonists (Goel et al., 2014). However, restoring the contractile phenotype of SMCs in intimal hyperplasia not only inhibits cell proliferation but also improves contractile protein expression and the suppression of cell migration and ECM production (Beamish et al., 2010, Rensen et al., 2007). Thus, using the MYH11-NLuc-tdTomato reporter cell line may identify drugs other than proliferation antagonists.
MYH11 is a specific protein expressed by SMCs and is a marker for the mature contractile phenotype. Mutation or reduced expression of MYH11 is associated with vascular disease (Owens et al., 2004, Pannu et al., 2007). Using CRISPR/Cas9 technology (Cong et al., 2013, Hou et al., 2013, Mali et al., 2013), we generated a MYH11-NLuc-tdTomato human embryonic stem cell (ESC) reporter cell line and used it in a high-throughput screen of 4,804 small molecules. In this screen, RepSox was identified as a potent small molecule that promoted NOTCH signaling and improved contractile SMC differentiation from human PSCs. SMCs generated by RepSox (RepSox-SMCs) demonstrated a more contractile phenotype compared with SMCs induced by PDGF-BB (P-SMCs), SMCs induced by TGF-β1 (T-SMCs), and SMCs induced by both TGF-β1 and PDGF-BB (PT-SMCs). RepSox also promoted synthetic to contractile phenotypic switching of primary human aortic smooth muscle cells (AoSMCs) in vitro and inhibited intimal hyperplasia in vivo.
Results
High-Throughput Screening for Contractile SMC Differentiation
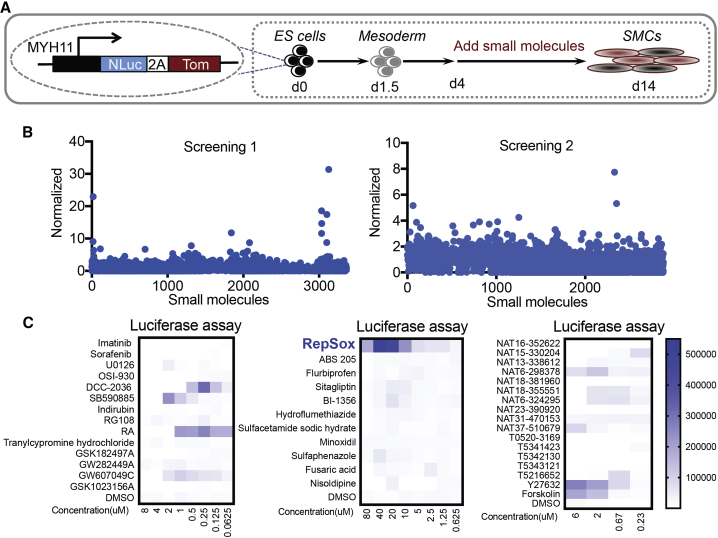
The MYH11-NLuc-tdTomato human ESC reporter line was generated by CRISPR/Cas9 technology (Figure S1). The reporter cell line was differentiated into mesoderm by E8BAC medium for 2 days (Zhang et al., 2017) and then treated with fibroblast growth factor 2 (FGF2) and bone morphogenetic protein 4 (BMP4) to further mature mesoderm for another 2 days. The cells were then passaged into 96-well plates and exposed to small molecules for 10 days using a customized robotic workstation (Figure 1A). The media were changed every other day and small molecules were added during each feeding. Among the 4,804 small molecules tested, 42 increased contractile SMC differentiation, as evidenced by the increased MYH11 promoter-driven luciferase activity (Figures 1B and 1C; Table S1). We then validated these hits and optimized their concentration. Among them, RepSox was the most effective at promoting MYH11 expression (Figure 1C) and was used for further optimizing contractile SMC differentiation.
Figure 1.
High-Throughput Screening
(A) Schematic of high-throughput screening for generating contractile smooth muscle cells and restenosis drug discovery. The MYH11-NLuc-Tom reporter cell line was differentiated into mesoderm by E8BAC medium (E8 medium [Chen et al., 2011] supplemented with 5 ng/mL BMP4, 25 ng/mL activin A, and 1 μM CHIR99021) for 2 days and then treated with 50 ng/mL FGF2 and 20 ng/mL BMP4 in E6 medium (E8 medium minus FGF2 and TGF-β1) for another 2 days. The cells were passaged at day 4 and seeded on the 96-well plate for screening (2 × 106 cells/plate). The small molecules were added to the medium from day 4 to day 14. d, day; ES, embryonic stem; SMCs, smooth muscle cells; Tom, tdTomato.
(B) Screening results. Two screens were performed with a total of 4,804 small molecules. The luciferase assay results of individual small molecules were normalized to the average reads of all samples for each batch. The small molecules were selected for further analysis when the normalized reads were greater than “average + 3× SD.”
(C) Optimizing the concentration of the selected small molecules.
RepSox Modulates NOTCH Signaling to Promote MYH11+ SMC Differentiation
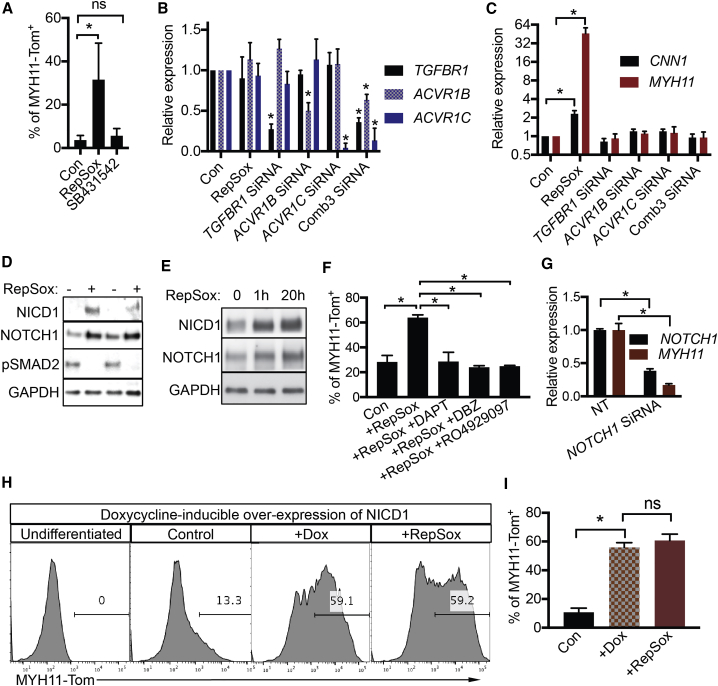
Previously, RepSox was shown to be a TGF-β signaling inhibitor (Ichida et al., 2009). However, inhibition or knockdown of TGF-β receptors failed to increase MYH11 expression (Figures 2A–2C), even though RepSox inhibited pSMAD2 during SMC differentiation (Figure 2D). The results suggested that RepSox was acting through another pathway to regulate SMC differentiation. Further investigation revealed that RepSox increased full-length NOTCH1 and its intracellular domain (NICD, activated form) expression (Figure 2D) as early as 1 h after RepSox treatment (Figure 2E). The NOTCH inhibitors DAPT, DBZ, and RO4929097 all eliminated the effect of RepSox on the increase in MYH11-Tom+ SMC differentiation (Figure 2F). Similarly, knockdown of NOTCH1 mRNA suppressed the increase in MYH11 expression (Figure 2G). In a gain-of-function experiment, the doxycycline-induced overexpression of NICD1 increased MYH11-Tom+ differentiation to levels similar to those obtained by RepSox (Figures 2H and 2I). Inhibition of TGF-β did not further enhance MYH11-Tom+ SMC differentiation when combined with overexpression of NOTCH signaling (Figure S2). Taken together, these data demonstrate RepSox acts through the NOTCH signaling pathway in promoting MYH11-positive SMC differentiation.
Figure 2.
RepSox Promotes NOTCH Signaling
(A) Flow-cytometric analysis of MYH11-Tom+ cells after treatment with RepSox (25 μM) or SB431542 (10 μM) from day 10 to day 14. Data are presented as mean ± SD, n = 3 independent experiments. ns, not significant; ∗p < 0.05, Student's t test.
(B) qPCR analysis of gene expression. Cells were treated with RepSox (25 μM) or small interfering RNA (siRNA). Comb3: Knockdown of TGFBR1, ACVR1B, and ACVR1C at the same time. Data are presented as mean ± SD, n = 3 independent experiments. ∗p < 0.05, Student's t test.
(C) qPCR analysis of CNN1 and MYH11 expression. Cells were treated with RepSox (25 μM) or siRNA. Comb3: Knockdown of TGFBR1, ACVR1B, and ACVR1C at the same time. Data are presented as mean ± SD, n = 3 independent experiments. ∗p < 0.05, Student's t test.
(D) Western blot. During smooth muscle cell differentiation, cells were treated with or without RepSox from day 10 to day 11.
(E) Western blot. During smooth muscle cell differentiation, cells were treated with RepSox for 1 or 20 h at days 10–11.
(F) Flow-cytometric analysis of MYH11-Tom+ cells after treatment with DMSO, RepSox (25 μM), DAPT (20 μM), DBZ (10 μM), or RO4929097 (10 μM) from day 10 to day 16. Data are presented as mean ± SD, n = 3 independent experiments. ∗p < 0.05, Student's t test.
(G) qPCR analysis of NOTCH1 and MYH11 expression. Cells were treated with RepSox and non-targeting control (NT)/siRNA at day 10. The RNA was isolated at day 14. Data are presented as mean ± SD, n = 3 independent experiments. ∗p < 0.05, Student's t test.
(H) Flow-cytometric analysis of MYH11-Tom+ cells. The cells were treated with doxycycline (1 μg/mL) to induce the expression of NICD1, or RepSox (25 μM) from days 10–16 or days 12–16.
(I) Statistical data for NICD1-induced MYH11-Tom+ cells. Data are presented as mean ± SD, n = 6 independent experiments. ns, not significant; ∗p < 0.05, Student's t test.
Optimization of RepSox-Induced SMC Differentiation in Fully Defined, Xeno-Free Medium
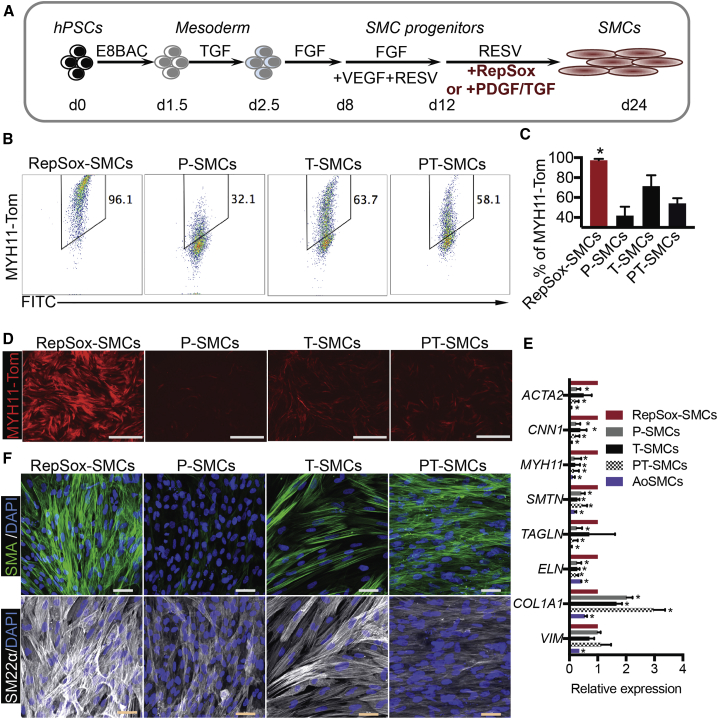
Previous SMC differentiation protocols used serum or serum replacement products that contain animal components (Bajpai et al., 2012, Cao et al., 2013, Cheung et al., 2012, Dash et al., 2015, Karamariti et al., 2013, Lin et al., 2019, Patsch et al., 2015, Wang et al., 2014, Wanjare et al., 2013, Yang et al., 2016, Zhang et al., 2011). Here, we performed a stepwise optimization to further improve SMC differentiation efficiency in completely defined, xeno-free medium (Figures S3 and 3A). After optimization (Figure 3A), human PSCs were differentiated into mesoderm in E8BAC medium (Zhang et al., 2017) for 36 h and then treated with TGF-β1 for another 18 h. FGF2 was used for another 5 days. Next, the cells were treated with FGF2, VEGFA, and RESV (a NOTCH agonist) to induce SMC progenitors from day 8 to day 12. RESV and RepSox were then used to further mature SMCs from day 12 to day 24. The optimized RepSox protocol generated >95% MYH11+ SMCs, while PDGF-BB and TGF-β1 treatment generated 30%–60% of MYH11+ SMCs (Figures 3B–3D). Compared with pericytes, these RepSox-SMCs expressed lower levels of mesenchymal markers, PDGF-RB and NG2, further confirming the SMC fate (Figures S4A and S4B). We then performed a comparison with a previous protocol that generated 80% MYH11+ SMCs (Cheung et al., 2012). Flow-cytometric analysis showed that RepSox-SMCs expressed much higher levels of MYH11 compared with Cheung's LM-SMCs (Figure S4C). qRT-PCR further confirmed that RepSox induced a 23-fold increase of MYH11 expression over that protocol (Figure S4D). qRT-PCR also revealed that RepSox-SMCs expressed higher levels of other structural genes, including CNN1, MYH11, SMTN, and ELN, compared with PDGF-BB- and/or TGF-β1-treated cells (Figure 3E). In contrast, the expression of collagen (COL1A1), a major ECM gene that is increased in the synthetic state (Wanjare et al., 2013, Yang et al., 2016), was lower in RepSox-SMCs compared with P-SMCs, T-SMCs, and PT-SMCs (Figure 3E). Immunostaining showed that RepSox-SMCs strongly expressed structural proteins SMA and SM22α (Figure 3F). We further measured elastin protein expression. Fastin Elastin Assay revealed that RepSox-SMCs produced higher elastin levels than PT-SMCs (Figure S4E). Finally, we tested whether these cells maintained SMC phenotype over long-term culture. We found that the expression of MYH11 could be maintained in RepSox-SMCs for at least 8 weeks after differentiation, while its expression was almost undetectable in PT-SMCs (Figures S4F and S4G). These RepSox-SMCs were less proliferative compared with PT-SMCs (Figure S4H), indicating a contractile phenotype.
Figure 3.
Molecular Characterization of SMCs
(A) Schematic of the optimized SMC differentiation protocol. Matrigel coating was used for all the following experiments. The MYH11-NLuc-tdTomato reporter human ESC line was differentiated into mesoderm by using E8BAC medium (E8 medium supplemented with 5 ng/mL BMP4, 25 ng/mL activin A, and 1 μM CHIR99021) for 36 h and then treated with 1.7 ng/mL TGF-β1 in E6 medium (E6 is defined as E8 medium minus FGF2 and TGF-β1) for 18 h. FGF2 (100 ng/mL) in E5 medium (E5 is defined as E6 minus insulin) was used for another 5 days. Next, the cells were treated with FGF2 (100 ng/mL), VEGFA (50 ng/mL), and RESV (5 μM, a NOTCH agonist) in E6 medium to induce SMC progenitors from day 8 to day 12. RESV in E6 medium supplemented with RepSox (25 μM), PDGF-BB (10 ng/mL), or TGF-β1 (1.7 ng/mL) was then used to further mature SMCs.
(B) Flow-cytometric analysis of MYH11-Tom+ cells. RepSox-SMCs, P-SMCs, T-SMCs, and PT-SMCs induced from day 12 to day 24.
(C) Statistical data for MYH11-Tom+ cells in each treatment group from (B). Data are presented as mean ± SD, n = 3 independent experiments. ∗p < 0.05, Student's t test.
(D) Live imaging of MYH11-Tom expression. Scale bars, 1 mm.
(E) qRT-PCR of SMCs. Data are presented as mean ± SD, n = 3 independent experiments. ∗p < 0.05, Student's t test. hPSCs, human pluripotent stem cells; Tom, tdTomato.
(F) Immunostaining of SMA and SM22α. Scale bars, 50 μm.
The RepSox protocol also worked robustly for human induced PSC (iPSC) lines 005B23.1 and PBMC-3-1, which are derived from skin punch fibroblasts and peripheral blood mononuclear cells, respectively. Eighty-seven percent to 98% of MYH11+, SMA+, or SM22α+ SMCs were generated from these two iPSC lines and non-targeted H1 human ESCs (Figures S5A and S5B). In addition, qRT-PCR revealed that the expression of the contractile genes and ECM genes were similar among iPSC- and ESC-derived SMCs (Figure S5C).
RepSox-SMCs Have a Contractile Phenotype
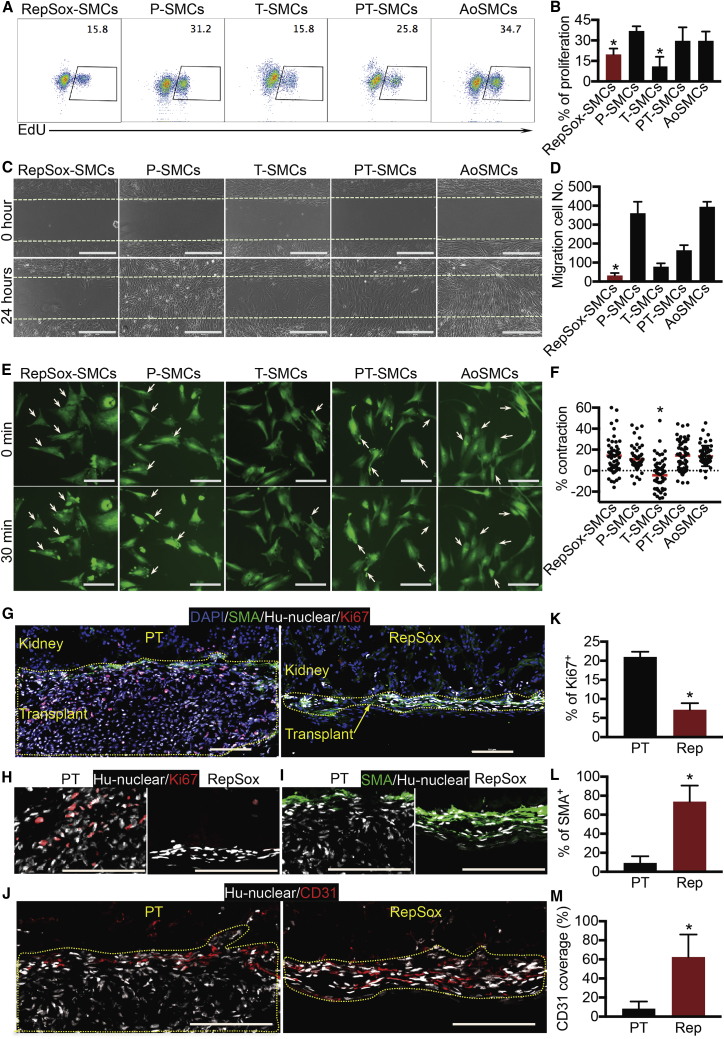
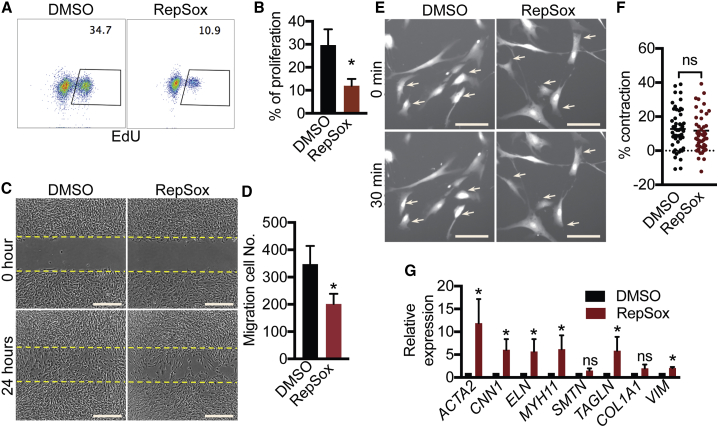
Since low proliferation and migration rates are characteristic of contractile SMCs, we characterized these properties in PSC-derived SMCs and AoSMCs. RepSox-SMCs and T-SMCs had lower proliferation rates than the other SMCs (Figures 4A and 4B). In addition, RepSox-SMCs showed the lowest migration rate compared with all the other SMC groups (Figures 4C and 4D). SMC contraction controls vascular tone and blood pressure (Brozovich et al., 2016). Thus, we measured carbachol-evoked cell contraction. Time-lapse imaging revealed that P-SMCs, PT-SMCs, RepSox-SMCs, and AoSMCs exhibited a 10%–20% change of cell-surface area after carbachol treatment (Figures 4E and 4F). However, the average T-SMC surface area actually expanded during treatment (Figures 4E and 4F), suggesting that T-SMCs do not have physiologically normal contractile properties. In summary, RepSox treatment produced a more contractile SMC phenotype including lower proliferation and migration rates.
Figure 4.
Functional Characterization of SMCs (H1 Cell-Derived)
(A) Click-iT 5-ethynyl-2′-deoxyuridine (EdU) analysis of cell proliferation.
(B) Statistical data of EdU-incorporated cells are presented as mean ± SD, n = 4 independent experiments. ∗p < 0.05 compared with P-SMCs and AoSMCs, Student's t test.
(C) Cell migration. Cells were imaged at 0 and 24 h. Scale bars, 400 μm.
(D) Statistical data of migration cells are presented as mean ± SD, n = 4 independent experiments. ∗p < 0.05 versus all the other SMCs, Student’s t test.
(E) Cell-contraction assay. Cells were imaged before and after treatment with 100 μM carbachol. Arrows indicate contracting cells. Scale bars, 100 μm.
(F) Statistical data of cell-surface changes are presented as mean ± SD, n = 65, 61, 56, 59, and 53 cells (from 3 independent experiments) for RepSox-SMCs, P-SMCs, T-SMCs, PT-SMCs, and AoSMCs, respectively. ∗p < 0.05 versus all the other SMCs, Student's t test.
(G) Kidney capsule experiment. PT-SMCs and RepSox-SMCs were transplanted under the kidney capsule of NBSGW mice. Triple immunostaining of anti-smooth muscle actin (SMA; labeling smooth muscle cells), anti-hu-nuclear (labeling human cells), and anti-Ki67 (labeling proliferating cells). Scale bars, 100 μm.
(H) Higher magnification of anti-hu-nuclear and anti-Ki67 staining. Scale bars, 100 μm.
(I) Higher magnification of anti-SMA and anti-hu-nuclear staining. Scale bars, 100 μm.
(J) Immunostaining of anti-hu-nuclear and anti-CD31 (labeling endothelial cells). Scale bars, 100 μm.
(K–M) Statistical data for percentage of Ki67+ cells (K), percentage of SMA+ cells (L), and CD31 coverage (M) of human cells under kidney capsule. Data are presented as mean ± SD, n = 3 mice for PT-SMCs and n = 5 mice for RepSox-SMCs. ∗p < 0.05, Student's t test.
To test whether RepSox-SMCs can maintain a contractile phenotype in vivo, we transplanted RepSox-SMCs and PT-SMCs under the kidney capsules of immune-deficient mice. Immunostaining of transplanted grafts showed that RepSox-SMCs had a lower proliferative rate compared with PT-SMCs (Figures 4G, 4H, and 4K). In addition, most PT-SMCs lost smooth muscle actin expression (Figures 4G, 4I, and 4L) and recruited few endothelial cells (Figures 4J and 4M; highlighted by yellow dashed line). In contrast, most RepSox-SMCs (75%) maintained smooth muscle α-actin expression (Figures 4G, 4I, and 4L) and were able to recruit more endothelial cells (Figures 4J and 4M). Thus, RepSox-SMCs maintained a more contractile phenotype than PT-SMCs after transplantation.
RepSox Inhibits Intimal Hyperplasia
Multiple pathways are shared between vascular SMC differentiation and the dedifferentiation observed in vascular disease (Beamish et al., 2010, Mack, 2011). Therefore, we hypothesized that screening for molecules that drive ESC differentiation to a mature, contractile SMC phenotype might also block the dedifferentiation and restore the contractile phenotype of primary SMCs, and that such molecules might prove useful for inhibiting intimal hyperplasia in vivo. Indeed, among the molecules identified in our screen (Figure 1C) UO126, Y27632, and retinoic acid have all been shown to inhibit neointimal hyperplasia previously (DeRose et al., 1999, Gulkarov et al., 2009, Sawada et al., 2000).
Cultured primary AoSMCs undergo a contractile to synthetic switch upon culture (Beamish et al., 2010, Owens et al., 2004), so we first tested whether RepSox would reverse this switch. RepSox treatment reduced cell migration and proliferation rates of AoSMCs (Figures 5A–5D) without affecting contraction ability (Figures 5E and 5F). In addition, RepSox treatment increased contractile gene expression (Figure 5G). Thus, RepSox improved the contractile phenotype of primary AoSMCs in vitro.
Figure 5.
RepSox Promotes a Contractile Phenotype of Cultured AoSMCs
(A) Click-iT EdU analysis of cell proliferation.
(B) Statistical data of EdU-incorporated cells are presented as mean ± SD, n = 4 independent experiments. ∗p < 0.05, Student's t test. AoSMCs (primary human aortic smooth muscle cells) were treated with DMSO or 100 μM RepSox for 2 days.
(C) Cell migration. AoSMCs were treated with DMSO or 100 μM RepSox for 2 days before the migration assay and one more day during the assay. Cells were imaged at 0 and 24 h during migration assay. Scale bars, 500 μm.
(D) Statistical data of cell migration are presented as mean ± SD, n = 6 samples from 3 independent experiments. ∗p < 0.05, Student's t test.
(E) Cell-contraction assay. AoSMCs were treated with DMSO or 100 μM RepSox for 2 days before adding carbachol. Cells were imaged before and after the treatment with 100 μM carbachol. Scale bars, 100 μm.
(F) Statistical data of cell-surface changes are presented as mean ± SD, n = 50 cells/group. ns, not significant by Student's t test. Arrows indicate contracting cells.
(G) qRT-PCR of SMCs. AoSMCs were treated with DMSO or 100 μM RepSox for 3 days. Data are presented as mean ± SD, n = 3 independent experiments. ns, not significant; ∗p < 0.05, Student's t test.
The clinically used anti-restenotic drugs rapamycin (or analogs) and paclitaxel also suppress endothelium proliferation and repair (Prasad et al., 2005, Zheng et al., 2014). To examine potential side effects of RepSox on endothelial cells in vitro, we performed a proliferation assay. The result demonstrated RepSox had significantly less effect on arterial endothelial cell proliferation compared with rapamycin (Figures S6A and S6B). In addition, RepSox did not increase apoptosis (Figures S6C and S6D) and did not compromise nitric oxide production (Figure S6E), an arterial specific function (Zhang et al., 2017).
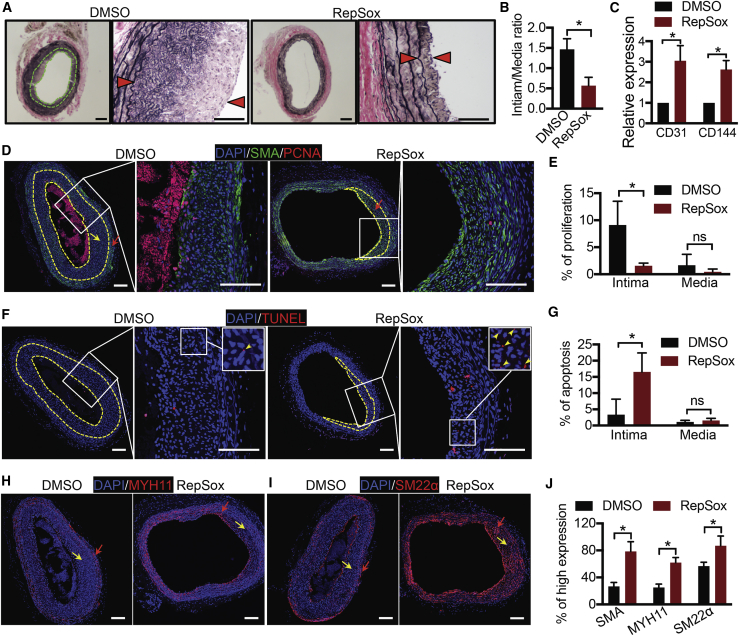
To test RepSox in vivo, we used a rat intimal hyperplasia model. RepSox or DMSO control was dissolved in a slow-released hydrogel and then applied to the outside of the injured artery segment immediately after balloon injury of the rat carotid artery (Chen et al., 2017). Carotid arteries were collected and sectioned 14 days after surgery. H&E staining revealed pronounced intimal hyperplasia in the control group (DMSO), which was reduced from 1.5 to 0.5 through treatment with RepSox (intima/media ratio, Figures 6A and 6B). To examine potential negative effects of RepSox on endothelial cells, we performed qPCR analysis for endothelial markers on treated arteries. RepSox increased endothelial cell marker expression compared with DMSO control-treated arteries (Figure 6C), suggesting that RepSox may not inhibit endothelium repair. To further understand how RepSox reduced intimal hyperplasia, we performed immunostaining. RepSox decreased proliferation and increased apoptosis in the intima but not in the media (Figures 6D–6G) and also increased the expression of contractile proteins SMA, MYH11, and SM22α (Figures 6D and 6H–6J). Thus, RepSox inhibited intimal hyperplasia in vivo.
Figure 6.
RepSox Inhibits Intimal Hyperplasia in Rat Balloon Injury Model
(A) Rat balloon injury experiment. H&E staining is shown. Arrowheads indicate the boundaries of the intima. Scale bars, 200 μm.
(B) Statistical data of intima/media ratio are presented as mean ± SD, n = 5 mice from 2 independent experiments. ∗p < 0.05, Student's t test.
(C) qRT-PCR of endothelial cell marker expression. Data are presented as mean ± SD, n = 3 mice. ∗p < 0.05, Student's t test.
(D) Double immunostaining of anti-SMA and anti-PCNA (labeling endothelial cells). Neointima is the area between the yellow dashed lines. Red arrows indicate the region with high SMA expression. Yellow arrows indicate the region with low SMA expression. Scale bars, 100 μm.
(E) Statistical data of proliferation are presented as mean ± SD, n = 3 mice. ns, not significant; ∗p < 0.05, Student's t test.
(F) TUNEL assay (labeling apoptotic cells). Neointima is the area between the yellow dashed lines. Scale bars, 100 μm.
(G) Statistical data of apoptosis are presented as mean ± SD, n = 3 mice. ns, not significant; ∗p < 0.05, Student's t test.
(H) Immunostaining of MYH11. Red arrows indicate the region with high MYH11 expression. Yellow arrows indicate the region with low MYH11 expression. Scale bars, 100 μm.
(I) Immunostaining of SM22α. Red arrows indicate the region with high SM22α expression. Yellow arrows indicate the region with low SM22α expression. Scale bars, 100 μm.
(J) Statistical data of SMA, MYH11, or SM22α expression. The “% of high expression” denotes the ratio (area of high expression)/(total area of intima and media). These are presented as mean ± SD, n = 3 mice. ∗p < 0.05, Student's t test.
Discussion
Vascular stent placement can abrade the endothelial cell layer and cause intimal hyperplasia and restenosis. In an attempt to reduce restenosis, drug-eluting stents have been developed to target either inflammation or cell proliferation (Pendyala et al., 2008). However, proliferation inhibitors currently used in stents, such as rapamycin and paclitaxel, are not cell-type specific and thus may inhibit endothelial repair (Prasad et al., 2005, Zheng et al., 2014). In an attempt to identify more cell-type-specific agents with fewer negative endothelial effects, we screened for drugs that reduce intimal hyperplasia by forcing differentiation of proliferative synthetic SMCs to a more mature, less proliferative contractile state. Interestingly, three compounds identified in our screen, UO126, Y27632, and retinoic acid, have all been shown to inhibit intimal hyperplasia in previous studies (DeRose et al., 1999, Gulkarov et al., 2009, Sawada et al., 2000); validating this as a suitable method for identifying inhibitors of intimal hyperplasia. Importantly, RepSox was identified in our assay as the most potent inducer of the contractile state, having not been described previously as such. In an in vivo artery balloon injury model, RepSox reduced neointimal formation, increased intimal apoptosis, and increased contractile protein expression in the neointima that did form (Figure 6). These results indicate potential as a very promising screening strategy for identifying additional candidate anti-restenotic drugs in larger libraries.
RepSox was reported previously to be a TGF-β signaling inhibitor and was used to replace Sox2 in reprogramming (Ichida et al., 2009), but our results suggest that RepSox regulated SMC differentiation via a TGF-β signaling-independent pathway. Our data show that RepSox promoted NOTCH signaling and inhibited TGF-β-SMAD2 signaling (Figure 2B). However, inhibition of TGF-β signaling alone failed to promote SMC differentiation to a contractile phenotype in vitro (Figures 2A–2C), nor did inhibition of TGF-β further enhance the contractile phenotype when combined with NOTCH signaling (Figure S2). Instead, at least in these in vitro conditions, NOTCH signaling alone was sufficient to promote the contractile state. However, the function of NOTCH signaling in intimal hyperplasia in vivo is controversial (Fouillade et al., 2012). TGF-β inhibition has been shown to inhibit intimal hyperplasia (Suwanabol et al., 2011), promote endothelial cell expansion (James et al., 2010), and promote arterial endothelial cell differentiation (Zhang et al., 2017); thus, our experiments cannot rule out that RepSox may be acting through distinct pathways in the more complex cellular environment in vivo. Finally, it is interesting to note that RepSox induced apoptosis in intima but not media layers (Figures 6F and 6G). NOTCH induces cell-cycle arrest followed by apoptosis (Chadwick et al., 2008). Given that RepSox reduced proliferation in intima (Figures 6D and 6E), it is possible that the apoptotic intima was caused by the cell-cycle arrest resulting from RepSox-activated NOTCH signaling.
The RepSox protocol described here produced SMCs in completely defined medium that were more mature than those reported previously. In a side-by-side comparison, both flow-cytometric analysis and qRT-PCR results revealed that RepSox-SMCs were expressed on levels an order of magnitude higher than MYH11 (a marker for the mature contractile phenotype) compared with a previous protocol that generated 80% MYH11+ SMCs (Cheung et al., 2012) (Figures S4C and S4D). In addition, previous protocols have used TGF-β1 and PDGF-BB, which have been reported to cause intimal hyperplasia in vivo (Muto et al., 2007, Nabel et al., 1993, Newby and Zaltsman, 2000, Raines, 2004, Suwanabol et al., 2011, Wolf et al., 1994). In contrast, RepSox promoted maturation in vitro and reduced intimal hyperplasia in vivo. Thus, RepSox-SMCs could reduce the risk of intimal hyperplasia if used in tissue-engineered vascular constructs.
Serum has been widely used in previous smooth muscle differentiation studies (Bajpai et al., 2012, Karamariti et al., 2013, Wang et al., 2014, Wanjare et al., 2013). However, serum suppresses the contractile phenotype of SMCs (Wanjare et al., 2013), suggesting that signals from serum may have compromised SMC maturation. Two potential signals could arise from PDGF-BB and insulin, both of which can be found in serum (Czarkowska-Paczek et al., 2006, Osei et al., 1993). PDGF-BB has been shown to promote the synthetic phenotype of SMCs as well as intimal hyperplasia (Muto et al., 2007, Newby and Zaltsman, 2000, Raines, 2004, Wanjare et al., 2013). Also, our data show that adding insulin from day 3 to day 8 reduced SMC differentiation (Figure S3F). In addition to being present in serum, insulin is one component of serum replacement used in previous protocols (Cao et al., 2013, Cheung et al., 2012, Patsch et al., 2015, Yang et al., 2016). Our differentiation medium is fully defined, and the differentiation could also be performed on a defined substrate (recombinant vitronectin) (Figure S3I). Thus, a fully defined xeno-free protocol will facilitate the generation of contractile SMCs and investigation of the molecular mechanisms underlying SMC differentiation. Finally, RepSox is a small molecule that is more stable and cheaper than growth factors previously employed; thus, our protocol is more suitable to large-scale applications and could be useful clinically.
Vascular SMCs have multiple origins in vivo (Sinha et al., 2014, Wang et al., 2015) including paraxial and lateral plate mesoderm, neural crest, second heat field, epicardium, pleural mesothelium, and nephrogenic stromal cells. In the mouse, a Cre-loxp lineage-tracing experiment demonstrated that AoSMCs are derived from a MEOX1-positive population (Wasteson et al., 2008). Because MEOX1 is a paraxial mesoderm marker, this suggests AoSMCs are derived from paraxial mesoderm. However, whether these MEOX1-derived SMC progenitors express other classical paraxial mesoderm markers in vivo is currently unknown, and they may represent a distinct subset of the paraxial mesoderm. The SMC mesoderm population we produced expressed both paraxial (MEOX1 and MSGN1) and lateral plate (ISL1 and NKX2.5) mesodermal markers (Figure S3E). Thus it is likely that this mesoderm is a mixture of both lateral plate and paraxial mesoderm, although it is also possible that MEOX1 SMC progenitors display a unique molecular signature.
Human PSCs offer a scalable, consistent, genetically defined source of SMCs and arterial endothelial cells (Bajpai et al., 2012, Cao et al., 2013, Cheung et al., 2012, Dash et al., 2016, Karamariti et al., 2013, Patsch et al., 2015, Wang et al., 2014, Wanjare et al., 2013, Yang et al., 2016, Zhang et al., 2011, Zhang et al., 2017) with applications in vascular disease modeling, drug discovery, and tissue engineering. Using the most active hit from our screen, a protocol was developed for differentiating contractile SMCs from human PSCs in fully defined, xeno-free medium. The protocol is efficient and works robustly for multiple iPSC and ESC lines. Such SMCs should also be useful in tissue-engineered vascular constructs, as their more mature state should reduce intimal hyperplasia, thereby reducing the likelihood of graft failure.
Experimental Procedures
Smooth Muscle Cell Differentiation
Human PSCs (H1) were cultured in E8 medium on a Matrigel-coated plate. On the day of differentiation, ESCs were dissociated by Accutase and plated on a Matrigel-coated plate at 1:4 ratios (1 × 105 cells/cm2). The cells were cultured in E8BAC medium for 36 h. At 36 h, the cells were passaged and seeded on a new Matrigel-coated plate (1.6 × 104 cells/cm2). The cells were treated with E6T medium for 18 h to induce the transient and medium-level expression of MEOX1. E5F medium was used to suppress MEOX1 expression for another 5 days (days 3–8). Next, the cells were treated with FVR medium to induce SMC progenitors from day 8 to day 12. E6R medium supplemented with RepSox (25 μM) was then used to further mature SMCs from day 12 to day 24. Cells were split (1 × 105 cells/cm2) at day 16 and further differentiated until day 24. For P-SMCs, T-SMCs, and PT-SMCs, RepSox was replaced by PDGF-BB, TGF-β1, or a combination of PDGF-BB and TGF-β1 from day 12 to day 24.
A more detailed protocol is provided in Supplemental Information.
Author Contributions
J.Z. designed and performed the experiments, analyzed the data, and wrote the paper. B.E.M. and M.D.P. performed the high-throughput screening. L.-W.G. and C.K. designed, and B.W. performed, rat balloon injury experiments. M.E.B. and W.J.B. designed, and M.E.B. and Y.Z. performed, kidney capsule experiments. S.W. provided technical support for cell culture. B.D. provided technical support for mouse experiments. M.F. wrote the algorithms for high-throughput screening. J.A.T. designed the experiments and revised the manuscript.
Acknowledgments
This work was supported by the National Institutes of Health (1UH2TR000506-01) (to J.A.T.) and (T32AI125231) (to M.E.B.). We thank Erin Syth for critical reading and editorial assistance.
Published: May 9, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.stemcr.2019.04.013.
Contributor Information
Jue Zhang, Email: juzhang@morgridge.org.
James A. Thomson, Email: jthomson@morgridge.org.
Supplemental Information
References
- Bajpai V.K., Mistriotis P., Loh Y.H., Daley G.Q., Andreadis S.T. Functional vascular smooth muscle cells derived from human induced pluripotent stem cells via mesenchymal stem cell intermediates. Cardiovasc. Res. 2012;96:391–400. doi: 10.1093/cvr/cvs253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beamish J.A., He P., Kottke-Marchant K., Marchant R.E. Molecular regulation of contractile smooth muscle cell phenotype: implications for vascular tissue engineering. Tissue Eng. Part B Rev. 2010;16:467–491. doi: 10.1089/ten.teb.2009.0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozovich F.V., Nicholson C.J., Degen C.V., Gao Y.Z., Aggarwal M., Morgan K.G. Mechanisms of vascular smooth muscle contraction and the basis for pharmacologic treatment of smooth muscle disorders. Pharmacol. Rev. 2016;68:476–532. doi: 10.1124/pr.115.010652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao N., Liang H., Huang J., Wang J., Chen Y., Chen Z., Yang H.T. Highly efficient induction and long-term maintenance of multipotent cardiovascular progenitors from human pluripotent stem cells under defined conditions. Cell Res. 2013;23:1119–1132. doi: 10.1038/cr.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick N., Fennessy C., Nostro M.C., Baron M., Brady G., Buckle A.M. Notch induces cell cycle arrest and apoptosis in human erythroleukaemic TF-1 cells. Blood Cells Mol. Dis. 2008;41:270–277. doi: 10.1016/j.bcmd.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Chen G., Gulbranson D.R., Hou Z., Bolin J.M., Ruotti V., Probasco M.D., Smuga-Otto K., Howden S.E., Diol N.R., Propson N.E. Chemically defined conditions for human iPSC derivation and culture. Nat. Methods. 2011;8:424–429. doi: 10.1038/nmeth.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Shi X., Wang B., Xie R., Guo L.W., Gong S., Kent K.C. Unimolecular micelle-based hybrid system for perivascular drug delivery produces long-term efficacy for neointima attenuation in rats. Biomacromolecules. 2017;18:2205–2213. doi: 10.1021/acs.biomac.7b00617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung C., Bernardo A.S., Trotter M.W., Pedersen R.A., Sinha S. Generation of human vascular smooth muscle subtypes provides insight into embryological origin-dependent disease susceptibility. Nat. Biotechnol. 2012;30:165–173. doi: 10.1038/nbt.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong L., Ran F.A., Cox D., Lin S., Barretto R., Habib N., Hsu P.D., Wu X., Jiang W., Marraffini L.A. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czarkowska-Paczek B., Bartlomiejczyk I., Przybylski J. The serum levels of growth factors: PDGF, TGF-beta and VEGF are increased after strenuous physical exercise. J. Physiol. Pharmacol. 2006;57:189–197. [PubMed] [Google Scholar]
- Dangas G., Kuepper F. Cardiology patient page. Restenosis: repeat narrowing of a coronary artery: prevention and treatment. Circulation. 2002;105:2586–2587. doi: 10.1161/01.cir.0000019122.00032.df. [DOI] [PubMed] [Google Scholar]
- Dash B.C., Jiang Z., Suh C., Qyang Y. Induced pluripotent stem cell-derived vascular smooth muscle cells: methods and application. Biochem. J. 2015;465:185–194. doi: 10.1042/BJ20141078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash B.C., Levi K., Schwan J., Luo J., Bartulos O., Wu H., Qiu C., Yi T., Ren Y., Campbell S. Tissue-engineered vascular rings from human iPSC-derived smooth muscle cells. Stem Cell Reports. 2016;7:19–28. doi: 10.1016/j.stemcr.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries M.R., Simons K.H., Jukema J.W., Braun J., Quax P.H. Vein graft failure: from pathophysiology to clinical outcomes. Nat. Rev. Cardiol. 2016;13:451–470. doi: 10.1038/nrcardio.2016.76. [DOI] [PubMed] [Google Scholar]
- DeRose J.J., Jr., Madigan J., Umana J.P., Prystowsky J.H., Nowygrod R., Oz M.C., Todd G.J. Retinoic acid suppresses intimal hyperplasia and prevents vessel remodeling following arterial injury. Cardiovasc. Surg. 1999;7:633–639. doi: 10.1016/s0967-2109(99)00041-1. [DOI] [PubMed] [Google Scholar]
- Fouillade C., Monet-Lepretre M., Baron-Menguy C., Joutel A. Notch signalling in smooth muscle cells during development and disease. Cardiovasc. Res. 2012;95:138–146. doi: 10.1093/cvr/cvs019. [DOI] [PubMed] [Google Scholar]
- Goel S.A., Guo L.W., Wang B., Guo S., Roenneburg D., Ananiev G.E., Hoffmann F.M., Kent K.C. High-throughput screening identifies idarubicin as a preferential inhibitor of smooth muscle versus endothelial cell proliferation. PLoS One. 2014;9:e89349. doi: 10.1371/journal.pone.0089349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulkarov I., Bohmann K., Cinnante K.M., Pirelli L., Yu P.J., Grau J.B., Pintucci G., Galloway A.C., Mignatti P. Topical mitogen-activated protein kinases inhibition reduces intimal hyperplasia in arterialized vein grafts. J. Surg. Res. 2009;154:150–156. doi: 10.1016/j.jss.2008.04.025. [DOI] [PubMed] [Google Scholar]
- Hou Z., Zhang Y., Propson N.E., Howden S.E., Chu L.F., Sontheimer E.J., Thomson J.A. Efficient genome engineering in human pluripotent stem cells using Cas9 from Neisseria meningitidis. Proc. Natl. Acad. Sci. U S A. 2013;110:15644–15649. doi: 10.1073/pnas.1313587110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichida J.K., Blanchard J., Lam K., Son E.Y., Chung J.E., Egli D., Loh K.M., Carter A.C., Di Giorgio F.P., Koszka K. A small-molecule inhibitor of tgf-Beta signaling replaces sox2 in reprogramming by inducing nanog. Cell Stem Cell. 2009;5:491–503. doi: 10.1016/j.stem.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James D., Nam H.S., Seandel M., Nolan D., Janovitz T., Tomishima M., Studer L., Lee G., Lyden D., Benezra R. Expansion and maintenance of human embryonic stem cell-derived endothelial cells by TGFbeta inhibition is Id1 dependent. Nat. Biotechnol. 2010;28:161–166. doi: 10.1038/nbt.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamariti E., Margariti A., Winkler B., Wang X., Hong X., Baban D., Ragoussis J., Huang Y., Han J.D., Wong M.M. Smooth muscle cells differentiated from reprogrammed embryonic lung fibroblasts through DKK3 signaling are potent for tissue engineering of vascular grafts. Circ. Res. 2013;112:1433–1443. doi: 10.1161/CIRCRESAHA.111.300415. [DOI] [PubMed] [Google Scholar]
- Lin H., Qiu X., Du Q., Li Q., Wang O., Akert L., Wang Z., Anderson D., Liu K., Gu L. Engineered microenvironment for manufacturing human pluripotent stem cell-derived vascular smooth muscle cells. Stem Cell Reports. 2019;12:84–97. doi: 10.1016/j.stemcr.2018.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack C.P. Signaling mechanisms that regulate smooth muscle cell differentiation. Arterioscler. Thromb. Vasc. Biol. 2011;31:1495–1505. doi: 10.1161/ATVBAHA.110.221135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali P., Yang L., Esvelt K.M., Aach J., Guell M., DiCarlo J.E., Norville J.E., Church G.M. RNA-guided human genome engineering via Cas9. Science. 2013;339:823–826. doi: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto A., Fitzgerald T.N., Pimiento J.M., Maloney S.P., Teso D., Paszkowiak J.J., Westvik T.S., Kudo F.A., Nishibe T., Dardik A. Smooth muscle cell signal transduction: implications of vascular biology for vascular surgeons. J. Vasc. Surg. 2007;45(Suppl A):A15–A24. doi: 10.1016/j.jvs.2007.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabel E.G., Shum L., Pompili V.J., Yang Z.Y., San H., Shu H.B., Liptay S., Gold L., Gordon D., Derynck R. Direct transfer of transforming growth factor beta 1 gene into arteries stimulates fibrocellular hyperplasia. Proc. Natl. Acad. Sci. U S A. 1993;90:10759–10763. doi: 10.1073/pnas.90.22.10759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newby A.C., Zaltsman A.B. Molecular mechanisms in intimal hyperplasia. J. Pathol. 2000;190:300–309. doi: 10.1002/(SICI)1096-9896(200002)190:3<300::AID-PATH596>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Osei K., Cottrell D.A., Adenuwon C.A., Ezenwaka E.C., Akanji A.O., O'Dorisio T.M. Serum insulin and glucose concentrations in people at risk for type II diabetes. A comparative study of African Americans and Nigerians. Diabetes Care. 1993;16:1367–1375. doi: 10.2337/diacare.16.10.1367. [DOI] [PubMed] [Google Scholar]
- Owens G.K., Kumar M.S., Wamhoff B.R. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol. Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- Pannu H., Tran-Fadulu V., Papke C.L., Scherer S., Liu Y., Presley C., Guo D., Estrera A.L., Safi H.J., Brasier A.R. MYH11 mutations result in a distinct vascular pathology driven by insulin-like growth factor 1 and angiotensin II. Hum. Mol. Genet. 2007;16:2453–2462. doi: 10.1093/hmg/ddm201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsch C., Challet-Meylan L., Thoma E.C., Urich E., Heckel T., O'Sullivan J.F., Grainger S.J., Kapp F.G., Sun L., Christensen K. Generation of vascular endothelial and smooth muscle cells from human pluripotent stem cells. Nat. Cell Biol. 2015;17:994–1003. doi: 10.1038/ncb3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendyala L., Jabara R., Shinke T., Chronos N., Robinson K., Li J., Hou D. Drug-eluting stents: present and future. Cardiovasc. Hematol. Agents Med. Chem. 2008;6:105–115. doi: 10.2174/187152508783955051. [DOI] [PubMed] [Google Scholar]
- Prasad C.K., Resmi K.R., Krishnan L.K., Vaishnav R. Survival of endothelial cells in vitro on Paclitaxel-loaded coronary stents. J. Biomater. Appl. 2005;19:271–286. doi: 10.1177/0885328205047397. [DOI] [PubMed] [Google Scholar]
- Raines E.W. PDGF and cardiovascular disease. Cytokine Growth Factor Rev. 2004;15:237–254. doi: 10.1016/j.cytogfr.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Rensen S.S., Doevendans P.A., van Eys G.J. Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Neth. Heart J. 2007;15:100–108. doi: 10.1007/BF03085963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada N., Itoh H., Ueyama K., Yamashita J., Doi K., Chun T.H., Inoue M., Masatsugu K., Saito T., Fukunaga Y. Inhibition of rho-associated kinase results in suppression of neointimal formation of balloon-injured arteries. Circulation. 2000;101:2030–2033. doi: 10.1161/01.cir.101.17.2030. [DOI] [PubMed] [Google Scholar]
- Sinha S., Iyer D., Granata A. Embryonic origins of human vascular smooth muscle cells: implications for in vitro modeling and clinical application. Cell. Mol. Life Sci. 2014;71:2271–2288. doi: 10.1007/s00018-013-1554-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwanabol P.A., Kent K.C., Liu B. TGF-beta and restenosis revisited: a Smad link. J. Surg. Res. 2011;167:287–297. doi: 10.1016/j.jss.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Hu J., Jiao J., Liu Z., Zhou Z., Zhao C., Chang L.J., Chen Y.E., Ma P.X., Yang B. Engineering vascular tissue with functional smooth muscle cells derived from human iPS cells and nanofibrous scaffolds. Biomaterials. 2014;35:8960–8969. doi: 10.1016/j.biomaterials.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Jacquet L., Karamariti E., Xu Q. Origin and differentiation of vascular smooth muscle cells. J. Physiol. 2015;593:3013–3030. doi: 10.1113/JP270033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanjare M., Kuo F., Gerecht S. Derivation and maturation of synthetic and contractile vascular smooth muscle cells from human pluripotent stem cells. Cardiovasc. Res. 2013;97:321–330. doi: 10.1093/cvr/cvs315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasteson P., Johansson B.R., Jukkola T., Breuer S., Akyurek L.M., Partanen J., Lindahl P. Developmental origin of smooth muscle cells in the descending aorta in mice. Development. 2008;135:1823–1832. doi: 10.1242/dev.020958. [DOI] [PubMed] [Google Scholar]
- Wolf Y.G., Rasmussen L.M., Ruoslahti E. Antibodies against transforming growth factor-beta 1 suppress intimal hyperplasia in a rat model. J. Clin. Invest. 1994;93:1172–1178. doi: 10.1172/JCI117070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Geng Z., Nickel T., Johnson C., Gao L., Dutton J., Hou C., Zhang J. Differentiation of human induced-pluripotent stem cells into smooth-muscle cells: two novel protocols. PLoS One. 2016;11:e0147155. doi: 10.1371/journal.pone.0147155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Lian Q., Zhu G., Zhou F., Sui L., Tan C., Mutalif R.A., Navasankari R., Zhang Y., Tse H.F. A human iPSC model of Hutchinson Gilford progeria reveals vascular smooth muscle and mesenchymal stem cell defects. Cell Stem Cell. 2011;8:31–45. doi: 10.1016/j.stem.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Zhang J., Chu L.F., Hou Z., Schwartz M.P., Hacker T., Vickerman V., Swanson S., Leng N., Nguyen B.K., Elwell A. Functional characterization of human pluripotent stem cell-derived arterial endothelial cells. Proc. Natl. Acad. Sci. U S A. 2017;114:E6072–E6078. doi: 10.1073/pnas.1702295114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng N., Ding X., Jahan R. Low concentration of rapamycin inhibits hemangioma endothelial cell proliferation, migration, and vascular tumor formation in mice. Curr. Ther. Res. Clin. Exp. 2014;76:99–103. doi: 10.1016/j.curtheres.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.