Abstract
Safe consumption spaces (SCS) are evidence-based interventions that reduce drug-related morbidity and mortality operating in many countries. However, SCS are yet to be widely implemented in the USA despite the escalating overdose epidemic. The aim of this multi-city study was to identify the factors associated with willingness to use a SCS among people who use drugs (PWUD) in Baltimore, Providence, and Boston, stratified by injection drug use status. Our secondary aim was to characterize the anticipated barriers to accessing SCS if they were to be implemented in these cities. PWUD were invited to complete a cross-sectional survey in 2017. The analysis was restricted to 326 opioid users (i.e., heroin, fentanyl, and non-medical opioid pill use). The majority (77%) of participants expressed willingness to use a SCS (Baltimore, 78%; Providence, 68%; Boston. 84%). Most respondents were male (59%), older than 35 years (76%), non-white (64%), relied on public/semi-public settings to inject (60%), had a history of overdose (64%), and recently suspected fentanyl contamination of their drugs (73%). A quarter (26%) preferred drugs containing fentanyl. Among injectors, female gender, racial minority status, suspicion of drugs containing fentanyl, and drug use in public/semi-public settings were associated with higher willingness to use a SCS; prior arrest was associated with lower willingness. Among non-injectors, racial minority status, preference for fentanyl, and drug use in public/semi-public settings were associated with higher willingness, whereas recent overdose held a negative association. The most commonly anticipated barriers to accessing a SCS in the future were concerns around arrest (38%), privacy (34%), confidentiality/trust/safety (25%), and cost/time/transportation (16%). These data provide evidence of high SCS acceptability among high-risk PWUD in the USA, including those who prefer street fentanyl. As SCS are implemented in the USA, targeted engagement efforts may be required to reach individuals exposed to the criminal justice system.
Keywords: Supervised injection, Substance use, Addiction, Overdose prevention
Globally, there is a growing need for the development, implementation, and evaluation of innovative and evidence-based interventions to reduce the burden of drug overdose mortality, particularly those that target high-risk populations. The overdose epidemic claimed more than 72,000 US lives in 2017 alone, driven increasingly in recent years by illicitly manufactured fentanyl and other synthetic opioids in the drug supply [1], the purity of which are highly volatile and unregulated due to the criminalization of manufacturing and selling drugs. In many states, fentanyl has overtaken the heroin supply and has also been found in street-level opioid pills, K2, and cocaine [2].
Safer consumption spaces (SCS) are places that PWUD can go to use previously purchased drugs in a controlled environment, and there they can receive medical care (e.g., wound/abscess care), harm reduction supplies (such as sterile syringes and naloxone), HIV testing, and referrals to drug treatment [3]. At these sites, which are also referred to as supervised injection facilities (SIF), medically supervised injection centres (MSIC), and overdose prevention sites (OPS), staff are equipped with naloxone in case of an overdose [4, 5]. SCS have existed for 30 years and currently operate legally in 11 countries worldwide, with no documented fatal overdoses recorded [4]. The majority of the peer-reviewed evidence base stems from Canada and Australia and focuses primarily on mitigating the risks associated with injection drug use. A study from Vancouver, Canada, demonstrated 35% decreases in overdose mortality after the implementation of a SIF, and other research has shown increases in drug treatment uptake among SIF clients vs. non-clients [6–8].
Despite the literature demonstrating that SCS comprise an evidence-based strategy to reduce overdose mortality, and a national study showing that they are publicly supported [5], there are no legal SCS in the USA. In the context of the current opioid epidemic, SCS may be beneficial in numerous ways. First, they could be used to reduce the burden of overdose among a broader range of PWUD, including individuals who use pills, smoke, snort or inject heroin and fentanyl, and polysubstance users; second, they provide cost savings to the health system through averted overdoses, blood-borne infections, skin and tissue infections, among others [9, 10]; and third, they may be especially effective in preventing overdoses attributable to fentanyl, which occur more rapidly than heroin due to its absorption properties and hence require swift naloxone administration [11]. SCS can also deliver drug checking programs, which allow PWUD to check the contents of their drugs prior to use in order to dose more safely; such programs have been implemented at multiple SIFs in Canada.
Several studies have examined willingness to use SIFs among people who inject drugs (PWID) in the USA; three quantitative studies showed high willingness among PWID in San Francisco (85%), Boston (91%), and Rhode Island (87%) [12–14]. In all of these studies, elevated willingness to use a SIF was observed in high-risk subgroups such as PWID engaging in public injection and speedball use (concomitant heroin and cocaine use) and individuals with complex morbidities and high health service needs. International studies have shown that SIFs serve the most socially and structurally vulnerable individuals and that reported willingness to use a SIF predicts actual attendance and use, though more studies that are inclusive of non-injectors are required [4, 7]. Building on this research, the aims of this multi-city study were to identify the factors associated with willingness to use a SCS among injectors and non-injectors and to characterize the barriers to future SCS access that are anticipated by people who use opioids non-medically.
Methods
The Fentanyl Overdose REduction Checking Analysis STudy (FORECAST) is a multi-phase study investigating the validity and feasibility of drug checking tools for reducing overdose [15–18]. The study was conducted in three northeastern cities in the USA with large and established opioid markets and high burdens of fentanyl mortality. Between June and October 2017, 334 PWUD were recruited to participate in a survey; the methods have been previously described [16]. The study recruited from Baltimore, Maryland; Boston, Massachusetts; and Providence, Rhode Island at a time where fentanyl overdose rates were rapidly escalating and yet legally sanctioned SCS programs were absent. In Baltimore, Maryland, a grassroots coalition called BRIDGES Coalition has been working to build capacity for legislative advocacy surrounding SCS [3]. In Boston, Massachusetts, a coalition called SIFMA in conjunction with the Boston Area Drug Users Union (BADUU) was conducting education, advocacy, and drug policy work around SIF, and they were joined by support from the Massachusetts Medical Society, which had done its own evaluation and public statement in support of a pilot [19]. No such efforts existed in Providence at the time of the study though community organizing began recently in 2018.
Targeted sampling based on geospatial mapping of data from the Baltimore City Police Department [20] was used to recruit PWUD (n = 175) in Baltimore; specifically, we used ArcMap v10.2.1 to create heat maps of drug arrests that occurred between May 2016 and April 2017. We visually identified eight zones with concentrated drug-related arrests. Data from these zones were imported to SAS Enterprise Guide where we calculated frequency distributions of drug-related arrests by day and time (hour of arrest). We then developed a sampling frame that consisted of 4 hour periods with high arrest counts in each zone from which shifts could be randomly selected. In contrast, PWUD in Boston (n = 80) and Providence (n = 79) were recruited through syringe service programs (SSP) and harm reduction services. After briefly explaining the study, study interviewers conducted a brief screening and an anonymous 30-min computer-assisted personal interview, with individuals who were eligible to participate and who provided oral informed consent. Eligibility criteria were as follows: recent (past 30 days) non-medical heroin, fentanyl, cocaine, methamphetamine, or opioid pill use; 18 years or older; and ability to speak English. Participants were compensated with 25 USD for completing the study procedures. The study was approved by the Johns Hopkins Bloomberg School of Public Health and the Rhode Island Hospital Institutional Review Boards.
Conceptual Framework
This analysis is informed by the risk environment framework, which situates an individual’s level of overdose risk within broader physical, social, economic, and policy environments [21–23]. Specifically, we focused on factors operating at the micro-risk environment such as encounters with law enforcement driven by macro-level policies. We also examined the physical environment of PWUD. Local and national surges in the supply of illicitly manufactured fentanyl may shape drug use preferences, behaviors, and associated risks. High rates of urban poverty and homelessness may also shape PWUD’s willingness to engage in a SCS and other harm reduction services.
Measures
The Baltimore and Providence survey defined SCS as “a place where it would be legal for people to safely inject, snort, or smoke, or otherwise consume drugs that they buy somewhere else. You would not be arrested while in the site. There would be staff on-site to respond to an overdose, and to provide basic medical care and referrals to health and social services upon request.” Due to the language being actively used by local grassroots organizers in Boston at the time of the study, the term "supervised injection facilities" was used in the Boston survey instead of SCS. The term “supervised injection facilities” was selected by SIFMA and BADUU in order to gain state-level political support since it was the dominant phrase used to name safe places where people could use drugs under supervision at the time (i.e., in 2016), with the understanding that such spaces, if implemented, would not exclude other types of drug use.
Willingness to use a SCS/SIF was measured directly after providing the definition using a four-point Likert scale indicating likelihood of use (very likely/somewhat likely/somewhat unlikely/very unlikely). The variable was collapsed into a binary outcome for analysis (likely vs. unlikely). Anticipated barriers that would “make it difficult” to access a SCS/SIF were also ascertained.
The survey also measured sociodemographic characteristics (age, gender, race, ethnicity, current homelessness, health insurance status, employment status, and involvement in the drug trade and arrest). We measured substance use and overdose history (lifetime and past 6 months), including by drug type and route of administration and experiencing and witnessing a fatal overdose. Items on fentanyl were adapted from previous studies [24, 25] and included suspicion of drugs containing fentanyl within the past 6 months and preference for drugs containing fentanyl. The survey also included items on rushing drug purchases, preparation or use due to policing (past year), settings where drugs were usually used (past 30 days), and using drugs alone. Drug use setting [26] was modeled after our previous work: public spaces for drug use were defined as using in a street or park; semi-public spaces were defined as building stairwells, abandoned buildings, public restrooms, in a vehicle, and at a shooting gallery; and private spaces were in the participant’s home or somebody else’s home. The survey ascertained any access to a variety of health services in the past 6 months (emergency room, drug treatment, health care provider, and SSP), whether they currently had naloxone, and interest in using fentanyl test strips (FTS).
Statistical Analyses
The current analysis was restricted to opioid users due to their high risk of experiencing fentanyl overdose (overall, N = 326; Baltimore, n = 169; Providence, n = 78; Boston, n = 79). After calculating the overall prevalence of all covariates, we used Pearson’s chi-squared tests to explore and describe city-specific differences in relevant covariates by willingness to use a SCS. In order to model the correlates of willingness to use an SCS, bivariate and multivariate logistic regression analyses with clustered variance for each study city were used. Analyses were stratified by injection status (i.e., injected drugs in the past 6 months; yes/no), given that these are distinct target populations reached by different health services (e.g., SSP). First, Pearson’s chi-squared tests were used to test differences in the outcome. The subset of correlates significant at the p < 0.15 level was reported and was considered for inclusion in multivariate logistic modeling. The bivariate associations differed in each stratum; thus, the variables included in each multivariate model differed, and we chose the most parsimonious model for each stratum. We applied clustered variances by city of recruitment. We next conducted stratified analysis, which modeled the correlates of SCS willingness separately for each study city using the same procedures. Complete case analysis was used across all models due to the low level of missingness. All analyses were conducted in Stata/SE 14.2 (StataCorp: College Station, TX, USA).
Results
Sociodemographic Characteristics
Table 1 describes the sociodemographic characteristics of the sample. Most participants were over the age of 35 (76%), male (59%), and non-white (64%). Most had attained at least a high school diploma or GED (69%), were homeless (69%), and were legally unemployed (87%). Over half of the sample had sold drugs in the past 3 months (57%). History of arrest in the past year was common overall (47%), though less common specifically for drug-related arrest (27%). The majority of participants cited that policing in an area caused them to rush drug purchases (73%) or the preparation or use of drugs (67%) in the last year. The city-specific differences in demographics and drug use have been previously described [16]. Notably, the PWUD recruited in Baltimore, Providence, and Boston were similar in distributions of age, gender, and education level but differed significantly by race, i.e., proportion who were black (Baltimore, 66%; Providence, 24%; Boston, 24%); Hispanic (Baltimore, 1%; Providence, 19%; Boston, 27%); currently homeless (Baltimore, 61%; Providence, 67%; Boston, 87%); participated in the drug trade (Baltimore, 50%; Providence, 60%; Boston, 71%); and arrested in the past year (Baltimore, 33%; Providence, 50%; Boston, 72%) (χ2 tests, p < 0.05).
Table 1.
Sociodemographic characteristics and willingness to use a safe consumption space (SCS) among adult opioid users in Baltimore, Providence, and Boston (N = 326)
| Total | Willingness to use SCS | ||||
|---|---|---|---|---|---|
| N = 326 | All sites N = 326 |
Baltimore n = 169 |
Providence n = 78 |
Boston n = 79 |
|
| n (col%) | n (row%) | n (row%) | n (row%) | n (row%) | |
| Overall | 250 (76.7) | 131 (77.5) | 53 (67.9) | 66 (83.5) | |
| Age | |||||
| Younger (< 35 years) | 78 (23.9) | 58 (74.4) | 31 (77.5) | 11 (55.0) | 16 (88.9) |
| Older (≥ 35 years) | 248 (76.1) | 192 (77.4) | 100 (77.5) | 42 (72.4) | 50 (82.0) |
| Gender | |||||
| Male | 192 (59.1) | 147 (76.6) | 74 (79.6) | 24 (75.0) | 22 (84.6) |
| Female | 133 (40.9) | 103 (76.9) | 57 (75.0) | 53 (67.9) | 44 (83.0) |
| Race/ethnicity category | |||||
| Black, Hispanic, other | 207 (63.5) | 159 (76.8) | 96 (75.6) | 27 (69.2) | 36 (87.8) |
| White | 119 (36.5) | 91 (76.5) | 35 (83.3) | 26 (66.7) | 30 (78.9) |
| Highest level of education completed | |||||
| < High school/GED | 127 (39.0) | 94 (74.0) | 52 (74.3) | 17 (63.0) | 25 (83.3) |
| High school diploma/GED/college/associates | 199 (61.0) | 156 (78.4) | 79 (79.8) | 36 (70.6) | 41 (83.7) |
| Homeless, currently | 224 (68.7) | 178 (79.5) | 83 (80.6) | 37 (71.2) | 58 (84.1) |
| Legally unemployed | 283 (86.8) | 222 (78.4) | 118 (79.7) | 41 (68.3) | 63 (84.0) |
| Sold drugs, past 3 months | 187 (57.4) | 141 (75.4) | 64 (76.2) | 30 (63.8) | 47 (83.9) |
| Law enforcement encounters^ | |||||
| Arrest | 151 (46.5) | 114 (75.5) | 43 (78.2) | 25 (64.1) | 46 (80.7) |
| Drug-related arrest | 89 (27.3) | 67 (75.3) | 28 (77.8) | 14 (70.0) | 25 (75.8) |
| Rushed drug purchases due to policing | 239 (73.3) | 141 (75.4) | 88 (79.3) | 41 (75.9)* | 61 (82.4) |
| Rushed preparing/using drugs due to policing | 219 (67.2) | 200 (78.1) | 83 (79.1) | 31 (68.9) | 57 (82.6) |
^Past year
*p < 0.05
**p < 0.01
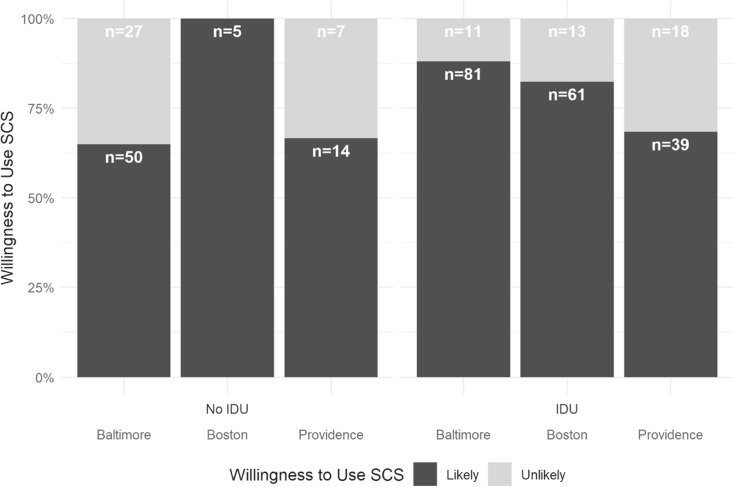
Overall, 77% of participants expressed a willingness to use SCS. Willingness was highest among PWUD in Boston (84%), followed by Baltimore (78%) and Providence (68%). However, non-injectors reported lower willingness to use SCS in Baltimore (Fig. 1). Willingness to use a SCS did not significantly differ in bivariate analysis by any of the sociodemographic factors or law enforcement encounters measured.
Fig. 1.
Willingness to use a safe consumption space among adult opioid users in Baltimore, Providence, and Boston by city and injection status (N = 326). IDU, injection drug use; SCS, safer consumption space
Substance Use and Overdose Risk
Patterns of substance use and overdose risk in the past 6 months are presented in Table 2. Due largely to the differences in recruitment methods, there were significant city differences in injection drug use (Baltimore, 54%; Providence, 73%; Boston, 86%; p < 0.001), smoking/snorting heroin (Baltimore, 53%; Providence, 36%; Boston, 20%; p < 0.001), cocaine snorting (Baltimore, 24%; Providence, 40%; Boston, 13%; p < 0.001), and methamphetamine use (Baltimore. 4%; Providence, 10%; Boston, 28%; p < 0.001). Pooled data revealed that the majority of participants reported injection drug use (66%), with people who had recently (within the past 6 months) injected drugs significantly more likely to report willingness to use a SCS (81%, p < 0.05). Heroin was the most commonly injected drug (61%), followed by speedball (40%) and cocaine (34%). Snorting or smoking heroin (41%) was less common. Smoking crack cocaine was much more prominent (73%) than snorting powdered cocaine (25%). Methamphetamine use was uncommon in our sample (11%). Participants who reported misuse of prescription benzodiazepine medications (43%) were significantly more likely to be willing to use a SCS (82%, p < 0.05) than those who did not. Polysubstance use (≥ 3 drugs) in the past 6 months was high (70%). The majority of participants reported recent use of a drug they suspected of containing fentanyl (73%), of whom 84% were concerned about such adulteration. A quarter (26%) of participants reported a preference for drugs with fentanyl, and this subgroup was significantly more likely to be willing to use a SCS (85%, p < 0.05). Alcohol use was reported by 68% of the sample.
Table 2.
Substance use and overdose risk factors and willingness to use a safe consumption space among adult opioid users in Baltimore, Providence, and Boston (N = 326)
| Willing to use SCS | |||||
|---|---|---|---|---|---|
| N = 326 | All sites N = 326 |
Baltimore n = 169 |
Providence n = 78 |
Boston n = 79 |
|
| n (col%) | n (row%) | n (row%) | n (row%) | n (row%) | |
| Overall | 250 (76.7) | 131 (77.5) | 53 (67.9) | 66 (83.5) | |
| Substance use# | |||||
| Injection drug use (IDU) | 216 (66.3) | 174 (80.6)* | 81 (88.0)** | 39 (68.4) | 54 (80.6) |
| Heroin | 199 (61.0) | 159 (79.9) | 70 (87.5)** | 38 (69.1) | 51 (79.7) |
| Cocaine | 110 (33.7) | 91 (82.7) | 41 (89.1)* | 17 (73.9) | 33 (80.5) |
| Heroin and cocaine (“speedball”) | 131 (40.2) | 107 (81.7) | 53 (85.5) | 15 (71.4) | 39 (81.2) |
| Heroin, snorted/smoked | 133 (40.8) | 96 (72.2) | 65 (73.0) | 19 (67.9) | 12 (75.0) |
| Non-medical prescription opioid pill use | 118 (36.2) | 96 (81.4) | 53 (81.5) | 22 (84.6)* | 21 (77.8) |
| Crack cocaine, smoked | 238 (73.0) | 187 (78.6) | 99 (76.7) | 39 (76.5)* | 49 (84.5) |
| Powdered cocaine, snorted | 82 (25.2) | 62 (75.6) | 33 (80.5) | 20 (64.5) | 9 (90.0) |
| Methamphetamine, any route | 37 (11.4) | 30 (81.1) | 6 (85.7) | 4 (50.0) | 20 (90.9) |
| Non-medical benzodiazepine use | 140 (42.9) | 115 (82.1)* | 51 (89.5)* | 25 (71.4) | 39 (81.2) |
| Thought drugs contained fentanyl | 239 (73.3) | 189 (79.1)* | 102 (81.6) | 29 (64.4) | 58 (84.1) |
| Concerned about drugs containing fentanyl | 272 (84.0) | 208 (76.5) | 103 (75.7) | 45 (70.3) | 60 (83.3) |
| Prefer drugs that contain fentanyl | 84 (25.9) | 71 (84.5)* | 45 (88.2)* | 8 (72.7) | 18 (81.8) |
| Alcohol use | 221 (67.8) | 166 (75.1) | 83 (74.1) | 39 (69.6) | 44 (83.0) |
| Overdose history and risk | |||||
| Overdosed, lifetime | 209 (64.1) | 164 (78.5) | 75 (81.5) | 32 (66.7) | 57 (82.6) |
| Overdosed ≥ 1 time# | 113 (34.7) | 86 (76.1) | 45 (77.6) | 13 (59.1) | 28 (84.8) |
| Usually used drugs alone | 158 (48.6) | 118 (74.7) | 74 (75.5) | 25 (65.8) | 19 (86.4) |
| Usual drug use setting& | |||||
| Public/semi-public | 196 (60.1) | 164 (83.7)** | 85 (86.7)** | 23 (69.7) | 56 (86.2) |
| i. Public | |||||
| Street or park | 108 (33.1) | 89 (82.4) | – | – | – |
| ii. Semi-public | |||||
| An abandoned building | 55 (16.9) | 45 (81.8) | – | – | – |
| Public bathroom | 18 (5.5) | 15 (83.3) | – | – | – |
| In a car or other vehicle | 4 (1.2) | 4 (100.0) | – | – | – |
| At a shooting gallery | 8 (2.5) | 8 (100.0) | – | – | – |
| Private residence | 128 (39.3) | 84 (65.6) | 45 (64.3) | 29 (67.4) | 10 (71.4) |
| At your home | 69 (21.2) | 47 (68.1) | – | – | – |
| At someone else’s home | 59 (18.1) | 38 (64.4) | – | – | – |
| Witnessed a fatal overdose | 138 (42.3) | 108 (78.3) | 56 (78.9) | 15 (65.2) | 37 (84.1) |
#Past 6 months
&Past 30 days
*p < 0.05
**p < 0.01
A third (35%) of the sample reported experiencing at least one opioid overdose in the past 6 months. Lifetime witnessing of a fatal overdose was common (42%). When asked what types of locations participants usually used their drugs, the majority listed public or semi-public spaces (60%). Participants reporting use in non-private spaces were significantly more likely to be willing to use a SCS (84%, p < 0.01). Nearly half of the sample reported usually using drugs alone (49%).
Access to Health Services
Access to health services are detailed in Table 3. The majority of participants currently had health insurance (89%), and almost half reported seeking care at an emergency room in the past 6 months (47%). Half of participants had been in some form of drug treatment program in the past 6 months (52%), with medically assisted treatment being the most common, followed by rehabilitation and drug detoxification programs (32%, 11%, and 11%, respectively). Roughly a quarter had recently received counseling, participated in a support group, or received mental health care (28%). Just over half of participants had recently gone to visit a health care provider outside of an emergency room (53%). Most had received opioid overdose response or naloxone training in the past year (70%), with nearly half reporting currently possessing naloxone (47%). Drug checking interest was high in the sample: 90% were interested in using take-home FTS, and 76% were interested in an on-site drug checking service. Willingness to use a SCS did not differ significantly by any health care access variable explored.
Table 3.
Access to health services and willingness to use a safe consumption space among adult opioid users in Baltimore, Providence, and Boston (N = 326)
| Willing to use SCS | |||||
|---|---|---|---|---|---|
| All sites N = 326 |
Baltimore n = 169 |
Providence n = 78 |
Boston n = 79 |
||
| n (col%) | n (row%) | n (row%) | n (row%) | n (row%) | |
| Overall | 250 (76.7) | 131 (77.5) | 53 (67.9) | 66 (83.5) | |
| Health insurance, current | 290 (89.0) | 219 (75.5) | 107 (75.4) | 48 (67.6) | 64 (83.1) |
| Emergency room (ER) visit# | 153 (46.9) | 124 (81.0) | 60 (82.2) | 28 (73.7) | 36 (85.7) |
| Drug treatment# | 170 (52.1) | 128 (75.3) | 53 (74.6) | 27 (67.5) | 48 (81.4) |
| Medication-assisted treatment | 105 (32.2) | 84 (80.0) | 38 (82.6) | 14 (70.0) | 32 (82.1) |
| Rehabilitation | 36 (11.0) | 27 (75.0) | 6 (66.7) | 13 (68.4) | 8 (100) |
| Detoxification | 35 (10.7) | 26 (74.3) | 5 (62.5) | 7 (77.8) | 14 (77.8) |
| Counseling/mental health/support group | 90 (27.6) | 64 (71.1) | 26 (63.4)* | 14 (66.7) | 24 (85.7) |
| Doctor/health provider visit (non-ER)# | 171 (52.5) | 137 (80.1) | 56 (77.8) | 33 (75) | 48 (87.3) |
| Received naloxone training^ | 227 (69.6) | 178 (78.4) | 88 (80.7) | 29 (63.0) | 54 (83.1) |
| Has naloxone, current | 151 (46.6) | 113 (74.8) | 46 (75.4) | 25 (64.1) | 42 (82.4) |
| Interest in take-home fentanyl test strips | 293 (89.9) | 226 (77.1) | 119 (78.3) | 47 (68.1) | 60 (83.3) |
| Interest in on-site drug checking service | 247 (75.8) | 193 (78.1) | 89 (78.8) | 47 (70.1) | 57 (85.1) |
p < 0.05 denoted in italics
#Past 6 months
^Past 12 months
*p < 0.05
**p < 0.01
Adjusted Correlates of Willingness to Use a SCS: Overall and by Injection Status
Pooled multivariate analysis revealed higher willingness to use a SCS among non-white PWUD (AOR = 1.47, p < 0.001), those who preferred drugs containing fentanyl (AOR = 1.82, p = 0.012) and public/semi-public drug use (AOR = 3.07, p < 0.001), and no significant associations with age, gender, injection drug use, overdose, or thinking drugs contained fentanyl (Table 4). We observed distinct correlates of willingness to use a SCS among injectors (n = 213) versus non-injectors (n = 109), as shown in Table 4. Among injectors, willingness to use SCS was independently associated with gender (female AOR = 2.16, p = 0.005), arrest in the past year (AOR = 0.46, p = 0.002), and suspicion that drugs contained fentanyl in the past 6 months (AOR = 1.74, p = 0.002). Among non-injectors, we observed a significant association between higher willingness to use SCS with increasing age (per 10 years, AOR = 1.18, p = 0.038), as well as those who reported preferring fentanyl (AOR = 2.72, p = 0.025). Lower odds of reported willingness was detected among individuals with recent overdose among non-injectors (AOR = 0.22, p < 0.001). Across strata, race and usual drug use setting were independently associated with willingness to use SCS. Non-white respondents were significantly more likely to express willingness to use SCS (AOR among injectors = 1.23, p = 0.01; AOR among non-injectors = 2.15, p < 0.001). Respondents usually using drugs in public or semi-public settings had over 3.5 times greater odds of being willing to use a SCS, whether they had injected in the past 6 months (OR 3.57, p = 0.005) or not injected (OR 3.52, p = 0.003).
Table 4.
Multivariate regression models of willingness to use a safe consumption space among adult opioid users by injection status in Baltimore, Providence and Boston
| Overall | Injection drug use, past 6 months | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| (N = 322) | Yes (N = 213) | No (N = 109) | |||||||
| AOR | 95% CI | p | AOR | 95% CI | p | AOR | 95% CI | p | |
| Age (in decades) | 1.20 | 0.9, 1.6 | 0.233 | 1.28 | 0.66, 2.52 | 0.463 | 1.18 | 1.01, 1.37 | 0.038 |
| Gender | |||||||||
| Male | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | ||||||
| Female | 1.42 | 0.9, 2.3 | 0.136 | 2.16 | 1.26, 3.67 | 0.005 | 0.83 | 0.62, 1.10 | 0.196 |
| Race/ethnicity | |||||||||
| Black, Hispanic, other | 1.47 | 1.5, 1.5 | < 0.001 | 1.23 | 1.05, 1.46 | 0.01 | 2.15 | 1.70, 2.74 | < 0.001 |
| White | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | ||||||
| Arrest, past year | 0.65 | 0.5, 0.8 | < 0.001 | 0.46 | 0.28, 0.76 | 0.002 | 0.83 | 0.59, 1.18 | 0.296 |
| Injection drug use# | 2.17 | 0.8, 5.87 | 0.127 | – | – | – | – | – | – |
| Crack use# | – | – | – | – | – | – | – | – | – |
| Non-medical benzodiazepine use# | – | – | – | – | – | – | – | – | – |
| Overdose# | 0.66 | 0.3, 1.5 | 0.325 | – | – | – | 0.22 | 0.11, 0.45 | < 0.001 |
| Thought drugs contained fentanyl | 1.19 | 0.8, 1.9 | 0.453 | 1.74 | 1.34, 2.25 | 0.002 | – | – | – |
| Prefer drugs that contain fentanyl | 1.82 | 1.1, 2.9 | 0.012 | 1.54 | 0.80, 2.98 | 0.196 | 2.72 | 1.13, 6.58 | 0.025 |
| Usual drug use setting& | |||||||||
| Private | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | ||||||
| Public and semi-public | 3.07 | 1.9, 4.9 | < 0.001 | 3.57 | 1.47, 8.63 | 0.005 | 3.37 | 1.53, 7.43 | 0.003 |
Multivariate logistic regression with clustered variance for city
p < 0.05 denoted in italics
#Past 6 months
&Past 30 days
Adjusted Correlates of Willingness to Use a SCS by Study Location
The correlates of SCS willingness differed by city in multivariate analysis (Table 5). In Baltimore, SCS willingness was higher among those who engaged in injection drug use (AOR = 3.86, p = 0.004), non-medical benzodiazepine use (AOR = 3.78, p = 0.012), and relied on public/semi-public drug use (AOR = 3.19, p = 0.007). In Providence, older age (AOR = 1.95, p = 0.032) and crack use (AOR = 2.96, p = 0.049) were positively associated with SCS willingness whereas no significant correlates emerged among PWUD in Boston.
Table 5.
Multivariate regression models of willingness to use a safe consumption space among adult opioid users by study location
| Baltimore | Providence | Boston | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N = 169 | N = 78 | N = 79 | |||||||
| AOR | 95% CI | p | AOR | 95% CI | p | AOR | 95% CI | p | |
| Age (in decades) | 1.24 | 0.82, 1.88 | 0.301 | 1.95 | 1.06, 3.59 | 0.032 | 0.64 | 0.3, 1.34 | 0.233 |
| Gender | |||||||||
| Male | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | ||||||
| Female | 0.90 | 0.39, 2.08 | 0.798 | 2.55 | 0.81, 8.01 | 0.109 | 0.98 | 0.24, 3.95 | 0.977 |
| Race/ethnicity | |||||||||
| Black, Hispanic, other | 1.68 | 0.52, 5.39 | 0.384 | 1.10 | 0.38, 3.2 | 0.859 | 2.97 | 0.67, 13.13 | 0.15 |
| White | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | ||||||
| Arrest, past year | – | – | – | – | – | – | 0.56 | 0.1, 3.02 | 0.498 |
| Rushed drug purchase/use due to policing | – | – | – | 1.99 | 0.58, 6.83 | 0.272 | – | – | – |
| Injection drug use# | 3.86 | 1.53, 9.72 | 0.004 | – | – | – | |||
| Crack use# | – | – | – | 2.96 | 1, 8.73 | 0.049 | – | – | – |
| Non-medical benzodiazepine use# | 3.78 | 1.33, 10.72 | 0.012 | – | – | – | – | – | – |
| Overdose# | – | – | – | – | – | – | – | – | – |
| Thought drugs contained fentanyl | 0.89 | 0.34, 2.33 | 0.814 | – | – | – | – | – | – |
| Prefer drugs that contain fentanyl | 1.75 | 0.53, 5.76 | 0.359 | – | – | – | – | – | – |
| Usual drug use setting& | |||||||||
| Private | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | ||||||
| Public and semi-public | 3.19 | 1.37, 7.39 | 0.007 | – | – | ||||
p < 0.05 denoted in italics
#Past 6 months
&Past 30 days
Anticipated Barriers to Accessing a SCS
Lastly, we asked participants on potential barriers that “may make it difficult” for them to access a SCS. Seventy-five participants (23%) identified no barriers (i.e., responded “none”); responses among the 251 PWUD who did identify potential barriers are shown in Table 6. Participants most commonly cited concerns about arrest as their chief barrier (38%), followed by issues of privacy (34%), as well as concerns about confidentiality, trust, or safety (24%). Logistical considerations (such as the cost and time on transportation or wait times) were reported by 16% of the sample. Smaller proportions cited a lack of interest (6%) and staffing concerns such as judgment, forced counseling, or disliking the staff (6%), or “dope sickness” (2%). Other barriers that did not fall into any of the above categories were reported by 4% of respondents, while 5% responded “do not know.” There were two significant city-specific differences: confidentiality/trust/safety was a higher concern among PWUD in Boston (p = 0.006), whereas logistical considerations such as cost and time of transportation and wait times were more prominent among PWUD in Baltimore (p = 0.002).
Table 6.
Anticipated barriers to accessing a safe consumption space among adult opioid users in Baltimore, Providence, and Boston (n = 251)
| Response (Select all that apply) | All sites n = 251 |
Baltimore n = 133 |
Providence n = 60 |
Boston n = 58 |
p |
|---|---|---|---|---|---|
| n (col%) | n (col%) | n (col%) | n (col%) | ||
| Concerns about arrest | 95 (37.9) | 46 (34.6) | 21 (35.0) | 28 (48.3) | 0.175 |
| Privacy | 85 (33.1) | 47 (35.3) | 22 (36.7) | 16 (27.6) | 0.507 |
| Confidentiality/trust/safety | 61 (24.3) | 28 (21.1) | 10 (17.7) | 23 (39.7) | 0.006 |
| Logistical considerations: cost and time of transportation, wait times | 41 (16.3) | 32 (24.1) | 5 (8.3) | 4 (6.9) | 0.002 |
| Lack of interest | 15 (6.0) | 9 (6.8) | 4 (6.7) | 2 (3.5) | |
| Judgment, forced counseling, disliking staff | 14 (5.6) | 8 (6.0) | 6 (10.2) | 0 (0.0) | 0.055 |
| “Dope sickness”/drug withdrawal | 6 (2.3) | 5 (3.8) | 1 (1.7) | 0 (0.0) | 0.269 |
| Other barriers | 11 (4.3) | 5 (3.8) | 3 (5.0) | 3 (5.2) | 0.876 |
| Do not know | 13 (5.2) | 6 (4.5) | 2 (3.3) | 5 (8.6) | 0.380 |
p < 0.05 denoted in italics
Discussion
This study examined willingness to use SCS among a diverse population of PWUD at high risk of opioid overdose from three urban areas of the US northeast. SCS are a life-saving and cost-effective structural intervention used in more than 11 countries to reduce the burden of fatal overdose, distribute naloxone, and increase access to a range of health and social services to PWUD. These data demonstrate high willingness to use SCS among PWUD indicating their potential for engaging PWUD and intervening on their risk environment to help avert the most extreme consequences of the opioid epidemic. While SCS are not legally sanctioned in the USA, a number of cities are advocating for their implementation [14, 27]. Our study adds to the nascent US-based literature and could be useful in informing future efforts around SCS implementation. Factors associated with higher SCS willingness among both injectors and non-injectors were reliance on public/semi-public spaces to use drugs driven strongly by the Baltimore sample; in contrast, race/ethnicity, arrest, perceptions of fentanyl, and overdose experiences held specific associations by injection status. The correlates of SCS willingness when the analysis was stratified by city differed, though the estimates were unstable due to the modest sample size in each location. Taken together, our findings show the opportunities that SCS provide in reaching the most structurally vulnerable PWUD at high risk of opioid overdose, particularly due to fentanyl, around the country. These data also highlight that geospatial differences in drug-related risk and uptake of harm reduction services can be anticipated.
The majority of PWUD relied on public/semi-public settings to use drugs, and in Baltimore, this group demonstrated higher willingness to use SCS compared with those who used in private settings, corroborating previous research [14]. Our data supports the potential of SCS in reducing the risks associated with public drug use and improper disposal of drug paraphernalia [28, 29]. The risks associated with public injecting are vast and include higher risk of overdose, rushed drug preparation/use due to fear of police harassment, and risky injection practices [13, 26]. Furthermore, the impacts of highly visible public drug use practices can be far-reaching in the wider community and magnify stigma towards PWUD. The capacity of SCS to reduce public drug use and associated harms for PWUD, and confer positive externalities for their broader community is a key strength of the intervention and has been documented in settings where SCS are well established [4]. Our data suggest that similar gains could be expected in the US setting. Support for SCS existed among non-injectors in our study, many of whom were racial minority, reported overdose risk factors, and relied on public/semi-public settings to use drugs. There were no associations between recent overdose and SCS willingness by city. However, we observed lower SCS willingness among non-injectors who had experienced overdose in the pooled analysis. Given the small number of non-injectors in Providence (n = 5) and Boston (n = 21), this finding requires reexamination in a larger and more representative cohort of PWUD. The SCS willingness gap between injectors and non-injectors in Baltimore was particularly striking and may reflect the historical lack of programmatic engagement of non-injectors—an underserved PWUD population. Fewer studies have assessed SCS willingness among non-injectors, but their clear risk of overdose underscores the imperative to consider them in programmatic and policy decisions around SCS [30]. As observed in the current study and elsewhere, non-injectors appear to report lower interest in SCS/SIF [4], demonstrating the importance of targeted outreach and being attentive in messaging. Given the high co-occurrence of homelessness and reliance on public drug use settings among PWUD [25, 31, 32], settings with high levels of both conditions may also consider broader structural interventions such as Housing First programs, which have been shown to reduce alcohol use [33, 34].
Fentanyl has substantially altered the overdose landscape in North America and in many settings worldwide. Three-quarters of our study sample perceived that their drugs contained fentanyl, which corroborates the pervasiveness of fentanyl observed in these drug markets, as well as epidemiologic trends in overdose deaths demonstrating that fentanyl dominates drug-related deaths [30, 35]. The potential lethality of fentanyl coupled with the high prevalence of using drugs alone (i.e., without the presence of a bystander to revive the person with naloxone during an overdose event, reported by half of our sample) renders this specific population of PWUD highly vulnerable to overdose. Perceptions around fentanyl held significant associations with willingness to use SCS; the odds of willingness was almost two times higher among PWID who thought their drugs contained fentanyl, which echoes recent findings of increased fear of overdose among PWUD observed by service providers [15]. However, the relationships did not remain significant when the analysis was disaggregated by study location; these findings require further research. Given the uncertainties around the composition of drugs purchased through non-medical avenues, there is growing interest in implementing drug checking programs to help PWUD understand the contents of their drugs. Placing drug checking programs within SCS may be a useful public health approach to the growing crisis. Non-injectors who preferred fentanyl had an almost threefold higher odds of willingness to use a SCS; this suggests that SCS interventions may be able to engage this highly vulnerable subset of PWUD.
The most common barrier to accessing a SCS anticipated by PWUD were concerns of being arrested. We also observed dramatically lower willingness to use a SCS among PWID in our study who had recently been arrested, though these associations were not significant when examined separately by study location. Relatedly, one in three PWUD was concerned about maintaining privacy and confidentiality. Drug law enforcement is a major structural determinant shaping the health and risk environment of PWID [21, 36]. Arrest and incarceration are well-documented risk factors for reduced frequenting of health services, heightened overdose and HIV risk, and perpetuating the social and structural marginalization of PWUD [26, 37–39]. The criminalization of drug use and subsequent policing strategies also consistently target racial minorities, often at the expense of their constitutional rights [40–42]. Decriminalization of drug possession would provide PWUD the highest level of legal protection against harmful police encounters, harassment, and arrest around SCS locations. Multiple calls to decriminalize non-violent drug offenses have been made by national (e.g., Law Enforcement Action Partnership, Drug Police Alliance) and international (e.g., World Health Organization, United Nations) organizations, as well as many experts in the field [43]. In the interim, key issues to consider include providing legal protections from being arrested while on site, which may be achieved through agreements with local police or through the placement of SCS within Law Enforcement Assisted Diversion (LEAD) areas. Protecting the anonymity of PWUD at SCS locations (e.g., rejecting models requiring identification cards and the use of video cameras) will also be critical for the success of SCS [12]. These data also inform the range of health needs among PWUD that could be met through the implementation of SCS. In addition to SSP and injection-related services that are commonly implemented [4], SCS could provide overdose training and naloxone distribution, drug-checking services and FTS distribution, and drug treatment in a low-threshold way that is acceptable to clients. Comprehensive and integrated models of SCS/SIF exist elsewhere [3, 27, 44, 45]. Our data highlight that paying attention to privacy, confidentiality, trust, and safety will be required to garner engagement in such services.
There are several limitations to consider when interpreting these findings. Owing to our recruitment methods, non-injectors were underrepresented in Providence and Boston. The city-specific multivariate analyses were underpowered, particularly among Providence and Boston samples, and should be interpreted with caution; however, this analysis adds to the small number of US-based studies on this topic. Other contextual differences such as local awareness building efforts around SCS could have impacted the levels of SCS interest between cities. The cross-sectional design of this study does not allow assessment of temporality. Survey data may be subject to social desirability and recall bias. Given high rates of overdose in the recruitment cities, the data may be subject to survivor bias.
The opioid epidemic has taken a devastating toll in the USA and elsewhere. The current crisis, largely driven by fentanyl, warrants bold and pragmatic solutions. Our study demonstrates high SCS willingness among PWUD at elevated risk of opioid overdose, including those with a preference for drugs containing fentanyl. Several US cities are in the process of implementing SCS to help curb the epidemic, providing opportunities for novel research. In order to successfully implement this life-saving and cost-effective intervention in the US context, significant outreach efforts will be required to engage subpopulations of PWUD, including non-injectors and individuals with a history of arrest, in concert with access to drug treatment, harm reduction programming, and criminal justice reform.
Acknowledgments
This work was supported by the Bloomberg American Health Initiative. T.C. Green is also supported by the COBRE on Opioids and Overdose funded by the National Institutes of Health (P20GM125507). S.T. Allen is also supported by the National Institutes of Health (K01DA046234). The funders had no role in study design, data collection or in analysis and interpretation of the results, and this paper does not necessarily reflect the views or opinions of the funding agencies. Dr. Sherman is an expert witness for plaintiffs in opioid litigation. We are grateful to the FORECAST study team, collaborators, and study participants. We also thank Rajani Gudlavalleti and Natanya Robinowitz for providing a locally appropriate definition of a safe consumption space.
Author Contributions
S.G. Sherman and T.C. Green conceived and supervised the parent study. J.N. Park and M. McKenzie managed the study implementation. J.N. Park and S. Rouhani completed the analyses with input from K. Morales, T.C. Green, B.D.L. Marshall and S.G. Sherman. J. N. Park led the writing with support from S. Rouhani and K.B. Morales. S.T. Allen provided analysis support for generating the sampling frame. B.D.L. Marshall contributed to survey development. All authors provided critical revisions and approved the final manuscript.
Compliance with Ethical Standards
The study was approved by the Johns Hopkins School of Public Health (#00000287) and the Rhode Island Hospital Institutional Review Boards (#1062206).
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and opioid-involved overdose deaths - United States, 2013-2017. MMWR Morb Mortal Wkly Rep. 2018;67(5152):1419–1427. doi: 10.15585/mmwr.mm675152e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drug Enforcement Administration . National Drug Threat Assessment. Washington D.C.: U.S. Department of Justice; 2017. [Google Scholar]
- 3.Sherman SG, Hunter K, Rouhani S. Safe drug consumption spaces: a strategy for Baltimore City. Baltimore, Maryland: Abell Foundation; 2017. [Google Scholar]
- 4.Potier C, Laprevote V, Dubois-Arber F, Cottencin O, Rolland B. Supervised injection services: what has been demonstrated? A systematic literature review. Drug Alcohol Depend. 2014;145:48–68. doi: 10.1016/j.drugalcdep.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 5.Barry CL, Sherman SG, McGinty EE. Language matters in combatting the opioid epidemic: safe consumption sites versus overdose prevention sites. Am J Public Health. 2018;108(9):1157–1159. doi: 10.2105/AJPH.2018.304588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marshall BD, Milloy MJ, Wood E, Montaner JS, Kerr T. Reduction in overdose mortality after the opening of North America’s first medically supervised safer injecting facility: a retrospective population-based study. Lancet. 2011;377(9775):1429–1437. doi: 10.1016/S0140-6736(10)62353-7. [DOI] [PubMed] [Google Scholar]
- 7.DeBeck K, Kerr T, Bird L, Zhang R, Marsh D, Tyndall M, Montaner J, Wood E. Injection drug use cessation and use of North America’s first medically supervised safer injecting facility. Drug Alcohol Depend. 2011;113(2–3):172–176. doi: 10.1016/j.drugalcdep.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vipler S, Hayashi K, Milloy MJ, Wood E, Nosova E, Kerr T, Ti L. Use of withdrawal management services among people who use illicit drugs in Vancouver, Canada. Subst Abuse Treat Prev Policy. 2018;13(1):27. doi: 10.1186/s13011-018-0164-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Irwin A, Jozaghi E, Bluthenthal RN, Kral AH. A cost-benefit analysis of a potential supervised injection facility in San Francisco, California, USA. J Drug Issues. 0(0):0022042616679829.
- 10.Irwin A, Jozaghi E, Weir BW, Allen ST, Lindsay A, Sherman SG. Mitigating the heroin crisis in Baltimore, MD, USA: a cost-benefit analysis of a hypothetical supervised injection facility. Harm Reduct J. 2017;14(1):29. doi: 10.1186/s12954-017-0153-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mars SG, Ondocsin J, Ciccarone D. Toots, tastes and tester shots: user accounts of drug sampling methods for gauging heroin potency. Harm Reduct J. 2018;15(1):26. doi: 10.1186/s12954-018-0232-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kral AH, Wenger L, Carpenter L, Wood E, Kerr T, Bourgois P. Acceptability of a safer injection facility among injection drug users in San Francisco. Drug Alcohol Depend. 2010;110(1–2):160–163. doi: 10.1016/j.drugalcdep.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bouvier BA, Elston B, Hadland SE, Green TC, Marshall BD. Willingness to use a supervised injection facility among young adults who use prescription opioids non-medically: a cross-sectional study. Harm Reduct J. 2017;14(1):13. doi: 10.1186/s12954-017-0139-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leon C, Cardoso L, Mackin S, Bock B, Gaeta JM. The willingness of people who inject drugs in Boston to use a supervised injection facility. Subst Abus. 2018;39(1):95–101. doi: 10.1080/08897077.2017.1365804. [DOI] [PubMed] [Google Scholar]
- 15.Glick JL, Christensen T, Park JN, McKenzie M, Green TC, Sherman SG. Stakeholder perspectives on implementing fentanyl drug checking - results from a multi-site study. Accepted in Drug and Alcohol Dependence. Drug and Alcohol Dependence. In press. 194:527–532. [DOI] [PubMed]
- 16.Sherman SG, Morales K, Park JN, McKenzie M, Marshall BDL, Green TC. Feasibility of implementing community-based drug checking services for people who use drugs in three United States cities: Baltimore, Boston and Providence. Int J Drug Policy. 2019;68:46–53. doi: 10.1016/j.drugpo.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Sherman SG, Park JN, Glick J, et al. FORECAST study summary report: Johns Hopkins Bloomberg School of Public Health: Baltimore; 2018.
- 18.Green TC, Park JN, Gilbert M, et al. A multi-site assessment of the limits of detection, sensitivity and specificity of three devices for public health-based drug checking of fentanyl in street-acquired samples. Under review. [DOI] [PubMed]
- 19.SIFMA NOW. Available at: http://www.sifmanow.org/. Accessed 11/01/2018.
- 20.Allen ST, Footer KHA, Galai N, Park JN, Silberzahn B, Sherman SG. Implementing targeted sampling: lessons learned from recruiting female sex workers in Baltimore, MD. J Urban Health. 2018. [DOI] [PMC free article] [PubMed]
- 21.Rhodes T, Singer M, Bourgois P, Friedman SR, Strathdee SA. The social structural production of HIV risk among injecting drug users. Soc Sci Med. 2005;61(5):1026–1044. doi: 10.1016/j.socscimed.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 22.Dasgupta N, Beletsky L, Ciccarone D. Opioid crisis: no easy fix to its social and economic determinants. Am J Public Health. 2018;108(2):182–186. doi: 10.2105/AJPH.2017.304187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ciccarone D. Fentanyl in the US heroin supply: a rapidly changing risk environment. Int J Drug Policy. 2017;46:107–111. doi: 10.1016/j.drugpo.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Macmadu A, Carroll JJ, Hadland SE, Green TC, Marshall BD. Prevalence and correlates of fentanyl-contaminated heroin exposure among young adults who use prescription opioids non-medically. Addict Behav. 2017;68:35–38. doi: 10.1016/j.addbeh.2017.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park JN, Weir BW, Allen ST, Chaulk P, Sherman SG. Fentanyl-contaminated drugs and non-fatal overdose among people who inject drugs in Baltimore, MD. Harm Reduct J. 2018;15(1):34. doi: 10.1186/s12954-018-0240-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hunter K, Park JN, Allen ST, Chaulk P, Frost T, Weir BW, Sherman SG. Safe and unsafe spaces: non-fatal overdose, arrest, and receptive syringe sharing among people who inject drugs in public and semi-public spaces in Baltimore City. Int J Drug Policy. 2018;57:25–31. doi: 10.1016/j.drugpo.2018.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kral AH, Davidson PJ. Addressing the nation’s opioid epidemic: lessons from an unsanctioned supervised injection site in the U.S. Am J Prev Med. 2017;53(6):919–922. doi: 10.1016/j.amepre.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 28.Wood E, Kerr T, Small W, Li K, Marsh DC, Montaner JS, Tyndall MW. Changes in public order after the opening of a medically supervised safer injecting facility for illicit injection drug users. CMAJ. 2004;171(7):731–734. doi: 10.1503/cmaj.1040774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stoltz JA, Wood E, Small W, Li K, Tyndall M, Montaner J, Kerr T. Changes in injecting practices associated with the use of a medically supervised safer injection facility. J Public Health (Oxf) 2007;29(1):35–39. doi: 10.1093/pubmed/fdl090. [DOI] [PubMed] [Google Scholar]
- 30.Mattson CL, O’Donnell J, Kariisa M, Seth P, Scholl L, Gladden RM. Opportunities to prevent overdose deaths involving prescription and illicit opioids, 11 states, July 2016-June 2017. MMWR Morb Mortal Wkly Rep. 2018;67(34):945–951. doi: 10.15585/mmwr.mm6734a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krieger MS, Yedinak JL, Buxton JA, Lysyshyn M, Bernstein E, Rich JD, Green TC, Hadland SE, Marshall BDL. High willingness to use rapid fentanyl test strips among young adults who use drugs. Harm Reduct J. 2018;15(1):7. doi: 10.1186/s12954-018-0213-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sutter A, Curtis M, Frost T. Public drug use in eight U.S. cities: health risks and other factors associated with place of drug use. Int J Drug Policy. 2019;64:62–69. doi: 10.1016/j.drugpo.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 33.Collins SE, Malone DK, Clifasefi SL, Ginzler JA, Garner MD, Burlingham B, Lonczak HS, Dana EA, Kirouac M, Tanzer K, Hobson WG, Marlatt GA, Larimer ME. Project-based Housing First for chronically homeless individuals with alcohol problems: within-subjects analyses of 2-year alcohol trajectories. Am J Public Health. 2012;102(3):511–519. doi: 10.2105/AJPH.2011.300403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsai J, Mares AS, Rosenheck RA. A multi-site comparison of supported housing for chronically homeless adults: “housing first” versus “residential treatment first”. Psychol Serv. 2010;7(4):219–232. doi: 10.1037/a0020460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Donnell JK, Halpin J, Mattson CL, Goldberger BA, Gladden RM. Deaths involving fentanyl, fentanyl analogs, and U-47700 - 10 states, July-December 2016. MMWR Morb Mortal Wkly Rep. 2017;66(43):1197–1202. doi: 10.15585/mmwr.mm6643e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burris S, Blankenship KM, Donoghoe M, et al. Addressing the “risk environment” for injection drug users: the mysterious case of the missing cop. Milbank Q. 2004;82(1):125–156. doi: 10.1111/j.0887-378X.2004.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Binswanger IA, Stern MF, Deyo RA, Heagerty PJ, Cheadle A, Elmore JG, Koepsell TD. Release from prison--a high risk of death for former inmates. N Engl J Med. 2007;356(2):157–165. doi: 10.1056/NEJMsa064115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beletsky L, Cochrane J, Sawyer AL, et al. Police encounters among needle exchange clients in Baltimore: drug law enforcement as a structural determinant of health. 20150808 DCOM- 20151030 (1541–0048 (Electronic)). 2015;105(9):1872–9. [DOI] [PMC free article] [PubMed]
- 39.Beletsky L, Grau LE, White E, Bowman S, Heimer R. The roles of law, client race and program visibility in shaping police interference with the operation of US syringe exchange programs. Addiction. 2011;106(2):357–365. doi: 10.1111/j.1360-0443.2010.03149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alexander M. The New Jim Crow: Mass Incarceration in the Age of Colorblindness. New York, NY: The New Press; 2012.
- 41.Cooper HL. War on drugs policing and police brutality. Subst Use Misuse. 2015;50(8–9):1188–1194. doi: 10.3109/10826084.2015.1007669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cooper HL, Fullilove M. Editorial: excessive police violence as a public health issue. J Urban Health. 2016;93(Suppl 1):1–7. doi: 10.1007/s11524-016-0040-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Csete J, Kamarulzaman A, Kazatchkine M, Altice F, Balicki M, Buxton J, Cepeda J, Comfort M, Goosby E, Goulão J, Hart C, Kerr T, Lajous AM, Lewis S, Martin N, Mejía D, Camacho A, Mathieson D, Obot I, Ogunrombi A, Sherman S, Stone J, Vallath N, Vickerman P, Zábranský T, Beyrer C. Public health and international drug policy. Lancet. 2016;387(10026):1427–1480. doi: 10.1016/S0140-6736(16)00619-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kerr T, MacPherson D, Wood E. Establishing North America’s first safer injection facility: lessons from the Vancouver experience. In: Stevens A, editor. Crossing Frontiers: International Developments in the Treatment of Drug Dependence. Brighton: Pavilion Publishing; 2008. [Google Scholar]
- 45.Anoro M, Ilundain E, Santisteban O. Barcelona’s safer injection facility-EVA: a harm reduction program lacking official support. J Drug Issues. 2003;33(3):689–711. doi: 10.1177/002204260303300309. [DOI] [Google Scholar]